Abstract
The role of 5-HT2 and opioid receptors in pudendal inhibition of bladder activity induced by intravesical infusion of saline or 0.25% acetic acid (AA) was investigated in anesthetized cats using methysergide (a 5-HT2 receptor antagonist) and naloxone (an opioid receptor antagonist). AA irritated the bladder and significantly (P<0.0001) reduced bladder capacity to 27.0±7.4% of saline control capacity. Pudendal nerve stimulation (PNS) at multiples of the threshold (T) intensity for inducing anal sphincter twitching restored bladder capacity to 60.1± 8.0% at 1–2T (P<0.0001) and 92.2±14.1% at 3–4T (P=0.001) of the saline control capacity. Methysergide (0.03–1 mg/kg, i.v.) suppressed low intensity (1–2T) PNS inhibition but not high intensity (3–4T) inhibition, and also significantly (P<0.05) increased control bladder capacity at the dosage of 0.3–1 mg/kg. During saline infusion without AA irritation, PNS significantly increased bladder capacity to 150.8±9.9% at 1–2T (P<0.01) and 180.4±16.6% at 3–4T (P<0.01) of the saline control capacity. Methysergide (0.1–1 mg/kg) significantly (P<0.05) increased saline control bladder capacity and suppressed PNS inhibition at the dosage of 0.03–1 mg/kg. After methysergide treatment (1 mg/kg), naloxone significantly (P<0.05) reduced control bladder capacity during AA infusion but had no effect during saline infusion. Naloxone also had no influence on PNS inhibition. These results suggest that 5-HT2 receptors play a role in PNS inhibition of reflex bladder activity and interact with opioid receptors in micturition reflex pathway. Understanding neurotransmitter mechanisms underlying pudendal neuromodulation is important for the development of new treatments for bladder disorders.
Keywords: 5-HT2 receptor, opioid receptor, pudendal, neuromodulation, cat
Introduction
Overactive bladder (OAB) is a symptom complex of urinary urgency, frequency and incontinence that can be treated by pudendal nerve or sacral neuromodulation (Peters et al., 2005, 2010; van Kerrebroek et al., 2007). Pudendal neuromodulation has been reported to be superior to sacral neuromodulation in patients with intractable OAB or painful bladder syndrome (PBS) (Peters et al., 2002, 2005). However, the mechanisms underlying neuromodulation therapies are currently still un-known. Understanding the mechanisms could potentially improve the efficacy of these therapies or develop new treatments for bladder disorders (Andersson, 2004; Andersson and Wein, 2004).
Our studies in cats (Tai et al., 2012; Zhang et al., 2012) have identified an involvement of opioid receptors in tibial nerve stimulation-induced inhibition of nociceptive bladder overactivity caused by intravesical acetic acid (AA) irritation. Activation of opioid receptors also plays a minor role in pudendal nerve stimulation (PNS)-induced inhibition of non-nociceptive reflex bladder activity in cats caused by saline distention of the bladder (Chen et al., 2010); but does not contribute to PNS inhibition of nociceptive bladder overactivity (Mally et al., 2013). Thus, neurotransmitters other than endogenous opioid peptides must mediate PNS inhibition of reflex bladder activity.
Previous studies in various species (cats, rats, guinea pigs) have raised the possibility that 5-hydroxtryptamine (5-HT) may function as an inhibitory or excitatory transmitter that modulates the micturition reflex pathway in the brain and spinal cord (de Groat, 2002; Ramage, 2006; Cheng and de Groat 2010; Mbaki et al., 2012) and that it also has an important role in the regulation of nociceptive mechanisms in the central nervous system (Basbaum and Fields, 1984). Thus, we have conducted a pharmacological study to examine the contribution of 5-HT to the inhibition of nociceptive and non-nociceptive bladder reflexes induced by PNS. We have used methysergide, a non-selective 5-HT2 receptor antagonist, which in a rat model of somatic nociception was effective after intrathecal administration in significantly reducing the antihyperalgesic effect of transcutaneous electrical nerve stimulation or mechanical joint manipulation (Radhakrishnan et al., 2003; Skyba et al., 2003). These results indicated that descending serotonin (5-HT) inhibitory mechanisms might be involved in neuromodulation of somatic nociception in the spinal cord.
In addition, intrathecal administration of methysergide to cats enhanced the axonal firing in the lateral funiculus of T11–T12 spinal segments elicited by electrical stimulation of bladder afferent axons in the pelvic nerve (Espey et al., 1998). These observations suggested that methysergide-sensitive 5-HT receptors generate a tonic inhibitory modulation of the ascending limb of the spinobulbospinal micturition reflex pathway (Espey et al., 1998). This modulatory input arises in the brain stem raphe nuclei where electrical (McMahon and Spillane, 1982; Morrison and Spillane, 1986) or chemical stimulation (Chen et al., 1993) has been shown to inhibit bladder reflexes. Therefore, the present study was undertaken to determine if methysergide-sensitive 5-HT receptors might be involved in PNS inhibition of nociceptive or non-nociceptive bladder reflexes.
Intravesical infusion of diluted (0.25%) AA was used in this study to irritate the bladder, activate the nociceptive bladder C-fiber afferents, and induce bladder overactivity in α- chloralose anesthetized cats, while saline infusion was used to distend the bladder, activate the non-nociceptive bladder Aδ-fiber afferents, and induce normal reflex bladder activity (Fowler et al., 2008; Häbler et al., 1990). PNS was employed as the antinociceptive stimulus to model the clinical use of pudendal neuromodulation in treating OAB or painful bladder syndrome (PBS) (Peters et al., 2002, 2005). Methysergide and naloxone (an opioid receptor antagonist) were administered intravenously to determine the role of 5-HT and opioid receptor mechanisms in the neuromodulation. WAY100635 (a 5-HT1A receptor antagonist) was used to exclude the possibility that methysergide affects the bladder activity through activation of 5-HT1A receptors.
Materials and Methods
The Animal Care and Use Committee at the University of Pittsburgh approved all protocols involving the use of animals in this study.
Experimental Setup
Experiments were conducted in a total of 20 cats (11 male, 9 female, 3.0–4.4 kg, Liberty Research Inc., Waverly, NY, USA) anesthetized initially with isoflurane (2–3% in oxygen) and maintained with α-chloralose (65 mg/kg i.v. with supplementation as necessary). Heart rate and blood oxygen level were monitored by a pulse oximeter (9847V, NONIN Medical, Inc., Plymouth, MN, USA) with the sensor attached to the tongue. Systemic blood pressure was monitored via a catheter in the right carotid artery. Drug and fluid were administered via the right cephalic vein, and airway access was secured with a tracheostomy tube.
The ureters were isolated via an abdominal incision, cut, and drained externally. The bladder was cannulated through the urethra with a double lumen catheter. One lumen was used to infuse saline or 0.25% AA at a rate of 0.5–2 ml/min, and the other lumen was attached to a pressure transducer to record the bladder pressure. A ligature was tied around the urethra to prevent leakage. The pudendal nerve was dissected from the right side via a 3–4 cm incision between the tail and the sciatic notch. A tripolar cuff electrode (NC223pt, MicroProbe, Inc., Gaithersburg, MD, USA) was applied around the nerve and connected to a stimulator (S88, Grass Medical Instruments, Quincy, MA, USA).
