Abstract
The recent discovery of a simple method for making induced pluripotent stem cells (iPSC) from human somatic cells was a major scientific advancement that opened the way for many promising new developments in the study of developmental and degenerative diseases. iPSC have already been used to model many different types of neurological diseases, including autism, schizophrenia, Alzheimer’s Disease and Parkinson’s Disease. Because of their pluripotent property, iPSC offer the possibility of modeling human development in-vitro. Their differentiation seems to follow the developmental timeline and obeys environmental cues. Clinically relevant phenotypes of neurodegenerative pathologies have also been observed using iPSC derived human neuronal cultures. Options for treatment are still some way off. Although some early research in mouse models has been encouraging, major obstacles remain for neural stem cell (NSC) transplantation therapy. However, iPSC now offer the prospect of an unlimited amount of human neurons or astrocytes for drug testing. The aim of this review is to summarize the recent progress in modeling neural development and neurological diseases using iPSC and to describe their applications for aging research and personalized medicine.
Keywords: Stem cells, neurodegeneration, neural development, human disease model, transplantation
1. Introduction
The recent discovery of a simple method for making induced pluripotent stem cells (iPSC) from somatic cells was a major scientific advancement that opened the way for many promising new developments in the study of human developmental and degenerative diseases. Sir John Gordon and Shinya Yamanaka have just been awarded the Nobel Prize in 2012 for Medicine for their contribution to the reprogramming technology, highlighting the significance of this scientific breakthrough. Since the publication of Dr. Yamanaka’s work on generation of induced pluripotent stem cells (iPSC), significant progress has already been made as human iPSCs have been used to model many different types of neurological diseases, including autism, schizophrenia, Alzheimer’s Disease and Parkinson’s Disease. The aim of this review is to summarize the recent progress in neurological disease modeling using iPSC and to describe their applications and implications in aging research. We will first describe the current methodology for generating neurons, then we will discuss the different in-vitro systems attempting to model neurodevelopmental and neurodegenerative diseases. We will also cover how these models could be applied in replacement therapy and medicinal drug development.
2. There is more than one way to derive human neurons
Currently, there are three different methods to derive neurons from somatic cells. Somatic cells such as skin fibroblasts can be reprogrammed to become iPSC and then differentiated into neurons and glia. They can also be transdifferentiated to become induced neural stem cells (iNSC), or directly transdifferentiated to become induced neurons (iN). It is useful to understand the potentials and limitations for each of these three methods, for their inherent properties and downstream applications are quite different. In this section, we will discuss these three different methods.
2.1 Generation of induced pluripotent stem cells (iPSC) from human somatic cells
The generation of iPSC was first developed by Yamanaka’s group. They performed a screen of 24 candidate genes, based on the hypothesis that these genes controlled embryonic stem cell identity. They narrowed the candidates down to four factors, Oct3/4, Sox2, Klf4 and c-Myc. When these factors were introduced to mouse embryonic fibroblasts (MEF) with virus, the MEFs were reprogrammed to iPSCs (Takahashi and Yamanaka, 2006). The cells were morphologically similar to the mouse embryonic stem cells and exhibited pluripotent stem cell markers, SSEA-3, SSEA-4, Tra-1-60 and Tra-1-81. The pluripotent potential of the iPSC was tested by teratoma assays, which showed that the iPSC can differentiate to the three different germ layers, namely ectoderm, mesoderm and endoderm. Following their initial discovery in the mouse, the group subsequently showed that the same four factors could turn human adult dermal fibroblasts to iPSC (Takahashi et al., 2007). Similarly, the human iPSCs have a morphology resembling the human embryonic stem cells, such that they grow in condensed clusters, with scanty cytoplasms, and they express pluripotent stem cell markers. Furthermore, they can be differentiated into cell types with three germ layers, for example, beta-III-tubulin positive neuron-like cells.
The reprogramming of human fibroblasts was reproduced by Yu et al. (Yu et al., 2007), using slightly different factors, Oct4, Sox2, Nanog and Lin28. It has also been shown that iPSC could be derived from other types of somatic cells, for example, pancreatic beta-cells (Stadtfeld et al., 2008), liver (Aasen et al., 2008), and keratinocytes (Aoi et al., 2008). However in some terminally differentiated cells, such as B-cell lymphocytes (Hanna et al., 2008) and neurons (Kim et al., 2011b), suppression of p53 is necessary to improve the reprogramming efficiency, suggesting that although cell type may not be restrictive for reprogramming, the efficiencies may be significantly different and some cell types may require additional factors.
2.2 Generation of induced neurons (iN) from human dermal fibroblasts
In addition to reprogramming somatic cells into iPSC, methods of transdifferentiation have been developed to directly reprogram one somatic cell type into another. Transdifferentiation is a process by which one cell type is transformed to take on the identity of another cell type. In contrast to reprogramming, where one cell type is first reprogrammed to the pluripotent state and then differentiated to the cell type of interest, transdifferentiation directly transforms one cell type into another (Slack, 2007). Some examples include retinal epithelial cells converted into muscle-like cells (Choi J Fau - Costa et al.), and mature B lymphocytes converted into macrophages (Xie et al., 2004). Douglas Melton’s group has used a similar approach to that applied in the discovery of the four factors in iPSC to identify the combination of transcription factors important to the development of islet cells, which when added to pancreatic exocrine cells, can transdifferentiate them into pancreatic islet cells in vivo (Zhou et al., 2008).
Several groups have shown that human fibroblasts can be transdifferentiated into neurons (Ambasudhan et al., 2011; Pang et al., 2011; Qiang et al., 2011). Vierbuchen et al., using a similar approach to that used by Yamanaka and Melton’s groups, screened 19 genes which were expressed in neurons specifically and involved in neural development or epigenetic modifications. They found that the Brn2 (also known as Pou3f2), Ascl1 and Myt1l transcription factors, which are key regulators of neural lineage, could convert mouse embryonic stem cells and postnatal mouse fibroblasts into neurons (Vierbuchen et al., 2010). With the addition of NeuroD1, a basic helix–loop–helix transcription factor, human dermal fibroblasts could also be transdifferentiated into neurons. The process took about 3 weeks. The neurons exhibited electrophysiologic properties, demonstrating that they could fire an action potential when stimulated with an electric current. When the iNs were co-cultured with mouse cortical cultures, they developed spontaneous activities and were sensitive to the GABAA receptor inhibitor Picrotoxin and to AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid), and the receptor blocker CNQX (Pang et al., 2011), demonstrating that these methods are sufficient to generate different subtypes of neurons.
