Abstract
Macrophages which infiltrate adipose tissue and secrete pro-inflammatory cytokines may be responsible for obesity-induced insulin resistance. However, why macrophages migrate into adipose tissue and become activated remains unknown, though some studies suggest this may be regulated by T and B lymphocytes. In the present study, we test whether T and B lymphocytes and NK cells are necessary for the obesity-induced activation of macrophages in adipose tissue.
NOD/SCID/IL2-receptor gamma-chain knockout (NSG) mice, which lack mature T and B lymphocytes and NK cells, were made obese by selectively reducing litters and weaning onto a high-fat diet. Mice were then maintained on the diet for 10-11 weeks. Adipose tissue from obese NSG mice had more activated macrophages than non-obese mice. These macrophages were found in “crown like structures” surrounding adipocytes, and expressed higher levels of the inflammatory cytokine TNFα. However, obesity did not impair glucose tolerance in the NSG mice.
These studies demonstrate that T and B lymphocytes and NK cells are not necessary for adipose tissue macrophage activation in obese mice. T and B lymphocytes and/or NK cells may be necessary for the development of obesity-induced impaired glucose tolerance.
Introduction
There is accumulating evidence that infiltration of adipose tissue by immune cells may play an important role linking obesity and diabetes (1-3). Obesity is associated with alterations in the immunobiology of adipose tissue, including an influx of T and B lymphocytes, NK cells, and macrophages, and a relative paucity of regulatory T lymphocytes (2, 4-8). However, the signals regulating the trafficking of these immune cells into and out of adipose tissue remain unclear. In obese immunocompetent mice, T and B lymphocytes enter adipose tissue prior to macrophages. A scheme has been proposed in which pro-inflammatory T lymphocytes (CD8+ and CD4+ Th1) promote macrophage migration and/or activation in adipose tissue, while regulatory T lymphocytes prevent the infiltration and activation of macrophages (9).
It is unclear what the mechanism is for obesity-associated insulin resistance. While some studies in obese mice and humans have demonstrated the importance of activated macrophages, NK cells, and CD8 T lymphocytes in the development of insulin resistance, other studies in immunodeficient mice have implied that T and B lymphocytes are not necessary (5, 10). Given the growing prevalence of type 2 diabetes, along with other comorbidities that are likely related to inflammation (e.g. cardiovascular disease, cancer), it is important to elucidate the mechanisms regulating obesity-associated adipose tissue inflammation. In the present study, we induced obesity in NOD/SCID/IL2-receptor gamma-chain knockout (NSG) mice, which lack T and B lymphocytes and NK cells. In these studies, we directly test whether T and B lymphocytes and NK cells are necessary for obesity to cause 1) adipose tissue macrophage activation, and 2) impaired glucose tolerance.
Methods and Procedures
Mouse models
All animal studies were approved by the CHLA IACUC, and performed in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ are NOD/SCID mice which lack an IL2 receptor gamma chain. They therefore lack mature lymphocytes and NK cells, and are used commonly for xenotransplant (11). Mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in the Children’s Hospital Los Angeles Animal Care Facility. In initial experiments, mice were randomized at the time of weaning to a high fat (60% calories from fat) or control diet (10% calories from fat, Research Diets, New Brunswick, NJ). Because of the modest degree of obesity attained with this model, an optimized model was developed, in which litters were selectively reduced from 5-7 mice down to 2 female mice at day of life #5 to maximize weight gain prior to weaning ((12) and Simerly, pers. comm). At day of life #28, remaining mice from these culled litters were weaned onto a high-fat diet (60% calories from fat). Control litters were kept at 5-7 mice/litter until weaning, and then the females put on a control diet (10% calories from fat).
C57Bl/6J mice were purchased from Jackson Laboratories. Litters were randomized to control or obese, selectively culled to 2 female mice, and placed on diets identical to the optimized model described above. All mice were housed 3-4 per cage and given free access to food and water. Physiological testing was done after 8-10 weeks on the diet, and then mice were sacrificed after 10-11 weeks on the diet.
Body Composition
After sacrifice, a subset of mice was sent frozen to University of Alabama, Birmingham for body composition measurements by chemical carcass analysis (13). Body fat was determined on carcasses after the stomach and intestines were removed. Fat weight was calculated as the weight lost during fat extraction.
