Abstract
Cancer stem-like cells (CSCs)/cancer-initiaiting cells (CICs) are defined as a small population of cancer cells that have self-renewal capacity, differentiation potential and high tumor-initiating ability. CSCs/CICs of ovarian cancer have been isolated by side population (SP) analysis, ALDEFLUOR assay and using cell surface markers. However, these approaches are not definitive markers for CSCs/CICs, and it is necessary to refine recent methods for identifying more highly purified CSCs/CICs. In this study, we analyzed SP cells and aldehyde dehydrogenese bright (ALDHBr) cells from ovarian cancer cells. Both SP cells and ALDHBr cells exhibited higher tumor-initiating ability and higher expression level of a stem cell marker, sex determining region Y-box 2 (SOX2), than those of main population (MP) cells and ALDHLow cells, respectively. We analyzed an SP and ALDHBr overlapping population (SP/ALDHBr), and the SP/ALDHBr population exhibited higher tumor-initiating ability than that of SP cells or ALDHBr cells, enabling initiation of tumor with as few as 102 cells. Furthermore, SP/ADLHBr population showed higher sphere-forming ability, cisplatin resistance, adipocyte differentiation ability and expression of SOX2 than those of SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells. Gene knockdown of SOX2 suppressed the tumor-initiation of ovarian cancer cells. An SP/ALDHBr population was detected in several gynecological cancer cells with ratios of 0.1% for HEC—1 endometrioid adenocarcinoma cells to 1% for MCAS ovary mucinous adenocarcinoma cells. Taken together, use of the SP and ALDHBr overlapping population is a promising approach to isolate highly purified CSCs/CICs and SOX2 might be a novel functional marker for ovarian CSCs/CICs.
Introduction
Cancer stem-like cells (CSCs)/cancer-initiating cells (CICs) are defined as small population of cancer cells that have the properties of high tumor initiating ability, self-renewal ability and differentiation ability [1]–[3]. Furthermore, CSCs/CICs are shown to be resistant to standard cancer therapies including chemotherapy and radiotherapy; therefore, CSCs/CICs are responsible for cancer relapse after treatment [4], [5]. Several approaches have been described to identify CSCs/CICs, including isolation by CSC/CIC-specific cell surface marker expression (e.g. CD44, CD133, CD166), detection of side population (SP) cell phenotype by Hoechst 33342 exclusion and detection of aldehyde dehydrogenase 1 (ALDH1) activity in the ALDEFLUOR assay [6]. However, the expression of cell surface markers, SP cells and the expression of ALDH1 are not related to tumor-initiating ability in some reports [7]–[9]. These observations thus suggest that these stem cell markers (cell surface markers, SP cells and ALDH1) are not functional and not necessary for maintenance of CSCs/CICs. These markers may not define high tumorigenic CSCs/CICs, and these markers are thus merely surrogate markers for CSCs/CICs. Therefore, functional non-surrogate marker which is essential for maintenance of CSCs/CICs is expected.
Ovarian cancer is one of the major malignancies and causes the death of more than one million people in the world every year [10]. In addition, most patients have miserable episodes of ascites, especially in advanced stages. To improve the clinical treatment of ovarian cancer, ovarian cancer stem cell research has emerged as a recent topic. CD44 cell surface marker, SP cells and ALDHBr cells have been reported as stem cell markers for gynecological malignancies using cell lines OVCAR3, HEC-1 and other lines and primacy samples [11]–[14], and CSC/CIC research may improve the outcome of advanced ovarian cancer patients.
To improve the methods for isolation of highly purified ovarian CSCs/CICs, we analyzed the combination of known ovarian CSC/CIC markers. We analyzed ovarian cancer cell lines by SP analysis and ALDEFLUOR assay and found that SP cells and ALDHBr cells were higher tumorigenic than those of main population (MP) cells and ALDHLow cells, respectively. We found that the overlapping population of SP cells and ALDHBr cells (SP/ALDHBr) were more highly tumorigenic. And we found that SOX2 was expressed in an SP/ALDHBr population at higher level, and gene knockdown of SOX2 abrogated the tumor-initiation of ovarian cancer cells. Therefore, SOX2 might be a novel functional marker for ovarian CSCs/CICs and SP/ALDHBr population is more suitable population for analysis of ovarian CSCs/CICs than SP cells or ALDHBr cells.
Materials and Methods
Ethics Statement
Mice were maintained and experimented on in accordance with the guidelines of and after approval by the Committee of Sapporo Medical University School of Medicine, Animal Experimentation Center under permit number 08-006. Any animal found unhealthy or sick were promptly euthanized. Immunohistochemical staining study was approved by Institutional Review Boards (IRB) of Sapporo Medical University Hospital. We obtained written informed consent from all patients according to the guidelines of the Declaration of Helsinki.
Cell lines and culture
Human ovarian cell lines (MCAS, HTBoA, OVCAR3, OVSAHO) and human endometrial carcinoma (HEC-1) cells were obtained from ATCC (Manassas, VA, USA). MCAS and HEC-1 cells were maintained in Minimun Essential medium (MEM) (Life Technologies, Grand Island, NY, USA). HTBoA and OVCAR3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St Louis, MO, USA). OVSAHO cells were maintained in RPMI1640 medium (Sigma-Aldrich). Each cell line was supplemented with 10% FBS and cultured in a humidified 5% CO2 incubator at 37°C.
Side population (SP) assay
Side population (SP) analysis was performed as described previously with some modifications [15], [16]. Hoechst 33342 (Lonza, Walkersville, MD, USA) dye was used at the concentration of 2.5 or 5.0 µg/ml in the presence or absence of verapamil (50 mM; Sigma-Aldrich) as an inhibitor of the ABC transporter. The cells were incubated at 37°C for 60 min or 90 min with continuous shaking. One million of stained cells were analyzed by FACS Aria II (BD Biosciences, San Jose, CA, USA). The Hoechst 33342 dye was excited at 357 nm and its fluorescence was analyzed using dual wave-lengths (blue, 402–446 nm; red, 650–670 nm).
ALDEFLUOR assay
Aldehyde dehydrogenase (ALDH) activity was detected using an ALDEFLUOR assay kit (StemCell Technologies) according to the manufacturer's protocol [17]. Cells were stained by bodipy-aminoacetaldehyde (BAAA) at 1.5 mM and incubated for 30 min at 37°C. An inhibitor of ALDH1, diethylamino- benzaldehyde (DEAB), at a10-fold molar excess was used as a negative control. One million of stained cells were analyzed by FACS Aria II. The brightly fluorescent ALDH1-expressing cells (ALDH1Br) were detected in the green fluorescence channel (520–540 nm).
