Abstract
Objective
Loss of BDNF-TrkB signaling results in obesity in both humans and mice; however, the neural circuit that mediates this effect is unknown. We tested the role of TrkB signaling dopamine-1 receptor expressing neurons in body weight regulation.
Design and Methods
Mice with a floxed allele of the TrkB gene were paired with mice expressing Cre-recombinase under control of the D1 promoter in order to conditionally knock out expression of TrkB receptors from D1-neurons.
Results
Deletion of TrkB receptors from D1 neurons results in obesity in chow fed mice due to increased feed efficiency. In contrast, loss of Trk B signaling in D1 neurons induced hyperphagia and hyperglycemia in mice maintained on high fat diet.
Conclusions
These findings indicate TrkB signaling in D1 neurons regulates body weight by distinct mechanisms for chow and high fat diet and may be important for defending the body against the development of obesity and obesity-related disorders.
Keywords: brain-derived neurotrophic factor (BDNF), tropomyosin-related Kinase B (Trk B), dopamine 1 receptor (D1R), feeding, body weight
Introduction
Brain-derived neurotrophic factor (BDNF) is a highly conserved neuotrophin that signals through the tropomyosin-related kinase B (TrkB) receptor 1. In addition to roles in neuronal development and synaptic plasticity, BDNF-TrkB signaling is appreciated as an important regulator of body weight. Mice heterogyzous for BDNF have marked obesity that can be reversed by intraventricular infusion of BDNF2. Mice with genetically reduced TrkB expression (~25% of normal) are hyperphagic and gain excessive weight on a high fat diet 3. Furthermore, genetic disruption of BDNF-TrkB signaling causes obesity in human subjects, thus confirming the importance of this pathway in human body weight regulation 4,5.
While BDNF signaling has been associated with control of feeding and body weight1, the specific sites of TrkB action that mediate this effect are unknown. TrkB is broadly expressed in the brain, including most areas of the hypothalamus as well as the mesolimbic reward pathway, a major regulator of reward processing 1. During studies examining the role of TrkB signaling in the action of cocaine 6, it was noted that loss of TrkB in dopamine-1 receptor (D1) neurons resulted in weight gain, indicating that D1 neurons may be an important site of TrkB action on body weight. D1 neurons are expressed in brain regions known to regulate food intake, including the ventral striatum, and several hypothalamic nuclei including the paraventricular and suprachiasmatic nuclei 7. Therefore, we conducted a rigorous analysis on the role of D1 neurons in BDNF-TrkB-related body weight effects by crossing mice with a loxP-flanked allele of the TrkB receptor 8 to a mouse line expressing Cre-recombinase under control of the D1-promotor (D1-Cre).
Methods and Procedures
Animals
Mice were housed in the University of Texas Southwestern Medical Center (UTSW) vivarium in a temperature-controlled environment (lights on: 06:00–18:00) with ad lib access to water and standard chow (SC; 4% fat diet #7001, Harlan-Teklad, Madison, WI). All animal procedures were performed in accordance with UTSW Institutional Animal Care and Use Committee guidelines. All mice used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the U.S. National Institutes of Health, and the specific protocols were approved by the Institutional Animal Care and Use Committee.
Conditional knockout mice in which lox-P sites were inserted into the Trk B gene (fTrkB) to create a floxed allele were previously reported 8. The fTrkB mouse line as then were bred to a mouse line expressing Cre-recombinase under the control of the dopamine 1 receptor (D1-Cre) mice (Gensat, EY262, http://www.gensat.org/cre.jsp) to specifically delete TrkB expression from D1-neurons. Selective loss of TrkB in D1 neurons was verified as reported previously 6.
Mice heterogyzous for fTrkB were bred with D1-cre(+) or D1-cre(−) mice resulting in four genotypes of littermate mice: wild-type mice with and without D1-Cre and fTrkB mice with and without D1-Cre. Previous work by our group has shown that the D1-cre transgene has no effect on body weight 9 and our analyses showed no significant differences between groups, thus the wild-type mice with or without D1-cre transgene were collapsed into one group for comparisons to the fTrkB x D1-cre (+/−) mice.
Long-Term Feeding Studies
Mice were weaned and genotyped at four weeks of age and then were individually housed for feeding studies. Mice were weighed at baseline and provided a measured amount of diet, either normal mouse chow (Global Diet #2016, Harlan-Teklad, Madison, WI) or a high fat diet (HFD; 42% fat, diet #TD88.137, Harlan-Teklad, Madison, WI), and food intake and body weight was monitored once a week for 8 weeks. For the chow fed group, the average starting body weights at 5 weeks of age were 15.8 g ± 0.8 (wild-type), 19.8 g ± 0.62 (fTrkB), 17.0 g ± 1.58 (fTrkBD1-Cre). For HFD fed mice, the average starting body weights at 5 weeks of age were 18.0 g ± 1.15 (wild-type), 19.6 g ± 1.28 (fTrkB), 20.5 g ± 1.56 (fTrkBD1-Cre).