Hormone assays
Plasma cytokines and hormones were measured using Milliplex mouse cytokine and adipokine kits (Millipore, Billerica, MA) in the Beckman Center for Immune Monitoring at the University of Southern California Norris Cancer Center, and analyzed with the Bio-Plex Suspension Array System (BIO-RAD, Hercules, CA). Adiponectin was measured using an ELISA (Millipore).
Flow cytometry
Mice were perfused with PBS with 0.1% heparin at sacrifice, and adipose tissue depots rapidly removed. Tissue was minced into small pieces in the presence of 0.004 units/mg tissue of Liberase TM (Roche) and incubated for 30 minutes with shaking at 37°C. Tissue was resuspended in 5% FBS in PBS and passed through a 70 μm nylon filter. The stromovascular fraction (SVF) was isolated by centrifugation at 500g for 10 minutes, washed twice, resuspended in buffer with Fc Block (BD Bioscience), and then stained with DAPI and labeled anti-CD45, anti-F4/80, anti-CD11c, anti-CD206, or isotype controls (BioLegend, San Diego). Cells were then analyzed in the Saban Research Institute Flow Cytometry Core on an LSR II Analyzer using FACSDiva software (Becton Dixon), and live leukocytes gated by DAPI and forward and side scatter. M1 macrophages were characterized as F4/80+ CD11c+ CD206−, while M2 macrophages were F4/80+ CD11c− CD206+ (6).
Immunohistochemistry
Paraformaldehyde fixed adipose tissue samples were embedded with paraffin, sliced, and mounted by the CHLA Pathology Core. Sections were subjected to antigen retrieval with Tris-EDTA steam at pH 8.0 for 30 min. Endogenous peroxidases were inactivated with 3-H2O2. Non-specific staining was blocked with 2.5% normal goat serum before staining with rat anti-mouse F4/80 (Clone: A3-1, Abcam, Cambridge, MA), and detected with the ImmPRESS reagent (Vector Laboratories Inc., Burlingame, CA) containing polymerized peroxidase labeled goat anti-rat immunoglobulin (mouse adsorbed). The reaction was detected with ImmPACT DAB (Vector Laboratories Inc.) and counterstained with Mayer’s hematoxylin. Images were acquired on a Zeiss Axioplan Microscope (20x/0.5 and 63x/1.25) with a SPOT QE Color Digital Camera.
Adipocyte area was measured by an automated routine with the Fiji distribution of ImageJ software (14). Each color image was divided by a 20 × 20 Gaussian-filtered copy to normalize intensity variations. Then the contrast was enhanced by saturating 1% of the pixels and equalizing the histogram. The image was then converted to 8-bit and a minimum filter (radius 3 pixels) and grayscale open operation (radius 10 pixels) were applied. The image was then coverted to binary by manual thresholding to include only cell outlines. In a few cells where the automated routine failed the cells were traced manually. Measurements were performed on three fields per mouse from four obese and four lean C57BL/6J mice and 6 obese and 6 lean NOD/SCID mice. Adipocyte area data from 477 to 1796 fat cells per group were analyzed using the percent relative cumulative frequency (PCRF) approach and EC50 values were calculated according to Riachi et al.(15).
Collected raw data from each mouse in an experimental group were pooled, sorted, and the cumulative frequency calculated. To allow comparisons between groups with different numbers of data points, the cumulative frequency values were converted to PRCFs. The curve of best fit was plotted through the combined data points from each group using non-linear regression (curve fit) analysis of log(adipocyte area) vs PCRF using GraphPad Prism Version 5. Goodness of fit was judged as an R2 value of greater than 0.99. F-tests were performed to test the hypothesis that 50th percentile values (data set mean) and slopes were significantly different between diet groups.
