Abstract
Objective
We sought to determine whether aerobic exercise training+weight loss (AEX+WL) would affect the expression of myostatin and its relationship with insulin sensitivity in a longitudinal, clinical intervention study
Design and Methods
Thirty-three obese sedentary postmenopausal women and men (n=17 and 16, age:61±1 yrs, BMI:31±1 kg/m2, VO2max:21.9 ± 1.0 ml/kg/min, X ± SEM) completed 6 months of 3d/wk AEX+WL. During an 80 mU·m−2·min−1 hyperinsulinemic-euglycemic clamp, we measured glucose utilization (M), myostatin, myogenin, and MyoD gene expression by real-time RT-PCR in vastus lateralis muscle at baseline and 2 hrs.
Results
Body weight (−8%) and fat mass (−17%) decreased after AEX+WL (P<0.001). FFM and mid-thigh muscle area by CT did not change but muscle attenuation increased (P<0.05). VO2max increased 14% (P<0.001). AEX+WL increased M by 18% (P<0.01). Myostatin gene expression decreased 19% after AEX+WL (P<0.05). Basal mRNA myostatin levels were negatively associated with M prior to the intervention (r=−0.43, P<0.05). Insulin infusion increased myoD and myogenin expression before and after AEX+WL (both P<0.001) but basal levels did not change. The insulin effect on myostatin expression was associated with the change in M after AEX+WL (r=0.56, P<0.005).
Conclusions
Exercise and weight loss results in a down-regulation of myostatin mRNA and an improvement in insulin sensitivity in obese older men and women.
Introduction
Myostatin is a member of the transforming growth factor Beta family of secreted growth factors and is a significant negative regulator of skeletal muscle development and size (1). Transcription factors that regulate muscle differentiation include myogenic differentiation factor (MyoD), which is responsible for stimulating myoblasts to enter differentiation and join the muscle lineage, and myogenin which mediates terminal differentiation of myoblasts (2). Knock-out in the myostatin (Mstn) gene has led to dramatic increases in muscle mass in animals (3, 4) and mutations has been documented in a child (5). In humans, resistive training either acute or chronic, reduces basal mRNA myostatin (6–12). One study showed a decrease in myostatin protein levels in middle-aged men after aerobic training (13), whereas two studies report significant reductions in myostatin mRNA levels after severe weight loss by gastric bypass surgery (14) (15). Thus, the effects of aerobic training and modest weight loss on these genes in skeletal muscle remain largely unaddressed, especially in older adults.
Animal model studies have demonstrated the importance of myostatin in glucose metabolism (16–18). Agouti lethal yellow and obese (Lepob/ob) myostatin knock-out mice have a reduction in body fat and improved glucose tolerance (16). Mstn−/− mice have increased glucose uptake measured by the hyperinsulinemic-euglycemic clamp (17) and when fed a high-fat diet, myostatin deficient mice also have improved muscle insulin sensitivity (17, 18). Myostatin knock-out mice have increased AMP-activated protein kinase, Akt, and phosphorylated Akt (19)which may explain the increased insulin sensitivity. Since in vivo insulin increases serine phosphorylation of Akt in healthy controls and men with impaired glucose tolerance (20), it is of interest to study the effect of insulin stimulation on myostatin in vivo. Based on the studies in the myostatin knock-out model and the limited evidence that exercise and weight loss can affect myostatin levels in humans, it was our interest to test the hypothesis that chronic aerobic exercise training+weight loss would reduce basal skeletal muscle myostatin protein and gene expression and be associated with improvements in insulin sensitivity. Therefore, the aim of this investigation was to examine glucose utilization and the basal and insulin-stimulated gene expression levels of myostatin, MyoD, and myogenin before and after a 6 month aerobic training+weight loss program in older, obese adults.
Methods and Procedures
Subjects were healthy, overweight and obese (body mass index, BMI >25 kg/m2; range of 25–46 kg/m2) aged 54–77 years. They were weight stable (<2.0 kg weight change in past year), sedentary (<20 min of aerobic exercise 2x/wk) and screened by medical history questionnaire, physical examination, and fasting blood profile. Individuals with untreated hypertension (blood pressure higher than 160/90 mm Hg) or hyperlipidemia (triglycerides ≥ 4.5 mM, cholesterol > 6.2 mM, LDL cholesterol > 4.3 mM) were referred to their doctor for therapy and allowed to enter if they were treated with a antihypertensive or lipid-lowering drug that did not affect glucose metabolism. All subjects were nonsmokers, showed no evidence of cancer, liver, renal or hematological disease, or other medical disorders. The women in the study were postmenopausal and had not menstruated for at least 1 year. In addition, all subjects underwent a screening Bruce graded treadmill test to exclude those with asymptomatic coronary artery disease.
