Abstract
Recent epidemiological evidence in children indicates that time spent outdoors is protective against myopia. Studies in animal models (chick, macaque, tree shrew) have found that light levels (similar to being in the shade outdoors) that are mildly elevated compared to indoor levels, slow form-deprivation myopia and (in chick and tree shrew) lens-induced myopia. Normal chicks raised in low light levels (50 lux) with a circadian light on/off cycle often develop spontaneous myopia. We propose a model in which the ambient illuminance levels produce a continuum of effects on normal refractive development and the response to myopiagenic stimuli such that low light levels favor myopia development and elevated levels are protective. Among possible mechanisms, elevation of retinal dopamine activity seems the most likely. Inputs from intrinsically-photosensitive retinal ganglion cells (ipRGCs) at elevated light levels may be involved, providing additional activation of retinal dopaminergic pathways.
Keywords: refractive development, myopia, illuminance, dopamine, animal models, lens-induced myopia, form-deprivation myopia
1. Introduction
Recent studies from numerous groups have reported that outdoor activity is protective against myopia development in children (Deng et al., 2010; Dirani et al., 2009; French et al., 2013; Guggenheim et al., 2012; Jones et al., 2007; Mutti et al., 2002; Rose et al., 2008a) and, in animal models of myopia, that elevated light levels slow the rate of myopia development (Ashby et al., 2009; Ashby and Schaeffel, 2010; Siegwart et al., 2012; Smith et al., 2012). These results raise the issue of the how ambient light levels may affect the emmetropization mechanism, including normal refractive development and the response to myopiagenic stimuli.
In comparison with illuminance levels outdoors, indoor lighting experienced by humans is typically less than 1,000 lux and often much less – in the range of 100 to 500 lux. This, of course, is far less than the light levels experienced outdoors during the daytime (130,000 lux and above in direct sun on a clear day, about 15,000 lux in the shade). Indeed, these are the levels that presumably were experienced by terrestrial vertebrate eyes throughout the evolution of the primate line. Most terrestrial creatures develop in a visual environment that ranges from high photopic light levels outdoors during the day to mesopic levels at dawn and dusk (or inside buildings) and scotopic levels at night unless artificial lighting is provided. Rather than considering outdoor illuminance levels to be “high” or “bright” or “elevated,” it is more appropriate to consider them as normal, and to consider “standard” indoor illuminance as low.
With the development of towns and cities, one may suppose that humans began to spend more time indoors, in lower-illuminance conditions; time spent indoors also appears to have increased with the development of indoor lighting and the development of non-agricultural indoor employment. Good visual acuity, needed for reading and other visual tasks that involve fine detail, is achieved with illuminances of approximately 100 lux – 500 lux (Norton et al., 2002). Based at least in part on the increased costs involved in providing light levels above this point, indoor lighting for humans, and the lighting provided in the vivaria housing many of the animals used in studies of refractive development, are in this same illuminance range (Feldkaemper et al., 1999; Li and Howland, 2003; Morgan et al., 2004; Norton and McBrien, 1992; Schmid and Wildsoet, 1997; Smith, III et al., 2001) and, rarely, up to 1000 lux (Bitzer et al., 2000). The emerging reports of the protective effects of outdoor activity on myopia suggest that it is important to systematically explore the effect of illuminance levels above the low photopic levels experienced indoors.
In this review we suggest, as have Cohen et al. (2011; 2012) that the effects of illuminance on the emmetropization mechanism may form a continuum from scotopic and low photopic light levels, which foster the development myopic refractive errors, to the much higher illuminance levels experienced in the outdoors that affect refractive development, keeping eyes slightly hyperopic, and reduce the impact of myopiagenic stimuli. Indeed, in a 1999 paper on the effect of light levels on form-deprivation myopia in chicks, Feldkaemper et al. (1999) concluded, “Experiments show that the eye becomes more sensitive to image degradation at low light, the human eye may also be more prone to develop myopia if the light levels are low during extended periods of near work.”
Although the amount of light reaching the retina is presumably the key factor, it is difficult to measure the μW/cm2 of the many visible wavelengths that enter through the pupil and reach the retina. For convenience, illuminance (light falling on a surface) is a more easily measured quantity, indicating the amount of visible light (lumens) reaching an area of a surface (square meters) and corrected for the spectral sensitivity of humans: the lux. Illuminance levels from the sun on a clear day are approximately 130,000 lux (Birmingham, Alabama). Higher levels have also been reported (Dharani et al., 2012). In the shade on a sunny day, lux measured at the ground is typically 15,000 to 25,000 lux. Outdoors on a cloudy day it ranges from 10,000 to 40,000 lux. By comparison, indoor illuminance (100–500 lux) is very low.
Of course, most eyes are not pointed constantly toward the sky, but are aimed roughly parallel to the ground and mostly receive light reflected from objects. Light reaching the retina in this manner is lower, sometimes considerably so. Changes in pupil diameter also can alter the retinal illuminance by over 1 log unit. That said, the illuminance in lux can serve as an indicator of the upper limit of available light. This review will examine the relatively few studies that have varied the illuminance levels above and (in animal studies) slightly below standard indoor levels. Even though these indoor illuminance levels are, in an evolutionary sense, “low”, they are the levels at which most human and animal observations have been made and therefore serve as a standard level. By comparison, outdoor illuminance levels and the levels used in a few animal studies are “elevated” and we will refer to them as such.
2. Human Studies
Normal refractive development
The effects of illuminance on human refractive development occur against a background of changing refractive state in the months and years after birth. At birth, refractive state, measured with cycloplegia, is broadly distributed, ranging from low myopia (−1 to −4 D) to high hyperopia (up to 8 D) with a mean refraction of low (2 D) to moderate (3.5 D) hyperopia (Chen et al., 2011; Cook and Glasscock, 1951). This may reflect genetic factors that determine the location of the focal plane (corneal and lens curvatures and spacing) and the axial length before there is guidance from the emmetropization mechanism (Siegwart, Jr. and Norton, 2011). Very quickly, however, the refractive distribution narrows (Mayer et al., 2001; Mutti et al., 2005; Sorsby et al., 1957; Stenstrom and trans.Woolf, 1948). Eyes that are more hyperopic at 3 months of age grow axially more than emmetropic eyes, moving the retina toward the focal plane (Mutti et al., 2005). At some point in infancy or early childhood, the majority of eyes become nearly emmetropic, typically achieving a low hyperopia that is easily cleared with accommodation (Borchert et al., 2011; Gwiazda et al., 1993a; Howland et al., 1993; Multi-ethnic Pediatric Eye Disease Study, 2010). However, the degree of low hyperopia achieved has implications for subsequent refractive development. Having less than 0.5 D at about 6 years of age is a risk factor for subsequently developing myopia (Hirsch, 1964) as is having less than 0.75 D at about 8 years of age (“third grade”) (Zadnik et al., 1999) or less than 0.75 D at 5 years of age for children with 2 myopic parents (Gwiazda et al., 2007). If, as suggested from animal studies (Section 3), exposure to outdoor light levels bias human refractive development toward remaining slightly more hyperopic, this hyperopia may provide a protective reserve against subsequent myopia development. Eyes that start to elongate and progress into myopia would start from a more hyperopic level, delaying the point at which they become myopic.
Myopia prevention
An important feature of being outdoors is that the illuminance levels are much higher than indoors. However, the number of hours spent outdoors among children and young adults seems quite variable depending on age, urban vs. rural location, ethnicity, and region of the world. Expressed as hours of outdoor activity per day (converted, in many cases, from hours per week presented in the reports) and based on responses to questionnaires, children living in Australia who are of European Caucasian ancestry have considerably more outdoor time (about 6 hours per day) and lower myopia prevalence, compared with children of East Asian ancestry (~ 4 hours per day) (French et al., 2013). Children in rural suburbs of Beijing have been reported to have just over 2 hours per day of outdoor activity whereas children in urban Beijing neighborhoods have about 1 hour per day (Guo et al., 2013). In southwest England, Guggenheim et al. (2012) considered three or more hours per day outdoors in summertime as “high.” Daily outdoor activity measures, also from questionnaires, have been reported to be as low as less than 0.5 hours per day in Taiwan (Wu et al., 2010) and in Singapore (Rose et al., 2008b).
