Abstract
Behavioral experience, in the form of skilled limb use, has been found to impact the structure and function of the central nervous system, affecting post-stroke behavioral outcome in both adaptive and maladaptive ways. Learning to rely on the less-affected, or non-paretic, body side is common following stroke in both humans and rodent models. In rats, it has been observed that skilled learning with the non-paretic forelimb following ischemic insult leads to impaired or delayed functional recovery of the paretic limb. Here we used a mouse model of focal motor cortical ischemic injury to examine the effects of non-paretic limb training following unilateral stroke. In addition, we exposed some mice to increased bimanual experience in the home cage following stroke to investigate the impact of coordinated dexterous limb use on the non-paretic limb training effect. Our results confirmed that skilled learning with the non-paretic limb impaired functional recovery following stroke in C56BL/6 mice, as it does in rats. Further, this effect was avoided when the skill learning of the non-paretic limb was coupled with increased dexterous use of both forelimbs in the home cage. These findings further establish the mouse as an appropriate model in which to study the neural mechanisms of recovery following stroke and extend previous findings to suggest that the dexterous coordinated use of the paretic and non-paretic limb can promote functional outcome following injury. Keywords: experience-dependent plasticity, learned nonuse, motor cortex, motor rehabilitation, stroke
1. Introduction
Stroke is among the leading causes of death and disability worldwide, with chronic upper limb impairment (usually unilateral) among the most common deficits reported in stroke survivors [1, 2]. Often this deficit presents as a loss of functional use of the hand or arm contralateral to the lesion locus (paretic body side; i.e, paretic limb). A natural response to loss of function is to develop alternative strategies to circumvent the problem. As a result, a common compensatory strategy among stroke survivors is to learn to rely on the less-affected, or non-paretic, limb for daily tasks involving skilled limb or hand use such as grasping and manipulating objects [3, 4]. However, the long-term neural and behavioral consequences of continued compensatory skill learning with the non-paretic limb use are not well understood.
Reliance on the non-paretic limb contributes to a learned non-use of the paretic limb [5, 6] and may limit long-term functional outcome following stroke. This is the basis of constraint-induced movement therapy (CIMT) [4, 7–10], whereby, in addition to intense rehabilitation, the non-paretic limb is bound for most waking hours, encouraging patients to use the paretic limb to complete daily tasks. While CIMT has been reported to significantly improve upper extremity deficits [11, 12], many stroke survivors continue to use their non-paretic limb for daily tasks when it is unbound [13], possibly affecting the rehabilitative potential of the paretic limb.
Unilateral motor cortex damage in the caudal forelimb representation area (CFA) of the rat results in contralateral-to-lesion forelimb impairments [14–24] and a reliance on the non-paretic body side [6, 17, 25–28], that resemble in many respects both the upper extremity impairments and learned non-use of the paretic limb observed in humans. Focused rehabilitative training of the paretic limb can improve motor function and structural and functional plasticity in remaining regions of cortex after stroke in rats [29– 36]. Following ischemic insult, contralesional cortex has been found to exhibit increased neuroplasticity [37, 38] that may facilitate the acquisition of new motor skills with the non-paretic limb [15, 17, 25]. Previously, our laboratory has reported that focused training of the non-paretic limb in a skilled reaching task impedes recovery of the paretic limb [39, 40] and disrupts functional reorganization in peri-infarct cortex that would otherwise contribute to improvements in functional outcome of the impaired limb [15, 40, 41], while promoting experience-driven plasticity in the contralateral-to-lesion cortex [17, 21, 22, 38, 42]. Rats that receive focused bilateral rehabilitative training on a skilled reaching task do not show the maladaptive effects and exhibit functional outcome that is similar to that of animals that receive focused rehabilitation of the paretic limb [40].
As they are inexpensive to house and offer many transgenic lines that are suitable for in vivo imaging, mice are an important tool in understanding the impact of behavioral training on functional and structural recovery following stroke. We have determined that C57BL/6 mice have long lasting forelimb impairments following focal ischemic insult of the CFA [43] and exhibit improved functional outcome and structural plasticity following focused rehabilitative training of the paretic limb [44]. While it is clear that mice are an effective model of upper limb impairment and functional rehabilitation following stroke, the effects of non-paretic limb training, and thus their usefulness for investigating neural mechanisms of learned non-use, has not been established. The present study investigated the impact of skilled non-paretic limb use in our mouse model of focal ischemic insult. In addition, we explored the impact of coordinated, dexterous bimanual limb use in the home cage on non-paretic limb training effects. Our current findings lay the groundwork for further studies that will explore the neural consequences of non-paretic skill learning in both contra- and ipsilesional cortices.
