Abstract
Achalasia is a primary esophageal motor disorder. The etiology is still unknown and therefore all treatment options are strictly palliative with the intention to weaken the lower esophageal sphincter (LES). Current established endoscopic therapeutic options include pneumatic dilation (PD) or botulinum toxin injection. Both treatment approaches have an excellent symptomatic short term effect, and lead to a reduction of LES pressure. However, the long term success of botulinum toxin (BT) injection is poor with symptom recurrence in more than 50% of the patients after 12 mo and in nearly 100% of the patients after 24 mo, which commonly requires repeat injections. In contrast, after a single PD 40%-60% of the patients remain asymptomatic for ≥ 10 years. Repeated on demand PD might become necessary and long term remission can be achieved with this approach in up to 90% of these patients. The main positive predictors for a symptomatic response to PD are an age > 40 years, a LES-pressure reduction to < 15 mmHg and/or an improved radiological esophageal clearance post-PD. However PD has a significant risk for esophageal perforation, which occurs in about 2%-3% of cases. In randomized, controlled studies BT injection was inferior to PD and surgical cardiomyotomy, whereas the efficacy of PD, in patients > 40 years, was nearly equivalent to surgery. A new promising technique might be peroral endoscopic myotomy, although long term results are needed and practicability as well as safety issues must be considered. Treatment with a temporary self expanding stent has been reported with favorable outcomes, but the data are all from one study group and must be confirmed by others before definite recommendations can be made. In addition to its use as a therapeutic tool, endoscopy also plays an important role in the diagnosis and surveillance of patients with achalasia.
Keywords: Achalasia, Pneumatic dilation, Botulinum toxin injection, Per oral endoscopic myotomy, Dysphagia, Laparoscopic cardiomyotomy
Core tip: Upper gastrointestinal-endoscopy is an important part in the diagnostic algorithm of achalasia. Although it does not have a high sensitivity in detection of early stage achalasia, it is essential to rule out pseudoachalasia. This updated review included the newest data on treatment and surveillance of achalasia patients with special emphasis on the new treatment option of per oral endoscopic myotomy, including all fulltext publications until January, 2013.
INTRODUCTION
Idiopathic achalasia is a rare primary esophageal motor disorder of unknown etiology, with an estimated incidence of 1 case per 100000 of the general population[1]. It represents a neurodegenerative disorder, in which neurons of the myenteric plexus become destroyed. Although major strides have been made in understanding the pathogenesis, including a probable autoimmune mediated destruction of inhibitory neurons caused by an unknown insult in genetically predisposed patients, the definite pathophysiology is still unknown[2].
Achalasia is characterized by a loss of function of the lower esophageal sphincter and the esophageal peristalsis. The classical features are incomplete relaxation of a frequently hypertensive lower esophageal sphincter (LES) and a lack of peristalsis in the tubular esophagus, which causes symptoms such as dysphagia, regurgitation, weight loss and chest pain.
The diagnosis of achalasia is suspected clinically on the basis of the symptoms mentioned above and confirmed by diagnostic tests, such as barium swallow, and esophageal manometry. However, an endoscopic examination is always necessary to distinguish primary achalasia from the secondary form, in cases of possible malignancy[3].
Since the underlying defect cannot be reversed, the treatment of achalasia remains palliative. Therefore, the aim of all current therapies is the improvement of the esophageal food passage by reducing the distal esophageal obstruction. Such improvement will lead to symptomatic relief of dysphagia, regurgitation, as well as weight gain.
This goal can be achieved by pharmacologic therapy, by endoscopic treatment with pneumatic dilatation (PD) or botulinum toxin (BT) injection, or by surgery. Recently, new therapy options such as stent implantation or peroral endoscopic myotomy (POEM) have been reported[4,5]. However, the efficacy of these treatment options varies and the recommendation for the best therapy is still controversial. Although pneumatic dilation and Heller myotomy seemed to be the most effective treatments for achalasia[6], the choice of treatment modality depends on multiple factors, such as patients’ characteristics, clinical presentation, local expertise and patients preference[7].
In addition, surveillance strategies remain a matter of debate. Despite an increased risk for malignancy there are no existing guidelines for surveillance of cancer or other complications such as esophagitis, peptic strictures or megaesophagus[8,9].
This review will provide an evidence-based approach for the use of endoscopic options for the diagnosis, treatment and surveillance of achalasia.
DIAGNOSTIC USE OF ENDOSCOPY
Endoscopy is one of the primary tools in the diagnosis of achalasia as the leading symptom of the disease is dysphagia. Esophago-gastroscopy, esophageal barium swallow and esophageal manometry are the standard diagnostic procedures in suspected achalasia. Although an endoscopic diagnosis can only be made in about 1/3 of all patients with achalasia, its sensitivity increases with progressive stages of disease[10]. Typically the resistance at the gastroesophageal junction is increased, but still relatively easy to pass with the endoscope. In advanced stages of achalasia the esophagus is dilated and contains retention of food or secretions[11]. The esophageal mucosa usually appears normal, although sometimes inflammation or ulceration caused by retained food can be demonstrated. The endoscopic examination is especially important to rule out other possible causes for the symptoms. These include esophageal and gastric tumors as well as stenosis caused by scarring or inflammatory conditions or by aberrant vascular patterns (e.g., dysphagia lusoria). Especially the esophagogastric junction, as well as the gastric cardia and the fundus, should be examined carefully for evidence of neoplasm, because gastric adenocarcinoma is the most common neoplasm associated with pseudoachalasia[12].
Furthermore, esophago-gastroscopy might be important for the detection and treatment of complications that can be a result of the disease itself such as megaesophagus or carcinoma, or of successful treatment for example reflux esophagitis or peptic stricture[13].
ENDOSCOPIC TREATMENT
The treatment options remain strictly palliative; therefore the primary goal of all therapies is the improvement of the esophageal food passage by reducing the distal esophageal obstruction. Such improvement will lead to symptomatic relief of dysphagia, regurgitation, as well as weight gain. Endoscopic treatments include mechanical rupture of the smooth muscle fibers of the LES and relaxation of the hypertensive lower esophageal sphincter by injection of botulinum toxin, an inhibitor of acetylcholine release from nerve endings[14] as well as novel reported endoscopic therapies such as stent placement, and POEM respectively[4,5].
ENDOSCOPIC INJECTION OF BT
Strictly speaking, botulinum toxin injection into the LES is a pharmacologic treatment, but it requires upper endoscopy for its application.
Botulinum toxin is a neurotoxin that leads to a blockade of the release of acetylcholine from vesicles of excitatory motor neurons. Therefore, it counteracts the loss of inhibitory input to the LES and helps to restore the LES to a lower resting pressure[15].
Botulinum neurotoxins are divided into seven subgroups, identified by the letters A-G. In clinical practice subtype A is most frequently used[16].
The application of BT is performed by prograde or retrograde injection into the LES using a standard sclerotherapy needle. The most common approach is the injection of 20-25 units BT-A diluted in 1 mL of saline, in each of the 4 lower esophageal sphincter quadrants approximately 1 cm above the Z-line into the bulging muscle (Figure 1)[17]. Whether the use of endoscopic ultrasound or manometry to identify the LES can achieve better clinical results has not been definitively established[18,19]. Botulinum toxin diffuses into the surrounding tissue of up to 10 mm, therefore absolute precision might not be necessary[18]. In two studies, instead of BT-A (Allergan Inc., Irvine, CA, United States), Dysport (Ipsen, Milan, Italy) was used at doses of 200-240 U and was equally effective[20]. BT injection is a safe method no more demanding than a routine endoscopy with no major complications. The most common side effect is retrosternal pain in up to 25% of patients[15]. It is an outpatient procedure and the patients can go home after they recover from sedation. The patients are allowed to drink in the recovery room and to eat soft foods later in the day. Symptomatic improvement occurs gradually and usually peaks 1-3 d later, although this may be delayed even further in the occasional patient[21].
Figure 1.
