Abstract
Overexpression of Toll-like Receptor-4 (TLR4) in human tumors often correlates with chemoresistance and metastasis. We found that TLR4 is overexpressed in the majority of clinical breast cancer (BC) samples and in 68% of the examined BC lines. TLR4 is activated by lipopolysaccharide (LPS) and other ligands including the widely-used drug paclitaxel (PXL). LPS is frequently used to show a tumor-promoting role of TLR4 although this bacterial component is unlikely to be found in BC environment. We reasoned that PXL-dependent activation of TLR4 is more relevant to BC chemoresistance that could be mediated by activation of the NF-κB pathway leading to upregulation of pro-survival genes. To test this hypothesis, we correlated TLR4 expression with resistance to PXL in two modified BC lines with either depleted or overexpressed TLR4 protein. Depletion of TLR4 in naturally overexpressing MDA-MB-231 cells downregulated pro-survival genes concomitant with 2–3 fold reduced IC50 to PXL in vitro and a 6-fold decrease in recurrence rate in vivo. Conversely, TLR4 overexpression in a negative cell line HCC1806 significantly increased expression of inflammatory and pro-survival genes along with a 3-fold increase of IC50 to PXL in vitro and enhanced tumor resistance to PXL therapy in vivo. Importantly, both tumor models showed that many PXL-upregulated inflammatory cytokines were co-induced with their receptors suggesting that this therapy induces autocrine tumor-promoting loops. Collectively, these results demonstrate that paclitaxel not only kills tumor cells but also enhances their survival by activating TLR4 pathway. These findings suggest that blocking TLR4 could significantly improve response to PXL therapy.
Keywords: TLR4, chemoresistance, breast cancer, paclitaxel
Introduction
Chemoresistance is the major obstacle to successful treatment of metastatic cancers. The two well-recognized mechanisms of chemoresistance are overexpression of drug transporters that decrease intracellular concentration of the drug (1) and preexisting mutations that decrease drug binding to target proteins (2). In addition to these intrinsic mechanisms, therapeutic efficacy can be reduced by chemotherapy-induced upregulation of inflammatory and pro-survival genes(3–5). The goal of this study was to delineate the latter mechanism that enables tumor cells to escape cytotoxic therapy through acquisition of a more aggressive phenotype.
Chemotherapy induction of tumor-promoting pathways can be illustrated by response of malignant cells to taxanes. Paclitaxel (PXL),the active component of taxanes, is a common drug used against various solid tumors (6). PXL induces apoptosis by over-stabilizing microtubules (7), which leads to cell arrest at the G2/M phase (8). Although the therapeutic utility of PXL has been improved by formulating it as nanoparticles(nab-PXL) (9), the overall response of breast cancer (BC) patient seven to this advanced therapy remains below 35% (10). The main reason for low response rate is tumor recurrence after cessation of therapy, which might relate to PXL-mediated induction of inflammatory mediators including TNF-α (11), IL-1β(12), IL-8(13), IL-6 (14)and VEGF-A (3, 4). In addition to inflammatory mediators, PXL also upregulates pro-survival proteins including XIAP (15), Bcl-2 (16), Akt (17), and Bcl-xL (4, 15). Cumulatively, these studies suggest that tumor cells respond to PXL by activating two conflicting pathways: one induced by over-stabilizing microtubules which leads to apoptosis, and the other induced by a currently undefined receptor that promotes tumor cell survival through upregulation of inflammatory and pro-survival genes.
Based on the transcriptional profile induced by PXL, we postulated that the likely candidate for transmitting PXL signaling is Toll-like Receptor-4 (TLR4). This hypothesis is based on multiple lines of evidence. First, PXL-induced pro-survival transcription largely overlaps with that of lipopolysaccharide (LPS), a natural ligand of TLR4 (18). PXL activates TLR4 by binding to human or mouse MD2, an adaptor protein that confers LPS responsiveness of the TLR4 cascade (19). Second, PXL activates several pro-oncogenic signaling including NF-κB (20), MAPK (21) and PI3K (22). This effect of PXL has been shown in many human cancers including breast (20), prostate (23), ovarian (17), colon (24), lung, and pancreatic (25)tumors. Third, PXL increases expression of inflammatory cytokines in both tumor lines (12, 20, 26) and human cancer patients (14). Fourth,TLR4 is likely to transducer PXL signals because anti-TLR4 siRNA downregulates the expression of inflammatory cytokines induced by PXL (15, 26). Lastly, overexpressed TLR4 in human tumors correlates with resistance to PXL therapy (26), recurrence (27), clinical stage and grade (28), and poor patient survival (17). Taken together, this evidence suggests that TLR4 expression in human BC contributes to resistance to PXL therapy due to transcriptional upregulation of pro-survival genes.
To test this hypothesis, we determined TLR4 expression in a broad panel of BC lines, correlated cell responsiveness to PXL with TLR4 expression, and generated two isogenic lines with either reduced or increased TLR4 expression. These new BC models enabled us to analyze the role of TLR4 in PXL resistance using a variety of in vitro and in vivo approaches. The results strongly suggest that TLR4-mediated increase of cytokines and their receptors protects tumors from chemotherapy through activation of autocrine loops in neoplastic cells that may, in turn, activate paracrine pathways in the tumor environment.
Materials and Methods
Materials
LPS derived from Escherichia coli 055:B5, TRI-reagent, and protease inhibitors were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM) and supplements were from Lonza (Basel, Switzerland). SB225002 and PD98059 were purchased from EMD Millipore (Billerica, MA).
Plasmids and study drugs
Human TLR4 CDS ligated into pCDNA3.1 plasmid (TLR4-pCDNA3.1) was purchased from Addgene (Cambridge, MA). TLR4 shRNA plasmid (TLR4-psiRNA) was purchased from InvivoGen (San Diego, CA). The pKT2-IRES-PURO plasmid was a generous gift from Dr. Wilber (Southern Illinois University, IL, USA). Paclitaxel albumin-bound nanoparticles (nab-PXL) was obtained from Abraxis BioScience (now Celgene), Los Angeles, CA. Paclitaxel (Taxol®) was obtained from Bristol-Myers Squibb, NY.
Antibodies
Primary antibodies were: goat anti-hTLR4 (Imgenex, CA); rabbit anti-Bcl-2, anti-Bcl-xL, anti-p-Akt and anti-Akt (Cell Signaling, MA); rabbit anti-p65, anti-p50 and anti-p-p50 (Santa Cruz Biotechnology, CA); rabbit anti-p-p65 (Epitomics, CA), rabbit anti-active caspase-3 (R&D Systems, MN), and mouse anti-β-actin (JLA20) (Developmental Studies Hybridoma Bank, IA). Secondary HRP-conjugated anti-goat, anti-rabbit, and anti-mouse antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Human BC tissues and cell lines
Human tumor and normal BC tissues were obtained from ILSBio (Chestertown, MD). Luciferase-tagged MDA-MB-231 and HCC1806 cells were cultured in 10% DMEM with standard additives at 37°C in 10% CO2. All cell lines were authenticated by ATCC and tested for mycoplasma using a kit from Roche Diagnostics GmbH (Penzberg, Germany).
Generation of stable sublines with modified TLR4
To suppress TLR4 expression, MDA-MB-231 cells were stably transfected with TLR4-psiRNA or scrambled shRNA followed by selection with zeocin (30µg/ml). To overexpress TLR4, HCC1806 line was transfected with human TLR4 CDS subcloned in pKT2-IRES-PURO vector or empty plasmid followed by selection with puromycin (1µg/ml). Changes in TLR4 expression were determined by RT-qPCR and Western blot. Modified sublines were designated as 231Cntrl and 231TLR4− or 1806Cntrl and 1806TLR4+ for MDA-MB-231 and HCC1806 sublines, respectively.
