Abstract
The perivascular microenvironment helps maintain stem cells in many tissues. We sought to determine if there is a perivascular niche for hair follicle stem cells. The association of vessels and follicle progenitor cells began by embryonic day 14.5 (E14.5), when nascent hair placodes had blood vessels approaching them. By birth, a vascular annulus stereotypically surrounded the Keratin 15 negative (K15−) stem cells in the upper bulge, and remained associated with the K15− upper bulge throughout the hair cycle. The angiogenic factor Egfl6 was expressed by the K15− bulge and localized adjacent to the vascular annulus, which was comprised of post-capillary venules. Although denervation altered the phenotype of upper bulge stem cells, the vascular annulus persisted in surgically denervated mouse skin. The importance of the perivascular niche was further suggested by the fact that vascular annuli formed around the upper bulge of de novo reconstituted hair follicles prior to their innervation. Together, these findings demonstrate that the upper bulge is associated with a perivascular niche during the establishment and maintenance of this specialized region of hair follicle stem cells.
INTRODUCTION
The hair follicle epithelium relies on resident stem cells to replenish differentiated cells incorporated into the growing hair shaft and inner root sheath. Furthermore, the follicle itself undergoes lifelong cycles of regression (catagen), rest (telogen), and stem cell-mediated regeneration (anagen). Keratin 15 expressing (K15+) bulge cells are a well defined stem cell population that participates in regenerating the anagen follicle during homeostasis and repairing the epidermis after wounding (Ito et al., 2005; Morris et al., 2004). Recently, it has become clear that the bulge region contains additional, phenotypically and spatially distinct subpopulations of follicle stem cells. Above the K15+ bulge and below the follicle isthmus is the K15− upper bulge that contains Gli1+ follicle stem cells (Brownell et al., 2011). Gli1+ upper bulge cells repeatedly regenerate the anagen follicle and are uniquely capable of becoming epidermal stem cells in healing skin wounds. Signaling from the surrounding microenvironment is important in regulating various subpopulations of bulge stem cells. For example, activation of stem cells in the hair germ at the base of the bulge is induced by signals from the underlying dermal papilla (Greco et al., 2009), and the Gli1+, K15− upper bulge cells receive Sonic hedgehog (Shh) signaling from adjacent sensory neurons (Brownell et al., 2011). Thus spatially restricted signaling factors appear to contribute to the regionalization and regulation of hair follicle stem cells. Identifying additional localized components of the follicle stem cell niche will be important in furthering our understanding of how the microenvironment regulates tissue specific progenitors.
The perivascular microenvironment regulates many cellular and developmental processes including stem cell and progenitor cell specification. Stem cells preferentially reside adjacent to blood vessels in many tissues, including the bone marrow (Kiel et al., 2005), brain (Tavazoie et al., 2008), testes (Yoshida et al., 2007) and adipose tissue (Tang et al., 2008). The epidermis and follicle epithelium are avascular, and depend on nutrients obtained via diffusion from closely associated dermal blood vessels. The dermal vasculature has been well characterized (Durward and Rudall, 1958), with anastomosing superficial and deep plexuses in the reticular dermis. Arising from these dermal arterioles and venules are arborizing capillary networks that richly envelope hair follicles. Interestingly, the superficial vascular plexus resides at the level of the hair follicle bulge, and an intrinsic relationship between dermal vessels and the hair follicle bulge has been suggested in studies demonstrating expression of a Nestin-GFP transgene in both tissues. (Amoh et al., 2007; Amoh et al., 2004). In addition to necessary trophic support of the skin, studies suggest that vasculature may regulate hair follicle cycling. Proper hair cycle progression is dependent on angiogenesis and remodeling of the cutaneous microvasculature (Mecklenburg et al., 2000). Moreover, chemotherapy drugs such as doxorubicin disrupt the hair-follicle-associated blood vessel network, and result in dystrophic hair follicles and alopecia (Amoh et al., 2007). Although ischemia likely contributes to these phenotypes, impaired stem cell regulation may also be involved. Identifying a population of follicle stem cells that associate with the perivascular microenvironment would support this hypothesis.