A growing number of human epidemiological studies in many countries have reported that time spent in outdoor activities is protective against myopia. Mutti et al. (2002) initially reported that myopia prevalence was inversely related to time spent participating in sports. Several subsequent studies also have shown that outdoor activity is inversely related to the development of myopia (Dirani et al., 2009; Guo et al., 2013; Jones et al., 2007; Jones-Jordan et al., 2011; Lee et al., 2013; Onal et al., 2007; Rose et al., 2008a; Sherwin et al., 2012b; Wu et al., 2010). Some studies have reported that it is the time outdoors, independent of physical activity, that is the important variable (Guggenheim et al., 2012; Rose et al., 2008a). Jones et al. (2007) found that children who spend more than 15 hours per week (2.1 hours per day) outdoors have only one-third the risk of becoming myopic as do children who spend less than 5 hours per week (0.7 hours per day) outdoors. In a review, Sherwin et al. (2012a) performed a meta-analysis on seven papers published between 2002 and 2010. They concluded that there was consistent evidence for a small reduction in the risk for being myopic related to the amount of time spent outside, such that each additional hour spent outside per week reduced the odds of being myopic by 2%. However, several studies have been published since then and, in agreement with Jones et al. (2007), suggest that the benefit of additional hours of daily outdoor exposure may be greater (French et al., 2013; Guggenheim et al., 2012). A separate issue is whether time spent outdoors also produces a small hyperopic shift in the refractions of emmetropic children (Rose et al., 2008a).
Myopia progression
In addition to being protective against becoming myopic, there is also evidence that outdoor activity may slow the progression of myopia in children who are already myopic, although some studies have not found a relationship (Jones-Jordan et al., 2012; Saw et al., 2000). Parssinen & Lyyra (1993) found that myopia progression was reduced in boys, but not girls, as the number of daily hours spent in outdoor activities increased. In a small intervention study in Hunan province, China (41 in intervention group, 39 in control group) myopia progression was compared over a 2-year period in myopic children aged 7 – 11 (Yi and Li, 2011). Based on questionnaires, the intervention group spent more time in outdoor activities (2.0 ± 0.34 hours per day) than the control group (0.9 ± 0.23 hours per day), less time in “middle vision” activities, and similar amounts of time in near-vision activities. Myopia progression in the intervention group (0.38 ± 0.15 D) was slowed significantly compared to progression in the control group (0.52 ± 0.19 D).
A larger study in Taiwan (Wu et al., 2013) compared refractive changes in children (7–11 years at baseline) at two suburban schools. At the intervention school, during recess periods totaling 1.3 hours per day, the classrooms were emptied and children were encouraged to go outdoors. Children at the control school had the same recess period durations, but did not have any special programs during recess. Both groups also had about 0.5 hours per day of outdoor physical education. Based on answers to a questionnaire, it appeared that children in both groups had an additional 1.3+ hours per day of outdoor activity. After 1 year, the number of children who became myopic was lower (8.4%) in the intervention group than in the control group (17.6%). The progression in already-myopic children who were not receiving atropine therapy (−0.28 ± 0.57 D) was not significantly slower than the progression in the control group (−0.37 ± 0.67 D).
Interestingly, Gwiazda et al. (2012) found seasonal differences in myopia progression in myopic children enrolled in the COMET study. The amount of progression was significantly greater in winter than in summer. They suggested that this might be due to more near work in the winter months and/or more outdoor activity in the summer. A similar result was reported by Fulk et al. (2000).
Taken together, it appears that exposure to outdoor activity is protective against myopia development and, in some studies, against myopia progression. Are the effects of outdoor activity on the development of myopia and on myopia progression due to the higher illuminance? The studies in animals reviewed in the next section suggest that exposure to elevated illuminance, by itself, is sufficient to affect normal refractive development and the progression of induced myopia.
3. Animal Studies
Over the past 35 years there has been extensive characterization of the emmetropization mechanism in animal models, examining normal refractive development and induced myopia produced with form deprivation or negative lens-wear. However, the majority of these studies have used “standard” colony lighting that, from the present perspective, is quite low, in the range of 100 – 500 lux. Currently, we have only a very limited understanding of ambient illuminance as a variable that may affect the performance of the emmetropization mechanism over a wide range of light levels from low photopic (1 lux) to moderately “high” light levels (15,000 lux) and none at full outdoor illuminance levels.
Refractive development
“Standard” colony lighting
The animal species that are most frequently used in studies of refractive development and induced myopia are diurnal: chick (Wallman et al., 1978; Wallman and Adams, 1987), monkey (rhesus macaque and marmoset) (Raviola and Wiesel, 1990; Smith, III et al., 1999; Troilo and Judge, 1993), tree shrew (Norton et al., 2010; Sherman et al., 1977), and guinea pig (Howlett and McFadden, 2006; Howlett and McFadden, 2007; Howlett and McFadden, 2009). Normal refractive development has been observed in light levels that have ranged upwards from 60 lux (Ehrlich et al., 1990) to 700 lux (Benavente-Perez et al., 2012; Li et al., 2000) but more typically have been in the 250 to 500 lux range (Ashby et al., 2010; Backhouse and Phillips, 2010; Callahan and Petry, 2000; Crewther and Crewther, 2002; Dong et al., 2011; Feldkaemper et al., 1999; Iuvone et al., 1978; Leech et al., 1995; McCarthy et al., 2007; Metlapally and McBrien, 2008; Smith et al., 2012; Vessey et al., 2005). The lights typically are on for 12 or 14 hours per day and off the remaining time. The illuminance levels during the light-off period, when specified, have been below 1 lux (McCarthy et al., 2007). Many studies have found that maintenance of normal circadian light/dark cycles is critically important (for a recent review, see (Stone et al., 2013)).
Refractive development in these baseline “standard” lighting conditions follows a similar pattern in all of these species; eyes initially are hyperopic when first exposed to visual images, which occurs at birth (monkeys, guinea pigs), hatching (chicks), or at eye opening (about 3 weeks postnatal in tree shrews) (Norton and McBrien, 1992). As in human infants, the range of refractive states at the onset of the emmetropization process is larger than is the case later in development (Bradley et al., 1999; Howlett and McFadden, 2007; Norton et al., 2006; Norton and McBrien, 1992; Pickett-Seltner et al., 1988; Troilo and Judge, 1993; Wallman et al., 1981). Active emmetropization occurs in weeks (chicks, guinea pigs, tree shrews) (Howlett and McFadden, 2007; Norton et al., 2010; Norton and McBrien, 1992; Pickett-Seltner et al., 1988; Wallman et al., 1981), or months (monkeys)(Bradley et al., 1999; Troilo and Judge, 1993); the eyes approach emmetropia, rapidly at first and then more slowly, but typically remain slightly hyperopic.
Effect of low light levels
There has been limited work exposing animals to low light levels (around 1 lux) on a normal lights on/off schedule that would be expected to maintain circadian rhythms (Meijer et al., 1990). Normal circadian patterns are extremely important; continuous “standard” illuminance produces corneal flattening in chicks that, despite axial elongation, causes the eyes to become hyperopic (Lauber, 1987; Li et al., 1995; Li et al., 2000). Continuous dim (10 lux) illuminance also produces eye enlargement (Lauber et al., 1961). When chicks are kept on light cycles of 12 h ON and 12 h OFF, Feldkaemper et al. (1999), using neutral density filters, found that low light levels (calculated at 0.5 lux) were myopiagenic. Cohen et al. (2011) followed the refractive development of chicks for 90 days in 3 levels of ambient illuminance: 50, 500, and 10,000 lux. After emmetropizing from hyperopia, the refractions of many of the chicks in the 50 lux level continued to decline until, by 90 days, the eyes were myopic; the animals in the higher illuminance conditions remained emmetropic. Importantly, these animals did not develop flattened corneas. It thus appears that low photopic light levels (1 – 50 lux) may affect normal refractive development such that eyes become less hyperopic than normal (as judged from colony rearing), and refractions can actually become myopic.
Darkness
In tree shrews that have emmetropized in standard colony lighting (100 – 300 lux at the cage floor) with a 14:10 light ON:OFF schedule, treatment with an 11-day period of complete darkness produced axial elongation and myopia (Norton et al., 2006). Corneal power was unaffected. It appears that darkness, after exposure to circadian light/dark cycles, produces increased axial elongation.
In chicks, rearing from near hatching in the dark produces elongated, hyperopic eyes (Gottlieb et al., 1987; Troilo and Wallman, 1991). The increased axial length is due to vitreous chamber elongation; the hyperopia occurs because there is substantial flattening of the cornea. However, it appears that the initial response is axial elongation and myopia (Gottlieb et al., 1987; Troilo and Wallman, 1991). This changes to hyperopia over time due to corneal flattening despite continued axial elongation. Monkeys (Guyton et al., 1989; Raviola and Wiesel, 1978) and tree shrews (McKanna et al., 1983) raised in complete darkness and that have not been allowed to at least partially emmetropize in light/dark conditions do not appear to develop myopia, perhaps because the emmetropization and/or circadian mechanisms have not been activated. It may be that visual experience in cyclic day/night conditions produces retinal maturation and activates the retinal “go” and “stop” signals (Rohrer and Stell, 1994) of the emmetropization mechanism. Subsequent continuous darkness may be myopiagenic because it disrupts the circadian rhythms and either produces “go” signals or removes “stop” signals. Taken together, the low light and dark-treatment experiments in chicks and tree shrews suggest that very low (less than 1 lux) light levels favor axial elongation that, if there are no corneal changes, results in myopia.