2.3 Generation of induced neural stem cells (iNSC)
In addition to turning fibroblasts into neurons directly by transdifferentiation, other researchers have demonstrated that it is possible to turn fibroblasts directly into neural stem cells (NSC). Several groups have reported transdifferentiation of mouse fibroblasts into NSCs with various combinations of transcription factors (Han et al., 2012; Kim et al., 2011a; Lujan et al., 2012; Ring et al., 2012; Thier et al., 2012). It seems that Sox2 is essential in these cocktails. Ring et al. showed that by introducing Sox2 alone, fibroblasts could turn into NSCs. The NSCs showed a self-renewal property, and could differentiate into neurons, astrocytes and oligodendrocytes. The neurons had proper electrical activities and could form synapses (Ring et al., 2012). Kim et al. used transient transfection of the four reprogramming factors, Oct4, Sox2, Klf4 and c-Myc (Kim et al., 2011a). They demonstrated that the iNSC derived from exogenous expression of Sox2, Klf4 and c-Myc, (which are normally expressed in NSC), were enough for transdifferentiation, and sustained expression was not necessary. Their iNSC could be maintained in culture up to 50 passages (Thier et al., 2012). Ring et al. showed that iNSC could also be made from human fetal fibroblasts. When the iNSCs were transplanted into the mouse brain, they did not form tumors. It is not known whether these methods could work with human adult fibroblasts. It is also not clear that the iNSC are patternable and have the potential to generate all the different kinds of neurons in the brain.
3. Application of pluripotent stem cells (PSC) in studying human neural development
Our understanding of development has been significantly aided by studies performed in lower organisms, for human development is difficult to study in vivo due to the inaccessibility of the human tissues. Because of their pluripotent property, the human PSC offer the possibility of modeling human development, assuming that they retain their developmental program in vitro. Recently, several lines of research suggest that PSC do partially recapitulate the developmental program and can self-organize to form three-dimensional structures mimicking the shape of an organ. Their differentiation seems to follow the developmental timeline and obey environmental cues. This suggests that they may be useful for disease modeling, organogenesis, and replacement therapy in transplantation applications.
3.1 Developmental milestones and patterns are recapitulated in the PSC
We have a very limited ability to study neural development directly in humans. The study of human subjects is limited by obvious physical and ethical constraints. With the use of genetic engineering in other species, from c-elegans to rodents, we have gained a general understanding of how the nervous system develops. Now, with the application of the PSC method we may finally be able to study the development of the human nervous system in a human PSC model. The key question is, do the cells derived from PSC mimick the basic principles of human neural development? There is some encouraging evidence showing that the PSC do recapitulate the time-line of neural development and that they also follow the endogenous guidance cue to arrive at their proper destination (Eiraku et al., 2008; Gaspard et al., 2008).
3.1.1 PSC form three-dimensional structures mimicking the laminar structure of the brain and follow the developmental time-line
Sasai’s group demonstrated that neurospheres formed by PSC when left growing in culture will over time lateralize into a three dimensional structure, similar to the six layers in the cortex. They noticed that N-cadherin+ neural progenitors first accumulate in the Sox1+ outer regions, then these progenitors gradually expanding inward. When the neurospheres were sectioned, the group observed by immunofluorescent staining that Reelin+ and Tbr1+/Reelin−/Bf1+ neurons appeared on days 7 and 8, while Ctip2+/ Emx1+ neurons were seen only on and after day 10. Brn2+/Bf1+ cells were substantially increased during days 10–13. These Brn2+ neurons also expressed other upper CP markers such as Cux1 and Satb2 (Eiraku et al., 2008). The group extended their study, by conducting a birth-date analysis, pulsing BrdU during days 8-14 and analyzing the BrdU positive cells on day 16. They found the birth of Reelin+ (layer I), Tbr1+/Bf1+ (layer VI), Ctip2+ /Emx1+ (layer V), and Brn2+/Tuj1+ (layer II/III) neurons peaked on days 8–10, 9 to 10, 10 to 11, and 12 to 13, respectively. These results demonstrated that the layer-specific neurons from mouse embryonic stem cell (mESC)-derived cortical progenitors were generated in the same temporal order previously observed in the embryonic mouse cortex.
More recent work from Sasai’s group suggests that human ESC have the ability to self-organize and form three-dimensional structures resembling the optic cup (Nakano et al., 2012). They observed that the human embryonic stem cell (hESC)-derived optic cup is larger than the mouse ESC-derived optic cup, likely due to species differences. The hESC-derived neural retina can form multilayered tissue, including both rods and cones. This work further confirms that the PSC can self-organize to form structures resembling the nervous system. This is a promising finding suggesting that it may be possible to use PSC for organogenesis in transplantation applications and disease modeling.
3.1.2 Transplantation of NSC shows guidance
Another line of work from Gaspard et al. using mouse embryonic stem cells (mESC), has demonstrated that the NSC derived from the mESC pass through the normal stages of developmental progression and can generate neurons that follow the developmental timeline(Gaspard et al., 2008). They differentiated the mESC with a sonic hedgehog inhibitor, cyclopramine, to drive the progenitors to the dorsal cortical fate. They then evaluated these pyramidal neurons to see if they express layer specific markers. Although they were able to observe lower layer specific markers over time, they were not able to detect markers associated with upper layers, suggesting that development of upper layer neurons requires in vivo conditions or a three dimensional cue.
Using a TAU (MAPT)-GFP knock-in ESC line, Gaspard et al. studied the axonal projections of grafted ESC derived neurons. After differentiating the TAU-GFP mESC for 12-17 days, the differentiated cells were grafted into the frontal cortex of neonatal mice. They noticed that the neurons had projections which followed cortical efferents, suggesting that the neurons generated had cortical identity. Furthermore, they observed that the projections were area specific. For example, transplanted cells in the cortex only projected to the visual and limbic area, but not to the motor or somatosensory cortices. These are striking findings which hint that the neural progenitors derived from the ESCs can differentiate into a variety of neurons and still contain the cues for proper projection to the target area. This could have ramifications for developing potential replacement therapy under optimal circumstances.
3.2 Modeling developmental neurological diseases
PSC lines modeling pediatric neurological disorders have been developed and demonstrated to be a useful addition to the current developmental disease model system. This topic has been reviewed elsewhere (Kunkanjanawan et al., 2011, Chailangkarn, 2012 #1600). We will cover a few of the studies as examples of successes, dilemmas and new opportunities.