The existence of multiple molecularly distinct populations of stem cells in the hair follicle bulge allowed us to assess progenitor subgroups for potential association with a perivascular niche. Using fluorescence immunostaining and 3D confocal imaging, a close spatial relationship was observed between blood vessels and the K15− stem cells in the upper bulge region. This association remained consistent throughout the hair cycle despite extensive vascular remodeling in the perifollicular adventitia. In contrast to the capillaries that surrounded most of the follicle, the K15− bulge was typically wrapped with a solitary venule annulus. During development, forming vessels approached the nascent follicle as early as embryonic day 14.5 (E14.5) and the vascular annulus was fully established by birth. In adult mouse skin, the K15− upper bulge expressed Egfl6, a signaling factor known to promote angiogenesis and attract vascular endothelial cells. Although the vascular annulus was not sufficient to maintain the K15− phenotype in the upper bulge of surgically denervated mouse skin, reconstituted hair follicles spontaneously formed vascular loops around the upper bulge, recapitulating the pattern of normal hair bearing skin. These findings identify a population of perivascular stem cells in the upper bulge and suggest that a venous vascular annulus is instrumental in the establishment, regionalization and regulation of hair follicle stem cells.
RESULTS
K15− cells in the upper bulge of the hair follicle associate with a vascular annulus
To investigate the possible association of vasculature with follicle stem cells, we used CD31 immunostaining of endothelial cells in thickly (20~80 μm) sectioned mouse skin to visualize the distribution of dermal blood vessels at multiple stages in development. Early in the developing dermis a capillary plexus forms via angiogenesis and continues to remodel throughout development. Nascent hair placodes at E14.5 lacked K15 expression, and blood vessels were observed extending up from the capillary plexus around the forming follicles (Figure 1a). By E16.5, vasculature was arranged circumferentially around the neck of the hair follicles (Supplemental Figure 1). In the growing follicle at E18.5, the upper hair peg uniformly expressed K15, and prominent blood vessels began to envelop the follicle neck in the developing upper bulge region (Figure 1b). By the day of birth a K15− domain had been specified in the upper bulge, and it was surrounded by a typical vascular annulus (Figure 1c). Staining with Sox9 to mark the bulge in perinatal hair follicles (Nowak et al., 2008; Vidal et al., 2005) confirmed that the K15− region corresponded to the upper bulge (Supplemental Figure 1b). Thus the upper bulge forms in the context of a vascular microenvironment, and K15− upper bulge stem cells undergo specification around birth, when a mature vascular annulus is present.
Figure 1. Upper bulge stem cells are associated with a vascular annulus.
CD31 and K15 immunostaining of vascular endothelial cells in mouse skin sections (40-80 μm thickness) at (a) E14.5, (b) E18.5 and (c) P0. CD31 and K15 co-stained adult skin with hair follicles in (d) anagen, (e) catagen, and (f) telogen. (g) Mean distance (μm, n=55) to nearest CD31 stained cell from keratinocytes in the mid K15− upper bulge, mid-infundibulum and mid K15+ bulge in telogen skin. (h) Whole mount CD31 immunostaining and autofluorescent hair shafts in adult ear skin viewed en face. (i) Egfl6 immunostaining in K15− upper bulge in telogen hair follicle. Arrowhead = follicle associated blood vessel. Error bars represent SD. * p<0.05 (F-test). Scale bar = 50 μm.
In postnatal mouse skin, follicles in all stages of the hair cycle were examined for their relationships to vessels. Despite the extensive angiogenesis and vascular remodeling that accompanies follicular growth and regression during the hair cycle (Mecklenburg et al., 2000), a prominent vascular annulus remained associated with the K15− upper bulge throughout the cycle (Figure 1d-f, Supplemental Figure 1c). 3D imaging and whole-mount staining showed a vascular annulus around the upper bulge of the hair follicle (Figure 1h). To confirm that upper bulge cells are preferentially positioned in close proximity to blood vessels, the distance between the closest CD31+ blood vessel and follicle cells in the midpoint of the K15− region was measured in 3D reconstituted images (n=55, Figure 1g). The distance between K15− cells and the nearest blood vessel was significantly shorter than that of midpoint cells of the infundibulum or K15+ bulge (p<0.05).
