Abstract
The prevalence of the metabolic syndrome (MetS) is higher among patients receiving atypical antipsychotics (AAPs) treatment, and even among AAPs, treatment with clozapine has been shown to be associated with a higher long-term incidence rate of MetS. Likewise, brain-derived neurotrophic factor (BDNF) deficiency has been reported to result in metabolic traits, such as increased food intake, hyperphagia and obesity, etc. In this study, we hypothesized that a functional polymorphism (Val66Met) in the BDNF gene may confer susceptibility to clozapine-induced MetS, potentially in a sex-specific manner, since an interaction between Val66Met polymorphism and sex was observed in our previous studies. A total of 199 schizophrenia patients being treated with clozapine were divided into two groups, MetS and non-MetS, based on the diagnostic criteria of the National Cholesterol Education Program's Adult Treatment Panel III. We genotyped the Val66Met polymorphism, and measured the serum levels of fasting glucose (GLU), triglyceride (TG) and high density lipoprotein cholesterol (HDL). There was a trend indicating a significant association between the homozygous Met/Met genotype and MetS in male patients (OR = 2.39; 95% CI: 1.05–5.41; p = 0.039; corrected p = 0.078). Among the six risk factors listed in the ATPIII criteria, we found a significant association between fasting GLU levels and Val66Met polymorphism in males (p = 0.005; corrected p = 0.03), but not in females (p = 0.65). Post-hoc analysis in males revealed that the Met/Met carriers had significant higher levels of fasting GLU than those with Val/Val or Val/Met genotypes (p = 0.007; corrected p = 0.042 and p = 0.002; corrected p = 0.012, respectively). In conclusion, we observed a weak association between the Val66Met polymorphism and clozapine-induced MetS in a sex-specific manner. While preliminary, such findings prompt further, large-scale longitudinal studies to replicate these findings.
Introduction
Antipsychotic drugs have been widely used to treat psychosis, particularly among patients with schizophrenia and bipolar disorder. Antipsychotics are likewise increasingly becoming accepted for managing of non-psychotic disorders. Over the past decade, atypical antipsychotics (AAPs) have been approved by the U.S. Food and Drug Administration and are now more frequently prescribed than typical antipsychotics. However, clinical observations have indicated that treatment with AAPs are associated with obesity and other components of metabolic syndrome (MetS), particularly abnormal glucose and lipid metabolism [1]. In 2003, the U.S. Food and Drug Administration (FDA) issued a warning associating AAPs with a connection to increased risk for diabetes and hyperglycemia.
Clozapine is an atypical antipsychotic often used for its demonstrated superiority in the treatment of refractory schizophrenia [2], [3]. However, clozapine exhibited a higher long-term incidence rate of MetS (up to 58.3%) as compared with other AAPs, using the National Cholesterol Education Program's Adult Treatment Panel III (ATP III) criteria [4], [5]. Accordingly, investigating the mechanisms that contribute to the high occurrence of clozapine-induced MetS may help facilitate the identification and clinical management of this significantly adverse effect.
Obesity, a status characterized by excessive adipose tissue, is one of the most important components of MetS [6]. In rodents, models of brain-derived neurotrophic factor (BDNF) disruption all exhibited increased food intake, obesity, and hyperphagia [7], [8], whereas calorie restriction in heterozygous BDNF +/− mice can increase expression of BDNF and reduce obesity [9], [10]. In humans, BDNF was found to be associated with eating disorders, such as restrictive anorexia nervosa [11], as well as antipsychotic-induced body weight gain [12], [13], [14], [15]. In a recent pharmacogenetic study, BDNF was reported to regulate the clozapine treatment response [16], suggesting that BDNF may be a pharmacological target of clozapine. Given these findings, it seems plausible that a deficiency in BDNF may be a potential biological mechanism that underlies clozapine-induced MetS.
At the molecular level, a common functional polymorphism of BDNF, leading to a valine to methionine substitution at codon 66, is known to alter the intracellular trafficking and activity-dependent secretion of BDNF [17]. Recently, the Val66Met polymorphism was identified as a risk factor for metabolic traits, such as obesity and insulin resistance in several human populations [12], [13], [14], [18], [19], [20], [21].