RT-PCR and RT-qPCR
RNA was extracted by TRI-reagent and reverse transcribed using Revert Aid cDNA synthesis kit (Fermentas, Canada). Primers were designed based on CDS of human targets found in NCBI database. All primer sequences are listed in supplementary Table S1. Targets were amplified for 35 cycles followed by gel analysis and imaging. For RT-qPCR, cells were treated with LPS (100ng/ml) or nab-PXL (10nM) followed by transcript analysis using Go TaqGreen Master Mix (Promega, WI) and Real-Time PCR machine (Applied BioSystems, CA). Data were normalized to β-actin and relative mRNA expression was determined using the ΔΔCt method (29).
Western blot analysis
Cells seeded in a 6-well plate at the density of 0.5×106 adhered overnight prior to treatment with 10nM nab-PXL for 0, 12, 24, and 48hrs followed by lysis in 150µl buffer containing 50mM Tris-HCl, pH 7.5, 150mM NaCl, 1mM EDTA, 1%Triton-X100, 0.1% SDS, and protease inhibitors. Lysates were cleared at 13,000rpm for 10min, boiled, separated on 12% SDS gels and transferred to a nitrocellulose membrane followed by overnight incubation with primary antibodies against TLR4, Bcl-2, Bcl-xL, NF-κB p50, p-p50, NF-κB p65, p-p65, Akt, p-Akt, ERK1/2, p-ERK1/2, or β-actin. Protein bands were visualized by a Fujifilm LAS-3000 camera after a 1hr-incubation with HRP-conjugated secondary antibodies and development with ECL (Pierce, IL).
Cytotoxic assay
Cells seeded at the density of 50,000 cells per well in 24-well plates were treated with 0–100nM of PXL, nab-PXL or plain medium. After 48hrs, viable cells were enumerated to determine the IC50. Each condition was tested in duplicate and reproduced thrice.
FACS analysis of apoptosis and cell cycle
Apoptotic cells were visualized using Annexin V detection kit according to the manufacturer’s instructions (BD Pharmingen, CA). Briefly, nab-PXL treated cells (10nM) for 48hrs were incubated with FITC conjugated-Annexin V and propidium iodide (PI). After a-15min incubation, samples were analyzed by a Becton Dickinson FACS Calibur flow cytometer. Cell cycle phase distribution was determined in 70% alcohol-fixed cells stained with 10µg/ml of PI, sorted by FACS and analyzed using the CellQuest software (Becton Dickinson).
Measurement of cytokine concentrations by ELISA
Conditioned medium (CM) was collected from MDA-MB-231 and HCC1806 lines treated with 10nM of nab-PXL or plain medium. Concentrations of IL-6, IL-8, and TNF-α were determined by ELISA kits purchased from Peprotech (Rocky Hill, NJ). All experiments were performed in duplicate and reproduced thrice. Results are presented as mean pg of cytokine ±S.D. normalized per 106 cells.
Animal studies
Tumor growth of orthotopically implanted cell lines was monitored as described previously (3, 4). Briefly, 4×106 cells suspended in 50% Matrigel were implanted into the mammary fat pad (MFP) of 4–6 week-old female SCID mice (Taconic, Hudson, NY). Every 2–3 days, perpendicular tumor diameters were measured by digital caliper and used to calculate tumor volume according to the formula: volume = Dd2π/6, where D equals larger diameter and d equals smaller diameter. Animal care was in accordance with institutional guidelines.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (La Jolla, CA). Results are expressed as mean ±S.D. Combination Index (CI) indicating synergism of two drugs was calculated by CompuSyn software (CompuSyn Inc., NY). Statistical significance for continuous variables and categorical covariants was determined by Student’s paired t-test and Chi-square test, respectively. P-values ≤0.05 were considered significant.
Results
TLR4 expression and function in human BC tissues and cell lines
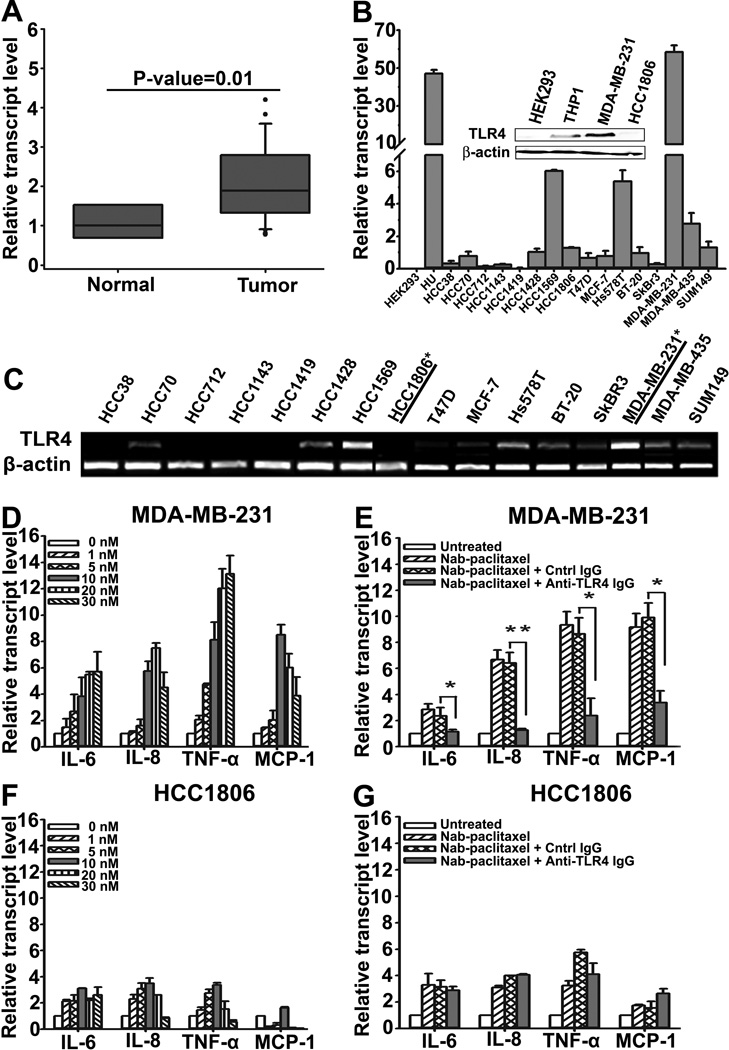
We first determined the frequency of TLR4 expression in human BC clinical samples and established lines. RT-qPCR analysis of 20 malignant breast tumors and 5 normal mammary tissues showed significantly(P-value=0.01) upregulated TLR4 expression in invasive BC as compared with non-malignant breast (Fig. 1A). Screening of BC lines by endpoint and RT-qPCR showed that 11 out of 16 (68%) lines had moderate to high TLR4 expression (Figs. 1B and 1C). The MDA-MB-231 line had the highest expression (53-fold above the level in negative lines) whereas HCC1806 line did not expressTLR4. Western blot confirmed TLR4 protein expression in MDA-MB-231 and HCC1806 lines (Fig. 1B, insert). These two lines were selected to represent TLR4-positive and -negative BC.
Figure 1. Frequency of TLR4 expression in human BC tumors and lines.
(A) Analysis of TLR4 mRNA in human normal breast (n=5) and tumors (n=20) by RT-qPCR. (B) RT-qPCR analysis of TLR4 mRNA in BC lines. Insert: Western blot of TLR4 protein expression in MDA-MB-231 and HCC1806 cells. HEK293 and THP1 represent lines with negative and positive TLR4 expression, respectively. (C) RT-PCR analysis of TLR4 mRNA expression in cell lines. Underline and asterisk indicates the cell lines to represent TLR4+ and TLR4− lines. (D, F) Activation of TLR4 pathway by paclitaxel inMDA-MB-231 and HCC1806 cells. RT-qPCR quantification of IL-6, IL-8, TNFα and MCP-1 in cells treated with nab-PXL (0–30nM) for 48hrs. (E, G) MDA-MB-231 and HCC1806 cells were pre-treated with control-IgG or anti-TLR4 antibody (4ug/ml) for 2hrs before nab-PXL treatment (10nM) and analyzed as in 1D. Data are presented as β-actin normalized mRNA expression ±S.D. from two experiments done in duplicate. The P-values represent * <0.05 and ** <0.01 vs. control as determined by Student’s paired t test.