3.2.1 Using iPSC to model a monogenetic neural developmental disorder: Rett Syndrome
Rett syndrome is a pediatric neural developmental disease, affecting almost exclusively females. It is mainly due to mutations in the methyl-CpG-binding protein-2 (MECP2) gene, located on the X chromosome. MECP2 binds to methylated DNA and is thought to regulate gene expression. Normal MECP2 protein is vital for life, as male infants with MECP2 mutation do not survive. The mouse model has contributed to our current understanding of MECP2 in Rett syndrome; however, the mouse model does not fully recapitulate the pathological features.
Muotri’s group reprogrammed dermal fibroblasts from patients with Rett Syndrome due to MECP2 mutation and found that the mutant neurons recapitulated several phenotypes of the disease. They had fewer synapses and less spines compared to the control. The neurons also seemed to have less connectivity compared to the control, as they had lower activity dependent calcium signaling and decreased frequency of spontaneous postsynaptic currents (Marchetto et al., 2010). This work demonstrates the possibility of studying synaptic and network dysfunction in iPSC autism models.
3.2.2 Reprogramming of FMR1 fibroblasts reveals that some disease epigenetic modifications are resistant to reprogramming
Fragile X is the leading cause of inheritable intellectual disability. It is due to trinucleotide CCG repeat in the 5′ untranslated region of the Fragile X Mental Retardation (FMR1) gene, resulting In the loss of FMR1 expression. FMR1 is a RNA binding protein, which trafficks RNAs from the nucleus to the synapses, and is required for normal neural development. The PSC model is an attractive way to study Fragile X, since the regulation of the early events of FMR1 silencing could not be reproduced using other animal models. Eiges et al. generated hESC with the trinucleotide expansion detected by preimplantation genetic diagnosis. They found that FMR1 was expressed by the ESC, and the promoter was unmethylated. However, the FMR1 promoter becomes methylated upon differentiation and is associated with the decrement of FMR1 protein (Eiges et al., 2007). Interestingly, two other groups reprogrammed somatic cells with the trinucleotide expansion to iPSC. They found that the FMR1 promoter was methylated and there was an absence of FMR1 expression (Urbach et al., 2010, Sheridan, 2011 #6). This suggests that the methylation at the promoter of FMR1 is due to the trinucleotide repeat and is resistant to the reprogramming process. Sheridan et al. went on to show that the FMR1 promoter is methylated and FMR1 expression was absent in the neurons and astroytes derived from the mutant iPSC. The inconsistency between the hESC and hiPSC lines derived from Fragile X carriers demonstrates some of the limitations and potentials of using hPSC in disease modeling.
4. Application of iPSC and transdifferentiated neurons in studying human neurodegenerative diseases
Even though neurodegenerative pathologies may take years to accumulate in the brain, clinically relevant phenotypes have been observed in a relatively short time within the in-vitro iPSC derived neuronal cultures. When iPSCs were differentiated the resulting cells expressed premature aging phenotypes, including accelerated senescence, shortening of telomeres and dysmorphic nuclei. Although prolonged culturing in vitro is problematic it is also possible to age the human PSC derivatives by injecting them into animal brains. The use of iPSC would not be recommended for the study of diseases which are highly affected by environmental factors and may not have a strong genetic contribution, since most of the epigenetic marks are erased during re-programming. However, human iPSC have been used successfully to study many different types of neurodegenerative diseases.
4.1 Modeling neurodegenerative diseases
4.1.1 Modeling Alzheimer’s disease with iPSC
Alzheimer’s disease (AD) is an age-related neurodegenerative disease. Patients develop gradual and insidious memory loss, which progresses to inability to perform daily activities. The pathology typically starts in the entorhinal cortex and hippocampus, then spreads to the cerebral cortex (Braak and Braak, 1991). However, recently a minor subtype was described which originates in the hippocampus (Murray et al., 2011). The pathological hallmarks are an accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles. The severity of the inclusions seems to correlate with disease severity, although the tangles correlate better than the plaques.
AD can be caused by mutations in Presenilin-1 (PS1), Presenilin-2 (PS2), or Amyloid precursor protein (APP). These mutations are inherited in an autosomal dominant fashion and thus are called familial AD (FAD). PS1 forms the catalytic component of the γ-secretase, which has multiple substrates, including APP. After cleavage by β-secretase (BACE), the C-terminus fragment of APP is cleaved by γ-secretase to form the Aβ peptide and AICD (APP intracellular domain). In the PS1 FAD mutations, the Aβ42 to Aβ40 ratio is elevated, in general either due to increased Aβ42 production or a decrease of Aβ40 production. The Aβ42/40 ratio has been observed in human fibroblasts and in mouse transgenic models. Both Aβ42 and Aβ40 are found in the amyloid plaques. However, it is thought that Aβ42 is more amyloidogenic, and its reduction may serve as a cerebral spinal fluid (CSF) biomarker for confirming an AD diagnosis, possibly due to the decreased removal of Aβ42 from the brain parenchyma (Hulstaert et al., 1999). In addition to the FAD mutations, patients with Down’s Syndrome also develop memory disorders and dementia in their 40s and 50s, presumably due to duplication of APP, located on chromosome 21. In fact, people with duplication of the APP gene also develop AD, likely due to the overproduction of APP (Rovelet-Lecrux et al., 2007).
iN cells have been generated from fibroblasts carrying the Presenilin-1 mutation (Qiang et al., 2011). These neurons exhibit an elevated Aβ42/40 ratio compared to the control. When treated with γ-secretase inhibitor, DAPT, these neurons have reduced secreted Aβ and reduced Aβ containing endosomes.
Israel et al. showed that neurons derived from iPSC originating from patients with APP duplication have elevated Aβ40 (Israel et al., 2012). They also showed that a patient with sporadic AD can have elevated Aβ40 and enlarged endosomes. Interestingly, they found an elevated level of phospho Tau, which is thought to be the precursor to neurofibrillary tangle formation, in lines with elevated Aβ. This phenomenon is associated with elevated activated GSKβ and could be alleviated by BACE inhibitor treatment, but not γ–secretase inhibitor treatment.
Shi et al. showed that neuronal cultures derived from Down’s Syndrome iPSC and ESC form extracellular plaques after three months in culture (Shi et al., 2012). They were thioflavin analog BTA1 positive. This evidence shows that the clinically relevant phenotypes could be observed in the in-vitro iPSC derived neuronal cultures. Even though these pathologies take years to accumulate in the brain, they could be seen in a relatively short amount of time in culture using these systems.
4.1.2 Modeling Huntington’s disease with iPSC
Huntington’s disease (HD) is caused by neurodegeneration of the basal ganglia, caused by an expanded CAG repeat in the Huntingtin gene.
