Abstract
Female rats exhibit greater intake and motivation to self-administer cocaine. In females but not males, isolation by itself is a stressor, which could lead to increased drug intake. Therefore, we hypothesized that social housing would buffer against stress and reduce the motivation to self-administer cocaine primarily in females. Male and female Sprague-Dawley rats were housed individually or in same-sex pairs. The individually housed rats and one of each pair were allowed to self-administer (SA) a low dose of cocaine (0.2 mg/kg/inf) on a fixed ratio (FR1) schedule for one week. Motivation for cocaine SA was measured for an additional 2 weeks on a progressive ratio schedule. Isolated females had greater cocaine-intake on the FR1 schedule and greater motivation to take cocaine than males. Pair-housing in females, but not males, attenuated the motivation to take cocaine. Isolated females, but not males, showed escalation of their motivation to take cocaine, which was attenuated by pair housing of females. Concluding, the motivation to take cocaine escalates in females but not males, and pair-housing of females attenuates this escalation.
Keywords: sex differences, social, addiction, escalation
The majority of drug users are men, but the gender gap is closing. Women escalate drug use more rapidly than men and can find it harder to quit [1,2]. In rodents sex differences in the impact of drugs of abuse have also been observed. Female rats are more sensitive to the rewarding effects of cocaine than male rats. Females develop a cocaine-induced conditioned place preference faster and at lower doses than males [3]. Self-administration studies using psychomotor stimulants find that females acquire SA more rapidly than males at low doses [4,5].
Drug intake increases as an individual transitions from infrequent to regular and finally compulsive drug use [6]. The escalation of drug use is a major component of the addiction process, encompassing both the increased intake as well as the increased time and energy devoted to acquiring the drug (DSM-IV). In animal models of this phenomenon, rats exposed to extended daily access show increased drug intake over time, in contrast to animals with short daily access [7]. Females are more sensitive to escalation after extended daily access [8]. Exposure to a progressive ratio (PR) schedule of reinforcement, in which the animal has to increase the effort for each subsequent infusion can also result in escalation of drug use; however, this has only been studied in male rats [9].
Stressors increase drug intake and enhance reinstatement of drug seeking [10,11], whereas positive stimuli (e.g., environmental enrichment) decrease drug intake [12,13]. Social housing can reduce the effects of stressors in both male and female rats [14–16] and pair or group housing can reduce intake of heroin and amphetamine in male rats [12,17].
Since isolation is a stressor especially for female rats and pair-housing has been shown to buffer the effects of stress [15,16,18], we hypothesized that pair housed females would have reduced motivation for cocaine compared to isolates, whereas the differences between housing groups would be less pronounced in males.
Male and female Sprague Dawley rats (age 42 days) were purchased from Harlan (Indianapolis, IN). After arrival they were housed individually (6 males, 6 females) or in same-sex pairs (12 males, 12 females (6 experimental animals and 6 partners for each sex)). They were housed in a 14 h light: 10 h dark cycle (lights off at 7.00 hr). Food and water were available ad libitum, and all experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines on laboratory animal use and care, using a protocol approved by the University Committee on Use and Care of Animals.
After 2 weeks of isolation or pair housing, all of the individually housed animals and one of each of the pairs received implants of indwelling intravenous jugular catheters connected to a back port. Catheters were constructed by gluing silastic tubing (Silastic tubing, 0.51 mm I.D. × 0.94 mm O.D., Dow Corning, Midland, MI) to an external guide cannula (22 gauge guide cannula; Plastics One, Roanoke, VA) using cranioplastic cement. A polypropylene mesh was secured to the bottom of the cannula using this same cement. The exterior part of the backport was protected by a stainless steel tube (8 by 9 mm). Rats received an injection of buprenorphine (0.02 mg/kg; s.c.) 30 minutes before they were anesthetized with isoflurane (5% isoflurane in oxygen). The free end of the silastic tubing of the catheter apparatus was inserted into the right jugular vein of the animal and secured using 4.0 silk sutures around the tubing and the venous tissue. The catheter port exited dorsally from the animal. After successful implantation, the animal’s catheter was flushed with 0.2 ml each of heparin (30 U/ml in 0.9% sterile saline) and gentamicin (3 mg/kg) to prevent clotting and infection, respectively. A dummy stylet was then inserted into the port opening. Two days after surgery, catheters were flushed with 0.2 ml of heparin (30 U/ml in 0.9% sterile saline) and gentamicin (3 mg/kg), and with gentamicin every day after that. Catheters were flushed with 0.1 ml of sterile saline prior to each session and with gentamicin (3 mg/kg) following each self-administration session and on weekends. Female’s estrous cycle stage was monitored via daily vaginal lavage and microscopic examination of cell types collected immediately prior to the self-administration session. Catheter patency was checked weekly using a solution of Pentothal® (thiopental sodium, 15mg/ml, 0.15–0.25ml) in sterile water.