SP and ALDEFLUOR dual staining
The cells were stained by Hoechst 33342 dye and then stained by BAAA. One million of SP and ALDEFLUOR-dual-stained cells were analyzed by FACS Aria II. The cells were divided into three groups according to ALDH intensity (ALDHBr (ALDH bright), ALDHMid (ALDH middle), ALDHLow (ALDH low)), then analyzed by SP assay.
Immunohistochemical staining
Immunohistochemical staining using formalin-fixed paraffin-embedded sections of surgically resected ovarian carcinoma tissue was performed as described previously [18]. Anti-ALDH1 mouse antibody was used at 250-times dilution. Anti-ABCG2 rabbit polyclonal antibody (Sigma-Aldrich) was used at 5 µg/ml. Peroxidase-labeled goat anti-rabbit polyclonal antibody (Nichirei, Tokyo, Japan) was used as manufacturer's protocol and visualized by DAB. Alkaline phosphatase-labeled goat anti-mouse polyclonal antibody (Nichirei) was used as manufacturer's protocol and visualized by New Fuchsin (Nichirei). Membrane brown staining was judged as positive staining for ABCG2, and cytoplasm red staining was judged as positive staining for ALDH1.
Xenograft transplantation
Sorted cells were collected and re-suspended at concentrations of 102–104 cells per 50 µl of PBS and then mixed with 50 µl of matrigel (BD Biosciences). The cell-matrigel mixture was injected in the subcutaneous space of 6-week-old non-obese diabetic/severe combined immune-deficiency (NOD/SCID) mice (NOD.CB17-Prdkcscid/J, Charles River Laboratory, Yokohama, Japan) under anaesthesia. Tumor growth was monitored weekly, and tumor volume was calculated by XY2/2 (X = long axis, Y = short axis).
Sphere formation assay
Spherical colony formation assay was performed using CSC Complete Recombinan Medium (Cell Systems Corporation, Kirkland, WA, USA). SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells were plated at 103 cells per well in 6-well ultra-low attachment plates (Corning Inc., Corning, NY, 14831) and cultured for 10 days. The morphology of the cells was assessed and pictures were taken under a light microscope every day. Round cell clusters larger than 100 µm were judged as spheres.
Cell viability assay
For cell viability assay, SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells were isolated. Then, the cells were plated at 1000 cells per 96-well plate for 1 day and then were treated with cisplatin for 3 days under several concentrations. Subsequently, the cell viability was investigated using the Premix WST-1 Cell Proliferation Assay System (Takara Bio Inc., Otsu, Japan) according to the manufacturer's protocol.
Adipocyte differentiation assay
For adipocyte differentiation assay, SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells were isolated. The cells were plated at 10000 cells per 24-well plate, and were grown in serum-reduced RPMI∶DMEM (1∶1) medium containing 0.5 mM trans-retinoic acid (Sigma-Aldrich) and 50 nM insulin (Sigma-Aldrich) for 2 days followed by the addition of adipose differentiation medium containing 170 nM insulin, 2 nM triiodothyronine and 0.5 mM rosiglitazone [19]. Cells were maintained in the medium for 5 days and neutral lipid accumulation was detected in 4% formaldehyde fixed cells using Oil red O (Sigma-Aldrich) staining. Lipid staining was observed using microscope, and lipid stained cells were counted.
SOX2 mRNA knockdown by siRNA
A SOX2 gene knockdown experiment was performed using small interfering RNA (siRNA). SOX2 siRNA (NM003106) and negative control siRNA were purchased from Life Technologies. MCAS cells were seeded into a 24-well plate, and transfections were carried out using Lipofectamine RNAi max (Life Technologies) in Opti-MEM according to the manufacturer's instructions. Fourty-eight hours later, the cells were analyzed for expression of SOX2, ALDH1A1 and ABCG2 by RT-PCR.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Gene knockdown of SOX2 was confirmed by RT-PCR. Isolation of RNA and RT-PCR analysis were performed as described previously [20]. The thermal cycling conditions were 94°C for 2 min, followed by 35 cycles of 15 sec at 94°C, 30 sec at 60°C, and 30 sec at 72°C. Primer pairs used for RT-PCR analysis were 5′-TGTTAGCTGATGCCGACTTG-3′ and 5′-TTCTTAGCCCGCTCAACACT-3′ for ALDH1A1 with an expected PCR product size of 154 base pairs (bps), 5′-CACCTTATTGGCCTCAGGAA-3′ and 5′- CCTGCTTGGAAGGCTCTATG-3′ for ABCG2 with an expected PCR product size of 206 bps, 5′-CATGATGGAGACGGAGCTGA-3′ and 5′-ACCCCGCTCGCCATGCTATT-3′ for SOX2 with an expected PCR product size of 410 bps, 5′-GCAGTCAACAGTCGAAGAAGG-3′, and 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) with an expected product size of 452 bps. GAPDH was used as an internal control.
Quantitative real-time PCR analysis (qPCR)
Quantitative real-time PCR was performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. SOX2 (Hs01053049_s1), ABCG2 (Hs00184979_m1), CD44 (Hs01075861_m1), PROM1 (Hs01009250_m1) and ABCB1 (Hs00184500_m1) primers and probes were designed by the manufacturer (TaqMan Gene expression assays; Applied Biosystems). Thermal cycling was performed using 40 cycles of 95°C for 15 seconds followed by 60°C for 1 min. Each experiment was done in triplicate, and normalized to the GAPDH gene as an internal control.
