Abstract
Background
Sleep loss produces abnormal increases in reward-seeking, though the mechanisms underlying this phenomenon are poorly understood. The present study examined the influence of one night of sleep deprivation on neural responses to a monetary reward task in a sample of late adolescents/young adults.
Methods
Using a within-subjects crossover design, 27 healthy, right-handed late-adolescents/young adults (16 females, 11 males; mean age 23.1 years) completed functional magnetic resonance imaging following a night of sleep deprivation and following a night of normal sleep. Participants’ recent sleep history was monitored using actigraphy for one week prior to each sleep condition.
Results
Following sleep deprivation, participants exhibited increased activity in the ventral striatum and reduced deactivation in medial prefrontal cortex during the winning of monetary reward, relative to the same task following normal sleep conditions. Shorter total sleep time over the five nights before the sleep deprived testing condition was associated with reduced deactivation in the medial prefrontal cortex during reward.
Conclusions
These findings support the hypothesis that sleep loss produces aberrant functioning in reward neural circuitry, increasing the salience of positively-reinforcing stimuli. Aberrant reward functioning related to insufficient sleep may contribute to the development and maintenance of reward dysfunction-related disorders, such as compulsive gambling, eating, substance abuse, and mood disorders.
Keywords: sleep, sleep deprivation, reward, functional magnetic resonance imaging
Introduction
Worldwide, more than one fifth of young adults report sleeping less than 7 hours per night (Steptoe et al. 2006). Short sleep is linked to a range of negative physical and psychological consequences in humans, including obesity, hypertension, anxiety/depression, and suicidality (Grandner et al. 2010). Sleep loss also appears to increase unhealthy pursuit of rewards. For example, following acute sleep deprivation, individuals make riskier decisions to optimize gains on gambling tasks (McKenna et al. 2007), and show an increased preference for more rewarding, fatty, high-carbohydrate foods (Nedeltcheva et al. 2009). Moreover, sleep disturbance predicts subsequent relapse in alcoholics (Brower et al. 1998), and in a subset of patients with bipolar disorder, a single night of experimental sleep deprivation induces mania (Colombo et al. 1999), a condition often characterized by impulsive pursuit of rewards. In parallel, rodent studies indicate that sleep deprivation produces increased self-administration of cocaine (Puhl et al. 2009) and ethanol (Aalto & Kiianmaa 1986).
The neural mechanisms linking sleep loss and increased reward pursuit are not clearly established. The existing literature, however, provides insights into why sleep loss may create a neural basis for impulsive, emotionally driven behavior. Prefrontal cortical networks, which are integral to decision making and emotion regulation, are some of the most susceptible to sleep loss (Goel et al. 2009). Functional neuroimaging studies have documented diminished prefrontal activity (Chee et al. 2008; Drummond et al. 1999; Mu et al. 2005) and metabolism (Thomas et al. 2000; Thomas et al. 2003; Wu et al. 1991; Wu et al. 2006) following sleep deprivation, although depending on task characteristics (i.e., task difficulty) prefrontal cortical activity sometimes shows compensatory increases in activity (Chee & Choo 2004; Drummond et al. 2000; Drummond et al. 2005). Yoo et al. (2007) reported that after one night of sleep deprivation, young adults exhibited heightened amygdala activation to negative emotional images, and reduced connectivity between the amygdala and the medial prefrontal cortex (mPFC). Others reported that increased distraction by emotional stimuli after sleep deprivation was accompanied by heightened amygdala activation and decreased functional coupling between the amygdala and prefrontal cognitive control regions (Chuah et al. 2010). In parallel, we have previously demonstrated heightened emotional reactivity in sleep deprived adults and adolescents (Franzen et al. 2009; Franzen et al. 2010) as indicated by greater pupil dilation responses to negative emotional stimuli. Taken together, these findings suggest that sleep deprivation may confer vulnerability to more emotionally driven behavior through a combination of reduced prefrontal and increased amygdala activation.
In humans, the processing of reward is mediated by a complex network of subcortical and prefrontal structures, including the ventral striatum (VS), ventral pallidum, midbrain, orbital frontal and anterior cingulate cortices (Berridge & Kringelbach 2008; Haber & Knutson 2010). The VS in particular has been shown to robustly activate when individuals anticipate receiving money (Ernst et al. 2004; Knutson et al. 2001; Liu et al. 2007; Rademacher et al. 2010), pleasant taste (O’Doherty 2004; O’Doherty et al. 2002), or even social rewards such as praise (Kirsch et al. 2003) and happy faces (Rademacher et al. 2010; Spreckelmeyer et al. 2009). The mPFC receives input from the VS and the insula, and is believed to help individuals anticipate and weigh benefits and losses associated with behavioral choices (Haber and Knutson, 2010). Although co-activation of the VS and mPFC often occurs during reward processing, this is not always the case. For example, in adolescents and adults with depression, a pattern of low VS and high mPFC activation is typically produced in response to reward (Forbes & Dahl 2012).