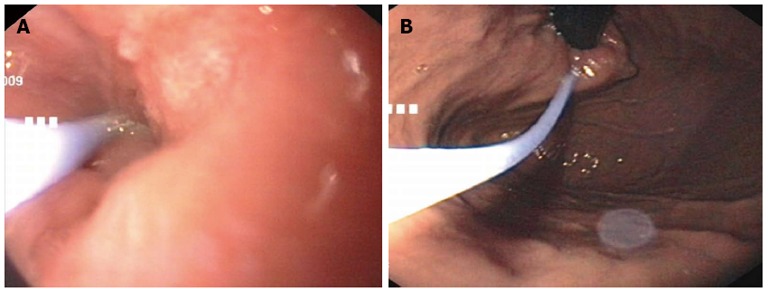
Endoscopic images of botulinum toxin injection. Injection with the standard sclerotherapy needle deep intramuscular in the region of the cardia. A: Prograde injection with an endoscopic view of the distal esophagus; B: Retrograde injection with a retroflexed view of the cardia.
The first clinical studies were conducted in the 1990th, after preliminary studies in piglets[22-24]. In these initial studies, patients were treated with endoscopic injection of botulinum toxin in comparison to placebo injection of saline with symptomatic improvement as well as a remarked reduction of the LES pressure after BT injection were demonstrated[25]. However, the clinical effect of botulinum neurotoxins is reversible, because of the regeneration of the presynaptic membrane[26]. Therefore, the efficacy of a single BT injection has been found to vary from 3 mo to 3 years. In numerous placebo-controlled trials, significant improvement of symptoms has been shown in approximately 75% (70%-90%) of the patients[27] (Table 1). Although, after 12 and 24 mo symptoms recurred in more than 50% and in nearly 100% of the patients, respectively[28-30]. Therefore, repeat injections are commonly required and nearly 75% of the initially responsive patients will respond to a second BT treatment. However, patients who failed to respond to initial BT injection respond to a second injection in less than 20%[31]. Furthermore, it is known that increasing the dose to 200 U BT does not improve the success rate whereas two injections of 100 U of BT 30 d apart seemed to be the most effective therapeutic schedule[32].
Table 1.
Efficacy of botulinum toxin injection in the treatment of achalasia
| Ref. | n | BT-dose U | Initial symptomatic response | Injection rate | Long-term symptomatic response | Follow up (mo) |
| Wehrmann et al[19] | 20 | 100 | 80% | 2.5 | 70% | 24 |
| Annese et al[20] | 36 | 100 | 90% | 0 | 78% | 6 |
| Pasricha et al[28] | 31 | 80 | 90% | 1.6 | 68% | 12 |
| Fishman et al[31] | 60 | 80 | 70% | 1.3 | 36% | 12 |
| Annese et al[32] | 38 | 100 | 82% | 1 | 68% | 24 |
| Gordon et al[100] | 16 | 80 | 75% | 1.25 | 58% | 7 |
| Cuillière et al[101] | 55 | 80 | 85% | 1.2 | 60% | 6 |
| Vaezi et al[102] | 22 | 100 | 64% | 1.1 | 32% | 12 |
BT: Botulinum toxin.
However, the long-term safety and efficacy are less certain[20]. It is known that repeated BT injections may lead to decreased effects due to the development of inhibitory antibodies[15] and there is some evidence that injection of BT into the LES is associated with increased difficulty of performing esophagomyotomy[33].
The long-term success of BT injection into the LES in patients with achalasia was highest in elderly patients (> 55 years), in patients with vigorous achalasia and those with an LES pressure not exceeding the upper normal level by 50% or more prior to treatment[34,35]. In fact, several investigators have speculated that the better long-term response to BT injections seen in the elderly might be explained by diminished nerve regeneration[36].
In summary, the advantages of this method are that it is simple, effective and relatively inexpensive, with no major side effects and excellent short-term results. Unfortunately this result only lasts for 6-9 mo on average in most patients and only half of them benefit for more than 1 year[37]. Because of its less invasive nature compared with other therapeutic alternatives Botox injection may be the preferred approach in the treatment of some patients with achalasia, such as elderly patients (Figure 2) or patients with multiple medical problems who are poor candidates for more invasive procedures as well as those unwilling to have either surgery or pneumatic dilatation[38]. Furthermore, BT injection might be a useful therapy in patients with atypical achalasia, or complex achalasia in whom it is unclear whether more invasive procedures such as pneumatic dilation or surgical myotomy are the correct therapy[39].
Figure 2.
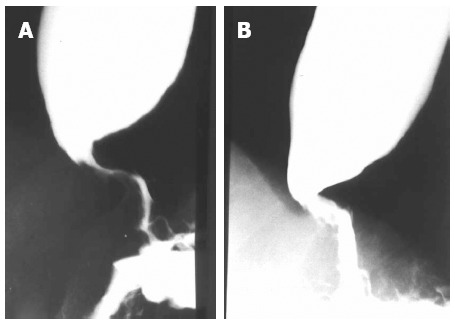
Radiologic image of the esophagus of an elderly patient. A: Before Botox injection; B: After Botox injection, with a decrease of the diameter in the area of the lower esophageal sphincter.
DILATION OF THE LES
Theoretically, there are two possible modalities used to dilate the LES in patients with achalasia: bougienage and pneumatic balloon dilation.
Although bougienage is a technique known to be highly effective in peptic or anastomotic strictures, it provides only temporary and incomplete symptom relief in patients with achalasia[40,41]. Therefore, the more forceful stretching of the LES with pneumatic balloon dilation that weakens the LES by tearing its muscle fibers is the preferred approach.
PD
Pneumatic dilatation has been a well established and proven treatment for achalasia for decades and is currently considered the most effective nonsurgical treatment option for achalasia[42].
Since the first description of treating achalasia with whale bone by Sir Thomas Willis in 1674, the aim of the procedure has principally remained the same. That is to rupture the hypertensive smooth muscle of the LES. In the past different kinds of balloons such as Witzel or Mosher balloons, with a remarkable variation in the methods of dilatation were used for the forceful dilation[43,44]. The procedure has become more standardized with the development of the so called Rigiflex balloon System (Boston Scientific Corporation, MA, United States), a low compliance polyethylene balloon available in 3 diameters (3.0, 3.5, and 4.0 cm) (Figure 3A). It is fixed on a flexible catheter that can be placed over an endoscopically placed guidewire with subsequent fluoroscopic monitoring of the balloon position across the LES. The rapid inflation of the balloon with air leads to stretching of the LES muscle fibers, resulting in at least partial rupture. In order to avoid radiation exposures, some centers monitor balloon position by direct endoscopic observation[45]. A pressure of up to 10-12 psi (average 7 psi) is used to inflate the balloon for 1-2 min until the waist of the balloon, which lies in the region of the LES, is completely elapsed (Figure 3B). The dilation protocols and follow-up varies among different investigators in the United States and Europe[13]. Some authors have used single dilation[46], others performed serial graded dilations on consecutive days or a few weeks apart with balloon sizes ranging from 3 to 4 cm[47-50] and a few European centers perform serial progressive dilations over several days, until the manometrically measured LES pressure is below 10-15 mmHg[51].
Figure 3.
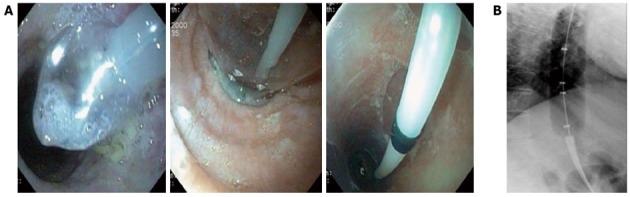
Pneumatic dilation with a rigiflex balloon. A: Endoscopic image; B: Radiologic images. The waist of the balloon lies in the region of the lower esophageal sphincter.
However, in the past numerous comparative studies found no significant different symptomatic response rates for the use of different balloon systems, or different length of inflation or peak pressures respectively, although previous studies could show that the use of a Rigiflex dilator and multiple dilations during the initial treatment might improve efficacy[13,43].