Results
CSCs/CICs were enriched in SP cells
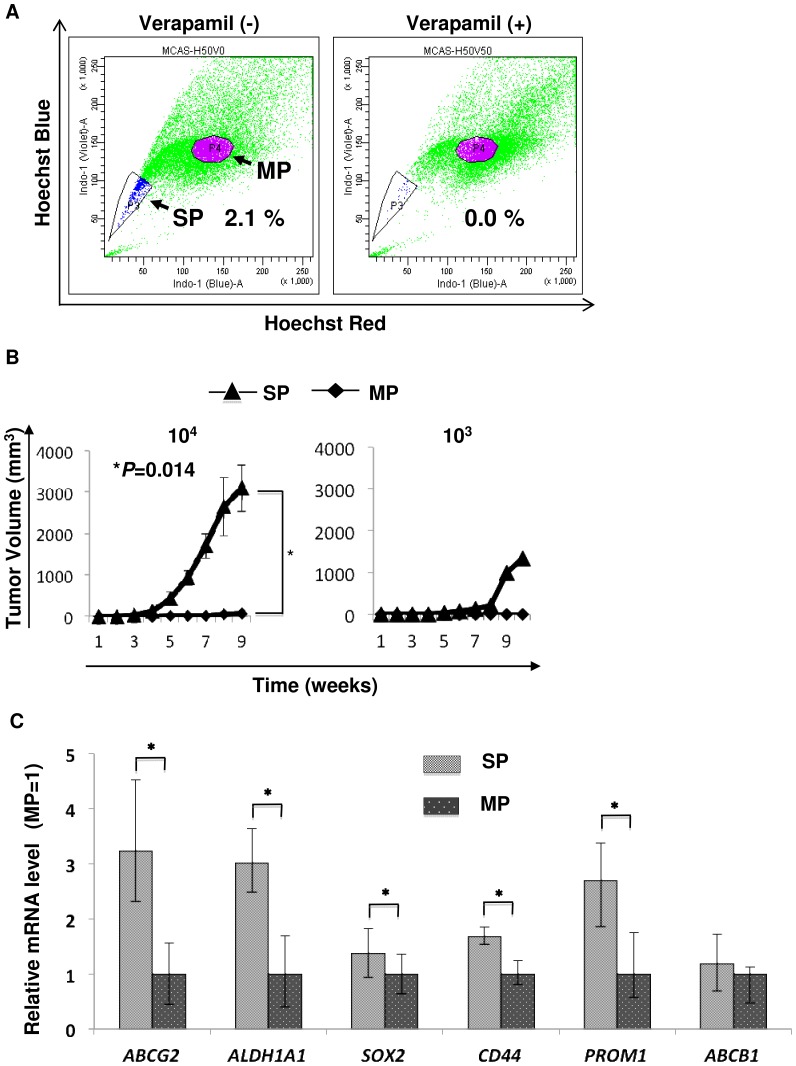
Ovarian CSCs/CICs have been isolated as SP cells from human and mice ovarian cancer line cells [11], [21]. We analyzed several gynecological caner cell lines including human ovarian cell lines (MCAS, HTBoA, OVCAR3, OVSAHO) and human endometrial carcinoma (HEC-1) cell line (Figure 1A and Figure S1). SP cells could be detected in all line cells and the SP cell ratio were ranged from 1.2% to 2.6%. Since there is no report describing human mucinous adenocarcinoma line cell MCAS, we thus further analyzed SP cells derived from MCAS. CSCs/CICs have high tumor-initiating ability [22], we thus injected serially diluted numbers of SP cells and MP cells into the backs of three NOD/SCID mice subcutaneously to examine the tumor-initiating ability. In all three mice, tumors were initiated with 104 SP cells, and tumors were initiated with 104 MP cells in 2 of the 3 mice. In one mouse, a tumor was initiated with 103 SP cells, while 103 MP cells did not initiate any tumor (Table 1). The size of tumors derived from SP cells was significantly larger than that of tumors derived from MP cells (Figure 1B). The expression levels of stem cell markers were investigated by qPCR, and SP cells derived from MCAS cells expressed higher levels of the stem cell markers SOX2, CD44 and PROM1 and the ABC transporter gene ABCG2, whereas ABCB1 was not (Figure 1C). These results indicate that CSCs/CICs were enriched in SP cells derived from MCAS cells. The results were reproduced in at least three independent experiments.
Figure 1. MCAS CSCs/CICs are enriched in SP cells.
A. Detection of SP cells from MCAS cells. MCAS ovarian mucinous adenocarcinoma cells were stained with Hoechst 33342 dye and analyzed. The percentage represents the ratio of SP cells. B. Tumor initiation of SP cells derived from MCAS cells. 103 and 104 SP and MP cells derived from MCAS cells were inoculated subcutaneously into the backs of NOD/SCID mice, and tumor growth was measured weekly. Data represent means ± SD. Differences between SP and MP cells were examined for statistical significance using Student's t-test. *P values. C. qPCR of CSC/CIC markers in MCAS SP and MP cells. Data represent means ± SD. Asterisks indicate significant difference. *P<0.05. t-test.
Table 1. Summary of tumor initiation incidence.
| MCAS Cell | Tumor initiation (injected cell number)* | ||
| 102 | 103 | 104 | |
| SP/ALDHBr cells | 5/5 | 5/5 | n.d. |
| SP cells | 0/3 | 1/3 | 3/3 |
| ALDHBr cells | 0/5 | 0/5 | 5/5 |
| MP cells | 0/3 | 0/3 | 2/3 |
| ALDHLow cells | 0/5 | 0/5 | 2/5 |
| MP/ALDHLow cells | 0/5 | 0/5 | 0/5 |
Tumor-initiating abilities were evaluated at day 70 post cell injection. Tumor-initiation/injection.
n.d.: not determined.