Reward-related activity in the striatum may also be influenced by sleep loss. Using a gambling task, Venkatraman et al. (2007) reported abnormally increased VS activity in sleep deprived young adults during high-risk decision-making, and during the winning of money (Venkatraman et al. 2011). Another recent paper indicated that sleep deprived young adults exhibited a bias toward more positive evaluations of emotional images, with concomitant abnormally elevated activity in the amygdala and striatum (i.e., putamen) (Gujar et al. 2011). Together, these early studies suggest that sleep deprivation may create a neural sensitivity to a spectrum of rewarding stimuli.
Connections between sleep loss and reward functioning are particularly pertinent in adolescents and young adults, given their heightened vulnerability to the effects of sleep deprivation (Philip et al. 2004) and increased risk for both mood disorders and substance abuse problems (Kessler et al. 2005). These disorders are frequently characterized by the co-occurrence of sleep disturbance and altered reward functioning (American Psychiatric Association 2000).
In this study we utilized functional magnetic resonance imaging (fMRI) and a well-validated monetary reward card-guessing paradigm (Forbes et al. 2009) to examine the impact of acute sleep deprivation upon activity in reward neural circuitry in healthy late adolescents/young adults. We also examined whether variation in recent sleep history had measurable associations with reward responding when participants were tested following normal sleep and sleep deprived conditions. Based on research linking sleep loss with elevated striatal reactivity to rewarding stimuli (Venkatraman et al. 2007; Venkatraman et al. 2011; Gujar et al. 2011) we hypothesized that sleep deprivation would result in increased VS activity to reward. Based on literature suggesting that sleep deprivation causes diminished prefrontal activity (Chee et al. 2008; Drummond et al. 1999; Mu et al. 2005), we expected to see less mPFC activation to reward relative to performance under normal sleep conditions. In addition, we hypothesized that reduced sleep duration on the five nights prior to each testing condition would be associated with increased VS and less relative mPFC activity to reward.
Methods
Participants
Participants included 27 right-handed, healthy adults (16 women, 11 men) age 18–25 years (mean: 23.1, SD = 1.6). Participants were free of current or past sleep, psychiatric, or major medical disorders as determined using the Structured Clinical Interview for DSM-IV (First et al. 1995), a locally-developed Structured Interview for DSM-IV Sleep Disorders, and a medical history questionnaire. Additional exclusion criteria included: positive pregnancy test; a bedtime that regularly fell outside the hours of 10:00 pm to 1:00 am, and a wake up time that regularly fell outside the hours of 6:00 am to 9:00 am, as determined by self-report; use of more than 2 caffeinated or alcoholic beverages per day and inability to abstain from caffeine and alcohol for one day prior to and during participation in both experimental conditions; regular use of tobacco; colorblindness; or have metallic foreign objects within body. The study was approved by the Institutional Review Board at the University of Pittsburgh, and all individuals provided written informed consent before participation.
Procedures
Using a within-subjects crossover design, participants completed two experimental conditions, sleep deprivation and normal sleep, in counterbalanced order. Conditions were separated by at least one week to allow sufficient recovery sleep for those participants who completed the sleep deprivation condition first. Preceding each experimental night, participants completed a laboratory adaptation night with polysomnography (PSG), during which they slept according to their reported habitual sleep/wake times. On the first adaptation night, additional sensors were attached to assess for periodic limb movements and sleep disordered breathing; 10 or more events per hour of sleep were exclusionary. The following evening, participants returned to the lab. In the normal sleep condition, participants retired to bed at their habitual bedtime. In the sleep deprivation (SD) condition, participants were not allowed to sleep, and were under constant behavioral and PSG monitoring to ensure continued wakefulness. Participants underwent fMRI testing the following morning, 1.5–3 hours after their habitual wake time. This resulted in a total sleep deprivation of between 25.5 and 27 hours.
The sleeping habits of participants were monitored for one week prior to both experimental sleep conditions using sleep diaries and Actiwatch-64 actigraphs (Mini Mitter Co. 2001) worn on the participant’s non-dominant wrist. Twenty-three out of 27 participants supplied actigraphy before the normal sleep condition, while 21 supplied data before the sleep deprivation condition. The remaining participants were not included in actigraphy analyses because they provided incomplete data (<5 nights). Actigraphy provides an objective estimate of the sleep/wake cycles via gross motor movement, and has been shown to correlate well (80–90% agreement) with PSG in the determination of sleep and wakefulness (Morgenthaler et al. 2007). Activity data were summed into 60-second epochs and analyzed using the Actiware 5.59 software package (Respironics Inc. 2011). This software classifies epochs as sleep or wake using a validated algorithm and calculates a variety of sleep-related variables, including minutes of total sleep time.