Self-administration was performed in standard operant chambers (Med Associates, Inc., Georgia, VT) where the animals could nose poke into the active hole for cocaine or in an inactive hole, which had no consequences. Rats were connected to the infusion syringe via a swivel mounted to a counter balanced arm, which allowed animals to move freely in the testing environment.
All self-administration sessions occurred daily for 5 days a week (weekends off) between 9.00 and 15.00 hr. FR1: One week after surgery, animals were transported to the SA chambers and allowed to nose-poke for cocaine (0.2 mg/kg/inf) for 2 hours, or a maximum of 100 infusions, using a FR1 schedule of reinforcement. Each nose poke in the active hole resulted in a 50-µl infusion of cocaine HCL delivered over 2.8 seconds accompanied by the activation of a stimulus light in the active hole. Each infusion during this portion of the test was followed by a 5-second timeout period, during which time nose pokes were recorded yet had no consequences. Animals were subjected to the FR1 schedule for 5 consecutive days. PR: All animals were then transferred to a PR schedule of reinforcement for 2 weeks. The PR schedule escalated through an exponential series of response ratios: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208…. adapted from Richardson and Roberts [19]. Reaching a higher final ratio (i.e., breaking point, BP) indicates that the animal is more motivated for cocaine, as they are willing to work harder to obtain each subsequent infusion. The number of infusions, nose pokes in the active hole, nose pokes in the inactive hole, and BP were recorded. The PR session terminated after 6 hours, or if 1 hour elapsed without an infusion.
Statistical analyses were performed with SPSS (version 18.0). For the FR1 data, the number of infusions and nose-pokes in the active and inactive hole were analyzed with repeated measures ANOVA, with day as within-subject variable and sex and housing as between-subject variables. For the PR data, BP, number of infusions, and nose-pokes in the active and inactive hole were analyzed with day and week as within-subject variable and sex and housing as between subject variables. Sphericity assumed modeling, with Greenhouse-Geisser and Huynh-Feldt adjustments, was applied [20]. Acquisition of cocaine self-administration was considered the first of three consecutive days in which the animal had twice as many nose-pokes in the active hole compared to the inactive hole. Percentage of animals per group that acquired was analyzed using a Kaplan-Meier survival analysis.
No significant group differences were found in the acquisition of cocaine self-administration (Chi square (Mantel-Cox) = 3.237, p=0.356). Average days to acquire per group ± SEM: individual males, 1.7 ± 0.49; paired males, 4.7 ± 1.98; individual females, 2.8 ± 0.75; paired females, 2.8 ± 1.33.