2. Materials and Methods
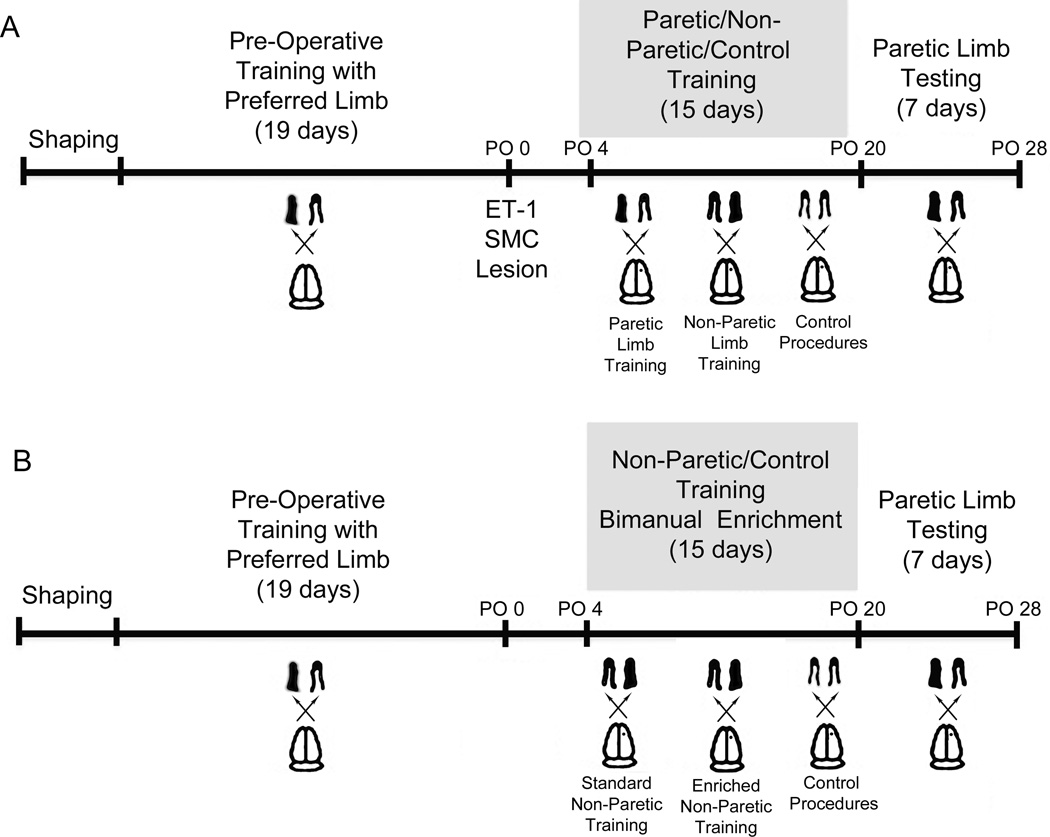
Experimental designs are summarized in Fig. 1.
Fig. 1.
Experimental Design. In Experiment 1 (A), mice were given ET-1 induced ischemic lesions of the contralateral SMC following task acquisition. During postoperative training, mice trained with either the paretic (Par) or non-paretic (NonPar) limb on a reaching task or received control procedures. Following training, all mice were assessed on the Pasta Matrix Reaching Test using their paretic limb. Experiment 2 (B) was similar to Experiment 1. However, during post-operative training all mice were forced to use their non-paretic limb for reaching or received control procedures. In addition, half of the mice were given home cage enrichment that encouraged bimanual dexterous forelimb use (BE) versus standard housing conditioning (ST).
2.1. Subjects
A total of 94 well-handled, 3-month-old male C57BL/6 mice were housed in groups of four with standard housing supplementation (a small piece of PVC pipe, a cardboard roll, and small wooden objects for chewing) on a 12:12 light/dark cycle. Animals were maintained on a restricted feeding schedule (2.5–3g/mouse/day) to prevent satiation and promote reaching performance. Daily food restriction was monitored and adjusted such that no animal lost more than 10% of their free feeding weight, established prior to experimental procedures. In Experiment 1, 39 mice received intracortical infusions of the vascoconstricting peptide, endothelin-1 (ET-1), and 15 mice received a sham surgery consisting of intracortical infusion of 0.9% sterile saline. Six of the mice receiving ET-1 died during recovery from perioperative anesthesia and were consequently not included in the study. The surviving mice were separated into one of three groups on post-op day 5: paretic-trained (Par), non-paretic trained (NonPar), or Control (for all conditions, lesion: n = 11, sham: n = 5). Groups were matched on pre-operative performance levels.
In Experiment 2, 33 mice reached appropriate pre-operative reaching levels (defined in 2.3) and received intracortical infusion of ET-1. Five mice died during recovery from perioperative anesthesia. One additional mouse was excluded from the study because of severe motor deficits observed 48 hours after surgery. No sham operates were used in Experiment 2. Following surgery, mice were separated into one of four groups (matched on pre-operative performance): bimanual home cage enriched control (BE Control; n = 5), BE non-paretic limb trained (BE NonPar; n = 9), standard housed control (ST Control; n = 5), and ST NonPar (n = 8). Enrichment procedures are described below (see 2.4). Animal use was in accordance with a protocol approved by the University of Texas at Austin Animal Care and Use Committee.
2.2. Intracortical infusion of ET-1
Following pre-operative motor skill learning, mice received intracortical infusion of ET-1 as described previously [43]. Briefly, mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed into a stereotaxic frame (Stoelting, Wood Dale, IL). Lidocaine (2 mg/kg, s.c.) was injected into the scalp, and a midline incision was made. A small burr hole was drilled over the center of the forelimb region of SMC (0.3 mm anterior to Bregma; 1.5 mm from midline) contralateral to the preferred reaching paw. A total of 3 µl of ET-1 (American Peptide; 320 pmol, 0.2µg/µl in sterile saline) was injected into layer V of the motor cortex at a depth of 800µm below the surface of the cortex over the course of 10 minutes (1 µl at a time). The burr hole was then filled with gelfoam and covered with UV curing dental cement (Wave A2; Southern Dental Industries, Victoria, Australia). The wound was sutured and covered with antibiotic ointment. All mice were permitted to fully awaken in a heated chamber before receiving buprenorphine (3 mg/kg at 0.015 mg/mL in sterile saline, s.c.) and returning to the home cage. Mice in the sham group (Experiment 1) received an identical surgery with 3 µl of 0.9% sterile saline in the place of ET-1.
2.3. Behavioral methods: Pasta Matrix Reaching Test
Animals were trained on the Pasta Matrix Reaching Test (Figure 1A) as described previously [43]. Briefly, mice were trained to reach through a small slit found in the center wall of a Plexiglas chamber to break pieces (3.2 cm in height, 1mm in diameter) of vertically oriented, uncooked capellini pasta (De Cecco brand, Fratelli De Cecco di Filippo Fara San Martino S.p.A., Italy). Pasta pieces were located 2 mm apart in a heavy-duty plastic block outside of the reaching chamber. Half of each pasta piece extended above the block. This is a skilled reaching task that requires the animal to adjust its reach trajectory to obtain pasta pieces that are located increasingly further from the reaching aperture.