We next determined the functionality of TLR4 in these lines. Cells were treated with 100ng/ml of LPS followed by quantitative analysis of TLR4 targets such as IL-6, IL-8, TNF-α and MCP-1. LPS induced ∼100-fold higher response in MDA-MB-231 as compared with HCC1806 cells. The effect in MDA-MB-231 cells was mediated specifically via TLR4 because94% of the response was suppressed by an anti-hTLR4 antibody (Fig. S1). In contrast, a minor response in HCC1806 cells was TLR4-independent suggesting that other TLRs might be responsible for this effect. Indeed, HCC1806 cells express functional TLR2 and TLR9 (Fig. S2) that might weakly respond to TLR4 ligands.
We next asked whether MDA-MB-231 and HCC1806 differentially respond to PXL, a mimetic of LPS (30, 31). Cells were treated for 48hrs with nab-PXL followed by RT-qPCR analysis of IL-6, IL-8, TNF-α and MCP-1 mRNA. As shown in Fig. 1D, TLR4+ MDA-MB-231 cells responded to PXL in a dose-dependent manner with robust upregulation of cytokines ranging from a 6.5-fold for IL-8 (<0.01) to 9-fold for TNF-α (<0.05). This effect was mediated by TLR4 as indicated by a 95% reduction in cytokine expression in the presence of anti-hTLR4 antibody (Fig. 1E). Minor increase in cytokines was also noted in HCC1806 cells, albeit this response was not affected by anti-hTLR4 antibody (Figs. 1F, 1G).
TLR4 expression in BC lines correlates with resistance to PXL
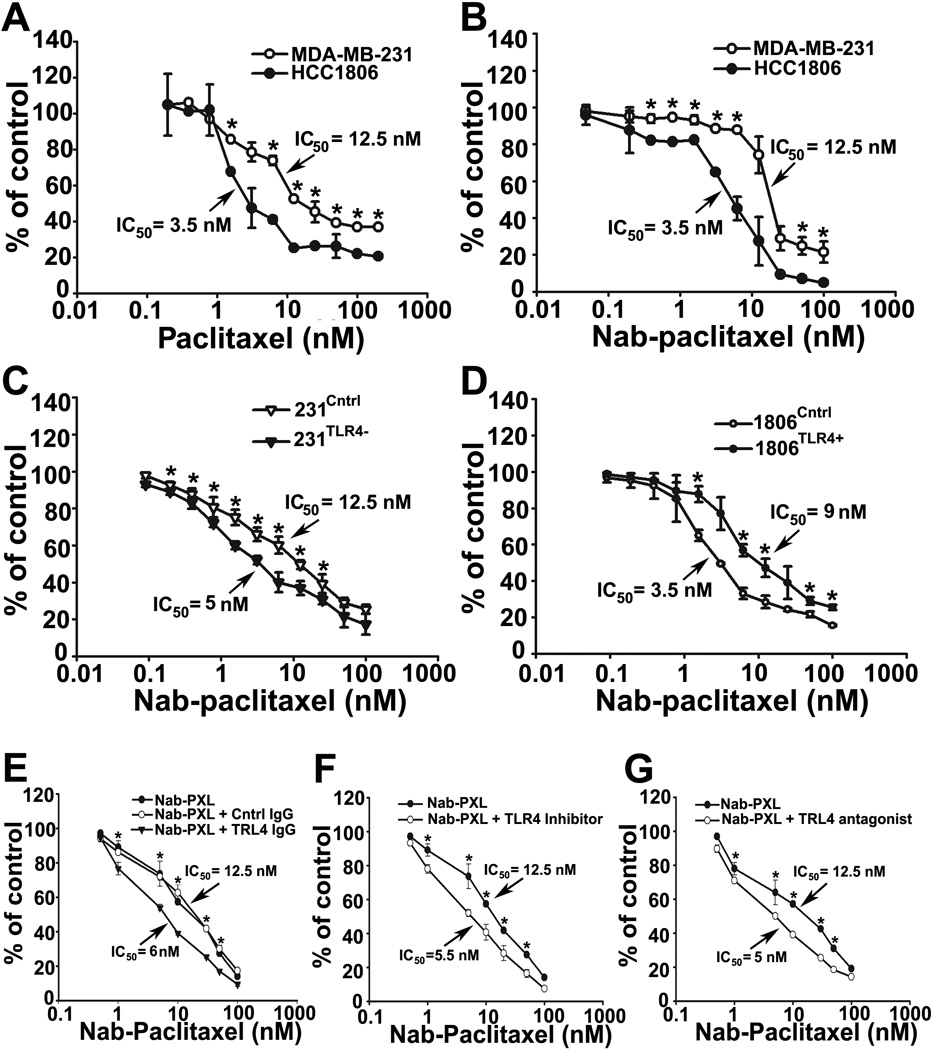
Functional TLR4 may significantly enhance inflammation in the tumor environment leading to increased chemoresistance (32, 33), and metastasis(34). To test whether PXL-induced inflammatory responses protect cells from chemotherapy, we measured IC50 of PXL in TLR4+ MDA-MB-231 and TLR4-negative HCC1806 cells. As shown in Figs. 2A and 2B, TLR4+ MDA-MB-231, was 4-fold more resistant to PXL as compared with HCC1806 (IC50 of 12.5 vs.3.25nM; P-value 0.003). This difference was equally observed in PXL and nab-PXL treated cells indicating that this effect is formulation-independent. We also tested four additional lines with either high or low TLR4 expression. As shown in supplementary Table S2,the mean IC50 for lines enriched withTLR4 was 2-fold higher (P-value=0.037) than for those with low level of this receptor. This finding supported the role of TLR4 in chemoresistance although the firm conclusion could be reached only by comparing genetically identical lines.
Figure 2. TLR4-positive and negative lines significantly differ in sensitivity to PXL.
MDA-MB-231 and HCC1806 cells were treated with PXL (A) or nab-PXL (B) at indicated concentrations (0–100nM) for 48hrs followed by calculating IC50. (C, D) 231Cntrl, 231TLR4− cells, 1806Cntrl and 1806TLR4+ cells were treated and analyzed as described under 2A. (E) MDA-MB-231 cells were pretreated with 4µg/ml of anti-TLR4 or control IgG for 2hrs followed by analysis described under 2A. (F, G) Cells were pretreated with a TLR4 inhibitor TAK-242 (10uM) or with an LPS antagonist LPS-EKUltra for 2hrs followed by analysis described under 2A. Data are presented as percent of viable cells ±S.D. from three experiments done in triplicate. The P-values represent * <0.05 vs. control as determined by Student’s paired t-test.
To this end, we generated and functionally characterized isogenic derivatives of MDA-MB-231 and HCC1806 with differential TLR4 expression. Compared with controls, the selected MDA-MB-231 clones had 80% reduced TLR4 whereas HCC1806 clones had 10,000-fold higher TLR4 expression (Fig. S3). Modified TLR4 expression corresponded to changes in both LPS and PXL-induced upregulation of inflammatory cytokines. Downstream TLR4 mRNA and protein targets were significantly (50–60%) reduced in 231TLR4− and 5–25-fold upregulated in 1806TLR4+ lines treated with TLR4 ligands. These clones designated 231TLR4− or 1806TLR4+,along with their controls 231Cntrl and 1806Cntrl,were used to analyze the TLR4 role in PXL resistance.