CSCs/CICs were enriched in ALDHBr cells
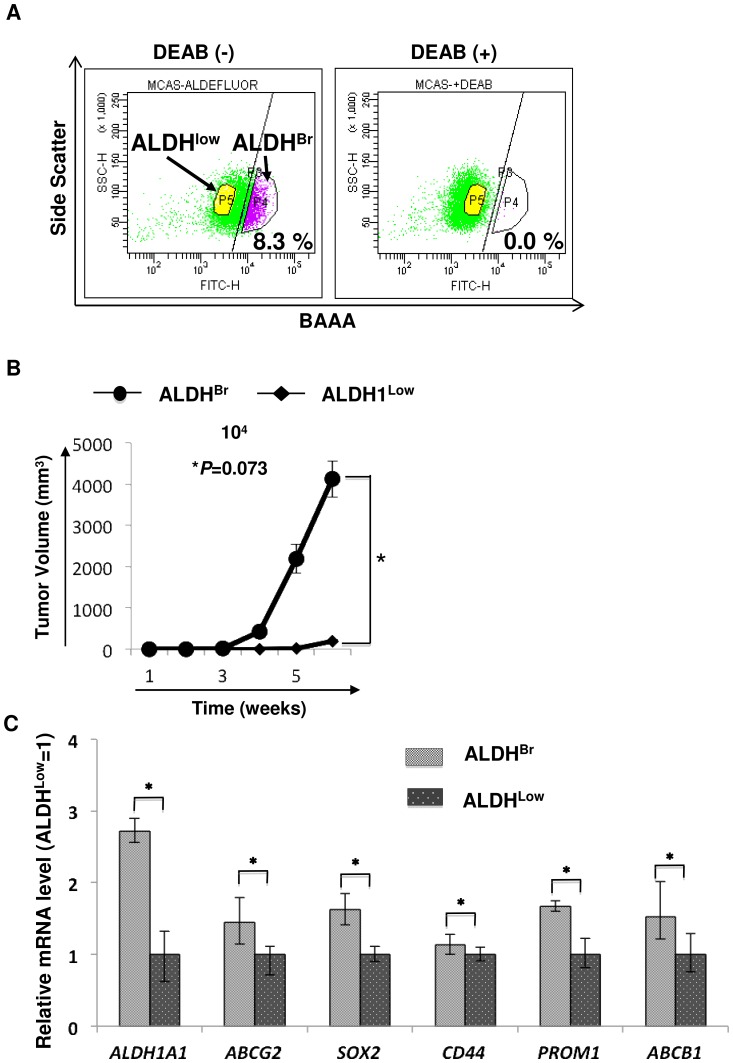
CSCs/CICs could be isolated as ALDHBr cells by the ALDEFLUOR assay [23]. We therefore examined whether CSCs/CICs can be successfully isolated by the ALDEFLUOR assay. MCAS, HTBoA, OVCAR3, OVSAHO and HEC-1 cells were analyzed by the ALDEFLUOR assay and we found that the ratio of ALDHBr cells was 8.1% to 11.3% (Figure 2A and Figure S1). ALDHBr cells and ALDHLow cells derived from MCAS were sorted and injected into the backs of five NOD/SCID mice to examine the tumor-initiating ability. In all five mice, tumors were initiated with 104 ALDHBr cells, while tumors were initiated with 104 ALDHLow cells in only 2 of the 5 mice (Table 1). The size of tumors derived from ALDHBr cells was significantly larger than that of tumors derived from ALDHLow cells (Figure 2B). The expression levels of stem cell markers were investigated by qPCR. ALDHBr cells derived from MCAS cells expressed higher levels of the stem cell markers SOX2, CD44 and PROM1, ALDH1A1, and the ABC transporter gene ABCG2 and ABCB1 (Figure 1C). These results indicate that CSCs/CICs were also enriched in ALDHbr cell derived from MCAS cells. The results were reproduced in at least three independent experiments.
Figure 2. MCAS CSCs/CICs are enriched in ALDHBr cells.
A. Detection of ALDHBr cells from MCAS cells. MCAS ovarian mucinous adenocarcinoma cells were stained with BAAA and analyzed. The percentage represents the ratio of ALDHBr cells. Inhibitor indicate ALDH1 inhibitor (diethylamino- benzaldehyde (DEAB)). B. Tumor initiation of ALDHBr cells derived from MCAS cells. 104 ALDHBr and ALDHLow cells derived from MCAS cells were inoculated subcutaneously into the backs of NOD/SCID mice, and tumor growth was measured weekly. Data represent means ± SD. Differences between ALDHBr and ALDHLow cells were examined for statistical significance using Student's t-test. *P values. C. qPCR of CSC/CIC markers in MCAS SP and MP cells. Data represent means ± SD. Asterisks indicate significant difference. *P<0.05. t-test.
SP and ALDEFLUOR dual analysis
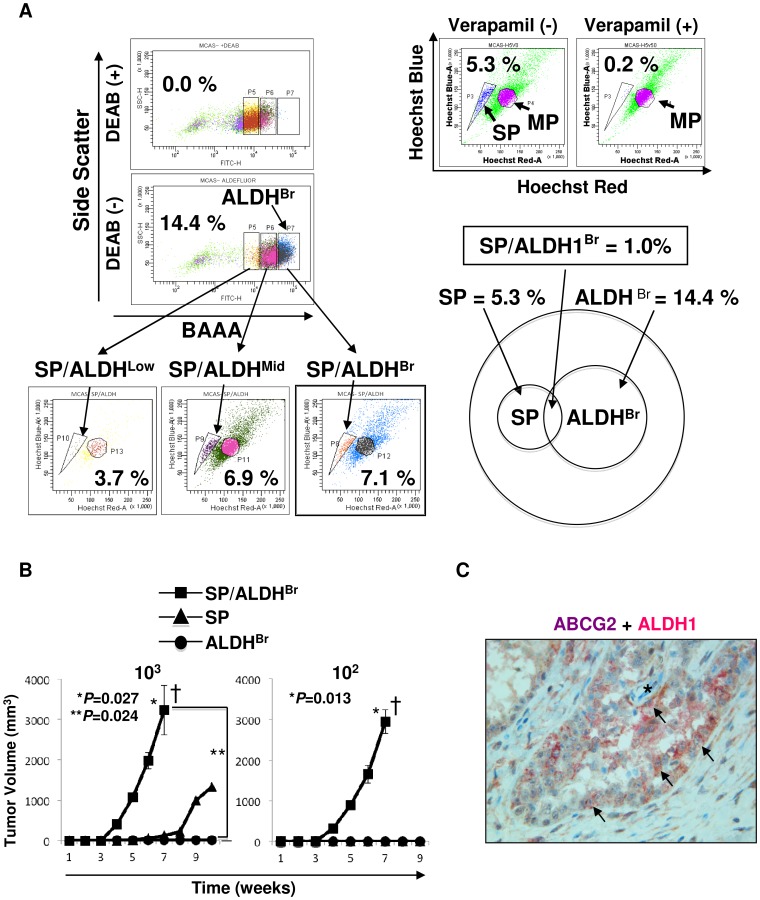
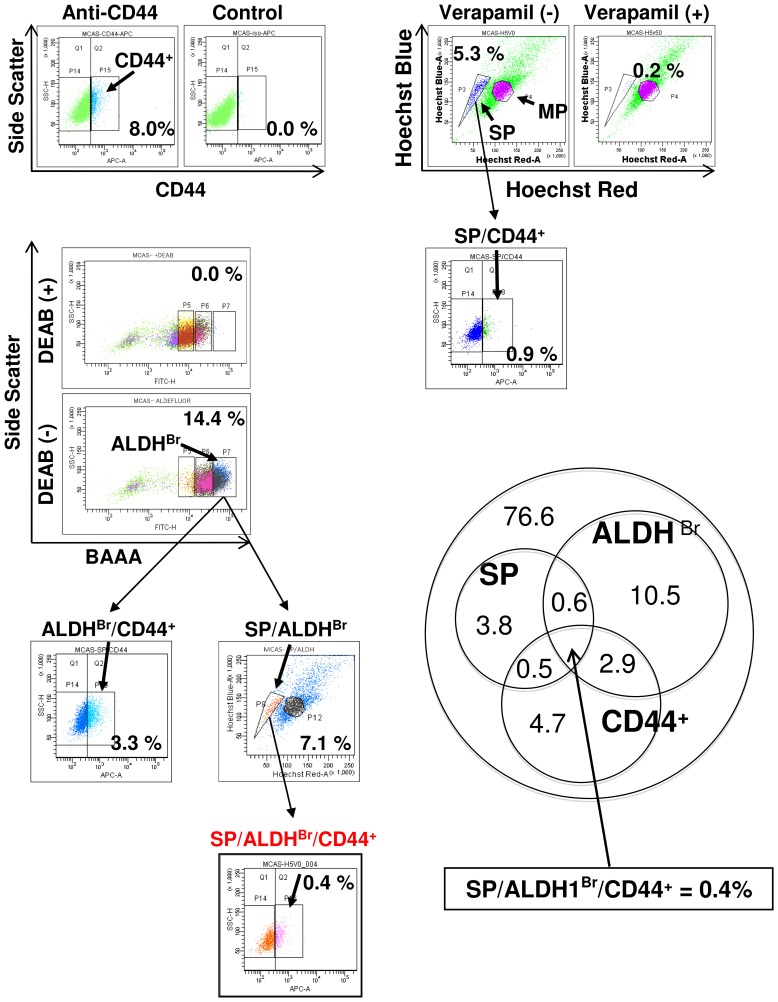
SP cells and ALDHBr cells show greater efflux of Hoechst 33342 dye and higher expression level of aldehyde dehydrogenese, which are different phenotypes, and hematopoietic stem cells were isolated as an SP and ALDHBr population in a previous study [24]. We therefore investigated the overlapping population of SP cells and ALDHBr cells. After staining the MCAS cells with Hoechst 33342 dye, the cells were washed and then stained with ALDEFLUOR reagent and analyzed. In this experiment, the ratio of SP cells was 5.3% and the ratio of ALDHBr cells was 14.4%. 7.1% of ALDHBr cells showed SP population, and 6.9% of ALDHMid cells showed SP population, and only 3.7% of ALDHLow cells showed SP population (Figure 3A). ALDHBr cells exhibited partial overlapping, and only 1.0% of total cells (7.1% of 14.4% population) expressed both SP cell phenotype and ALDHBr phenotype (Figure 3A).
Figure 3. SP and ALDEFLUOR dual assay.