Prior to training, all animals underwent shaping procedures in which they were acclimated to the testing apparatus and limb preference was determined. Animals were placed individually in reaching chambers once daily for three to five days. During this time, the matrix stage (containing the pasta pieces) was completely filled, allowing animals to reach pasta pieces with both limbs. Each daily trial lasted for 10 minutes or until the mouse reached a minimum of 10 times. A reach was defined as extending the limb through the reaching aperture such that the entire forepaw was outside the chamber walls. Limb preference was determined when a minimum of 70% of an animal’s reaches were made with either the right or left forelimb. Shaping trials continued once daily until each animal’s limb preference was determined.
Following shaping procedures, mice were trained on the Pasta Matrix Reaching Test to establish the skill. During training, only half of the matrix stage was filled with pasta (contralateral to the preferred limb), forcing the mice to use their preferred to limb to reach for pasta pieces. Each daily trial consisted of 15 minutes or 100 reaches, whichever occurred first. Mice were trained for a total of 19 days, at which point performance was considered to be stable for all mice. To establish performance level, the average number of successful retrievals (i.e., number of pasta pieces broken) was calculated for the final three days of training. Previous results suggest that each mouse is only physically able to reach a maximum of 18 pieces of pasta in the matrix [43]. Eighty-seven mice (54 in Experiment 1; 33 in Experiment 2) reached criterion performance, defined as 9 successful retrievals, and received ET-1 or sham lesions (post-op day 0). On post-op day 4, performance of the affected limb was assessed on the Pasta Matrix Reaching Test. On post-op day 5, mice were separated into their respective groups (see 2.1) for post-operative training. For both experiments, Par and NonPar groups received daily training sessions (15 minutes or 100 reaches in length) of the paretic or non-paretic limb. Control mice were placed into reaching chambers with no matrix stage and ate a comparable amount of pasta broken into small nibblets. These training procedures took place for a total of 15 consecutive days.
Following post-operative training, all mice were assessed with their paretic limb on the Pasta Matrix Reaching Test with procedures identical to those during pre-operative training. Assessment took place over 7 consecutive days.
2.4. Bimanual home cage enrichment
Animals in the enriched groups (Experiment 2) received daily home cage enrichment that maximized novel dexterous bimanual forelimb use. Enrichment consisted of 10 pieces of 1.25 in long pasta and 6 sunflower seeds (in-shell) per mouse in addition to two square chewing blocks (5/8 mm3) per cage per week. The dexterous manner in which rodents handle and consume long pasta pieces has been documented previously[43, 45–47]. It was noted in the current study that mice often shelled the sunflower seeds before consuming them as evidenced by empty shells found in the cages each morning, purportedly involving the dexterous use of both forelimbs. All other mice received a similar amount of pasta broken into small nibblets and shelled sunflower seeds. The square blocks were chosen for the enrichment condition as a novel shaped object that was distinct from those that the mice previously experienced in the baseline housing condition, which included round or smooth-edged wooden gnawing objects. Instead of square blocks, mice in the un-enriched condition received two round chew toys (5/8 mm in diameter) per cage per week, which were similar to those gnawing objects available during pre-operative baseline housing conditions. While mice in both conditions were assumed to use both limbs to manipulate the food and gnawing objects, the conditions of the bimanual enrichment were intended to promote experience with new bimanual handling patterns. Both enrichment and control cages consumed all of their allotted pasta and sunflower seeds (either shelled or unshelled) each day, and the wooden objects were observed to have been gnawed upon during weekly replacement. All animals received either enrichment or control procedures concurrent with daily training sessions as described in 2.3. Following 15 days of post-operative training, paretic limb assessment commenced as described above. No enrichment (beyond the standard housing supplementation described in 2.1) was available to any mouse during paretic limb assessment.
2.5. Tissue processing and lesion analysis
Twenty-four hours after the final testing session (i.e., 27 days after ET-1 administration), mice were euthanized with an overdose of sodium pentobarbital (euthasol, 175 mg/kg, i.p.) and perfused intracardially with 0.1M phosphate buffer (PB) and 4% paraformaldehyde. Following perfusion, brain tissue was removed and stored in 4% paraformaldehyde at 4°C for a minimum of 72 hours before being sliced into 40 µm thick sections using a vibratome. Every sixth section was mounted onto gelatin-coated slides and Nissl stained with toluidine blue.
Neurolucida software was used to estimate lesion volume. Coronal sections were viewed at a magnification of 17x. The cortical areas of 10 coronal sections from approximately 2 mm anterior to 1.5 mm posterior to Bregma, each 240 µm apart, were measured by tracing cortical boundaries of both contralesional and ipsilesional cortex. The SMC fell within the area of tissue measured and no lesion extended beyond these boundaries. Cortical volume was estimated with the Cavalieri method, by multiplying the sum of section areas by the distance between sections[48, 49]. Lesion volume was calculated as the difference between contralesional and ipsilesional cortex.
2.6. Statistical analyses
SPSS software was used to conduct repeated-measures analyses of variance (ANOVAs) for Pasta Matrix Reaching Test performance, with Day as a within-subjects variable and Group as a between subjects variable. Post hoc analyses with a least significant difference (LSD) correction were conducted as necessary. A one-way ANOVA was conducted to compare the lesion extents of sham and ET-1 groups. In Experiment 1, there were no statistical differences between the performances of different sham groups (p > 0.1); all sham mice in Experiment 1were combined for statistical analyses. Likewise, there were no statistical differences between the performance levels of the two control groups in Experiment 2 (p > 0.1); mice in these groups were combined for statistical analyses. An α level of 0.05 was considered significant for all comparisons.