The technique of graded balloon dilation starting with 3.0-cm Rigiflex balloon as the initial dilator and progressing to 3.5-cm and 4.0-cm balloon in absence of response to previous balloon size seems to be the safest approach[52]. Following dilation, radiologic esophagograms with water-soluble contrast agents are frequently performed to rule out serious complications; whereas others do not recommend a routine esophagogram in the absence of symptoms suggestive of a perforation, such as chest pain often with radiation to the back or to the shoulder, followed in one third of patients by vomiting and shortness of breath[53].
Transmural perforation, mostly located just above the cardia along the left side of the esophagus where there is an anatomic area of weakness. The perforation rate reported in different studies ranges between 0%-5% with a mean range of 2%-3% (Table 2). In the review of Katzka et al[43] in which 29 studies of pneumatic dilation in achalasia were evaluated the overall perforation rate was 2% of which only 1% required surgery.
Table 2.
Initial effiacy of pneumatic dilation in the treatment of achalasia
| Ref. | Dilator-system | n | Symptomatic response | Perforation rate |
| Chuah et al[52] | Rigiflex | 32 | 91% | 3% |
| Eckardt et al[61] | Brown-McHardy | 54 | 78% | 2% |
| Wehrmann et al[62] | Rigiflex | 40 | 88% | 3% |
| Csendes et al[73] | Mosher | 39 | 65% | 5% |
| Stark et al[103] | Brown-McHardy | 10 | 100% | 0% |
| Parkman et al[104] | Brown-McHardy | 123 | 88% | 2% |
| Coccia et al[105] | Rider-Moeller | 16 | 75% | 0% |
| Bourgeois et al[106] | Rider-Moeller | 53 | 80% | 4% |
| Gelfand et al[107] | Rigiflex | 24 | 83% | 0% |
| Vaezi et al[108] | Rigiflex | 20 | 75% | 5% |
| Rai et al[109] | Rigiflex | 56 | 89% | 0% |
The mortality rate (5%-6%) after transmural perforation due to pneumatic dilation is usually caused by the development of mediastinitis or bleeding into the mediastinum[54]. In general, conservative treatment with fluid resuscitation, gastric decompression, and antibiotics, best combined with an immediate endoscopic closure of the perforation, is a possible option[43,52]. Complications following pneumatic dilation, if recognized and treated promptly, were not associated with adverse, long-term sequelae[50]. Multiple dilations, the use of inflation pressures > or = 11 psi or a large balloon (4 cm) at initial dilation as well as older age (> 65 years) seemed to be risk factors for esophageal perforation[55]. Although suspected by early observations, a hiatus hernia, a diverticulum of the esophagus and vigorous achalasia do not increase this risk[56]. Other minor complications include esophageal mucosal tears, bleeding, intramural hematomas, aspiration and diverticula at the cardia[50,56]. Post procedural fever usually resolves spontaneously without the use of antibiotics, and in approximately 15% of patients severe but self- limited chest pain occurs[50,55].
Furthermore, some patients will develop reflux when measured by 24-h esophageal pH monitoring. Although severe complications of gastro esophageal reflux disease such as peptic stricture, or Barrett esophagus are rare, 15%-45% of the patients will complain of heartburn responding to proton pump inhibitor treatment[57,58].
The only absolute contraindication for pneumatic dilatation is poor cardiopulmonary status or other comorbid illness preventing surgery, if a transmural perforation might occur[17].
Outcome of PD
Initial success rates are high with up to 85% of patients reporting symptom improvement after one month. Table 2 summarizes the results of several studies according to the short-term symptomatic success rate of PD.
A recently published review of 21 studies using Rigiflex balloons demonstrated that the initial success rates depends on the balloon size, with larger balloons showing better outcomes. Success rates of 74%, 80% and 90% were achieved when using balloon sizes of 30, 35 and 40 mm, respectively[45].
However, a decline in success rates over time was consistently found. For example, researchers achieved success rates of 74% at 6 mo, 68% at 12 mo and 58% after 36 or more months. If patients are observed for more than 10 years, only 40%-60% will remain asymptomatic after a single PD. Therefore, repeated on demand PD might be necessary and long term remission can be achieved with this approach in up to 90% of the patients[47,50,58].
Nevertheless, it must be considered that the patients with frequent PD are exposed to potentially serious complications such as esophageal perforation, intramural hematoma or aspiration and the uncertain durability of symptom free intervals between dilations[59,60]. Therefore, it is important to predict which patient is less likely to respond or will have an early recurrence of symptoms. In fact, patients older than 40 years generally have better outcomes following dilation than those who are younger[61,62]. Further positive predictive factors are a LES-pressure of < 15 mmHg or a LES pressure reduction of more than 50% in comparison to the pre-dilation LES pressure[63,64]. By contrast, a wide esophagus, the use of small balloon sizes, an incomplete obliteration of the balloon waist during the procedure, a failed response to one or two dilations, type I or III patterns of achalasia in high resolution manometry, poor esophageal clearance on a timed barium swallow and younger male patients have been shown to predict a poor treatment response[63,65-67].
A recently published study reported a new predictor of treatment success by measuring the distensibility of the esophagogastric junction with an endoscopic functional luminal imaging probe (EndoFLIP®). Even when LES pressure was low, esophagogastric junction distensibility could be reduced, which was associated with impaired emptying and recurrent symptoms[68]. Although it must be considered that even if LES pressure is not an optimal predictor, it still remains a valuable measure in clinical practice.
In summary, PD is safer than commonly thought and very effective even in the long term, although multiple dilations will be needed over a lifetime in most patients. The technique of graded balloon dilation starting with 3.0 cm Rigiflex balloon as the initial dilator and progressing to 3.5 and 4.0 cm balloon in absence of response to previous balloon size seems to be the safest approach[69]. Patients not responding to three serial dilations are less likely to respond to repeated dilations and should be offered surgery.
Comparative trials between various treatment modalities
The review of six randomized controlled trials comparing PD to BT injection in patients with primary achalasia demonstrated no significant difference in symptomatic remission and the mean esophageal pressure within 4 wk of the initial intervention. However, in the long term (> 6 mo) PD was more effective[30]. The combination of both treatments does not improve the outcome[35].
In summary, BT injection has similar efficacy as pneumatic dilatation in achieving an initial improvement in dysphagia. It can also be effective in some patients with tortuous megaesophagus and vigorous achalasia, but serial injections are required to sustain relief and its long term efficacy is inferior to PD[70]. Furthermore, serial BT injection is more costly than PD dilation, if the life-expectancy is > 2 years[71].
The role of PD in comparison to surgical myotomy is less clear. The difficulty in comparing both therapies is due to the lack of prospective randomized studies with a long follow up (> 5 years) in a large population and the lack of standardized technique of balloon dilation.
In the past years meta-analyses have favored surgery as the best treatment to achieve long-term success[42,72]. However, these analyses mostly included retrospective studies of different sizes and quality and did not include approaches with on demand repeat dilations. In fact, a repeated dilation was the negative endpoint in some of the studies.
Until recently, only one randomized study existed. The study by Csendes et al[73], in which conventional cardiomyotomy plus Dor fundoplication was compared with the pneumatic dilation using the so-called “Mosher Bag”, reported symptomatic response rates 5 years after treatment of 95%, and 65% in the surgical and PD group, respectively. However, the technique used for the pneumatic dilation was possibly suboptimal and a later published long-term follow-up of the same patient group showed that the results of the surgery were less favorable after more than 15 years of observation, with only 75% of patients being in sustained remission.
Last year, the results of a European multicenter study were published[74]. In one study arm, patients were treated (n = 94) with PD, starting with a 30 mm Rigiflex balloon, followed 1 to 3 wk later by dilation with the use of a 35-mm balloon. All patients thus underwent at least two dilations. If the Eckardt score 4 wk later was greater than 3, a third dilation was performed, with the use of a 40-mm balloon. The other group (n = 104) received a laparoscopic Heller cardiomyotomy with antireflux technique (LHM). Both treatments had comparable therapeutic success at 2 years, with 86% of the patients achieving symptomatic relief with PD and LHM, respectively. Furthermore, there was no significant difference in the LES pressure or esophageal emptying, as assessed by the height of barium-contrast column in both groups. Although age was not an overall predictor for therapeutic success for treatment, similar to previous investigations, an inferior symptomatic response of PD in patients with age < 40 years was observed.