Based on the findings from abovementioned studies, we hypothesized that the BDNF Val66Met polymorphism may confer susceptibility to clozapine-induced MetS. To test this potential association, we conducted a pharmacogenetic study to investigate the association between the Val66Met polymorphism and MetS in a population of Han Chinese under long-term clozapine treatment. Taking into consideration the interaction between the Val66Met polymorphism and sex observed in our previous studies [22], [23], we also aimed to evaluate potential differences in the effect of this variant on males and females.
Methods
Ethics Statement
This study was reviewed and approved by the ethics committee of the Shanghai Mental Health Center. All participants provided written informed consent prior to inclusion in this project, and were treated in accordance with the Declaration of Helsinki. The assessment of participants’ capacity to provide consent was based on their (1) ability to communicate a reasoned choice regarding participation; (2) ability to understand relevant information regarding the study, including consequences of participation for the participant’s own situation (such as health condition) and consequences of the alternatives to participation; (3) ability to comprehend the nature of the situation and its likely consequences; and (4) ability to manipulate information rationally. Next of kin, carer takers, or guardians consented on the behalf of participants whose capacity to consent was compromised.
Participants
A total of 199 unrelated Han Chinese schizophrenia patients (143 males and 56 females, aged 55.0±7.4 and 55.9±5.2, respectively) were recruited from the Inpatient Psychiatry Unit at Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. The inclusion criteria for patients consisted of six conditions: (1) patients had been diagnosed with schizophrenia according to the DSM–IV, with the diagnoses either made or reviewed by experienced psychiatrists; (2) patients were free from MetS before receiving clozapine, based on the medical records; (3) patients were receiving clozapine treatment alone or in conjunction with typical antipsychotics, but not atypical ones as other atypical antipsychotics (e.g. olanzapine, quentipine) may potentially enhance the risk of MetS [2]; (4) patients had been receiving clozapine for more than 24 months [24], [25]; (5) patients had maintained a stable condition for more than six months before entry into the study; and (6) patients had no other diagnosed psychiatric disorders aside from schizophrenia.
Diagnosis of clozapine-induced MetS
A cross-section assessment of metabolic parameters was performed to determine the prevalence of MetS based on the ATPIII definition, which comprises the best criteria for diagnosing MetS in a Chinese population [26]. MetS was diagnosed in the presence of any three of the following: (1) a waist circumference ≥90 cm in Chinese men and ≥80 cm in Chinese women [27]; (2) triglyceride (TG) ≥1.7 mmol/l; (3) high density lipoprotein cholesterol (HDL) <1.0 mmol/l in men and <1.3 mmol/l in women; (4) blood pressure ≥130/85 mmHg; or (5) fasting glucose≥5.6 mmol/l [28].
Metabolic parameters analysis
Waist circumference was measured between the lower rib margin and the iliac crest, after a normal expiratory breath. Serum fasting GLU, TG, and HDL levels were measured using an automatic Biochemical Analyzer (HITACHI 7170A, Hitachi, Ltd, Tokyo, Japan). Overnight fasting blood samples were drawn between 7:00 and 7:30 a.m. from all patients.
Genotyping
The Val66Met polymorphism, also known as rs6265 (G/A), is located at Chr.11:27679926 based on National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?rs=6265). Indentified from HapMap-HCB (Han Chinese in Beijing) database, the more common A allele of rs6265 encodes the Met, while the G allele encodes Val.
In this study, the Val66Met polymorphism was amplified independently by PCR and genotyped by direct sequencing using an ABI PRISM 3730 Genetic Analyzer (Perkin-Elmer Applied Biosystems). Genotyping was carried out according to the method described previously by Zhang et al. [29]. PCR amplification was performed in a volume of 25 µL containing a primer pair (Forward: 5′-AAACATCCGAGGACAAGGTG-3′; Reverse: 5′-CCTCATGGACATGTTTGCAG-3′). PCR primers were also used for sequencing. Sequencing results were handled by the DNAStar (DNAstar Inc. USA) and the original sequencing chromatograms of each sample were further checked manually.