3. Results
3.1. Non-paretic limb training impedes functional outcome following ischemic stroke
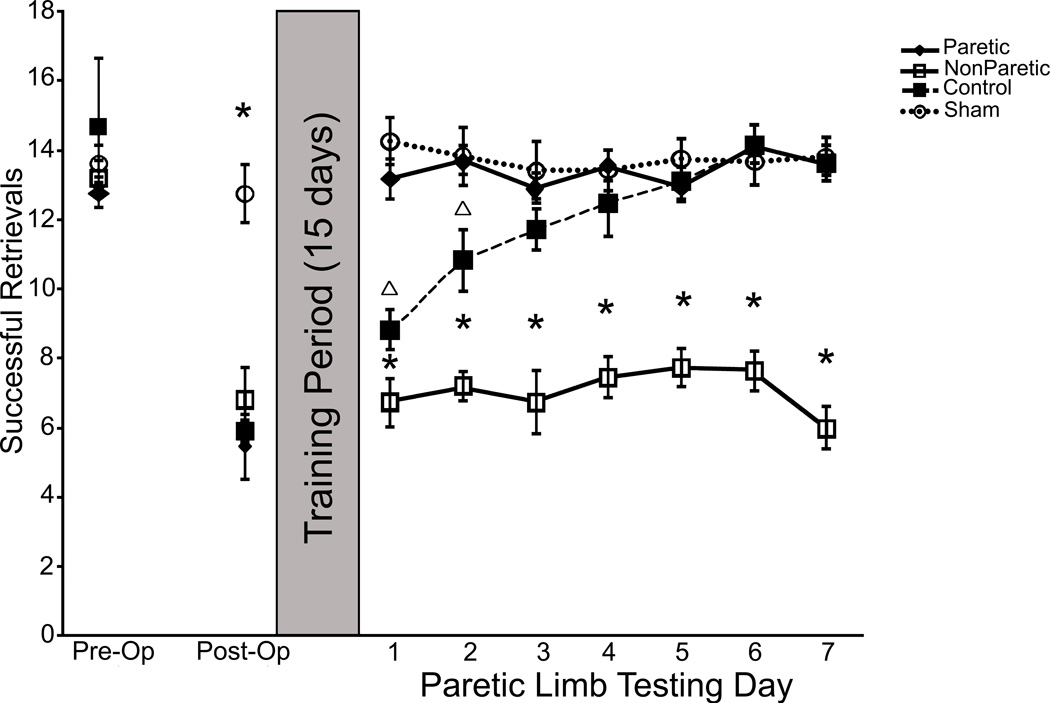
Subjects were matched on pre-operative performance on the Pasta Matrix Reaching Test. All animals exhibited similar initial deficits in reaching performance (around a 40% decrease in successful reaching) after ischemic lesions (Fig. 2). Following 15 days of reach training procedures with either the paretic (Par) or non-paretic (NonPar) limb or control procedures, the reaching performance of the paretic limb was assessed in all animals. During initial post-training assessment, the performance of Sham and Par groups resembled pre-operative levels. Control and NonPar groups exhibited performance levels that were similar to that of the initial post-operative assessment. Over seven consecutive days of testing the paretic limb, Control mice gradually began to display behavioral outcome that resembled pre-operative performance levels. The performance of NonPar mice did not improve with seven days of paretic limb assessment and remained at initial post-operative levels. A repeated measures ANOVA confirmed a significant Group x Day interaction (F(23,352) = 9.04, p < 0.001). Post hoc analyses with an LSD correction revealed significant differences between NonPar animals and all other groups (p < 0.001 for all comparisons). In addition, there was a significant difference between Control and Sham groups (p < 0.03). No other post hoc comparisons reached statistical significance (p > 0.1 for all comparisons).
Fig. 2.
Experiment 1: Pasta Matrix Reaching Test. Mice receiving rehabilitative training of the paretic limb (Par) during the training period exhibited functional recovery of the paretic limb during testing. Control mice received no skilled training during the training period. With paretic limb assessment, control mice improved their performance to reach pre-operative levels. Mice that received focused training of the non-paretic limb (NonPar) during the training period did not exhibit recovery during paretic limb assessment. NonPar mice did not experience any improvement of the paretic limb during the experiment. Significant differences (p < 0.05) from all other groups is denoted by *. Significant differences (p < 0.05) from only Par and Sham groups is denoted by Δ.
3.2.2. Bimanual home cage enrichment (BE) ameliorates non-paretic limb training effects following ischemic lesion
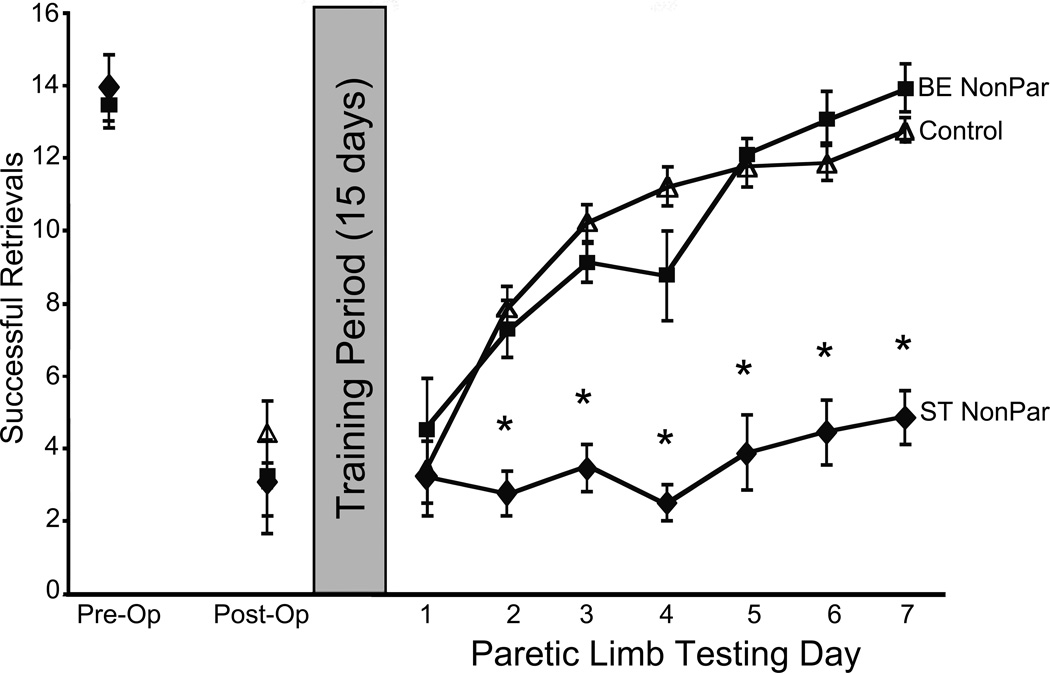
Experiment 2 assessed the value of dexterous coordinated use of both forelimbs in the home cage to functional outcome following non-paretic limb training. Following ET-1-induced ischemic infarcts, all groups exhibited similar deficits in reaching performance, with an approximate 50% decrease in successful reaching. Following 15 days of either standard housing or bimanual home cage enrichment and non-paretic or control training procedures, paretic limb performance was assessed for all mice over seven consecutive days (Fig. 3). Paretic limb assessment revealed that mice receiving bimanual home cage enrichment exhibited performance levels that were similar to those of Control mice. Both BE NonPar and Control groups improved their reaching performance over seven days of assessment, ultimately achieving performance levels that resembled pre-operative performance. ST NonPar mice did not improve their reaching performance with paretic limb assessment. Performance of ST NonPar mice remained similar to the initial deficit following stroke. A repeated measures ANOVA confirmed a significant Group x Day interaction (F(16, 192) = 9.361, p < 0.001). Post hoc analyses with an LSD correction revealed that the performance of ST NonPar mice was significantly different from both BE NonPar and Control mice (p < 0.001 for both comparisons). No other comparisons reached statistical significance (p > 0.1).