Because of its monogenetic nature, several groups have modeled HD with iPSC (An et al., 2012; Park et al., 2008; The†Hd†iPsc†Consortium, 2012). An et al. found that the neural stem cells derived form the HD iPSC were more vulnerable to oxidative stress than control iPSC. These defects could be reversed when the HD iPSC CAG repeat was corrected by genetic engineering (An et al., 2012).
The HD consortium found that lines with higher CAG repeats had more severe pathological phenotypes than the lines with less CAG repeats (The†Hd†iPsc†Consortium, 2012). They found that the NSCs with higher CAG repeat did not form functional neurons and gradually died, as compared to NSCs with lower CAG repeats. They also found morphological changes in the neural progenitor cells (NPC) with HD lines, in that they had less binding in phalloidin, suggesting that they had changes in the actin cytoskeleton. And they noted that the NPCs from the HD lines had a decreased ATP/ADP ratio, suggesting compromise in the energy metabolism.
Because of the autosomal dominant nature of HD, there is extensive pre-implantation screening for it during in-vitro fertilization, so that the embryos in the blastocyst stage which carry the long CAG repeat might potentially become available for research purposes. These embryos could be used for the generation of embryonic stem cell lines for HD research. Although iPSC and hESC are similar, the hESC could be used as a comparison to the iPSC lines.
4.1.3. Modeling ALS with iPSC
ALS (Amyotrophic Lateral Sclerosis), also known as Lou Gehrig’s disease, is a neurodegenerative disease with symptoms primarily due to the demise of motor neurons. This disease strikes people in their 40s, starting with weakness such as difficulty buttoning shirts, and slurring of speech. As the disease progresses, the weakness spreads to involve proximal muscles, resulting in difficulty ambulating, lifting weight, chewing and swallowing. Ultimately, patients die of respiratory distress from the failure of the diaphragms. The disease originates with the motor neurons in the spinal cord, and about 10% of ALS is familial. About 1% of all ALS cases are caused by a Superoxide dismutase 1 (SOD1) mutation. It is not clear how SOD1 causes ALS. There is evidence suggesting that the sick astrocytes in the spinal cord may poison the motor neurons (Clement et al., 2003). Patients’ symptoms can progress quickly; from the time of diagnosis to death can be as short as two years.
When astrocytes derived from ALS iPSC, were co-cultured with the neurons, they showed a toxic effect on motor neurons, but not the interneurons (Di Giorgio et al., 2008; Di Giorgio et al., 2007). Furthermore, this process may be due to activation of NOX2 to produce superoxide in the astrocytes, and could be mitigated by treatment with NOX2 inhibitor, apocynin (Marchetto et al., 2008). These results suggest a non-cell autonomous effect that is specific to the motor neurons and that finding treatments to protect the motor neuron loss in ALS could potentially ameliorate the disease (Di Giorgio et al., 2008).
Multiple other causes of familial type of ALS have been found, such as FUS/TLS, TDP43, and most recently c9ORF. Many of these genes involve RNA binding, and potentially the regulation of gene expression. It will be very interesting to see if the neurons derived from fibroblasts carrying these other genetic causes show similar phenotypes as the SOD1.
4.1.4. Modeling Machado-Joseph disease with iPSC
Machado-Joseph disease or Spinocerebellar ataxia type 3 (SCA3) is a rare, autosomal dominant neurodegenerative disease, which causes progressive cerebellar ataxia. It is caused by abnormal CAG expansion in the ATXN3 gene. Koch et al. generated iPSC from the skin cells of patients with Machado-Joseph disease (Koch et al., 2011). They found that neurons differentiated from the iPSC formed insoluble protein aggregates upon stimulation. The authors hypothesized that calcium dependent activation of proteases causes the cleavage of ATXN3 leading to protein aggregation as a result. When they stimulated the neuronal culture, they observed elevation of ATXN3 cleavage and ATXN3 insoluble aggregates. This process was dependent on the strength of the stimulation, as the aggregation correlated with the concentration of L-glutamine, and the depolarization induced calcium influx through voltage-gated calcium channels. Interestingly, the authors did not observe inclusion bodies or increased cell death. This combination of observations suggests that the excitation induced protein aggregation is an early event, which could lead to the development of inclusion bodies and cell death when the neurons could no longer tolerate the burden of aggregated proteins.
4.2 Modeling aging with iPSC
We provided examples above for using iPSC to model age-related neurodegeneration, indicating that it is possible to observe what was thought to be age-dependent phenotypes in only a few weeks in vitro. Research has also shown that diseases caused by accelerated aging can be modeled with iPSC. Hutchinson–Gilford progeria syndrome (HGPS) is a rare and fatal premature aging disease in humans, which is characterized by premature arteriosclerosis and degeneration of vascular smooth muscle cells (SMCs). HGPS is caused by a single point mutation in the lamin A (LMNA) gene, resulting in the generation of progerin, which is a truncated splicing mutant of lamin A. The accumulation of progerin leads to various aging-associated nuclear defects including disorganization of nuclear lamina and loss of heterochromatin.
Liu et al. reprogrammed dermal fibroblasts from patients with HGPS. They found that progerin was expressed in the patient fibroblasts; however, it was not expressed in the embryonic stem cells, nor in the iPSC, consistent with the theory that progerin expression is cell type specific and reprogramming resets its expression level (Liu et al., 2011). When HGPS iPSCs were differentiated to SMC, progerin expression was upregulated and increased over time with passaging. In addition, the HGPS SMC exhibited premature aging phenotypes, including accelerated senescence, shortening of telomeres and dysmorphic nuclei. The group performed a proteomic analysis using MudPit and found that progerin interacted with DNA-dependent protein kinase catalytic subunit (DNAPKcs). Interestingly, they also observed that there was down regulation of DNAPKcs in HGPS cells, which is dependent on the accumulation of progerin in differentiated cells. DNAPKcs may be important in the disease pathogenesis, for knockdown of DNAPKcs also recapitulated premature aging. Liu et al. demonstrated that aging could potentially be accelerated by causing mutations in genes which control aging, suggesting that such manipulations of the aging process could potentially accelerate the manifestation of what was once thought to be age-related phenotypes. If accelerated aging is feasible, such process would make the iPSC based models more powerful for modeling age-dependent diseases.