A. Summary of SP and ALDEFLUOR dual assay. MCAS cells were stained by Hoechst 33342 dye and then stained by BAAA and analyzed. The cells were divided into three groups according to ALDH intensity (ALDHBr (ALDH bright), ALDHMid (ALDH middle), ALDHLow (ALDH low)), then analyzed by SP assay. The ratio of ALDHBr cells was 14.4%, and the ratio of SP cells was 5.3%. The ratios of SP cells in ALDHBr cells, ALDHMid cells and ALDHLow cells were 7.1%, 6.9% and 3.7%, respectively. The ratio of SP/ALDHBr cells in total cells was 1.0%. B. Tumor initiation of SP/ALDHBr, SP and ALDHBr cells. 102 and 103 SP/ALDHBr, SP and ALDHBr cells derived from MCAS cells were inoculated subcutaneously into the backs of NOD/SCID mice, and tumor growth was measured weekly. Data represent means ± SD. Differences between SP/ALDHBr and SP cells or ALDHBr cells were examined for statistical significance using Student's t-test. *P values. Daggers indicate mice death due to tumor. C. Immunohistochemical staining of ABCG2 and ALDH1. Ovarian carcinoma tissue was stained by anti-ABCG2 antibody and anti-ALDH1 antibody. Brown membrane staining indicates ABCG2 and cytoplasm pink staining indicates ALDH1. Asterisk indicates vessel, and arrows indicate ABCG2 and ALDH1 double-positive ovarian carcinoma cells. Magnification, ×400.
SP and ALDHbr cells have high tumor-initiating ability
The tumor-initiating ability of SP and ALDHBr (SP/ALDHBr) cells was examined by injecting 103 and 102 cells, respectively, into NOD/SCID mice. Surprisingly, tumors were initiated with 103 SP/ALDHBr cells and with as few as 102 SP/ALDHBr cells in all mice (Table 1). Furthermore, tumors derived from SP/ALDHBr cells showed significantly faster growth than that of tumors derived from SP cells and ALDHBr cells (Figure 3B). These results indicate that SP/ALDHBr cells are extremely enriched with CICs/CSCs.
We performed immunohistochemical staining to detect SP/ALDHBr population in primary human ovarian carcinoma tissue. We used anti-ABCG2 antibody to detect SP cells, since SP cells are known to express higher level of ABCG2. As shown in figure 3C, dual positive (ABCG2-positive and ALDH1-positive) ovarian cancer cells were detectable in ovarian carcinoma tissue. Interestingly, some dual-positive cells exist next to vessels, might be indicating ovarian CSCs/CICs exist in vascular niche. SP/ALDHBr cells were detected also from other ovarian serous adenocarcinoma line cells (OVSAHO, OVCAR3 and HTBoA) and an endometrial cell line (HEC-1), and the ratios of SP/ALDHBr cells were 0.1% to 0.8% (Figure S1).
SP/ALDHBr cells have stem cell phenotypes
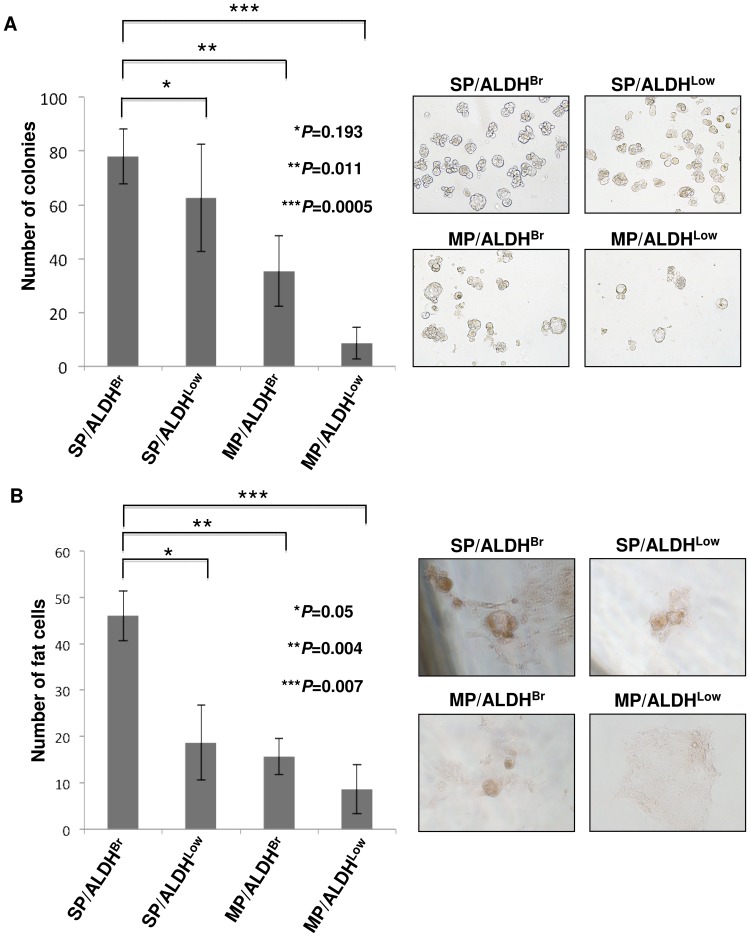
The SP/ALDHBr population was compared with other populations by the sphere forming assay. SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells were isolated from MCAS cells and cultured in an ultra-low attachment condition for the sphere forming assay. SP/ALDHBr cells exhibited higher sphere formation efficiency than that of MP/ALDHBr cells and MP/ALDHLow cells (Figure 4A). The difference between SP/ALDHBr cells and SP/ALDHLow cells was not significant; however, SP/ALDHBr cells tend to have higher sphere formation efficiency than that of SP/ALDHLow cells.