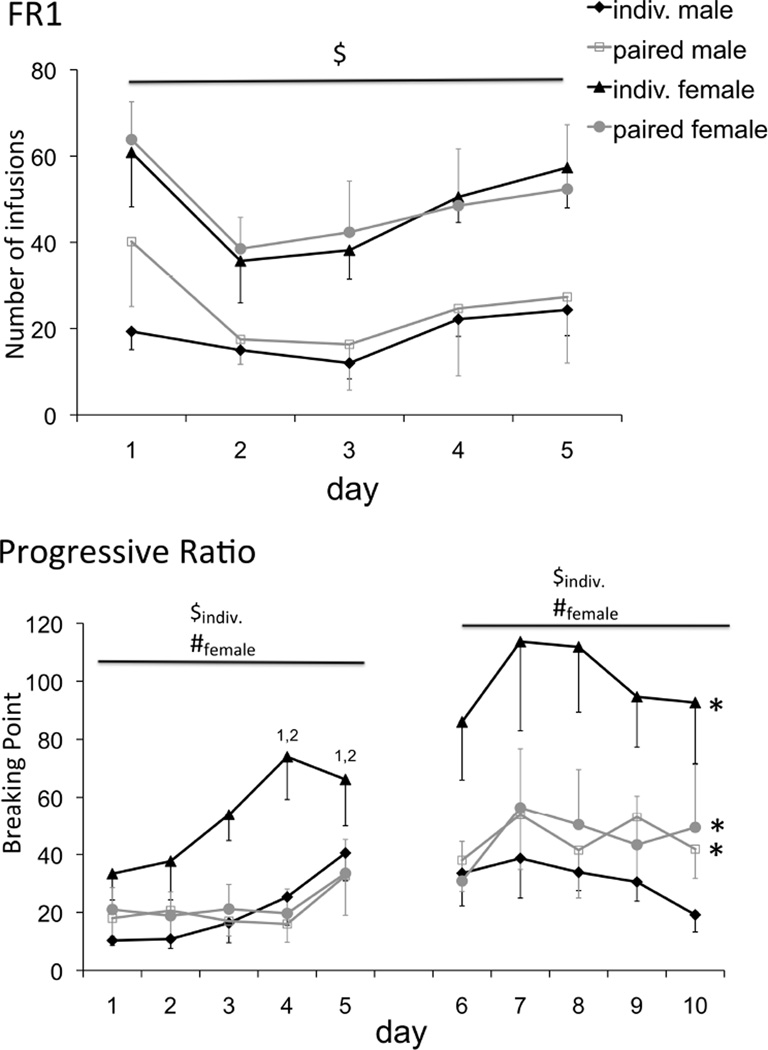
The number of infusions that animals self-administered on the FR1 schedule differed between males and females (F1,20=9.510, p=0.006). As illustrated in Figure 1A, individually housed females had more infusions than their male counterparts (p=0.025). The sex difference was not significant for pair-housed rats (p=0.067). Even though a significant effect of day was found (F1,20=8.910, p≤0.001), pos-hoc analysis showed no significant differences in cocaine-intake between days for any of the groups. (Figure 1A). When the number of active nose-pokes was analyzed there was again a significant effect of time (F4,80=13.297, p≤0.001) and sex (F1,20=8.931, p=0.007). On subsequent individual group comparisons, only the isolated males and females differed from each other with the females having more nose-pokes than males, although there was a trend for the paired males and females to differ (isolated: p=0.042; paired: p=0.053). No significant effects were found for the number of inactive nose-pokes (Table 1).
Figure 1.
A) Number of infusions during the 5 days on the FR1 schedule of reinforcement. Isolated females had significant more infusions over the 5-day period than their counterpart males (* p≤ 0.05). B) The average breaking points per week reached on the PR schedule of reinforcement. Significant difference between week 1 and 2 (*p≤ 0.05), significant sex differences within housing conditions for the specified housing condition($p≤ 0.05), and significant differences between individually and pair housed rats within sex for the specified sex (#p≤ 0.05). Significantly different from day 1 and 2 for the isolated females (1,2 p≤ 0.05).
Table 1.
Mean number of infusions, active/inactive nose-pokes (± SEM) during the FR1 and PR, and mean breaking points (± SEM) reached on the PR.