4.3 Synapses, neuronal connections, axonal transport, can these be demonstrated?
As mentioned before, neurons derived from iPSC or direct transdifferentiation from fibroblasts (iN cells) harbor many of the neuronal properties. These differentiated neurons contain sodium and potassium channels, can fire action potentials when stimulated, and exhibit properties of spontaneous activities (Israel et al., 2012; Marchetto et al., 2010; Qiang et al., 2011). From the transplantation work by Gaspard et al., it is even possible to study differentiation into different subtypes of neurons and axon guidance (Gaspard et al., 2008). The differentiation of neurons does seem to follow developmental timelines. Therefore, it is not surprising that the neurons derived from three-week culture closely resemble the expression profile of human embryonic brains (Israel et al., 2012). Human neurons are distinct from other typical model systems such as rodents, in that they can be over a meter long. Therefore, using hPSC to generate human neurons to study axonal transport could be particularly fruitful, since the mechanisms of regulation in humans could potentially be different.
4.4 Limitations of PSC for modeling neurodegenerative diseases
4.4.1 Erasure of epigenetic marks during reprogramming
For neurodegenerative diseases, epigenetic modifications arising from environmental exposure to toxins, lifestyle factors, and co-morbidities could be significant in disease modification and manifestation. However, several studies suggest that during re-programming most of the epigenetic marks are erased (Elden et al., 2010; Stadtfeld et al., 2010). This prevents modeling of any epigenetic changes related to environmental factors that occur during an individual’s lifetime. Therefore, the use of iPSC would not be recommended for the study of diseases which are highly affected by environmental factors and may not have a strong genetic contribution (Grskovic et al., 2011). The status of the preservation of epigenetic marks is still unknown in the iN and iNSC system. If the epigenetic marks are preserved during the transdifferentiation during the making of iN and iNSC, then these systems would be superior than the iPSC for studying the epigenetic contribution to diseases. This issue has yet to be resolved.
4.4.2 Aging as a factor for disease modeling
The majority of neurodegenerative diseases are age-dependent. Age makes a contribution to disease development and manifestation for a variety of reasons. Some of these involve environmental factors, possibly causing the epigenetic changes just discussed, that accumulate over a lifetime. Because so many of these factors might contribute to disease development, it is very difficult, if not impossible, to model aging itself using PSC. Prolonged culturing of PSC in vitro is prone to contamination. Current culture conditions are poorly controlled for long-term culture and could be compromised by excessive cell death. It may be possible, however, to age the human PSC derivatives by injecting them into animal brains. Protocols for injecting NSC into rodent brains have been reported by multiple groups (Kriks et al., 2011; Muotri et al., 2005; Tabar et al., 2005); therefore, this seems to be highly feasible. It is difficult, though, to track these cells in vivo, and new methods need to be developed to observe them. It is also difficult to interpret the results, for there may be different read outs due to the varied genetic backgrounds of the recipient animals. This is another area that needs further development.
5. Application of PSC in therapeutic development
As PSCs hold the promise to become any cell type of the body, this potentially could be applied in development of therapy. It has been proposed that PSC derived transplantable cells would be considered autologous (self to self); therefore, long-term immuno-supression may not be necessary. Furthermore, these patient-specific cells could be used for drug-development to screen for drugs that are most suitable to the patient in terms of their efficacy and toxicity profile. In this section we will discuss the evidence for using PSC derivatives in transplantation and drug screening.
5.1 Transplantation as a therapy for neurodegenerative diseases
Although some early research in mouse models has been encouraging, major obstacles remain for NSC transplantation therapy. Neurodegenerative diseases by their nature tend to spread over time, making it difficult to target specific regions for therapy and suggesting the importance of early intervention.
5.1.1 Transplantation therapy in Alzheimer’s disease
Alzheimer’s Disease pathology generally originates in the entorhinal cortex and the hippocampus, but then spreads to other regions of the brain as the disease progresses (Braak and Braak, 1991). Therefore, transplantation as a therapy for Alzheimer’s disease does not seem likely to be an effective solution once the disease has begun to spread. However, in recent work by Blurton-Jones et al., they showed that NSCs slowed the disease progression in an AD mouse model.
Blurton-Jones et al. transplanted GFP labeled NSCs into the hippocampal region bilaterally in triple transgenic mice (3×Tg-AD) that express pathogenic forms of the amyloid precursor protein and presenilin (Blurton-Jones et al., 2009). The group observed that deficits in cognitive function in the aged 3×Tg-AD mice, measured by Morris Water Maze and context-dependent novel object recognition, were improved with transplantation without altering Aß or tau pathology. The authors found that there was a significant increase in hippocampal synaptic density, mediated by brain derived neurotrophic factor (BDNF). The authors found that BDNF was secreted by the NSCs, because when BDNF was knocked down by shRNA in the NSCs, and the behavioral improvement in the 3×Tg-AD mice could no longer be observed. These results are intriguing, because they suggest that even in a widespread pathology, a neurodegenerative disease might possibly be modulated by stem cell transplantation therapy. This might offer reasons to hope that stem cell therapy may provide a regenerative approach to combat AD. Further studies are warranted to fully evaluate the efficacy, toxicity and tumor formation potential of such a therapy.
5.1.2 Transplantation therapy in Parkinson’s disease
The hallmark of Parkinson’s disease pathology is loss of dopaminergic neurons in the substansia nigra, causing extrapyramidal movement disorders, such as tremors, bradykinesia and rigidity. Because the cell loss seems to be more restricted to one area, there has been a great interest in transplanting neurons derived from iPSC using various Parkinson’s disease mouse models. Multiple clinical trials transplanting fetal ventral mesencephalic (fVM) tissue into the striatum demonstrated that such transplantation is feasible. Patients in general benefited from the transplantation and required less medication; however some developed significant dyskinesia as a side effect (Lindvall and Björklund, 2004). Recently, in a follow up evaluation using fMRI, three patients were shown to have a normal level of dopamine in the striatum, but a low serotonergic level in the non-cholinergic neurons, highlighting the fact that the neuronal loss in PD is not limited to dopaminergic neurons alone (Politis et al., 2012). Although the initial symptoms of Parkinson’s disease seem to be limited to motor impairments, neurodegeneration also involves other areas as patients age. Studies from Braak and Braak show that Parkinson’s disease may start outside of the midbrain (Braak and Del Tredici, 2008; Del Tredici et al., 2002). However, stem cell therapy could still be warranted if significant improvement of motor symptoms could be demonstrated.