Figure 4. Characterization of SP/ADLHBr cells.
A. Sphere formation assay. The numbers of colonies from four fractions (SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow) were evaluated at day 7. Data represent means ± SD. The differences were examined for statistical significance using Student's t-test. *P values. Representative images of spheres are shown (×100). B. Adipocyte differentiation assay. The cells were cultured under existence of trans-retinoic acid followed by adipocyte differentiation medium. Oli Red O-positive adipocytes were counted. Data represent means ± SD. The differences were examined for statistical significance using Student's t-test. *P values. Representative images of Oil Red O-staining are shown (×200). Red-staining indicate adipocyte differentiation.
CSCs/CICs have been described to have pluripotency [25], we thus analyzed the adipocyte differentiation ability of SP/ALDHBr cells (Figure 4B). Isolated SP/ALDHBr, SP/ALDHLow, MPALDHBr and MP/ALDHLow population were cultured in an adypocyte differentiation condition. SP/ALDHBr cells derived from MCAS cells revealed highest adipocyte differentiation ability compared with SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells derived from MCAS cells.
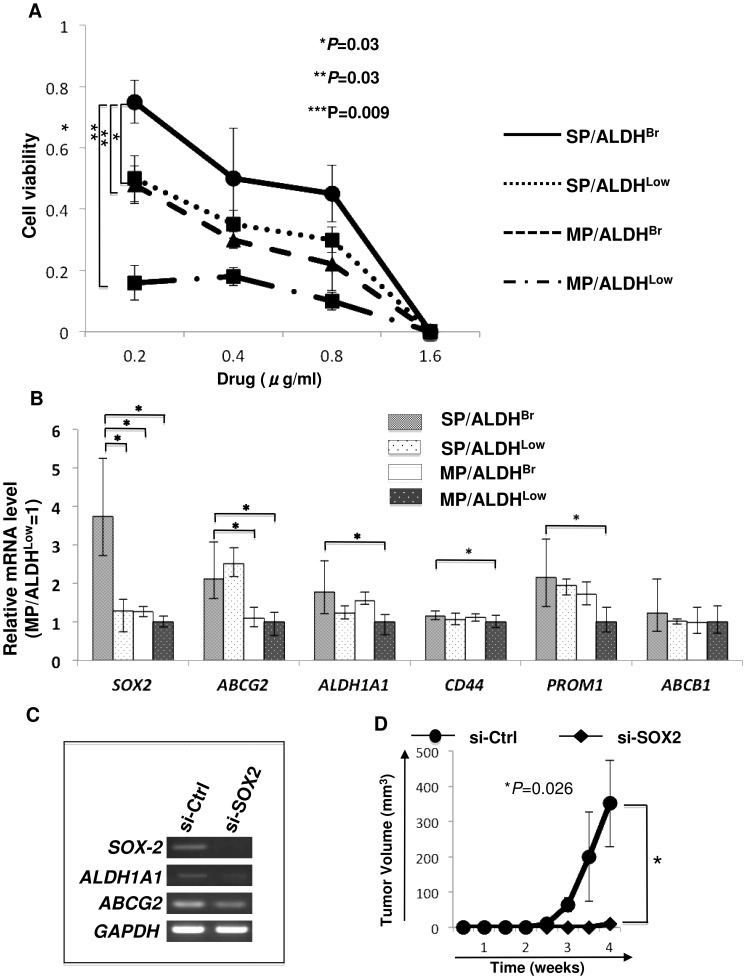
CSCs/CICs are resistant to chemotherapy [4], we thus analyzed drug resistance of SP/ALDHBr cells. Since cisplatin is a key drug for ovarian carcinoma chemotherapy, we used cisplatin. Isolated SP/ALDHBr, SP/ALDHLow, MPALDHBr and MP/ALDHLow population were cultured in an existence of cisplatin. SP/ALDHBr cells significantly higher cisplatin resistance compared with SP/ALDHLow cells, MP/ALDHBr cells and MP/ALDHLow cells. And, MP/ALDHLow cells exhibited highest sensitivity to cisplatin (Figure 5A).
Figure 5. Charasterization of SP/ALDHBr cells.
A. Cell viability assay. The cells were cultured under existence of serially diluted cisplatin. The viable cells were analyzed by WST-1 kit. Y-axis indicates the viability of cells. Data represent means ± SD. The differences were examined for statistical significance using Student's t-test. *P values. B. qPCR analysis. The expression of stem cell markers was examined using SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells. Data represent means ± SD. Asterisks indicate significant difference. *P<0.05. t-test. C. SOX2 knockdown suppress the expressions of ALDH1A1 and ABCG2. SOX2 mRNA was knocked down by siRNA. Two days after transfection of SOX2 siRNA, the expressions of ALDH1A1 and ABCG2 were investigated by RT-PCR. GAPDH was used as an internal control. Control siRNA (si-Cont) transfected cells were used as negative control. D. SOX2 knock down suppress the tumor-initiation. SOX2 mRNA was knocked down by siRNA. Ten thousand si-SOX2 and control siRNA (si-Cont) transfected cells were inoculated subcutaneously into the backs of NOD/SCID mice, and tumor growth was measured weekly. Data represent means ± SD. Differences were examined for statistical significance using Student's t-test. *P values.
