Abstract
Background
Metabolic syndrome (MetS)—a cluster of cardiovascular risk factors—is linked with cognitive decline and dementia. However, the brain changes underlying this link are presently unknown. In this study, we tested the relationship between MetS, cerebral blood flow (CBF), white matter hyperintensity (WMH) burden and gray matter (GM) volume in cognitively healthy late middleaged adults. Additionally, we assessed the extent to which MetS was associated with cognitive performance.
Methods and Results
Late middle-aged adults from the Wisconsin Registry for Alzheimer’s Prevention (N=69, mean age=60.4 yrs) underwent a fasting blood draw, arterial spin labeling perfusion MRI, T1-weighted MRI, T2FLAIR MRI, and neuropsychological testing. MetS was defined as abnormalities on 3 or more factors, including: abdominal obesity, triglycerides, HDL-cholesterol, blood pressure, and fasting glucose.
Mean GM CBF was 15% lower in MetS compared to controls. Voxel-wise image analysis indicated that the MetS group had lower CBF across a large portion of the cortical surface, with the exception of medial and inferior parts of the occipital and temporal lobes. The MetS group also had lower immediate memory function; a mediation analysis indicated this relationship was partially mediated by CBF. Among the MetS factors, abdominal obesity and elevated triglycerides were most strongly associated with lower CBF.
Conclusions
The results underscore the importance of reducing the number of cardiovascular risk factors for maintaining CBF and cognition in an aging population.
Keywords: cerebral blood flow, brain, magnetic resonance imaging, metabolic syndrome, cognitive function
Introduction
Metabolic syndrome (MetS) refers to a cluster of cardiovascular risk factors that often occur together and reflect an increased risk for type 2 diabetes. Five core risk factors contribute to the clinical identification of MetS: abdominal obesity, high triglycerides, low HDL cholesterol, hypertension, and high fasting glucose (1). A diagnosis of MetS is made when three or more factors are present. It is estimated that 34% of American adults have MetS, and while the condition is increasingly identified in younger adults, the prevalence of MetS increases sharply in middle age(2). At the same time, the US population is aging(3), a phenomenon accompanied by an increased prevalence of dementia, in particular Alzheimer’s disease (AD). The current epidemic of MetS in middle age and the projected epidemic of AD in old age converge in findings that MetS and individual MetS factors occurring in middle-age are associated with increased risk of cognitive decline and AD(4–6). In fact, several studies suggest that midlife may be a critical period when cardiovascular risk factors influence cognitive aging trajectories (4, 7, 8).
While several potentially complementary mechanisms have been proposed for linking MetS and its comprising factors to cognitive decline(9), little is known about the midlife brain changes that occur in people with MetS that may adversely impact cognition beyond normal aging. The factors comprising MetS are known cerebrovascular risk factors and abnormal triglycerides, HDL, blood pressure, and obesity have been linked to decreases in cerebral blood flow (CBF)(10–13). Recent studies have also identified global and regional CBF reductions in people with mild cognitive impairment (MCI) and AD(14), suggesting cerebral hypoperfusion as a possible mechanism for neural damage and cognitive decline.
Thus, the goal of the present study was to assess the extent to which MetS is associated with CBF differences in midlife and examine the role of cerebral perfusion as a potential mechanism for structural brain alteration and cognitive decline. Middle-aged participants were recruited from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) cohort (15) to undergo magnetic resonance imaging (MRI) sensitive to CBF, and brain scans that index regional gray matter (GM) volume and white matter lesion burden. We hypothesized that people with MetS would have lower CBF, lower GM volume, higher WMH burden, and lower cognitive performance compared to people without MetS. We also expected that differences between the MetS group and controls in cognition, GM volume and WMH lesion load would be explained by differences in CBF.
Methods
Subjects
Seventy-five participants were recruited from WRAP, a registry of cognitively normal adults who are followed longitudinally and comprise a cohort whose members either have a family history of late onset AD or no family history of AD(15). A positive family history was defined as having one or both parents with autopsy-confirmed or probable AD as defined by NINCDSADRDA research criteria (16). The inclusion criteria for this study consisted of: normal cognitive function determined by neuropsychological evaluation, no contraindications for MRI and a subsequent normal MRI scan, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., myocardial infarction, or recent history of cancer), and no history of head trauma. All participants underwent a fasting blood draw, MRI, and neuropsychological testing. Four participants were excluded because of abnormal radiological findings from the reviewing radiologist (HAR). Two participants were excluded because of scan artifact, leaving 69 participants in the study. Subject demographics can be found in Table 1. The University of Wisconsin Institutional Review Board approved all study procedures and each participant provided signed informed consent before participation.