| indiv. male | paired male | indiv. female | paired female | |||
|---|---|---|---|---|---|---|
| FR1 | infusions | 18.6 (3.49) | 25.2 (11.26) | 48.5 (7.74)$ | 49.1 (10.28) | |
| active pokes | 25.0 (4.79) | 34.5 (14.33) | 62.8 (9.36)$ | 70.2 (16.98) | ||
| inactive pokes | 5.2 (0.85) | 8.3 (4.90) | 12.5 (3.99) | 37.7 (25.54) | ||
| PR | Breaking point | week 1 | 20.7 (5.16) | 20.9 (8.38) | 53.0 (9.66)$ | 22.8 (8.50)# |
| week 2 | 31.1 (6.07) | 45.6 (15.58)* | 99.8 (19.14)*$ | 46.0 (15.79)*# | ||
| infusions | week 1 | 5.5 (0.85) | 5.4 (1.22) | 9.1 (0.65) | 5.7 (1.33) | |
| week 2 | 7.0 (0.95)* | 8.1 (1.38)* | 11.6 (0.82)* | 8.0 (1.76)* | ||
| active pokes | week 1 | 84.1 (23.61) | 87.0 (34.91) | 232.8 (42.61)$ | 98.0 (41.15)# | |
| week 2 | 132.1 (27.40) | 200.9 (66.76) | 485.7 (95.24)*$ | 230.0 (85.38)*# | ||
| inactive pokes | week 1 | 7.9 (1.32) | 11.6 (3.39) | 15.8 (3.42) | 15.8 (12.27) | |
| week 2 | 11.3 (2.52) | 6.1 (2.46) | 15.0 (7.26) | 16.6 (7.78) | ||
Significant difference between week 1 and 2 (*p≤ 0.05), significant sex differences within housing conditions ($p≤ 0.05), and significant differences between individually and pair housed rats within sex (#p≤ 0.05).
Responding on a PR schedule varied by week (F1,20=22.376, p<0.001), day (F4,80=4.154, p=0.009), and sex (F1,20=6.268, p=0.021), with a week by day interaction (F4,80=4.380, p=0.006) and a sex by housing interaction (F1,20=5.485, p=0.03). As illustrated in Figure 1B, individually housed females showed higher BP’s than pair-housed females and male counterparts, with an increase in the motivation to take cocaine over time (see also table 1). In both the first and the second weeks, individually housed females had higher BP’s than paired females (resp. p=0.014 and p=0.019). In addition, individually housed females showed higher BP’s than their male counterparts during both weeks 1 and 2 (resp. p=0.009 and p=0.003). Individually housed females and pair-housed males and females had a higher BP during week 2 compared to week 1 (resp. p≤0.001, p=0.04, and p=0.045). BP of isolated females increased during the first week, with higher BP on day 4 and 5 than on day 1 (p=0.010 and p=0.040, resp.) and day 2 (p=0.023 and p=0.036, resp.). The other groups did not differ across days within a week.
The number of infusions also changed by week (F1,20=44.003, p<0.001) with a day by week interaction (F4,80=3.271, p=0.015). No group differences were found, but all groups showed an increase in number of infusions between the first and the second week on the PR schedule (indiv. males: p=0.025; paired males: p=0.001; indiv. females: p=0.001; paired females: p=0.009)(Table 1).
Active pokes also varied by the week (F1,20=24.378, p<0.001), day (F4,80=4.439, p=0.004), and by sex (F1,20=7.127, p=0.015), with a week by day interaction (F4,80=5.207, p=0.002) and a sex by housing interaction (F1,20=5.121, p=0.035)(Table 1). Only females showed a significant increase in active nose-pokes between weeks 1 and 2 (individual: p≤0.001; paired: p=0.027), paired males showed a trend towards significance (p=0.055). A sex difference was only observed in isolated animals, with males having a lower number of nose-pokes compared to females (week 1: p=0.009; week 2: p=0.003). Isolated females poked significantly more in the active hole than pair-housed females during both week 1 and 2 (p=0.016 and p=0.023 resp.) No significant main or interaction effects were found for the number of inactive pokes (Table 1). The stage of the estrous cycle did not affect cocaine self-administration behavior on either schedule of reinforcement.
Housing conditions affected the escalation of the motivation to self-administer cocaine in female, but not male, rats. Pair-housing of female rats with a same-sex partner attenuated the motivation to take cocaine relative to isolates, whereas in males, irrespective of housing conditions, the motivation did not change over time during 2 weeks of self-administration on a PR schedule of reinforcement. In addition, a sex difference was found with isolated females showing greater motivation for cocaine than their male counterparts. Our data confirm the higher risk for escalation of drug use in females compared to males; although in isolated rats only.