The rate of complications as well as the frequency of induction of gastroesophageal reflux was similar in both groups. This data suggests that PD and LHM have equal efficacy, given that PD is performed with at least two dilations.
Not surprisingly, the only study comparing BT injections with laparoscopic cardiomyotomy showed an inferiority of BT. The 1-year remission rate was 53% in the BT group and 90% in the myotomy group and 2 years later only 34% of the patients treated with BT and 88% of the operated patients were in clinical remission[75].
NEW ENDOSCOPIC THERAPEUTIC APPROACHES
POEM
POEM is a new endoscopic treatment for achalasia. Ortega et al[76] first reported an endoscopic myotomy in the treatment of achalasia using a needle knife to cut the inner circular muscle fibers of the LES by cutting directly through the mucosa during endoscopy.
After this small study with excellent results the method fell into oblivion, until Pasricha et al[77] reported a technique of endoscopic submucosal method on a pig model. Afterwards Inoue et al[78] described a clinical application of the modified Pasricha technique as POEM. This approach involves endoscopic dissection of the esophageal submucosal space (under CO2 insufflation) to gain access to LES muscle fibers. The semicircumferent dissection starts approximately 6-13 cm proximal to the esophagogastric junction and is extended 2 cm into the stomach. Circular muscle bundles are then dissected, leaving the longitudinal muscle layer intact. Inoue et al[78] could show a significant improvement of dysphagia and reduction of LES pressure after this intervention, although the mean postinterventional LES pressure was still high at 20 mmHg. Most recently, several centers are using the POEM technique and reported excellent short term results and no “serious” complications, although pneumomediastinitis, C-reactive protein elevation are common and long term results are required[78-80]. A short overview of the results to date is given by Table 3.
Table 3.
Results of peroral endoscopic myotomy for the treatment of patients
| Ref. | n | Mean age (yr) | Myotomy length (cm) | Follow-up (mo) | Symptomatic response (Eckardt score before/after POEM) | LES-tone (before/after POEM, mmHg) |
| Inoue et al[5] | 17 | 41 | 8 | 5 | 10/1.3 | 52/20 |
| von Renteln et al[80] | 16 | 45 | 12 | 3 | 8.8/1.1 | 27/12 |
| Swanström et al[110] | 5 | 64 | 7 | 1 | Not quantified | Not measured |
| Costamagna et al[111] | 11 | 32 | 10 | 1 | 7.1/1.1 | 45/17 |
| Chiu et al[112] | 16 | 48 | 11 | 6 | 5.5/0 | 44/30 |
Only full text publications are considered. LES: Lower esophageal sphincter; POEM: Peroral endoscopic myotomy.
The procedure is promoted as less invasive than surgical myotomy, but it still requires general anesthesia and is not less time consuming than a laparoscopic approach. It is a sophisticated and demanding technique even for experienced endoscopists and so far has shown suboptimal results for lowering LES pressure compared in comparison to the published results with surgery. Furthermore revisional surgery might be more difficult because the space between the submucosal and muscular layers might become inflamed and scarred[81].
In summary, it is a very interesting approach but long term results as well as a comparison of POEM with other treatment modalities in randomized controlled studies are required and it’s use should only be applied in the context of clinical trials.
Stenting
Another novel therapeutic approach is temporary esophageal stenting. Recently, a strategy of using retrievable stents has been successfully applied in the treatment of benign esophageal strictures[82,83]. A few studies, from a single Chinese study group reported a symptomatic benefit with the use of self expanding metal stents in patients with achalasia. In this endoscopic approach a partially covered, self-expanding metal stent (SEMS) with a diameter of 20, 25 or 30 mm was applicated in unsedated patients with achalasia. It was kept in place for 1 wk and then it was removed endoscopically. The best results after 10 years were shown in patients treated with a 30 mm stent. The clinical remission rate was 86%, 27%, 13%, 0%, in 30 mm SEMS, 25 mm SEMS, 20 mm SEMS and PD, respectively[84,85]. In contrast, other study groups could not confirm these results and complications, such as stent migration, chest pain and reflux esophagitis have been reported, with a mortality and morbidity of 33% and 50% respectively[86,87].
Ethanolamine oleate injection
Case reports from southern Europe[88,89] and Iran[90] reported a good response after endoscopic injection of the sclerosing agent ethanolamine oleate in the cardia. As a possible mechanism inflammatory destruction of the LES is discussed. Symptom relief as well as improved esophageal emptying has been demonstrated. However, the reported number of cases is very small and the longest follow up was 17 mo.
USE OF ENDOSCOPY FOR SURVEILLANCE
In patients with achalasia surveillance is important for several reasons. First, treatment success needs to be documented by objective parameters. Second, regular follow-up enables the clinician to detect symptomatic recurrences at an early stage and, third, endoscopic surveillance has the potential for early recognition of late complications, such as esophageal squamous cell cancer, megaesophagus or reflux esophagitis.
Objective evaluation of treatment success at least with a structured symptom orientated questionnaire and esophageal manometry, or better with additional timed barium esophagogram and endoscopy should be performed early (4-12 wk) after the initial intervention. Some centers even perform esophageal manometry intraoperatively or immediately after pneumatic dilation[91,92]. A post-dilation LES resting pressure of < 10-15 mmHg is generally considered to be predictive of a good long-term response[61,62]. However, falsely elevated LES pressure could be measured immediately after disruption of the LES due to associated edema. In the immediate post interventional period endoscopy is less important, but it is useful for further surveillance. Endoscopy might have a role in the detection or prevention of long-term complications. Up to 10% of all patients with long-standing achalasia (more than 10 years after first diagnosis) develop progressive enlargement of the esophagus, which can lead to a sigmoid-shaped esophagus and/or megaesophagus[93] (Figure 4). This complication more frequently develops in patients who remain ineffectively treated for years. If these morphological changes are only recognized at an advanced stage, esophageal resection may be the only remaining therapeutic option[13].
Figure 4.
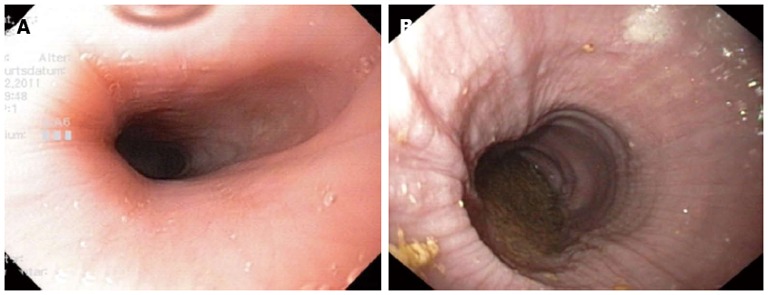
Endoscopic images of patients with achalasia. A: Early achalasia; B: Advanced achalasia with a megaesophagus, hyperplasia of the esophageal epithelium.
In addition, the risk of esophageal cancer in achalasia patients is estimated to be approximately 30-fold higher than in the general population[8,9]. Especially in male achalasia patients, a substantially greater risk for both squamous cell carcinoma and adenocarcinoma of the esophagus has been shown, whereas the risk in female patients could not be evaluated due to the small numbers[94].
The first prospective evaluation of esophageal cancer risk in a large cohort of achalasia patients with long-term follow-up demonstrated an increased rate of esophageal cancer. The mean age at cancer diagnosis was 71 years, after a mean of 11 years (range 2-23 years) following initial diagnosis, and a mean of 24 years (range 10-43 years) after symptom onset. Although, most neoplastic lesions remained undetected until an advanced stage, despite structured endoscopic surveillance, the authors suggested such a surveillance strategy in patients with longstanding achalasia[95].