Thirty-three men (n=16) and women (n=17) met all study criteria and enrolled into the aerobic exercise+weight loss (AEX+WL) intervention. The Institutional Review Board of the University of Maryland approved all methods and procedures. Each participant provided written informed consent to participate in the study.
Study Protocol
Subjects received instruction in maintaining a weight-stable, Therapeutic Lifestyle Changes (TLC) diet (21), by a registered dietitian (RD) one day/week for 6–8 weeks, prior to baseline testing. Subjects were weight stable on the TLC diet prior to baseline testing, and were instructed to maintain the TLC diet throughout the study.
AEX+WL Program
Subjects exercised at the Baltimore VA Medical Center GRECC exercise facility three times a week for 6 months using treadmills and elliptical trainers. Each exercise session included a 5–10 minute stretching and warm-up phase and a 5–10 minute cool-down phase. For the first 3–4 weeks, subjects exercised on the treadmill at ~50–60% heart rate reserve for 20–30 continuous minutes as tolerated. Initially, time was progressed to 50 min. The duration remained for 50 min throughout the remainder of the intervention. Then, intensity was progressed to >60% VO2max and up to 80% VO2max. This progression of intensity typically took 4–6 weeks based on each subject’s exercise tolerance. Exercise intensity, prescribed as a target heart rate range, was monitored using chest-strap heart rate monitors (Polar Electro Inc., Lake Success, NY). There were no rest periods during the exercise sessions. All sessions were supervised by exercise physiologists.
All subjects also attended weekly weight loss classes led by a RD for instruction in the TCL diet. Compliance was monitored by 7-day food records (or 24 hour recalls) using the American Diabetes Association exchange list system. Individuals were instructed to restrict their caloric intake by 500 kcal/d. The average compliance to the exercise sessions and weight loss classes was greater than 75%.
VO2max and Body Composition
VO2max was measured using a continuous treadmill test protocol (22). Height (cm) and weight (kg), waist and hip circumference were measured to calculate body mass index (BMI) and waist-to-hip ratio (WHR). Fat mass, lean tissue mass and bone mineral content (BMC) (fat-free mass= lean+BMC) were determined by dual-energy X-ray absorptiometry (DXA) (Prodigy, LUNAR Radiation Corp., Madison, WI). A single computed tomography (CT) scan at L4–L5 region using a Siemens Somatom Sensation 64 Scanner (Fairfield, CT) was used to determine visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) areas and analyzed using MIPAV (NIH Image Analysis Program). A second scan at the mid-thigh was used to quantify muscle area, total fat area, low density lean tissue area, and muscle attenuation in Hounsfield units (HU) of both the right and left legs (22).
Metabolic Testing
All subjects were weight stabilized (±2%) for at least two weeks prior to metabolic testing before and after the interventions and were provided with a eucaloric diet for two days before the clamp by a RD to control nutrient intake (22). All testing was performed in the morning after a 12-hr overnight fast. At the end of the 6 month program, subjects were asked to continue the aerobic training 3 d/wk during the final testing period and the glucose clamps were performed 36–48 hours after the last bout of exercise.
Oral glucose tolerance test (OGTT)
Blood samples were drawn before and at 30-min intervals for 2 h after ingestion of 75 g of glucose. Plasma glucose concentrations were measured using the glucose oxidase method (2300 STAT Plus, YSI, Yellow Springs, OH). Plasma insulin was measured by RIA (Linco Research, St. Charles, MO). Glucose and insulin total area under the curve (GAUC and IAUC, respectively) were calculated by the trapezoidal method.
Hyperinsulinemic-euglycemic Clamps with Skeletal Muscle Biopsies
Peripheral tissue sensitivity to exogenous insulin was measured using the hyperinsulinemic-euglycemic clamp technique (23). Arterialized blood was obtained from a dorsal heated hand vein (24). For the assessment of basal glucose and insulin levels, three arterialized blood samples were drawn at 10-min intervals. Blood samples were obtained every 5 and 10 min thereafter for the determination of plasma glucose and insulin levels. A 10 min priming and continuous infusion of insulin (80 mU·m−2·min−1, Humulin, Eli Lilly Co., Indianapolis, IN) was performed for 180 min with a continuous infusion of 20% glucose solution starting at 10 min. The plasma glucose levels during each clamp period (10–180 min) did not differ before and after the interventions and averaged 5.13±0.06 vs. 5.10±0.07 mmol/l which was 95±0.8% of the desired goal with a coefficient of variation (CV) of 5.9±0.3% with insulin levels of 1062±26 pmol/l in all clamps (n=64). One male did not undergo the glucose clamp procedure.