Locomotor activity
Individually housed animals were kept in their home cage, and locomotor activity was measured for 120 minutes by photocell beams linked to computer data acquisition software (San Diego Instruments, San Diego, CA).
Metabolic Parameters/ELISA
At the conclusion of the feeding studies, mice were fasted for ~3 hours, then rapidly killed by cervical dislocation, decapitated, and trunk blood was collected. Blood was spun at 10,000x g for 20 min at 4°C and the serum was collected and stored at −20°C. Aliquots of serum were transferred to the UTSW Metabolic Core for measurement of metabolic parameters (total cholesterol, triglycerides, glucose, and HDL). Other aliquots were used for ELISA analysis of insulin (#90060 Crystal Chem, Downers Grove, Illinois).
Statistics
The data are presented as mean ± SEM. GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA) were used to perform all statistical analyses. For long-term feeding studies, comparisons between groups were made by Two-W\way ANOVA followed by a Bonferroni’s post-hoc analysis to determine group differences. For metabolic parameters, One-way ANOVAs followed by Tukey’s post-hoc analyses were used for comparisons of feed efficiency, glucose, total cholesterol, triglycerides, and insulin. P< 0.05 was considered to be statistically significant.
Results
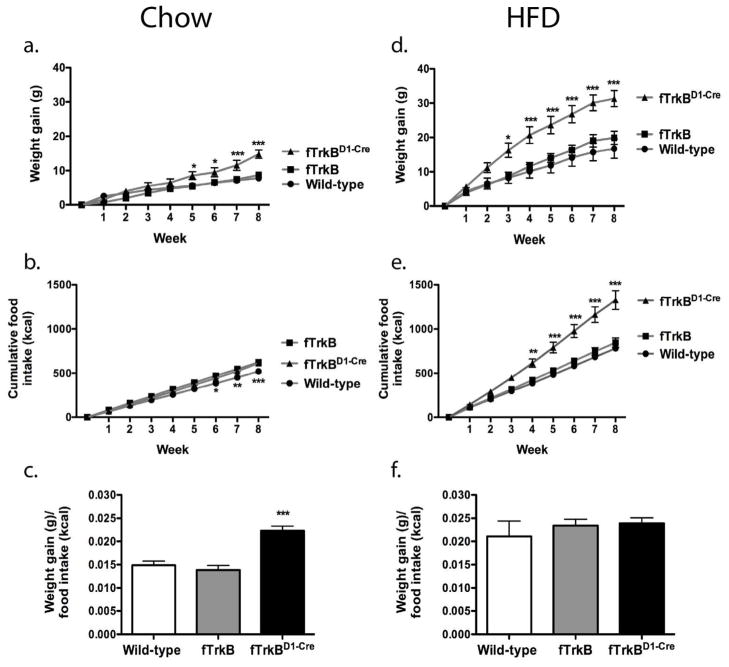
Wild-type (non-loxP-flanked TrkB with or without D1-Cre), floxed-TrkB without D1-Cre (fTrk) and floxed-TrkB with D1-Cre (fTrkBD1-Cre) littermate male mice were individually housed and monitored for food intake and body weight for eight weeks on both chow and a 42% high fat diet (HFD). On regular chow, while no difference in body weight was noted between wild-type and fTrkB mice, fTrkBD1-Cre mice gained over 6 g more in body weight over the course of the eight week experiment (Fig. 1a). Analysis of cumulative food intake demonstrated no difference between fTrkB and fTrkBD1-Cre groups (Fig. 1b). A small, but statistically significant, increase in chow consumed was observed between both fTrkB and fTrkBD1-Cre groups compared to wild-type mice, suggesting that creation of the floxed allele affects neurophysiology to promote a small increase in food intake without altering body weight gain.
Figure 1. Trk B signaling dopamine 1 receptor neurons regulates body weight.
Male wild-type, fTrkB, and, fTrkBD1-Cre littermates were placed on chow or HFD for eight weeks after weaning. In chow-fed mice, loss of Trk B signaling in D1 neurons increased weight gain (a; significant interaction of genotype x time [F(16,162)=3.82, p<0.0001] by Two-way ANOVA), and cumulative calorie intake (b; significant genotype x time interaction [F(16,162)=2.56, p=0.0015]. In mice fed HFD, loss of Trk B sigaling in D1 neurons increased weight gain (d; significant genotype x time interaction [F(16,225)=2.66, p=0.0007] and cumulative calorie intake (e; significant genotype x time interaction [F (16,225)=6.58, p<0.0001]. Comparison of feed efficiency (weight gain/cumulative calorie intake) demonstrates that loss of Trk B signaling in D1 neurons increases feed efficiency on chow (c; One-way ANOVA, F(2,18)=18.46, p<0.0001), but not HFD (f; One-way ANOVA, F(2,25)=0.542, p=0.588). N = 8 (wild-type/chow), 8 (fTrkB/chow), 5 (fTrkB D1- Cre/chow), 7 (wild-type/HFD), 11 (fTrkB/HFD), 10 (, fTrkBD1-Cre/HFD). *P < 0.05, **P < 0.01, ***P < 0.001. Data presented as mean ± S.E.M.