Taken together, these data demonstrate a consistent spatial relationship between K15− follicle stem cells residing in the upper bulge and a vascular annulus associated with each follicle.
The K15− upper bulge expresses Egfl6
Egfl6 is a member of the EGF repeat superfamily that can induce angiogenesis and promotes migration of endothelial cells (Chim et al., 2011). Because previous studies showed focal Egfl6 expression within the hair follicle (Fujiwara et al., 2011), we speculated that Egfl6 might be expressed by the upper bulge to mediate its association with the vascular annulus. Indeed, immunostaining revealed that Egfl6 was expressed specifically by the K15− stem cells in the upper bulge where it appeared to be preferentially deposited on the exterior surface of the telogen follicle (Figure 1i), consistent with its localization to basement membranes. The expression pattern and known functions of Egfl6 suggest that it may signal to, and regulate, the adjacent vascular annulus.
The vascular annulus is composed of venules
There are three major types of blood vessels in the dermis: arterioles, capillaries and venules. These vessel types vary in size, structure, function, and biomarker expression. As the vascular annulus was too large in diameter to be a capillary, we wanted to know if the annulus was an arteriole or venule. In adult tissues, ephrinB2 is a genetic marker of arterial smooth muscle and endothelial cells (Shin et al., 2001; Wang et al., 1998). Using immunostaining for CD31 and beta-galactosidase in skin from ephrinB2-tau-lacZ transgenic mice, we were able to identify dermal arterioles. Whereas arterioles in the superficial and deep vascular plexuses co-stained with the two markers, the vascular annulus surrounding the upper bulge was consistently negative for ephrinB2 (Figure 2a), suggesting it is a venule. Consistent with prior reports, parts of the upper follicle also stained for ephrinB2 (Egawa et al., 2009). To confirm the venous nature of the annulus, we used an antibody to EphB1 that stains venous endothelial cells in the skin (W.L. and Y.M., personal observation to be described elsewhere; Supplemental Figure 2). In wild type mouse skin the vascular annulus consistently stained for EphB1 (Figure 2b). These results indicate that the blood vessel annulus surrounding the upper bulge is composed of venules, and that K15− follicle stem cells exist in a perivenous microenvironment.
Figure 2. The vascular annulus is composed of venules.
(a) Telogen follicles in back skin from ephrinB2-tau-lacZ mice immunostained for CD31 and β-galactosidase. Arterioles label for both markers (arrow), whereas the vascular annulus only labels for CD31 (arrowhead). EphrinB2 also stains the follicle (*). (b) Wild type telogen follicles immunostained for CD31 and EphB1. Venules, including the vascular annulus, label for both markers (arrow), whereas arterial vessels only label with CD31+ (arrowhead). Scale bar = 50 μm.