Even if the latest American Society of Gastrointestinal Endoscopy guidelines correctly state that there are still “insufficient data to support routine endoscopic (cancer) surveillance for patients with achalasia”[96], endoscopic surveillance might be beneficial in particular if one considers that cancer is not the only late complication of this disease. Therefore, most experts favor some form of endoscopic surveillance in patients with achalasia if the disease has been present for more than 10-15 years[97,98]. It could be considered that chromoendoscopy or narrow band imaging might be superior for early detection of neoplastic lesions, but further studies are needed to compare these techniques with standard endoscopy.
Another long-term complication that requires careful attention is the development of clinically significant gastro-esophageal reflux disease (GERD), which occurs in up to 25% of patients with achalasia who are followed up for > 15 years[99]. GERD-related findings range from reflux esophagitis and peptic strictures to Barrett’s esophagus, which in rare instances may progress to esophageal adenocarcinoma. In our practice follow-up visits are recommended biannually. The patients undergo structured interviews using a scoring system (Eckardt score) for the symptoms and upper gastrointestinal (GI)-endoscopy to detect reflux-esophagitis or the development of a megaesophagus. If achalasia has been present for more than 10 years the follow-up interval is shortened to annual intervals.
However, further studies are needed to determine whether such surveillance strategies will improve the overall outcome.
CONCLUSION
Upper GI-endoscopy is an important part in the diagnostic algorithm of achalasia. Although it does not have a high sensitivity in detection of early stage achalasia, it is essential to rule out pseudoachalasia.
Treatment remains palliative as the neuronal defect of the disease seems to be irreversible. Therefore, the primary goal of all therapies is the improvement of the esophageal passage by disruption of the LES and the prevention of long-term complications. The most effective endoscopic therapy is graded pneumatic dilation with Rigiflex balloons, whereas the endoscopic injection of Botulinum toxin injection is mostly reserved for old patients or those with major comorbid illnesses preventing surgery. A new promising technique might be POEM although long-term results and comparison of POEM to PD and LHM are needed.
Treatment with a temporary self expanding stent are reported by one group who reported a better long term effect than PD, but the results of PD were poor in this study and the data must be confirmed by others before this method can be recommended. In addition, multiple complications such as stent migration, bleeding and chest pain can occur with this technique.
Most experts favor some form of endoscopic surveillance in patients if achalasia has been present for more than 10-15 years. However, no guidelines exist and further studies are needed to determine whether and which surveillance strategies will improve overall outcome.
Footnotes
P- Reviewer Holscher AH S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–374. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Gockel HR, Schumacher J, Gockel I, Lang H, Haaf T, Nöthen MM. Achalasia: will genetic studies provide insights? Hum Genet. 2010;128:353–364. doi: 10.1007/s00439-010-0874-8. [DOI] [PubMed] [Google Scholar]
- 3.Gockel I, Eckardt VF, Schmitt T, Junginger T. Pseudoachalasia: a case series and analysis of the literature. Scand J Gastroenterol. 2005;40:378–385. doi: 10.1080/00365520510012118. [DOI] [PubMed] [Google Scholar]
- 4.Zhu YQ, Cheng YS, Tang GY, Li MH, Zhao JG, Li F. Comparison of temporary stent insertion with pneumatic dilation of the same diameter in the treatment of achalasia patients: a retrospective study. J Gastroenterol Hepatol. 2010;25:499–505. doi: 10.1111/j.1440-1746.2009.06107.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, Satodate H, Odaka N, Itoh H, Kudo S. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–271. doi: 10.1055/s-0029-1244080. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt AJ, Eckardt VF. Achalasia: Should pneumatic dilation be the primary treatment strategy? Nat Rev Gastroenterol Hepatol. 2010;7:188–190. doi: 10.1038/nrgastro.2010.33. [DOI] [PubMed] [Google Scholar]
- 7.Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15:3969–3975. doi: 10.3748/wjg.15.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porschen R, Molsberger G, Kühn A, Sarbia M, Borchard F. Achalasia-associated squamous cell carcinoma of the esophagus: flow-cytometric and histological evaluation. Gastroenterology. 1995;108:545–549. doi: 10.1016/0016-5085(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 9.Streitz JM, Ellis FH, Gibb SP, Heatley GM. Achalasia and squamous cell carcinoma of the esophagus: analysis of 241 patients. Ann Thorac Surg. 1995;59:1604–1609. doi: 10.1016/0003-4975(94)00997-l. [DOI] [PubMed] [Google Scholar]
- 10.Howard PJ, Maher L, Pryde A, Cameron EW, Heading RC. Five year prospective study of the incidence, clinical features, and diagnosis of achalasia in Edinburgh. Gut. 1992;33:1011–1015. doi: 10.1136/gut.33.8.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeckxstaens GE. Achalasia. Best Pract Res Clin Gastroenterol. 2007;21:595–608. doi: 10.1016/j.bpg.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Fackler W, Rice TW, Richter JE, Achkar E, Goldblum JR. The pathogenesis of pseudoachalasia: a clinicopathologic study of 13 cases of a rare entity. Am J Surg Pathol. 2002;26:784–788. doi: 10.1097/00000478-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt AJ, Eckardt VF. Treatment and surveillance strategies in achalasia: an update. Nat Rev Gastroenterol Hepatol. 2011;8:311–319. doi: 10.1038/nrgastro.2011.68. [DOI] [PubMed] [Google Scholar]
- 14.Dunaway PM, Wong RK. Achalasia. Curr Treat Options Gastroenterol. 2001;4:89–100. doi: 10.1007/s11938-001-0051-1. [DOI] [PubMed] [Google Scholar]
- 15.Richter JE. Achalasia - an update. J Neurogastroenterol Motil. 2010;16:232–242. doi: 10.5056/jnm.2010.16.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gui D, Rossi S, Runfola M, Magalini SC. Review article: botulinum toxin in the therapy of gastrointestinal motility disorders. Aliment Pharmacol Ther. 2003;18:1–16. doi: 10.1046/j.1365-2036.2003.01598.x. [DOI] [PubMed] [Google Scholar]
- 17.Classen M, Tytgat GN, Lightdale CJ, editors . Gastroenterological Endoscopy. 2nd ed. Stuttgart: Georg Thieme Verlag; 2010. p. 467. [Google Scholar]
- 18.Hoffman BJ, Knapple WL, Bhutani MS, Verne GN, Hawes RH. Treatment of achalasia by injection of botulinum toxin under endoscopic ultrasound guidance. Gastrointest Endosc. 1997;45:77–79. doi: 10.1016/s0016-5107(97)70306-7. [DOI] [PubMed] [Google Scholar]
- 19.Wehrmann T, Schmitt T, Dietrich CF, Caspary WF, Seifert H. Manometrically-guided endoscopic injection of botulinum toxin for esophageal achalasia: a pilot trial. Z Gastroenterol. 2000;38:899–903. doi: 10.1055/s-2000-10294. [DOI] [PubMed] [Google Scholar]