Because BDNF-TrkB signaling has previously been implicated in the consumption of palatable foods, we next analyzed the effect of HFD on body weight in our three groups. We did not observe a difference in body weight between wild-type and fTrkB groups, but again fTrkBD1-Cre mice gained significantly more than the other two groups (Fig. 1d). Analysis of cumulative food intake demonstrated no significant difference in HFD intake between the wild-type and fTrkB groups, but a large increase in cumulative HFD consumption in fTrkBD1-Cre mice (Fig. 1e).
While body weight homeostasis was affected by loss of TrkB signaling in D1-neurons, it was not clear if the weight gain was a result of increased calorie intake or reduced energy expenditure. To begin to answer this question, we calculated feed efficiency (body weight gain/cumulative calorie intake) for all groups of mice during the eight-week period on both diets, taking into account the above food intake data. Compared to wild-type and fTrkB groups, fTrkBD1-Cre mice displayed a significant increase in feed efficiency on chow (Fig. 1c). Importantly, no difference in locomotor activity was noted between fTrkB and fTrkBD1-Cre groups (2152 ± 124 vs. 2581 ± 252, p > 0.05), suggesting that reduced energy expenditure, and not a change in locomotor activity, is the primary cause of weight gain. In contrast, no difference in feed efficiency was noted between the three groups on HFD (Fig. 1f), indicating that increased calorie consumption was the primary cause of the obesity observed in fTrkBD1-Cre mice fed HFD.
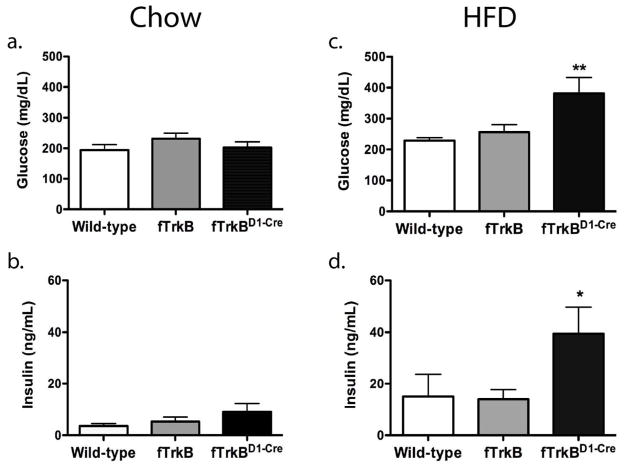
Obesity is associated with increased risk for developing several metabolic disorders including type II diabetes and metabolic syndrome. Therefore at the end of the study, we performed a comprehensive metabolic profile on all three groups to determine if loss of TrkB signaling in D1R neurons predisposed to these metabolic disorders. Despite a significant weight gain in chow-fed fTrkBD1-Cre mice (Fig. 1a), no effect of genotype was observed on glucose or insulin levels in chow fed mice (Fig. 2a and 2b). In contrast, loss of TrkB signaling in D1 neurons induced significant elevations in both fasting glucose and fasting insulin levels in fTrkBD1-Cre mice (Fig. 2c and 2d) suggesting insulin resistance. No significant effect of genotype was observed on fasting levels of triglycerides, total cholesterol, or high-density lipoprotein cholesterol (data not shown). Therefore, TrkB signaling in D1 neurons defends against the development of HFD-induced weight gain and associated conditions such as insulin resistance.
Figure 2. Loss of Trk B signaling in dopamine-1 receptor neurons exacerbates high fat diet-induced insulin resistance.
One-Way ANOVAs indicated no significant differences between groups for plasma glucose or insulin after consuming regular chow (2a and 2b, respectively). A One-way ANOVA reported fTrkBD1-Cre mice having significantly higher plasma glucose after consuming high-fat diet than both the fTrkB mice and the wild-type mice (2c; F(2,22)=5.90, p=0.0089) and also significantly different plasma insulin after consuming high-fat diet (2d; F(2,25)=3.539, p=0.04).