Statistical analysis
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used to perform either the t-test or chi-square test in order to compare demographic characteristics between the MetS and non-MetS groups. The online program SHEsis (http://analysis.bio-x.cn) [30] was used to test Hardy-Weinberg equilibrium. Odds ratios were used to measure the association of MetS risk with the alleles and genotypes of the Val66Met polymorphism. Unconditional logistic regression models were used to obtain maximum-likelihood estimates of the odds ratios (ORs) and their 95% confidence intervals (CIs). Analysis of the relationship between the individual metabolic parameters (dependent variable) and the Val66Met genotypes (independent variable) was performed with ANCOVA. Variables that affected metabolic parameters (i.e. age, sex, duration of clozapine treatment, clozapine doses) were included as covariates. To correct for multiple testing using the Bonferroni test, corrected p values were set at an uncorrected p value multiplied by k (independent significance tests). All p values are two-tailed, and p values below 0.05 were considered statistically significant after Bonferroni correction.
Results
MetS was found in 86/199 of the patients (43.2%), with 40.0% prevalence (57/143) in males and 51.8% (29/56) in females. Results showed no difference between the MetS and non-MetS groups in terms of age, sex, duration of clozapine treatment, and clozapine dose. Patients with MetS had notably higher BMI, waist circumference, fasting GLU, fasting TG, fasting HDL, SBP and DBP than those without MetS. There was no significant difference in either allele or genotype frequencies between the schizophrenia patients with and without MetS. Further analyses based on sex stratification revealed a marginal association of the homozygous Met/Met genotype with MetS in male patients (OR = 2.39; 95% CI: 1.05–5.41; p = 0.039; corrected p = 0.078) (Table 1).
Table 1. Distribution of Val66Met genotype and allele in schizophrenic patients with or without MetS.
| Genotype distribution (%) | Odds ratio (95% CI) | p c | Allele (%) | p c | ||||||
| MetS group | n | Val/Val | Val/Met | Met/Met | n | Val | Met | |||
| Total (Males, Females) | 86 | 20 (23.3) | 45 (52.3) | 21 (24.4) | 1.71 (0.84–3.45) a | 0.15 | 172 | 85 (49.4) | 87 (50.6) | 0.52 |
| 0.89 (0.45–1.75) b | 0.73 | |||||||||
| Males | 57 | 11 (19.3) | 29 (50.9) | 17 (29.8) | 2.39 (1.05–5.41) a | 0.039 | 114 | 51 (44.7) | 63 (55.3) | 0.10 |
| 1.35 (0.59–3.07) b | 0.54 | |||||||||
| Females | 29 | 9 (31.0) | 16 (55.2) | 4 (13.8) | 0.70 (0.17–2.95) a | 0.73 | 58 | 34 (58.6) | 24 (41.4) | 0.19 |
| 0.27 (0.07–1.17) b | 0.10 | |||||||||
| non-MetS group | ||||||||||
| Total (Males, Females) | 113 | 24 (21.2) | 71 (62.8) | 18 (15.9) | 226 | 119 (52.7) | 107 (47.3) | |||
| Males | 86 | 21 (24.4) | 52 (60.5) | 13 (15.1) | 172 | 94 (54.7) | 78 (45.3) | |||
| Females | 27 | 3 (11.1) | 19 (70.4) | 5 (18.5) | 54 | 25 (46.3) | 29 (53.7) | |||
The Odds ratio was calculated for MetS group homozygous for Met allele a (Met/Met vs. Val/Val+Val/Met), and homozygous or heterozygous for Met allele b (Met/Met+Val/Met vs Val/Val).
p values were not corrected for multiple test.