The vascular annulus associates with perineural upper bulge stem cells
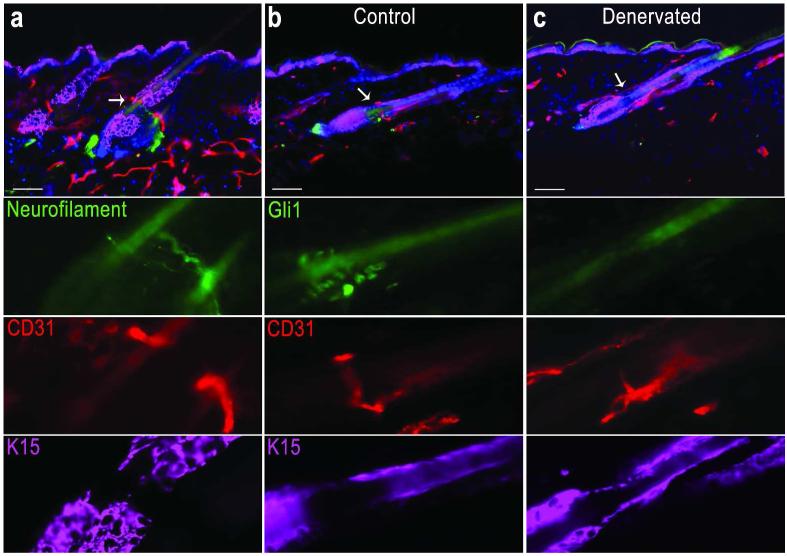
Prior studies have shown that the Gli1+ K15− phenotype in the upper bulge is dependent on contact with sensory nerves, and these stem cells become Gli1− K15+ after surgical denervation in Gli1LacZ/+ mice (Brownell et al., 2011). As circumferential nerve endings wrapped hair follicles in the same region as the vascular annulus (Figure 3a), we sought to test if denervation might also alter the perivascular niche. As was expected, CD31 immunostaining identified a close relationship between upper bulge Gli1+ K15− cells and the vascular annulus in Gli1LacZ/+ telogen mouse skin (Figure 3b). We performed unilateral surgical ablation of the dorsal cutaneous nerves in 6 week old Gli1LacZ/+ mice, and collected back skin 2 weeks after denervation. In control skin, the vascular annulus was associated with the Gli1+ K15− upper bulge (Figure 3b). In denervated skin, upper bulge Gli1 expression was absent and K15 was now expressed, however the vascular annulus was unperturbed (Figure 3c). The same result, including a persistent vascular annulus and lack of follicle innervation, was seen in skin analyzed 7 months after denervation (data not shown). These results demonstrate that K15− upper bulge stem cells reside in both a perineural and a perivascular microenvironment. Moreover, they demonstrate that despite altering the molecular signature of the upper bulge, denervation of the follicle leaves the perivascular niche and long-term maintenance of hair follicles undisturbed.
Figure 3. The vascular annulus persists in denervated skin.
Immunostaining in telogen skin, (a) the K15− upper bulge associates with both CD31 marked blood vessels and neurofilament marked nerves. (b) In Gli1lacZ/+ mouse hair follicles, Gli1+ K15− upper bulge stem cells associate with the CD31+ vascular annulus. (c) In surgically denervated skin from the same mouse, 2 weeks post denervation, the vascular annulus remains present although the upper bulge has become Gli1− and K15+. Scale bar = 50 μm.
Vascular annulus reforms in regenerated hair follicles in reconstituted mouse skin
The invariant association of upper bulge stem cells and the vascular annulus suggests a follicular dependence on the perivascular niche. To determine if the upper bulge associates with a perivascular niche in follicles that form in a context other than normal development, we examined the vasculature around follicles that were generated de novo in adult skin. New follicles in reconstituted mouse skin were created by mixing primary keratinocytes and dermal fibroblasts from neonatal mouse skin in a silicon-grafting chamber implanted into the back skin of an athymic nude mouse (Weinberg et al., 1993). The reconstituted follicles lack the regular orientation and patterning of normal mouse skin. Nonetheless, by three weeks after grafting, the vasculature associated with the reconstituted follicles recapitulated a typical vascular annulus in the upper bulge region (Figure 4a, b). Consistent with a role in establishing associations with vasculature, Egfl6 was expressed in the upper bulge region of the newly reconstituted follicles at the location of the vascular annulus (Figure 4c, d). Of note, reconstituted follicles in the center of the graft were not innervated, whereas those on the margin of the graft had associated nerves (Supplemental Figure 3). In innervated follicles near the graft margin, the upper bulge had specified a K15− region that coincided with the placement of the vascular annulus (Figure 4a). In the non-innervated center of the graft, follicles lacked a K15− domain. Nonetheless a vascular annulus formed adjacent to Egfl6-expressing cells in the upper bulge region (data not shown). The recreation of this association in de novo follicles strongly suggests a requirement for the perivascular microenvironment in the generation and maintenance of upper bulge stem cells.