After validating functionality of an ectopically expressed TLR4, we used the modified lines to determine differences in cytotoxic response to PXL. Figs. 2C and 2D show that 231TLR4− cells were more sensitive to nab-PXL (2.5 fold; P<0.04) than 231Cntrlcells, whereas1806TLR4+ line was nearly 3-fold more resistant to PXLthan1806Cntrl cells. In addition to genetic manipulation, the role of TLR4 in conferring resistance to PXL was also demonstrated by a monospecific anti-hTLR4 antibody (Fig. 2E), specific intracellular and extracellular TLR4 inhibitors, TAK-242 and LPS-EKultra (Figs. 2F, 2G). These structurally unrelated agents targeting TLR4 share the capacity to increase response to PXL by 2.0 to 2.5 fold. Collectively, these data show that TLR4 expressed in human tumor cells regulates both expression of inflammatory cytokines and sensitivity to PXL.
TLR4 promotes survival of PXL-treated tumor cells by increasing levels of anti-apoptotic proteins
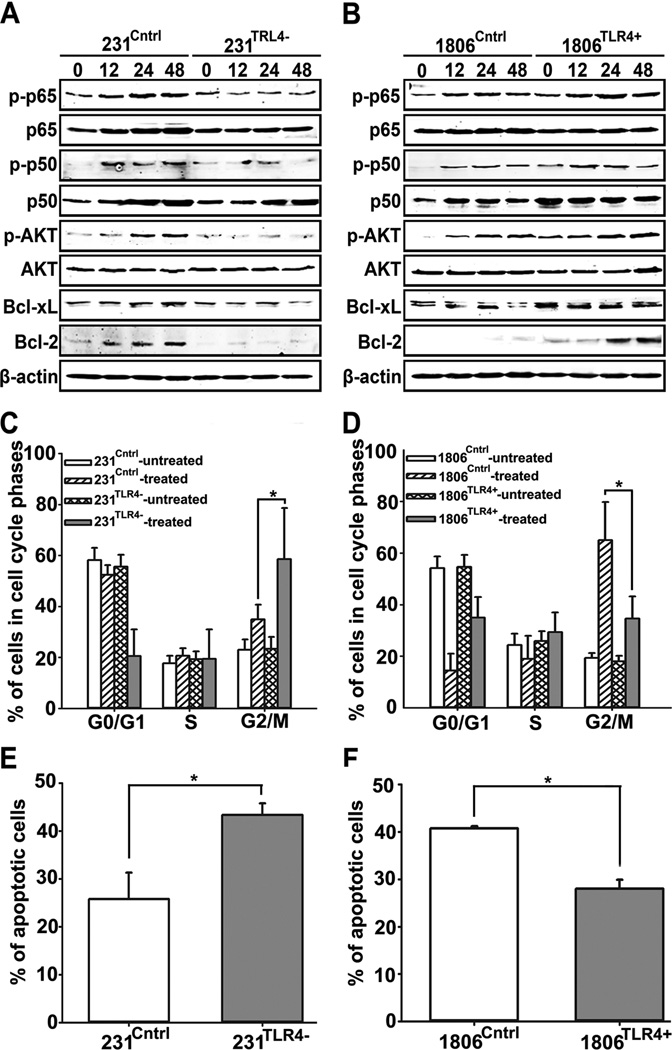
Increased chemoresistance typically correlates with upregulation of pro-survival proteins (17, 26, 32). We therefore hypothesized that TLR4 promotes survival of PXL-treated cells by activating NF-κB pathway known to transcribe pro-survival genes (35, 36). To test this hypothesis, cells were treated with 10nM of nab-PXL for 0–48hrs followed by Western blot analysis of phosphorylated and total p50, p65, Akt, Bcl-xL, and Bcl-2. Depletion of TLR4 in MDA-MB-231 substantially decreased expression of both phosphorylated and non-phosphorylated p50 and p65, as well as, p-Akt, Bcl-xL and Bcl-2 (Fig. 3A). Conversely, TLR4 overexpression in HCC1806 cells increased phosphorylation of NF-κB concomitant with upregulation of Bcl-2 and Bcl-xL (Fig. 3B).
Figure 3. TLR4 mediates the response of BC cells to nab-PXL.
(A, B) Western blot analysis of NF-κB p-p50, NF-κB p50, NF-κB p-p65, NF-κB p65, p-Akt, Akt, Bcl-2, Bcl-xL and β-actin proteins in 231Cntrl, 231TLR4−, 1806Cntrl, and 1806TLR4+ cells treated with nab-PXL (10nM, 48hrs). (C, D) Cell cycle analysis by FACS of PI-stained cells treated with nab-PXL for 48hrs. Data are presented as mean percentage of cells in different stages of cell cycle ±S.D. from three experiments. (E, F) Apoptosis of 231Cntrl, 231TLR4−, 1806Cntrl, and 1806TLR4+ cells treated with nab-PXL for 48hrs determined by FACS measurement of% Annexin-and PI-positive cells. Data are presented as mean percentage of apoptotic cells ±S.D. from three experiments. Asterisks indicate P-values <0.05 vs. control as determined by Student’s unpaired t-test.
We next examined the consequences of TLR4-dependent biochemical changes in major pro-survival proteins for developing resistance to PXL therapy. PXL induces apoptosis by over-stabilizing microtubules which leads to cell arrest in the G2/M phase (1). Therefore, we hypothesized that TLR4 might alter distribution of tumor cells through cell cycle phases. To test this hypothesis, cells were treated with nab-PXL (10nM) for 48hrs, stained with PI and analyzed for phase distribution by FACS. As shown in Fig. 3C, TLR4 knockdown significantly increased the number of cells arrested at the G2/M phase as evidenced by 58% of 231TLR4− cells detected in this phase after PXL treatment as compared with 35% in the control line(P<0.05). An opposite distribution was observed in the HCC1806 model (65% in control vs. 34% in 1806TLR4+, Figs. 3D and S4).
These results suggested that tumor cells with reduced TLR4 are more susceptible to PXL-induced apoptosis as compared with TLR4-overexpressing cells. To test this hypothesis, we quantified PXL-induced apoptosis in MDA-MB-231 and HCC1806 lines. Cells were treated as described above followed by quantification of apoptotic cells identified by double-staining with PI and Annexin V. Fig.3E shows that% of apoptotic cells significantly increased in 231TLR4− as compared with 231Cntrl (43% vs. 29%, P<0.05). An identical experiment in HCC1806 model independently confirmed causalitybetweenTLR4 and drug-induced apoptosis: only 28% of 1806TLR4+ cells were apoptotic as compared with 40% in 1806Cntrl (Fig. 3F; P<0.05). Similar results were obtained by identifying apoptotic cells using anti-active caspase-3 antibody. As shown in Fig. S4, 43% of 231TLR4− cells were positive for caspase-3 as compared with 20% in control line (2.2-fold increase, P<0.05). Similarly, 1806TLR4+ cells showed a 2.1-fold decreased apoptosis compared with 1806Cntrl cells (P<0.05). These results suggest that PXL-mediated activation of the TLR4 pathway concordantly diminishes the number of cells arrested in G2/M phase and those undergoing apoptosis. This, in turn, may significantly increase tumor recurrence after cessation of therapy.