qPCR
To compare the relative number of macrophages infiltrating adipose tissue in mice, RNA was extracted from fat depots in a subset of mice (parametrial in NSG mice, epididymal in C57Bl/6 mice) with RNEasy Mini Kits (Qiagen). Fat depots were rinsed and then snap frozen prior to extraction. One μg of total RNA was reverse transcribed to cDNA with High Capacity 1st Strand Synthesis Kit (Applied Biosystems). The expression of selected genes was measured with rtPCR in at least duplicate, using 25 ng of cDNA, Power SYBR Green PCR Master Mix (Applied Biosystems) and 200 nM primers generated using NCBI Primer-BLAST. Primers used were: β-actin (forward: 5′-TCATGAAGTGTGACGTTGACATCCGT-3′; reverse: 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′); F4/80 (forward: 5′-CTTTGGCTATGGGCTTCCAGTC-3′; reverse 5′-GCAAGGAGGACAGAGTTTATCGTG-3′); CD11c (forward: 5′-ACACAGTGTGCTCCAGTATGA-3′; reverse: 5′-GCCCAGGGATATGTTCACAGC-3′); Arg-1 (forward: 5′-CTCCAAGCCAAAGTCCTTAGAG-3′; reverse: 5′-AGGAGCTGTCATTAGGGACATC-3′); and IL-10 (forward: 5′-GCTCTTACTGACTGGCATGAG-3′; reverse: 5′-CGCAGCTCTAGGAGCATGTG-3′).
Gene expression levels were quantified using the ABI 7900HT Sequence Detection System with the following thermal profile: 10 minutes at 95.0 followed by 40 repeats of 95.0, 15 sec and 60.0, 1 minute, and a final dissociation stage of 95.0 for 15 sec, 60.0 for 15 sec, and 95.0 for 15 sec. Transcript levels were quantified using the ΔΔCT method using β-actin as a control, and presented as fold increase over tissue from non-obese animals.
Physiological tests
At 12-14 wks of age, mice underwent either glucose (IPGTT) or insulin (ITT) tolerance tests. IPGTTs were performed on 6-hour fasted mice with 1 mg/kg of glucose. Blood glucose was measured from tail nick on a FreeStyle Lite (Abbott Diabetes Care, Inc, Alameda, CA). Blood for insulin was taken at t=30 minutes from the submandibular plexus, and insulin measured using an ELISA (Crystal Chem Inc., Downers Grove, IL). ITT’s were performed on non-fasted mice using 0.75 units/kg of regular human insulin (Novo Nordisk, Bagsvaerd, Denmark), and glucose was measured by tail nick as above.
Calculations and Statistics
Glucose area-under-the-curves were calculated during IPGTT and ITT using the trapezoidal rule. Percent body fat was calculated as the fat weight divided by the carcass weight after removal of stomach and intestine. Unpaired t-tests were used to compare obese and non-obese groups for normally distributed data, and Wilcoxon sum rank test for non-normal data. All normal data are presented as mean±SD.
Results
Litter size reduction and high-fat diet cause obesity in NSG mice
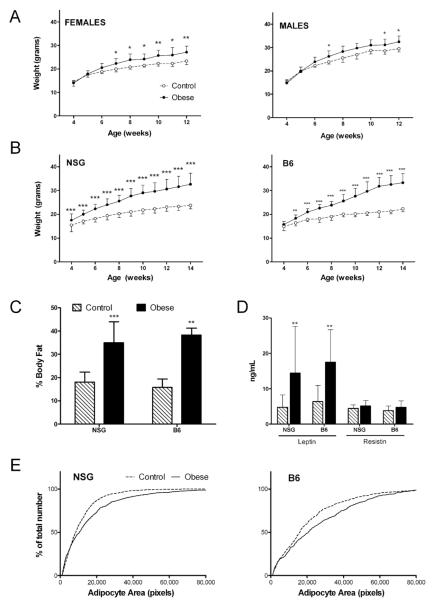
Obesity was induced in NSG and B6 mice by weaning them onto a high fat (60% of calories from fat) or control (10% of calories from fat) diet. In initial experiments, male and female NSG mice were randomized to these diets at time of weaning (3-4 weeks old). High-fat fed NSG mice were heavier than controls (obese males were 10% heavier than controls, p=0.027, obese females were 16% heavier than controls, p=0.009, Figure 1A). However, since the fat-fed NSG mice exhibited only modest obesity compared to fat-fed B6 mice and had no impairment in glucose tolerance (not shown), we selectively reduced litters on the fifth day of life to maximize obesity in subsequent experiments. Female NSG and B6 mice were used for this optimized obesity model. At the time of weaning, mice from the culled NSG and B6 litters were ~14% and 6% heavier, respectively, than mice from unculled litters (Figure 1B). These mice were then placed on a high-fat diet and by 12 wks of age were substantially heavier than non-obese mice; obese NSG mice were on average 33% heavier, while obese B6 mice were on average 51% heavier than non-obese mice. Obese NSG mice had approximately twice the body fat percentage as non-obese mice, similar to obese B6 mice (Figure 1C). Overall, the selective reduction of litters coupled with high fat diet yielded NSG mice with similar degree of obesity and adiposity as similarly treated C57Bl/6 mice.