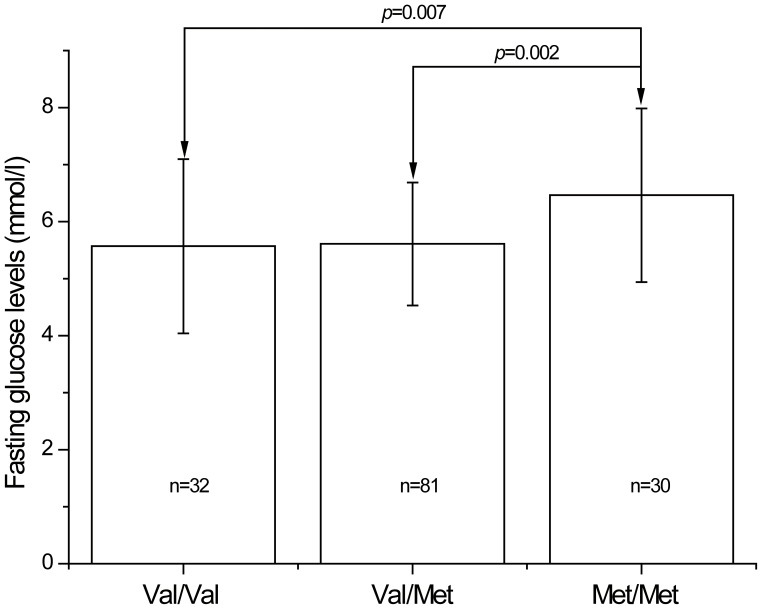
Among the six risk factors listed in the ATPIII criteria, a significant association was found between fasting GLU levels and the Val66Met polymorphism, but the significance did not survive after Bonferroni correction (F 6, 192 = 2.24, p = 0.04; corrected p = 0.24) (Table 2). The mean ± standard deviation fasting GLU levels (mmol/l) of Val/Val, Val/Met and Met/Met carriers were 5.7±1.4, 5.6±1.1 and 6.2±1.5, respectively. After stratification based on sex, results did indicate a significant association between fasting GLU levels and Val66Met polymorphism in males (p = 0.005; corrected p = 0.03), but not in females (p = 0.65) (Table 3). Post-hoc analysis in males further revealed that the Met/Met carriers had significantly higher levels of fasting GLU than those with Val/Val or Val/Met genotypes (p = 0.007; corrected p = 0.042 and p = 0.002; corrected p = 0.012, respectively) (Figure 1).
Table 2. BDNF Val66Met polymorphism and individual parameters of 199 subjects.
| Val/Val (n = 44) | Val/Met (n = 116) | Met/Met (n = 39) | p a | |
| Waist circumference (cm) | 88.0 (5.9) | 86.9 (6.4) | 87.0 (6.9) | 0.76 |
| Fasting GLU (mmol/l) | 5.7 (1.4) | 5.6 (1.1) | 6.2 (1.5) | 0.04 |
| Fasting TG (mmol/l) | 1.6 (0.8) | 1.6 (0.9) | 1.3 (0.5) | 0.22 |
| Fasting HDL (mmol/l) | 1.0 (0.5) | 1.2 (0.5) | 1.0 (0.3) | 0.11 |
| SBP (mm Hg) | 119.4 (9.1) | 118.7 (10.0) | 120.0 (9.9) | 0.89 |
| DBP (mm Hg) | 77.8 (5.3) | 76.8 (5.7) | 76.8 (6.3) | 0.36 |
Data were presented as mean (S.D.).
p values were adjusted for age, gender, duration of clozapine treatment and drug dose, and not corrected for multiple test.
Table 3. BDNF Val66Met polymorphism and individual parameters in males and females.
| Males, mean (S.D.) | Females, mean (S.D.) | |||||||
| Val/Val (n = 32) | Val/Met (n = 81) | Met/Met (n = 30) | p a | Val/Val (n = 12) | Val/Met (n = 35) | Met/Met (n = 9) | p a | |
| Waist circumference | 89.1 (6.0) | 89.1 (4.9) | 88.7 (5.4) | 0.87 | 85.0 (4.6) | 81.9 (6.7) | 81.2 (7.3) | 0.13 |
| Fasting GLU (mmol/l) | 5.6 (1.5) | 5.6 (1.1) | 6.5 (1.5) | 0.005 | 5.9 (1.2) | 5.6 (1.2) | 5.2 (0.8) | 0.65 |
| Fasting TG (mmol/l) | 1.6 (0.9) | 1.5 (0.9) | 1.3 (0.6) | 0.52 | 1.6 (0.5) | 1.8 (1.1) | 1.1 (0.3) | 0.09 |
| Fasting HDL (mmol/l) | 1.1 (0.4) | 1.2 (0.4) | 1.0 (0.4) | 0.10 | 1.0 (0.6) | 1.2 (0.5) | 1.3 (0.3) | 0.07 |
| SBP (mm Hg) | 119.5 (10.1) | 118.3 (10.2) | 119.8 (10.1) | 0.82 | 119.2 (6.0) | 119.4 (9.8) | 120.6 (8.8) | 0.92 |
| DBP (mm Hg) | 77.8 (5.4) | 76.8 (5.9) | 76.5 (6.7) | 0.15 | 77.9 (5.4) | 76.9 (5.4) | 77.8 (5.1) | 0.56 |
p values were adjusted for age, duration of clozapine treatment, and drug dose, and not corrected for multiple test.