5.1.3 Transplantation therapy in Amyotrophic Lateral Sclerosis (ALS)
Multiple studies demonstrated that in ALS, astrocytes could actively damage the motor neurons, causing neuronal demise (Clement et al., 2003; Di Giorgio et al., 2008; Di Giorgio et al., 2007; Marchetto et al., 2008). Leopore et al. showed that transplantation of healthy glial restricted progenitors (GRP) derived from human fetal tissue into the cervical cord of SOD1G93A rats led to significant improvement in their survival, with reduced motor neuron loss and decline in fore-limb motor and respiratory physiological function (Lepore et al., 2011; Lepore et al., 2008). The transplanted GRP initially expressed mainly Nestin, and later in vivo it became glial fibrillary acidic protein (GFAP) positive, which is a glial marker. These results suggest that the transplantation of NSC or astrocytes derived from NSC could be a viable therapy for treating devastating neurodegenerative diseases such as ALS.
5.1.4 Source of cell types for transplantation
The most ideal cell type for transplantation is probably highly dependent on the disease etiology and the therapeutic goals of each of the study designs. However, it is important to ensure that the cells are highly pure and of GMP (Good Manufacturing Practice) quality. We have developed a method, which enriches neurons, neural stem cells and glial progenitors, based on cell surface signatures (Yuan et al., 2011). It is also important to know the likelihood of survival and integration of the different cell types. We investigated this problem, by comparing the efficiency of grafting sorted NSC, differentiated neuronal culture and sorted neurons into rat spinal cord. We found that the sorted neurons had the lowest and the NSC had the highest rate of survival (unpublished data).
5.1.5 Transplantation of stem cells in clinical trials
NSC derived from human spinal cord has already been in a clinical safety study in ALS patients. Neuralstem completed its Phase I NSC transplantation in ALS patients, and is now proceeding with recruitment of Phase II trial (http://www.clinicaltrials.gov/ct2/show/NCT01348451). A highly publicized first FDA approved clinical trial using oligodendrocyte progenitors derived from hESC for spinal cord injury sponsored by Geron was halted after one year of the study. The study was stopped not due to adverse effects, but strictly as a business decision. The four patients who received transplantation are still being followed. There were no reports of any changes in their symptoms, nor of any adverse effects (http://clinicaltrials.gov/ct2/show/study/NCT01217008). Advanced Cell Technology is currently evaluating hESC derived retinal pigmented epithelium (RPE) in an FDA approved Phase I/II trial for the treatment of age-related macular degeneration (AMD) (http://clinicaltrials.gov/ct2/show/study/NCT01217008).
5.2. Drug screening
Until now, researchers did not have the resources to acquire an unlimited amount of human neurons or astrocytes for drug testing. However, iPSC models have demonstrated that such drug testing is now possible.
Currently the cell types used for drug screening, whether of chemicals or small organic molecules, have included animal cells or immortalized cell lines overexpressed with mutant proteins. For example, in Alzheimer’s disease, the typical cell lines used are CHO (Chinese Hamster Ovary) or neuroblastoma SH5YSY lines overexpressed with Presenilin-1 or APP mutations. These cells are easy to grow and they are scalable for screening a large number of compounds. However, these cell lines may not share the same behaviors as neurons or astrocytes, which are the primary cell types at risk in neurodegenerative diseases. Furthermore, the overexpression of these mutations may not truly reflect the cellular responses to compounds the body would have at a physiological level. The use of iPSC offers an alternative model that can more accurately recapitulate the in-vivo conditions of the disease, improving the efficacy of the drug screen, making it more likely that candidate drugs which have performed well in-vitro will also function in-vivo.
Several reviews have been written regarding the potential applications of bioengineered stem cells for development of therapy (Ebert et al., 2012, Almad, 2012 #1608, Grskovic, 2011 #1173). Recent work by Lee et al. utilized neural crest precursors derived from iPSC generated from patients with familial dysautonomia (FD) to demonstrate the feasibility of large scale-screening of a chemical library, including 6912 compounds (Lee et al., 2012). The authors identified eight compounds that rescued the expression of inhibitor of kappa light polypeptide gene enhancer in B-cells (IKBKAP), which is deficient in FD, and the remediation of the cellular phenotype. The authors found one of the eight compounds induced IKBKAP transcription through modulation of intracellular cAMP levels.
Peng et al. evaluated 44 known compounds, examining the response to rotenone-induced toxicity to dopaminergic neurons derived from iPSC (Peng et al., 2013). Of the 44 compounds, 16 were found to be protective. This offers the potential that such a screen could be expanded to a large, high-throughput screen, in post-mitotic neurons.
6. Discussion
6.1 Implications of iPSC in development and aging research
It is surprising that many of the phenotypes associated with neurodegenerative diseases could be observed in relatively young neurons, after a few months in culture, though it often takes many decades for them to appear in humans. Our understanding of neurodegenerative diseases has been shaped by the fact that, by the time symptoms have developed, there are only a small number of healthy neurons left alive. Neurodegeneration is a slow process, which begins ten to twenty years before the onset of disease (Bateman et al., 2012). Yet some of the abnormalities have been observed as early as in utero in the familial form of Alzheimer’s disease (Cataldo et al., 2001). These disease related phenotypes could represent the beginning or early developmental stage of the disease. Pluripotent stem cells offer a new opportunity to study these early events and the mechanism of disease progression.
6.2 Implications of iPSC in personalized disease modeling and medicine
Up until now, disease modeling has been involved with known genetic causes. Modeling sporadic diseases, where the genetic causes are usually multiple and unclear, has not been feasible. It is now possible to model sporadic diseases using iPSC. These patient derived cells embody the combined genetic risk factors which contribute to patients’ development of disease. For example, diseases such as autism, where multiple defective genes may be involved in the development of disease, could potentially be modeled using iPSC by reprogramming patient fibroblasts. The iPSC method will also save time required to develop the models. For example, a traditional animal model would take years to make in polygenetic diseases, for it would require several rounds of targeting to get all of the mutations into the germline of the animal.
It is also possible to create iPSC models for individuals and study how that person could develop disease. This could become a tool for assessing risk factors for the development of disease. Such information could be useful in modification of risk factors, so that individual’s future development of disease could be avoided. The personalized disease model-in-a-dish, could also potentially be used for testing drug responses and toxicity, to avoid treatment failure and adverse events.