Monetary Reward Task
To probe patterns of neural activity in response to monetary reward, we used a card guessing fMRI paradigm developed by Forbes et al. (2009) and based on the work of Delgado et al. (2000). The block design paradigm consisted of pseudorandom presentation of trials wherein participants played a card guessing game and received either positive or negative (i.e., win or loss) feedback for each trial. All participants were told that their performance on the game would determine the monetary reward received at the end of the study, earning $1 for each correct guess and losing $0.50 for each incorrect guess. Participants were unaware of the fixed outcome probabilities associated with each block until the entire study protocol was completed, at which time they were debriefed and compensated $10 for completion of each reward task. During each trial, participants were given 3 seconds to guess, via button press, whether the value of a visually presented card would be higher or lower than 5 (index and middle finger, respectively). After a choice was made, the numerical value of the card was presented for 500 ms and followed by appropriate feedback, with a green, upward arrow indicating a correct guess (win $1), and a red, downward arrow indicating an incorrect guess (loss of $0.50), for an additional 500 ms. Upon receiving positive feedback, participants were required to respond via button press to collect the money for that trial (to simulate an acquisition behavior). A crosshair was then presented for 3 seconds, for a total trial length of 7 seconds. Each block consisted of 5 trials, with three “reward” blocks featuring predominantly positive feedback (80% correct guesses) and three loss blocks with predominantly negative feedback (80% incorrect guesses), interleaved with three control blocks. During sensorimotor control blocks, participants were instructed to simply make alternating button presses during the presentation of an ‘x’ (3 seconds), which was followed by an asterisk (500 ms) and a yellow circle (500 ms), and then a crosshair (3 seconds). Each block was preceded by a 2 second instruction of “Guess Number” (for positive or negative feedback blocks) or “Press Button” (for control blocks), resulting in a total block length of 37 seconds and a total task length of five minutes and fifteen seconds.
MRI Data Acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Trio MRI scanner at the University of Pittsburgh Magnetic Resonance Research Center. Visual stimuli were presented by projecting images onto a rear projection screen at the subject's chest and viewed through a mirror attached to the head coil. Stimulus presentation and registration of responses was controlled by a computer running E-prime (Psychology Software Tools; Pittsburgh, PA). Participants’ button press responses and reaction times were recorded using an RF-shielded response box and cable connected to the computer. Structural T1-weighted volumetric scans were obtained during the first scanning session using a magnetization prepared gradient echo (MP-RAGE) sequence: 192 1.0-mm axial slices; flip angle 9°; field of view=256x192mm; TR=2200msec; TE=3.29msec; matrix=256x192. BOLD images were acquired with a gradient echo EPI sequence with 39 3-mm axial slices; flip angle 90°; field of view=205mm; TR=2000ms; TE=25ms; matrix=64x64.
fMRI Data Analysis
Data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Data were realigned and unwarped, spatially normalized into a standard stereotactic space (Montreal Neurologic Institute, MNI; http://www.bic.mni.mcgill.ca), resampled to 2mm3 voxels, and smoothed using a 6mm FWHM Gaussian kernel. We used a two-level random-effects general linear model to analyze fMRI data. At the first level, individual whole brain statistical maps were constructed to evaluate the difference between the two feedback conditions (reward and loss) and the control condition, as well as between the reward and loss conditions. Single-subject data were modeled using a box-car function convolved with the canonical hemodynamic response function (HRF). Movement parameters from the realignment stage were included as covariates of no-interest. These contrasts were then brought forward to second level, paired sample t-test analyses. Our primary focus was on the reward-control and loss-control contrasts within each region of interest (ROI) (tested for sleep deprived > normal sleep and normal sleep > sleep deprived). We then examined the reward-loss contrast to disambiguate whether our primary findings were unique to rewarding outcomes, and not simply just a product of receiving positive or negative outcome feedback. As in Forbes et al. (2010) we used an ROI approach focused on the striatum and medial prefrontal cortex (mPFC). For our ventral striatal ROI, the WFU PickAtlas (Wake Forest University, Winston-Salem, NC) was used to construct 8mm radius bilateral spheres centered at MNI coordinates x=9, y=9, z=−8, and x=−9, y=9, z=−8, based on recent meta-analysis of basal ganglia functional connectivity (Di Martino et al. 2008; Postuma & Dagher 2006). For the mPFC ROI, we constructed a 25mm sphere centered on coordinates x=0, y=44, z=18 capturing anterior portions of the cingulate gyrus, and medial segments of Brodmann’s Areas 9 and 10, as in Forbes et al. (2010) We also conducted exploratory whole-brain paired sample t-tests comparing reward-control and loss-control contrasts across sleep conditions. Finally, we used regression analyses to examine relationships between activity on the reward-control contrast in the striatal and mPFC ROIs and mean total sleep time derived from actigraphy in the five days prior to each fMRI scan. Order of sleep deprivation condition was controlled for in all second level analyses.
We employed a voxelwise threshold of p<0.05 for all analyses. To correct for multiple voxelwise comparisons within each ROI, we computed clusterwise extent thresholds using the AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) with 1000 Monte Carlo simulations, a validated technique for ROI analyses (Ward 2000). These simulations indicated that cluster sizes of 53 voxels in the VS, and 148 voxels in the mPFC (at p=0.05), would result in an overall family-wise error rate of 0.05. Because the exploratory whole-brain analyses were not part of a priori hypotheses, these were given a more liberal threshold (voxelwise p<0.005 with an extent threshold of 20 voxels).