Fig. 3.
Experiment 2 Pasta Matrix Reaching Test. Home cage bimanual enrichment (BE NonPar) ameliorated the effects of non-paretic limb training (ST NonPar). BE NonPar mice performed similarly to Control mice. As in Experiment 1, ST NonPar mice did not show evidence of improved paretic limb performance during assessment. Significant differences (p < 0.05) from all other groups is denoted by *.
3.1.1. Lesion volume and reconstruction
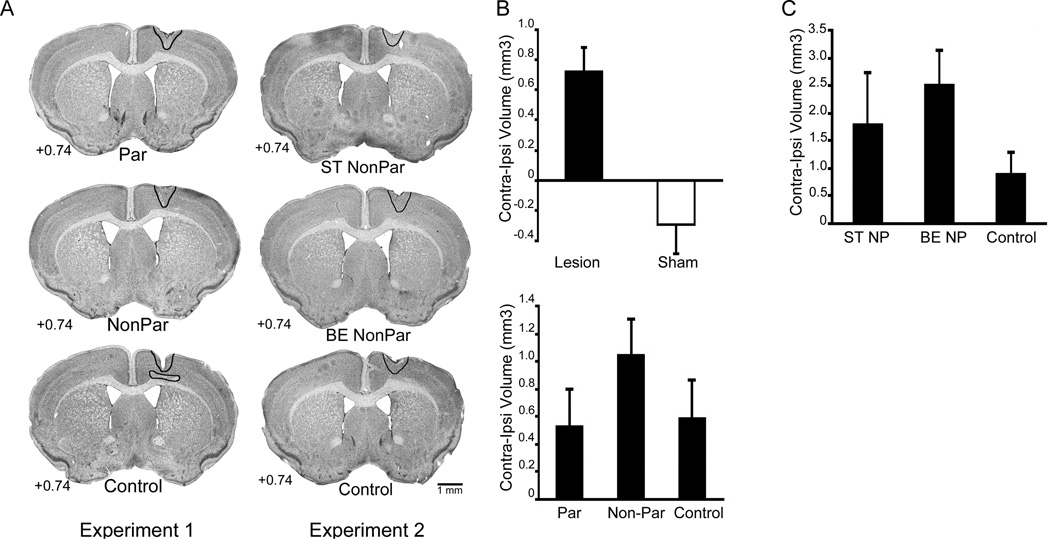
Representative images of Nissl stained tissue from both experiments are presented in Fig. 4A. Average lesion extent is outlined for all groups. ET-1 infusion resulted in damage in all mice, while sham procedures did not affect cortical volume. In Experiment 1, ET-1 infusion produced damage to the forelimb representation area of motor cortex extending in an approximately 1 mm radius from the infusion site (Figure 4A). Reconstruction of lesion placement and extent for Experiment 2 indicated that lesion placement was similar to that of Experiment 1, though the total lesion volume (contralateral-ipsilateral volume difference) tended to be larger (Figure 4A). No damage was observed in underlying white matter or striatum in either experiment.
Fig. 4.
Lesion Analysis. Representative images of Nissl stained sections from Experiments 1 and 2 (A). In Experiment 1 (A left) Par, NonPar, and Control mice exhibit similar damage. Mice in Experiment 2 (A right) exhibited lesion sizes that were slightly larger than those in Experiment 1, though lesion sizes between ST NonPar, BE NonPar, and Control groups were similar. Representative lesion sizes in both experiments are indicated in black outline. (B top) ET-1 infusion, as estimated by contralateral-ipsilateral cortical volume difference, resulted in lesion sizes of approximately 0.8 mm3 in Experiment 1. Sham procedures did not cause cortical damage. Within ET-1 lesioned animals (B bottom), there were no differences between training groups in lesion volume (p > 0.1). In Experiment 2 (C), there were no statistically significant differences in lesion sizes between groups (p > 0.1).
As seen in Figure 4B (top), in Experiment 1 lesions (n = 33) resulted in a significant loss of ipsilesional cortical volume (as estimated by contralateral-ipsilateral volume difference) compared to sham operates (F(1,46) = 15.383, p < 0.001). There was no significant difference in interhemispheric volume differences between the two lesion groups (p > 0.1; Figure 4B bottom). In Experiment 2, there was no statistical difference in lesion volume between Control (n = 10), ST NonPar (n = 8), and BE NonPar (n = 9) groups (p > 0.1; Figure 4C).