PXL-activated TLR4 upregulates both inflammatory ligands and receptors potentially creating pro-survival autocrine loops
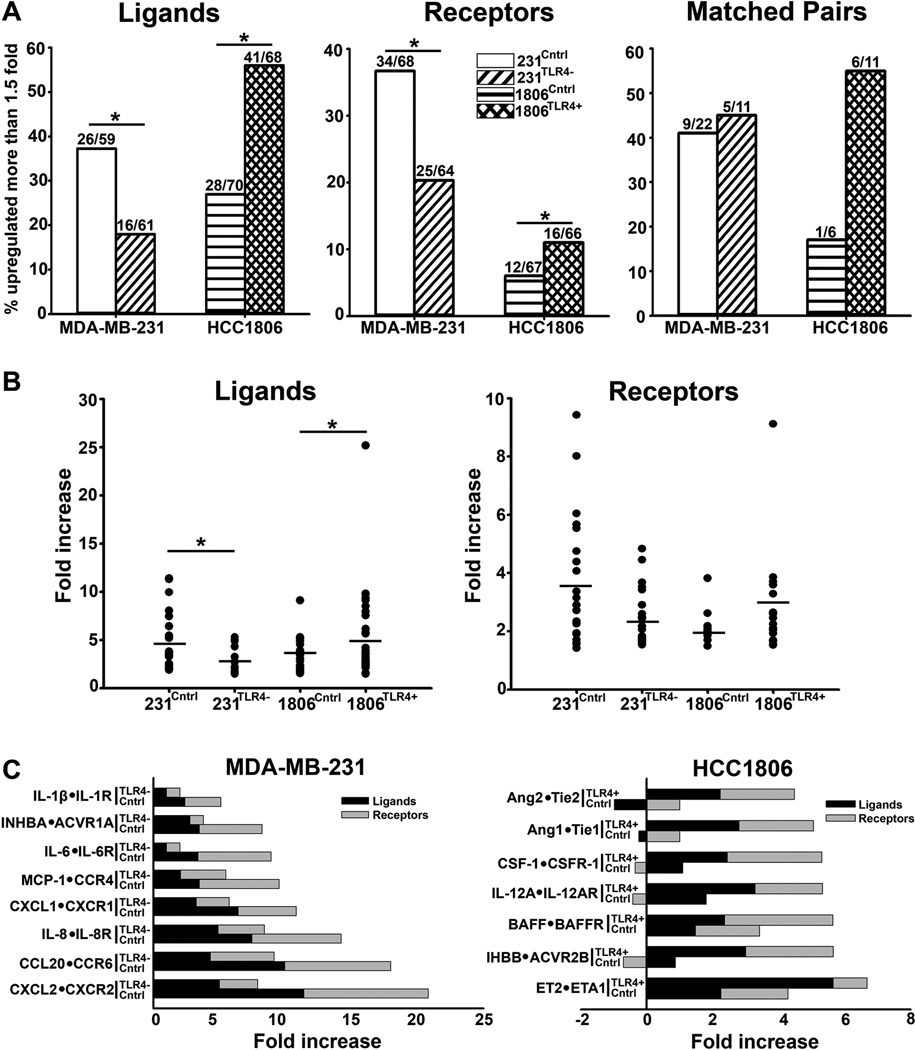
In macrophages, the hallmark of LPS activated-TLR4 signaling is coincident induction of inflammatory cytokines and matching receptors. Generation of these autocrine loops amplifies intracellular pathways that enhance proliferation, migration, and survival of pathogen-fighting immune cells. Because PXL is an LPS mimetic(30), we hypothesized that a similar effect can be produced by taxanes on TLR4+ cancer cells. To test this hypothesis, we compared the expression of both cytokines and matching receptors in control and PXL-treated MDA-MB-231 and HCC1806 cell lines. Cells were treated with nab-PXL followed by RT-qPCR analysis of ∼100 inflammatory cytokines and matching receptors. Using this approach, we identified a substantial number of targets upregulated by nab-PXL by at least 1.5 fold (Fig. 4A). 1806Cntrl line lacking TLR4 was the least responsive line with 17.9% of upregulated receptors whereas the broadest responses (50% upregulated targets) were obtained in the 231Cntrl cells that have the highest expression of TLR4. This response was TLR4-dependent because both the number of targets and the degree of their upregulation correlated with TLR4 expression in both MDA-MB-231 and HCC1806 models(Figs. 4A, 4B). Importantly, some targets became detectable only after exposure to PXL(Tables S3, S4) suggesting that this therapy not only intensifies inflammation, but also qualitatively changes the tumor environment.
Figure 4. PXL upregulates inflammatory cytokines and receptors in TLR4+ lines.
(A) Number of inflammatory genes upregulated >2 fold in231Cntr, 231TLR4,1806Cntr and 1806TLR4+ cells in PXL-treated(10nM, 48hrs) vs. untreated cells. Asterisks * and ** indicate P-values<0.05 and <0.01 vs. control determined by Chi-square test. (B) Fold increase of upregulated inflammatory genes in 231Cntrl, 231TLR4−,1806Cntrl, and 1806TLR4+ lines after PXL treatment. Asterisks * indicate P-values <0.05 vs. control as determined by Mann-U-Whitney test comparing number of ligands and receptors upregulated >1.5-fold in 231Cntrl vs. 231TLR4− cells. (C) Main cytokines and corresponding receptors upregulated >2.0 fold in 231Cntrl, 231TLR4, 1806Cntrl and 1806TLR4+ cells.
Significantly, several upregulated cytokines in both BC models were co-induced with matching receptors, potentially creating autocrine growth-promoting loops (Figs. 4A, 4C). This is best illustrated by the changes in HCC1806 line in which ectopic expression of TLR4 increased the number of matching pairs by 328% (16.6% and 54.5% in 1806Cntrl and 1806TLR4+, respectively; Fig. 4A). Using this profiling, we identified several new ligand-receptor pairs induced by TLR4 as indicated by significant differences between the control and TLR4-manipulated lines(Fig. 4C, P<0.05). In the 231Cntrl line, the highest upregulated pairswere: CXCL2•CXCR2 (11.40±1.89 and 9.43±1.60 fold increase for ligand and receptor, respectively), CCL20•CCR6 (9.95±1.53 and 8.02±0.62 fold), and IL-8•IL-8R (7.46±0.55 and 6.76±1.93 fold). Pairs upregulated in 1806TLR4+ cells included CSF1•CSFR1, BAFF•BAFFR and INHBB•ACVR2B. This study, therefore, suggests that treatment with nab-PXL creates a broad inflammatory response that fundamentally alters the tumor milieu, and consequently, the response to therapy.