Figure 1. Obesity phenotype of fat-fed NSG mice.
(A) Body weight of female and male NSG mice weaned onto high fat (closed circles, n=8) and control (open circles, n=4) diet at 3-4 weeks of age. (B) Body weight of female control (n=41) and obese (n=37) NSG mice and female control (n=13) and obese (n=14) B6 mice, on optimized diet-induced obesity protocol with selective culling of litters (see Methods for details). (C) Body fat measured by chemical carcass analysis of control (hatched bars, n=13) or obese (solid bars, n=10) NSG and B6 (n=3/diet group) mice. (D) Plasma leptin and resistin concentration in NSG (n=23 control and n=19 obese) and B6 (n=6 control and n=10 obese) mice. (E) Percent relative cumulative frequency of adipocyte size by area in obese and control NSG (n=6) and B6 (n=4) mice. *p<0.05, **p<0.01, ***p<0.001.
Obesity in NSG and B6 mice was associated with increased plasma levels of the obesity-related hormone leptin (p<0.01 for both strains, Figure 1D). Resistin and adiponectin levels (not shown) were not different between obese and non-obese mice of either strain, perhaps due to the relatively young age of the mice (~3 months old). The lack of effect of fat-feeding on adiponectin has been reported in other similar studies of fat-fed female B6 mice (for example, (16)). Obese mice of both strains had larger mean adipocyte size than their control-fed counterparts, consistent with the diet-induce obesity phenotype (Figure 1E).
Obese NSG mice accumulate activated adipose tissue macrophages
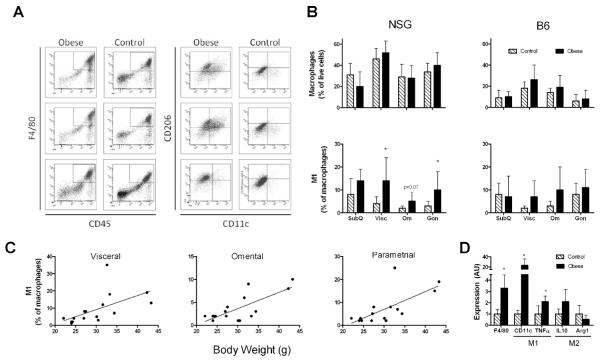
To determine whether activated macrophages accumulate in the adipose tissue of obese NSG mice, we analyzed the macrophages in the SVF from multiple fat pads using flow cytometry (Fig 2). Obesity did not significantly alter the percentage of macrophages in adipose tissue from obese NSG and B6 mice, but it was associated with a higher expression of CD11c (Fig 2A). M1 “classically” activated macrophages, characterized by surface expression of CD11c and absence of CD206 (4), were significantly increased in the visceral and parametrial adipose depots from obese NSG mice, but not in any adipose depots from obese B6 mice. Further, the percentages of M1 adipose tissue macrophages in NSG mice were correlated with body weight in the visceral, omental, and parametrial fat pads (r2 = 0.34, 0.56, and 0.49, respectively; p<0.05 for all correlations, Figure 2C), though not in the subcutaneous fat pad (not shown). In contrast, M1 activation did not correlate with body weight in any fat pads from B6 mice (not shown). These flow cytometry findings were confirmed by measuring gene expression of macrophage markers in RNA isolated from whole parametrial adipose tissue; F4/80, a gene expressed by all macrophages (17), was significantly higher in adipose tissue from obese compared to non-obese NSG mice (p=0.025, Fig 2D). CD11c and TNFα were expressed at significantly higher levels in fat from obese than from non-obese NSG mice (Fig 2C). On the other hand, expression of arginase-1 and IL10— markers of M2 “alternatively” activated macrophages—was not different between obese and non-obese NSG mice.
Figure 2. Obesity induces accumulation of activated macrophages in adipose tissue.