Stimulation Protocol and Drug Administration
Based on our previous studies (Chen et al., 2010), uniphasic rectangular pulses (5 Hz frequency, 0.2 ms pulse width) were delivered to the pudendal nerve via the cuff electrode to inhibit bladder activity. The intensity threshold (T) for inducing anal sphincter twitching was determined by gradually increasing the stimulation intensity. Then, multiples (1–2 T or 3–4 T) of the threshold intensity were used in the pharmacological experiments.
Initially a cystometrogram (CMG) was performed with saline infusion to determine the control bladder capacity that was defined as the bladder volume threshold to induce a large amplitude (>30 cm H2O) and long duration (>20 sec) bladder contraction. Then, multiple saline CMGs were repeated to evaluate the reproducibility. Once the control bladder capacity was determined during saline infusion, pharmacological studies were performed in three experimental groups.
In the first experimental group (N=8 cats), 0.25% AA was infused into the bladder during repeated CMGs in order to activate nociceptive bladder C-fiber afferents and induce bladder overactivity. Four CMGs were performed during AA infusion: (1) control CMG without PNS, (2) CMG during 1–2T PNS, (3) CMG during 3–4T PNS, (4) control CMG without PNS to determine any post-stimulation effect (Fig.1A). Then, cumulative doses (0.01, 0.03, 0.1, 0.3, and 1 mg/kg, i.v.) of methysergide [1-Methyl-d-lysergic acid-(1-hydroxybut-2-yl) amide, Sigma-Aldrich Corp., St. Louis, MO, USA] were administered. To determine the dose response, the four CMGs (control, 1–2T, 3–4T, and control) were repeated starting 10 minutes after administering each dose of methysergide. Following the last dose of methysergide (1 mg/kg), naloxone (1 mg/kg, i.v, Sigma-Aldrich, St. Louis, MO, USA) was given to investigate the interaction between 5-HT2 and opioid receptors. Five minutes after administering naloxone, the four CMGs were performed again to examine the naloxone effect.
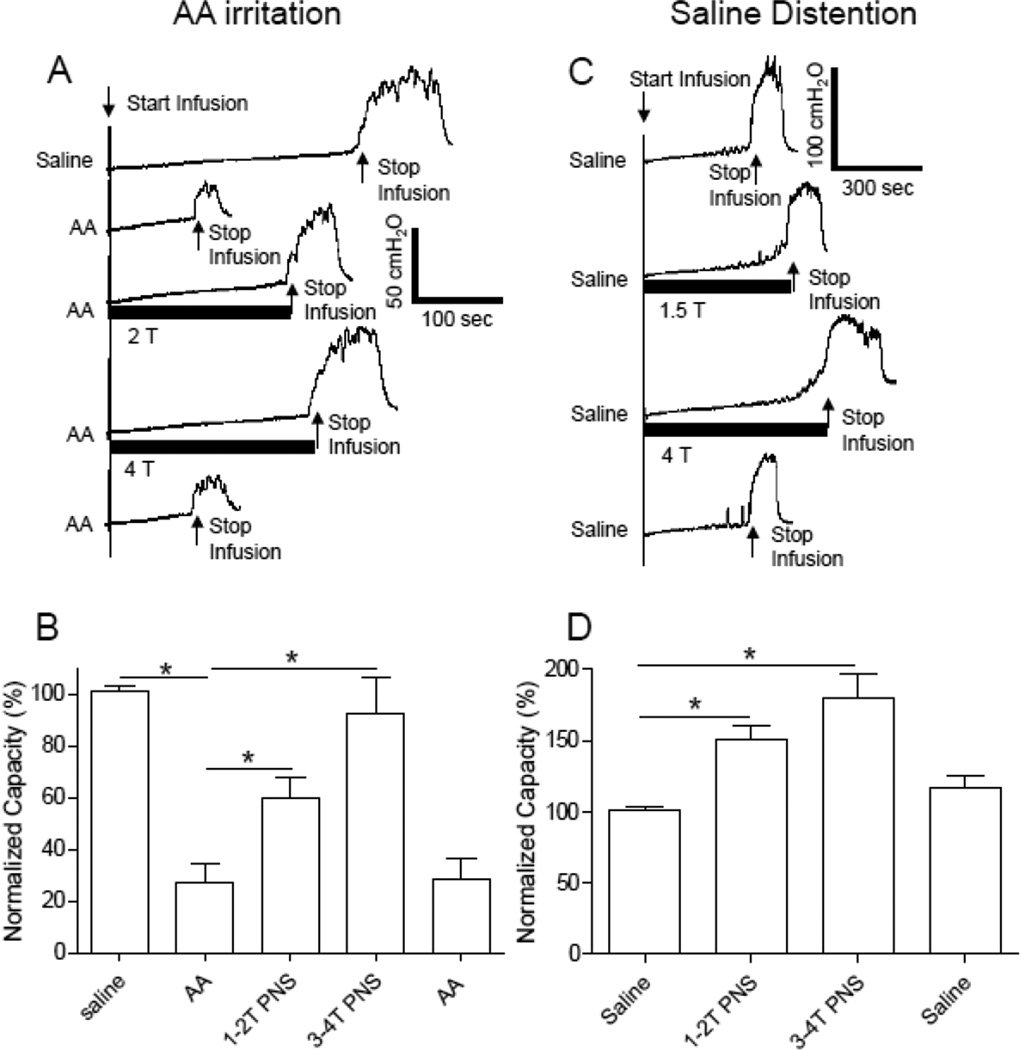
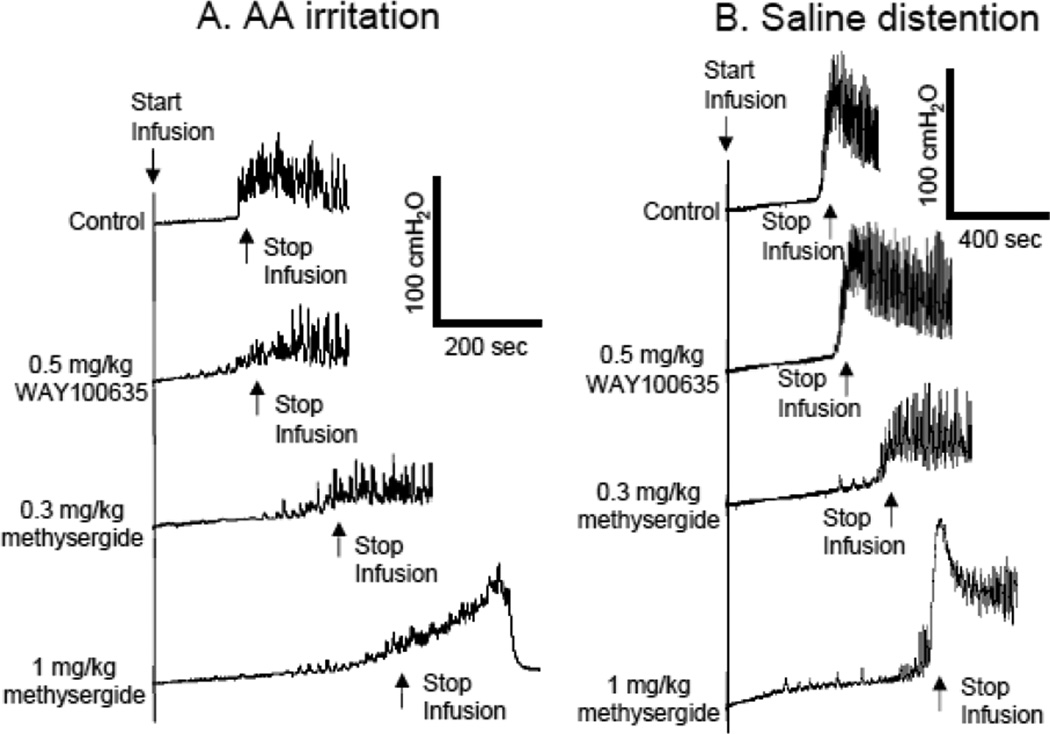
Figure 1.