Analysis of Blood Samples
Blood samples were collected for serum or plasma (heparin) by blood draw and placed in test tubes. For the serum samples, blood was allowed to clot by leaving it undisturbed at room temperature for 30 minutes. For the plasma, samples were placed in prechilled test tubes containing 1.5 mg EDTA/ml of blood and centrifuged at 2,000 × g for 10 minutes at 4°C. The liquid component (serum or plasma) was immediately transferred into clean polypropylene tubes. Aliquots were stored at −80°C until analysis for plasma insulin by RIA (22) and for serum myostatin by ELISA. Serum myostatin was measured in duplicate using Myostatin ELISA kit according to the manufacturer’s instructions (ALPCO, Cat# K1012). Samples were read using VERSAMAX microtiter plate reader at 450 nm against 620 nm as a reference. A 4-parameter-algorithm was used to calculate the standard curve and the concentrations of myostatin in human serum were determined. The coefficient of variance between replicates was below 15%.
Skeletal Muscle Biopsies and Analysis
Prior to the start of the clamp procedure and at 120-min during the glucose clamp, vastus lateralis muscle biopsies were taken from each subject under local anesthesia using a 5mm Bergstrom needle for the measurement of basal and insulin-stimulated gene expression. Three women had basal and not insulin-stimulated muscle biopsies. Muscle was immediately freeze-clamped and stored at −80°C. Approximately 50–80 mg of muscle was used for RNA isolation and 100mg of muscle was used for tissue lysate preparation. There was sufficient muscle sample of 15 individuals for the western blot analyses.
RNA Extraction and Reverse Transcription and Real-time RT-PCR
Total RNA was extracted from skeletal muscle using TRIzol® Reagent (Invitrogen, Cat# 15596-018) as specified by the manufacturer. The RNA pellet was resuspended in RNAsecure Resuspension solution (Cat. #7010, Ambion Inc.) and RNA concentrations were measured in a spectrophotometer.
One μg of total RNA for each sample was reverse transcripted into first strand cDNA using Transcriptor First Strand cDNA Synthesis Kit (Cat# 04 896 866 001, Roche Applied Science) according to the detailed manufacturer’s protocol. The random primer was used as the primer and the RT reaction was carried out at 10 min at 25°C and then 55°C for 30 min in 20μl volume. The reaction was inactivated by incubating at 85°C for 10 min and stopped by placing the tube on ice. An RT control (master mix without RT enzyme) was performed.
Quantitative Real-time PCR (qPCR) and data analysis were performed in a LightCycler 480 Real-Time PCR System with LightCycler® 480 software (Roche Applied Science). LightCycler 480 Multiwell plate 384 (Cat# 04 729 748 001), LightCycler 480 Probes Master kit (Cat# 04 887 301 001,) and Taqman gene expression primer/probe set (Applied Biosystems, Foster City, CA.) were used. Each qPCR reaction was carried out in a final volume of 10μl, consisting of 2μl 1:4 diluted template cDNA, 5μl LightCycler 480 Probes Master. 0.5μl Taqman gene expression primer and probe mix, and 2.5μl nuclease-free water. Water instead of cDNA served as the no template control. According to the manufacturer’s instruction, the qPCR protocol was adopted for all samples: after incubation at 95°C for 10 min to activate the DNA polymerase, 45 cycles of 95°C for 10s and 60°C for 30s each were performed to facilitate the PCR reaction. 36B4 served as an internal control for normalization. Data acquisition occurred at real time during the annealing/elongation incubation at 60°C. All samples were amplified in triplicate from the same RNA preparation. Gene expression data were analyzed by Roche LightCycle 480 system Software version 1.5 advanced relative quantification program. Data is presented as the mean ± SEM of three determinations for each sample and the normalized ratio of Target/Reference.