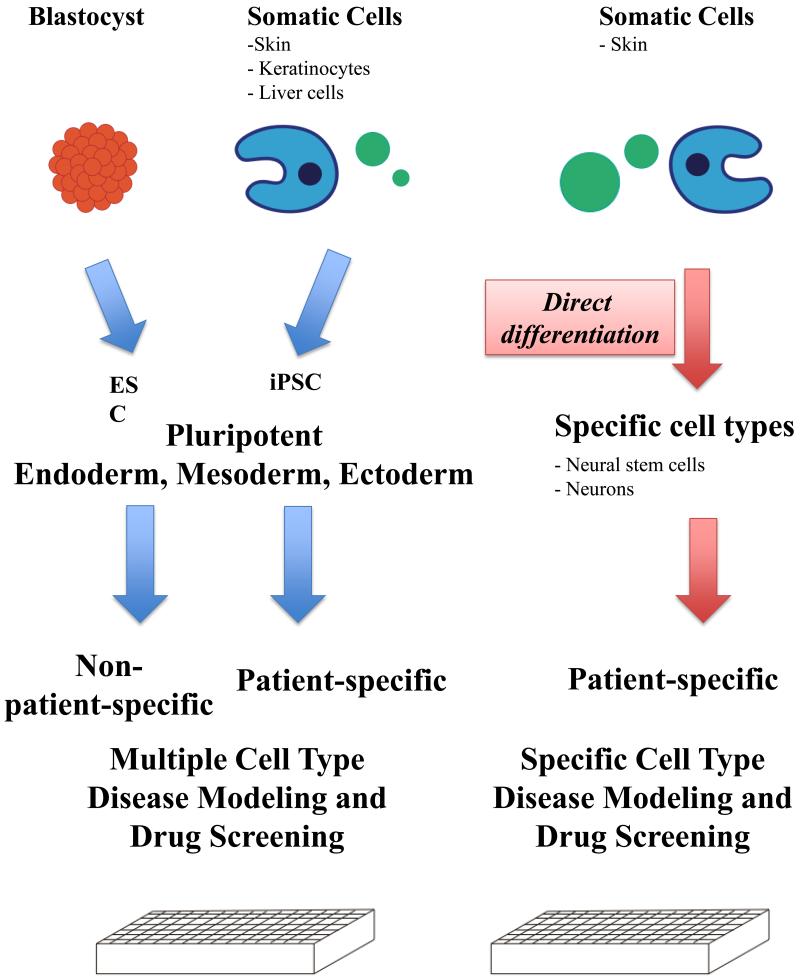
Now that there are multiple methods to derive the cell type of interest, the method of choice is highly dependent on the research question and the efficiency of the method (Figure 2). The main advantage of iPSC is that these cells are by definition pluripotent stem cells; therefore, they have the potential to differentiate into all the different types of cells of the human body. This allows the researcher to perform studies on experimental paradigms involving multiple types of cells. For example, one could study drug toxicity in different end organs, such as liver cells and cardiac myocytes. However, getting from the iPSC to useful terminally differentiated cells could take months, involving both reprogramming and differentiation processes. Direct conversion of one type of somatic cell, such as the fibroblast, into another type such as neurons, would speed up the generation of the desired terminally differentiated population. However, neurons do not live alone in the brain; therefore, this method has very specific and limited utility. Conversion of the fibroblast into a neural stem cell (NSC), which could give rise to both the neuron and the glia, is preferable if the research requires both neurons and the glia. In the past few years, multiple groups have demonstrated that each of these cell types can be derived, providing a set of diverse and useful tools for researchers.
Figure 2.
Overview of the current methods of bioengineered stem cells. The ESCs are derived from blastocysts, whereas the iPSC, iN and iNSC are generated from somatic cells. Both the ESC and iPSC have the potential to generate different cell types, whereas iNSC and induced neurons derived through direct conversion are fate restricted. These differences in the methodology and the cells of origin have implications on how these cells could be used. For example, the disease mechanisms and therapies developed from cells derived through the reprogramming or transdifferentiation methods have the potential to be specific to the individual. The direct conversion methods result in model systems that limit the research in specific cells, whereas the pluripotent stem cells allow the study of multiple cell types.
These engineered cells are derived from individuals, allowing researchers to study phenomena associated with that specific individual. This could be very useful, for example, when attempting to determine why some individuals develop disease while others do not.
8. Conclusions
The past few years have been an exciting time for stem cell biology. The discovery of induced pluripotent stem cells (iPSC) has given new impetus to the stem cell field. The hope is that this will ultimately lead to the creation of individualized patient models which can predict disease and assist in its treatment. We are already seeing the effect of whole genome sequencing on personalized treatment in cancer patients. Perhaps stem cells may also have such an effect on the way we prevent, diagnose and treat our patients in the future.
Highlights.
Induced pluripotent stem cells, induced neurons and induced neural stem cells can be generated from human fibroblasts.
Bioengineered stem cells form three-dimensional brain structures and follow guidance cues
Human induced pluripotent stem cells and neurons can be used to model diseases in neural development and neurodegeneration
Bioengineered stem cell models can be used for development of therapies.
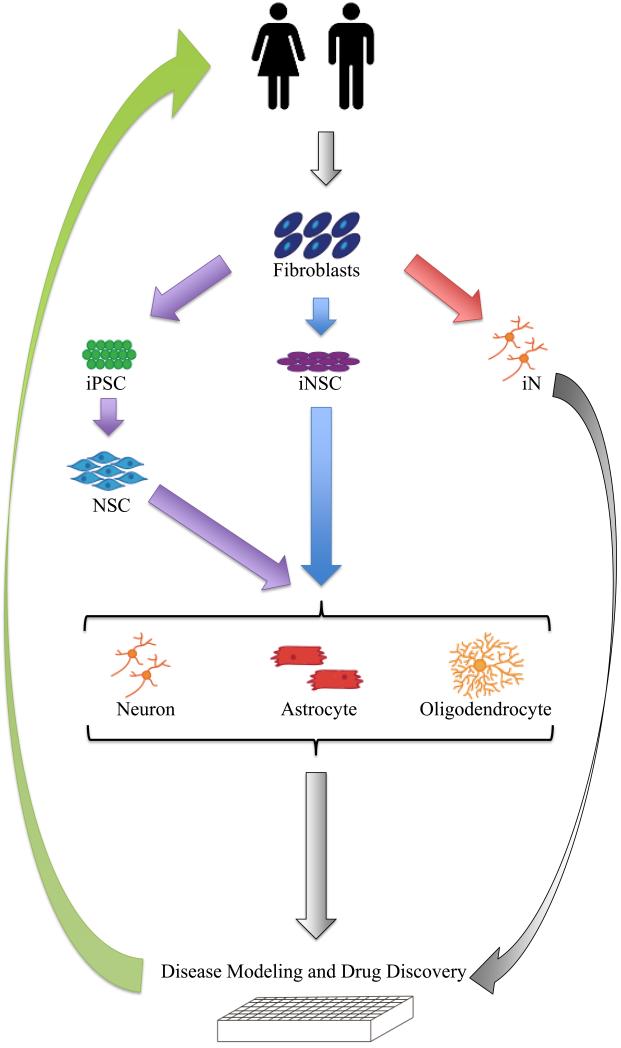
Figure 1.