Effect of “elevated” light levels
The limited number of studies that have examined refractive development in light levels above 1000 lux have found that “elevated” light levels (compared with standard colony lighting) slow the normal decrease in hyperopia so that normal or control eyes are more hyperopic than they would have been if exposed to standard colony lighting. The normal chicks that Cohen et al. (2011) raised in “high intensity” (10,000 lux) light became over 1 D hyperopic, compared with chicks raised in 500 lux, starting by 30 days of exposure and persisting through the end of treatment after 90 days. The refractive difference occurred because the refractions of the “high intensity” animals stabilized at a hyperopic level while the refractions of the 500 lux animals continued to decline toward emmetropia.
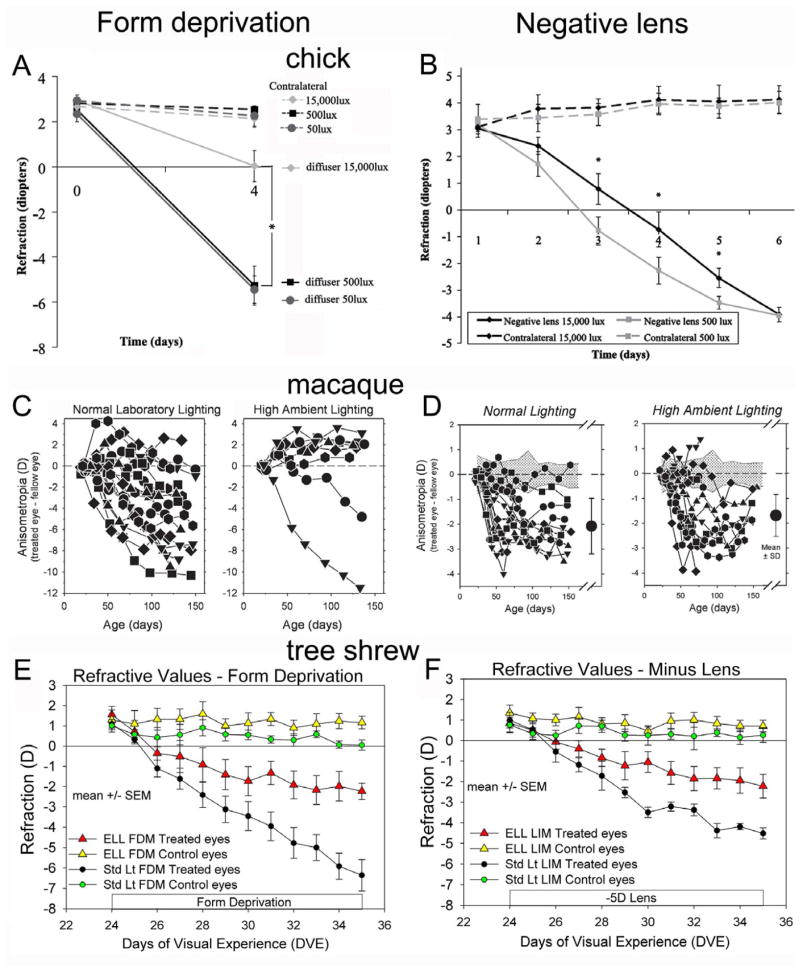
Normal eyes of juvenile tree shrews, and untreated control eyes of monocularly form-deprived or negative-lens treated animals (Fig. 1E & F) that were exposed for approximately 7.5 hours per day to “elevated” light levels of nearly 15,000 lux became hyperopic, compared to normal and control eyes exposed to standard colony lighting (100 – 300 lux) (Siegwart et al., 2012). Control eyes of a monocular form-deprived group of tree shrews that were exposed to the elevated light levels for 11 days were hyperopic compared to standard light control eyes (1.2 ± 0.3 D vs. 0.1 ± 0.3 D). These control eyes remained hyperopic when the animals were returned to standard colony lighting for 21 additional days. Control eyes of monocularly form-deprived rhesus monkeys exposed to 25,000 lux for 6 hours per day for many weeks became hyperopic compared to the control eyes of animals exposed to standard colony lighting (15 – 630 lux) (Smith et al., 2012). A hyperopic shift was not found in the control eye of newly hatched chicks treated for 5 days with 15,000 lux, 6 hours per day (Fig. 1A & B) (Ashby et al., 2009; Ashby and Schaeffel, 2010), suggesting that longer treatment periods and/or older animals may be needed for elevated light levels to affect normal refractive development.
Fig. 1.
Effects of “elevated” light levels (“high ambient lighting”) on the response to form deprivation and negative (minus) lens wear in chick (Ashby et al., 2009; Ashby and Schaeffel, 2010), macaque monkey (Smith et al., 2012; Smith et al., 2013), and tree shrew (Siegwart et al., 2012). A, C and E: In all three species, “elevated” light levels (ELL) slowed the rate of myopia development in response to form deprivation when compared with standard (200 – 600 lux) colony lighting. B and F: In chicks and tree shrews ELL slowed the initial rate of negative lens-induced myopia. In macaque monkeys (D), no effect from elevated light levels was found. Chick: A. refractive values for the treated and the control eyes exposed to 50 lux, 500 lux or 15,000 lux for 6 h per day. B. exposure to 500 or 15,000 lux for 5 hours per day; Macaque: the refractive difference (treated – control eyes) of animals exposed colony lighting or to to 18 – 28,000 lux from metal halide lamps (filtered to remove wavelengths below 360 nm) for 6 h per day. Tree shrew: treated and control eye refractive values comparing animals exposed to “standard” colony lighting (100 – 300 lux) or elevated light levels (ELL): 15,000 lux from compact fluorescent lamps for 7.75 h per day. In tree shrews, the untreated control eyes in both ELL groups were hyperopic compared with control eyes in standard colony lighting. A, B, C, and D, copyright Association for Research in Vision and Ophthalmology. Reproduced with permission.
Although the number of studies and the number of animals studied is small, it appears in three species that mildly elevated light levels, comparable to those experienced in the shade outside on a sunny day, slow the gradual decrease in hyperopia so that eyes remain slightly (approximately 1 D) more hyperopic than in standard indoor lighting. If outdoor activity in children has a similar effect, it would, on average, slightly increase the modest hyperopia that normally exists. To the extent that myopiagenic environmental stimuli (such as blur occurring during near work) may produce myopia, these eyes would begin myopia progression from a more hyperopic refractive state, which might reduce their final myopic refractions.
Development of induced myopia
“Standard” colony lighting conditions
Until recently, most studies of axial elongation and myopia produced with either form deprivation or with negative lenses occurred in the same indoor lighting conditions used to examine “normal” refractive development. These have provided the baseline of information about the functioning of the emmetropization mechanism. Monocular form deprivation is typically produced by holding a translucent diffuser (resembling the material of a Ping-Pong ball or “frosted” light bulb) in front of the eye with a mask (Hung et al., 1995; Lu et al., 2006; Schaeffel et al., 1988), a Velcro ring around the eye (Kee et al., 2001), or a goggle frame attached to a pedestal permanently installed on the top of the head (Howlett and McFadden, 2006; Siegwart and Norton, 1994). This reduces image contrast, especially for high spatial frequencies and prevents focused images from forming on the retina. The result is axial elongation, primarily enlargement of the vitreous chamber that moves the retina behind the focal plane, producing an induced myopia (form-deprivation myopia). The amount of myopia typically increases with the duration of the form deprivation and substantial amounts of myopia (more than 10 D) can result (McBrien and Norton, 1992; Smith et al., 1987; Wallman and Adams, 1987).