(A) Representative dot plots from visceral adipose tissue stromovascular fractions of three obese and control NSG mice. Live cells were gated based on DAPI and side scatter. Macrophages were defined as F4/80 and CD45 positive, and M1 and M2 determined based on expression of either CD11c or CD206. (B) Quantification of macrophages of various adipose depots from flow cytometry (n=4-8 per depot). (C) Correlation between percentage of M1 macrophages and body weight in NSG mice (n=16). See text for statistics. (D) Expression of selected macrophage M1 activation (left) and M2 alternatively activation (right) genes from parametrial adipose tissue of NSG mice (n=4 per diet group), measured by qPCR. (control = hatched bars, obese = solid bars). *p<0.05
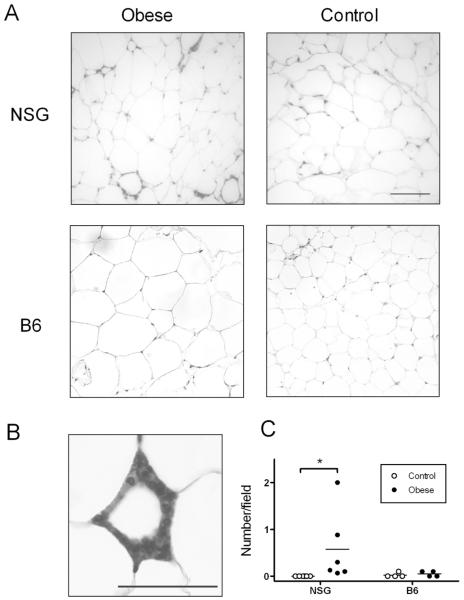
Macrophages have been shown to surround adipocytes in obese mice and humans, forming “crown-like structures” (CLS), which are observed only rarely in the lean state (18). Adipose tissue from obese NSG mice contained more CLSs than non-obese NSG mice (p=0.03, Figure 3C). However, consistent with our flow cytometry results, obese B6 mice did not have increased numbers of CLSs compared to non-obese mice.
Figure 3. Immunohistochemical analysis of adipose tissue macrophages.
(A) Representative 20x fields of parametrial adipose tissue from obese (left) and control (right) NSG (top) and B6 (bottom) mice. Macrophages labeled with F4/80, calibration bar is 100 μm (see methods for staining procedures and microscope model). (B) Representative CLS from an obese NSG mouse labeled with F4/80. Photo taken at 63x magnification, calibration bar is 25 μm. (C) Quantification of macrophages found in crown like structures (CLS) in parametrial fat pads of NSG (n=6/group) and B6 (n=4/group) mice. CLSs were counted by a blinded observer in 15 fields at 20x magnification.
Obesity does not impair glucose tolerance in NSG mice
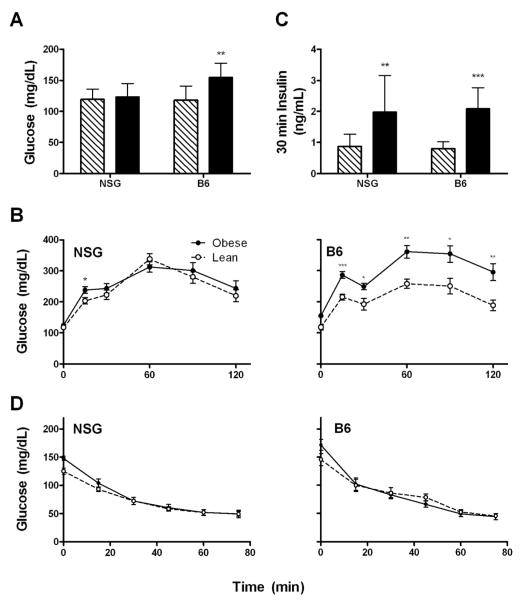
To determine whether obesity would impair glucose physiology in obese NSG mice, we performed intraperitoneal glucose tolerance tests (IPGTTs) and insulin tolerance tests (ITTs). While obesity had no effect on fasting glucose or glucose tolerance in the NSG mice, it caused a fasting hyperglycemia and impaired glucose tolerance in the B6 mice (Figure 4A&B). Both obese mouse strains had higher 30 minute insulin response during the IPGTT, implying a degree of insulin resistance (Figure 4C); however, obesity did not alter insulin sensitivity quantified during the ITT for either strain (Figure 4D). Thus, obesity did not significantly impair glucose homeostasis in the NSG mouse as it did in the B6 mouse.