Results
Actigraphy Data
Participants slept a mean of 402.3 minutes (SD = 56.2) per night over the 5 nights prior to the rested experimental condition, and 413.0 minutes (SD=43.8) per night prior to the sleep deprivation condition. Mean total sleep time prior to the two experimental conditions did not significantly differ, t (18)= −1.49, p=0.13.
fMRI Data
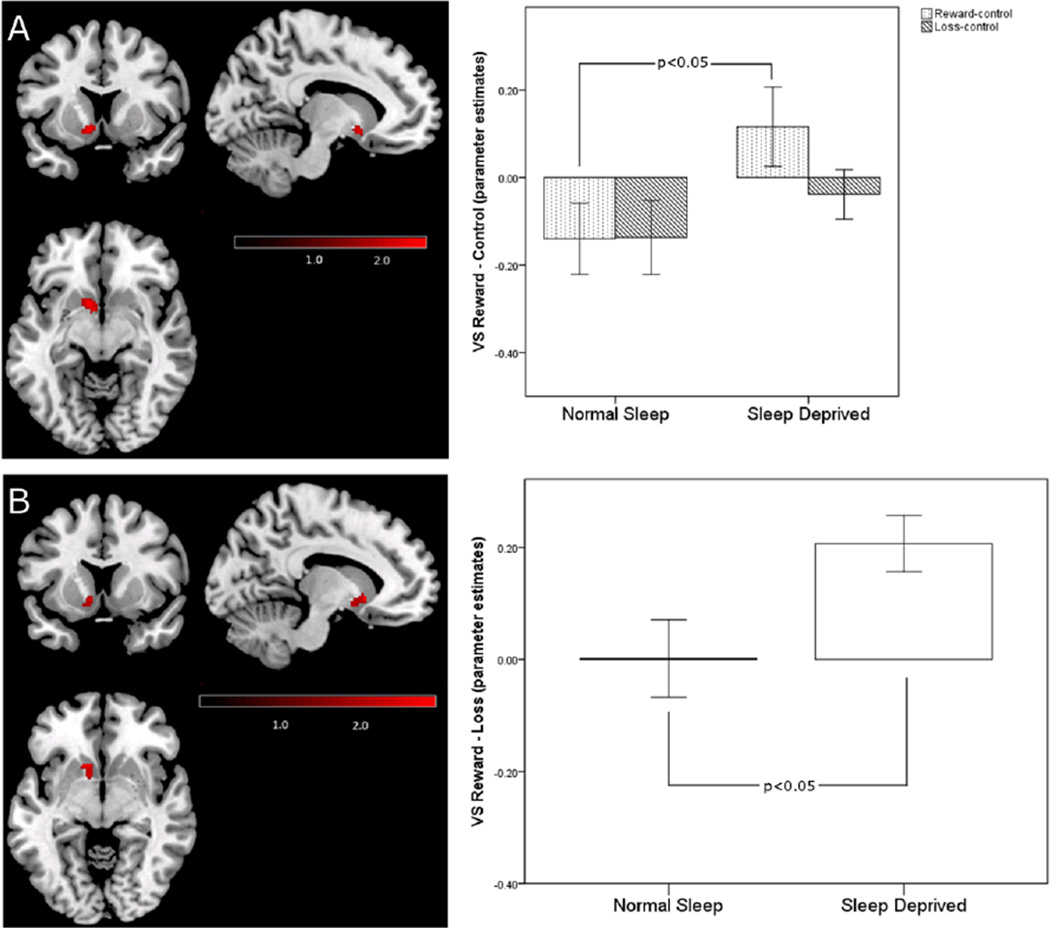
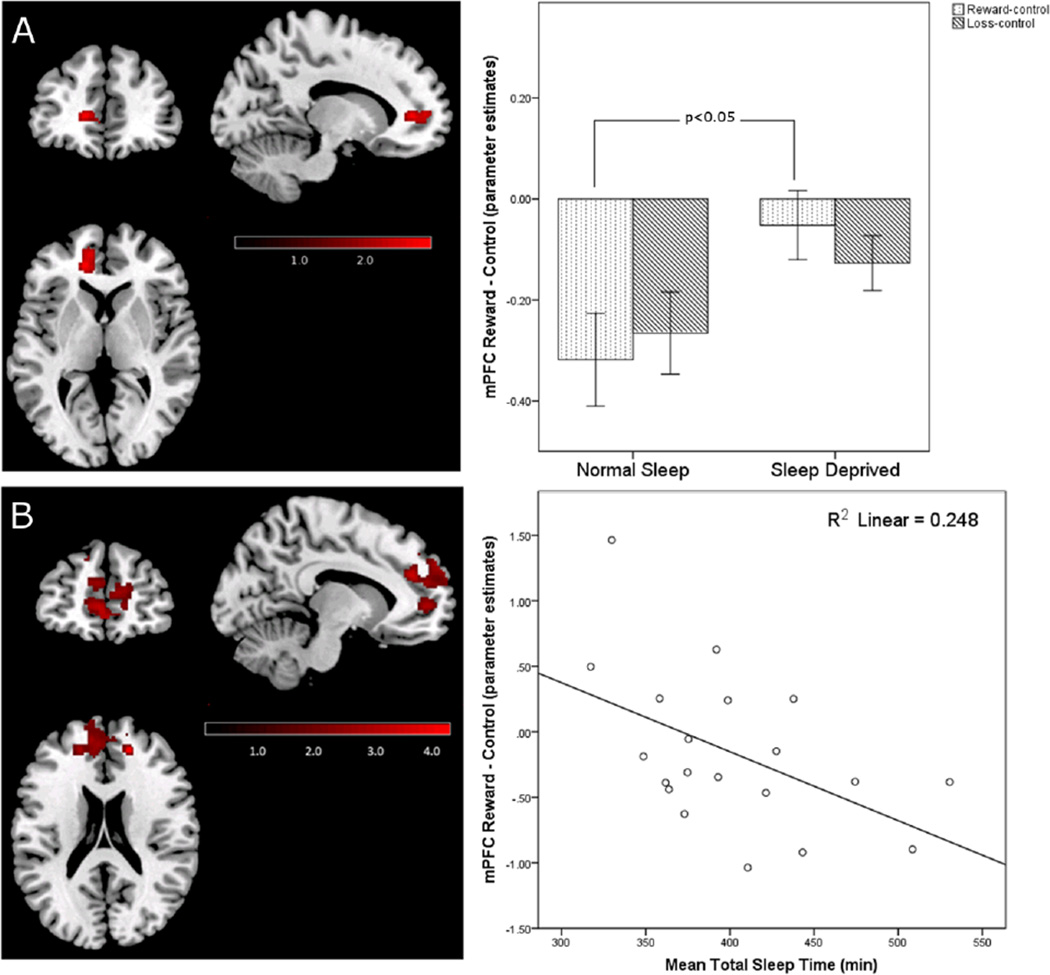
Participants exhibited significantly greater VS activity during reward blocks under sleep deprived than under normal sleep conditions (Figure 1A). This increase in activity to reward during sleep deprivation was also present when the loss blocks were used as the contrast (Figure 1B), suggesting that sleep deprivation selectively increases VS sensitivity to rewarding outcomes. In contrast to our hypothesis, sleep deprived participants did not show significantly decreased activity in the mPFC to winning rewards. In the normal sleep condition, participants exhibited significantly more mPFC activity to the control blocks than to the reward blocks (i.e., the mPFC was relatively deactivated during the reward blocks), which was significantly reduced following sleep deprivation, (i.e., more equivalence in mPFC activation across the control and reward blocks) (Figure 2A).This difference in the reward-control contrast within the mPFC across sleep conditions was not detected in the reward-loss contrast, suggesting that a failure to deactivate the mPFC to the reward or loss blocks may be a general effect of sleep deprivation, rather than an effect specific to winning rewards. For the loss-control contrast, no significant differences were detected between the normal sleep and sleep deprivation conditions in the VS or mPFC.
Figure 1.
A) Significantly greater activity to reward following sleep deprivation compared to the normal sleep condition in the left VS (t (25)=2.60, p=0.005, k=61 [peak voxel: x=−10, y=10, z=−8 in MNI space]). The bar graph represents mean blood-oxygen-level-dependent (BOLD) signal change in each sleep condition during reward and loss blocks relative to control blocks across all voxels within the cluster; the error