Pudendal nerve stimulation (PNS) inhibited bladder overactivity caused by acetic acid (AA) irritation (A and B) and normal bladder activity induced by saline distention (C and D). PNS duration is indicated by the black bar under the bladder pressure trace. T – PNS threshold intensity for inducing anal sphincter twitching. A. Repeated CMGs during AA infusion (2 ml/min). PNS: 5 Hz, 0.2 ms, T = 1.2 V. B. Normalized bladder capacity during AA irritation (N=8 cats). C. Repeated CMGs during saline infusion (2 ml/min). PNS: 5 Hz, 0.2 ms, T = 1.2 V. D. Normalized bladder capacity during saline infusion (N=8 cats). * indicates a significant difference (P<0.05).
In the second experimental group of cats (N=8), repeated saline CMGs were continued without AA irritation. The drug testing protocol was same as the first experimental group, but was performed during saline instead of AA infusion.
In the third experimental group of cats (N=4), PNS was not applied. WAY100635 was administered in a dose (0.5 mg/kg, i.v.) to maximally antagonize the 5-HT1A receptors, followed by 2 cumulative doses (0.3 and 1 mg/kg, i.v.) of methysergide. A control CMG was performed after each dose of drug during either AA infusion (N=2 cats) or saline infusion (N=2 cats).
Data Analysis
For the repeated CMG recordings, bladder capacities were measured and normalized to the measurement of the first saline control CMG in the same animal so that the results from different animals could be compared. Repeated measurements in the same animal under the same experimental conditions were averaged. The results from different animals were reported as mean ± standard error (SE). Statistical significance (P<0.05) was detected by Student t-test, one-way ANOVA followed by Dunnett post-hoc test, or two-way ANOVA followed by Bonferroni post-hoc test.
Results
PNS inhibition of reflex bladder activity
Intravesical infusion of 0.25% AA irritated the bladder, activated nociceptive bladder C-fiber afferents, and significantly (P<0.0001) reduced bladder capacity to 27.0±7.4 % of the saline control capacity (11.2±2.2 ml, N=8 cats) (Fig.1 A and B). PNS at 1–2T or 3–4T intensity suppressed AA-induced bladder overactivity and significantly increased bladder capacity to 60.6±8.0% (P<0.0001) or 92.2±14.1% (P=0.001) of saline control capacity, respectively (Fig.1 A and B). After termination of the PNS, bladder capacity returned to 28.5±7.6 % of saline control capacity (Fig.1B). The bladder capacities were significantly different between saline control and 1–2T PNS but not between saline control and 2–4T PNS (Fig.1B).