To analyze the molecular characteristics of SP/ALDHBr population, we performed qPCR (Figure 5B). ABCG2 mRNA was preferentially expressed in SP/ALDHBr cells and SP/ALDHLow cells. ALDH1A1 was expressed in SP/ALDHBr cells and MP/ALDHBr cells. These expression profiles are consistent with the fact that the SP cell phenotype depends on the expression of ABCG2 and the ALDHBr cell phenotype depends on the expression of ALDH1A1. SOX2 mRNA was expressed at highest level in SP/ALDHBr cells but not in SP/ALDHLow cells, MP/ALDHBr cells or MP/ALDHLow cells (Figure 5B), indicating that the SP/ALDHBr population preferentially include a stem cell population. Since SOX2 has a role in the maintenance of lung CSCs/CICs [26], we investigated the relation of SOX2 and expressions of ABCG2 and ALDH1A1. The expressions of ABCG2 and ALDH1A1 were reduced in SOX2 mRNA knocked down cells (Figure 5C). Furthermore, SOX2 knocked down cells showed lower tumor-initiation than that of control siRNA transfected MCAS cells (Figure 5D). Therefore, these results suggest that SP/ALDHBr population express high level of stem cell gene SOX2, which may have role in the maintenance of CSCs/CICs and expressions of ABCG2 and ALDH1A1.
The expression levels of CD44, a representative marker for ovarian CSCs/CICs [27], [28], was similar in all populations (Figure 5B). We therefore investigated the relation of SP cells, ALDHBr cells and CD44-positive (CD44+) cells. The ratio of CD44+ cells was 8.0%. The ratio of SP/CD44+ overlapping population was 0.9%, and the ratio of ALDHBr/CD44+ overlapping population was 3.3%. Furthermore, the ratio of SP/ALDHBr/CD44+ overlapping population was 0.4% (Figure 6).
Figure 6. SP, ALDEFLUOR and CD44 triple staining.
MCAS cells were stained by Hoechst 33342 dye, BAAA and anti-CD44 antibody, and analyzed. The ratios of SP, ALDHBr, CD44+, SP/ALDHBr, SP/CD44+, ALDHBr/CD44+ and SP/ALDHBr/CD44+ cells were 5.3%, 14.4%, 8.0%, 1.0%, 0.9%, 3.3% and 0.4%, respectively.
Discussion
The concept of CSCs/CICs was proposed long time ago [29]. Leukemia stem cells have been isolated from acute leukemic cells [30], [31], and the first CSC/CIC population was isolated from breast carcinoma with the combination of CD44 and CD24 expression [32]. In the following works, CSCs/CICs were successfully isolated in several malignancies. However, since the molecular mechanisms of CSCs/CICs are still elusive, accurate markers for CSCs/CICs are still unknown. Therefore, improvements in methods for isolation of CSCs/CICs are still needed.
Combination methods with double or triple markers and with markers and ALDEFLUOR assay have been reported. The populations of ALDHBr and CD44+/CD24− cells exhibited partial overlapping, and the ALDHBr/CD44+/CD24− population showed higher tumor-initiating ability than that of the ALDHBr population or CD44+/CD24− population [17]. The ALDHBr/CD44+ population and ALDHBr/CD133+ population derived from human primary colon carcinoma exhibited higher tumor-initiating ability than that of ALDHBr, CD44+ and CD133+ populations [33]. These findings indicate that the expressions of CSC/CIC markers are partially overlapped and that the overlapped population is highly enriched with CSCs/CICs. Indeed, our results also showed similar overlapping of ALDHBr cells and SP cells, and the overlapping population exhibited higher tumor-initiating ability. In an ovarian cancer study, ALDHBr cells were more enriched in CD44+ cells than in use of the CD133+ cells [23], but the ALDHBr and CD44+ overlapping was partial. We identified SP/ALDHBr/CD44+ overlapping population from MCAS cells (Figure 6). Therefore, use of the overlapping population of SP/ALDHBr/CD44+ cells may be a better approach to identify CSC/CIC populations.
Glioma stem cells have been described to differentiate into endothelial cells [34]. Malignant methotelioma stem cells have been described to differentiate into endothelial cells, neural cells and adipocytes [25]. In this study, we confirmed that SP/ALDHBr cells have higher adipocyte differentiation ability. Therefore, ovarian cancer stem cells might have potential to differentiate into different lineage cells, suggesting that ovarian cancer stem cells are immature state. SOX2, a key factor for cell reprogramming [35], was expressed in SP/ALDHBr cells at highest level. And knockdown of SOX2 reduced the expressions of ABCG2 and ALDH1A1. Thus SOX2 might have a role to sustain immature state of ovarian cancer stem cells.
CSCs/CICs are described to be resistant to chemotherapy [4]. Indeed SP/ALDHBr cells showed higher cisplatin resistance than did SP/ALDHLow cells, MP/ALDHBr cells and MP/ALDHLow cells. Several mechanisms of drug resistance have been described, such as CSCs/CICs are dormant state, CSCs/CICs express higher levels of transporters, CSCs/CICs express higher levels of inhibitor of apoptosis proteins (IAPs) and so on. In this study, only SP/ALDHBr population expressed both ABCB1 and ABCG2 transporters among SP/ALDHBr, SP/ALDHLow, MP/ALDHBr and MP/ALDHLow cells (Figure 6B). Thus, higher expressions of transporters might be one mechanisms of drug resistance of SP/ALDHBr cells.
Dual SP and ALDHBr cells in hematologic stem cells have been reported [24]. The overlapping population of ALDHBr cells and CD133+ cells has prominent tumorigenicity [36]. However, SP/ALDHBr cells have not been reported in solid tumors yet. SP cell phenotype represents the efflux of Hoechst 33342 dye due to the expression of ABC transporter, ABCG2, which may be involved in drug resistance [37]. ALDHBr cells represent the higher expression of ALDH1, which may be involved in detoxification [38]. SP cells and ALDHBr cells thus have different molecular properties, and the overlapping of SP cells and ALDHBr cells were partial. And we found SP and ALDHBr overlapping population was the highest CSCs/CICs enriched population which exhibited higher sphere formation, higher tumor-initiation, higher adipocyte differentiation ability, higher drug resistance and higher expression level of SOX2, a representative marker of CSCs/CICs, which is related to the tumor-initiating ability of CSCs/CICs [26]. Therefore, SP/ALDHBr population is the better source of CSCs/CICs than SP cells or ALDHBr cells that have been previously described. We found that SOX2 is expressed at high level in SP/ALDHBr cells, and knockdown of SOX2 suppressed the expressions of ALDH1A1 and ABCG2, and suppressed tumor-initiation. Thus, SOX2 might has role in the maintenance of both SP cell and ALDHBr cell population, and also has role in the maintenance of ovary CSCs/CICs.