Figure 4. The vascular annulus forms around reconstituted hair follicles.
Reconstituted mouse skin was generated on the back of adult nude mice. Vasculature (CD31) and K15 expression were examined three weeks after transplantation. (a, b) A vascular annulus was consistently associated with the upper bulge region of the reconstituted follicles (arrowheads). At the periphery of the graft (a), the vascular annulus coincided with K15− upper bulge (arrows). (c) Egfl6 immunostaining in upper bulge of reconstituted hair follicle. Scale bar = 50 μm.
DISCUSSION
Stem cell fate, function and maintenance are highly influenced by signals from their local microenvironment. In many tissues a perivascular stem cell niche is well defined. We systematically examined the patterning of the dermal vasculature during skin development and hair cycle homeostasis, and identified a characteristic perivascular niche associated with a subset of hair follicle stem cells residing in the K15− upper bulge (Figure 5). Importantly, we found that upper bulge stem cells are specified in close association with vessels, they remain associated with a venule annulus throughout the postnatal hair cycle and after skin denervation, and the vascular annulus is recapitulated around the upper bulge of de novo reconstituted hair follicles. The consistent and persistent nature of this association in different contexts strongly suggests a necessary regulatory relationship between the perivascular microenvironment and upper bulge stem cells. Intriguingly, the upper bulge specifically expresses Egfl6, a signaling molecule with angiogenic properties that can influence endothelial cell migration. This suggests a reciprocal mechanism whereby a molecularly distinct subset of bulge cells may create a microenvironment that influences dermal vasculature, thus maintaining the perivascular stem cell niche.
Figure 5. A schematic model of perivascular hair follicle stem cells.
In adult mouse skin a venous vascular annulus remains associated with Gli1+ K15− stem cells in the upper bulge throughout the hair cycle, despite dramatic remodeling of other perifollicular vasculature. Upper bulge stem cells are known to be spatially, molecularly and functionally distinct from other follicle stem cells, and formation of the vascular annulus appears to coincide with specification and regionalization of the upper bulge. The unique expression of the angiogenic factor Egfl6 by the upper bulge further suggests a specialized association with a perivascular microenvironment for these stem cells. SG = sebaceous gland. DP = dermal papilla.
Upper bulge stem cells in the perivascular niche are also regulated by a perineural microenvironment (Brownell et al., 2011). Nerves induce Gli1 expression in the upper bulge by Shh signaling, and are required to maintain the unique ability of upper bulge cells to become epidermal stem cells after being recruited into healing skin wounds. Interestingly, nerves appear to be dispensable for continued follicle regeneration in the hair cycle. Thus the upper bulge is specified and is maintained in a neurovascular microenvironment, but follicle stem cell properties continue to manifest after denervation, in the perivascular environment alone. Moreover, the upper bulge that forms in de novo reconstituted follicles expresses Egfl6 and recruits a vascular niche in the absence of follicle innervation. Together these observations suggest a critical need for the perivascular microenvironment for the specification and maintenance of upper bulge stem cells.
Vasculature is also necessary to supply oxygen and nutrients to cells in the skin, and disruption of vasculature results in ischemic necrosis. It has been speculated that stem cells may preferentially associate with vasculature in order to insure their access to essential nutrients. However, the vascular annulus associated with the upper bulge is a venous vessel, suggesting it would carry blood with lower oxygen and nutrient contents than arterioles or capillaries. The venous nature of the vessels that associate with the stem cell niche could imply that relative hypoxia and low nutrients are part of the regulatory microenvironment. Alternatively, molecules specifically generated by venules could regulate the follicle stem cells. While angiocrine factors have been found to regulate adult stem cells (Butler et al., 2010; Sugiyama et al., 2006) and cancer stem cells (Beck et al., 2011), it is not clear if the signals originate from arterioles, venules, or both. Moreover, it is not known if the perivascular stem cells observed in other organs also associate exclusively with venules. Having demonstrated that venules associate with a subpopulation of hair follicle stem cells, it will be interesting to learn if this is consistent across other perivascular niches, or if arteriolar signals may regulate stem cells in other tissues.