During saline infusion CMGs without AA irritation, PNS significantly increased bladder capacity to 150.8±9.9% at 1–2T (P<0.01) and 180.4±16.6% at 3–4T (P<0.01) of saline control capacity (11.6±1.0 ml, N=8 cats) (Fig.1 C and D). After termination of the PNS, bladder capacity returned to 116.4±9.4% of saline control capacity (Fig.1D).
Dose-dependent effect of methysergide on PNS inhibition of reflex bladder activity
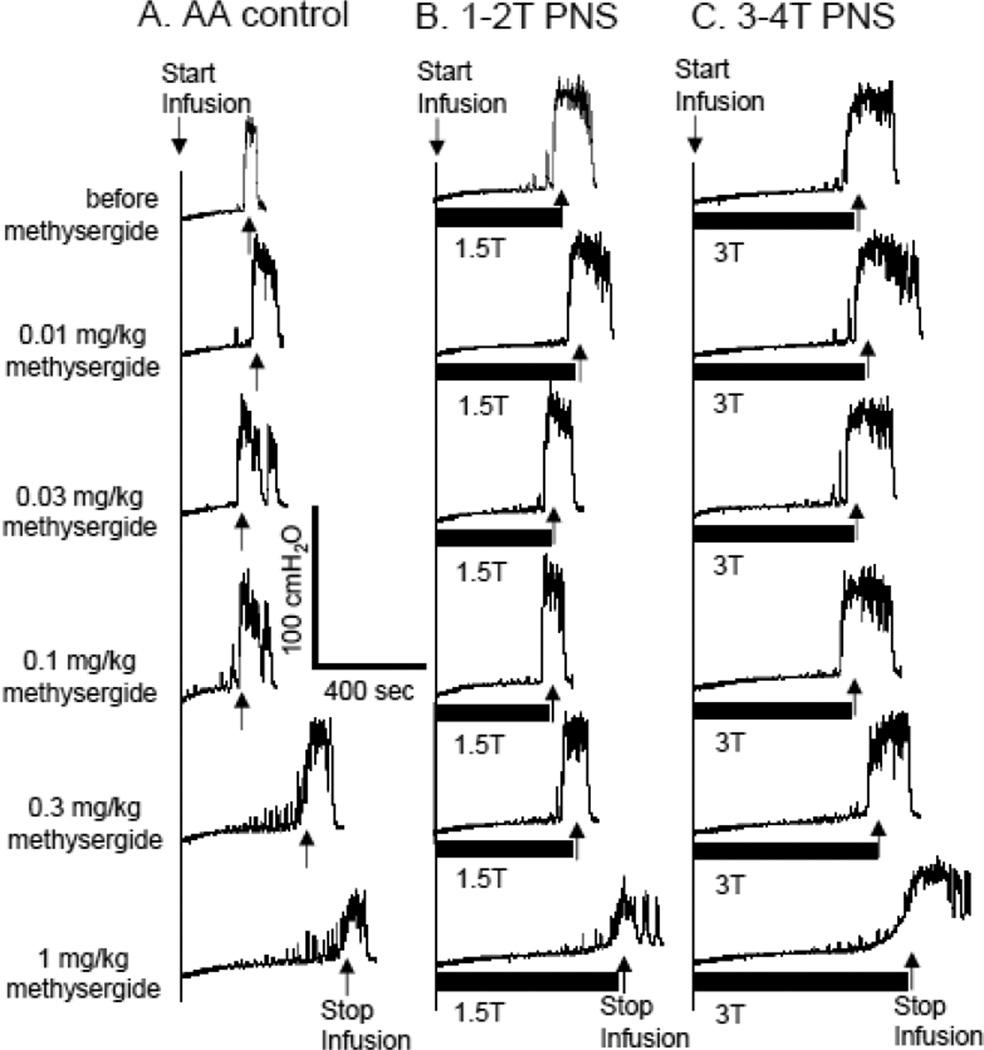
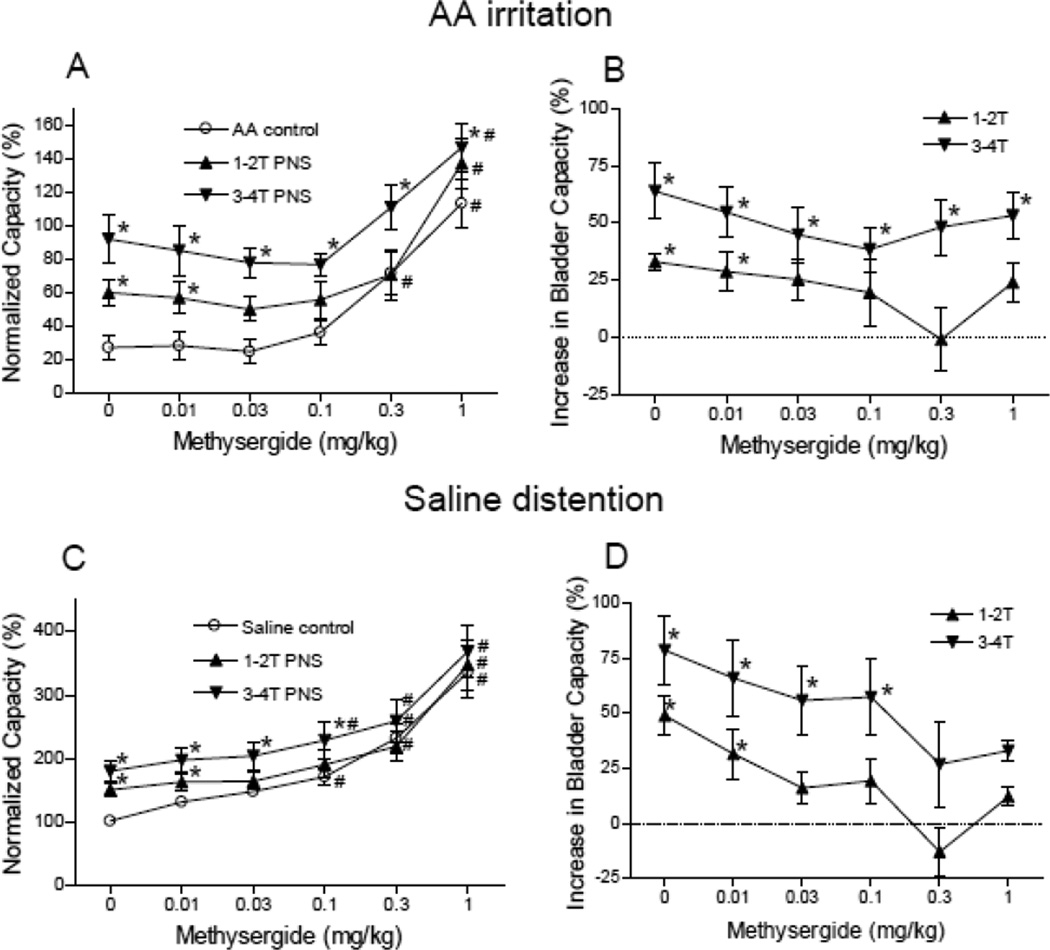
The effect of methysergide on PNS inhibition of reflex bladder activity is dependent on the drug dosage and the PNS intensity (Figs.2–4). During AA irritation, methysergide (0.3–1 mg/kg) significantly (P<0.05) increased bladder capacity in the absence of stimulation (Fig.2A and Fig.4A) and at doses of 0.03–1 mg/kg also significantly (P<0.05) suppressed the pudendal inhibition induced by low intensity (1–2T) PNS (Fig.4 A and B). However, the high intensity (3–4T) PNS could still significantly (P<0.05) increase bladder capacity even at the largest dosage (1 mg/kg) of methysergide (Fig.4 A and B).
Figure 2.
Dose dependent effect of methysergide on pudendal nerve stimulation (PNS)-induced inhibition of bladder overactivity caused by acetic acid (AA) irritation. The CMGs were performed in sequence from left to right in figures A–C and from top to bottom in each figure. PNS duration is indicated by black bar under bladder pressure trace. T – PNS threshold intensiy for inducing anal sphincter twitching. A. Control CMGs without PNS. B. CMGs during 1–2T PNS. C. CMGs during 3–4T PNS. PNS: 5 Hz, 0.2 ms, T = 0.5 V. Infusion rate = 1 ml/min.
Figure 4.
Summarized results of dose-dependent effect of methysergide on PNS inhibition. A–B. During acetic acid (AA) irritation (N= 8 cats). PNS: 5 Hz, 0.2 ms, T = 0.5–1.6 V. C–D. During saline distention (N= 8 cats). PNS: 5 Hz, 0.2 ms, T = 0.22–5 V. * indicates significantly (P<0.05) different from AA control (A and B) or saline control (C and D). # indicates significantly (P<0.05) different from the bladder capacity measured before methysergide treatment (i.e. at 0 mg/kg of methysergide) for each CMG condition. B and D show the increase in bladder capacity after subtracting the control bladder capacity at each dosage of methysergide.
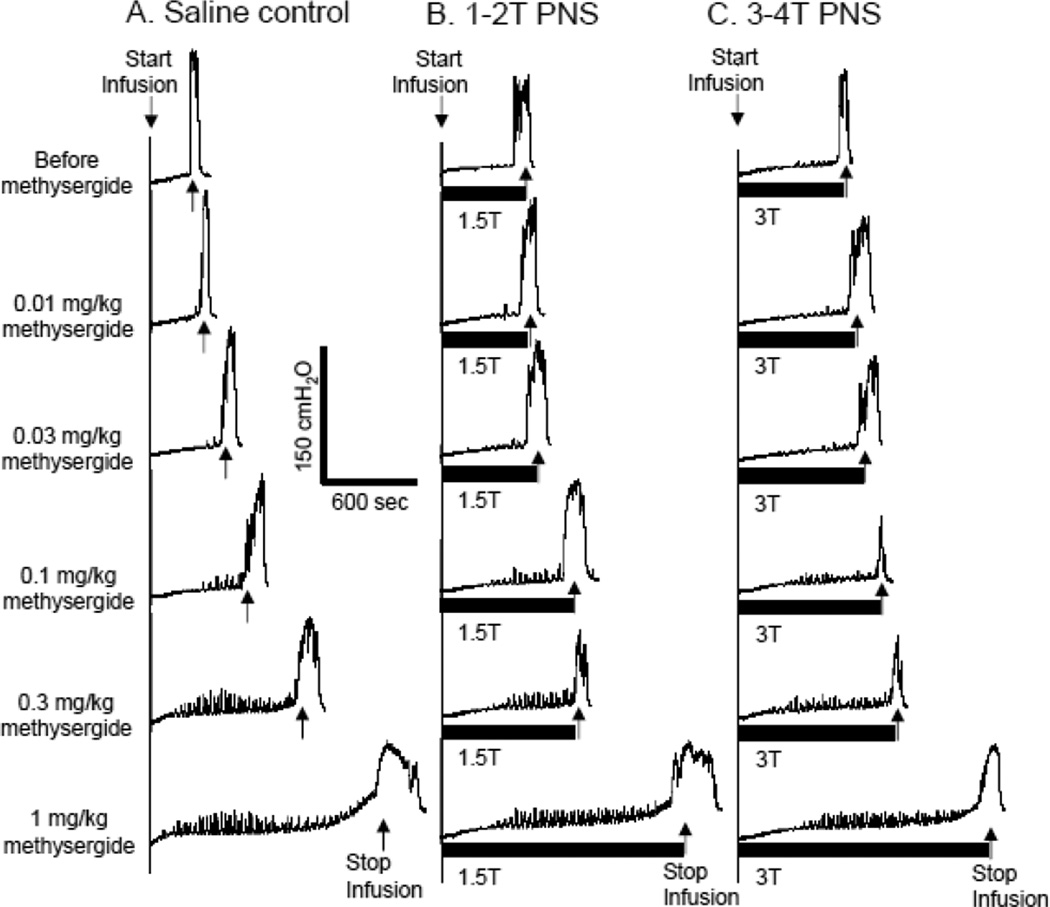
During saline infusion CMGs without AA irritation, methysergide (0.1–1 mg/kg) significantly (P<0.05) increased bladder capacity in the absence of stimulation (Fig.3A and Fig.4C) and also significantly (P<0.05) suppressed the pudendal inhibition induced by low intensity (1–2T) PNS at dosages of 0.03–1 mg/kg and the inhibition induced by high intensity (3–4T) PNS at dosages of 0.3–1 mg/kg (Fig.4 C and D).