bars represent standard error of the mean. B) Significantly greater activity to win-loss following sleep deprivation compared to the normal sleep condition in the left VS (t (25)=2.66, p=0.007, k=62 [peak voxel: x=−12, y=16, z=−4 in MNI space]). The bar graph represents mean BOLD signal change in each sleep condition during reward blocks relative to loss blocks across all voxels within the cluster; the error bars represent standard error of the mean. Within-group differences to reward across sleep conditions were significant using a voxelwise threshold of p<0.05 corrected for family wise error using AlphaSim Monte Carlo simulation.
Figure 2.
A) Significantly increased activation of left mPFC to reward-control following sleep deprivation compared to the normal sleep condition (t (25)=2.92, p=0.002, k=184 [peak voxel: x=−14, y=42, z=4 in MNI space]). The bar graph represents mean BOLD signal change in each sleep condition during reward and loss blocks relative to control blocks across all voxels within the cluster; the error bars represent standard error of the mean. The loss bars are included for illustrative purposes, and are not significantly different between sleep conditions. Within-group differences to reward across sleep conditions were significant using a voxelwise threshold of p<0.05 corrected for family wise error using AlphaSim Monte Carlo simulation. B) Cluster within the mPFC region of interest mask showing a significant negative relationship with recent sleep history (i.e., actigraphy-derived mean total sleep time in five nights prior to the experimental night in the laboratory preceding the fMRI scan) during reward trials under the sleep deprived condition (bilateral rostral anterior cingulate cortex; t (21)=4.06, p<0.001, k=1436 [peak voxel: x=18, y=44, z=20 in MNI space]). The scatterplot represents mean total sleep time before the sleep deprivation condition plotted against mean BOLD signal change during reward relative to control blocks across all voxels in the highlighted cluster within the mPFC. Regression analyses were significant using a voxelwise threshold of p<0.05 corrected for family wise error using AlphaSim Monte Carlo simulation.