Figure 4. Physiological measures of obese and control mice.
(A) Fasting glucose (B) Serum insulin level 30 minutes post intraperitoneal glucose load, and (C) glucose levels during intraperitoneal glucose tolerance tests (NSG: n=14 obese, 17 control; B6: n=9 obese, 8 control). (D) Glucose concentrations during insulin tolerance tests (NSG: n=13 obese, 15 control; B6: n=9 obese, 5 control).
Plasma cytokines are not altered by obesity in NSG mice
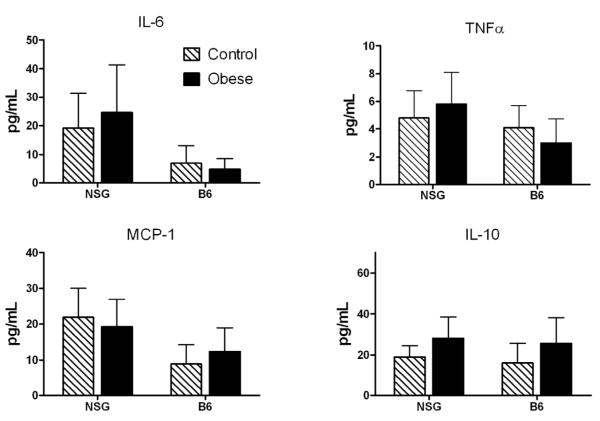
Cytokines have been shown to cause insulin resistance in immunocompetent mice. Therefore, we measured various cytokines in our mice to determine which might be altered by obesity in immunodeficient NSG mice. There was no effect of obesity on MCP-1, IL-6, IL-10 (Fig. 5), VEGF, PAI-1, IL-13, IL-12(p70), IL-4, IL-1b, or IFNγ (not shown). TNFα tended to be higher in obese NSG mice than in non-obese, though the difference did not reach statistical significance (p=0.10). TNFα production by parametrial adipose depots ex vivo also was not different between obese and non-obese mice of either strain (not shown).
Figure 5. Obesity does not substantially alter cytokine levels in NSG or B6 mice.
Plasma cytokine levels of NSG (23 controls/19 obese) and B6 (6 controls/9 obese) mice.
Discussion
As the world-wide prevalence of obesity has risen dramatically over recent decades, there is increased interest in understanding the mechanisms linking obesity and its complications. Obesity is associated with a low level of chronic inflammation, which may arise in part from immune cell infiltration into adipose tissue (19). Multiple subsets of immune cells are found in the adipose tissue of obese animals and humans, including macrophages, T and B lymphocytes, and NK cells. However, it has been difficult to determine how these cells interact with each other and with adipocytes, and how they might contribute to obesity-related complications such as insulin resistance. In the present study, we tested whether macrophages would infiltrate into the adipose tissue of obese NSG mice, which lack both T and B lymphocytes and NK cells. We further tested whether these mice would develop impaired glucose physiology.
We determined that NSG mice, which had been made obese by a combination of selective litter size reduction and high fat diet, had increased accumulation of activated macrophages in their adipose tissue. These results demonstrate for the first time that T and B lymphocytes and NK cells are not necessary for the obesity-related accumulation of activated macrophages in adipose tissue. These results contrast with a study by Nishimura et al., which demonstrated that the selective depletion of CD8+ T lymphocytes prevented the obesity-related infiltration of macrophages into adipose tissue. However, an alternative explanation proposed by Lumeng et al., is that the balance of pro- and anti-inflammatory T lymphocytes may regulate the adipose tissue infiltration and activation of macrophages. Pro-inflammatory T lymphocytes could stimulate accumulation of macrophages, while anti-inflammatory regulatory T lymphocytes could prevent macrophage influx and activation (9). The obese B6 mice did not accumulate activated macrophages in adipose tissue, likely reflecting that the anti-inflammatory effects of the regulatory T lymphocytes had not yet been counterbalanced by pro-inflammatory T lymphocytes. Since the NSG mouse is missing T and B lymphocytes and NK cells, it is a somewhat artificial model, which lacks this balance between lymphocyte populations. However, the present results show that the “default state” of obese adipose tissue without any T lymphocytes promotes macrophage infiltration and activation. Thus, it is clear from our experiments that the non-immune components of obese adipose tissue can actively contribute to recruitment and activation of macrophages, independent of lymphocytes. Our results are consistent with the finding that obesity stimulates macrophage accumulation in adipose tissue of Rag2 knockout mice, which lack most mature T and B lymphocytes (5).