Table 1.
Sample demographics and cognitive factor scores between the control and MetS groups controlling for age.
| Demographic | All N = 69 | Controls n = 40 | MetS n = 29 | Statistic | p-value |
|---|---|---|---|---|---|
| Women, n (%) | 43 (62.3) | 27 (67.5) | 16 (55.2) | χ2=1.09 | 0.297 |
| Family History, n (%) | 42 (60.9) | 21 (52.5) | 21 (72.4) | χ2=2.80 | 0.094 |
| APOE4 Carriers, n (%) | 27 (39.1) | 17 (42.5) | 10 (34.5) | χ2=0.45 | 0.501 |
| Age, M (SD) | 60.4 (6.1) | 58.9 (5.8) | 62.6 (5.8) | t=2.65 | 0.011* |
| Education, M (SD) | 16.4 (2.5) | 17.2 (2.4) | 15.4 (2.4) | t=3.03 | 0.003** |
|
| |||||
| Cognitive Factor Score, M (SE) | |||||
|
| |||||
| Speed and Flexibility | 0.09 (0.09) | 0.27 (0.12) | −0.09 (0.14) | F(1, 65) = 3.71 | 0.058 |
| Working Memory | 0.10 (0.133) | 0.10 (0.18) | 0.10 (0.21) | F(1, 66) = 0.00 | 0.98 |
| Verbal Learning and Memory | 0.10 (0.11) | 0.18 (0.15) | 0.03(0.18) | F(1, 66) = 0.41 | 0.52 |
| Immediate Memory | −0.06 (0.11) | 0.22 (0.15) | −0.33 (0.17) | F(1, 66) = 5.58 | 0.021* |
p < 0.05;
p < 0.01
Cognitive testing
As part of their participation in WRAP, participants received at least one comprehensive neuropsychological assessment(15). On average, neuropsychological testing occurred within ten months of the MRI scan (M= 0.82 years, SD = 0.60 years). Time between testing and MRI did not significantly differ between the MetS group and the control group, t = 0.91, p = .37. The neuropsychological battery(15) tested cognitive function in the domains of memory, attention, executive function, verbal ability, and visuospatial ability. We analyzed four cognitive factor scores that were determined from a factor analytic study of the WRAP neuropsychological battery and adapted from work published in Dowling et al.(17): Immediate Memory (Rey Auditory Verbal Learning Test(18), Trials 1 and 2), Verbal Learning & Memory (Rey Auditory Verbal Learning Test(18), Trials 3–5 and Delayed Recall Trial), Working Memory (Wechsler Adult Intelligence Scale – 3rd edition(19), Digit Span, Arithmetic, and Letter-Numbering Sequencing subtests), Speed & Flexibility (interference trial from the Stroop Test(20), and Trail Making Test A and B(21)). These factors were selected for analysis because they represent domains of cognitive skill that may be affected in preclinical stages of AD (22). The speed and flexibility factor score was unavailable for one participant. Cognitive analyses used unadjusted factor scores but with age as a covariate.
MetS factors
MetS was determined according to consensus criteria published in 2009(1). The criteria include the following: abdominal obesity > 102cm for men, >88cm for women; triglycerides ≥ 150 mg/dL; HDL cholesterol < 40 mg/dL in men, and < 50 mg/DL in women; blood pressure ≥ 130/85 mmHg; fasting glucose ≥ 100 mg/dL. The use of medication to treat high blood pressure, elevated triglycerides, elevated glucose or low HDL also indicated the presence of the respective MetS factor. Participants who exceeded criteria on 3 or more factors (n = 29) were included in the MetS group while the rest of the sample (n = 40) were considered controls. The percentages of participants that met criteria on each factor in the MetS group and the control group are listed in Table 2.
Table 2.
Distribution of MetS factors in the sample (n, %)
| All (N = 69) | MetS (n = 29) | Controls (n = 40) | |
|---|---|---|---|
|
|
|||
| High Waist | 30, 43% | 23, 79% | 7, 17% |
| High Triglycerides | 19, 28% | 14, 48% | 5, 13% |
| Low HDL | 29, 42% | 21, 72% | 8, 20% |
| Hypertension | 42, 61% | 27, 83% | 15, 38% |
| High Glucose | 26, 37% | 21, 72% | 5, 13% |
Brain Imaging Acquisition
MR scanning was performed on a General Electric 3.0 Tesla Discovery MR750 (Waukesha, WI) MRI system with an 8-channel head coil and parallel imaging (ASSET).
