Abstract
In the present study, we examined the relationship between developmental modulation of socioaffective brain systems and adolescents’ preoccupation with social evaluation. Child, adolescent, and adult participants viewed cues indicating that a camera was alternately off, warming up, or projecting their image to a peer during the acquisition of behavioral-, autonomic-, and neural-response (functional MRI) data. Believing that a peer was actively watching them was sufficient to induce self-conscious emotion that was stronger in adolescents than in children and adults. Autonomic arousal was uniquely heightened in adolescents. These behavioral patterns were paralleled by emergent engagement of the medial prefrontal cortex (MPFC) and striatum-MPFC connectivity during adolescence, which are thought to promote adolescent-motivated social behavior. These findings demonstrate that adolescents’ self-consciousness is related to age-dependent sensitivity of brain systems critical to socioaffective processes. Further, unique interactions between the MPFC and striatum may provide a mechanism by which social-evaluation contexts influence adolescent behavior.
Keywords: adolescence, embarrassment, evaluation, fMRI, medial prefrontal cortex, self-consciousness, social
Adolescence is a phase of the human lifecourse defined by immense social change. Given that adolescents spend more time with peers relative to children and adults (Brown, 2004), a unique feature of adolescent behavior is heightened attunement to, concern over, and reaction to perceived instances of peer evaluation. During adolescence, reported concern over social evaluation rises sharply from childhood (Westenberg, Drewes, Goedhart, Siebelink, & Treffers, 2004), reported daily self-consciousness peaks (Rankin, Lane, Gibbons, & Gerrard, 2004), and adolescents more frequently interpret themselves as being the target of social evaluation (e.g., imaginary audience behavior (Elkind & Bowen, 1979)).
An emerging viewpoint in neurodevelopmental research is that dynamic features of brain development are consequential to unique aspects of behavior that emerge over the lifecourse (Casey, Tottenham, Liston, & Durston, 2005; Somerville, Jones, & Casey, 2010). Despite the primary role of actual or perceived social evaluation in adolescents’ daily lives and well-being, little is known about the biological mechanisms that accompany phenomenological shifts in adolescent social concern. The present study sought to test the hypothesis that experiential, autonomic, and socioaffective brain responses would change nonlinearly from pre-adolescence to post-adolescence, even under minimal conditions – simply being looked at by a peer.
The current study focused on developmental modulation of the response properties and connectivity of the medial prefrontal cortex (MPFC). The MPFC is commonly engaged by social and emotional processes (Amodio & Frith, 2006; Roy, Shohamy, & Wager, 2012), and is a key node in neuroscientific models of the development of the adolescent self-concept (Sebastian, Burnett, & Blakemore, 2008). Given that the MPFC shows dynamic structural and connectivity-based maturation throughout the adolescent years (Shaw et al., 2008), we sought to evaluate the neurodevelopmental features of MPFC response and connectivity during instances of experimentally induced social evaluation. Sixty-nine human participants ranging in age from 8 to 22.9 years completed self-report, autonomic arousal (galvanic skin response; GSR) and functional brain imaging (fMRI) measures to test a) whether adolescents experience heightened emotional and autonomic responses to instances of peer evaluation; b) whether these responses extend to social anticipatory contexts; c) whether such a behavioral profile is paralleled by distinct recruitment and connectivity patterns of the MPFC in adolescents; and d) whether such potential effects subside or persist into early adulthood. Analyses utilized age as a continuous variable to test for linear, quadratic (U or inverted-U shaped), and asymptotic (change during childhood and adolescence stabilizing into adulthood) effects on responses to evaluation and anticipation periods.
Methods
Participants
N=69 healthy participants 8.0–22.9 years of age completed fMRI scanning. Participant volunteers were recruited from the New York City metropolitan area (for demographics, see Table S1 available online). See Supplementary Materials for inclusion and exclusion criteria. Participants provided informed written consent (parental consent and subject assent for minors) approved by the Institutional Review Board of Weill Cornell Medical College.