Our results demonstrate that obesity does not impair glucose tolerance in NSG mice, despite the activated macrophage accumulation in adipose tissue. In contrast, the B6 mice made obese by a similar protocol exhibited impaired glucose tolerance. Since control-fed NSG mice exhibited worse glucose tolerance than control B6 mice, direct comparisons of glucose tolerance between the two strains are not possible; however, qualitatively it appears that the NSG mice were able to fully compensate for the high-fat diet while the B6 mice were not. Obesity-induced glucose intolerance is caused by insulin resistance coupled with a beta cell impairment, which renders them unable to fully compensate for the increased insulin requirements. Surprisingly, we were unable to detect insulin resistance in the obese B6 mice, despite the fact that they had increased insulin secretion during the IPGTT and impaired glucose tolerance. It is possible that insulin resistance was not detected in the ITT because the standard insulin doses used (0.75 U/kg) led to a substantial glucose suppression, and even severe hypoglycemia in some animals (<30 mg/dL). Thus, if the glucose suppression was near maximal, differences between groups may have been obscured. Also, counterregulatory hormonal responses could have also confounded the results. In any case, these data do show that the NSG mice were resistant to obesity-induced glucose intolerance; future studies will be needed to address whether this effect was due to improved insulin sensitivity, beta cell function, or both.
Our results are in agreement with other studies which have demonstrated that T or B lymphocytes may contribute to obesity-induced adipose tissue inflammation and glucose intolerance (6, 8, 10). In fact, diet-induced obesity was shown to cause a rapid infiltration of T lymphocytes into adipose tissue, possibly mediated by the chemokine SDF1α, while macrophage infiltration was much slower and was detected well after insulin resistance was initially detected (20). In contrast, Winer et al. demonstrated that macrophages infiltrate visceral adipose tissue of mice within 2 weeks of switching to a high fat diet, and this occurs before a detectible increase in T or B lymphocytes (21). Depletion of T lymphocytes by injection of F(ab’)2 anti-CD3 antibody improved insulin sensitivity (10). Further, the local elimination of T lymphocytes by injection of anti-CD3 antibody into an epididymal fat pad systemically reversed obesity-related insulin resistance in young mice (22). On the other hand, selectively increasing Treg lymphocytes can reverse both adipose tissue macrophage infiltration and insulin resistance (10, 23). Recent data show that B cells may contribute to adipose tissue T cell and macrophage activation leading to insulin resistance and glucose intolerance (21).
Our results are consistent with evidence that NK or NKT cells may have a role in obesity-related insulin resistance. Rag2 knockout mice made obese by a high fat diet exhibited adipose tissue infiltration of NK cells and insulin resistance (5). Visceral adipose tissue, which is epidemiologically more closely related to insulin resistance, had more NK cells than subcutaneous adipose tissue in obese humans (24). Obese mice lacking NKT cells had fewer adipose tissue macrophages and better glucose tolerance than wildtype mice, while mice with NKT cell activated with α-galactosylceramide had increased adipose tissue macrophage infiltration and impaired glucose tolerance (7). Thus, the available evidence suggests that T lymphocytes, macrophages, and NK and NKT cells may all contribute in interdependent ways to obesity-related insulin resistance.
Since rats made obese using selective litter size reduction developed insulin resistance (12), we believe that it is unlikely that the selective culling protocol would itself prevent obesity-induced insulin resistance. In fact, the selective reduction of litters mimics the rapid weight gain in infants who consume excessive calories, a known risk factor for later obesity (25). The use of selective culling coupled with a high fat diet also more closely mimics the current epidemic of obesity caused by excessive caloric intake and decreased physical activity. Thus, although the obesity in this model is more modest than common genetic models (e.g. ob/ob mice), it is likely more physiologically relevant to the human disease.