Figure 1. Fasting levels of GLU in males classified according to the BDNF Val66Met genotypes.
Each column represents the mean±SD. Met/Met homozygous individuals had significantly higher levels of fasting GLU than those with Val/Val or Val/Met genotypes (corrected p = 0.042 and corrected p = 0.012, respectively).
Discussion
BDNF is a member of the neurotrophin family, which regulates various neurodevelopmental processes, such as neuronal differentiation, neurite outgrowth and neuronal survival [31]. Altered BDNF signaling has been shown to involved in a variety of peripheral and central nervous system disorders, including dementia, amyotrophic lateral sclerosis, depression and schizophrenia, etc [32]. A recent study implicated BDNF in the control of GLU, lipid and antioxidant metabolism [33], which is considered an anorexigenic signal in the central control of food intake [34]. By contrast, heterozygous knock-out mice for BDNF showed hyperphagia and obesity [35]. BDNF then seems to play similar roles in neuronal development and the regulation of energy homeostasis [36], and accordingly has been conceptually viewed not only as a neurotrophin, but also a metabotrophic factor [33].
Several studies reported that serum concentrations decreased in subjects with MetS, and that a supplement of BDNF could reduce body weight [37], [38], [39], [40]. However, opposing results have also been reported [6], [41]. The Met allele has been known to significantly reduce activity-dependent secretion of BDNF as compared with the Val allele [17].The Met/Met genotype thus results in considerable low-activity of BDNF protein. In the present study, we observed a trend towards an association of the Met/Met genotype with clozapine-induced MetS in males. This finding is in agreement with the association between low BDNF concentration and MetS. Another major finding of this study is the association of the Val66Met polymorphism with clozapine-induced increased fasting GLU levels. BDNF has been reported to influence fasting GLU levels and insulin sensitivity [36], and decreased levels of the serum BDNF were found in the patients with MetS and type 2 diabetes mellitus [37], [42]. Mice with BDNF haploinsufficiency exhibited obesity and elevated levels of GLU [10], whereas systemic peripheral administration of BDNF contributed to the improvement of GLU metabolism and prevented the development of diabetes [43], [44]. In humans, there is a significant correlation between increased GLU levels and the BDNF Met allele in the general population [45]. Insulin is critical for the body’s use of GLU as energy, and insulin resistance (IR) is a condition in which the body produces insulin but does not use it effectively, leading to increased levels of GLU. Clinical and preclinical studies have both documented that clozapine can result in marked IR [46]. Burghardt et al. reported that the BDNF Met allele alone and in combination with AAP medications is associated with higher IR values [21]. Similarly IR is well established as the major pathogenic feature of MetS [47]. Taken together, we assume that the BDNF Val66Met genotype confers susceptibility to MetS by decreasing insulin action in the peripheral tissues.
In this study, we found that the impact of the Met/Met genotype on MetS and increased levels of GLU is only seen in male patients. To our best knowledge, this is the first study to evaluate the sexual variation in the effects of the Val66Met polymorphism on MetS. Our previous studies also indicated that the Val66Met polymorphism may have sex-specific characteristics [22], [23]. A recent preclinical study showed that BDNF heterozygous mice have complex brain region-specific changes in neurotrophins and their receptors differ gender-specifically [48]. This finding suggested that BDNF-TrkB signaling may be controlled in a sex-specific manner. Therefore, our findings in the present study imply that the effects of BDNF on MetS may be dependent on sex. The following question then arises naturally: do sex steroids contribute to this effect? Further studies are required for clarification, given the limited sample size and scope of this study.