Discussion
To our knowledge, this is the first evidence for a TrkB signaling role in feed efficiency and also identifies D1-neurons as a critical site of TrkB action on body weight. Furthermore, our data indicate that TrkB signaling in D1-neurons differentially regulates body weight depending on the diet, suggesting that BDNF-TrkB signaling may integrate dietary information differentially into coordinated metabolic responses that defend against obesity and obesity-related disorders such as insulin resistance. Such a possibility is in line previous reports that BDNF expression is differentially regulated by macronutrients. One report found that glucose infusion causes a rapid increase in BDNF mRNA in the ventromedial hypothalamus 8 while consumption of HFD reduces BDNF mRNA in the ventral tegmental area after 60 minutes 10.
Still unknown is the site of BDNF production that regulates TrkB signaling on D1-neurons. Dopaminergic neurons originating from the VTA are one likely candidate. As noted above, BDNF levels within the VTA are decreased after consumption of HFD and selective reduction of BDNF in the VTA increases consumption of HFD 10. The observation that HFD reduces BDNF mRNA levels in the ventral tegmental area is consistent with our findings that complete loss of TrkB signaling in D1-neurons leads to a dramatic increase of the highly palatable HFD. It has previously been hypothesized that loss of BDNF could affect reward processing in the ventral striatum leading to a “reward-deficiency syndrome” that promotes compensatory overeating (reviewed in 1). Indeed, deletion of TrkB from D1-neurons increases the conditioned place preference for cocaine 6, indicating that BDNF-TrkB signaling in D1 neurons does affect reward sensitivity. These observations suggest a model in which calorically dense foods, like HFD, promote overconsumption by reducing expression of BDNF within dopaminergic neurons and subsequent TrkB signaling in D1-neurons. Another possible source is the hypothalamus. Central infusion of BDNF induces neuronal activity in several hypothalamic nuclei and reduction of BDNF expression in the basomedial hypothalamus increases food intake and body weight 8.
Finally, it is important to identify the intracellular signaling pathways that mediate the effect of TrkB signaling on body weight in D1-neurons. Research in the fields of drug addiction and stress implicates multiple consequences of TrkB signaling in D1-neurons on neuroplasticity, including alterations in CREB activity, ERK signaling and AMPA receptor trafficking 11–13. These new insights will help determine the role of neural adaptations in body weight regulation and protection from obesity and related disorders.
What is already known about this subject
mutations in the genes encoding brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin-related kinase B (TrkB) have been shown to result in obesity in both humans and mice.
What this study adds
This study identifies dopamine 1 receptor neurons as a key site of action for TrkB receptor signaling in maintaining body weight.
TrkB signaling in dopamine 1 neurons differentially affects body weight depending on the diet: mice fed high fat diet eat significantly more while mice fed chow are more efficient at storing energy.
Acknowledgments
This work was funded by the following grants: DK081185-01, DK081182-01, MH084058-01A1, Disease Oriented Clinical Scholars Program, and NARSAD Young Investigator Award (ML). The funding sources had no role in the following: study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We would like to thank Gensat for use of mouse lines.
BLM conceived and carried out experiments, analyzed data, generated figures and wrote the manuscript. MLK and LFP conceived experiments and wrote the manuscript. ML conceived experiments, analyzed data, and wrote the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Footnotes
Conflict of Interest Statement- ML has done consulting work for MARS, Inc. BLM, MKL, and LFP have no conflicts of interest to report. I verify that, as corresponding author, I have in my possession, and will keep on file, ICMJE COI forms from all coauthors, and that I have compiled a COI statement that fully and accurately reflects what I and each co-author have stated on our ICMJE COI forms and have included it in the acknowledgements section of our manuscript.
References
- 1.Cordeira J, Rios M. Weighing in the role of BDNF in the central control of eating behavior. Molecular neurobiology. 2011;44:441–448. doi: 10.1007/s12035-011-8212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. The EMBO journal. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nature neuroscience. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, Keogh JM, et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes. 2006;55:3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature neuroscience. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 6.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, et al. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He XP, Kotloski R, Nef S, Luikart BW, Parada LF, McNamara JO. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43:31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Mason BL, Lee C, Nishi A, Elmquist JK, Lutter M. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiology & behavior. 2012;106:201–210. doi: 10.1016/j.physbeh.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordeira JW, Frank L, Sena-Esteves M, Pothos EN, Rios M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Dabrowska J, Hazra R, Rainnie DG. Synergistic activation of dopamine D1 and TrkB receptors mediate gain control of synaptic plasticity in the basolateral amygdala. PloS one. 2011;6:e26065. doi: 10.1371/journal.pone.0026065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. The European journal of neuroscience. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tropea TF, Kabir ZD, Kaur G, Rajadhyaksha AM, Kosofsky BE. Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Frontiers in psychiatry/Frontiers Research Foundation. 2011;2:67. doi: 10.3389/fpsyt.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]