Task
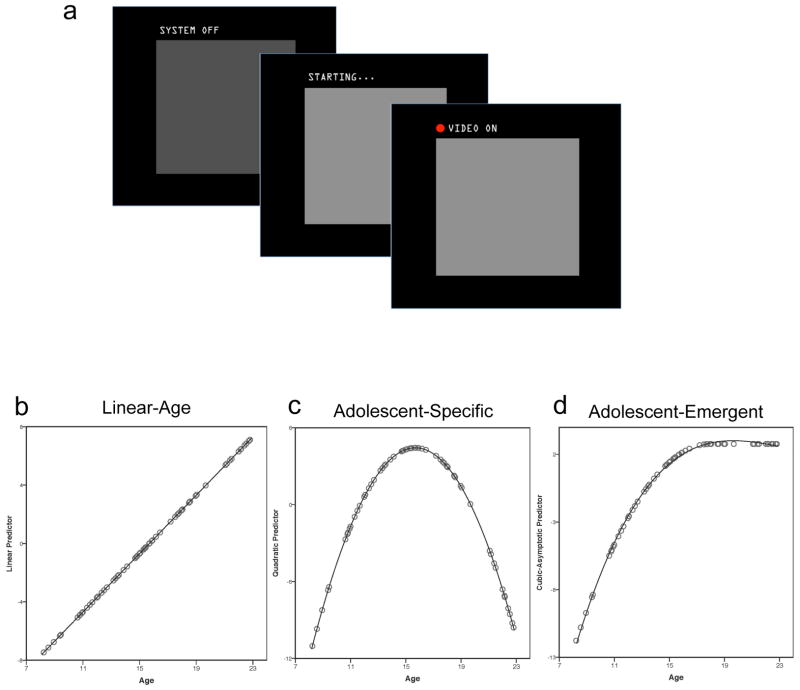
Participants were led to believe that a small, one-way video camera was embedded in the head coil of the fMRI scanner. They were instructed that this was a novel technology, and that the experimenters wished to test it during their experimental session by cycling through its settings (off, warming up, on) several times. Participants were instructed to passively view the screen and monitor the video camera’s status. Cued by low-level changes in the screen display, the supposed video camera cycled between three phases (Figure 1a): “Off” (resting baseline condition), “Starting…” and would turn on at any moment (anticipation condition), or “On” and ostensibly project their image to be viewed by a peer (evaluation condition).
Figure 1.
a. Participants were led to believe that a one-way video camera was embedded in the head coil of the fMRI scanner. During different blocks of the experiment, participants believed that a camera was projecting an image of their face in real-time (‘evaluation’ condition, right), ‘starting’ while evaluation was absent yet imminent (‘anticipation’ condition, middle), or off (left). b–d. Linear (b), quadratic (c), and asymptotic (d) age patterns under investigation. Plots of predictor variables, with each dot representing a participant.
Participants were instructed that a same-sexed peer of similar age would monitor the video feed during the participant’s scan, and could see the participant’s face in real-time whenever the camera was “on”. They were told the camera was a one-way projection, and thus should not expect to view a peer. Although there was, in fact, no camera, all participants had completed a separate peer interaction task immediately prior to this study (Jones et al., 2011) which conveniently made the cover story more believable. Though it cannot be ruled out that the prior task influenced the present findings, the two tasks were held consistent across all participants and each participant had a short break between the two studies.
The task was structured as a block design that pseudorandomly alternated between rest, anticipation, and evaluation conditions. Participants saw a total of 12 blocks, four of each condition (rest, anticipation, evaluation). To reduce predictability, block length varied in duration between 16 and 38 seconds. Across the task, participants spent an equal total duration viewing anticipation and evaluation conditions (total per condition: 92 seconds), and the mean duration of anticipation and evaluation blocks was matched (mean: 23 seconds). Participants viewed the resting baseline “camera off” stimulus for a total of 126 seconds, in blocks averaging 31.5 seconds duration.
Measures
Emotion
Immediately following the task, participants were asked to rate the extent to which they experienced the following emotions: Happiness, Excitement, Nervousness, Worry, Fear, and Embarrassment. Participants rated each emotion category by bisecting a continuous line with anchors “Not at all” (far left) and “Extremely” (far right). Ratings were completed for anticipation and evaluation phases separately and were not acquired for the rest blocks when participants believed the camera was off.
Skin conductance
Skin conductance (GSR) was sampled simultaneously for N=62 of the participants, with usable data acquired from N=56 participants (see Supplementary Materials for exclusion criterion and Table S1 for demographics). An MRI compatible skin conductance recording system (GSR100C Biopac, Goleta, CA) together with AcqKnowledge 4.0 (Biopac; Goleta, CA) software continuously sampled skin conductance data at 100 Hertz.
Neuroimaging
Participants were scanned with a General Electric Signa 3.0 Tesla MRI scanner (General Electric, Milwaukee, WI) with a quadrature head coil. See Supplementary Materials for structural and functional acquisition sequences.