Our observations reveal that the developing hair follicle bulge is surrounded by vasculature from its earliest stages. Moreover, by E16.5 the developing bulge region is enveloped by both vessels and neuronal processes (Supplemental Figure 1a’). Thus, hair follicle stem cells form and ultimately reside in a neurovascular microenvironment. During the same period of development, myelinated sensory nerves in the deeper dermis direct vascular remodeling and arterial differentiation so that arterioles and small arteries align with nerve branches (Mukouyama et al., 2002). In contrast, nerve endings at the developing bulge associate with a capillary network that remodels to form a venule annulus. Either the nerves and vessels independently localize to the developing bulge via environmental patterning signals, or follicle-associated nerve endings direct venous remodeling and differentiation. Considering that a vascular annulus forms around the bulge of de novo regenerated follicles in the absence of nerves, patterning by a common ontology seems more likely. Investigating these alternatives, along with any role the vascular annulus may play in nerve maintenance at the bulge, will require additional study.
There is growing evidence that the bulge contains multiple distinct subpopulations of hair follicle stem cells. Mechanisms must exist to establish and maintain stem cell domains within the bulge. Signals from adjacent structures such as the dermal papilla, sensory nerves, and the arrector pili muscle have been posited to be involved in establishing stem cell domains within the bulge (Greco and Guo, 2010). Now the vascular annulus must also be considered as a potential regionalizing influence. There are multiple reasons why the upper bulge would need spatial regulation. First, the upper bulge lies on a functional border. The upper bulge contains stem cells that are quiescent during telogen and are recruited to regenerate the follicle during anagen. Immediately above the upper bulge is the isthmus that contains proliferative progenitor cells that do not normally contribute to the adult follicle. Signals from the vascular annulus may help maintain the disparate characteristics of these two adjacent keratinocyte populations. Furthermore, fate mapping of Gli1 and Lgr5 follicle stem cells show preferential retention of the long-term stem cells in the upper bulge region (Brownell et al., 2011; Jaks et al., 2008). Thus the vascular annulus is positioned appropriately to provide a niche that regulates the long-term maintenance of self-renewing follicle stem cells.
Based on these data, we propose that a venous vascular annulus forms around the upper bulge of follicles in mouse skin and participates in the establishment and maintenance of a specialized stem cell population. We also observe that Egfl6 is specifically expressed by the upper bulge stem cells found in the perivascular niche and is a candidate for sustaining the vascular microenvironment. Future studies will hopefully identify the essential angiocrine factors that regulate follicle stem cell behavior. Understanding the requisite components of the stem cell niche will have far reaching implications on tissue engineering and developing stem cell based therapies.
MATERIALS AND METHODS
Animals
Normal fetal and postnatal FVB mice were obtained after overnight mating. 3 embryos were analyzed for each time point. E0.5 was regarded as noon on the first day after the mating. P0 was the morning of the day of birth. FVB, Gli1LacZ/+ mice (Bai et al., 2002), and EphrinB2-tau-LacZ mice (Wang et al., 1998) were bred and housed in a pathogen-free facility at the NCI, Bethesda, MD, and used in experiments in accordance with institutional guidelines.
Immunofluorescent staining and image analysis
Skin was fixed in 4% paraformaldehyde over-night at 4 °C and then cryoprotected over-night in 30% sucrose, embedded in OCT and frozen. 12 μm to 80 μm sections were obtained. Sections were fixed in 4% paraformaldehyde for 15 min before incubation in 10% serum in 0.1% PBT for 1 hour, then in primary antibody overnight at 4 °C. Alexa Fluor conjugated secondary antibodies (1:2000, Invitrogen, California, US) were used to detect the signals. The primary antibodies used are as following: rat anti CD31 (BD Pharmingen™, California, US), rabbit anti CD31, chicken anti K15, rabbit anti K14 (Covance, Virginia, US), goat anti EphB1, rabbit anti sox9 (Santa Cruz, California, US), rabbit anti beta-galactosidase (ebioscience, California, US), rabbit anti Egfl6 (a kind gift from Dr. Fiona Watt (Fujiwara et al., 2011)). Confocal images were acquired with Zeiss LSM 710 Confocal system (Carl Zeiss Inc, Thornwood, NY). Whole mount staining followed the online protocol as described before (Li and Mukouyama, 2011).