PXL-activated cytokine-receptor autocrine loops contribute to drug resistance through increased phosphorylation of AKT and ERK1/2
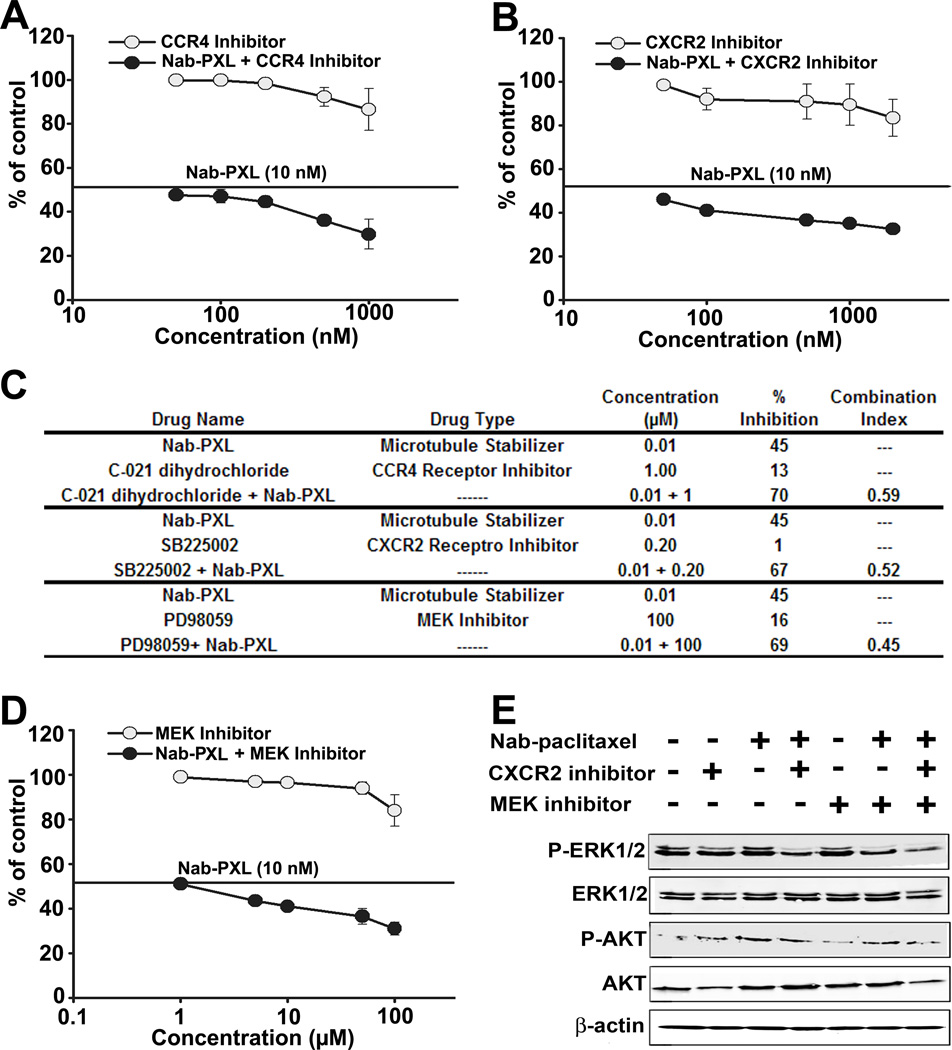
We next examined whether drug-upregulated cytokine-receptor pairs contribute to chemoresistance. We tested whether neutralizing antibodies or specific pharmacological inhibitors of cytokines or receptors increase tumor cell sensitivity to PXL. Out of 7 most upregulated pairs in MDA-MB-231 (Fig. 4C), we assessed the effect of interrupting a putative autocrine loop for 5 pairs (IL-6, IL-8, MCP-1, CXCL1 and CXCL2). While neutralization of IL-6 or IL-8 had no significant effect on nab-PXL toxicity, blockade of CCR4 and CXCR2 receptors was highly synergistic with PXL treatment (Figs. 5A, 5B). Analysis of synergism of blocking CCR4 and CXCR2 by CompuSyn software yielded highly significant Combination Index (CI) values for both inhibitors of 0.59 and 0.52, respectively (Fig. 5C). Both pathways activate MEK that phosphorylates ERK1/2(37). Indeed, combination of MEK inhibitor PD98059 (100µM) with nab-PXL was highly synergistic (CI=0.45) resulting in 69% inhibition compared with ∼50% caused by nab-PXL alone (Figs. 5C and 5D). Synergy with nab-PXL was also evident by enhanced phosphorylation of ERK1/2 and AKT (Fig. 5E). Collectively, these results indicate that CXCL1/2•CXCR2 and MCP-1•CCR4 loops functionally contribute to survival of tumor cells during chemotherapy.
Figure 5. Inhibition of CXCR2 and CCR4 receptors synergistically increases sensitivity to nab-PXL in MDA-MB-231 cells.
Cells were pretreated with 0–1µM of a CXCR2 inhibitor SB225002 (A) or a CCR4 receptor inhibitor C021 dihydrochloride(B) followed by 48hrs treatment with nab-PXL(10nM). IC50 was calculated as described in legend for Fig. 2.(C) Table demonstrating the synergy between nab-PXL and inhibitors of CCR4, CXCR2,or MEK as represented by measured as CI. (D) MDA-MB-231 cells were treated MEK inhibitor (0–100 uM) and nab-PXL (10nM) for 48hrs followed by calculating IC50. Data are presented as percentage of viable cells vs. control ±S.D. from two experiments done in triplicate. (E) MDA-MB-231 cells were pretreated with CXCR2 (1µM) and MEK inhibitors (50µM) alone or in combination for 2hrs followed by nab-PXL (10nM) for 48hrs. Phosphorylation of ERK1/2 and AKT was analyzed by Western blot.
Expression of TLR4 in BC cells dictates their responses to PXL therapy in vivo
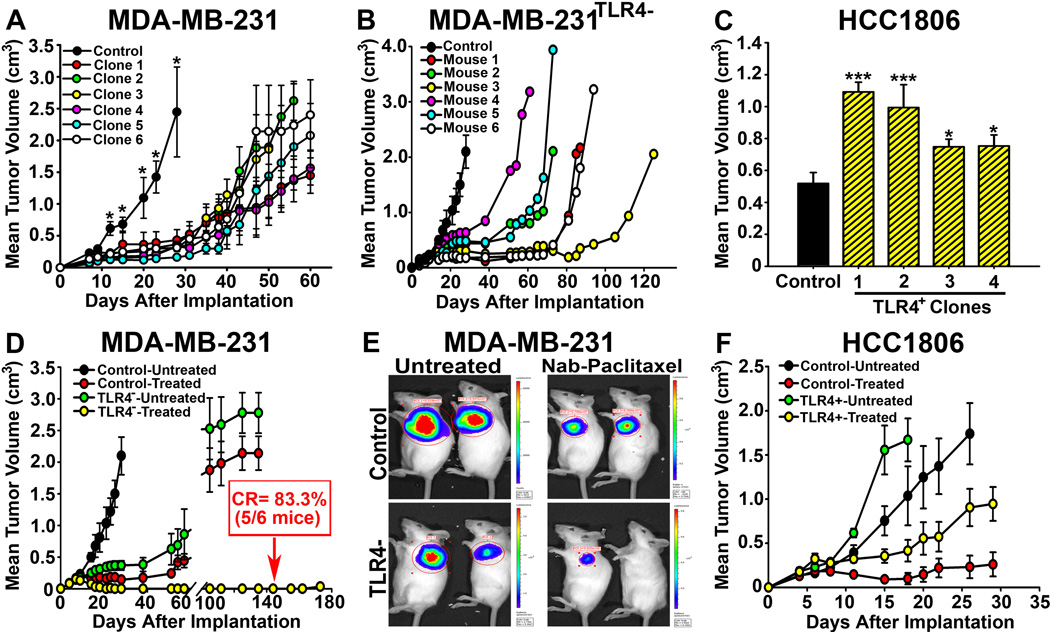
Substantial differences in susceptibility of BC lines to PXL in vitro suggested a prominent role of TLR4 in chemoresistance in vivo. To test this hypothesis, we measured tumor growth rate of orthotopically implanted lines with differential TLR4 expression. All 6 tested clones of 231TLR4− grew substantially slower than control 231Cntrl line (Fig. 6A). The period prior to exponential tumor growth of 231TLR4− clones doubled in most mice with some requiring>110 days compared with only 10–15 days in control mice (Fig. 6B). Expectantly, all 1806TLR4+ clones (n=4) grew much faster than control lines (P>0.02; Fig. 6C).
Figure 6. TLR4 determines sensitivity to nab-PXL in breast cancer modelsin vivo.
(A) The growth of 231TLR4− and control lines implanted in SCID mice was monitored twice weekly. Each point represents the mean tumor volume ±S.E and the asterisks indicate P-values >0.05. (B) Growth of a231TLR4− clone demonstrating significant delay in establishing tumor mass in all mice per group (n=6) compared with controls. Each line represents tumor growth in individual mouse. (C) Growth of HCC1806TLR4+ and HCC1806Cntrl tumorsin SCID mice. TLR4 significantly increased tumor growth in all clones with * and *** indicating P-values <0.05 and <0.001, respectively. (D) 231TLR4− and control tumors of 150 mm3 were treated with 10mg/kg of nab-PXL i.v. for 8 days. Five out 6 mice (83.3%) bearing 231TLR4− tumors (yellow circles) had complete response (CR) while 0% CRs was achieved in all other groups. (E) Bio-imaging of representative tumors from groups described in D.(F) Tumor growth of control and TLR4+overexpressing HCC1806 lines.