The strength of this study is that the patients recruited for the study were all subject to long-term hospitalization, under clozapine treatment, with the maximum control over drug compliance, and receiving the same amounts of daily diet and exercise. Alongside these strengths, however, we would be remiss in not noting some marked limitations to this study. First, the size of our sample is small, especially for the purposes of sex stratification, and accordingly our findings should be viewed as preliminary until replicated and independently verified. Second, our subjects are chronic patients, and other AAP treatment prior to this study may have already influenced the risk for MetS, lessening the effects attributable to clozapine that the patients are currently being treated with. Third, patients’ baseline metabolic parameters prior to clozapine treatment and their previous antipsychotic agents were unknown, which may potentially confound the results obtained in this study. Lastly, the ideal pharmacogenetic study design is a longitudinal, prospective, randomized and parallel-control clinical trial. However, our study was cross-sectionally designed rather than longitudinally, therefore we cannot affirm that some subjects in the non-MetS group did not develop MS after the investigation. To avoid the negative consequences of this potential bias, it is important to replicate this data using larger studies that are better designed to find conclusive and not simply suggestive evidence of the associations that we noted in the present study.
In summary, in this study we tested for the first time the relationship between the BDNF Val66Met polymorphism and MetS in patients with schizophrenia under long-term clozapine treatment. We concluded that BDNF appears to have a weak association with clozapine-induced MetS, and this effect is only evident in male patients. Large-scale longitudinal studies should be conducted to replicate these findings and offer more conclusive evidence.
Acknowledgments
We are deeply grateful to all participants. We thank these three anonymous reviewers for their insightful comments.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81000581), the Shanghai Science & Technology Development Foundation (12140904200), the China Postdoctoral Science Foundation (2013M530410) and the National Key Clinical Disciplines at Shanghai Mental Health Center (OMA-MH, 2011-873). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pramyothin P, Khaodhiar L (2010) Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 17: 460–466. [DOI] [PubMed] [Google Scholar]
- 2.Asenjo Lobos C, Komossa K, Rummel-Kluge C, Hunger H, Schmid F, et al.. (2010) Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev: CD006633. [DOI] [PMC free article] [PubMed]
- 3. McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, et al. (2006) Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 163: 600–610. [DOI] [PubMed] [Google Scholar]
- 4. De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, et al. (2008) Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res 101: 295–303. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, et al. (2013) Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull 39: 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee IT, Lee WJ, Tsai IC, Liang KW, Lin SY, et al. (2012) Brain-derived neurotrophic factor not associated with metabolic syndrome but inversely correlated with vascular cell adhesion molecule-1 in men without diabetes. Clin Chim Acta 413: 944–948. [DOI] [PubMed] [Google Scholar]
- 7. Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, et al. (1999) Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A 96: 15239–15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray J, Yeo GS, Cox JJ, Morton J, Adlam AL, et al. (2006) Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55: 3366–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fox EA, Byerly MS (2004) A mechanism underlying mature-onset obesity: evidence from the hyperphagic phenotype of brain-derived neurotrophic factor mutants. Am J Physiol Regul Integr Comp Physiol 286: R994–1004. [DOI] [PubMed] [Google Scholar]