A T1-weighted volume was acquired in the axial plane with a 3D fast spoiled gradient-echo (3D EFGRE FSPGR) sequence using the following parameters: TI = 450 ms; TR = 8.1 ms; TE = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 × 156 mm, FOV = 260 mm; slice thickness = 1.0 mm.
A T2-weighted fluid attenuated inversion recovery (FLAIR) sequence was acquired in the sagittal plane using the following parameters: TI = 1868 ms; TR = 6000 ms; TE = 123 ms; flip angle = 90°; acquisition matrix = 256 x 256 x 100 mm, FOV = 256 mm; slice thickness = 2.0 mm yielding a voxel resolution of 1 mm x 1 mm x 2 mm.
Resting CBF assessments were made using background-suppressed pseudo-continuous ASL (pcASL)(23, 24), featuring a 3D fast spin echo spiral sequence that utilizes a stack of variabledensity spiral 4ms-readout and 8 interleaves. Scan parameters were TR = 6000 ms; TE = 21 ms; FOV = 240 x 240 x 160 mm; slice thickness = 4 mm no gap; matrix size=128 x 128; NEX=3; and labeling RF amplitude=0.24 mG. Multi-slice spin labeling was implemented using a single coil that eliminates off-resonance errors(25). Post-labeling delay was 1525 ms for 40 participants and 2025 ms for 29 participants and was entered as a covariate in all analyses utilizing pcASL scans. The distribution of inversion time was not different between MetS and controls. The pcASL scan included 3 averaged acquisitions, each consisting of a control image subtracted from a labeled image. The sequence also included a fluid-suppressed proton density (PD) acquisition, with the same imaging sequence/image slab location as the pcASL but without the RF labeling preparation, for CBF flow quantitation and image registration. In order to reduce variability in the CBF assessment, participants fasted for a minimum 4-hours prior to scan, abstaining from food, tobacco, caffeine, and medications with vasomodulatory properties. We have previously reported excellent test-retest reliability (r > 0.95) of this pcASL procedure(26).
pcASL processing
To derive quantitative CBF maps, sensitivity maps were first created that represent image sensitivity to water at each voxel and are a function of the PD image, saturation time, T1 image and assumed tissue water concentration. CBF is then calculated using the density of brain tissue, the labeling efficiency, the post-labeling delay, the labeling duration, the T1 of arterial blood, the density of water in the blood, and the signal intensities in the labeled and control images. Equations can be found in Xu et al.(26)
The averaged quantified CBF maps were brought into normalized space by first registering the PD map to the T1 volume and applying the derived transformation matrix to the CBF map using SPM8 (www.fil.ion.ucl.ac.uk/spm), bringing the CBF maps into the space of the T1 volumes. In a similar fashion the T1 volume was then normalized to the Montreal Neurological Institute (MNI) standard space and the derived transformation matrix applied to the CBF map. Finally, the normalized CBF images were smoothed with a 8mm kernel in SPM8.
To derive mean gray matter CBF, the ICBM probabilistic GM map available in SPM (thresholded at 0.30) was applied to the CBF maps in MNI space and the mean voxel value was extracted using MarsBaR (http://marsbar.sourceforge.net). Mean CBF values were scaled to 50 ml/min/100g.
Between and within subject noise was accounted for by using a reference cluster as a covariate in all voxel-wise analyses and mean CBF statistical analyses(27). The reference cluster consisted of 549, 2 x 2 x 2 mm voxels centered in the left middle temporal gyrus, in a region where there was no difference in CBF between the MetS group (M = 35.13, SE = 1.68) and the control group (M = 37.92, SE = 1.42), F = 1.52, p = 0.22. The region was derived via a data-driven method developed for normalizing [(18)F]FDG Positron Emission Tomography scans(28). The raw values from the reference region were extracted with MarsBaR.
T1-weighted volumetric
Processing of the T1-weighted images was performed using a six-class segmentation processing stream in SPM8. Processing involved bias correction and iterative normalization and segmentation of the original anatomic images(29) into distinct tissue classes (gray matter, white matter, cerebrospinal fluid, skull, fat tissue, and image background) using spatial prior information. GM tissue segments were normalized to MNI template space via a 12-parameter affine transformation and nonlinear deformation (with a warp frequency cutoff of 25). The segmented and normalized GM maps were “modulated”, which involves scaling the final GM maps by the amount of contraction or expansion required to warp the images to the template. The final result was a GM probability map for each participant in which the total amount of GM remained the same as in the original images. The spatially normalized GM maps were smoothed using an 8-mm Gaussian kernel before being entered into the statistical analysis. The volume of the SPM8 GM segmentation was divided by intracranial volume (ICV) to create a total GM volume ratio variable.