Surgical denervation of cutaneous nerves
Dorsal cutaneous nerves were severed by microsurgery as described (Maurer et al., 1998). The denervated right dorsal skin and the sham treated left dorsal skin was separated by a midline incision.
Hair reconstitution assay
This was performed as per published protocols (Lichti et al., 2008; Woo et al., 2012). Briefly, primary mouse keratinocytes (7× 106 to 10 × 106) and primary mouse dermal cells (1 × 106) were isolated from 0-2 day postnatal pups skin, they were combined and transferred to a silicon grafting chamber implanted onto the back skin of an athymic nude mouse. After 3 weeks, the grafts were removed for analysis.
Supplementary Material
ACKNOWLEDGEMENT
We thank Ms. Carole Yee and Mr. James Perna for technical assistance, Dr. Mark Udey, for discussion and proof reading, and the CCR Confocal Microscopy Core Facility for assistance in confocal imaging. This research is supported by the NIH Intramural Research Program, Center of Cancer Research, National Cancer Institute.
Abbreviations
- K15
Keratin 15
- Egfl6
EGF-like-domain, multiple 6
- Shh
Sonic hedgehog
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Aki R, Amoh Y, Li L, Katsuoka K, Hoffman RM. Nestin-expressing interfollicular blood vessel network contributes to skin transplant survival and wound healing. J Cell Biochem. 2010;110:80–6. doi: 10.1002/jcb.22512. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol. 2007;127:11–5. doi: 10.1038/sj.jid.5700486. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A. 2005a;102:5530–4. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, et al. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci U S A. 2004;101:13291–5. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Yang M, Li L, Reynoso J, Bouvet M, Moossa AR, et al. Nestin-linked green fluorescent protein transgenic nude mouse for imaging human tumor angiogenesis. Cancer Res. 2005b;65:5352–7. doi: 10.1158/0008-5472.CAN-05-0821. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chim SM, Qin A, Tickner J, Pavlos N, Davey T, Wang H, et al. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. J Biol Chem. 2011;286:22035–46. doi: 10.1074/jbc.M110.187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durward A, Rudall KM. Chapter 9: The vascularity and patterns of growth of hair follicles. In: Montagna W, Ellis RA, editors. The biology of hair growth. Academic Press; New York: 1958. p. 189. 1958. [Google Scholar]
- Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa S. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J Invest Dermatol. 2009;129:2386–95. doi: 10.1038/jid.2009.105. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–89. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–69. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–94. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–9. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A. 2003;100:9958–61. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mukouyama YS. Whole-mount immunohistochemical analysis for embryonic limb skin vasculature: a model system to study vascular branching morphogenesis in embryo. J Vis Exp. 2011 doi: 10.3791/2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle. 2011;10:830–9. doi: 10.4161/cc.10.5.14969. [DOI] [PubMed] [Google Scholar]
- Maurer M, Peters EM, Botchkarev VA, Paus R. Intact hair follicle innervation is not essential for anagen induction and development. Arch Dermatol Res. 1998;290:574–8. doi: 10.1007/s004030050354. [DOI] [PubMed] [Google Scholar]
- Mecklenburg L, Tobin DJ, Muller-Rover S, Handjiski B, Wendt G, Peters EM, et al. Active hair growth (anagen) is associated with angiogenesis. J Invest Dermatol. 2000;114:909–16. doi: 10.1046/j.1523-1747.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol. 2001;230:139–50. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–88. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchugonova A, Duong J, Zhang N, Konig K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem. 2011;112:2046–50. doi: 10.1002/jcb.23122. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–51. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH, et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–36. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–46. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–6. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.