If a negative-power lens is used instead of form deprivation, the lens shifts the focal plane away from the cornea, creating refractive hyperopia or, if the animals are young and have not reached emmetropia, increasing the amount of hyperopia. The result is an increase in the rate of axial elongation that moves the retina away from the cornea (Hung et al., 1995; Schaeffel et al., 1988; Shaikh et al., 1999). This reduces the refractive hyperopia to the point that the lens-wearing eye’s refraction returns to an age-appropriate level of hyperopia. If treatment is monocular, the treated eye’s refractive state matches that of the untreated fellow control eye (the treated eye has “compensated” for the lens); if the lens is removed, the eye is myopic (lens-induced myopia, LIM). The rate of this refractive compensation varies across species as does the normal emmetropization process. As shown in Fig. 1A & B, it is quite rapid in chicks, a bit slower in tree shrews (Fig. 1E & F) and considerably slower in monkeys (Fig. 1C & D). In both form deprivation myopia (FDM) and negative lens-induced myopia (LIM), there are minimal changes in corneal shape or in the power of the crystalline lens (Pickett-Seltner et al., 1987; Qiao-Grider et al., 2010; Schaeffel et al., 1988; Siegwart, Jr. and Norton, 1998).
A number of studies that have compared form deprivation and negative lens treatments have suggested that the underlying mechanisms (presumably in the retina) are not identical (Choh et al., 2006; Kee et al., 2001; Nickla and Totonelly, 2011). One important difference between these treatments is that a negative lens, worn continuously, is an extremely powerful stimulus that guides the eye to with-the-lens emmetropia. In contrast, form deprivation does not offer a target so there is no guidance toward a target; it removes the possibility of achieving with-the-lens emmetropia. However, changes in the sclera produced by both treatments are virtually identical: increased growth in the inner cartilaginous layer in chick (Rada and Matthews, 1994), remodeling and, in mammals, loss of extracellular matrix (Gentle et al., 2003; Guo et al., 2011; Norton and Rada, 1995), and increased viscoelasticity (Siegwart, Jr. and Norton, 1999).
Elevated light levels
Just as exposure to “elevated” light levels slows the gradual decrease in hyperopia in control and normal eyes, it appears to also reduce the rate at which myopiagenic stimuli produce axial elongation and, hence, refractive myopia. Reports are available in three species, chick, macaque monkey, and tree shrew (Fig. 1).
With regard to form deprivation, periods of 5 – 7.5 hours of elevated light levels (15,000 to 28,000 lux) have been found to reduce the amount of myopia produced over a fixed period of time compared with form deprivation myopia produced in standard colony lighting in chicks (Fig. 1A) (Ashby et al., 2009), macaque monkeys (Fig. 1C) (Smith et al., 2012) and tree shrews (Fig. 1E) (Siegwart et al., 2012). In tree shrews, we found that light levels of 15,000 lux presented for 7.5 hours per day (the remaining daytime period in standard colony lighting [100 – 300 lux]) with a 10-hour lights-OFF period, produced a nearly 40% slowing of the rate of myopia development in response to form deprivation during an 11 day treatment period (Fig 1E).
When combined with negative lens wear, elevated light levels also slowed the rate at which the eyes compensated for the lens in chicks (Fig. 1B) (Ashby and Schaeffel, 2010) and tree shrews (Fig. 1F) (Siegwart et al., 2012). The rate of myopia development was reduced, but the eyes eventually compensated fully for the lenses (tree shrew data not shown). In tree shrews the average time to full compensation was nearly doubled (17.5 days vs. 9 days in standard lighting). In infant macaque monkeys, similarly elevated light levels did not slow the response to negative lenses (Fig. 1D)(Smith et al., 2013).
The eventual full compensation to negative lenses in “elevated” light conditions, coupled with the absence of slowing in rhesus monkeys, has raised questions about whether elevated light levels exposure will be useful as a treatment for slowing myopia progression in children. We suggest that these questions ignore important differences between continuous negative lens wear and the environmental visual stimuli that appear to contribute to axial elongation and myopia in children (Gwiazda et al., 1993b; Gwiazda et al., 2003; Smith III, 2013). Further, we suggest that it is the slowing of the rate of response to negative lenses that holds promise for future treatments.
A negative lens produces refractive hyperopia and continuous negative lens wear is a test applied to a normal emmetropization mechanism in animals. There is a refractive target, defined by the power of the lens, and one would not expect “elevated” light levels, similar to outdoor light levels, to significantly alter the endpoint. In contrast, if hyperopic defocus is the environmental cue that produces elongation in children, there is no refractive target. If the hyperopia is caused by under-accommodation (Gwiazda et al., 1993b), the under-accommodation continues as the eye elongates, so that defocus continues to occur rather than decreasing as with compensation for a negative lens. Similarly, if the elongation and myopia progression is caused by hyperopic defocus in the peripheral visual field (Hoogerheide et al., 1971), continued elongation of the eye causes it to become more prolate in shape, which should actually increase the peripheral hyperopic defocus. In both situations, the final amount of myopia is determined by the rate of myopia development, over the years that myopia progresses, not by a refractive target, which with negative lens wear is the power of the lens.
It is for this reason that the clinical studies that have used optical or pharmacological treatments to slow myopia progression in children (Bedrossian, 1979; Chua et al., 2006; Gwiazda et al., 2003; Shih et al., 2001; Siatkowski et al., 2004; Smith III, 2013) have measured the success of the treatments by the decrease in the rate of myopia progression. The reasoning is that if the treatment is continued throughout the years until myopia progression ceases, the final amount of myopia will be reduced. That the rate of progression also is slowed in two species in the case of negative lens wear and in all three species in response to form deprivation suggests that elevated light levels produce a slowing effect on the response to myopiagenic stimuli. If a safe regime of elevated light levels were devised that reduced the rate of myopia progression in children by a similar amount and applied throughout the period of myopia progression, the final amount of myopia might be substantially reduced.
4. Possible Mechanisms
Blur, and/or vitamin D levels
Several potential mechanisms have been suggested to explain the protective effects of outdoor activity against myopia in children. To the extent that hyperopic defocus on the retina from near targets contributes to axial elongation and myopia development in children, being outside with few nearby objects could remove that stimulus. In addition, the pupils would be expected to be smaller in the higher outdoor light levels, increasing the depth of focus and further reducing blur. These factors may contribute to the protective effect in children. However, in cage-housed animals with ample nearby stimuli, elevated light levels produce a hyperopic shift in normal and control eyes (Siegwart et al., 2012; Smith et al., 2012) and slow the response to negative-lens wear (Ashby and Schaeffel, 2010; Siegwart et al., 2012). Thus the elevated light effect can occur despite the presence of nearby objects.
The possible role of reduced blur outdoors in slowing myopia progression has not been assessed in human studies. However, the consistent slowing of myopia development in form deprived animals, in which reduced pupil size cannot reduce the amount form deprivation, suggests that this is not a necessary component. In addition, elevated light levels in negative-lens treated animals do not prevent the eyes from eventually fully compensating for the lenses, suggesting that the reduced pupil size does not dramatically affect the amount of blur; rather elevated light levels slow the rate of response to the blur in tree shrews and chicks.
Could vitamin D be involved? Sunlight produces increased blood levels of vitamin D. Mutti et al. (2011) found polymorphisms within the vitamin D receptor (VDR) that appeared to be associated with low to moderate amounts of myopia in Caucasian subjects, leading to the possibility that elevation of vitamin D levels might be protective against myopia development. However, in a small sample of children, vitamin D levels did not appear to be associated with the amount of myopia (Mutti and Marks, 2011). In a study of tree shrews, oral vitamin D3 supplementation that dramatically raised blood serum levels for vitamin D did not affect the refractions of the control eyes and did not affect the development of myopia induced with either a negative lens or form deprivation (Siegwart et al., 2011). Taken together, studies thus far suggest that the roles of fewer nearby objects, smaller pupils, and vitamin D levels may be minimal.
Retinal dopamine activity
The release of dopamine from dopaminergic retinal neurons (i.e., activation of dopaminergic pathways) has long been known to be stimulated by light (Witkovsky, 2004). Dopamine activity (synthesis, turnover, and release) is higher during the day and lower at night on a circadian rhythm (Brainard and Morgan, 1987; Doyle et al., 2002; Iuvone et al., 1978). In addition, dopaminergic activation has been implicated in the control of myopia development in animals (Iuvone et al., 1991; McCarthy et al., 2007; Rohrer et al., 1993; Schaeffel et al., 1995; Stone et al., 1989; Stone et al., 1990). Thus, as reviewed recently, retinal dopaminergic activation seems very likely to play a role in the protective effects of outdoor activities in children and the effects of elevated light levels in the animal studies (Stone et al., 2013).
Until recently, studies of the role of dopamine have mostly focused on 1) the mechanisms that alter retinal sensitivity and function involved with switching between scotopic (rod mediated) visual function and photopic (cone-dominated) visual function and 2) the mechanisms involved in establishing and maintaining circadian rhythms of sleep vs. wakefulness. These studies have provided important information, particularly about the role of dopamine and melatonin in modulating retinal function in the transition between states. Rarely, however, have studies been concerned with visual function from low to high photopic (outside) conditions and/or with considerations of seasonal changes in the length of the day (the photoperiod).