Figure 3.
Dose dependent effect of methysergide on pudendal nerve stimulation (PNS)-induced inhibition of normal bladder activity induced by saline distention. The CMGs were performed in sequence from left to right in figures A–C and from top to bottom in each figure. PNS duration is indicated by black bar under bladder pressure trace. T – PNS threshold intensiy for inducing anal sphincter twitching. A. Control CMGs without PNS. B. CMGs during 1–2T PNS. C. CMGs during 3–4T PNS. PNS: 5 Hz, 0.2 ms, T = 0.6 V. Infusion rate = 2 ml/min.
Effect of WAY100635 on methysergide-induced inhibition of reflex bladder activity
Prior to methysergide treatment, WAY100635 in a dose (0.5 mg/kg, i.v.) that should maximally block 5-HT1A receptors (Tai et al., 2006) did not change bladder capacity during either AA infusion CMG (Fig.5A, N=2 cats) or saline infusion CMG (Fig.5B, N=2 cats). Following administration of WAY100636, methysergide (0.3–1 mg/kg) still inhibited reflex bladder activity and increased bladder capacity (100–200%) during either AA (Fig.5A) or saline infusion (Fig.5B).
Figure 5.
WAY100635 failed to block the bladder inhibition induced by methysergide treatment. A. CMGs during acetic acid (AA) infusion. B. CMGs during saline infusion. Infusion rate = 2 ml/min.
Effect of naloxone on reflex bladder activity and PNS inhibition after methysergide treatment
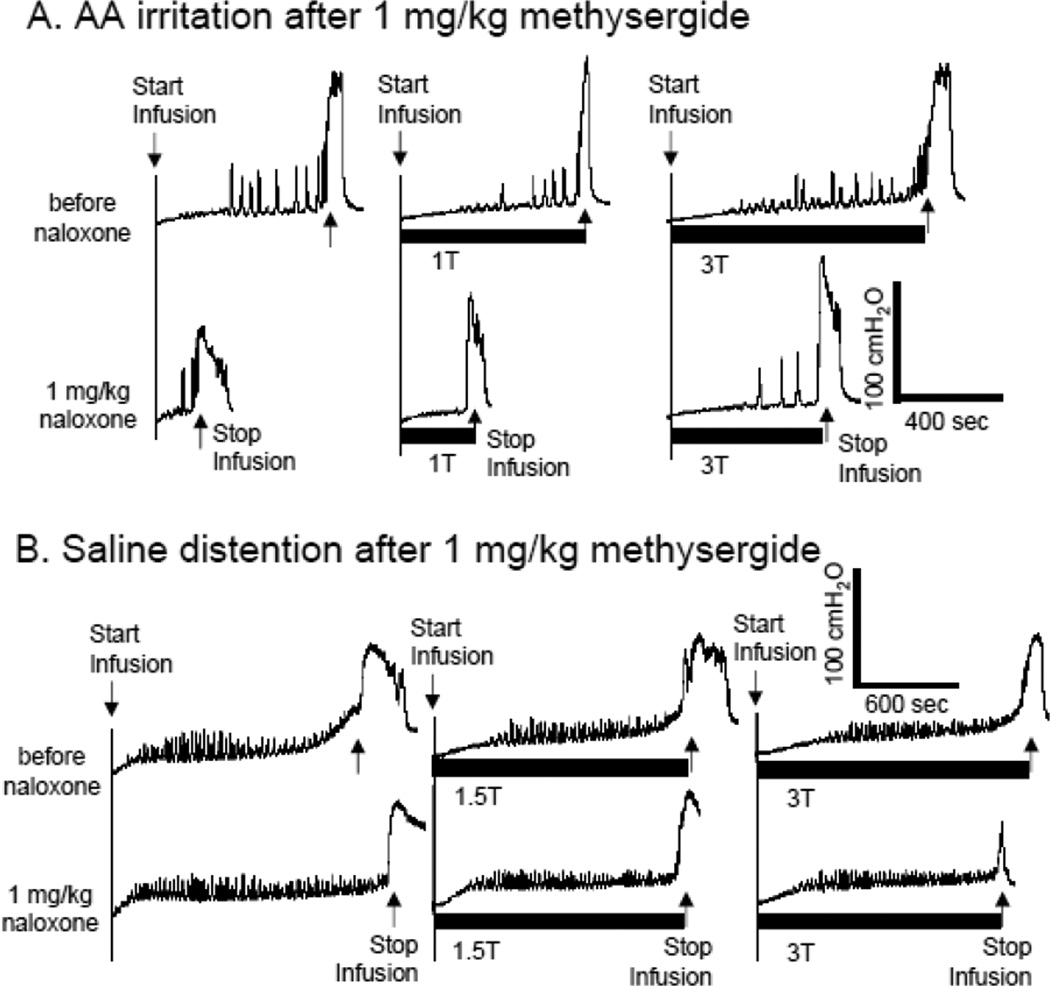
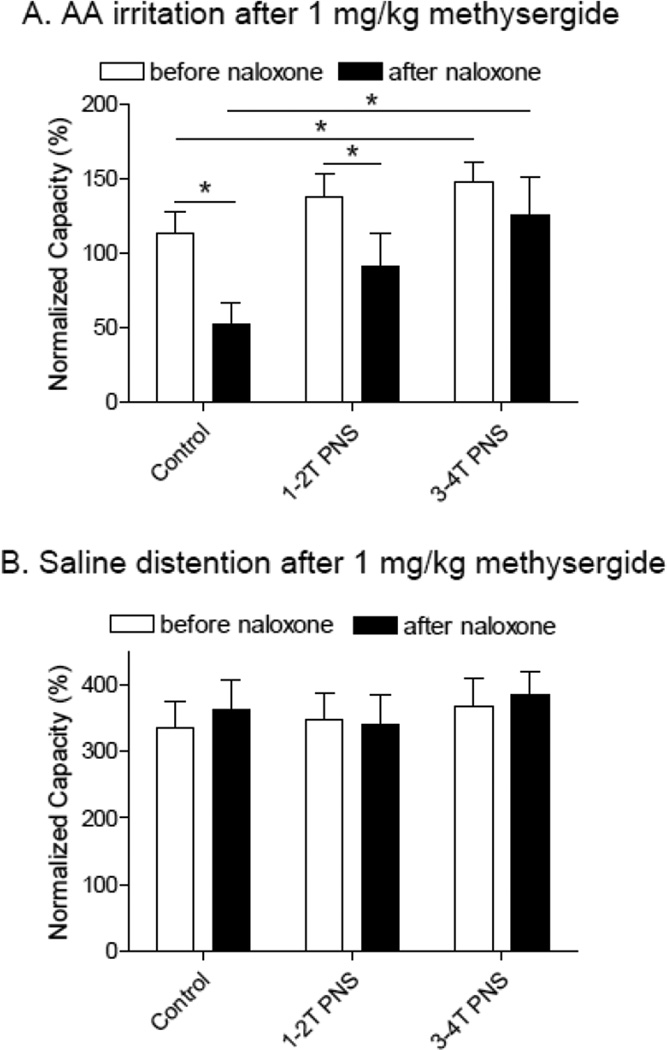
After the last dose (1 mg/kg) of methysergide, naloxone (1 mg/kg, i.v.) significantly (P<0.05) reduced bladder capacity from 116.8±16.5% to 52.5±14.2% of saline control capacity during AA irritation (Fig.6A and Fig.7A), but did not change the bladder capacity during saline infusion (Fig.6B and Fig.7B).