Data analysis
Age effects
Statistical analysis of each dependent variable (self-report, GSR, fMRI activity, fMRI connectivity) assessed the significance of three continuous age predictors, each assessing a distinct pattern of age-dependent change (Figure 1b–d): a) linear-age predictor with increasing age was modeled as a mean-centered linear age variable; b) adolescent-specific predictor to detect U- or inverted-U effects for which adolescents differ from both children and adults, modeled as a quadratic function (calculated by squaring the linear-age predictor; the quadratic peak fell at 15.94 years in the present sample); and c) adolescent-emergent predictor, that shows rapid change throughout adolescence and persists in magnitude into adulthood, which was modeled with a mean-centered asymptotic predictor calculated by generating a quadratic function peaking at 18 years of age and asymptoting (retaining the maximum value) for adult ages. The adolescent-emergent predictor closely mimicked a truncated cubic function for which the inflection point was fixed at 18 years of age.
Because the three predictors naturally share variance, group analyses consisting of a single ‘competing’ statistical model incorporating all age predictors are statistically invalid. Given that the objective of the present study was to assess age influences on self-conscious emotion and associated neural activity, every dependent variable was submitted to a triad of group statistical tests, each incorporating one continuous age predictor. From the triad of analyses, every age predictor that reached statistical significance is reported in the main Results section, and is represented by a fit-line in Figures. Full analysis of variance (ANOVA) results for every age predictor are reported in Supplementary Materials. This approach mitigates model instability caused by multicollinearity, and the need to engage in potentially biased experimenter choices regarding the importance of the three age predictors (e.g., choosing a testing order in stepwise group regressions, or choosing to orthogonalize one predictor with respect to another). Though this approach does not permit direct quantitative comparison of the three age patterns, we believe it provides the most efficacious and unbiased method of identifying the age predictor(s) that explain variance in the variables of interest.
Emotion
Self-reported emotion ratings were scored by recording the distance from the far left anchor at which the participant bisected the line, with a greater value indicating greater endorsement of the emotion category. Raw scores were proportionalized by dividing each score by the total line length and by the sum of all measurements for that participant.
Statistical analyses were conducted in IBM SPSS Statistics 19.0. A factor analysis indicated three latent variables evident in the self-report ratings corresponding to Anxiety, Positive Arousal, and Embarrassment (see SOM-R). For each of the three emotion variables, a group ANOVA tested for effects of task phase (anticipation, evaluation) and each of the three age predictors on self-reported emotion. If age effects but no significant effects of task phase were observed, data were averaged across task phases for post-hoc analyses that evaluated the specificity of age effects to the Anticipation and/or Evaluation phases. Significant age effects were plotted for inspection of distribution, possible outliers, and directionality. Given the focus of the present manuscript on developmental differences in task-evoked emotion, findings for the embarrassment ratings that yielded significant developmental differences are reported in the main text. Results for emotion ratings that did not show significant developmental differences are reported in Supplementary Materials for completeness. To account for independent tests of the three emotion variables (Anxiety, Positive Arousal, and Embarrassment), each statistical test is interpreted using an adjusted critical α=0.0167 (α=0.05 Bonferroni corrected for three tests).
Skin conductance analysis
Skin conductance analysis in N=56 usable participants was performed using AcqKnowledge 4.0 software and IBM SPSS Statistics 19.0. Skin conductance analyses focused on changes in response slope, or skin conductance level (SCL) per block. This standard analysis for block design data (Dawson, Schell, & Filion, 2001) measures the signal habituation rate during a task block, such that larger value corresponds to less habituation, indicative of autonomic arousal maintenance throughout the block. See Supplementary Materials for slope calculation methods.
Group analyses tested for effects of task phase (anticipation, evaluation) and age on GSR and included baseline GSR as a covariate of no interest to account for task-independent variance in GSR reactivity across participants. Group analyses were conducted as described above, with task time (first half of the experiment, second half of the experiment) additionally included as a within-subjects factor given the strong tendency for GSR effects to habituate over time (Andreassi, 2006; Dawson et al., 2001).
Neuroimaging
Functional imaging data were preprocessed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996). See Supplemental Materials for details on preprocessing and first-level task-based general linear modeling (GLM). Following GLM estimation for each participant, random effects group analysis consisted of a triad of linear mixed effects group models with regressors representing dummy-coded variables representing task phase (anticipation, evaluation), participant, and each age predictor (Fig. 1b–d). Results yielded group statistical maps representing the main effect of task phase (anticipation vs. evaluation), the main effect of each age predictor, and interactions of task phase by age predictor. Given the present focus on brain-behavior parallels, the present manuscript retains focus on age effects that persist for both anticipation and evaluation conditions as observed for embarrassment and GSR findings. However, a number of brain regions demonstrated significant age by task phase interactions indicating differential age modulation for anticipation and evaluation conditions. These regions and descriptions of age patterns are reported in Supplementary Table 2.