Tissue Lysate Preparation and Western Immunoblot Analyses
In a 15 individuals (n=7 women and n=8 men), a fasting/basal muscle samples in obtained before and after AEX+WL were lyophilized for 48 hrs and then dissected free of obvious connective tissue, fat and blood. Microdissected muscle (~5 mg) was homogenized in complete RIPA buffer (Santa Cruz Cat# sc-24948) for 1 min on ice. The homogenate was incubated on ice with shaking for 30 minutes and then centrifuged at 14,000 rpm for 15 min at 4°C. Total protein concentration was determined using BCA protein assay kit (Pierce Cat# 23227). Thirty μg of protein were denatured at 100°C for 5min and separated on a 10% SDS-PAGE gel (Bio-Rad Cat# 345-0009) along with molecular weight markers and subsequently transferred to PVDF membranes. The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween20 (TBS-T) for 1 h at room temperature, then incubated overnight at 4°C with primary antibodies (MSTN, NVUS Cat# H00002660-M07 and GAPDH, Santa Cruz, Cat# sc-25778) diluted according to the manufacturer. After TBS-T for 15min x3 washes, the membranes were incubated for 1 hr at room temperature with secondary antibody, anti-mouse IgG TrueBlot conjugated with Horseradish Peroxidase (HRP) (eBioscience, Cat# 18-8817) and goat anti-rabbit IgG conjugated with Horseradish Peroxidase (HRP). Detection was performed using the ECL Plus Western Blotting Detection System (GE Healthcare, Cat# PRN2132). The emitted light was captured on X-OMATTM Blue XB film (Kodak, Cat# 1776699). Blots were imaged using a personal density scanning imager (PDSI; Molecular Dynamics), and protein band densities were determined using the Image QuaNT software 5.0 (Molecular Dynamics). GAPDH served as an internal reference and was used to correct the difference in sample concentration and loadings. The whole normal skeletal muscle lysate (NUVS Cat# NB820-59169) served as a positive control and an external calibrator to counteract variations in Western blotting efficiency. Target/Reference ratio was normalized using the calibrator on each Blot.
Statistical Analyses
For the hyperinsulinemic-euglycemic clamps, the mean concentrations of glucose and insulin were calculated for each sample time point. The trapezoidal rule was used to calculate the integrated response over 30-min intervals from 30–180 min for each subject during the clamp. The integrated response was divided by its time interval to compute mean concentrations for glucose and insulin during the clamp. Glucose utilization (M) for 30-min intervals was calculated from the amount of glucose infused after correction for glucose equivalent space (glucose space correction). For one male who did not undergo post DXA, the FFM change was estimated from the mean change in order to calculate M expressed per FFM. The CT mid-thigh measurements were not different between the right and left legs so the values of the right leg were used in the statistical analyses.
Differences between pre-intervention and post-intervention measures of variables were determined using a paired t-test. Univariate regression analyses were used to determine predictors of glucose utilization. Statistical significance was set at P<0.05. Data were analyzed by SPSS statistical software (SPSS Inc., Chicago) and expressed as mean ± SEM.
Results
Body weight decreased 8% (P<0.001) accompanied by an absolute decrease in percent body fat of 4% (P<0.001), a 17% decrease in total fat mass (P<0.001) and no change in FFM or mid-thigh muscle area (Table 1). After AEX+WL, mid-thigh subcutaneous fat decreased (P<0.001), low density lean tissue area decreased (P<0.05), and muscle attenuation increased (P<0.05). VO2max (l/min) increased 14% after AEX+WL (P<0.001).
Table 1.
Physical and metabolic characteristics before and after the intervention (n=33).
| Before | After | |
|---|---|---|
|
| ||
| Age (yr) | 61 ± 1 yrs | |
| Weight (kg) | 90.8 ± 2.8 | 83.7 ± 2.7‡ |
| BMI (kg/m2) | 31.2 ± 0.7 | 28.3 ± 0.7‡ |
| % Body fat | 39.2 ± 1.6 | 34.8 ± 1.8‡ |
| Fat mass (kg) | 35.5 ± 1.9 | 29.3 ± 1.8‡ |
| Fat-free mass (kg) | 55.4 ± 2.3 | 54.9 ± 2.3 |
| VO2max (l/min) | 2.00 ± 0.11 | 2.26 ± 0.11‡ |
| Visceral fat area (cm2) | 163.0 ± 16.9 | 137.8 ± 14.1† |
| Subcutaneous abdominal fat (cm2) | 357.5 ± 23.5 | 306.8 ± 24.8‡ |
| Mid-thigh muscle area (cm2) | 90.7 ± 6.1 | 92.6 ± 5.2 |
| Mid-thigh subcutaneous fat (cm2) | 118.7 ± 11.6 | 102.2 ± 9.6‡ |
| Mid-thigh low density lean tissue (cm2) | 22.9 ± 1.5 | 21.5 ± 1.6* |
| Mid-thigh muscle attenuation (HU) | 36.2 ± 2.6 | 38.8 ± 3.0* |
| Fasting plasma glucose (mmol/l) | 5.4 ± 0.1 | 5.2 ± 0.1 |
| Fasting plasma insulin (pmol/l) | 87 ± 7 | 69 ± 5‡ |
| Glucose AUC (mmol/l/min) | 1271 ± 51 | 1212 ± 42* |
| Insulin AUC (pmol/l/min) | 68,607 ± 5499 | 57,127 ± 4521† |
| M (μmol·kgFFM−1·min−1) | 48.0 ± 3.29 | 56.5 ± 2.9† |
Values are means ± SEM. Area under the curve from the OGTT. Significantly different before vs. after the intervention:
P<0.05;
P<0.01;
P<0.001.