- 10. Duan W, Guo Z, Jiang H, Ware M, Mattson MP (2003) Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology 144: 2446–2453. [DOI] [PubMed] [Google Scholar]
- 11. Ribases M, Gratacos M, Fernandez-Aranda F, Bellodi L, Boni C, et al. (2005) Association of BDNF with restricting anorexia nervosa and minimum body mass index: a family-based association study of eight European populations. Eur J Hum Genet 13: 428–434. [DOI] [PubMed] [Google Scholar]
- 12. Zhang XY, Zhou DF, Wu GY, Cao LY, Tan YL, et al. (2008) BDNF levels and genotype are associated with antipsychotic-induced weight gain in patients with chronic schizophrenia. Neuropsychopharmacology 33: 2200–2205. [DOI] [PubMed] [Google Scholar]
- 13. Zai GC, Zai CC, Chowdhury NI, Tiwari AK, Souza RP, et al. (2012) The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry 39: 96–101. [DOI] [PubMed] [Google Scholar]
- 14. Tsai A, Liou YJ, Hong CJ, Wu CL, Tsai SJ, et al. (2011) Association study of brain-derived neurotrophic factor gene polymorphisms and body weight change in schizophrenic patients under long-term atypical antipsychotic treatment. Neuromolecular Med 13: 328–333. [DOI] [PubMed] [Google Scholar]
- 15. Zhang XY, Tan YL, Zhou DF, Cao LY, Wu GY, et al. (2007) Serum BDNF levels and weight gain in schizophrenic patients on long-term treatment with antipsychotics. J Psychiatr Res 41: 997–1004. [DOI] [PubMed] [Google Scholar]
- 16. Zhang JP, Lencz T, Geisler S, DeRosse P, Bromet EJ, et al. (2013) Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophrenia Research 146: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, et al. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. [DOI] [PubMed] [Google Scholar]
- 18. Ma XY, Qiu WQ, Smith CE, Parnell LD, Jiang ZY, et al. (2012) Association between BDNF rs6265 and obesity in the Boston Puerto Rican Health Study. J Obes 2012: 102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu L, Xi B, Zhang M, Shen Y, Zhao X, et al. (2010) Associations of six single nucleotide polymorphisms in obesity-related genes with BMI and risk of obesity in Chinese children. Diabetes 59: 3085–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedel S, Horro FF, Wermter AK, Geller F, Dempfle A, et al. (2005) Mutation screen of the brain derived neurotrophic factor gene (BDNF): identification of several genetic variants and association studies in patients with obesity, eating disorders, and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 132B: 96–99. [DOI] [PubMed] [Google Scholar]
- 21. Burghardt KJ, Pop-Busui R, Bly MJ, Grove TB, Taylor SF, et al. (2012) The influence of the brain-derived neurotropic factor Val66Met genotype and HMG-CoA reductase inhibitors on insulin resistance in the schizophrenia and bipolar populations. Clin Transl Sci 5: 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yi Z, Zhang C, Wu Z, Hong W, Li Z, et al. (2011) Lack of effect of brain derived neurotrophic factor (BDNF) Val66Met polymorphism on early onset schizophrenia in Chinese Han population. Brain Res 1417: 146–150. [DOI] [PubMed] [Google Scholar]
- 23. Lu W, Zhang C, Yi Z, Li Z, Wu Z, et al. (2012) Association between BDNF Val66Met polymorphism and cognitive performance in antipsychotic-naive patients with schizophrenia. J Mol Neurosci 47: 505–510. [DOI] [PubMed] [Google Scholar]
- 24. Ryu S, Oh S, Cho EY, Nam HJ, Yoo JH, et al. (2011) Interaction between genetic variants of DLGAP3 and SLC1A1 affecting the risk of atypical antipsychotics-induced obsessive-compulsive symptoms. Am J Med Genet B Neuropsychiatr Genet 156B: 949–959. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Zhang W, Yi Z, Lu W, Wu Z, et al.. (2013) Influence of polymorphisms in genes SLC1A1, GRIN2B, and GRIK2 on clozapine-induced obsessive-compulsive symptoms. Psychopharmacology (Berl) doi: 10.1007/s00213-013-3137-2. [DOI] [PubMed]
- 26. Zhou H, Guo ZR, Yu LG, Hu XS, Xu BH, et al. (2010) Evidence on the applicability of the ATPIII, IDF and CDS metabolic syndrome diagnostic criteria to identify CVD and T2DM in the Chinese population from a 6.3-year cohort study in mid-eastern China. Diabetes Res Clin Pract 90: 319–325. [DOI] [PubMed] [Google Scholar]