Given dynamic changes in MPFC morphology and connectivity (Shaw et al., 2008), and motivated social behavior (Steinberg, 2004) from childhood to adulthood, group connectivity analyses sought to assess putative age modulation of coupling between the MPFC and systems of the brain critical to motivated behavior, such as the striatum (Robbins & Everitt, 1996). A whole-brain psychophysiological interaction (PPI) analyses (Friston et al., 1997) was carried out to identify selective MPFC task-based functional coupling that could be subsequently queried for age effects. See Supplementary Materials for first-level PPI modeling methods. Random effects group analysis regressed voxelwise PPI parameter estimates against each of the three age patterns of interest (e.g., Fig. 1b–d). Resultant maps identified regions of the brain whose MPFC signal coupling during evaluation contexts fit each of the three age patterns.
All brain imaging findings considered statistically significant exceeded correction for multiple comparisons to preserve α≤0.05 by using a p-value/cluster size combination stipulated by Monte Carlo simulations run in the Clustsim subroutine. The search space of the simulation constituted the spatial coverage obtained for functional images (42,341 voxels acquired; whole-brain coverage minus much of the occipital lobe). Thus, all imaging findings achieve p<0.05, corrected thresholding for the full acquisition space.
Significant age effects were plotted for inspection of distribution, possible outliers, and directionality by extracting parameter estimates for each participant from a 6mm spherical ROI about the cluster peak. These parameter estimates were also used for analyses to test possible sex differences, the relationship between dependent measures, and were used to rule out potential age confounds in signal-to-noise ratio and motion (see Supplementary Materials).
Relationship between variables
Bivariate correlational analyses were conducted to quantify the degree of shared variance between self-reported embarrassment, GSR, and fMRI measures. Partial correlation analyses controlling for embarrassment and GSR assessed whether reported age effects in neural response remain significant when controlling for experiential measures. Results of this analysis (see Supplementary Materials) verified that the observed age differences could not be solely explained by covarying experiential differences across participants.
Results
The social evaluation task elicited self-conscious emotion (e.g., increased ratings of embarrassment) (Keltner & Haidt, 1999) and physiological arousal in adolescents. Repeated ANOVAs including task phase (anticipation, evaluation) and each age predictor indicated that the adolescent-emergent age predictor yielded a significant main effect on embarrassment ratings (asymptotic: F(1,67)=6.07, p=0.0163; η2 partial=0.083; Bonferroni-adjusted critical α=0.0167, see SOM-R; Figure 2), with the adolescent-specific age predictor yielding trend-level prediction of embarrassment ratings (quadratic: F(1,67)=5.52, p=0.022; η2 partial=0.076; Bonferroni-adjusted critical α=0.0167, see SOM-R). The estimated age of peak embarrassment ratings is 17.2 years.
Figure 2.
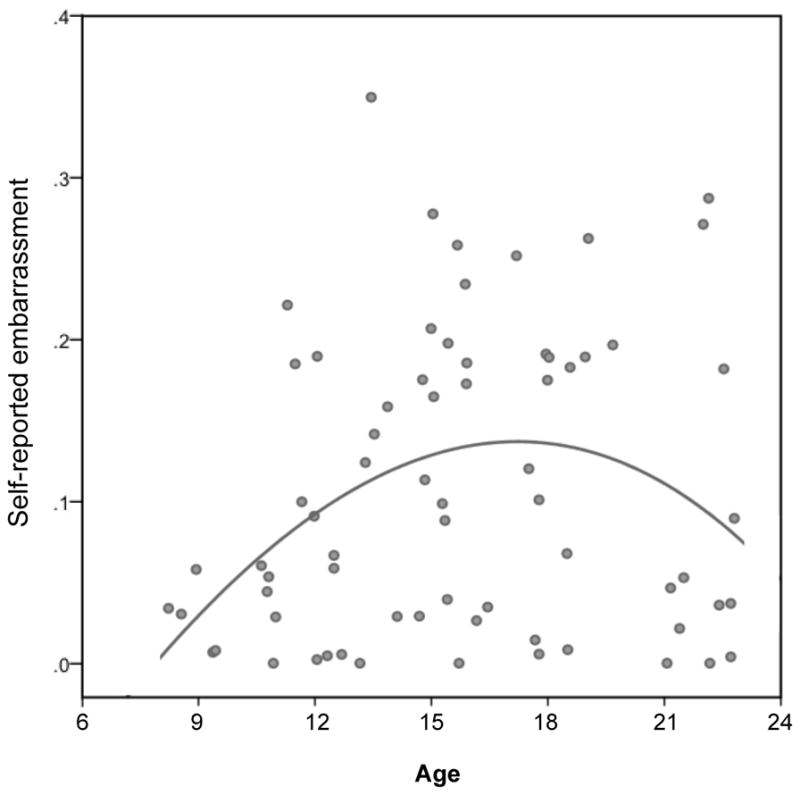
Scatterplot of embarrassment ratings response to evaluation and anticipation conditions (collapsed) by age. The fit line was derived from the adolescent-emergent predictor.