At baseline, 19 subjects had normal glucose tolerance, 11 had impaired glucose tolerance, and 3 had unconfirmed diabetes (25). Fasting plasma glucose concentrations did not change after AEX+WL but fasting insulin levels were reduced (P<0.001, Table 1). Glucose AUC during the OGTT decreased 5% (P<0.05) and insulin AUC during the OGTT decreased 17% (P<0.01). M increased 18% (P<0.001) after AEX+WL.
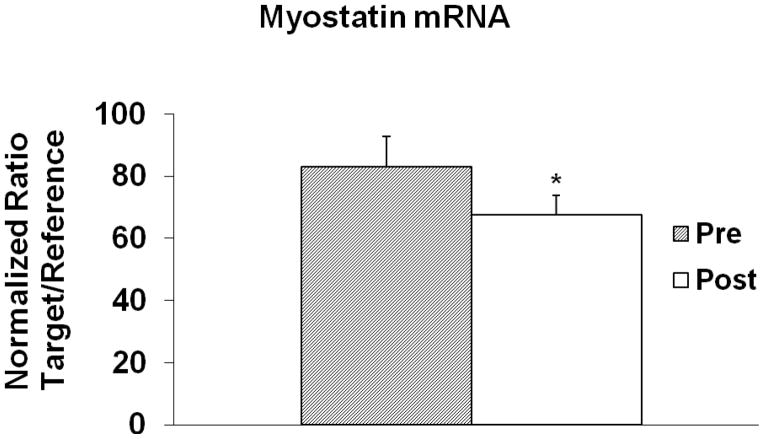
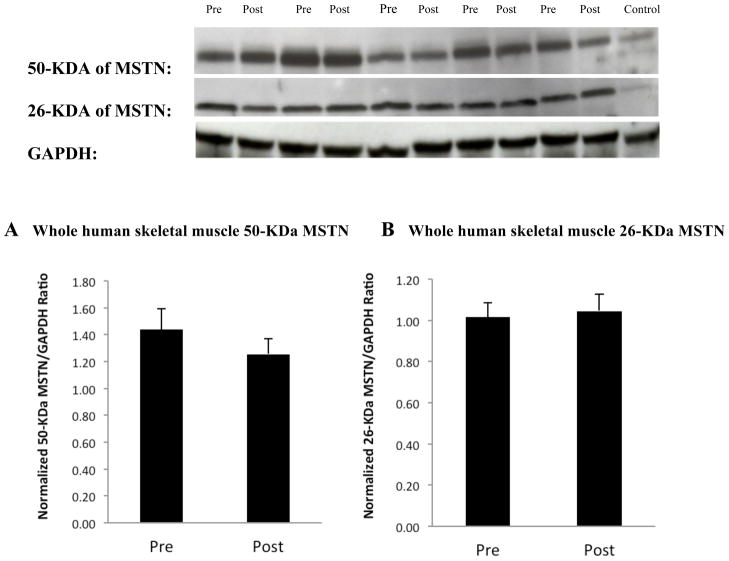
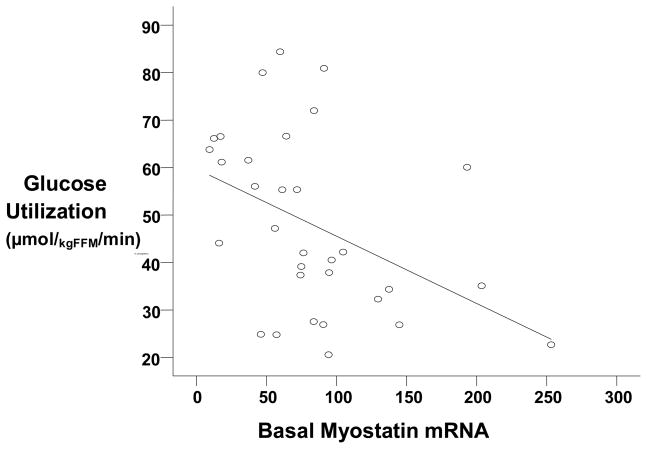
Basal myostatin mRNA levels decreased 19% after AEX+WL (83.0 ± 9.7 vs. 67.5 ± 6.4 AU, P<0.05, Figure 1) but myostatin protein levels did not change significantly for 26-kDa (1.29 ± 0.10 vs. 1.34 ± 0.10 AU) and 50-kDa (1.44 ± 0.16 vs. 1.26 ± 0.11 AU), (Figure 2). Basal myostatin mRNA levels were negatively associated with M prior to the intervention (r=−0.43, P<0.05, Figure 3). There was a trend toward a difference in the change in myostatin mRNA levels with insulin stimulation before vs. after AEX+WL (−7.2 ± 4.7 vs. 6.0 ± 7.5 AU, P=0.10). The insulin effect on myostatin gene expression was associated with the change in M after AEX+WL (r=0.56, P<0.005). Serum myostatin tended to decrease after AEX+WL (7.25 ± 3.71 vs. 6.10 ± 2.44 ng/ml, P=0.07). In an examination of each gender, we observed a decrease in skeletal muscle 50-kDa myostatin protein levels in women (1.75 vs. 1.22 AU, P<0.05) and a decrease in serum myostatin in men (9.02 vs. 6.48 ng/ml, P<0.05). There were no associations between age and BMI and changes in skeletal muscle protein or gene expression.