ICV was calculated to scale for differences in head size in the GM and WMH analyses using a “reverse brain masking” method(30). First, summing the gray, white and CSF ICBM probability maps created an ICV probability map. Then, the inverse deformation field resulting from unified segmentation on each subject image was applied to the ICV probability map, in order to produce an ICV mask in native space. A threshold of 90% was applied to this subject specific ICV probability map and the total volume was extracted.
T2FLAIR Processing
Total WMH lesion volume was calculated using the lesion segmentation toolbox in SPM8(31). The toolbox seeds lesions based on spatial probability from T1 images and hyperintense outliers on T2FLAIR images. The initial threshold was set at 0.30 and is used to create the binary conservative lesion belief map from the GM lesion belief map. Next, a growth algorithm grows these seeds from the conservative lesion belief map toward a probabilistic liberal lesion belief map from GM, WM, and CSF. Lastly, we used a threshold of 1.00 on the resulting lesion belief map. The resulting WMH volume was divided by ICV to give a ratio (WMHr). For voxel-wise WMH analysis, probability lesion belief maps were normalized to MNI space and smoothed with a 10mm Gaussian kernel.
Statistical analyses
With the exception of the voxel-wise image analysis conducted in SPM8, statistical analyses were carried out in IBM SPSS version 20.0 (Chicago, IL). Demographic differences between groups were assessed with t-tests for continuous variables and χ2 tests for categorical variables. The effect of APOE4 and parental family history on the brain indices and cognitive function were assessed using ANCOVA. Age was used as a covariate in all tests involving WMHr, GM volume, or cognition.
To test the hypothesis that MetS is associated with changes in CBF, WMH, GM volume, and cognitive factor scores, ANCOVA was utilized. Each model used MetS status entered as the independent variable and a brain measure or cognitive factor score as the dependent variable. As a follow-up analysis, the sum of present MetS factors was used in linear regression to predict CBF in order to better understand how the clustering of MetS factors affects CBF. To determine the regional voxel-wise brain differences in CBF, WMHs and GM volume between MetS and control, ANCOVA was utilized in SPM8. To minimize type 1 error, all voxel-wise analyses controlled for multiple comparisons using FWE p < .05. Age, sex, and ICV were used as covariates in WMH and GM volume voxel-wise analyses. Age, sex, reference region, and inversion time were used as covariates in voxel-wise CBF analyses. Voxel-wise GM volume analyses were restricted using an absolute threshold of 0.10. CBF voxel-wise analyses were restricted using a GM mask created by thresholding the ICBM probabilistic GM map by 0.3. WMH voxel-wise analyses were restricted using a WM mask created by thresholding the ICBM probabilistic WM map by 0.3.
The hypothesis that differences in CBF were associated with differences in WMHr, GM volume ratio, and cognitive factor scores was tested using linear correlations between reference cluster adjusted CBF, WMHr, GM volume ratio, and cognitive factor scores partialing out age and inversion time.
Next, to test the extent to which MetS factors affected CBF, we used a single linear regression model with each MetS factor status (abdominal obesity, triglycerides, HDL cholesterol, blood pressure, and fasting glucose) entered as an independent variable and mean CBF as the dependent variable controlling for the reference cluster and inversion time. Collinearity was assessed within the linear regression model using the tolerance statistic, which represents the proportion of variance explained by a factor that is not related to the other factors in the model (32).
Lastly, the hypothesis that CBF would mediate differences in cognition found between MetS and controls was tested using mediator analysis(33) performed in the PROCESS macro for SPSS (www.afhyes.com). CBF was adjusted for reference cluster and inversion time and age was used as a covariate in the mediation model. The direct and indirect effects were calculated and significance was determined using bootstrapping (k = 5000) with 95% confidence intervals.
Results
Demographic and Brain Measures
There were no differences between the MetS and the control group on sex, APOE4, or parental family history. MetS participants were 3.6 years older on average and had 1.8 fewer years of education than controls. Participant demographics between control and MetS groups are given in Table 1. With increasing age, WMHr increased and GM volume ratio decreased. Age did not have an effect on CBF in this age range. APOE4 carriers did not differ in WMHr or CBF compared to non-APOE4 carriers. APOE4 carriers had marginally lower GM volume ratio compared to non-carriers. Parental family history did not have an effect on brain indices in this sample. Table 3 shows the results of the tests performed with brain indices. In addition, no associations were noted between CBF, WMHr, or GM volume ratio.