Figure 6.
Effect of naloxone on bladder activity and pudendal nerve stimulation (PNS)-induced inhibition after methysergide treatment. PNS duration is indicated by black bar under bladder pressure trace. T – PNS threshold intensity for inducing anal sphincter twitching. A. CMGs during acetic acid (AA) infusion. PNS: 5 Hz, 0.2 ms, T = 1.6 V. Infusion rate = 1 ml/min. B. CMGs during saline infusion. PNS: 5 Hz, 0.2 ms, T = 0.6 V. Infusion rate = 2 ml/min.
Figure 7.
Naloxone effect on bladder activity and pudendal inhibition after methysergide treatment. A. During acetic acid (AA) infusion (N= 7 cats). B. During saline infusion (N=8 cats). * indicates a significant difference (P<0.05).
Naloxone treatment did not influence PNS inhibition during AA irritation. After methysergide treatment, only high intensity PNS (3–4T) could significantly (P<0.05) increase bladder capacity and this effect was similar before and after naloxone treatment (Fig.6A and Fig.7A). During saline infusion, PNS was ineffective in methysergide treated cats before and after naloxone treatment (Fig.6B and Fig.7B).
Discussion
This study revealed that intravenous administration of methysergide, a non-selective 5-HT2 receptor antagonist, significantly increased bladder capacity during both AA and saline infusion CMGs (Figs.2A, 3A and 4), partially suppressed the PNS inhibition of AA irritation-induced bladder overactivity and completely suppressed PNS inhibition of saline distention-induced bladder activity (Figs.2–4). WAY100635, a selective 5-HT1A receptor antagonist, did not influence bladder capacity or alter the effects of methysergide (Fig.5). After methysergide treatment, naloxone did not influence PNS inhibition. However, naloxone significantly reduced bladder capacity during AA irritation but not during saline distention (Figs.6–7). Based on these results, we conclude: (1) blockade of 5-HT2 receptors increases the storage function of the bladder but does not alter the magnitude of the micturition reflex triggered by either nociceptive (AA irritation) or non-nociceptive (saline distention) stimulation of bladder afferents, (2) activation of 5-HT2 receptors plays a partial role in PNS inhibition of nociceptive bladder overactivity and a major role in the inhibition of non-nociceptive bladder activity. (3) activation of opioid receptors is not important in PNS inhibition, but an interaction between opioid and 5-HT2 receptor mechanisms does influence the regulation of bladder capacity.
Methysergide not only blocks 5-HT2 receptors (Pytliak et al., 2011; Skyba et al., 2003) but also acts on 5-HT1A receptors and is a weak 5-HT7 receptor antagonist (Krobert and Levy, 2002; Thomas et al., 2002). Some studies reported methysergide as a 5-HT1A agonist (Dahlöf and Maassen Van Den Brink, 2012; Scrogin et al., 2000), while others reported it is an antagonist (Skyba et al., 2003). Because activation of 5-HT1A receptors can inhibit bladder overactivity induced by AA irritation or chronic spinal cord injury in cats (Tai et al., 2006; Thor et al., 2002), activation of this type of receptor might have contributed to the effects of methysergide. However this seems unlikely because pretreatment with WAY100635, a 5-HT1A antagonist, did not alter the effects of methysergide (Fig.5).
An action of methysergide on 5-HT2 receptors seems to be the most likely explanation for the effects of the drug, because in rats activation of 5-HT2A receptors enhances reflex bladder activity, a 5-HT2A antagonist suppresses the bladder reflex in rats, and activation of 5-HT2C receptors has an inhibitory effect (Ramage, 2006; Mbaki et al., 2012; Mbaki and Ramage, 2008). If these receptors have similar functions in cats, block of 5-HT2A and 5-HT2C receptors could account, respectively, for the effect of methysergide on bladder capacity and PNS inhibition. On the other hand, the possibility that the effects of methysergide are due to targeting 5-HT7 receptors cannot be excluded, because a study in rats (Read et al., 2003) showed that SB-266970 (a selective 5-HT7 receptor antagonist) administered intracerebroventricularly inhibited bladder activity. Further investigation is warranted to clarify the role of 5-HT7 and 5-HT2 receptor subtypes in the effects of methysergide on reflex bladder activity in the cat.
A previous study in cats (Espey et al., 1992) showed that intrathecal administration of methysergide excited the bladder and reduced bladder capacity. These effects were attributed to removal of tonic serotonergic inhibition on the ascending limb of the spinobulbospinal micturition reflex pathway (Espey et al., 1998). This result suggests that the opposite effect (i.e., an increased bladder capacity) observed in the present experiments after intravenous administration of methysergide must be due to an action at another site in the central nervous system, e.g., in the brain. This possibility is consistent with the histochemical data showing that 5-HT2A and 5-HT2C receptors are located in various brain regions known to be involved in micturition (Clemett et al., 2000; Cornea-Hébert et al., 1999; Fay and Kubin, 2000). Furthermore, intracerebroventricular administration of α-methyl-5-hydroxytryptamine (a 5-HT2 receptor agonist) excited the bladder and reduced bladder capacity, suggesting an excitatory role of 5-HT2 receptors in the brain (Ishizuka et al., 2002). Intracerebroventricular administration of SB-269970 or SB656104 (selective 5-HT7 receptor antagonists) (Read et al., 2003) also significantly inhibited bladder activity and increased bladder capacity in rats. Thus block of 5-HT2 or 5-HT7 receptors in the brain could account for the increased bladder capacity elicited in our experiments by i.v. administration of methysergide.
In our study, methysergide (0.3–1 mg/kg) completely eliminated the increase in bladder capacity induced by both low (1–2T) and high (3–4T) intensity PNS during saline CMGs (Fig.4B) and also eliminated this effect induced by low (1–2T) intensity PNS in AA irritated bladders (Fig.4D), indicating a possible role of 5-HT2 receptors in PNS inhibition. It is known that the descending 5-HT pathway from raphe nuclei in the brainstem can inhibit nociceptive afferent input via activation of inhibitory interneurons in the spinal cord (Basbaum and Jessell, 2000; Millan, 2002). Therefore, it is possible that the pudendal afferent firing might be transmitted to the brain and activate the raphe nuclei that drives the descending 5-HT pathway to activate 5-HT2 excitatory receptors on inhibitory spinal interneurons (GABAergic/glycinergic) that in turn suppress the micturition reflex. This possibility is supported by various evidence: (1) activation of presynaptic 5-HT2A receptors on the GABAergic interneuron can enhances GABA release (Fink and Göthert, 2007), (2) 5-HT2A receptors are present on the GABAergic or glycinergic neurons in the spinal dorsal horn (Wang et al., 2009; Xie et al., 2012) and contribute to spinal antinociception (Liu et al., 2007; Song et al., 2011), and (3) spinal 5-HT2A receptors are involved in the antinociceptive effect induced by transcutaneous electrical nerve stimulation (Radhakrishnan et al., 2003). However, methysergide did not completely block PNS inhibition in the AA irritated bladder raising the possibility that pudendal neuromodulation is mediated in part by non-5-HT mechanisms. Our previous studies in cats (Chen et al., 2010, Larson et al., 2011) revealed that metabotropic glutamate receptor 5 and opioid receptors are also involved in PNS inhibition. Therefore, it seems that PNS activates multiple neurotransmitter pathways in order to inhibit the micturition reflex.