Figure 1.
Basal myostatin mRNA levels before and after AEX+WL (n=33). *P<0.05
Figure 2.
Western blot analysis of myostatin protein in human skeletal muscle. (A) The 50-kDa precursor form of myostatin and (B) The small amounts of the 26-kDa mature myostatin were examined by western blotting analysis (upper panel) and the obtained results were quantitatively analyzed (lower panel, n=33). GAPDH served as a reference and was used to correct the difference in sample concentration and loadings. The whole normal human skeletal muscle lysate served as a positive control and an external calibrator to counteract variations in western blotting efficiency. MSTN: myostatin.
Figure 3.
Relationship between basal myostatin mRNA and glucose utilization, M (n=32) (r=−0.43, P<0.05).
Basal MyoD (2.59 ± 0.11 vs. 2.60 ± 0.09 AU) and myogenin (2.39 ± 0.09 vs. 2.39 ± 0.10 AU) mRNA levels did not change significantly after AEX+WL. However, insulin infusion increased myoD mRNA before (+2.62 ± 0.19 AU) and after (+2.71 ± 0.25 AU) AEX+WL (both P<0.001). Likewise, hyperinsulinemia increased myogenin gene expression before and after AEX+WL (+0.71 ± 0.09 and +0.84 ± 0.16 AU, respectively, both P<0.001). The increase in myoD and myogenin expression during hyperinsulinemia was similar before and after AEX+WL.
Discussion
Our results indicate that a down regulation of skeletal muscle mRNA content of myostatin occurs after an aerobic exercise and weight loss program in obese men and women. Basal MyoD and myogenin mRNA and myostatin protein expression did not significantly change after the intervention.
Satellite cells are important in the mechanisms involved in the muscles’ response to exercise training. Once the cells are activated, they proliferate and fuse together with pre-existing fibers to regenerate muscle tissue. Activated satellite cells can express the myogenic factors including MyoD, myogenin, and myostatin. Myostatin, a negative regulator of muscle cell growth, represses myoD and myogenin protein expression (26). Because of the importance of these genes in muscle growth, it is conceivable that an exercise training program could modify the expression of these genes.
To our knowledge, there are no studies examining the effects of aerobic training and weight loss on skeletal muscle basal myostatin, MyoD, or myogenin levels in older men and women. We found a significant decrease in myostatin gene expression after AEX+WL Although we found a 10% decrease in 50-kDa myostatin protein levels, this was not significant but is similar in magnitude to the 12% decrease in myostatin gene expression. We did not observe any significant changes in 26-kDa myostain protein, MyoD or myogenin mRNA expression after AEX+WL. Others report downregulation of myostatin mRNA without changing myostatin protein levels in conditions such as sepsis in rodents (27). A 6 month aerobic training program in 10 middle-aged men resulted in a decrease in myostatin protein levels (13). An acute cycling bout increased mRNA myostatin by two-fold, MyoD by 3-fold, and myogenin by <1-fold in endurance trained subjects (28). The significant decrease in basal myostatin mRNA after the AEX+WL in our study is consistent with studies utilizing resistive exercise (RT) as the mode of training whereby acute bouts of resistive exercise (6–10) and 9 weeks of RT in older men (11) reduce muscle myostatin mRNA. We have also observed a significant decrease in myostatin mRNA expression in the paretic muscle of stroke survivors after 12 weeks of high intensity RT (12). One study has reported an increase in myostatin expression after an acute resistive session (29). In addition, an acute bout of RT can reduce (6) or not change (8) myogenin levels and increase MyoD mRNA expression (30) in young but not elderly subjects (31). Although MyoD and myogenin gene expression increased after 6 weeks of RT in young males (32), we found no change in MyoD or myogenin after a longer aerobic exercise program.