It is well established that light triggers dopamine synthesis and release by dopaminergic amacrine cells (some in the inner nuclear layer of the retina, some with interplexiform processes); activity of the retinal dopaminergic system is higher in the day (Witkovsky, 2004). However, there have not been systematic studies of retinal dopamine levels and levels of dopamine metabolites in animals exposed to illuminance levels ranging from low photopic to the “elevated” levels that have been shown to slow myopia development. One study found that light-triggered dopamine release plateaus at around 100 lux (Brainard and Morgan, 1987). In preliminary studies, we have found that mid-day retinal levels of the dopamine metabolite dihydroxyphenylacetic acid (DOPAC), generally accepted as an indicator of dopamine release, were 30% higher in our “elevated” light-level condition (15,000 lux 7.75 hours per day, n=4) than in standard colony lighting (100 – 300 lux, n=4). Thus, light levels above those examined previously may produce further elevations in retinal dopaminergic activity.
A role for the retinal dopaminergic system in the control of eye growth has been explored in numerous studies (Iuvone et al., 1991; McCarthy et al., 2007; Rohrer et al., 1993; Schaeffel et al., 1995; Stone et al., 1989; Stone et al., 1990). DOPAC levels are reduced in chick and monkey eyes developing form-deprivation and negative-lens-induced myopia (Iuvone et al., 1989; Schaeffel et al., 1994). In addition, apomorphine, a non-specific dopamine agonist, reduced the amount of induced myopia in both chicks and macaque monkeys (Iuvone et al., 1992; Nickla et al., 2010; Rohrer et al., 1993; Schmid and Wildsoet, 2004; Stone et al., 1989). Interestingly, chicks raised in low light that became myopic also showed lower dopamine release (Cohen et al., 2012). Studies using selective dopamine agonists and antagonists, overwhelmingly in chicks, have implicated the dopamine D2 receptor system as playing a critical role. D2 receptor activation has been found to slow axial elongation and induced myopia development (Ashby and Schaeffel, 2010; McCarthy et al., 2007; Nickla et al., 2010; Rohrer et al., 1993). The elevated light level effect (slowed response to negative lenses) is mediated by the D2 receptor pathway in chicks; intravitreal administration of the D2 antagonist, spiperone, in ascorbic acid vehicle blocked the protective effect of ELL (Ashby and Schaeffel, 2010). However, in a line of guinea pigs that develops spontaneous myopia, Jiang et al. (2012) found that activation of the D1 receptor pathway appears to reduce axial elongation and myopia. They also found that D2 agonists increased axial elongation and myopia. Although we would like to think that all vertebrates use similar mechanisms to achieve something as fundamental as emmetropia, it may be that other species differences, such as the inner layer of cartilage in the sclera of birds vs. the all-fibrous sclera of eutherian mammals, may have dictated differing retinal mechanisms.
An additional factor may participate in activation of retinal dopaminergic pathways as illuminance levels rise, activation of intrinsically photoresponsive retinal ganglion cells (ipRGCs). These recently discovered neurons contain melanopsin and directly respond to light (peak response near 490 nm), become active in photopic illuminance levels, and demonstrate relatively little fatigue (Gamlin et al., 2007). ipRGCs have been found to synapse on dopaminergic amacrine cells (Zhang et al., 2008). Although this may not be as strong an input as that provided through the traditional photoreceptor/bipolar cell pathway (Cameron et al., 2009) it does provide a way, in addition to that pathway, for increasing light levels to produce activation of the retinal dopaminergic pathways.
5. Illuminance as a Continuous Variable
The research reviewed in the previous sections leads us to suggest that ambient light levels act as a continuous variable that, as light levels rise through the photopic range, has an increasing impact on the emmetropization mechanism (Fig. 2). The effect of rising illuminance is to shift the endpoint of normal refractive development towards hyperopia and to slow the response to myopiagenic stimuli. In this model it is assumed that illuminance varies on a circadian cycle with a period of low light (night) and a period of high light (day), which is known to be important for normal emmetropization (Lauber et al., 1961; Lauber, 1991; Li and Howland, 2003; Stone et al., 2013).
Fig. 2.
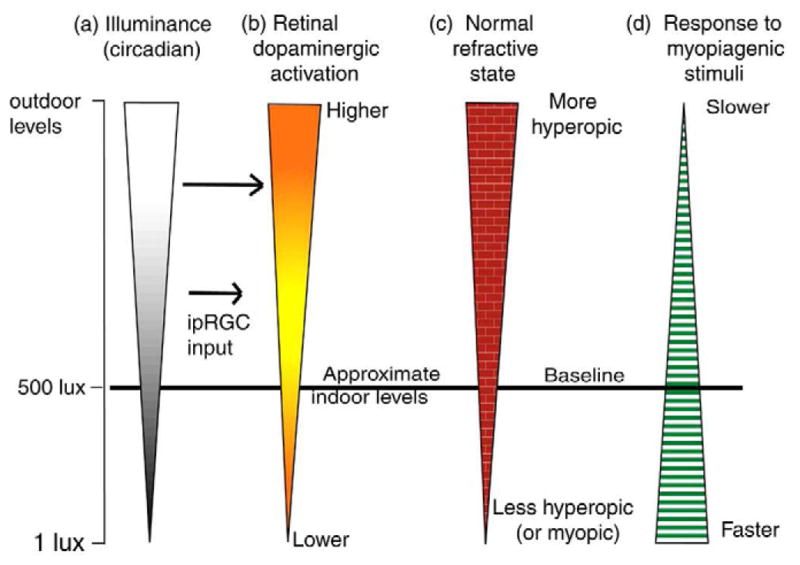
Schematic illustration of our hypothesis that ambient light levels produce a continuum of effects on normal refractive development and the response to myopiagenic stimuli. Left vertical panel (a): light levels rising from low (1 lux) through standard indoor illuminance levels (500 lux) to “high” (outdoor levels). The wider bar indicates higher illuminance. Most animal studies have examined animals at around 500 lux, providing baseline information about refractive development and responses to myopiagenic stimuli. Second panel (b): low (below standard indoor levels) daytime illuminance levels produce low activity of retinal dopaminergic amacrine cells through inputs from the photoreceptor/bipolar cell pathway. As illuminance rises, ipRGC input and traditional inputs raise the retinal dopaminergic activity levels in a graded manner. Third panel (c) suggests the effect of illuminance levels (via dopaminergic activation) on normal refractive development. At low light levels, the refractive endpoint of normal development is lower hyperopia or myopia. Increasing levels to and above baseline alter the endpoint toward more hyperopia. The fourth panel (d) suggests that the rate of response of the emmetropization mechanism to myopiagenic stimuli (form deprivation, hyperopic defocus) is inversely related to illuminance levels; high illuminance levels slow the rate of myopia development and low levels raise the response rate.
The relevant range of illuminance values that affect refractive development and the response to myopiagenic stimuli is not yet known. We, somewhat arbitrarily, place the low end at 1 lux (Fig. 2(a)), in the human mesopic range, because it is known that normal circadian sleep-wakefulness cycles are maintained in tree shrews at 1 lux (Meijer et al., 1990). This is somewhat lower than the light level (50 lux) at which Cohen et al. (2011) found many normal chick eyes gradually became myopic. It is over an order of magnitude lower than most lighting indoors and in most vivaria. Thus, studies conducted at an illuminance level of 500 lux (approximately) in Fig. 2 (baseline) have provided most of the human and animal data on refractive development and response to myopiagenic stimuli reviewed in the previous sections. At this baseline illuminance level, we suggest that retinal dopaminergic activity (Fig. 2(a)), though higher during the light cycle than in the dark (Brainard and Morgan, 1987), is relatively low. Although ipRGCs directly respond to light at this level, the strength of their effect on dopaminergic amacrine (interplexiform) cells is small (Cameron et al., 2009); input to dopaminergic neurons arrives via inputs from on-bipolar cells driven by photoreceptors (Hokoc and Mariani, 1987; Yazulla and Zucker, 1988) even though the dendrites of the ipRGCs extend into the off sublamina of the inner plexiform layer (Dumitrescu et al., 2009). This is consistent with findings in nob1 mice, which have an ON pathway defect, have low retinal dopamine and DOPAC levels in both light and darkness, and are more susceptible to form-deprivation myopia (Pardue et al., 2008).