4. Discussion
We have previously established C57BL/6 mice as a reliable model for sensorimotor deficits following focal ischemic insult [43], with behavioral deficits resembling those produced by similar lesions in rats [14, 15, 17] and chronic impairments observed in humans (e.g., [50]). In this study, we have further developed our mouse model by exploring the impact of non-paretic limb training following focal insult. Our results indicate that the mouse, much like rats and humans, exhibits impaired functional recovery of the paretic limb following skilled use of the non-paretic limb after insult. We have further shown that the maladaptive effects of non-paretic limb training can be ameliorated with increased coordinated bimanual forelimb use in the home cage, suggesting that peri-lesion plasticity can be maintained and stimulated by minimal, unskilled, dexterous forelimb use. These results are in concert with previous findings that skilled bilateral training in rats [39, 40, 51] and humans [52, 53] improves functional outcome and extend these findings by suggesting that bilateral limb use does not have to involve focused training, but simply coordinated, dexterous activity.
Mouse models of stroke are becoming increasingly important with the availability of transgenic lines, the affordability of housing, and the ease of in vivo imaging with the species. Because mice share homologies with rat and primate forelimb movements and motor system organization [32, 54–57], they are a strong candidate for modeling upper limb impairment following stroke. Previously, we have demonstrated that ET-1 ischemic lesions of SMC in mice produce behavioral deficits that are similar to those observed in the well-established rat model[25, 39]. Following ET-1 infusion, mice exhibit impaired skilled reaching abilities, asymmetrical responsiveness to tactile stimulation [43], and motor map reorganization [44], similar to that previously observed in rats and primates.
In both the rat model and in human rehabilitation studies, skilled use of the non-paretic limb (forelimb in the rat) results in impaired functional outcome of the paretic limb. The current study establishes that this finding is also consistent in our mouse model. In fact, non-paretic training following ischemic stroke appears to not only impair, but to prevent functional recovery, as mice show no improvement in performance with seven days of focused paretic limb assessment (see Fig. 3 and 6). The current findings cannot be explained by lesion size as both paretic and non-paretic trained animals had similar lesion volumes assessed at the termination of paretic limb assessment. Therefore, non-paretic limb training interferes with functional recovery of the paretic limb, possibly by impeding on neural plasticity in peri-lesion cortex.
We have previously explored the impact of skilled bilateral limb training following similar insults in rats [40]. Our results indicated that focused training of both limbs prevents the maladaptive effects of non-paretic limb training after stroke. These results suggested that functional recovery is possible, even with a reliance on the non-paretic limb, as long as the paretic limb is also utilized in a focused rehabilitative fashion. However, humans often rely on their non-paretic limb to perform daily activities. This compensation both precedes and supersedes focused paretic limb rehabilitation, which is typically limited in frequency and duration. In the current study, we found that enhancing bimanual dexterous forelimb use for daily activities, such as eating, in mice was sufficient to avoid the deleterious effects of skill learning with the non-paretic limb [58, 59] and promote better functional outcome. Therefore, increasing coordinated bimanual limb use in daily activities may be an effective therapy to promote functional outcome without the inconvenience and discomfort of binding the non-paretic limb.
Following injury, the remaining cortex undergoes time-dependent degenerative and regenerative cascades that impact functional outcome [60]. Rehabilitative training interacts with this naturally occurring plasticity to impact both the structure and function of the central nervous system. Skilled unimanual training increases dendritic arborization [17, 25, 61, 62], the number of synapses per neuron [22, 63], and induces LTP-like mechanisms [64, 65] in contralateral-to-training motor cortex. In mice, skilled reaching training on the Pasta Matrix Reaching Test induces rapid synaptic remodeling observed in vivo [66]. When combined with the growth permissive and inhibitive post-stroke environment, skilled rehabilitative training induces motor map plasticity [31, 32, 44], dendritic plasticity [25, 38, 67], and improved motor function [30, 34]. These effects are time-dependent [68, 69] and often occur in areas of remaining ipsilesional cortex (i.e., peri-infarct cortex), which has been found to be especially important for behavioral outcome following injury. There is a correlation between behavioral outcome and movement related activation of the peri-infarct cortex in humans [70]. In mice, the area of peri-infarct cortex that is closest to the lesion is particularly dynamic, with the greatest synaptic turnover found in areas within several µm of the lesion core [71].
Learned non-use may result, in part, because learning with the paretic limb somehow impedes or impairs the potential of residual cortex to mediate better function in the paretic limb. ET-1 induced lesions of the SMC facilitate learning the with non-paretic limb, possibly as a result of degeneration-triggered processes that facilitate synaptic changes that underlie learning [17, 22, 25, 72]. With this facilitated learning of the non-paretic limb comes additional, maladaptive plasticity including decreased neuronal activation that is associated with the initiation of plasticity in remaining ipsilesional cortex [40].
The neural basis of the present effects are poorly understood. We have previously demonstrated that the maladaptive effects of non-paretic training (our model for learned non-use) are mediated through interhemispheric connections of SMC, as contralesional cortex and transcallosal fibers are required for the appearance of non-paretic training effects. It has been suggested that learning to compensate with this limb exaggerates interhemisphere disruption that may occur following stroke [51]. This hypothesis is supported by human research whereby unilateral injury results in abnormalities in interhemispheric activity associated with decreased behavioral outcome [73–77]. It is possible that by increasing coordinated bimanual forelimb use in the home cage, we have increased interhemispheric communication and perhaps connectivity, preserving the neuroplastic capabilities of peri-infarct cortex. It is also feasible that increased bimanual limb use during non-paretic limb training periods preserves the function of peri-infarct cortex by stimulating neuronal activation associated with the initiation of neural plasticity and prevents maladaptive plasticity that results from non-paretic limb training from impeding on the structural rehabilitation of peri-infarct cortex. Coordinated bimanual limb use does not rescue damaged tissue in peri-infarct cortex as lesion sizes are similar between enriched and un-enriched animals.