Recent papers (17, 18) provide evidence that myostatin knockout mice have increased insulin sensitivity and glucose uptake. Mstn −/− mice have increased muscle mass, lower adipose tissue mass and lower basal skeletal muscle and whole body lipid oxidation with improved insulin signaling by increased Akt phosphorylation during insulin (17). Myostatin mice with the loss of function of both alleles (MstnLn/Ln) have improved glucose disposal rate compared to Mstn+/Ln on a high fat diet (18). In addition, TNFα expression is lower in both muscle and adipose tissue and treatment with recombinant myostatin increases TNFα and results in insulin resistance (18). Treatment of mice with a soluble activin receptor type IIB (an endogenous signaling receptor for myostatin) suppresses hepatic glucose production and increases glucose uptake (33). These animal models provide clear mechanistic evidence of the detrimental effects of myostatin on insulin sensitivity. We are the first to provide human evidence that skeletal muscle myostatin mRNA may be associated with insulin resistance in obese adults although circulating myostatin levels have been examined (13). Our results are consistent with a study in middle-aged men whereby plasma myostatin protein levels were inversely correlated with insulin sensitivity measured during an intravenous glucose tolerance test (13). Some potential molecular mechanisms responsible for increased insulin sensitivity in myostatin knock-out mice are increased AMP-activated protein kinase activity in skeletal muscle, increased Glut4 levels, Akt and phosphorylated Akt (19). Treatment of human myoblasts with myostatin for 24 hours decreases the phosphorylation of Akt by approximately 50% (34). During insulin stimulation in vivo, p-Akt increases in adults as a mechanism for increased insulin sensitivity (20) which could suggest that myostatin might change during hyperinsulinemia. Our results also show that the insulin effect on myostatin gene expression was associated with the change in insulin sensitivity after the intervention suggesting that those individuals with the greatest change in myostatin mRNA during hyperinsulinemia had the greatest improvement in insulin sensitivity after AEX+WL. Thus, alterations in insulin signaling may be one mechanistic link with changes in myostatin during hyperinsulinemia in humans although this cannot be addressed in the present investigation. Furthermore, myostatin-knock out mice have an increase in carbohydrate utilization for energy and increase in respiratory exchange ratio (17). Together these would also suggest that the increased carbohydrate oxidation and decrease in fat oxidation during a clamp (22, 35) would align with a potential change in myostatin during insulin. More work is needed to further advance our understanding of myostatin’s role in insulin action in vivo.
There are a several limitations in our study that deserve discussion. First, we recognize that our study cannot distinguish whether aerobic exercise alone (described above in one study) or the weight loss contributed to the changes in myostatin that we observed. There are only two papers that have examined the effects of weight loss on myostatin (14) (15) but neither examined MyoD or myogenin. Both groups of investigators measured myostatin gene expression before and after severe weight loss induced by gastric bypass surgery (body weight decreased by 38% in n=6 and 45% in n=3) in morbidly obese subjects. They reported significant reductions in myostatin mRNA levels (14) (15). Given the large degree of weight loss in these two studies, it is unknown whether the modest (8%) decrease in weight in our current study would have such an effect on myostatin. Additional studies of weight loss alone and aerobic exercise training alone on myostatin would be valuable. Although we observed a 10% decrease in myostatin protein levels, the reduced sample size in this measure could have limited our ability to detect a statistical significant change after AEX+WL. In addition, our study results are only applicable to older overweight and obese adults.
We found that myostatin mRNA expression is significantly reduced concomitant with improved insulin sensitivity after a six-month aerobic exercise training program combined with modest weight loss in obese adults. Further studies are necessary to directly address changes in fuel utilization and the relationship to myostatin signaling. The role of exercise training and myostatin inhibition in the mechanism for improvements in insulin sensitivity also warrants additional investigation.
Acknowledgments
Our appreciation is extended to the women and men who participated in this study. We are grateful to Lyndon J. Joseph, Ph.D., Andrew P. Goldberg, M.D., Leslie Katzel, M.D., Ph.D., the nurses, dieticians, research assistants Melissa Gray, Carol St. Clair, Lynda Robey and Gretchen Zietowski, of the Division of Gerontology and GRECC for their assistance to this project. This study was supported by funds from: the Baltimore VA Medical Research Service, VA Research Career Scientist Award (ASR), VA Merit Award, VA Career Development Award (JB), Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC), NIH grants RO1-AG19310, RO1-AG20116, K01 AG021457, P30 AG028747, P30 DK072488, P60 DK079637, and M01 RR016500.