When illuminance decreases below baseline, toward 1 lux, retinal dopaminergic activation from photoreceptors will be lower and input from ipRGCs may be decreased or absent. We suggest that the effect of reduced dopaminergic activation may affect normal refractive development (Fig. 2(c)) so that the endpoint may not be the small hyperopia found in most children and animals in baseline lighting conditions, but often will be lower so that normal eyes become less hyperopic and perhaps even myopic (Cohen et al., 2011; Cohen et al., 2012). Also, we suggest the lowered retinal dopaminergic activation produces an increased rate of response (Fig. 2. (d)) to myopiagenic stimuli (form deprivation; hyperopic defocus); the rate of myopia development is faster in animals and in children in keeping with the results seen in nob1 mice (Pardue et al., 2008).
As illuminance increases above standard baseline indoor levels toward outdoor levels (Fig. 2(a)), activation of the retinal dopaminergic system increases (Fig. 2(b) ), both from “traditional” photoreceptor/bipolar-cell connections (Cameron et al., 2009) and, increasingly, from slowly-adapting ipRGCs (Gamlin et al., 2007; Morgan and Boelen, 1996; Zhang et al., 2008). This affects the response of the emmetropization mechanism, altering the course of normal refractive development (Fig. 2(c)) so that the refractive endpoint of normal eyes is increasingly hyperopic. This is not to suggest a dramatic hyperopic shift, but one in the range of 1 – 2 D above emmetropia. This process also slows the response (Fig. 2(d) ), in animals, to form deprivation and negative lens wear; in children we suggest that it also slows the response to environmental myopiagenic hyperopic defocus. The upper illuminance point in our model, “outdoor levels,” is purposely vague because there are not yet studies that have systematically examined the impact of illuminance levels above 15,000 – 25,000 lux. It is possible that the emmetropization mechanism may not need additional light levels to optimize its performance, an upper limit may occur so that illuminance levels above 15,000 lux (shade on a sunny day) may be as effective as 130,000 lux (direct sunlight).
This model is novel primarily in that it extends upward the range of illuminance levels over which increased illuminance produces increased retinal dopaminergic activation beyond the apparent ceiling encountered by Brainard and Morgan (1987). It also includes a potential role for ipRGCs. Missing from the model is any suggestion of the specific mechanism by which retinal dopaminergic activation produces its effects on normal refractive development and the rate of response to myopiagenic stimuli. The emmetropization mechanism normally operates as a feedback system in which refractive error either increases or decreases the axial elongation rate of the eye. Refractive hyperopia acts to increase the elongation rate and refractive myopia acts to slow it. The rate at which this occurs is set by the gain of the feedback loop, which can vary across eyes. This is reflected in the differing rates of myopia development seen with form deprivation, which creates an “open loop” condition in which the mechanism operates without visual feedback. Given that elevated light levels consistently slow the rate of axial elongation and myopia in form deprived animals, it appears that a primary impact of elevated illuminance is on the gain of this system. The complete compensation to negative lenses suggests that the “set point” is not strongly affected. To the extent that an increased rate of axial elongation from myopiagenic conditions can be thought of as reflecting a retinally-generated “go” signal, the effect of high illuminance could be characterized as reducing the strength of the “go” signal.
6. Concluding Comments
In this review, we have tried to integrate information from human epidemiological studies, from investigations using animals models of refractive development and myopia, and from studies of retinal circuitry to suggest ways in which illuminance levels may impact normal refractive development and the response to environmental myopiagenic stimuli. We recognize that the resulting model is incomplete and, no doubt, contains some incorrect conclusions. The intersection of illuminance levels, circadian pathways, and refractive development appears to be a promising area for research. We propose our model not as a complete story, but as a way, we hope, to help stimulate and structure future investigations both in children and in animals.
Highlights.
Reviews the effects of outdoor light levels on human refractive development and myopia
Reviews the effects of low and high light levels on animal models of refractive development and induced myopia
Examines possible mechanisms for the effect of elevated illuminance
Proposes a model of how elevated illuminance may act through retinal dopaminergic pathways to promote hyperopia and slow the rate of myopia development
Acknowledgments
Supported by NIH grants R01 EY005922 and P30 EY003039 (Core). We thank Alexander H. Ward for participation in the tree shrew elevated light level study and in preliminary dopamine studies and Dr. Michael R. Frost for helpful comments on the manuscript and assistance in figure preparation. We also thank two anonymous reviewers for their insightful and helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby R, Kozulin P, Megaw PL, Morgan IG. Alterations in ZENK and glucagon RNA transcript expression during increased ocular growth in chickens. Mol Vis. 2010;16:639–49. [PMC free article] [PubMed] [Google Scholar]
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–54. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–53. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Backhouse S, Phillips JR. Effect of Induced Myopia on Scleral Myofibroblasts and In Vivo Ocular Biomechanical Compliance in the Guinea Pig. Investigative Ophthalmology & Visual Science. 2010;51:6162–71. doi: 10.1167/iovs.10-5387. [DOI] [PubMed] [Google Scholar]
- Bedrossian RH. The effect of atropine on myopia. Ophthalmol Rochester. 1979;86:713–9. doi: 10.1016/s0161-6420(79)35455-0. [DOI] [PubMed] [Google Scholar]
- Benavente-Perez A, Nour A, Troilo D. The Effect of Simultaneous Negative and Positive Defocus on Eye Growth and Development of Refractive State in Marmosets. Investigative Ophthalmology & Visual Science. 2012;53:6479–87. doi: 10.1167/iovs.12-9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitzer M, Feldkaemper M, Schaeffel F. Visually induced changes in components of the retinoic acid system in fundal layers of the chick. Exp Eye Res. 2000;70:97–106. doi: 10.1006/exer.1999.0762. [DOI] [PubMed] [Google Scholar]
- Borchert MS, Varma R, Tarczy-Hornoch K, Cotter S, Mckean-Cowdin R, Azen S, Tielsch JM, Friedman DS, Repka MX, Katz J. Risk factors for hyperopia and myopia in preschool children: The Multi-Ethnic Pediatric Eye Disease Study and the Baltimore Pediatric Eye Disease Study. ARVO Meeting Abstracts. 2011;52:2507. [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG. Emmetropization in the rhesus monkey (Macaca mulatta): birth to young adulthood. Invest Ophthalmol Vis Sci. 1999;40:214–29. [PubMed] [Google Scholar]
- Brainard GC, Morgan WW. Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Res. 1987;424:199–203. doi: 10.1016/0006-8993(87)91211-x. [DOI] [PubMed] [Google Scholar]
- Callahan TL, Petry HM. Psychophysical measurement of temporal modulation sensitivity in the tree shrew (Tupaia belangeri) Vision Res. 2000;40:455–8. doi: 10.1016/s0042-6989(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, Lucas RJ. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci. 2009;29:761–7. doi: 10.1111/j.1460-9568.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xie A, Hou L, Su Y, Lu F, Thorn F. Cycloplegic and noncycloplegic refractions of Chinese neonatal infants. Invest Ophthalmol Vis Sci. 2011;52:2456–61. doi: 10.1167/iovs.10-5441. [DOI] [PubMed] [Google Scholar]
- Choh V, Lew MY, Nadel MW, Wildsoet CF. Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res. 2006;46:1070–9. doi: 10.1016/j.visres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, Tan D. Atropine for the treatment of childhood myopia. Ophthalmol. 2006;113:2285–91. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res. 2011;92:40–6. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, Solomon AS. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Am J Ophthalmol. 1951;34:1407–13. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG. Refractive compensation to optical defocus depends on the temporal profile of luminance modulation of the environment. NeuroReport. 2002;13:1029–32. doi: 10.1097/00001756-200206120-00010. [DOI] [PubMed] [Google Scholar]
- Deng L, Gwiazda J, Thorn F. Children’s refractions and visual activities in the school year and summer. Optom Vis Sci. 2010;87:406–13. doi: 10.1097/OPX.0b013e3181da8a85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharani R, Lee CF, Theng ZX, Drury VB, Ngo C, Sandar M, Wong TY, Finkelstein EA, Saw SM. Comparison of measurements of time outdoors and light levels as risk factors for myopia in young Singapore children. Eye (Lond) 2012;26:911–8. doi: 10.1038/eye.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, Rose KA, Mitchell P, Saw SM. Outdoor activity and myopia in Singapore teenage children. Br J Ophthalmol. 2009;93:997–1000. doi: 10.1136/bjo.2008.150979. [DOI] [PubMed] [Google Scholar]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, Zhou X. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011;17:2824–34. [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002;83:211–9. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. J Comp Neurol. 2009;517:226–44. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D, Sattayasai J, Zappia J, Barrington M. Effects of selective neurotoxins on eye growth in the young chick. Ciba Found Symp. 1990;155:63–84. doi: 10.1002/9780470514023.ch5. [DOI] [PubMed] [Google Scholar]