Treatment with nab-PXL (10mg/kg) produced drastically different results in isogenic lines with modified TLR4 expression (Figs. 6D–F). Within few weeks after PXL treatment, 100% of control mice bearing MDA-MB-231 tumors had recurrence. In sharp contrast, only 1 out of 6 mice (17%) with 231TLR4− tumors had recurrence 127 days post-treatment while rest of the group was disease-free for experimental duration of 6 months (P>0.0001 vs. control, Fig. 6D). Consistently, 100% of mice with 1806TLR4+ clones recurred immediately upon cessation of therapy whereas 40% of mice with tumors lacking TLR4 had no palpable masses after chemotherapy(Fig. 6F). The mean inhibition in the latter group was 95% as compared with 10% inhibition in TLR4-overexpressing group (P>0.001, Fig. 6F). These results unequivocally show that TLR4 plays a major role in determining the response of tumor cells to PXL therapy.
Discussion
TLR4 has been previously shown to be overexpressed in a variety of human cancers (17, 38) and to correlate with tumor progression (39), recurrence (27), metastasis (38, 40), and resistance to PXL therapy (17, 26). However, a pro-tumorigenic effect of TLR4 was mainly demonstrated using LPS (25, 41), a bacterial component that is absent in epithelial malignancies outside of the gastrointestinal tract. More relevant to cancer research is use of PXL, an LPS mimetic (42)and a widely-administrated chemotherapeutic drug(43). PXL-mediated resistance to therapy has been previously shown in ovarian cancer cells in vitro(15, 26). Here, we present extensive evidence demonstrating the chemoresistance-promoting role of TLR4 in breast cancer using both in vitro and in vivo models. We show that TLR4 is overexpressed in clinical BC, and it is specifically responsible for mediating chemoresistance to PXL as evident in increasing IC50 in tumor cells in vitro and causing recurrence in BC in vivo.
TLR4 is considered as a “double-edge sword” with some studies showing its contribution to chemoresistance (17) and others arguing its role in enhancing anti-cancer immunity (44, 45). Here, we show in two distinct BC models that activatedTLR4 pathway drastically diminishes therapeutic efficacy as evidenced by reduced apoptosis of treated cells in vitro and prominent recurrence of treated tumors in vivo. This effect is mediated by NF-κB-mediated transcription of inflammatory and pro-survival genes (Figs. 3, 4). These findings indicate that TLR4 affords tumor evasion from PXL therapy rather than enhancing its cytotoxic effect.
While this conclusion is consistent with the evidence from ovarian cancer models (17, 26, 32), our study demonstrates several new findings underscoring the functional significance of TLR4 in BC. We show that TLR4 is broadly expressed in clinical BC as well as the in majority (68%) of examined BC lines (Fig. 1). We also show that TLR4 is functional in human cancer cells as evidenced by upregulated cytokine expression after treatment with either LPS or PXL(Fig. 1). Previous reports suggested that only mouse TLR4-MD2 complex transduces PXL signaling (18, 19). In contrast, we found that both PXL and nab-PXL phosphorylates NF-κB in TLR4+ human cancer cells followed by up to 15-fold upregulation of multiple targets induced in dose- and time-dependent manners. These data indicate that despite the biochemical difference between human and mouse TLR4-MD2 complexes (42), PXL can enhance chemoresistance of TLR4+ breast tumors in a clinical setting.
The specificity of TLR4 in mediating tumor-promoting PXL effects is shown here by genetic manipulation of TLR4 in two BC lines and by reduced cytotoxicity and cytokine expression following treatment with a monospecific anti-hTLR4 antibody, an extracellular LPS lipid antagonist, and an intracellular inhibitor cyclohexene TAK-242. The fact that several structurally unrelated TLR4 inhibitors significantly increase sensitivity of tumor cells to PXL strongly argues for important role of this receptor in conferring resistance. This is also supported by the evidence that PXL promoted resistance in TLR4+ cells regardless of their genetic background (Table S2) suggesting that TLR4 expression could be an independent prognostic marker for resistance to therapy.
Mechanistically, TLR4-dependent chemoresistance could be mediated by pro-survival autocrine loops some of which have been identified in this study (Tables S3, S4). Although PXL-mediated increases in some cytokines have been previously reported in both human BC patients (14, 46)and experimental BC models (3, 4), this is the first study that analyzed co-expression of inflammatory ligands and receptors in a TLR4-dependent manner. Two TLR4-regulated pathways that seem to be important in this regard are MCP-1•CCR4 and CXCL−1/−2•CXCR1/2. This is consistent with large body of evidence that shows strong correlations between MCP-1(47), CCR4(48), CXCL−1/−2(49), and CXCR1/2(50) with chemoresistance and metastasis. Importantly, in vivo cytokine-activated pathways have the potential to promote tumor cell survival through both autocrine and paracrine loops. This could explain the discrepancy between 2–3 fold shift in IC50 to nab-PXL in tumor cells in vitro(Fig. 2) and drastic 83% reduction in recurrence observed in TLR4-depleted tumors in vivo (Fig. 6). This evidence strongly suggests that therapy-induced activation of TLR4 pathway promotes tumor cell survival not only through autocrine loops, but also by activating secondary pathways in host cells in the tumor environment.
In summary, we show that TLR4 increases resistance to PXL in human BC cells by activating the NF-κB pathway leading to transcription of inflammatory genes that alter the tumor environment through autocrine and paracrine loops. TLR4 signaling in tumor cells may significantly reduce therapeutic efficacy by promoting chronic inflammation, angiogenesis, and recovery of damaged cells. Importantly, this pathway may also promote metastasis. This study, therefore, suggests that blocking TLR4 signaling during PXL therapy is essential for increasing the responsiveness of primary tumors and preventing relapse at secondary sites.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by NIH grant R01CA140732 and by grants from the Illinois William E. McElroy Foundation awarded to S. Ran.
List of Abbreviations
- BC
breast cancer
- CDS
complementary DNA sequence
- DMEM
Dulbecco’s modified Eagle’s medium
- HRP
horseradish peroxidase
- FACS
fluorescence-activated cell sorter
- IC50
inhibitory concentration-50
- IL-6
interleukin 6
- IL-8
interleukin 8
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- NF-κB
nuclear factor kappa-light-chain-enhancer
- PBS
phosphate buffer saline
- PI3K
phosphatidylinositol-3-kinase
- PXL
paclitaxel
- nab-PXL
nanoparticle albumin-bound paclitaxel
- RT-(q)PCR
reverse transcription (quantitative)polymerase chain reaction;TLR4 -Toll like receptor-4
- TNFα
tumor necrosis factor alpha
- SEM
standard error of the mean
- VEGF-A
Vascular Endothelial Growth Factor-A
Footnotes
Disclosure of Potential Conflicts of Interest: Authors declare no conflicting interests.
Authors’ Contributions
S. Rajput performed cell assays, RT-PCR, RT-qPCR, FACS, ELISA, apoptosis analyses and assisted in writing the manuscript. L. Volk-Draper assisted in tissue culture, generation of cell line derivatives, Western blots, cytotoxic assays, and animal studies. S. Ran conceived the hypothesis, designed all experiments, and wrote the manuscript.