We showed in cats (Chen et al., 2010; Mally et al., 2013) that naloxone (i.v.) excites the bladder and significantly reduces bladder capacity during saline infusion, but has no effect on the bladder during AA infusion, indicating that tonic enkephalinergic inhibition is only active during saline infusion. However, after methysergide treatment the naloxone effect on bladder activity was reversed, i.e. the bladder capacity was reduced during AA infusion but not during saline infusion (Figs.6–7). These results indicate that there is an interaction between 5-HT2 and opioid receptor mechanisms in the micturition reflex pathway. A similar interaction is thought to occur in the control of nociception where it is believed that activity in raphe nucleus-spinal 5-HT pathways excites spinal opioid peptide containing interneurons to induce an antinociceptive effect (Marek, 2003; Wigdor et al., 1987).
The unmasking by methysergide treatment of a naloxone effect in AA irritated bladders (Fig.6A and Fig.7A) suggests that a methysergide-sensitive 5-HT2 receptor mechanism suppresses tonic enkephalinergic inhibition of the C-fiber afferent mediated spinal micturition reflex activated by bladder irritation, and that the tonic inhibition emerges after blocking the 5-HT2 receptors. Conversely, the loss of the naloxone excitatory effect after methysergide treatment (Fig.6B and Fig.7B) in saline distended non-irritated bladders where micturition is dependent on a spinobulbospinal reflex triggered by Aδ-fiber afferent suggests that tonic enkephalinergic inhibition is maintained in this condition by activation of 5-HT2 receptors and lost after block of these receptors.
Our previous study which showed that naloxone partially reduces PNS inhibition during saline infusion (Chen et al, 2010) but has no effect on PNS inhibition during AA infusion (Mally et al., 2013) is consistent with above hypothesis that enkephalinergic inhibition is active during saline distension but not during AA irritation. Because PNS inhibition during saline infusion is naloxone and methysergide-sensitive, it seems reasonable to conclude that the inhibition has both an opioid and serotonergic component. Furthermore, because methysergide completely blocks the inhibition, it is possible that the opioid component is activated by the serotonergic pathway as proposed for the serotonergic-opioid control of nociception mentioned above. The persistence of PNS inhibition in AA irritated bladders after combined methysergide-naloxone treatment indicates that a component of PNS inhibition in the bladder irritated condition is mediated by other mechanisms in additional to the putative serotonergic-opioid pathway.
Methysergide is an FDA approved drug that is used for the treatment of migraine and other vascular headaches as well as severe diarrhea caused by carcinoid syndrome. The clinical doses of methysergide are 1–2 mg (t.i.d), which is about 0.016–0.033 mg/kg for a 60 kg adult (Weintraub, 1985; Whewell, 1966). Although it is difficult to directly compare drug doses in different species (Sharma and McNeill, 2009), it is clear that higher doses of methysergide are required in cats to inhibit bladder activity (0.1–0.3 mg/kg, Fig.4 A and C), suppress the cough reflex (1–1.5 mg/kg) (Kamei et al., 1986), ot to block serotonergic effects on motor (1–2 mg/kg) (Marley and Vane, 1967; Millhorn, 1986) and cardiovascular reflexes (0.5–1 mg/kg) (McCall and Harris, 1987; Orer et al., 1999). The lower methysergide dose in humans might reflect species differences or targeting different receptors at different locations (i.e., peripheral versus central nervous system) (Sharma and McNeill, 2009). Since methysergide is an FDA approved drug, it is feasible to conduct a clinical study to test its efficacy in OAB patients. Our study not only reveals the involvement of 5-HT2 receptors in PNS inhibition of bladder activity, but also demonstrates a potential new use of an FDA approved drug for OAB treatment. Thus, understanding the neurotransmitter mechanisms underlying pudendal neuromodulation therapy may be important for developing new drugs for OAB or PBS (Andersson, 2004; Andersson and Wein, 2004).
Highlights.
Pudendal neuromodulation inhibits nociceptive bladder overactivity
5-HT2 receptor mechanism is involved in pudendal neuromodulation
5-HT2 receptor plays an excitatory role in micturition reflex
Opioid mechanism interacts with 5-HT2 mechanism in micturition reflex
5-HT2 receptor could be a new target for pharmacotherapy of overactive bladder
Acknowledgement
This study is supported by the National Institute of Health under Grants DK-068566, DK-090006 and DK-091253.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology. 2004;63(3) Suppl 1:32–41. doi: 10.1016/j.urology.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Jessell TM. The Perception of Pain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principle of neural science. 4th ed. New York, NY: McGraw-Hill Medical; 2000. pp. 404–419. [Google Scholar]
- 5.Chen ML, Shen B, Wang J, Liu H, Roppolo JR, deGroat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol. 2010;224:282–291. doi: 10.1016/j.expneurol.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SY, Wang SD, Cheng CL, Kuo JS, de Groat WC, Chai CY. Glutamate activation of neurons in CV-reactive areas of cat brain stem affects urinary bladder motility. Am J Physiol. 1993;265:F520–F529. doi: 10.1152/ajprenal.1993.265.4.F520. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CL, de Groat WC. The role of 5HT1A receptors in control of lower urinary tract function in anesthetized rats. Am J Physiol Renal Physiol. 2010;289:F771–F778. doi: 10.1152/ajprenal.00266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localization of the 5-HT2C receptor protein in the rat CNS. Neuropharmacol. 2000;39:123–132. doi: 10.1016/s0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 9.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Dahlöf C, Maassen Van Den Brink A. Dihydroergotamine, ergotamine, methysergide and sumatriptan - basic science in relation to migraine treatment. Headache. 2012;52:707–714. doi: 10.1111/j.1526-4610.2012.02124.x. [DOI] [PubMed] [Google Scholar]
- 11.de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59(Suppl 5A):30–36. doi: 10.1016/s0090-4295(01)01636-3. [DOI] [PubMed] [Google Scholar]
- 12.Espey MJ, Du HJ, Downie JW. Serotonergic modulation of spinal ascending activity and sacral reflex activity evoked by pelvic nerve stimulation in cats. Brain Res. 1998;798:101–108. doi: 10.1016/s0006-8993(98)00401-6. [DOI] [PubMed] [Google Scholar]
- 13.Espey MJ, Downie JW, Fine A. Effect of 5-HT receptor and adrenoceptor antagonists on micturition in conscious cats. Eur J Pharmacol. 1992;221:167–170. doi: 10.1016/0014-2999(92)90788-6. [DOI] [PubMed] [Google Scholar]
- 14.Fay R, Kubin L. Pontomedullary distribution of 5-HT2A receptor-like protein in the rat. J Comp Neurol. 2000;418:323–345. [PubMed] [Google Scholar]
- 15.Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 16.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka O, Gu B, Igawa Y, Nishizawa O, Pehrson R, Andersson KE. Role of supraspinal serotonin receptors for micturition in normal conscious rats. Neurourol Urodyn. 2002;21:225–230. doi: 10.1002/nau.10043. [DOI] [PubMed] [Google Scholar]