Given the significant adolescent-emergent effects, embarrassment ratings were further queried for modulation by task phase. The adolescent-emergent age predictor yielded a trend-level effect for the anticipation condition (F(1,67)=3.57, p=0.063; η2 partial=0.051) and a significant effect for the evaluation condition (F(1,67)=7.14, p=0.009; η2 partial=0.096), suggesting consistency in age effects on embarrassment in both conditions but a more robust age difference during the evaluation phase. There was no main effect of task phase on embarrassment ratings (F(1,67)=0.093, p=0.76; Bonferroni-adjusted critical α=0.0167).
Skin conductance data yielded a quadratic age by time interaction (F(1,53)=10.34, p=0.002; η2 partial=0.163). Post-hoc analyses isolating the first half of the experiment indicated an adolescent-specific age effect which can be described as greater autonomic arousal (less habituation of slope) rising into adolescence and subsiding into adulthood (F(1,53)=9.40, p=0.003; η2 partial=0.151; Supplementary Figure 1). The estimated age of peak GSR response was 14.38 years. There were no significant effects of task phase or age during the second half of the experiment (p’s>0.2). Significant adolescent-specific age effects during the first half were evident for anticipation and evaluation phases when tested separately (anticipation: F(1,53)=4.77, p=0.033; η2 partial=0.083), evaluation: (F(1,53)=6.95, p=0.011; η2 partial=0.12). These results suggest that minimal social evaluative contexts are sufficient to induce heightened self-conscious emotion and physiological arousal that peaks in mid-adolescence.
A triad of a voxelwise whole-brain mixed model ANOVAs were conducted with the repeated factor of task phase (anticipation, evaluation) and each age predictor serving as a continuous covariate of interest. Guided by the behavioral findings, fMRI analyses focused on revealing neural activations that were similarly engaged by anticipation and evaluation phases, and differentially active as a function of the age predictors (Figure 1b–d). A single region of the brain located in the medial prefrontal cortex (xyz=−13,53,6; 72 3×3×3mm voxels; Brodmann area 32/10; mean cluster statistic F(1,67)=11.84; p<0.05 corrected; Figure 3a) was significantly related to the adolescent-emergent age predictor. The estimated age of peak in MPFC activity was 15.25 years. The identical, single region was also identified in an analysis of adolescent-specific age effects at p<0.05, corrected thresholding, albeit smaller in size (mean cluster statistic F(1,67)=10.38, 30 voxels). No regions demonstrated significant linear-age effects at whole-brain corrected thresholding. To summarize, mirroring the levels of experienced embarrassment and arousal, the MPFC demonstrated an elevated response in adolescents both during anticipation and evaluation conditions that partially retained its activity strength into young adulthood. Age differences in MPFC activity are plotted in Figure 3b for descriptive purposes.
Figure 3.
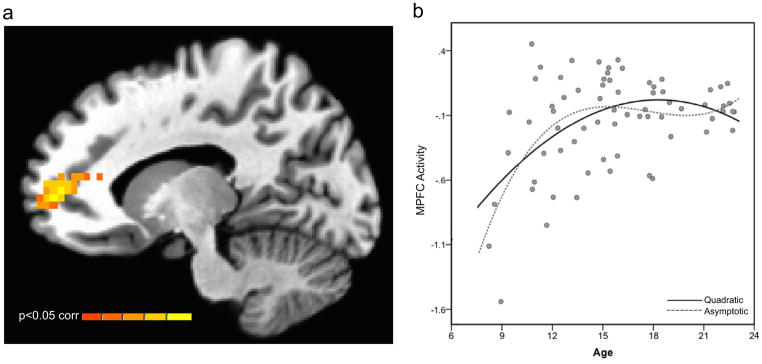
Age differences in task-based functional brain activity. The statistical map identified functional activity showing heightened engagement of the medial prefrontal cortex (MPFC) through adolescence (relative to childhood) that persists into adulthood. The image threshold was p < .05, corrected for acquisition space. The scatter plot (b) shows MPFC responses in the evaluation and anticipation conditions (collapsed) as a function of participants’ age, for descriptive purposes. The solid fit line was derived from the adolescent-emergent predictor, and the dashed line was derived from the adolescent-specific predictor.