Table 3.
Relationship between brain measures and age, APOE, parental family history, and MetS
| White Matter Hyperintensity Ratio (WMHr)
|
Gray Matter (GM) Ratio
|
Cerebral Blood Flow (CBF)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative Group | Positive Group | Negative Group | Positive Group | Negative Group | Positive Group | |||||||
|
|
|
|
||||||||||
| M, SE | M, SE | statistic | p-value | M, SE | M, SE | statistic | p-value | M, SE | M, SE | statistic | p-value | |
| Age | - | - | r(67) = 0.30 | 0 .012* | - | - | r(67) = −0.61 | 0.00** | - | - | r(65) = −0.13 b | 0.29 |
| APOE4 | 0.102, 0.025 | 0.092, 0.031 | F(1, 66) = 0.06a | 0.8 | 0.487, 0.004 | 0.475, 0.005 | F(1, 66) = 3.69a | 0.059 | 50.07, 0.87 | 49.90, 1.10 | F(1, 65) = 0.01 b | 0.91 |
| Family History | 0.108, 0.031 | 0.092, 0.025 | F(1, 66) = 0.16 a | 0.69 | 0.485, 0.005 | 0.481, 0.004 | F(1, 66) = 0.32 a | 0.57 | 51.00, 1.04 | 49.36, 0.84 | F(1, 65) = 1.51 b | 0.22 |
| MetS | 0.067, 0.025 | 0.141, 0.030 | F(1, 66) = 3.47 a | 0.067 | 0.482, 0.004 | 0.483, 0.005 | F(1, 66) = 0.02 a | 0.88 | 52.98, 0.66 | 45.90, 0.78 | F(1, 65) = 46.97 b | 0 .000** |
WMHr was calculated by dividing total WMH volume by intracranial volume (ICV). GM ratio was calculated by dividing GM volume by ICV. Means are adjusted;
controlling for age;
controlling for reference cluster and inversion time;
p < 0.05;
p < 0.01
WMH
While controlling for age, WMHr was marginally higher in the MetS group compared to the control group (Table 3). Voxel-wise analyses of WMH probability maps indicated no regional differences between MetS and controls.
GM Volume
The MetS and control groups did not differ on GM volume ratio controlling for age (Table 3). A voxel-wise analysis of GM comparing MetS to controls revealed no significant regional volume differences between the two groups.
CBF
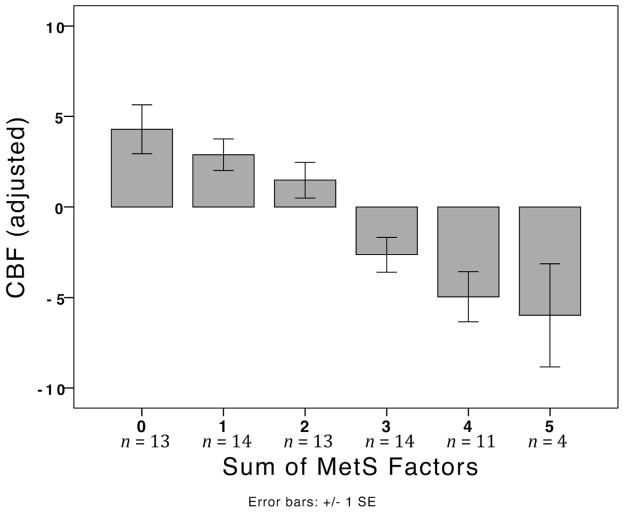
Total GM CBF was 15% lower in the MetS group compared to the control group (Table 3). As shown in Figure 1, possessing more MetS factors was associated with increasingly lower CBF, r(65) = 0.31, p < 0.001.
Figure 1.
Mean CBF is displayed by groups defined by the number of MetS factors present in an individual. CBF is adjusted by reference cluster and inversion time.
In order to determine how total GM CBF was predicted by the MetS factors, all of the factors were entered as independent variables into a linear regression model with CBF as the dependent variable. The resulting model (Table 4) shows that waist and triglycerides were significant predictors and glucose was near significance. Collinearity analysis in this model demonstrated that each factor largely had an independent effect on CBF that was not related to the other factors.
Table 4.