As peri-infarct cortex undergoes remodeling following injury, and contralesional homotopic cortex is likely responsible for mediating changes in non-paretic limb use, these two locations offer the most fruitful exploration of neural mechanisms supporting the adaptive and maladaptive effects of rehabilitative training. Stroke induces both vascular and dendritic plasticity in a coordinated fashion in peri-infarct cortex [71, 78], and non-paretic limb training has been found to decrease neural activation associated with initiating neural plasticity in peri-infarct cortex [40]. Clearly, interhemispheric disruption impedes neural plasticity in peri-infarct cortex. One potential mechanism of non-paretic training effects is interference with dendritic and vascular plasticity in peri-infarct cortex as a result of increased plastic responses in contralesional cortex. Additional research is needed to assess the neural mechanisms that support both the maladaptive effects of non-paretic limb training and the amelioration of those effects by bimanual dexterous limb use in the home cage.
It should be noted that in the current study, our ischemic insult produced different sized lesions between Experiment 1 and Experiment 2, with the lesions in Experiment 2 being somewhat larger on average that those in Experiment 1. It is important to realize that the size of lesions produced by ET-1 infusion are often small and the lesion sizes in both experiments fall within the normal range of what we have previously observed with this technique[43]. While the lesion-induced decrements in behavior were somewhat different between experiments, with animals in Experiment 1 exhibiting a 40% decrease in reaching performance and animals in Experiment 2 exhibiting a 50% decrease, the response of all animals to non-paretic limb training was similar. That is, in both experiments training the non-paretic limb reduced functional improvements in the paretic limb assessment. Further, the amelioration of the non-paretic limb training effect was detected in Experiment 2, following slightly larger lesions. As the larger lesion is associated with a larger impact on performance, it could be argued that effective rehabilitative strategies in Experiment 2 would also be effective in Experiment 1 and therefore do not limit our conclusions in the present study.
The current study is not without limitations. We assessed paretic limb performance for seven days following non-paretic training conditions. Additional research is also needed to determine the persistence of non-paretic training effects. In addition, the behavioral experience of our mice was limited in complexity, intensity, and variety and therefore does not accurately mimic the range of human bimanual experience. It is important to further explore the impact of intensity and variety of both behavioral experience and training on adaptive and maladaptive effects of post-stroke behavioral training. Finally, it would be useful to assess the generalizability of our findings to other disorders that present unilaterally or asymmetrically such as traumatic brain injury and Parkinson’s disease. Our present results suggest that therapeutic strategies focused on increasing coordinated bimanual limb use, and minimizing reliance on the non-paretic limb, will result in the most effective functional outcome following injury. The current findings also raise the possibility that the effects of paretic limb training might be further improved by its combination with bimanual enrichment training, although this effect was not explicitly tested as we did not have a paretic limb trained enrichment group. A better understanding of both the neural mechanisms that support functional rehabilitation and maladaptive behavioral outcomes, and the generalizability of our findings, may lead to optimal applications of rehabilitative strategies, including CIMT, cortical stimulation [79– 81], behavioral experience [82, 83], and focused behavioral training, to promote more effective behavioral recovery following injury.
Highlights.
We studied the effect of non-paretic limb training on paretic limb outcome in mice.
Non-paretic limb training was found to impede functional outcome of the paretic limb.
Home-cage bimanual limb use attenuated maladaptive effects of non-paretic training.
Potential neural mechanisms that support functional outcome are considered.
Acknowledgments
Sources of Funding
This work was supported by the National Institute of Health (NS056389; NS078791 by TAJ and NS076275 by ALK). The NIH was not involved in any aspect of study design or analysis nor composition of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Boerma T, Fat DM. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub E, Uswatte G, Mark VW, Morris DMM. The learned nonuse phenomenon: implications for rehabilitation. Europa medicophysica. 2006;42:241–256. [PubMed] [Google Scholar]
- 5.Mark VW, Taub E. Constraint-induced movement therapy for chronic stroke hemiparesis and other disabilities. Restorative Neurol Neurosci. 2004;22:317–336. [PubMed] [Google Scholar]
- 6.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 7.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 8.Mark VW, Taub E, Morris DM. Neuroplasticity and constraint-induced movement therapy. Europa medicophysica. 2006;42:269–284. [PubMed] [Google Scholar]
- 9.Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–1377. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- 10.Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following Constraint-Induced Movement therapy. Phys Med Rehabil Clin N Am. 2003;14:S77–S91. doi: 10.1016/s1047-9651(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 11.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA: the journal of the American Medical Association. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE Trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22:486–493. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobkin BH. Training and exercise to drive poststroke recovery. Nature clinical practice.Neurology. 2008;4:76–85. doi: 10.1038/ncpneuro0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Allred RP, Adkins DL, Woodlee MT, Husbands LC, Maldonado MA, Kane JR, Schallert T, Jones TA. The vermicelli handling test: a simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 2008;170:229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the "unaffected" forelimb and training-induced dendritic structural plasticity in the motor cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:8597–8606. doi: 10.1523/JNEUROSCI.22-19-08597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro AJ. The effects of cortical ablations on digital usage in the rat. Brain Res. 1972;37:173–185. doi: 10.1016/0006-8993(72)90665-8. [DOI] [PubMed] [Google Scholar]
- 19.Farr TD, Whishaw IQ. Quantitative and qualitative impairments in skilled reaching in the mouse (Mus musculus) after a focal motor cortex stroke. Stroke. 2002;33:1869–1875. doi: 10.1161/01.str.0000020714.48349.4e. [DOI] [PubMed] [Google Scholar]
- 20.Gilmour G, Iversen SD, O'Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav Brain Res. 2004;150:171–183. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Jones TA, Schallert T. Subcortical deterioration after cortical damage: effects of diazepam and relation to recovery of function. Behav Brain Res. 1992;51:1–13. doi: 10.1016/s0166-4328(05)80306-7. [DOI] [PubMed] [Google Scholar]