Given the powerful influence of social evaluation on motivated and affective behaviors in adolescence (Steinberg, 2008), psychophysiological interaction analyses tested the extent to which the MPFC demonstrates differentially selective connectivity during evaluation periods as a function of age (see Supplementary Materials for methodological details). Resultant statistical maps revealed MPFC coupling with the dorsal striatum that significantly fits an asymptotic age pattern (left caudate xyz=−8,20,6; 35 3×3×3 voxels; mean cluster statistic F(1,67)=10.24; p<0.05, corrected; Figure 4). No other activations were observed for this analysis. No regions demonstrated significant linear-age or adolescent-specific (quadratic) effects at whole-brain corrected thresholding. Thus, the transition from childhood through adolescence predicts the emergence of MPFC-striatal coupling during evaluation contexts, a pattern that persists into adulthood.
Figure 4.
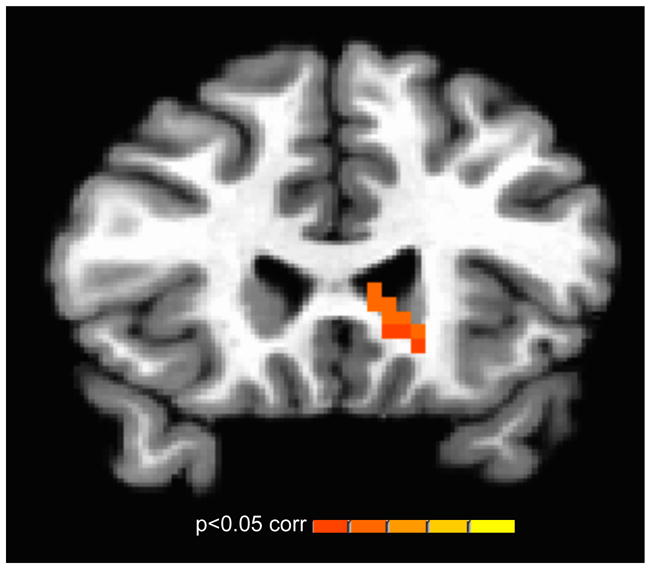
Age differences in task-dependent functional connectivity. Whole-brain connectivity analysis identified selective functional coupling between the MPFC and striatum during evaluation, which was greater in adolescence (relative to childhood) and persists into adulthood. The image threshold was p < .05, corrected for acquisition space.
Discussion
Using a simulated a social evaluation task, we observed that being watched by a peer was sufficient to generate nonlinear changes from childhood to young adulthood in self-conscious emotion and related physiological indices of emotional arousal. The nonlinear pattern of embarrassment and skin conductance findings support the hypothesis that even subtle social-evaluative contexts - and anticipation of them - lead to heightened self-conscious emotion and arousal during adolescence. Self-conscious emotion rose during adolescence and stabilized into adulthood, while arousal (skin conductance) levels showed maximal levels during adolescence.
There are numerous factors that are thought to converge during adolescence and contribute to the central role of social evaluation in adolescents’ everyday experience. On one hand, the transition to adolescence typically marks a rise in the frequency and intensity of peer interaction that has been documented in humans and animals (Cairns, Leung, Buchanan, & Cairns, 1995; Primus & Kellogg, 1989) and reflected high rates of digital communication (Wang, Iannotti, & Nansel, 2009). Such a shift in investment likely reflects a heightened motivation for peer acceptance, rendering social evaluation contexts increasingly salient to adolescents.
The present study evaluated whether adolescents instantiate unique neural response patterns to instances of social evaluation by peers that parallel phenomenological shifts in social sensitivity. We observed that behavioral shifts in adolescent social sensitivity are accompanied by nonlinear changes in MPFC response magnitude and selective MPFC-striatal connectivity, which sharply rise from late childhood into adolescence and partially subside into early adulthood.