- 20.Annese V, Bassotti G, Coccia G, D’onofrio V, Gatto G, Repici A, Andriulli A. Comparison of two different formulations of botulinum toxin A for the treatment of oesophageal achalasia. The Gismad Achalasia Study Group. Aliment Pharmacol Ther. 1999;13:1347–1350. doi: 10.1046/j.1365-2036.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 21.Dughera L, Chiaverina M, Cacciotella L, Cisarò F. Management of achalasia. Clin Exp Gastroenterol. 2011;4:33–41. doi: 10.2147/CEG.S11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasricha PJ, Ravich WJ, Kalloo AN. Effects of intrasphincteric botulinum toxin on the lower esophageal sphincter in piglets. Gastroenterology. 1993;105:1045–1049. doi: 10.1016/0016-5085(93)90947-b. [DOI] [PubMed] [Google Scholar]
- 23.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Treatment of achalasia with intrasphincteric injection of botulinum toxin. A pilot trial. Ann Intern Med. 1994;121:590–591. doi: 10.7326/0003-4819-121-8-199410150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Rollan A, Gonzalez R, Carvajal S, Chianale J. Endoscopic intrasphincteric injection of botulinum toxin for the treatment of achalasia. J Clin Gastroenterol. 1995;20:189–191. doi: 10.1097/00004836-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Pasricha PJ. Pharmacologic therapy in treating achalasia. Gastrointest Endosc Clin N Am. 2001;11:311–24, vii. [PubMed] [Google Scholar]
- 26.Ma J, Shen J, Lee CA, Elsaidi GA, Smith TL, Walker FO, Rushing JT, Tan KH, Koman LA, Smith BP. Gene expression of nAChR, SNAP-25 and GAP-43 in skeletal muscles following botulinum toxin A injection: a study in rats. J Orthop Res. 2005;23:302–309. doi: 10.1016/j.orthres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Richter JE. Update on the management of achalasia: balloons, surgery and drugs. Expert Rev Gastroenterol Hepatol. 2008;2:435–445. doi: 10.1586/17474124.2.3.435. [DOI] [PubMed] [Google Scholar]
- 28.Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long-term outcome and predictors of response. Gastroenterology. 1996;110:1410–1415. doi: 10.1053/gast.1996.v110.pm8613045. [DOI] [PubMed] [Google Scholar]
- 29.Allescher HD, Storr M, Seige M, Gonzales-Donoso R, Ott R, Born P, Frimberger E, Weigert N, Stier A, Kurjak M, et al. Treatment of achalasia: botulinum toxin injection vs. pneumatic balloon dilation. A prospective study with long-term follow-Up. Endoscopy. 2001;33:1007–1017. doi: 10.1055/s-2001-18935. [DOI] [PubMed] [Google Scholar]
- 30.Leyden JE, Moss AC, MacMathuna P. Endoscopic pneumatic dilation versus botulinum toxin injection in the management of primary achalasia. Cochrane Database Syst Rev. 2006;(4):CD005046. doi: 10.1002/14651858.CD005046.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Fishman VM, Parkman HP, Schiano TD, Hills C, Dabezies MA, Cohen S, Fisher RS, Miller LS. Symptomatic improvement in achalasia after botulinum toxin injection of the lower esophageal sphincter. Am J Gastroenterol. 1996;91:1724–1730. [PubMed] [Google Scholar]
- 32.Annese V, Bassotti G, Coccia G, Dinelli M, D’Onofrio V, Gatto G, Leandro G, Repici A, Testoni PA, Andriulli A. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597–600. doi: 10.1136/gut.46.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CD, Stival A, Howell DL, Swafford V. Endoscopic therapy for achalasia before Heller myotomy results in worse outcomes than heller myotomy alone. Ann Surg. 2006;243:579–84; discussion 584-6. doi: 10.1097/01.sla.0000217524.75529.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Onofrio V, Miletto P, Leandro G, Iaquinto G. Long-term follow-up of achalasia patients treated with botulinum toxin. Dig Liver Dis. 2002;34:105–110. doi: 10.1016/s1590-8658(02)80238-9. [DOI] [PubMed] [Google Scholar]
- 35.Mikaeli J, Bishehsari F, Montazeri G, Mahdavinia M, Yaghoobi M, Darvish-Moghadam S, Farrokhi F, Shirani S, Estakhri A, Malekzadeh R. Injection of botulinum toxin before pneumatic dilatation in achalasia treatment: a randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:983–989. doi: 10.1111/j.1365-2036.2006.03083.x. [DOI] [PubMed] [Google Scholar]
- 36.Neubrand M, Scheurlen C, Schepke M, Sauerbruch T. Long-term results and prognostic factors in the treatment of achalasia with botulinum toxin. Endoscopy. 2002;34:519–523. doi: 10.1055/s-2002-33225. [DOI] [PubMed] [Google Scholar]
- 37.Pasricha PJ, Ravich WJ, Hendrix TR, Sostre S, Jones B, Kalloo AN. Intrasphincteric botulinum toxin for the treatment of achalasia. N Engl J Med. 1995;332:774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 38.Bruley des Varannes S, Scarpignato C. Current trends in the management of achalasia. Dig Liver Dis. 2001;33:266–277. doi: 10.1016/s1590-8658(01)80718-0. [DOI] [PubMed] [Google Scholar]
- 39.Katzka DA, Castell DO. Use of botulinum toxin as a diagnostic/therapeutic trial to help clarify an indication for definitive therapy in patients with achalasia. Am J Gastroenterol. 1999;94:637–642. doi: 10.1111/j.1572-0241.1999.00927.x. [DOI] [PubMed] [Google Scholar]
- 40.McJunkin B, McMillan WO, Duncan HE, Harman KM, White JJ, McJunkin JE. Assessment of dilation methods in achalasia: large diameter mercury bougienage followed by pneumatic dilation as needed. Gastrointest Endosc. 1991;37:18–21. doi: 10.1016/s0016-5107(91)70614-7. [DOI] [PubMed] [Google Scholar]
- 41.Mandelstam P, Dillon M. Role of bougienage in the management of achalasia--need for reappraisal in the light of recent studies. J Clin Gastroenterol. 1990;12:3–4. [PubMed] [Google Scholar]
- 42.Wang L, Li YM, Li L. Meta-analysis of randomized and controlled treatment trials for achalasia. Dig Dis Sci. 2009;54:2303–2311. doi: 10.1007/s10620-008-0637-8. [DOI] [PubMed] [Google Scholar]
- 43.Katzka DA, Castell DO. Review article: an analysis of the efficacy, perforation rates and methods used in pneumatic dilation for achalasia. Aliment Pharmacol Ther. 2011;34:832–839. doi: 10.1111/j.1365-2036.2011.04816.x. [DOI] [PubMed] [Google Scholar]
- 44.Vela MF, Richter JE, Khandwala F, Blackstone EH, Wachsberger D, Baker ME, Rice TW. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–587. doi: 10.1016/s1542-3565(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 45.Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long-term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10:3322–3327. doi: 10.3748/wjg.v10.i22.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629–633. doi: 10.1136/gut.2003.029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.West RL, Hirsch DP, Bartelsman JF, de Borst J, Ferwerda G, Tytgat GN, Boeckxstaens GE. Long term results of pneumatic dilation in achalasia followed for more than 5 years. Am J Gastroenterol. 2002;97:1346–1351. doi: 10.1111/j.1572-0241.2002.05771.x. [DOI] [PubMed] [Google Scholar]
- 48.Chan KC, Wong SK, Lee DW, Mui WL, Chan AC, Ng EK, Wu JC, Sung JJ, Chung SC. Short-term and long-term results of endoscopic balloon dilation for achalasia: 12 years’ experience. Endoscopy. 2004;36:690–694. doi: 10.1055/s-2004-825659. [DOI] [PubMed] [Google Scholar]
- 49.Kadakia SC, Wong RK. Graded pneumatic dilation using Rigiflex achalasia dilators in patients with primary esophageal achalasia. Am J Gastroenterol. 1993;88:34–38. [PubMed] [Google Scholar]
- 50.Zerbib F, Thétiot V, Richy F, Benajah DA, Message L, Lamouliatte H. Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol. 2006;101:692–697. doi: 10.1111/j.1572-0241.2006.00385.x. [DOI] [PubMed] [Google Scholar]