- 19.Kamei J, Hosokawa T, Yanaura S, Hukuhara T. Effects of methysergide on the cough reflex. Japan J Pharmacol. 1985;42:450–452. doi: 10.1254/jjp.42.450. [DOI] [PubMed] [Google Scholar]
- 20.Krobert KA, Levy FO. The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol. 2011;589:5833–5843. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu FY, Xing GG, Qu XX, Xu IS, Han JS, Wan Y. Roles of 5-hydroxytryptamine (5-HT) receptor subtypes in the inhibitory effects of 5-HT on C-fiber responses of spinal wide dynamic range neurons in rats. J Pharmacol Exp Ther. 2007;321:1046–1053. doi: 10.1124/jpet.106.115204. [DOI] [PubMed] [Google Scholar]
- 23.Mally AD, Matsuta Y, Zhang F, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Role of opioid and metabotropic glutamate 5 receptors in pudendal inhibition of bladder overactivity in cats. J Urol. 2013 doi: 10.1016/j.juro.2012.09.095. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marek GJ. Behavioral evidence for mu-opioid and 5-HT2A receptor interactions. Eur J Pharmacol. 2003;474:77–83. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
- 25.Marley E, Vane JR. Tryptamines and spinal cord reflexes in cats. Br J Pharmacol Chemother. 1967;31:447–465. doi: 10.1111/j.1476-5381.1967.tb00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbaki Y, Ramage AG. Investigation of the role of 5-HT2 receptor subtypes in the control of the bladder and the urethra in the anaesthetized female rat. Br J Pharmacol. 2008;155:343–356. doi: 10.1038/bjp.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbaki Y, Gardiner J, McMurray G, Ramage AG. 5-HT2A receptor activation of the external urethral sphincter and 5-HT2C receptor inhibition of micturition: a study based on pharmacokinetics in the anaesthetized female rat. Eur J Pharmacol. 2012;682:142–152. doi: 10.1016/j.ejphar.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 28.McCall RB, Harris LT. Sympathetic alterations after midline medullary raphe lesions. Am J Physiol. 1987;253:R91–R100. doi: 10.1152/ajpregu.1987.253.1.R91. [DOI] [PubMed] [Google Scholar]
- 29.McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res. 1982;234:237–249. doi: 10.1016/0006-8993(82)90865-4. [DOI] [PubMed] [Google Scholar]
- 30.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 31.Millhorn DE. Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J Physiol. 1986;381:169–179. doi: 10.1113/jphysiol.1986.sp016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison JFB, Spillane K. Neuropharmacological studies on descending inhibitory controls over the micturition reflex. J Auton Nerv Syst. 1986;17(Suppl.):393–397. [Google Scholar]
- 33.Orer HS, Clement ME, Barman SM, Zhong S, Gebber GL, McCall RB. Role of serotonergic neurons in the maintenance of the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol. 1996;270:R174–R181. doi: 10.1152/ajpregu.1996.270.1.R174. [DOI] [PubMed] [Google Scholar]
- 34.Peters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Rev Urol. 2002;4(Suppl 1):S36–S43. [PMC free article] [PubMed] [Google Scholar]
- 35.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn. 2005;24:643–647. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 36.Peters KM, Killinger KA, Boguslawski BM, Boura JA. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn. 2010;29:1267–1271. doi: 10.1002/nau.20823. [DOI] [PubMed] [Google Scholar]
- 37.Pytliak M, Vargová V, Mechírová V, Felšöci M. Serotonin receptors - from molecular biology to clinical applications. Physiol Res. 2011;60:15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- 38.Radhakrishnan R, King EW, Dickman JK, Herold CA, Johnston NF, Spurgin ML, Sluka KA. Spinal 5-HT2 and 5-HT3 receptors mediate low, but not high, frequency TENS-induced antihyperalgesia in rats. Pain. 2003;105:205–213. doi: 10.1016/s0304-3959(03)00207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol. 2006;147(Suppl. 2):S120–S131. doi: 10.1038/sj.bjp.0706504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Read KE, Sanger GJ, Ramage AG. Evidence for the involvement of central 5-HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol. 2003;140:53–60. doi: 10.1038/sj.bjp.0705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scrogin KE, Johnson AK, Brooks VL. Methysergide delays the decompensatory responses to severe hemorrhage by activating 5-HT1A receptors. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1776–R1786. doi: 10.1152/ajpregu.2000.279.5.R1776. [DOI] [PubMed] [Google Scholar]
- 42.Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106:159–168. doi: 10.1016/s0304-3959(03)00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152:1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptors in tibial nerve inhibition of nociceptive and nonnociceptive bladder reflexes in cats. Am J Physiol Renal Physiol. 2012;302:F1090–F1097. doi: 10.1152/ajprenal.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tai C, Miscik CL, Ungerer TD, Roppolo JR, de Groat WC. Suppression of bladder reflex activity in chronic spinal cord injured cats by activation of serotonin 5-HT1A receptors. Exp Neurol. 2006;199:427–437. doi: 10.1016/j.expneurol.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Thomas DR, Atkinson PJ, Hastie PG, Roberts JC, Middlemiss DN, Price GW. [3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacol. 2002;42:74–81. doi: 10.1016/s0028-3908(01)00151-4. [DOI] [PubMed] [Google Scholar]
- 48.Thor KB, Katofiasc MA, Danuser H, Springer J, Schaus JM. The role of 5-HT1A receptors in control of lower urinary tract function in cats. Brain Res. 2002;946:290–297. doi: 10.1016/s0006-8993(02)02897-4. [DOI] [PubMed] [Google Scholar]
- 49.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, Lycklama á Nijholt AA, Siegel S, Jonas U, Fowler CJ, Fall M, Gajewski JB, Hassouna MM, Cappellano F, Elhilali MM, Milam DF, Das AK, Dijkema HE, van den Hombergh U. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Wang YY, Wei YY, Huang J, Wang W, Tamamaki N, Li YQ, Wu SX. Expression patterns of 5-HT receptor subtypes 1A and 2A on GABAergic neurons within the spinal dorsal horn of GAD67-GFP knock-in mice. J Chem Neuroanat. 2009;38:75–81. doi: 10.1016/j.jchemneu.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Weintraub MI. Methysergide (Sansert) treatment in acute stroke. Community pilot study. Angiology. 1985;36:137–142. doi: 10.1177/000331978503600301. [DOI] [PubMed] [Google Scholar]
- 52.Whewell J. Methysergide in prophylaxis of migraine: a clinical trial in general practice. Br Med J. 1966;2:394–395. doi: 10.1136/bmj.2.5510.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wigdor S, Wilcox GL. Central and systemic morphine-induced antinociception in mice: contribution of descending serotonergic and noradrenergic pathways. J Pharmacol Exp Ther. 1987;242:90–95. [PubMed] [Google Scholar]
- 54.Xie DJ, Uta D, Feng PY, Wakita M, Shin MC, Furue H, Yoshimura M. Identification of 5-HT receptor subtypes enhancing inhibitory transmission in the rat spinal dorsal horn in vitro. Mol Pain. 2012;8:58. doi: 10.1186/1744-8069-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang F, Mally AD, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol. 2012;302:F1576–F1582. doi: 10.1152/ajprenal.00107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]