Actigraphically-derived total sleep time during the five days preceding the sleep deprivation condition was negatively associated with magnitude of reward-related activity in a sub-region of the mPFC, the rostral anterior cingulate cortex (ACC) (Figure 2B). Thus, less sleep prior to the sleep deprived session was associated with greater ACC activation during reward-control. This effect was not observed with respect to the normal sleep condition. We did not find relationships between prior sleep history and reward-related activation in the VS under either experimental sleep condition.
Our exploratory whole-brain analyses revealed two subcortical regions that showed greater activation to reward-control following sleep deprivation relative to the normal sleep condition: the left ventral pallidum and the right parahippocampal gyrus (Table 1). Regions that were more activated to loss-control following sleep deprivation relative to the normal sleep condition included the right lingual gyrus, right anterior cingulate gyrus, and cerebellum. Conversely, regions that were more activated to reward-control following the normal sleep relative to the sleep deprivation condition included the right inferior parietal lobe, bilateral superior parietal lobe, and right middle frontal gyrus. In the loss-control contrast, participants showed greater activity during the normal sleep relative to sleep deprived condition in the right fusiform gyrus and left inferior parietal lobe.
Table 1. Wholebrain comparisons of reward and loss following sleep deprivation and normal sleep.
Wholebrain paired-samples t-test analyses (reward – control, and loss – control) with a voxelwise threshold of p <0.005 and a cluster extent threshold of 20 voxels. Each line in the table represents the voxel of peak activity difference between the sleep conditions within the specified region. Abbreviations: BA = Brodmann area; k = cluster size in voxels.
| MNI Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region | BA | k | x | y | z | t | P value |
| Reward - Control | |||||||
| Sleep Deprivation > Normal Sleep | |||||||
| Left Ventral Pallidum | 21 | −24 | −8 | −6 | 3.37 | 0.001 | |
| Right Parahippocampal Gyrus | 27 | 26 | −12 | −20 | 3.26 | 0.001 | |
| Sleep Deprivation < Normal Sleep Controls | |||||||
| Right Inferior Parietal Lobe | 40 | 40 | 36 | −58 | 34 | 3.78 | <0.001 |
| Right Superior Parietal Lobe | 7 | 28 | 32 | −58 | 48 | 3.04 | 0.002 |
| Right Middle Frontal Gyrus | 9 | 24 | 38 | 18 | 26 | 3.06 | 0.001 |
| Left Superior Parietal Lobe | 7 | 21 | −34 | −56 | 50 | 2.99 | 0.002 |
| Loss - Control | |||||||
| Sleep Deprivation > Normal Sleep | |||||||
| Right Lingual Gyrus | 18 | 137 | 28 | −44 | −4 | 4.73 | <0.001 |
| Right Cingulate Gyrus | 24 | 40 | 18 | 14 | 34 | 3.72 | <0.001 |
| Cerebellum | 26 | −30 | −44 | −26 | 3.71 | <0.001 | |
| Left Lingual Gyrus | 18 | −30 | −54 | 0 | 3.57 | <0.001 | |
| Sleep Deprivation < Normal Sleep | |||||||
| Right Fusiform Gyrus | 19 | 23 | 34 | −68 | −10 | 3.56 | <0.001 |
| Left Superior Parietal Lobe | 7 | 24 | −36 | −58 | 54 | 3.28 | 0.001 |
Discussion
Our findings demonstrate important relationships between sleep and activity in reward neural circuitry during the winning of reward. Following one night of sleep deprivation, participants showed significantly elevated VS activity to winning small monetary rewards. This result is consistent with other recent neuroimaging studies that have examined the effects of sleep deprivation on reward-related functioning. Two studies by Venkatraman and colleagues (Venkatraman et al. 2007; Venkatraman et al. 2011) used a more complex event-related gambling task that involved weighing monetary risks and probabilities, while the task we employed assessed activity during blocks where a fixed sum of money was won or lost. Despite their differences in design, both studies identified increased sensitivity to reward in the VS following a night of sleep deprivation. A third study also found greater striatal activity following sleep deprivation (Gujar et al. 2011), though in that case the rewarding stimuli were positive emotional images. This small collection of findings is particularly interesting in light of recent work demonstrating hyperstimulation of striatal D2 receptors following sleep deprivation (Volkow et al. 2009). Reward is a key determinant of goal-directed behavior and incentive-based learning; aberrant functioning in reward neural circuitry, resulting in over-valuation of positively-reinforcing stimuli, could lead to impaired decision-making and abnormally increased pursuit of reinforcers.
Contrary to our hypothesis, we found evidence of increased activation in the mPFC to control blocks relative to reward blocks following normal sleep, a pattern that was significantly attenuated following sleep deprivation. In addition to its role in reward-related decision making, the mPFC, in concert with the posterior cingulate gyrus, precuneus, and inferior parietal cortex constitutes the default-mode network (Raichle et al. 2001). This network is consistently found to be deactivated during performance of cognitive tasks, but active during mind-wandering (Mason et al. 2007) and self-reflective thought (Uddin et al. 2007). In our task, it is perhaps not surprising that participants would show greater activation in the mPFC during an uninteresting control block relative to a block in which reward-related decisions are made and outcomes are processed. Fairly equivalent activation of this region following sleep deprivation may represent a failure to properly deactivate the default network during active cognitive portions of the task. Interestingly, a recent paper indicated that the default-mode network is abnormally active following sleep deprivation (Gujar et al. 2010). Increased activity of this network during active decision-making may produce increased mind-wandering or thoughts about self, and ultimately interfere with the ability to regulate pursuit of rewards.