The higher cocaine intake on the FR1 schedule of reinforcement, found in females relative to male intake, corroborates previous results from our laboratory and others [4,5], although no sex differences in acquisition/cocaine intake on a FR1 schedule have also been reported [21]. Pair housing did not affect cocaine intake on the FR1 schedule, which is similar to findings by Bozarth et al. [17]. This indicates that when cocaine is easily available, such as on an FR1 schedule, social housing is not able to affect intake of a low dose of cocaine. However, when the effort required for each infusion increases, as on a PR schedule, the social environment modulates the motivation for taking cocaine. The low dose of cocaine employed in the present study produced modest BP’s, which may have obscured potential protective effects of pair housing in males, in contrast to the higher BP’s associated with the escalation of cocaine intake in females.
Escalation of drug use over time is one of the DSM-IV addiction criteria, and this has been modeled in several self-administration paradigms. Extended access self-administration of cocaine (6 hrs/day) has been shown to result in an escalation of drug intake in rats [22,23]. Self-administration of cocaine on a PR schedule, when there is limited prior training, also results in escalation of drug use [9], encompassing both DSM-IV criteria of escalation of intake and the increased effort expended for obtaining the drug. In the current study the dose of cocaine was lower than in some other studies, suggesting that at a low dose males do not develop escalation of cocaine intake on a PR schedule. Evidence for a dose effect on escalation of drug intake was also found in long-access self-administration (LgA), with escalation of drug use only occurring for males on a relatively high dose of cocaine (2 mg/kg/inf) and not on a lower dose of cocaine (0.5 mg/kg/inf) [23]. Although Gipson et al. have shown escalation of cocaine intake in LgA males on a low dose of cocaine (0.1 mg/kg/inf) [13]. In this later study animals were food restricted and this can increase drug intake [24], and may have led to an escalation of cocaine intake that would not typically occur in non-food restricted animals. In these food restricted males, social housing and environmental enrichment were able to prevent escalation of cocaine use [13]. Food restriction is a stressor [25]. Thus, the ability of a positive social environment to protect against addiction may be greater in more sensitive populations, such as stressed males and females. The fact that females in the current study did show escalation of cocaine intake on Page 10 of 17 the low dose of 0.2 mg/kg/inf is consistent with the human situation where women show a faster escalation than men [2].
Summarizing, pair-housing differentially affect the motivation to self-administer cocaine in male and female rats, decreasing motivation in females but not males. Isolation of females but not males results in an escalation of drug intake, which is also attenuated by pair housing in females only.
Highlights.
Male and female rats were either isolated or housed in same sex pairs
Rats were allowed to self-administer a low dose of cocaine
We find isolated females showed increased motivation for cocaine
Motivation for cocaine was higher in females than males
Escalation of cocaine intake was prevented by pair housing in females
Acknowledgements
Financial support for this research was contributed by NIH grant R01-DA012677 to JBB and R21DA032747 to CW and JBB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug, use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med. 2010;7:402–413. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- 3.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 5.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 6.Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, et al. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- 7.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DCS, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, You Z-B, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 12.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 13.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214:557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, et al. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 15.Westenbroek C, Snijders TAB, den Boer JA, Gerrits M, Fokkema DS, Ter Horst GJ. Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Hormones and Behavior. 2005;47:620–628. doi: 10.1016/j.yhbeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, Den Boer JA. Gender-specific effects of social housing in rats after chronic mild stress exposure. ProgNeuropsychopharmacolBiolPsychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 17.Bozarth M, Murray A, Wise R. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacology Biochemistry and Behavior. 1989;33:903–907. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- 18.Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58:1085–1089. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 19.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 20.Quintana SM, Maxwell SE. A Monte Carlo comparison of seven ε-adjustment procedures in repeated measures designs with small sample sizes. Journal of Educational and Behavioral Statistics. 1994;19:57–71. [Google Scholar]
- 21.Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Research. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 23.Mantsch JR, Yuferov V, Mathieu-Kia A-M, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- 24.Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 25.Duclos M, Bouchet M, Vettier A, Richard D. Genetic differences in hypothalamic-pituitary-adrenal axis activity and food restriction-induced hyperactivity in three inbred strains of rats. J Neuroendocrinol. 2005;17:740–752. doi: 10.1111/j.1365-2826.2005.01367.x. [DOI] [PubMed] [Google Scholar]