In summary, SP/ALDHBr cells comprise a more highly CSC/CIC- enriched popuration than do SP cells or ALDHBr cells, and further analysis of SP/ALDHBr cells should lead to elucidation of the molecular mechanisms of CSCs/CICs.
Supporting Information
SP and ALDEFLUOR dual assay. OVCAR3, OVSAHO, HTBoA and HEC-1 cells were analyzed by SP and ALDEFLUOR dual assay. Percentages indicate the ratios of ALDHBr, SP and SP/ALDHBr cells.
(TIF)
Acknowledgments
The authors thank Ms. Eri Saka for technical assistance.
Funding Statement
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant Nos. 16209013, 17016061 and 15659097) for Practical Application Research from the Japan Science and Technology Agency, and for Cancer Research (15-17 and 19-14) from the Ministry of Health, Labor and Welfare of Japan, Ono Cancer Research Fund (to NS) and Takeda Science Foundation (to YH). This work was supported in part by the National Cancer Center Research and Development Fund (23-A-44). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rosen JM, Jordan CT (2009) The increasing complexity of the cancer stem cell paradigm. Science 324: 1670–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghaffari S (2011) Cancer, stem cells and cancer stem cells: old ideas, new developments. F1000 Med Rep 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirohashi Y, Torigoe T, Inoda S, Morita R, Kochin V, et al. (2012) Cytotoxic T lymphocytes: Sniping cancer stem cells. Oncoimmunology 1: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275–284. [DOI] [PubMed] [Google Scholar]
- 5. Rich JN (2007) Cancer stem cells in radiation resistance. Cancer Res 67: 8980–8984. [DOI] [PubMed] [Google Scholar]
- 6. Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, et al. (2012) Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J [DOI] [PubMed] [Google Scholar]
- 7. Lehmann C, Jobs G, Thomas M, Burtscher H, Kubbies M (2012) Established breast cancer stem cell markers do not correlate with in vivo tumorigenicity of tumor-initiating cells. Int J Oncol 41: 1932–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, et al. (2008) CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest 118: 2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burkert J, Otto WR, Wright NA (2008) Side populations of gastrointestinal cancers are not enriched in stem cells. J Pathol 214: 564–573. [DOI] [PubMed] [Google Scholar]
- 10. Pecorelli S, Favalli G, Zigliani L, Odicino F (2003) Cancer in women. Int J Gynaecol Obstet 82: 369–379. [DOI] [PubMed] [Google Scholar]
- 11. Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, et al. (2006) Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A 103: 11154–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng S, Yang X, Lassus H, Liang S, Kaur S, et al. (2010) Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One 5: e10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Balch C, Chan MW, Lai HC, Matei D, et al. (2008) Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 68: 4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friel AM, Sergent PA, Patnaude C, Szotek PP, Oliva E, et al. (2008) Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle 7: 242–249. [DOI] [PubMed] [Google Scholar]
- 15. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183: 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoda S, Hirohashi Y, Torigoe T, Morita R, Takahashi A, et al. (2011) Cytotoxic T lymphocytes efficiently recognize human colon cancer stem-like cells. Am J Pathol 178: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, et al. (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1: 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michifuri Y, Hirohashi Y, Torigoe T, Miyazaki A, Kobayashi J, et al. (2012) High expression of ALDH1 and SOX2 diffuse staining pattern of oral squamous cell carcinomas correlates to lymph node metastasis. Pathol Int 62: 684–689. [DOI] [PubMed] [Google Scholar]
- 19. Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, et al. (2007) The generation of adipocytes by the neural crest. Development 134: 2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakatsugawa M, Hirohashi Y, Torigoe T, Asanuma H, Takahashi A, et al. (2009) Novel spliced form of a lens protein as a novel lung cancer antigen, Lengsin splicing variant 4. Cancer Sci 100: 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vathipadiekal V, Saxena D, Mok SC, Hauschka PV, Ozbun L, et al. (2012) Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One 7: e29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hirohashi Y, Torigoe T, Inoda S, Takahashi A, Morita R, et al. (2010) Immune response against tumor antigens expressed on human cancer stem-like cells/tumor-initiating cells. Immunotherapy 2: 201–211. [DOI] [PubMed] [Google Scholar]
- 23. Wang YC, Yo YT, Lee HY, Liao YP, Chao TK, et al. (2012) ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol 180: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 24. Pierre-Louis O, Clay D, Brunet de la Grange P, Blazsek I, Desterke C, et al. (2009) Dual SP/ALDH functionalities refine the human hematopoietic Lin-CD34+CD38− stem/progenitor cell compartment. Stem Cells 27: 2552–2562. [DOI] [PubMed] [Google Scholar]
- 25. Varghese S, Whipple R, Martin SS, Alexander HR (2012) Multipotent cancer stem cells derived from human malignant peritoneal mesothelioma promote tumorigenesis. PLoS One 7: e52825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakatsugawa M, Takahashi A, Hirohashi Y, Torigoe T, Inoda S, et al. (2011) SOX2 is overexpressed in stem-like cells of human lung adenocarcinoma and augments the tumorigenicity. Lab Invest 91: 1796–1804. [DOI] [PubMed] [Google Scholar]
- 27. Shi MF, Jiao J, Lu WG, Ye F, Ma D, et al. (2010) Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol Life Sci 67: 3915–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang L, Mezencev R, Bowen NJ, Matyunina LV, McDonald JF (2012) Isolation and characterization of stem-like cells from a human ovarian cancer cell line. Mol Cell Biochem 363: 257–268. [DOI] [PubMed] [Google Scholar]
- 29. Clevers H (2011) The cancer stem cell: premises, promises and challenges. Nat Med 17: 313–319. [DOI] [PubMed] [Google Scholar]
- 30. Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, et al. (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367: 645–648. [DOI] [PubMed] [Google Scholar]
- 31. Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3: 730–737. [DOI] [PubMed] [Google Scholar]
- 32. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100: 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, et al. (2009) Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 69: 3382–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, et al. (2010) Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468: 829–833. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 36. Silva IA, Bai S, McLean K, Yang K, Griffith K, et al. (2011) Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res 71: 3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, et al. (1997) Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 3: 1337–1345. [DOI] [PubMed] [Google Scholar]
- 38. Ma I, Allan AL (2011) The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev 7: 292–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SP and ALDEFLUOR dual assay. OVCAR3, OVSAHO, HTBoA and HEC-1 cells were analyzed by SP and ALDEFLUOR dual assay. Percentages indicate the ratios of ALDHBr, SP and SP/ALDHBr cells.
(TIF)