We also found an association between decreased total sleep time before the sleep deprivation condition and activation of mPFC to reward, suggesting that chronically reduced sleep duration may produce increased activation of the default-mode network during reward-related decision making and reward processing. We did not, however, find relationships between VS activity and recent sleep history. Thus, while both acute sleep deprivation and recent sleep history may be associated with increased default-mode network activity during reward-related processing, only acute sleep deprivation was found to be associated with elevation of VS activity to winning reward. The specific dose of sleep loss necessary to elevate VS activity will require further study.
Whole-brain analyses revealed elevated activity in the ventral pallidum and parahippocampal gyrus to reward-control after sleep deprivation relative to normal sleep. Both regions are implicated in the striatal-cortical reward circuit (Haber & Knutson 2010), and thus parallel our ROI findings of increased sensitivity of reward neural circuitry following sleep deprivation. Regions subserving attentional control were more activated after normal sleep compared to following sleep deprivation. This is consistent with previous studies indicating reduced activity (Tomasi et al. 2009) and metabolism (Thomas et al. 2000) in attentional control circuitry following sleep deprivation. Together with our a priori region of interest analyses, these findings support a role for sleep deprivation in disturbing the balance between subcortical reward circuitry and cortical attentional control circuitry in healthy young adults.
Although this study featured only healthy late adolescents and young adults, our findings provide one possible neural framework for considering how combinations of chronic and acute sleep loss might lead to chronic alterations in reward neural circuitry, contributing to the development of clinical conditions in which sleep and reward disturbances co-occur. Bipolar disorder provides a vivid example of this type of clinical condition. In this disorder, increasing sleep disturbance often signals, or potentially precipitates, a progression into mania (Jackson et al. 2003) a mood state frequently characterized by both reduced sleep and increased pursuit of reward (e.g., risky sexual behaviors, substance use, reckless use of money) (Goodwin & Jamison 2007). Interestingly, structural and functional abnormalities have been identified in reward-related neural circuitry in those with bipolar disorder (Phillips et al. 2008). Thus, in such individuals, sleep loss may tax neural circuits that are already compromised, leading to a spiral of severely dysregulated emotional and reward functioning. Similarly, in other conditions characterized by unhealthy reward pursuit, including compulsive gambling, binge eating, and substance dependence, sleep loss may further disrupt malfunctioning reward neural circuitry.
Limitations
There are several limitations of this study. This study examined the effects of only a single acute dose of sleep deprivation on reward-related neural activity. For many young adults, sleep loss typically occurs in a more moderate, chronic form, and it is not known whether chronic sleep restriction would incur the same VS increases to reward that we detected with our acute deprivation model. We did, however find that naturalistically incurred short sleep duration prior to the sleep deprivation condition was associated with relatively increased medial-prefrontal default-mode network activity during reward. This suggests that chronic short sleep, and not just total sleep deprivation, has significant influence on how the brain processes reward. Another potential limitation of this study is the use of a block design task that did not allow for separate analysis of anticipatory and acquisition phases of reward, a distinction that may be important for the underlying neural circuitry involved (Rademacher et al. 2010). Nonetheless, abnormally elevated VS activity following sleep deprivation has previously been documented during both the anticipation (Venkatraman et al. 2007) and outcome (Venkatraman et al. 2011) portions of a gambling task. Lastly, while we believe that our finding of reduced deactivation of mPFC following sleep deprivation suggests an intrusion of the default mode network into the decision making portion of our task, it is difficult to assess this clearly with the current design. Future studies of reward and sleep deprivation might consider including a non-reward related, pure cognitive trial type in their tasks; this would allow for examination of default mode activation during a task condition in which such structures should be relatively dormant.
Conclusions
In conclusion, this study found that compared to a night of sleep, acute sleep deprivation in late adolescents and young adults led to increased activity in VS and relatively increased activation of mPFC during the winning of monetary reward. In addition, reduced sleep during the five nights prior to the sleep deprivation condition was associated with increased activation in mPFC, especially in the ACC, during reward-control. These findings support the idea that acute sleep loss may result in increased sensitivity to rewarding stimuli in the VS, while both acute sleep loss and chronically short sleep may hamper one’s ability to disengage the default-mode network. Together, these factors may provide a neural basis for heightened reward pursuit and risky decision making. We studied a narrow age range of late adolescents/young adults in this study, and therefore comparisons to other younger or older individuals is warranted. Adolescents may be at particular risk for problems in reward-related function and behavior as a consequence of sleep loss. Adolescence is marked by both high levels of chronic sleep deprivation (Millman 2005), and hypersensitivity to rewards (Ernst et al. 2005), the latter believed to be mediated by an imbalance in the development of the striatum and prefrontal cortex (Van Leijenhorst et al. 2010). It is possible that acute sleep loss in this group would have particularly salient effects on reward circuitry. It will also be important for future studies to investigate whether altered activity in reward neural circuitry following sleep deprivation is associated with both laboratory and real life measures of abnormal risk-taking and reward pursuit.