- Feldkaemper M, Diether S, Kleine G, Schaeffel F. Interactions of spatial and luminance information in the retina of chickens during myopia development. Exp Eye Res. 1999;68:105–15. doi: 10.1006/exer.1998.0590. [DOI] [PubMed] [Google Scholar]
- French AN, Morgan IG, Mitchell P, Rose KA. Patterns of myopigenic activities with age, gender and ethnicity in Sydney schoolchildren. Ophthalmic Physiol Opt. 2013 doi: 10.1111/opo.12045. [DOI] [PubMed] [Google Scholar]
- Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–54. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278:16587–94. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Fugate-Wentzek LA, Wallman J. Different visual deprivations produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, Pourcain BS, Williams C. Time Outdoors and Physical Activity as Predictors of Incident Myopia in Childhood: A Prospective Cohort Study. Investigative Ophthalmology & Visual Science. 2012;53:2856–65. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Frost MR, Siegwart JT, Jr, Norton TT. Scleral Gene Expression Signatures in Tree Shrew in Response to Three Myopiagenic Visual Conditions: Minus Lens, Form Deprivation, and Darkness. Invest Ophthalmol Vis Sci. 2011;52 ARVO E-Abstract 6299. [Google Scholar]
- Guo Y, Liu LJ, Xu L, Lv YY, Tang P, Feng Y, Meng M, Jonas JB. Outdoor activity and myopia among primary students in rural and urban regions of Beijing. Ophthalmol. 2013;120:277–83. doi: 10.1016/j.ophtha.2012.07.086. [DOI] [PubMed] [Google Scholar]
- Guyton DL, Greene PR, Scholz RT. Dark-rearing interference with emmetropization in the Rhesus monkey. Invest Ophthalmol Vis Sci. 1989;30:761–3. [PubMed] [Google Scholar]
- Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vision Sci. 1993a;8:337–44. [Google Scholar]
- Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993b;34:690–4. [PubMed] [Google Scholar]
- Gwiazda JE, Deng L, Manny RE COMET Study Group. Seasonal differences in the progression of myopia in COMET children. Investigative Ophthalmology & Visual Science. 2012;53 doi: 10.1167/iovs.13-13029. ARVO E-abstract 2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda JE, Deng L, Thorn F, Gwiazda JD. The impact of parental myopia and children’s refractions at 5 years on the development of myopia in children by 15 years of age. Invest Ophthalmol Vis Sci. 2007;48 ARVO E-Abstract 2382. [Google Scholar]
- Hirsch MJ. Predictability of refraction at age 14 on the basis of testing at age 6 - interim report from the Ojai longitudinal study of refraction. Am J Optom Arch Am Acad Optom. 1964;41:567–73. doi: 10.1097/00006324-196410000-00001. [DOI] [PubMed] [Google Scholar]
- Hokoc JN, Mariani AP. Tyrosine hydroxylase immunoreactivity in the rhesus monkey retina reveals synapses from bipolar cells to dopaminergic amacrine cells. J Neurosci. 1987;7:2785–93. doi: 10.1523/JNEUROSCI.07-09-02785.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerheide J, Rempt F, Hoogenboom WP. Acquired myopia in young pilots. Ophthalmologica. 1971;163:209–15. doi: 10.1159/000306646. [DOI] [PubMed] [Google Scholar]
- Howland HC, Waite S, Peck L. Early focusing history predicts later refractive state: a longitudinal photorefractive study. Optical Society of America. 1993;3:210–3. [Google Scholar]
- Howlett MH, McFadden SA. Form-deprivation myopia in the guinea pig (Cavia porcellus) Vision Res. 2006;46:267–83. doi: 10.1016/j.visres.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res. 2007;47:1178–90. doi: 10.1016/j.visres.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Howlett MH, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009;49:219–27. doi: 10.1016/j.visres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995;1:761–5. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–2. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: Development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–71. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–7. [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Apomorphine inhibits development of myopia in visually-deprived infant rhesus monkeys. Invest Ophthalmol Vis Sci. 1992;33(Suppl):254. [Google Scholar]
- Jiang Liqin, Long Keli, Zhou Xiangtian, Qu Jia. Reciprocal activities of two type dopamine receptors determine the myopia development. Invest Ophthalmol Vis Sci. 2012;53 E-Abstract 3437. [Google Scholar]
- Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–32. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Jordan LA, Mitchell GL, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Twelker JD, Sims JR, Zadnik K. Visual activity before and after the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 2011;52:1841–50. doi: 10.1167/iovs.09-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Jordan LA, Sinnott LT, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Twelker JD, Zadnik K. Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Investigative Ophthalmology & Visual Science. 2012 doi: 10.1167/iovs.11-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee CS, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–83. [PubMed] [Google Scholar]
- Lauber JK. Review: Light-induced avian glaucoma as an animal model for human primary glaucoma. Journal of Ocular Pharmacology. 1987;3:77–100. doi: 10.1089/jop.1987.3.77. [DOI] [PubMed] [Google Scholar]
- Lauber JK. Review: avian models for experimental myopia. J Ocular Pharmacol. 1991;7:259–76. [PubMed] [Google Scholar]
- Lauber JK, Shutze JV, McGinnis J. Effects of exposure to continuous light on the eye of the growing chick. Proc Soc Exp Biol Med. 1961;106:871–2. doi: 10.3181/00379727-106-26505. [DOI] [PubMed] [Google Scholar]
- Lee YY, Lo CT, Sheu SJ, Lin JL. What factors are associated with myopia in young adults? A survey study in Taiwan military conscripts. Investigative Ophthalmology & Visual Science. 2013 doi: 10.1167/iovs.12-10480. [DOI] [PubMed] [Google Scholar]
- Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol Opt. 1995;15:351–6. [PubMed] [Google Scholar]
- Li T, Howland HC. The effects of constant and diurnal illumination of the pineal gland and the eyes on ocular growth in chicks. Invest Ophthalmol Vis Sci. 2003;44:3692–7. doi: 10.1167/iovs.02-0990. [DOI] [PubMed] [Google Scholar]
- Li T, Howland HC, Troilo D. Diurnal illumination patterns affect the development of the chick eye. Vision Res. 2000;40:2387–93. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–9. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- Lu F, Zhou X, Zhao H, Wang R, Jia D, Jiang L, Xie R, Qu J. Axial myopia induced by a monocularly-deprived facemask in guinea pigs: A non-invasive and effective model. Exp Eye Res. 2006;82:628–36. doi: 10.1016/j.exer.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2001;119:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri) Vision Res. 1992;32:843–52. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan IG. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84:100–7. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- McKanna JA, Casagrande VA, Norton TT, Marsh WL. Dark-reared tree shrews do not develop lid-suture myopia. Invest Ophthalmol Vis Sci. 1983;24(Suppl):226. [Google Scholar]
- Meijer JH, Daan S, Overkamp GJ, Hermann PM. The two-oscillator circadian system of tree shrews (Tupaia belangeri) and its response to light and dark pulses. J. Biol. Rhythms. 1990;5:1–16. doi: 10.1177/074873049000500101. [DOI] [PubMed] [Google Scholar]
- Metlapally S, McBrien NA. The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. J Vis. 2008;8:1–12. doi: 10.1167/8.3.1. [DOI] [PubMed] [Google Scholar]
- Morgan I, Kucharski R, Krongkaew N, Firth SI, Megaw P, Maleszka R. Screening for differential gene expression during the development of form-deprivation myopia in the chicken. Optom Vis Sci. 2004;81:148–55. doi: 10.1097/00006324-200402000-00013. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Boelen MK. A retinal dark-light switch: A review of the evidence. Vis Neurosci. 1996;13:399–409. doi: 10.1017/s0952523800008087. [DOI] [PubMed] [Google Scholar]
- Multi-ethnic Pediatric Eye Disease Study. Prevalence of myopia and hyperopia in 6- to 72-month-old african american and Hispanic children: the multi-ethnic pediatric eye disease study. Ophthalmol. 2010;117:140–7. doi: 10.1016/j.ophtha.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Marks AR. Blood levels of vitamin D in teens and young adults with myopia. Optom Vis Sci. 2011;88:377–82. doi: 10.1097/OPX.0b013e31820b0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Moeschberger ML, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. 2005;46:3074–80. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children’s refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- Mutti DO, Cooper ME, Dragan E, Jones-Jordan LA, Bailey MD, Marazita ML, Murray JC, Zadnik K The CLEERE Study Group. Vitamin D receptor (VDR) and Group-Specific Component (GC, vitamin D-binding protein) polymorphisms in myopia. Investigative Ophthalmology & Visual Science. 2011;52:3818–24. doi: 10.1167/iovs.10-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93:782–5. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010;91:715–20. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr Darkness causes myopia in visually experienced tree shrews. Invest Ophthalmol Vis Sci. 2006;47:4700–7. doi: 10.1167/iovs.05-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Amedo AO, Siegwart JT., Jr The effect of age on compensation for a negative lens and recovery from lens-induced myopia in tree shrews (Tupaia glis belangeri) Vision Res. 2010;50:564–76. doi: 10.1016/j.visres.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, Corliss DA, Bailey JE. In: The Psychophysical Measurement of Visual Function. Norton TT, Corliss DA, Bailey JE, editors. Amsterdam; Boston: Butterworth-Heinemann; 2002. pp. xv–361. [Google Scholar]
- Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri) Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- Norton TT, Rada JA. Reduced extracellular matrix accumulation in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- Onal S, Toker E, Akingol Z, Arslan G, Ertan S, Turan C, Kaplan O. Refractive errors of medical students in Turkey: one year follow-up of refraction and biometry. Optom Vis Sci. 2007;84:175–80. doi: 10.1097/OPX.0b013e3180335c52. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–12. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parssinen O, Lyyra A-L. Myopia and Myopic progression among schoolchildren: a three-year follow-up study. Invest Ophthalmol Vis Sci. 1993;34:2794–802. [PubMed] [Google Scholar]
- Pickett-Seltner RL, Sivak JG, Paternak JJ. Experimentally induced myopia in chicks: morphometric and biochemical analysis during the first 14 days after hatching. Vision Res. 1988;28:323–8. doi: 10.1016/0042-6989(88)90160-5. [DOI] [PubMed] [Google Scholar]
- Pickett-Seltner RL, Weerheim J, Sivak JG, Pasternak J. Experimentally induced myopia does not affect post-hatching development of the chick lens. Vision Res. 1987;27:1779–82. doi: 10.1016/0042-6989(87)90106-4. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., III Nature of the refractive errors in rhesus monkeys (Macaca mulatta) with experimentally induced ametropias. Vision Res. 2010;50:1867–81. doi: 10.1016/j.visres.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Matthews AL. Visual deprivation upregulates extracellular matrix synthesis by chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1994;35:2436–47. [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978;17:485–8. [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. Neural control of eye growth and experimental myopia in primates. Ciba Found Symp. 1990;155:22–38. doi: 10.1002/9780470514023.ch3. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D(2)-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–53. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-β) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–61. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmol. 2008a;115:1279–85. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw SM. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008b;126:527–30. doi: 10.1001/archopht.126.4.527. [DOI] [PubMed] [Google Scholar]
- Saw SM, Nieto FJ, Katz J, Schein OD, Levy B, Chew SJ. Factors related to the progression of myopia in Singaporean children. Optom Vis Sci. 2000;77:549–54. doi: 10.1097/00006324-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of retinal dopamine/melatonin system in experimental refractive errors in chickens. Vision Res. 1995;35:1247–64. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–57. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Hagel G, Bartmann M, Kohler K, Zrenner E. 6-Hydroxy dopamine does not affect lens-induced refractive errors but suppresses deprivation myopia. Vision Res. 1994;34:143–9. doi: 10.1016/0042-6989(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Contrast and spatial-frequency requirements for emmetropization in chicks. Vision Res. 1997;37:2011–21. doi: 10.1016/s0042-6989(97)00014-x. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–47. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Shaikh AW, Siegwart JT, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–15. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Norton TT, Casagrande VA. Myopia in the lid-sutured tree shrew (Tupaia glis) Brain Res. 1977;124:154–7. doi: 10.1016/0006-8993(77)90872-1. [DOI] [PubMed] [Google Scholar]
- Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmol. 2012a;119:2141–51. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Sherwin JC, Hewitt AW, Coroneo MT, Kearns LS, Griffiths LR, Mackey DA. The Association between Time Spent Outdoors and Myopia Using a Novel Biomarker of Outdoor Light Exposure. Investigative Ophthalmology & Visual Science. 2012b;53:4363–70. doi: 10.1167/iovs.11-8677. [DOI] [PubMed] [Google Scholar]
- Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79:233–6. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- Siatkowski RM, Cotter S, Miller JM, Scher CA, Crockett RS, Novack GD. Safety and efficacy of 2% pirenzepine ophthalmic gel in children with myopia: a 1-year, multicenter, double-masked, placebo-controlled parallel study. Arch Ophthalmol. 2004;122:1667–74. doi: 10.1001/archopht.122.11.1667. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Norton TT. Goggles for controlling the visual environment of small animals. Lab Animal Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The susceptible period for deprivation-induced myopia in tree shrew. Vision Res. 1998;38:3505–15. doi: 10.1016/s0042-6989(98)00053-4. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. Perspective: how might emmetropization and genetic factors produce myopia in normal eyes? Optom. Vis Sci. 2011;88:E365–E372. doi: 10.1097/OPX.0b013e31820b053d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT, Ward AH, Norton TT. Moderately elevated fluorescent light levels slow form deprivation and minus lens-induced myopia development in tree shrews. Invest Ophthalmol Vis Sci. 2012;53 ARVO E-abstract 3457. [Google Scholar]
- Siegwart JT, Jr, Herman CK, Norton TT. Vitamin D3 supplement did not affect the development of myopia produced with form deprivation or a minus lens in tree shrews. Invest Ophthalmol Vis Sci. 2011;52 ARVO E-Abstract 6298. [Google Scholar]
- Smith EL., III Optical treatment strategies to slow myopia progression: Effects of the visual extent of the optical treatment zone. Exp Eye Res. 2013 doi: 10.1016/j.exer.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, III, Bradley DV, Fernandes A, Hung LF, Boothe RG. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42:1146–52. [PubMed] [Google Scholar]
- Smith EL, Harwerth RS, Crawford MLJ, von Noorden GK. Observations on the effects of form deprivation on the refractive status of the monkey. Invest Ophthalmol Vis Sci. 1987;28:1236–45. [PubMed] [Google Scholar]
- Smith EL, III, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic Physiol Opt. 1999;19:90–102. [PubMed] [Google Scholar]
- Smith EL, Hung LF, Arumugam B, Huang J. Negative Lens-Induced Myopia in Infant Monkeys: Effects of High Ambient Lighting. Investigative Ophthalmology & Visual Science. 2013;54:2959–69. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, Huang J. Protective Effects of High Ambient Lighting on the Development of Form-Deprivation Myopia in Rhesus Monkeys. Investigative Ophthalmology & Visual Science. 2012;53:421–8. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsby A, Benjamin B, Davey JB, Sheridan M, Tanner JM. Emmetropia and its aberrations. Med Res Counc Spec Rep Ser. 1957;293:1–69. [PubMed] [Google Scholar]
- Stenstrom S. Investigation of the variation and the correlation of the optical elements of human eyes. In: Woolf D, translator. Am J Optom Arch Am Acad Optom. Vol. 25. 1948. pp. 218–32. [PubMed] [Google Scholar]
- Stone RA, Lin T, Iuvone PM, Laties AM. Myopia and the Control of Eye Growth. Vol. 155. New York: Wiley & Sons; 1990. Postnatal control of ocular growth: dopaminergic mechanisms; pp. 45–57. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989;86:704–6. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013 doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Res. 1993;33:1311–24. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Troilo D, Wallman J. The regulation of eye growth and refractive state: an experimental study of emmetropization. Vision Res. 1991;31:1237–1250. doi: 10.1016/0042-6989(91)90048-a. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005;46:3922–31. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–63. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI, Trachtman J. The eyes of young chicks grow toward emmetropia. Invest Ophthalmol Vis Sci. 1981;20:557–61. [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Hu CH, Yang YH. Effects of outdoor activities on myopia among rural school children in Taiwan. Ophthalmic Epidemiol. 2010;17:338–42. doi: 10.3109/09286586.2010.508347. [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor Activity during Class Recess Reduces Myopia Onset and Progression in School Children. Ophthalmol. 2013 doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Zucker CL. Synaptic organization of dopaminergic interplexiform cells in the goldfish retina. Vis Neurosci. 1988;1:13–29. doi: 10.1017/s0952523800000997. [DOI] [PubMed] [Google Scholar]
- Yi JH, Li RR. Influence of near-work and outdoor activities on myopia progression in school children. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:32–5. [PubMed] [Google Scholar]
- Zadnik K, Mutti DO, Friedman NE, Qualley PA, Jones LA, Qui P, Kim HS, Hsu JC, Moeschberger ML. Ocular predictors of the onset of juvenile myopia. Invest Ophthalmol Vis Sci. 1999;40:1936–43. [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–6. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]