Disease-in-a-dish model. Human dermal fibroblasts can be generated from skin biopsies. These fibroblasts subsequently can be converted to specific cells of interest, such as neurons and glia, that may be particularly vulnerable in neurodevelopmental or neurodegenerative diseases through either reprogramming to iPSC or direct conversion to NSC or neurons. These methods allow researchers to study not only disease mechanisms, but also use for development of therapies. The information gathered from these models can be further confirmed or applied towards the diagnosis or design of therapies for patients.
Acknowledgements
Due to limitation of space, the authors apologize the omission of other relevant manuscripts in the topics covered in this review. This work was supported by U01AG10483.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio, A Gonzalez, F., Vassena, R., Bilic J, Pekarik V, Tiscornia G, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotech. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct Reprogramming of Adult Human Fibroblasts to Functional Neurons under Defined Conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S, Melov S, Ellerby LM. Genetic Correction of Huntington’s Disease Phenotypes in Induced Pluripotent Stem Cells. Cell Stem Cell. 2012;11:253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of Pluripotent Stem Cells from Adult Mouse Liver and Stomach Cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- Cataldo A, Rebeck GW, Ghetri B, Hulette C, Lippa C, Van Broeckhoven C, van Duijn C, Cras P, Bogdanovic N, Bird T, et al. Endocytic disturbances distinguish among subtypes of Alzheimer’s disease and related disorders. Ann Neurol. 2001;50:661–665. [PubMed] [Google Scholar]
- Choi J Fau - Costa ML, Costa Ml Fau - Mermelstein CS, Mermelstein Cs Fau - Chagas C, Chagas C Fau - Holtzer S, Holtzer S Fau - Holtzer H, Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Liang P, Wu JC. Induced pluripotent stem cells as a disease modeling and drug screening platform. J Cardiovasc Pharmacol. 2012;60:408–416. doi: 10.1097/FJC.0b013e318247f642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiges R, Urbach A, Malcov M, Frumkin T, Schwartz T, Amit A, Yaron Y, Eden A, Yanuka O, Benvenisty N, et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- Grskovic M, Javaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells √¢,Ç,Äù opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Hîng S, Arȧzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct Reprogramming of Fibroblasts into Neural Stem Cells by Defined Factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, et al. Direct Reprogramming of Terminally Differentiated Mature B Lymphocytes to Pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, Deyn PPD, Bancher C, Cras P, Wiltfang J, Mehta PD, et al. Improved discrimination of AD patients using OE≤-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52:1555–1555. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011a;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lengner CJ, Kirak O, Hanna J, Cassady JP, Lodato MA, Wu S, Faddah DA, Steine EJ, Gao Q, et al. Reprogramming of Postnatal Neurons into Induced Pluripotent Stem Cells by Defined Factors. STEM CELLS. 2011b;29:992–1000. doi: 10.1002/stem.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, Doerr J, Ladewig J, Mertens J, Tuting T, et al. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480:543–546. doi: 10.1038/nature10671. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson/’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkanjanawan T, Noisa P, Parnpai R. Modeling Neurological Disorders by Human Induced Pluripotent Stem Cells. Journal of Biomedicine and Biotechnology. 20112011 doi: 10.1155/2011/350131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Ramirez CN, Kim H, Zeltner N, Liu B, Radu C, Bhinder B, Kim YJ, Choi IY, Mukherjee-Clavin B, et al. Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotech. 2012;30:1244–1248. doi: 10.1038/nbt.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, O’Donnell J, Kim AS, Williams T, Tuteja A, Rao MS, Kelley LL, Campanelli JT, Maragakis NJ. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PLoS One. 2011;6:e25968. doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. Neurotherapeutics. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G-H, Barkho BZ, Ruiz S, Diep D, Qu J, Yang S-L, Panopoulos AD, Suzuki K, Kurian L, Walsh C, et al. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature. 2011;472:221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A Model for Neural Development and Treatment of Rett Syndrome Using Human Induced Pluripotent Stem Cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10:785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011 doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I-H, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-Specific Induced Pluripotent Stem Cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Liu Q, Rao MS, Zeng X. Using Human Pluripotent Stem Cell,ÄìDerived Dopaminergic Neurons to Evaluate Candidate Parkinson,Äôs Disease Therapeutic Agents in MPP+ and Rotenone Models. Journal of Biomolecular Screening. 2013 doi: 10.1177/1087057112474468. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Bj√∂rklund A, Lindvall O, Piccini P. Serotonin Neuron Loss and Nonmotor Symptoms Continue in Parkinson,Äôs Patients Treated with Dopamine Grafts. Science Translational Medicine. 2012;4:128ra141. doi: 10.1126/scitranslmed.3003391. [DOI] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct Reprogramming of Mouse and Human Fibroblasts into Multipotent Neural Stem Cells with a Single Factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Frebourg T, Tuominen H, Majamaa K, Campion D, Remes AM. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2007;78:1158–1159. doi: 10.1136/jnnp.2006.113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Kirwan P, Smith J, MacLean G, Orkin SH, Livesey FJ. A human stem cell model of early Alzheimer’s disease pathology in Down syndrome. Sci Transl Med. 2012;4:124ra129. doi: 10.1126/scitranslmed.3003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JMW. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8:369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Brennand K, Hochedlinger K. Reprogramming of Pancreatic OE≤ Cells into Induced Pluripotent Stem Cells. Current Biology. 2008;18:890–894. doi: 10.1016/j.cub.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabar V, Panagiotakos G, Greenberg ED, Chan BK, Sadelain M, Gutin PH, Studer L. Migration and differentiation of neural precursors derived from human embryonic stem cells in the rat brain. Nat Biotech. 2005;23:601–606. doi: 10.1038/nbt1088. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- The†Hd†iPsc†Consortium Induced Pluripotent Stem Cells from Patients with Huntington’s Disease Show CAG-Repeat-Expansion-Associated Phenotypes. Cell Stem Cell. 2012;11:264–278. doi: 10.1016/j.stem.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier M, W^rsd^rfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, N^then MM, et al. Direct Conversion of Fibroblasts into Stably Expandable Neural Stem Cells. Cell Stem Cell. 2012;10:473–479. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential Modeling of Fragile X Syndrome by Human Embryonic Stem Cells and Induced Pluripotent Stem Cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T. Stepwise Reprogramming of B Cells into Macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yuan SH, Martin J, Elia J, Flippin J, Paramban RI, Hefferan MP, Vidal JG, Mu Y, Killian RL, Israel MA, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6:e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to [bgr]-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]