Footnotes
Competing Interests
The authors have no competing interests.
Clinical Trials #: NCT00882141
References
- 1.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 2.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 3.Grobet L, Martin LJ, Poncelet D, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 4.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 6.Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA. Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol. 2009;106:1720–1729. doi: 10.1152/japplphysiol.00087.2009. [DOI] [PubMed] [Google Scholar]
- 7.Dennis RA, Przybyla B, Gurley C, et al. Aging alters gene expression of growth and remodeling factors in human skeletal muscle both at rest and in response to acute resistance exercise. Physiol Genomics. 2008;32:393–400. doi: 10.1152/physiolgenomics.00191.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deldicque L, Atherton P, Patel R, et al. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol. 2008;104:371–378. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288:E1110–E1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E43–E51. doi: 10.1152/ajpendo.00504.2007. [DOI] [PubMed] [Google Scholar]
- 11.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med. 2003;228:706–709. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 12.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42:416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc. 2010;42:2023–2029. doi: 10.1249/MSS.0b013e3181e0b9a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milan G, Dalla Nora E, Pilon C, et al. Changes in muscle myostatin expression in obese subjects after weight loss. J Clin Endocrinol Metab. 2004;89:2724–2727. doi: 10.1210/jc.2003-032047. [DOI] [PubMed] [Google Scholar]
- 15.Park JJ, Berggren JR, Hulver MW, Houmard JA, Hoffman EP. GRB14, GPD1, and GDF8 as potential network collaborators in weight loss-induced improvements in insulin action in human skeletal muscle. Physiol Genomics. 2006;27:114–121. doi: 10.1152/physiolgenomics.00045.2006. [DOI] [PubMed] [Google Scholar]
- 16.McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. doi: 10.1172/JCI13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. doi: 10.1371/journal.pone.0004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C, McFarlane C, Lokireddy S, et al. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia. 2011;54:1491–1501. doi: 10.1007/s00125-011-2079-7. [DOI] [PubMed] [Google Scholar]
- 20.Storgaard H, Song XM, Jensen CB, et al. Insulin signal transduction in skeletal muscle from glucose-intolerant relatives of type 2 diabetic patients [corrected] Diabetes. 2001;50:2770–2778. doi: 10.2337/diabetes.50.12.2770. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein AH, Appel LJ, Brands M, et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26:2186–2191. doi: 10.1161/01.ATV.0000238352.25222.5e. [DOI] [PubMed] [Google Scholar]
- 22.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity. 2006;14:1064–1072. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 24.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976;41:565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes A. Diagnosis and Classification of Diabetes Mellitus. Diabetes. 2009;32:S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durieux AC, Amirouche A, Banzet S, et al. Ectopic expression of myostatin induces atrophy of adult skeletal muscle by decreasing muscle gene expression. Endocrinology. 2007;148:3140–3147. doi: 10.1210/en.2006-1500. [DOI] [PubMed] [Google Scholar]
- 27.Smith IJ, Aversa Z, Alamdari N, Petkova V, Hasselgren PO. Sepsis downregulates myostatin mRNA levels without altering myostatin protein levels in skeletal muscle. J Cell Biochem. 2010;111:1059–1073. doi: 10.1002/jcb.22796. [DOI] [PubMed] [Google Scholar]
- 28.Coffey VG, Zhong Z, Shield A, et al. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- 29.Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36:574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 30.Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab. 2006;290:E849–E855. doi: 10.1152/ajpendo.00299.2005. [DOI] [PubMed] [Google Scholar]
- 31.Drummond MJ, Fujita S, Abe T, Dreyer HC, Volpi E, Rasmussen BB. Human muscle gene expression following resistance exercise and blood flow restriction. Med Sci Sports Exerc. 2008;40:691–698. doi: 10.1249/MSS.0b013e318160ff84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Heinichen M, Wirth K, Schmidtbleicher D, Steinacker JM. Response of growth and myogenic factors in human skeletal muscle to strength training. Br J Sports Med. 2008;42:989–993. doi: 10.1136/bjsm.2007.045518. [DOI] [PubMed] [Google Scholar]
- 33.Akpan I, Goncalves MD, Dhir R, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes. 2009;33:1265–1273. doi: 10.1038/ijo.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 35.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]