Linear regression model with MetS factors predicting mean CBF. R = 0.69, F = 11.65, p < 0.001
| Standardized coefficients | Collinearity | |||
|---|---|---|---|---|
| Beta | t | p | tolerance | |
| High Waist | −0.34 | −3.33 | 0.001** | 0.79 |
| High Triglycerides | −0.32 | −3.38 | 0.001** | 0.94 |
| Low HDL | −0.13 | −1.36 | 0.18 | 0.86 |
| Hypertension | −0.09 | −0.93 | 0.36 | 0.83 |
| High Glucose | −0.19 | −1.89 | 0.064 | 0.81 |
Controlling reference cluster and inversion time;
p < 0.01; type III sums of squares
A voxel-wise CBF analysis showed that the MetS group had lower CBF in the medial and lateral aspects of frontal and parietal lobe GM, and lateral areas of the temporal and occipital lobe GM (Figure 2).
Figure 2.
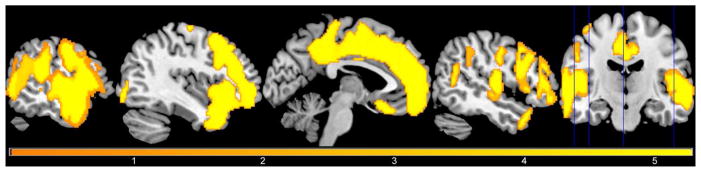
Participants with metabolic syndrome showed significantly lower CBF in large portions of the cortical surface of the frontal and parietal lobes, and the lateral and superior portions of the temporal and occipital lobes. Voxel-wise results are shown here at p < 0.05, FWE corrected, controlling for age, sex, and reference cluster. The color of the overlay reflects the size of the t-statistic.
Cognition
The MetS group (M = −0.33, SE = 0.17) had a significantly lower immediate memory factor score compared to the control group (M = 0.22, SE = 0.15), F(1, 66) = 5.58, p = 0.021. The speed and flexibility factor score was marginally lower for the MetS group (M = −0.092, SE = 0.140) compared to the control group (M = 0.267, SE = 0.117), F(1, 65) = 3.71, p = 0.058.
Total GM CBF, WMHr, and GM volume ratio were also used to predict cognitive function controlling for age. Correlation analysis showed that participants with lower CBF had lower immediate memory factor scores (r = 0.37, p = 0.002). There was a trend of higher WMHr being associated with lower immediate memory factor scores (r = −0.22, p = 0.070). None of the other factor scores were correlated with CBF, WMHr, or GM volume ratio.
Finally, based on the finding that MetS participants differed from controls on both CBF and immediate memory, we conducted a mediation analysis that tested the extent to which CBF mediated the relationship between MetS and lower immediate memory function. We found that CBF was a significant mediator between MetS and immediate memory (Figure 3). The direct effect of MetS on immediate memory was estimated to be r = −0.17, while the indirect effect of MetS on immediate memory accounted by CBF was estimated to be r = −0.41; 95 % CI [−0.82, −0.08].
Figure 3.
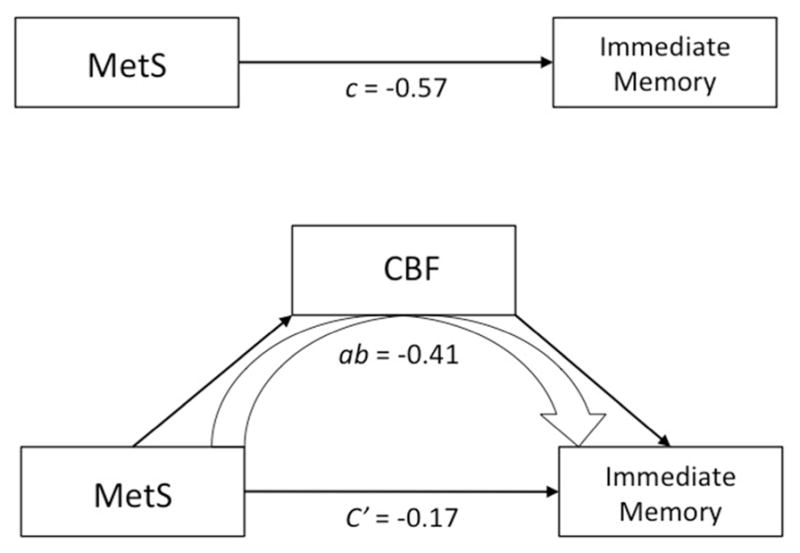
The first model displays the total effect, c, between MetS and immediate memory. The second uses CBF as a mediator that is partially accounting for the effect between MetS and immediate memory. The indirect effect, ab = −0.41, is the portion of the effect accounted for by CBF. Significance of the mediation was determined using bootstrapping (k = 5000) with 95% confidence intervals of the indirect effect [−0.82, − 0.08]. Age, reference cluster, and inversion time were controlled.