One weakness of this study is that the mice were made obese for a relatively short time (< 3 months). Although this resulted in a substantial increase in adiposity, the mice in both strains exhibited only a modest increase in leptin levels, and no significant elevations in cytokines that are generally elevated in obesity. However, the obese B6 mice developed fasting hyperglycemia and significant glucose intolerance in this time period, demonstrating that even this relatively short term of obesity can lead to physiological impairments in susceptible mice. Another caveat is that fat fed B6 mice generally develop increased adipose tissue macrophage infiltration somewhere between 5 and 10 weeks on the diet, so it is possible that this short diet period did not lead to maximal adipose tissue macrophage infiltration. However, our primary outcome of interest was macrophage infiltration in the NSG mice, which was clearly observed even at this early time point. Finally, it is also possible that the use of female mice contributed to the mild obesity phenotype, as female C57Bl/6 mice are less susceptible to insulin resistance than males (26). Thus, further studies with male NSG mice made obese for a longer period of time may help to elucidate whether the absence of lymphocytes will prevent insulin resistance and glucose intolerance in the face of chronic obesity.
In summary, we have developed a novel murine model of immune deficiency and obesity. Our results show that T and B lymphocytes and NK cells are not necessary for the accumulation of activated macrophages in adipose tissue of obese mice. It appears that adipocytes themselves are likely responsible for the obesity-related macrophage activation, possibly due to secretion of adipokines such as leptin (27) or MCP-1 (28). Surprisingly, the albeit modest degree of obesity observed in the mice lacking T and B lymphocytes and NK cells did not lead to glucose intolerance, suggesting that T and B lymphocytes and/or NK cells may play a necessary role in obesity-induced diabetes.
Acknowledgements
The authors would like to thank Renee Traub-Workman and Donna Foster for expert animal handling, Jason P. Yun for assistance performing experiments, G. Esteban Fernandez for assistance with microscopy, Jemily Malvar for statistical help, and Robertson Parkman for valuable critique of the manuscript. This work was supported by the National Cancer Institute (CA139060 to S.D.M.), the TJ Martell Foundation, and the Children’s Cancer Research Fund, a California non-profit organization.
Abbreviations Used in this Article
- CLS
Crown-like structure
- IPGTT
Intraperitoneal glucose tolerance test
- ITT
Insulin tolerance test
- NSG
NOD/SCID/IL2-receptor gamma-chain knockout
Footnotes
Disclosure
The authors have no conflicts of interest to disclose.
Reference List
- 1.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384(4):482–5. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 7.Ohmura K, Ishimori N, Ohmura Y, et al. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2010;30(2):193–9. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- 8.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nat Med. 2009;15(8):846–7. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 10.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 12.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65(Suppl 3):83–9. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- 13.Dobush GR, Ankney CD, Krementz DG. Canadian Journal of Zoology. 1985;63:1917–20. [Google Scholar]
- 14.Rasband w. s. ImageJ. U.S. National Institutes of Health; Bethesda, MD: 2012. pp. 1997–2012. http://imagej.nih.gov/ij/ [Google Scholar]
- 15.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol. 2004;82(12):1075–83. doi: 10.1139/y04-117. [DOI] [PubMed] [Google Scholar]
- 16.Townsend K, Lorenzi M, Widmaier E. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33(2):176–88. doi: 10.1007/s12020-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 17.Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA, Ravasi T. The mononuclear phagocyte system revisited. Journal of Leukocyte Biology. 2002;72(4):621–7. [PubMed] [Google Scholar]
- 18.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 20.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28(7):1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 21.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilan Y, Maron R, Tukpah AM, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc Natl Acad Sci U S A. 2010;107(21):9765–70. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Rourke RW, Metcalf MD, White AE, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33(9):978–90. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekelund U, Ong KK, Linne Y, et al. Association of Weight Gain in Infancy and Early Childhood with Metabolic Risk in Young Adults. J Clin Endocrinol Metab. 2007;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 26.Thakker GD, Frangogiannis NG, Bujak M, et al. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291(5):H2504–H2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan T, Li L. Molecular mechanism underlying the inflammatory complication of leptin in macrophages. Molecular Immunology. 2010;47(15):2515–8. doi: 10.1016/j.molimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J Biol Chem. 2009;284(45):31223–35. doi: 10.1074/jbc.M109.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]