- 51.Hulselmans M, Vanuytsel T, Degreef T, Sifrim D, Coosemans W, Lerut T, Tack J. Long-term outcome of pneumatic dilation in the treatment of achalasia. Clin Gastroenterol Hepatol. 2010;8:30–35. doi: 10.1016/j.cgh.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Chuah SK, Hu TH, Wu KL, Hsu PI, Tai WC, Chiu YC, Lee CM, Changchien CS. Clinical remission in endoscope-guided pneumatic dilation for the treatment of esophageal achalasia: 7-year follow-up results of a prospective investigation. J Gastrointest Surg. 2009;13:862–867. doi: 10.1007/s11605-009-0804-z. [DOI] [PubMed] [Google Scholar]
- 53.Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med. 2011;19:66. doi: 10.1186/1757-7241-19-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanuytsel T, Lerut T, Coosemans W, Vanbeckevoort D, Blondeau K, Boeckxstaens G, Tack J. Conservative management of esophageal perforations during pneumatic dilation for idiopathic esophageal achalasia. Clin Gastroenterol Hepatol. 2012;10:142–149. doi: 10.1016/j.cgh.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 55.Nair LA, Reynolds JC, Parkman HP, Ouyang A, Strom BL, Rosato EF, Cohen S. Complications during pneumatic dilation for achalasia or diffuse esophageal spasm. Analysis of risk factors, early clinical characteristics, and outcome. Dig Dis Sci. 1993;38:1893–1904. doi: 10.1007/BF01296115. [DOI] [PubMed] [Google Scholar]
- 56.Metman EH, Lagasse JP, d’Alteroche L, Picon L, Scotto B, Barbieux JP. Risk factors for immediate complications after progressive pneumatic dilation for achalasia. Am J Gastroenterol. 1999;94:1179–1185. doi: 10.1111/j.1572-0241.1999.01062.x. [DOI] [PubMed] [Google Scholar]
- 57.Kadakia SC, Wong RK. Pneumatic balloon dilation for esophageal achalasia. Gastrointest Endosc Clin N Am. 2001;11:325–46, vii. [PubMed] [Google Scholar]
- 58.Karamanolis G, Sgouros S, Karatzias G, Papadopoulou E, Vasiliadis K, Stefanidis G, Mantides A. Long-term outcome of pneumatic dilation in the treatment of achalasia. Am J Gastroenterol. 2005;100:270–274. doi: 10.1111/j.1572-0241.2005.40093.x. [DOI] [PubMed] [Google Scholar]
- 59.Gockel I, Junginger T, Bernhard G, Eckardt VF. Heller myotomy for failed pneumatic dilation in achalasia: how effective is it? Ann Surg. 2004;239:371–377. doi: 10.1097/01.sla.0000114228.34809.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckardt VF, Kanzler G, Westermeier T. Complications and their impact after pneumatic dilation for achalasia: prospective long-term follow-up study. Gastrointest Endosc. 1997;45:349–353. doi: 10.1016/s0016-5107(97)70142-1. [DOI] [PubMed] [Google Scholar]
- 61.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 62.Wehrmann T, Jacobi V, Jung M, Lembcke B, Caspary WF. Pneumatic dilation in achalasia with a low-compliance balloon: results of a 5-year prospective evaluation. Gastrointest Endosc. 1995;42:31–36. doi: 10.1016/s0016-5107(95)70239-3. [DOI] [PubMed] [Google Scholar]
- 63.Ghoshal UC, Rangan M. A review of factors predicting outcome of pneumatic dilation in patients with achalasia cardia. J Neurogastroenterol Motil. 2011;17:9–13. doi: 10.5056/jnm.2011.17.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dağli U, Kuran S, Savaş N, Ozin Y, Alkim C, Atalay F, Sahin B. Factors predicting outcome of balloon dilatation in achalasia. Dig Dis Sci. 2009;54:1237–1242. doi: 10.1007/s10620-008-0493-6. [DOI] [PubMed] [Google Scholar]
- 65.Alderliesten J, Conchillo JM, Leeuwenburgh I, Steyerberg EW, Kuipers EJ. Predictors for outcome of failure of balloon dilatation in patients with achalasia. Gut. 2011;60:10–16. doi: 10.1136/gut.2010.211409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chawda SJ, Watura R, Adams H, Smith PM. A comparison of barium swallow and erect esophageal transit scintigraphy following balloon dilatation for achalasia. Dis Esophagus. 1998;11:181–17; discussion 181-17;. doi: 10.1093/dote/11.3.181. [DOI] [PubMed] [Google Scholar]
- 68.Rohof WO, Hirsch DP, Kessing BF, Boeckxstaens GE. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328–335. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 69.Guardino JM, Vela MF, Connor JT, Richter JE. Pneumatic dilation for the treatment of achalasia in untreated patients and patients with failed Heller myotomy. J Clin Gastroenterol. 2004;38:855–860. doi: 10.1097/00004836-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Ghoshal UC, Chaudhuri S, Pal BB, Dhar K, Ray G, Banerjee PK. Randomized controlled trial of intrasphincteric botulinum toxin A injection versus balloon dilatation in treatment of achalasia cardia. Dis Esophagus. 2001;14:227–231. doi: 10.1046/j.1442-2050.2001.00189.x. [DOI] [PubMed] [Google Scholar]
- 71.Panaccione R, Gregor JC, Reynolds RP, Preiksaitis HG. Intrasphincteric botulinum toxin versus pneumatic dilatation for achalasia: a cost minimization analysis. Gastrointest Endosc. 1999;50:492–498. doi: 10.1016/s0016-5107(99)70071-4. [DOI] [PubMed] [Google Scholar]
- 72.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 73.Csendes A, Braghetto I, Henríquez A, Cortés C. Late results of a prospective randomised study comparing forceful dilatation and oesophagomyotomy in patients with achalasia. Gut. 1989;30:299–304. doi: 10.1136/gut.30.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boeckxstaens GE, Annese V, des Varannes SB, Chaussade S, Costantini M, Cuttitta A, Elizalde JI, Fumagalli U, Gaudric M, Rohof WO, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med. 2011;364:1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 75.Zaninotto G, Annese V, Costantini M, Del Genio A, Costantino M, Epifani M, Gatto G, D’onofrio V, Benini L, Contini S, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239:364–370. doi: 10.1097/01.sla.0000114217.52941.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortega JA, Madureri V, Perez L. Endoscopic myotomy in the treatment of achalasia. Gastrointest Endosc. 1980;26:8–10. doi: 10.1016/s0016-5107(80)73249-2. [DOI] [PubMed] [Google Scholar]
- 77.Pasricha PJ, Hawari R, Ahmed I, Chen J, Cotton PB, Hawes RH, Kalloo AN, Kantsevoy SV, Gostout CJ. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy. 2007;39:761–764. doi: 10.1055/s-2007-966764. [DOI] [PubMed] [Google Scholar]
- 78.Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519–525. doi: 10.1016/j.thorsurg.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Stavropoulos SN, Harris MD, Hida S, Brathwaite C, Demetriou C, Grendell J. Endoscopic submucosal myotomy for the treatment of achalasia (with video) Gastrointest Endosc. 2010;72:1309–1311. doi: 10.1016/j.gie.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 80.von Renteln D, Inoue H, Minami H, Werner YB, Pace A, Kersten JF, Much CC, Schachschal G, Mann O, Keller J, et al. Peroral endoscopic myotomy for the treatment of achalasia: a prospective single center study. Am J Gastroenterol. 2012;107:411–417. doi: 10.1038/ajg.2011.388. [DOI] [PubMed] [Google Scholar]
- 81.Gutschow CA, Hölscher AH. Myotomy for esophageal achalasia - laparoscopic versus peroral endoscopic approach. Endoscopy. 2010;42:318–319. doi: 10.1055/s-0029-1244071. [DOI] [PubMed] [Google Scholar]
- 82.Holm AN, de la Mora Levy JG, Gostout CJ, Topazian MD, Baron TH. Self-expanding plastic stents in treatment of benign esophageal conditions. Gastrointest Endosc. 2008;67:20–25. doi: 10.1016/j.gie.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 83.Repici A, Conio M, De Angelis C, Battaglia E, Musso A, Pellicano R, Goss M, Venezia G, Rizzetto M, Saracco G. Temporary placement of an expandable polyester silicone-covered stent for treatment of refractory benign esophageal strictures. Gastrointest Endosc. 2004;60:513–519. doi: 10.1016/s0016-5107(04)01882-6. [DOI] [PubMed] [Google Scholar]