Conclusions
The metabolic syndrome is increasingly recognized as contributing to adverse health outcomes, including increased risk for cardiovascular disease, type 2 diabetes and, more recently, cognitive decline(6). The purpose of this study was to examine the effect of MetS on brain health and cognition in middle-aged adults. In particular, the study aimed to determine the effect of MetS on CBF, and determine the extent to which CBF is associated with structural gray and white matter alterations and cognitive differences. We found that CBF is compromised in MetS, and both MetS and CBF are related to lower memory performance. Furthermore, the results of the mediation analysis indicate that CBF partially mediates the relationship between MetS and memory performance.
While several studies point toward midlife cardiovascular risk in predicting cognitive decline and dementia later in life (4, 7, 8), little is known about the midlife brain changes that may underlie such cognitive changes. Cardiovascular risk factors are associated with decreased cerebral perfusion (10–13). As an extension of these findings, our study found that CBF is compromised in participants with MetS. Indeed, having an increasing number of MetS factors was associated with decreasing cerebral perfusion.
Regarding the individual contributions of the metabolic risk factors making up MetS, lower CBF was most robustly associated with abdominal obesity, and to a lesser extent triglycerides and fasting glucose. This finding is in line with recent studies suggesting that obesity may be the major underlying pathophysiology in metabolic syndrome(34). Larger sample sizes will be needed in future studies to further parse the independent effects of factors.
A voxel-wise comparison of CBF between MetS and controls showed that perfusion was lower in medial and lateral frontal and parietal lobes, and lateral areas of the temporal and occipital lobes. These brain regions are supplied primarily by branches of the internal carotid arteries. While Iit is unknown whether our participants were showing lower CBF due to differences in neural requirements, or due to vascular insufficiency. , the incidence of occlusion and atherosclerosis is more common in the carotid system than the vertebral-basilar system(34), suggesting that the pattern of reduced CBF observed in MetS was due to arterial disease rather than a decrease in metabolic function. Furthermore, among patients with transient ischemic attack and stroke, metabolic syndrome is associated with more severe intracranial arterial stenosis and diabetes is more frequent with stenosis of the internal carotid arteries than in the vertebral-basilar system(35). The interaction between MetS risk factors and the structural and hemodynamic factors that vary by vascular location needs to be investigated.
Given the increased interest in altered brain metabolism in aging, and Alzheimer’s disease in particular(35–37), it will be of paramount importance to understand the mechanisms that underlie the observed differences in CBF found in this study. Further studies utilizing newly developed MR technology, for example PCVIPR (3D phase contrast vastly undersampled isotropic projection reconstruction) will provide flow measurements from large vessels, and will help clarify the contribution of flow to perfusion measurements and other blood based MR imaging (e.g. fMRI and resting BOLD). Likewise, complementary imaging using FDG-PET is expected to shed light on the relationship between cardiovascular risk factors, in particular altered insulin signaling, and altered metabolic demand.
In consideration of the effect of MetS on structural brain changes, we found little evidence for an effect of MetS on either white matter lesion load or gray matter volume. Analyses using measures from the whole brain, and those using a voxel-wise approach, did not suggest that MetS at mid-life is associated with significant structural brain alteration.
While MetS has been associated with cognitive decline in older adults(38), this study shows that cognitive differences can be measured as early as midlife. Additionally, for the first time, this study significantly identified CBF as a partial mediator between MetS and cognitive performance. While suggestive, further work will be needed to determine whether lower CBF is responsible for the decreased immediate memory performance or is a consequence of subtle neural injury which underlies both the reduced CBF demand and altered cognitive performance.
Immediate memory as measured on initial trials of word list learning reflects the operation of multiple cognitive processes, including auditory verbal working memory, strategic processing, and semantic encoding(39). These processes, as well as cognitive speed and flexibility, are relevant to the broad category of executive function, and current findings suggest that, at least in midlife, the cognitive footprint of MetS and/or lowered CBF may be most readily apparent on aspects of memory that are dependent on executive skills.