- 22.Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- 23.Voorhies AC, Jones TA. The behavioral and dendritic growth effects of focal sensorimotor cortical damage depend on the method of lesion induction. Behav Brain Res. 2002;133:237–246. doi: 10.1016/s0166-4328(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 24.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage in female rats: forelimb behavioral effects and dendritic structural plasticity in the contralateral homotopic cortex. Exp Neurol. 2004;190:433–445. doi: 10.1016/j.expneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski DA, Jones TA, Schallert T. Pruning of dendrites and restoration of function after brain damage: Role of the NMDA receptor. Restorative Neurol Neurosci. 1994;7:119–126. doi: 10.3233/RNN-1994-7207. [DOI] [PubMed] [Google Scholar]
- 28.Schallert T, Kozlowski DA, Humm JL, Cocke RR. Use-dependent structural events in recovery of function. Adv Neurol. 1997;73:229–238. [PubMed] [Google Scholar]
- 29.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008;22:250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science (New York, N.Y.) 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 32.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- 34.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 35.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci U S A. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu JE, Donlan N, Kleim JA, Jones TA. Protein synthesis inhibition in the perilesion cortex disrupts functional recovery and structural plasticity induced by rehabilitative training after unilateral cortical infarct in rats. 2007 Neuroscience Meeting Planner. 2007 [Google Scholar]
- 37.Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res. 1996;733:142–148. doi: 10.1016/0006-8993(96)00792-5. [DOI] [PubMed] [Google Scholar]
- 38.Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol. 1999;414:57–66. [PubMed] [Google Scholar]
- 39.Allred RP, Maldonado MA, Hsu And JE, Jones TA. Training the "less-affected" forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurol Neurosci. 2005;23:297–302. [PubMed] [Google Scholar]
- 40.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2008;210:172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allred RP, Adkins DL, Kim SY, Cappellini CH, Tennant K, Donlan N, Jones TA. Disruption of perilesion motor maps by learning with the ipilesional limb after unilateral sensorimotor cortex lesions. 2009 Society for Neuroscience Meeting Planner. 2009 [Google Scholar]
- 42.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J Neurosci Methods. 2009;181:18–26. doi: 10.1016/j.jneumeth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tennant KA, Adkins DL, Scalco MD, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. Skill learning induced plasticity of motor cortical representations is time and age-dependent. Neurobiol Learn Mem. 2012;98:291–302. doi: 10.1016/j.nlm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHISHAW IQ, DRINGENBERG HC, PELLIS SM. Spontaneous Forelimb Grasping in Free Feeding by Rats - Motor Cortex Aids Limb and Digit Positioning. Behav Brain Res. 1992;48:113–125. doi: 10.1016/s0166-4328(05)80147-0. [DOI] [PubMed] [Google Scholar]
- 46.Allred RP, Adkins DL, Woodlee MT, Husbands LC, Mónica A, Maldonado Kane JR, Schallert T, Jones TA. The Vermicelli Handling Test: A simple quantitative measure of dexterous forepaw function in rats. J Neurosci Methods. 170:229–244. doi: 10.1016/j.jneumeth.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whishaw IQ, Coles B. Varieties of paw and digit movement during spontaneous food handling in rats: Postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav Brain Res. 1996;77:135–148. doi: 10.1016/0166-4328(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 48.Henery CC, Mayhew TM. The cerebrum and cerebellum of the fixed human brain: efficient and unbiased estimates of volumes and cortical surface areas. J Anat. 1989;167:167–180. [PMC free article] [PubMed] [Google Scholar]
- 49.Mayhew TM. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- 50.Krakauer JW. Arm function after stroke: from physiology to recovery. Semin Neurol. 2005;25:384–395. doi: 10.1055/s-2005-923533. [DOI] [PubMed] [Google Scholar]
- 51.Allred RP, Cappellini CH, Jones TA. The "good" limb makes the "bad" limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- 53.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 55.Whishaw IQ, Gorny B. Arpeggio and fractionated digit movements used in prehension by rats. Behav Brain Res. 1994;60:15–24. doi: 10.1016/0166-4328(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 56.Whishaw IQ, Coles BL. Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav Brain Res. 1996;77:135–148. doi: 10.1016/0166-4328(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 57.Tennant KA, Adkins DL, Donlan NA, Asay AL, Thomas N, Kleim JA, Jones TA. The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cerebral cortex (New York, N.Y.: 1991) 2011;21:865–876. doi: 10.1093/cercor/bhq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeBow SB, Davies MLA, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–1026. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 59.Maclellan CL, Grams J, Adams K, Colbourne F. Combined use of a cytoprotectant and rehabilitation therapy after severe intracerebral hemorrhage in rats. Brain Res. 2005;1063:40–47. doi: 10.1016/j.brainres.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 61.Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. doi: 10.1016/0028-3932(89)90090-0. [DOI] [PubMed] [Google Scholar]
- 62.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–314. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 63.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science (New York, N.Y.) 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 65.Monfils M-, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 66.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, Nudo RJ. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Experimental brain research.Experimentelle Hirnforschung.Experimentation cerebrale. 2006;169:106–116. doi: 10.1007/s00221-005-0129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cramer SC, Shah R, Juranek J, Crafton KR, Le V. Activity in the peri-infarct rim in relation to recovery from stroke. Stroke. 2006;37:111–115. doi: 10.1161/01.STR.0000195135.70379.1f. [DOI] [PubMed] [Google Scholar]
- 71.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–1291. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- 72.Jones TA, Bury SD, Adkins-Muir DL, Luke LM, Allred RP, Sakata JT. Importance of behavioral manipulations and measures in rat models of brain damage and brain repair. ILAR J. 2003;44:144–152. doi: 10.1093/ilar.44.2.144. [DOI] [PubMed] [Google Scholar]
- 73.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 74.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 75.Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol (Lond) 2009;587:725–726. doi: 10.1113/jphysiol.2008.166926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain: a journal of neurology. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- 77.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 80.Bashir S, Mizrahi I, Weaver K, Fregni F, Pascual-Leone A. Assessment and modulation of neural plasticity in rehabilitation with transcranial magnetic stimulation. PM & R: the journal of injury, function, and rehabilitation. 2010;2:S253–S268. doi: 10.1016/j.pmrj.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]