- 84.Li YD, Tang GY, Cheng YS, Chen NW, Chen WX, Zhao JG. 13-year follow-up of a prospective comparison of the long-term clinical efficacy of temporary self-expanding metallic stents and pneumatic dilatation for the treatment of achalasia in 120 patients. AJR Am J Roentgenol. 2010;195:1429–1437. doi: 10.2214/AJR.10.4407. [DOI] [PubMed] [Google Scholar]
- 85.Cheng YS, Li MH, Chen WX, Chen NW, Zhuang QX, Shang KZ. Selection and evaluation of three interventional procedures for achalasia based on long-term follow-up. World J Gastroenterol. 2003;9:2370–2373. doi: 10.3748/wjg.v9.i10.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Palma GD, lovino P, Masone S, Persico M, Persico G. Self-expanding metal stents for endoscopic treatment of esophageal achalasia unresponsive to conventional treatments. Long-term results in eight patients. Endoscopy. 2001;33:1027–1030. doi: 10.1055/s-2001-18933. [DOI] [PubMed] [Google Scholar]
- 87.Mukherjee S, Kaplan DS, Parasher G, Sipple MS. Expandable metal stents in achalasia--is there a role? Am J Gastroenterol. 2000;95:2185–2188. doi: 10.1111/j.1572-0241.2000.02301.x. [DOI] [PubMed] [Google Scholar]
- 88.Terruzzi V, Minoli G. Endoscopic injection of ethanolamine as a treatment for achalasia: a first report. Gastrointest Endosc. 1997;45:540–542. [PubMed] [Google Scholar]
- 89.Moretó M, Ojembarrena E. Treatment of achalasia by injection of botulinum toxin or sclerosants? Endoscopy. 2000;32:361–362. [PubMed] [Google Scholar]
- 90.Niknam R, Mikaeli J, Mehrabi N, Mahmoudi L, Elahi E, Shirani S, Malekzadeh R. Ethanolamine oleate in resistant idiopathic achalasia: a novel therapy. Eur J Gastroenterol Hepatol. 2011;23:1111–1115. doi: 10.1097/MEG.0b013e328349647e. [DOI] [PubMed] [Google Scholar]
- 91.Mattioli S, Ruffato A, Lugaresi M, Pilotti V, Aramini B, D’Ovidio F. Long-term results of the Heller-Dor operation with intraoperative manometry for the treatment of esophageal achalasia. J Thorac Cardiovasc Surg. 2010;140:962–969. doi: 10.1016/j.jtcvs.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 92.Endo S, Nakajima K, Nishikawa K, Takahashi T, Souma Y, Taniguchi E, Ito T, Nishida T. Laparoscopic Heller-Dor surgery for esophageal achalasia: impact of intraoperative real-time manometric feedback on postoperative outcomes. Dig Surg. 2009;26:342–348. doi: 10.1159/000244512. [DOI] [PubMed] [Google Scholar]
- 93.Mattioli S, Di Simone MP, Bassi F, Pilotti V, Felice V, Pastina M, Lazzari A, Gozzetti G. Surgery for esophageal achalasia. long-term results with three different techniques. Hepatogastroenterology. 1996;43:492–500. [PubMed] [Google Scholar]
- 94.Zendehdel K, Nyrén O, Edberg A, Ye W. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am J Gastroenterol. 2011;106:57–61. doi: 10.1038/ajg.2010.449. [DOI] [PubMed] [Google Scholar]
- 95.Leeuwenburgh I, Scholten P, Alderliesten J, Tilanus HW, Looman CW, Steijerberg EW, Kuipers EJ. Long-term esophageal cancer risk in patients with primary achalasia: a prospective study. Am J Gastroenterol. 2010;105:2144–2149. doi: 10.1038/ajg.2010.263. [DOI] [PubMed] [Google Scholar]
- 96.Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–580. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Dunaway PM, Wong RK. Risk and surveillance intervals for squamous cell carcinoma in achalasia. Gastrointest Endosc Clin N Am. 2001;11:425–34, ix. [PubMed] [Google Scholar]
- 98.Clemente G. The choice of fundoplication after myotomy for achalasia. Arch Surg. 2006;141:612; author reply 612–613. doi: 10.1001/archsurg.141.6.612-b. [DOI] [PubMed] [Google Scholar]
- 99.Csendes A, Braghetto I, Burdiles P, Korn O, Csendes P, Henríquez A. Very late results of esophagomyotomy for patients with achalasia: clinical, endoscopic, histologic, manometric, and acid reflux studies in 67 patients for a mean follow-up of 190 months. Ann Surg. 2006;243:196–203. doi: 10.1097/01.sla.0000197469.12632.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gordon JM, Eaker EY. Prospective study of esophageal botulinum toxin injection in high-risk achalasia patients. Am J Gastroenterol. 1997;92:1812–1817. [PubMed] [Google Scholar]
- 101.Cuillière C, Ducrotté P, Zerbib F, Metman EH, de Looze D, Guillemot F, Hudziak H, Lamouliatte H, Grimaud JC, Ropert A, et al. Achalasia: outcome of patients treated with intrasphincteric injection of botulinum toxin. Gut. 1997;41:87–92. doi: 10.1136/gut.41.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vaezi MF, Richter JE, Wilcox CM, Schroeder PL, Birgisson S, Slaughter RL, Koehler RE, Baker ME. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut. 1999;44:231–239. doi: 10.1136/gut.44.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stark GA, Castell DO, Richter JE, Wu WC. Prospective randomized comparison of Brown-McHardy and microvasive balloon dilators in treatment of achalasia. Am J Gastroenterol. 1990;85:1322–1326. [PubMed] [Google Scholar]
- 104.Parkman HP, Reynolds JC, Ouyang A, Rosato EF, Eisenberg JM, Cohen S. Pneumatic dilatation or esophagomyotomy treatment for idiopathic achalasia: clinical outcomes and cost analysis. Dig Dis Sci. 1993;38:75–85. doi: 10.1007/BF01296777. [DOI] [PubMed] [Google Scholar]
- 105.Coccia G, Bortolotti M, Michetti P, Dodero M. Prospective clinical and manometric study comparing pneumatic dilatation and sublingual nifedipine in the treatment of oesophageal achalasia. Gut. 1991;32:604–606. doi: 10.1136/gut.32.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bourgeois N, Coffernils M, Buset M, Gelin M, Deltenre M, Panzer JM, Cremer M. Management of dysphagia in suspected esophageal motor disorders. Dig Dis Sci. 1991;36:268–273. doi: 10.1007/BF01318194. [DOI] [PubMed] [Google Scholar]
- 107.Gelfand MD, Kozarek RA. An experience with polyethylene balloons for pneumatic dilation in achalasia. Am J Gastroenterol. 1989;84:924–927. [PubMed] [Google Scholar]
- 108.Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol. 1999;94:1802–1807. doi: 10.1111/j.1572-0241.1999.01209.x. [DOI] [PubMed] [Google Scholar]
- 109.Rai RR, Shende A, Joshi A, Mathur A, Nijhawan S. Rigiflex pneumatic dilation of achalasia without fluoroscopy: a novel office procedure. Gastrointest Endosc. 2005;62:427–431. doi: 10.1016/j.gie.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 110.Swanström LL, Rieder E, Dunst CM. A stepwise approach and early clinical experience in peroral endoscopic myotomy for the treatment of achalasia and esophageal motility disorders. J Am Coll Surg. 2011;213:751–756. doi: 10.1016/j.jamcollsurg.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Costamagna G, Marchese M, Familiari P, Tringali A, Inoue H, Perri V. Peroral endoscopic myotomy (POEM) for oesophageal achalasia: preliminary results in humans. Dig Liver Dis. 2012;44:827–832. doi: 10.1016/j.dld.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 112.Chiu PW, Wu JC, Teoh AY, Chan Y, Wong SK, Liu SY, Yung MY, Lam CC, Sung JJ, Chan FK, et al. Peroral endoscopic myotomy for treatment of achalasia: from bench to bedside (with video) Gastrointest Endosc. 2013;77:29–38. doi: 10.1016/j.gie.2012.08.018. [DOI] [PubMed] [Google Scholar]