There are a few limitations that should be noted. This study is cross-sectional, and longitudinal studies are needed to determine cognitive decline. This study recruited participants from an established registry for Alzheimer’s research, potentially limiting the generalizability of these results. Another limitation is the sample size. While we successfully measured the independent contributions of individual risk factors, a larger sample would provide more power to observe the effects of single risk factors. Additionally, the sample size relative to the heterogeneity of WMH volumes and regional distribution may have restrained WMH analyses. The sample size also precluded analyses on the effects of medication, which is an interesting area of study and expected to shed light on the efficacy of interventions for cognitive decline. At the same time, it should be noted that one of the strongest predictors of CBF was abdominal obesity, which is the only factor lacking a drug intervention. Determining the effect of medications, and accounting for their use in statistical models may better reveal the effects of some of the MetS factors on CBF. The cross-sectional design and sample size also limited any analysis on the duration of MetS or consisting factors, which could modulate the results presented in this study. Finally, we consistently observed effects of MetS across analyses; although, type 1 error is possible and these results will need replication in additional samples.
In summary, this study suggests that reducing the number of metabolic risk factors may be important in preserving CBF and cognitive health.
Acknowledgments
The authors gratefully acknowledge Nancy Davenport-Sis, Amy Hawley, and the support of researchers and staff at the Waisman Center, University of Wisconsin-Madison, for their assistance in recruitment, data collection, and data analysis. Above all, we wish to thank our dedicated volunteers for their participation in this research.
Funding Sources
This project was supported by the Alzheimer’s Association, NIRG-09-132626, and in part by the National Institute on Aging (R01 AG027161 [MAS], ADRC P50 AG033514 [SA]), and the University of Wisconsin Institute for Clinical and Translational Research, funded through a National Center for Research Resources/National Institutes of Health Clinical and Translational Science Award, 1UL1RR025011. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. GRECC MS # 2012-XX. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures
None
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Ervin RB. National health statistics reports; no 13. National Center for Health Statistics; Hyattsville, MD: 2009. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. 2009. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention and The Merck Company Foundation. The State of Aging and Health in America, 2007. The Merck Company Foundation; Whitehouse Station, N.J: 2007. [Google Scholar]
- 4.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 5.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease. Neurology. 2006;67:843–7. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu W, Qiu C, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–7. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisardi V, Solfrizzi V, Seripa D, et al. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9:399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Rogers RL, Meyer JS, McClintic K, Mortel KF. Reducing Hypertriglyceridemia in Elderly Patients with Cerebrovascular Disease Stabilizes or Improves Cognition and Cerebral Perfusion. Angiology. 1989;40:260–9. [PubMed] [Google Scholar]
- 11.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI. Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann Neurol. 2012 doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- 12.Carlsson CM, Xu G, Wen Z, et al. Effects of Atorvastatin on Cerebral Blood Flow in Middle-Aged Adults at Risk for Alzheimer’s Disease: A Pilot Study. Curr Alzheimer Res. 2011 doi: 10.2174/156720512803251075. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willeumier KC, Taylor DV, Amen DG. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity (Silver Spring) 2011;19:1095–7. doi: 10.1038/oby.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–9. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–9. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24:742–56. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2. Oxford University Press; New York: 1998. [Google Scholar]
- 19.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale. Psychological Corporation; 1997. [Google Scholar]
- 20.Trenerry MR. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; 1989. [Google Scholar]
- 21.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1993. [Google Scholar]
- 22.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–31. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 23.Ye FQ, Frank JA, Weinberger DR, McLaughlin AC. Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST) Magn Reson Med. 2000;44:92–100. doi: 10.1002/1522-2594(200007)44:1<92::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med. 2005;54:366–72. doi: 10.1002/mrm.20556. [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Rowley HA, Wu G, et al. Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3. 0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer’s disease. NMR Biomed. 2010;23:286–93. doi: 10.1002/nbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tosun D, Mojabi P, Weiner MW, Schuff N. Joint analysis of structural and perfusion MRI for cognitive assessment and classification of Alzheimer’s disease and normal aging. Neuroimage. 2010;52:186–97. doi: 10.1016/j.neuroimage.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yakushev I, Hammers A, Fellgiebel A, et al. SPM-based count normalization provides excellent discrimination of mild Alzheimer’s disease and amnestic mild cognitive impairment from healthy aging. Neuroimage. 2009;44:43–50. doi: 10.1016/j.neuroimage.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Keihaninejad S, Heckemann RA, Fagiolo G, Symms MR, Hajnal JV, Hammers A. A robust method to estimate the intracranial volume across MRI field strengths (1. 5T and 3T) Neuroimage. 2010;50:1427–37. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774–83. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 32.O’brien R. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality & Quantity. 2007;41:673–90. [Google Scholar]
- 33.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 35.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry. 2002;159:738–45. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 37.Craft S, Zallen G, Baker LD. Glucose and memory in mild senile dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1992;14:253–67. doi: 10.1080/01688639208402827. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–8. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage. 2011;54:1530–9. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]