Though the present study was optimized to detect such age differences across the whole brain (except for the occipital lobe), nonlinear age effects were highly circumscribed to the MPFC. The specificity of effects to the MPFC converges with widely supported theories of the MPFC’s key role in social cognition and emotional valuation processes (Amodio & Frith, 2006; Blakemore, 2008). These findings extend existing accounts of MPFC function to suggest that it maintains persistent representation in even the most minimal of social evaluative contexts – being looked at.
Although the MPFC is frequently conceptualized as specialized for social cognition, emerging theoretical viewpoints have noted common recruitment of the MPFC during contexts that draw on affective valuation and assessment of significance to the self (Krienen, Tu, & Buckner, 2010; Roy et al., 2012) – which are often, but not exclusively, ‘social’. Indeed, the nonlinear age differences we observed were also evident in anticipatory situations during which participants believed they were not being viewed would be imminently, indicating that explicit evaluation was not necessary to invoke adolescent self-consciousness and its neural correlates. Based on this view, we propose that MPFC activity in the present study serves to incorporate salient contextual cues (in this case, imminent or perceived social evaluation) with emotional valuation processes. Thus, emergent heightened magnitude of MPFC activity in adolescence could result in assignment of heightened emotional value and self-relevance to instances of supposed social evaluation. This conceptualization is consistent with prior findings indicating that MPFC response to positive and negative social feedback is exaggerated in individuals for whom social feedback is particularly salient, i.e., individuals with low self-esteem (Somerville, Kelley, & Heatherton, 2010).
Robust MPFC signaling paired with increasing connectivity between MPFC and striatal regions could provide a mechanism by which peer evaluation contexts come to increasingly modulate adolescent motivated behavior. Not only does social concern serve as a motivating force that drives adolescents to seek out social bonds (Steinberg & Morris, 2001), adolescents are more prone to engage in suboptimal choice behaviors when with peers (e.g., risky driving; (Gardner & Steinberg, 2005). The striatum serves a key role in incorporating motivational, control, and contextual signals to facilitate context-dependent learning and behavior (Alexander, DeLong, & Strick, 1986). Though tentative, the observed pattern of MPFC-striatal connectivity might selectively upregulate motivational signaling, effectively compelling adolescent behavior toward action or approach when being evaluated by peers. While consistent with extant models of peer influence on adolescent decision-making (Lenhart, Ling, Campbell, & Purcell, 2010; Somerville & Casey, 2010; Steinberg, 2008), the current study illuminates a key role played by the MPFC in maintaining a representation of peer evaluation and its emotional qualities, while selective connectivity with the caudate may provide a means for integrating signals relevant to social context with motivational systems that govern goal-directed behavior.
It should be noted that the age effects observed for behavioral measures (embarrassment and GSR) partially correspond with the age-related changes observed for the brain imaging measures. All measures showed a robust influx of response from childhood into adolescence, but measures diverged into young adulthood: GSR levels also showed declining magnitude into early adulthood, whereas the other measures demonstrate a partial no decline from adolescence to adulthood. Future research with a broader age range is warranted to determine whether the divergence of age patterns into early adulthood is reliable.
A second feature of the reported findings is that physiological, MPFC, and self-conscious emotion demonstrate common maximal responding during adolescence, as indicated by analyses solving for the peak age of response using fit-line equations. Future research may assess whether the particular convergence of measures during adolescence plays a functionally significant role in promoting social sensitivity. There are also subtle and intriguing differences between the peak ages for GSR (14.38 years), MPFC activity (15.25 years), and self-reported embarrassment (17.2 years). Each measure contains its own profile of measurement error, so comparing the particular timing differences between variables should be interpreted cautiously. However, these findings provoke speculation that social sensitivity resonates in physiological and neural indices at an earlier age than when these emotions are most strongly labeled as self-conscious per se. Though even young children are capable of understanding embarrassment (Seidner, Stipek, & Feshbach, 1988), the current findings suggest that the process of attributing such physiological patterns as ‘embarrassment’ might not manifest to later in adolescence, perhaps due to perspective taking skills that continue to improve throughout adolescence ((Crone & Dahl, 2012; Dumontheil, Apperly, & Blakemore, 2010)) that may scaffold simulation of negative consequences of potential social transgressions that serve as a foundation of embarrassment (Keltner & Haidt, 1999). A complementary explanation is that though social evaluations are arousing and across different stages of adolescence, they might be experienced as less specifically embarrassing in early and mid-adolescence relative to late adolescence. Comprehensive studies with broader emotion measures will be needed to address these possibilities.