References
- 1.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–164. doi: 10.1016/S1470-2045(04)01411-1. [DOI] [PubMed] [Google Scholar]
- 3.Volk LD, Flister MJ, Bivens CM, Stutzman A, Desai N, Trieu V, et al. Nab-paclitaxel efficacy in the orthotopic model of human breast cancer is significantly enhanced by concurrent anti-vascular endothelial growth factor A therapy. Neoplasia. 2008;10:613–623. doi: 10.1593/neo.08302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volk LD, Flister MJ, Chihade D, Desai N, Trieu V, Ran S. Synergy of nab-paclitaxel and bevacizumab in eradicating large orthotopic breast tumors and preexisting metastases. Neoplasia. 2011;13:327–338. doi: 10.1593/neo.101490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosjean J, Kiriakidis S, Reilly K, Feldmann M, Paleolog E. Vascular endothelial growth factor signalling in endothelial cell survival: a role for NFkappaB. Biochem Biophys Res Commun. 2006;340:984–994. doi: 10.1016/j.bbrc.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 6.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 7.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5(Suppl 6):S3–S6. [PubMed] [Google Scholar]
- 9.Damascelli B, Cantu G, Mattavelli F, Tamplenizza P, Bidoli P, Leo E, et al. Intraarterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007): Phase II study of patients with squamous cell carcinoma of the head and neck and anal canal: preliminary evidence of clinical activity. Cancer. 2001;92:2592–2602. doi: 10.1002/1097-0142(20011115)92:10<2592::aid-cncr1612>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 11.Kirikae T, Ojima I, Kirikae F, Ma Z, Kuduk SD, Slater JC, et al. Structural requirements of taxoids for nitric oxide and tumor necrosis factor production by murine macrophages. Biochem Biophys Res Commun. 1996;227:227–235. doi: 10.1006/bbrc.1996.1494. [DOI] [PubMed] [Google Scholar]
- 12.White CM, Martin BK, Lee LF, Haskill JS, Ting JP. Effects of paclitaxel on cytokine synthesis by unprimed human monocytes, T lymphocytes, and breast cancer cells. Cancer Immunol Immunother. 1998;46:104–112. doi: 10.1007/s002620050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee LF, Haskill JS, Mukaida N, Matsushima K, Ting JP. Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol Cell Biol. 1997;17:5097–5105. doi: 10.1128/mcb.17.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang AC, Su QB, Wu FX, Zhang XL, Liu PS. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur J Clin Invest. 2009;39:157–164. doi: 10.1111/j.1365-2362.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, et al. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 17.Silasi DA, Alvero AB, Illuzzi J, Kelly M, Chen R, Fu HH, et al. MyD88 predicts chemoresistance to paclitaxel in epithelial ovarian cancer. Yale J Biol Med. 2006;79:153–163. [PMC free article] [PubMed] [Google Scholar]
- 18.Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, Nishijima M. Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem. 2000;275:2251–2254. doi: 10.1074/jbc.275.4.2251. [DOI] [PubMed] [Google Scholar]
- 19.Zimmer SM, Liu J, Clayton JL, Stephens DS, Snyder JP. Paclitaxel binding to human and murine MD-2. J Biol Chem. 2008;283:27916–27926. doi: 10.1074/jbc.M802826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaks-Zilberman M, Zaks TZ, Vogel SN. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine. 2001;15:156–165. doi: 10.1006/cyto.2001.0935. [DOI] [PubMed] [Google Scholar]
- 21.Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007;67:4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Qu Y, Niu XL, Sun WJ, Zhang XL, Li LZ. Autocrine production of interleukin-8 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cytokine. 2011;56:365–375. doi: 10.1016/j.cyto.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Pei Z, Lin D, Song X, Li H, Yao H. TLR4 signaling promotes the expression of VEGF and TGFbeta1 in human prostate epithelial PC3 cells induced by lipopolysaccharide. Cell Immunol. 2008;254:20–27. doi: 10.1016/j.cellimm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Tang XY, Zhu YQ, Wei B, Wang H. Expression and functional research of TLR4 in human colon carcinoma. Am J Med Sci. 2010;339:319–326. doi: 10.1097/MAJ.0b013e3181cef1b7. [DOI] [PubMed] [Google Scholar]
- 25.Ikebe M, Kitaura Y, Nakamura M, Tanaka H, Yamasaki A, Nagai S, et al. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J Surg Oncol. 2009;100:725–732. doi: 10.1002/jso.21392. [DOI] [PubMed] [Google Scholar]
- 26.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–4363. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Reyes S, Fernandez JM, Gonzalez LO, Aguirre A, Suarez A, Gonzalez JM, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2010;60:217–7726. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang JJ, Wu HS, Wang L, Tian Y, Zhang JH, Wu HL. Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J Gastroenterol. 2010;16:2881–2888. doi: 10.3748/wjg.v16.i23.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6:928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 32.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Alvero AB, Silasi DA, Mor G. Inflammation, cancer and chemoresistance: taking advantage of the toll-like receptor signaling pathway. Am J Reprod Immunol. 2007;57:93–107. doi: 10.1111/j.1600-0897.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 34.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doan HQ, Bowen KA, Jackson LA, Evers BM. Toll-like receptor 4 activation increases Akt phosphorylation in colon cancer cells. Anticancer Res. 2009;29:2473–2478. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang Y, Yuan J, Qin W, Liu F, Wang F, et al. Toll-like receptor 4 ligation confers chemoresistance to docetaxel on PC-3 human prostate cancer cells. Cell Biol Toxicol. 2012;28:269–277. doi: 10.1007/s10565-012-9221-2. [DOI] [PubMed] [Google Scholar]
- 37.Fukazawa H, Noguchi K, Murakami Y, Uehara Y. Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol Cancer Ther. 2002;1:303–309. [PubMed] [Google Scholar]
- 38.Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, Del Casar JM, Lamelas ML, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee CH, Wu CL, Shiau AL. Toll-like receptor 4 signaling promotes tumor growth. J Immunother. 2010;33:73–82. doi: 10.1097/CJI.0b013e3181b7a0a4. [DOI] [PubMed] [Google Scholar]
- 40.Liao SJ, Zhou YH, Yuan Y, Li D, Wu FH, Wang Q, et al. Triggering of Toll-like receptor 4 on metastatic breast cancer cells promotes alphavbeta3-mediated adhesion and invasive migration. Breast Cancer Res Treat. 2011;133:853–863. doi: 10.1007/s10549-011-1844-0. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Reyes S, Fernandez JM, Gonzalez LO, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2010;60:217–226. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawasaki K, Nogawa H, Nishijima M. Identification of mouse MD-2 residues important for forming the cell surface TLR4-MD-2 complex recognized by anti-TLR4-MD-2 antibodies, and for conferring LPS and taxol responsiveness on mouse TLR4 by alanine-scanning mutagenesis. J Immunol. 2003;170:413–420. doi: 10.4049/jimmunol.170.1.413. [DOI] [PubMed] [Google Scholar]
- 43.Wick N, Saharinen P, Saharinen J, Gurnhofer E, Steiner CW, Raab I, et al. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol Genomics. 2007;28:179–192. doi: 10.1152/physiolgenomics.00037.2006. [DOI] [PubMed] [Google Scholar]
- 44.Molavi O, Ma Z, Hamdy S, Lavasanifar A, Samuel J. Immunomodulatory and Anticancer Effects of Intra-Tumoral Co-Delivery of Synthetic Lipid A Adjuvant and STAT3 Inhibitor, JSI-124. Immunopharmacol Immunotoxicol. 2008;31:214–221. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 45.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 46.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egunsola AT, Zawislak CL, Akuffo AA, Chalmers SA, Ewer JC, Vail CM, et al. Growth, metastasis, and expression of CCL2 and CCL5 by murine mammary carcinomas are dependent upon Myd88. Cell Immunol. 2012;272:220–229. doi: 10.1016/j.cellimm.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li JY, Ou ZL, Yu SJ, Gu XL, Yang C, Chen AX, et al. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res Treat. 2012;131:837–848. doi: 10.1007/s10549-011-1502-6. [DOI] [PubMed] [Google Scholar]
- 49.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res. 2009;15:2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.