- 27. Bao YQ, Lu JX, Wang C, Yang M, Li HT, et al. (2008) Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 201: 378–384. [DOI] [PubMed] [Google Scholar]
- 28. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, et al. (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 29. Zhang W, Chen X, Gong W, Tang J, Tan L, et al. (2010) Common promoter variants of the NDUFV2 gene do not confer susceptibility to schizophrenia in Han Chinese. Behav Brain Funct 6: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi YY, He L (2006) SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci (vol 15, pg 97, 2005). Cell Research 16: 851–851. [DOI] [PubMed] [Google Scholar]
- 31. Kuipers SD, Bramham CR (2006) Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel 9: 580–586. [PubMed] [Google Scholar]
- 32. Travaglia A, La Mendola D, Magri A, Nicoletti VG, Pietropaolo A, et al. (2012) Copper, BDNF and Its N-terminal domain: inorganic features and biological perspectives. Chemistry 18: 15618–15631. [DOI] [PubMed] [Google Scholar]
- 33. Chaldakov GN, Tonchev AB, Aloe L (2009) NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Riv Psichiatr 44: 79–87. [PubMed] [Google Scholar]
- 34. Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG (2007) Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol 19: 974–982. [DOI] [PubMed] [Google Scholar]
- 35. Coppola V, Tessarollo L (2004) Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport 15: 2665–2668. [DOI] [PubMed] [Google Scholar]
- 36. Levin BE (2007) Neurotrophism and energy homeostasis: perfect together. Am J Physiol Regul Integr Comp Physiol 293: R988–991. [DOI] [PubMed] [Google Scholar]
- 37. Chaldakov GN, Fiore M, Stankulov IS, Hristova M, Antonelli A, et al. (2001) NGF, BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Arch Physiol Biochem 109: 357–360. [DOI] [PubMed] [Google Scholar]
- 38. Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, et al. (2001) Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 104: 1336–1342. [DOI] [PubMed] [Google Scholar]
- 39. Malik I, Danesh J, Whincup P, Bhatia V, Papacosta O, et al. (2001) Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet 358: 971–976. [DOI] [PubMed] [Google Scholar]
- 40. Pelleymounter MA, Cullen MJ, Wellman CL (1995) Characteristics of BDNF-induced weight loss. Exp Neurol 131: 229–238. [DOI] [PubMed] [Google Scholar]
- 41. Levinger I, Goodman C, Matthews V, Hare DL, Jerums G, et al. (2008) BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med Sci Sports Exerc 40: 535–541. [DOI] [PubMed] [Google Scholar]
- 42. Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, et al. (2007) Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50: 431–438. [DOI] [PubMed] [Google Scholar]
- 43. Yamanaka M, Itakura Y, Tsuchida A, Nakagawa T, Taiji M (2008) Brain-derived neurotrophic factor (BDNF) prevents the development of diabetes in prediabetic mice. Biomed Res 29: 147–153. [DOI] [PubMed] [Google Scholar]
- 44. Ono M, Itakura Y, Nonomura T, Nakagawa T, Nakayama C, et al. (2000) Intermittent administration of brain-derived neurotrophic factor ameliorates glucose metabolism in obese diabetic mice. Metabolism 49: 129–133. [DOI] [PubMed] [Google Scholar]
- 45. Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land SJ, et al. (2008) Brain-derived neurotrophic factor Val66Met and blood glucose: a synergistic effect on memory. Front Hum Neurosci 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cai J, Yi Z, Lu W, Fang Y, Zhang C (2013) Crosstalk between 5-HT2cR and PTEN signaling pathway in atypical antipsychotic-induced metabolic syndrome and cognitive dysfunction. Med Hypotheses 80: 486–489. [DOI] [PubMed] [Google Scholar]
- 47. Frisardi V, Solfrizzi V, Capurso C, Imbimbo BP, Vendemiale G, et al. (2010) Is insulin resistant brain state a central feature of the metabolic-cognitive syndrome? J Alzheimers Dis 21: 57–63. [DOI] [PubMed] [Google Scholar]
- 48. Hill RA, van den Buuse M (2011) Sex-dependent and region-specific changes in TrkB signaling in BDNF heterozygous mice. Brain Res 1384: 51–60. [DOI] [PubMed] [Google Scholar]