In conclusion, waiting to be looked at and believing one is being looked at were sufficient to induce nonlinear changes in self-conscious emotion and related physiological indices from childhood to young adulthood. Nonlinear differences in response in the MPFC, and MPFC-striatum connectivity, parallel this behavioral shift, and are proposed to influence adolescent social sensitivity. The functional properties of the MPFC are likely to be influenced by continued structural maturation (Shaw et al., 2008) and subcortical and cortical connections (e.g., (Asato, Terwilliger, Woo, & Luna, 2010) during adolescence. That said, future work will be needed to identify biological and experiential mechanisms that give rise to the functional differences illuminated by the present study. Together with other findings, this study bridges examinations of psychosocial development and neurodevelopmental science to inform how the emergent features of the adolescent social life can exert such a powerful influence over motivation, emotion, and well-being.
Supplementary Material
Acknowledgments
We acknowledge Frederico Laurenço, Juan Molina, Joe Moran, Alea Skwara, and Lucas Wandrey for their contributions to data collection and statistical analyses, and the resources and staff at the Biomedical Imaging Core Facility of the Citigroup Biomedical Imaging Center at Weill Cornell Medical College. Supported by K99 MH078713 (LHS) and the Mortimer D. Sackler, MD family.
Contributor Information
Leah H. Somerville, Weill Cornell Medical College, Harvard University
Rebecca M. Jones, Weill Cornell Medical College
Erika J. Ruberry, Weill Cornell Medical College
Jonathan P. Dyke, Weill Cornell Medical College
Gary Glover, Stanford University.
BJ Casey, Weill Cornell Medical College.
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial prefrontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreassi JL. Psychophysiology: Human behavior and physiological response. 5. Mahway, NJ: Lawrence Erlbaum; 2006. Electrodermal activity and behavior; pp. 259–288. [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Brown B. Adolescents’ relationship with peers. In: Lerner R, Steinberg L, editors. Handbook of adolescent psychology. 2. New York: Wiley; 2004. pp. 363–394. [Google Scholar]
- Cairns RB, Leung MC, Buchanan L, Cairns BD. Friendships and social networks in childhood and adolescence: Fluidity, reliability, and interrelations. Child Development. 1995;66:1330–1345. [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Science. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Cambridge, MA: Cambridge University Press; 2001. pp. 53–84. [Google Scholar]
- Dumontheil I, Apperly IA, Blakemore SJ. Online usage of theory of mind continues to develop in late adolescence. Developmental Science. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Elkind D, Bowen R. Imaginary audience behavior in children and adolescents. Developmental Psychology. 1979;15(1):38–44. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Ruberry EJ, Libby V, Glover G, Voss HU, Casey BJ. Behavioral and neural properties of social reinforcement learning. Journal of Neuroscience. 2011;31(37):13039–13045. doi: 10.1523/JNEUROSCI.2972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D, Haidt J. Social functions of emotions at four levels of analysis. Cognition and Emotion. 1999;13:505–521. [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortext responds to close others. Journal of Neuroscience. 2010;30(41):13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart A, Ling R, Campbell SB, Purcell K. Teens and mobile phones. Pew Internet & American Life Project. 2010 http://pewinternet.org/Reports/2010/Teens-and-Mobile-Phones.aspx.
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rankin JL, Lane DJ, Gibbons FX, Gerrard M. Adolescent self-consciousness: Longitudinal age changes and gender differences in two cohorts. Journal of Research on Adolescence. 2004;14(1):1–21. [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C, Burnett S, Blakemore SJ. Development of the self-concept during adolescence. Trends in Cognitive Sciences. 2008;12(11):441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Seidner LB, Stipek DJ, Feshbach ND. A developmental analysis of elementary school children’s concepts of pride and embarrassment. Child Development. 1988;59(2):367–377. doi: 10.1111/j.1467-8624.1988.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani N, Lerch JP, Eckstrand K, Lenroot R, Gotay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:1–6. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. S0278-2626(09)00112-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20(12):3005–3013. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Wang J, Iannotti RJ, Nansel TR. School bullying among US adolescents: Physical, verbal, relational, and cyber. Journal of Adolescent Health. 2009;45(4):368–375. doi: 10.1016/j.jadohealth.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberg PM, Drewes MJ, Goedhart AW, Siebelink BM, Treffers PDA. A developmental analysis of self-reported fears in late childhood through mid-adolescence: social-evaluative fears on the rise? Journal of Child Psychology and Psychiatry. 2004;45(3):481–495. doi: 10.1111/j.1469-7610.2004.00239.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.