Acknowledgements
We would like to thank Denise Duryea and the Neuroscience Clinical and Translational Research Center staff for their assistance in conducting this study.
Funding: This work was supported by National Institutes of Health grants [K01 MH077106 to PLF] [T32 HL082610 to BCM] [R01 MH076961 to MLP] and [UL1 RR024153].
Footnotes
Disclosure: All authors deny any conflicts of interest.
References
- Aalto J, Kiianmaa K. REM-sleep deprivation-induced increase in ethanol intake: Role of brain monoaminergic neurons. Alcohol. 1986;3:377–381. doi: 10.1016/0741-8329(86)90057-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders- Text revision. Washington, DC: American Psychiatric Association: 2000. [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcoholism, Clinical and Experimental Research. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Chee MWL, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. The Journal of Neuroscience. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. The Journal of Neuroscience. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah LYM, Dolcos F, Chen AK, Zheng H, Parimal S, Chee MWL. Sleep deprivation and interference by emotional distracters. Sleep. 2010;33:1305–1313. doi: 10.1093/sleep/33.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatric Research. 1999;86:267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Research. 2005;140:211–223. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0. New York: Biometric Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research review: Altered reward function in adolescent depression: what, when and how? The Journal of Child Psychology and Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology. 2009;80:300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ, Duryea DN, Wood A, Siegle GJ, Dahl RE. The emotional impact of sleep restriction in adolescents. Sleep. 2010;33S:A85. 2010. [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd ed. USA: Oxford University Press; 2007. [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Perlis ML, Pack AI. Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Medicine Reviews. 2010;14:239–247. doi: 10.1016/j.smrv.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. The Journal of Neuroscience. 2011;31:4466–4474. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. The unrested resting brain: Sleep deprivation alters activity within the default-mode network. Journal of Cognitive Neuroscience. 2010;22:1637–1648. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of Affective Disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. NeuroImage. 2003;20:1086–1095. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21:159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts S, Crone EA. What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. The Journal of Neuroscience. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly A, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Dickinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. Journal of Sleep Research. 2007;16:245–252. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Millman R. Excessive sleepiness in adolescents and young adults: causes, consequences, and treatment strategies. Pediatrics. 2005;115:1774–86. doi: 10.1542/peds.2005-0772. [DOI] [PubMed] [Google Scholar]
- Mini Mitter Co. Actiwatch 16/ Actiwatch 64/ Actiwatch L/ Actiwatch-Score Instruction Manual. Bend, OR: Mini Mitter Co. Inc.; 2001. [Google Scholar]
- Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Coleman J, Lee-Chiong T, Pancer J, Swick TJ. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- Mu Q, Nahas Z, Johnson KA, Yamanaka K, Mishory A, Koola J, Hill S, Horner MD, Bohning DE, George MS. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. The American Journal of Clinical Nutrition. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Philip P, Taillard J, Sagaspe P, Valtat C, Sanchez-Ortuno M, Moore N, Charles A, Bioulac B. Age, performance and sleep deprivation. Journal of Sleep Research. 2004;13:105–110. doi: 10.1111/j.1365-2869.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Puhl MD, Fang J, Grigson PS. Acute sleep deprivation increases the rate and efficiency of cocaine self-administration, but not the perceived value of cocaine reward in rats. Pharmacology Biochemistry and Behavior. 2009;94:262–270. doi: 10.1016/j.pbb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49:3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respironics Inc. Actiwatch Communication and Sleep Analysis Software. Murrysville, PA: 2011. [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Kircher T, Gründer G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Archives of Internal Medicine. 2006;166:1689–1692. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepinessIEffects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Wagner HN, Thorne DR, Popp KA, Rowland LM, Welsh AB, Balwinski SM, Redmond DP. Neural basis of alertness and cognitive performance impairments during sleepiness II. effects of 48 and 72 H of sleep deprivation on waking human regional brain activity. Thalamus & Related Systems. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang GJ, Fowler JS, Volkow ND. Impairment of attentional networks after 1 night of sleep deprivation. Cerebral Cortex. 2009;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah LYM, Huettel SA, Chee MWL. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LYM, Payne JW, Chee MWL. Sleep deprivation biases the neural mechanisms underlying economic preferences. The Journal of Neuroscience. 2011;31:3712–3718. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang G, Telang F, Fowler JS, Wang RL, Logan J, Wong C, Jayne M, Swanson JM. Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. NeuroImage. 2009;45:1232–1240. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000. [[Accessed March 1, 2011]]. Available at: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–2792. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Hazlett E, Sicotte N, Bunney WE. The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–162. [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep- a prefrontal amygdala disconnect. Current Biology. 2007;23:88–89. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]