Abstract
Many ocular processes show diurnal oscillations that optimize retinal function under the different conditions of ambient illumination encountered over the course of the 24 h light/dark cycle. Abolishing the diurnal cues by the use of constant darkness or constant light results in excessive ocular elongation, corneal flattening, and attendant refractive errors. A prevailing hypothesis is that the absence of the Zeitgeber of light and dark alters ocular circadian rhythms in some manner, and results in an inability of the eye to regulate its growth in order to achieve emmetropia, the matching of the front optics to eye length. Another visual manipulation that results in the eye growth system going into a “default” mode of excessive growth is form deprivation, in which a translucent diffuser deprives the eye of visual transients (spatial or temporal) while not significantly reducing light levels; these eyes rapidly elongate and become myopic. It has been hypothesized that form deprivation might constitute a type of “constant condition” whereby the absence of visual transients drives the eye into a similar default mode as that in response to constant light or dark. Interest in the potential influence of light cycles and ambient lighting in human myopia development has been spurred by a recent study showing a positive association between the amount of time that children spent outdoors and a reduced prevalence of myopia. The growing eyes of chickens and monkeys show a diurnal rhythm in axial length: Eyes elongate more during the day than during the night. There is also a rhythm in choroidal thickness that is in approximate anti-phase to the rhythm in eye length. The phases are altered in eyes growing too fast, in response to form deprivation or negative lenses, or too slowly, in response to myopic defocus, suggesting an influence of phase on the emmetropization system. Other potential rhythmic influences include dopamine and melatonin, which form a reciprocal feedback loop, and signal “day” and “night” respectively. Retinal dopamine is reduced during the day in form deprived myopic eyes, and dopamine D2 agonists inhibit ocular growth in animal models. Rhythms in intraocular pressure as well, may influence eye growth, perhaps as a mechanical stimulus triggering changes in scleral extracellular matrix synthesis. Finally, evidence shows varying influences of environmental lighting parameters on the emmetropization system, such as high intensity light being protective against myopia in chickens. This review will cover the evidence for the possible influence of these various factors on ocular growth. The recognition that ocular rhythms may play a role in emmetropization is a first step toward understanding how they may be manipulated in treatment therapies to prevent myopia in humans.
Keywords: emmetropization, circadian rhythms, choroid, axial length, form deprivation, sclera
1. Introduction
Many ocular processes show diurnal oscillations that serve to optimize retinal function under the different conditions of ambient illumination encountered over the course of the 24 h diurnal cycle of light and dark. These rhythms are conserved across phyla, and include changes in retinal circuitry, retinomotor movements in photoreceptors, transmitter synthesis and release, and changes in phototransduction (review: Cahill and Besharse, 1995). Some of these rhythms are endogenous, that is, they are controlled by a clock within the organism, and sometimes within the eye itself, and will free-run in constant darkness; others are driven by the exogenous cycles of light and dark. The seminal work by Jean Lauber and colleagues over five decades ago introduced the notion that circadian rhythms may be important in the control of ocular growth (Jensen and Matson, 1957; Lauber et al., 1961; Lauber and McGinnis, 1966; Lauber and Kinnear, 1979; Lauber and Kivett, 1981). They showed that eliminating the diurnal Zeitgeber (time-giver) by exposing chickens to constant light or darkness resulted in excessive eye growth and corneal flattening, which, depending on the duration of exposure, caused myopia or hyperopia. One interpretation is that emmetropization requires a normal light/dark cycle in order to synchronize ocular rhythms, and without this, a default growth mode is adopted. Many years later, a renewed interest in ocular diurnal rhythms and their influence on the eye growth controller was stimulated by parallel work in three labs, also using chicks: Weiss and Schaeffel (1993) described a diurnal rhythm in axial length that was altered in eyes growing excessively fast in response to form deprivation; Nickla et al. (1998a,b) and Papastergiou et al. (1998) found that, in addition, there was a rhythm in the thickness of the choroid, with a peak at around mid-night, coincident with the shortest axial length. More importantly, the phase relationships between these two rhythms changed during experimentally-induced changes in ocular growth rate. Another influential finding implicating ocular diurnal rhythms in eye growth came from Richard Stone and colleagues (Stone et al., 1989), who, again in chicks, found that retinal levels of dopamine, a diurnally-oscillating transmitter, were decreased in form deprived eyes, but only during the daytime when levels are normally highest, suggesting that form deprivation might constitute a type of “constant condition” similar to constant light or darkness. This hypothesis was supported by later work showing that stimuli that may function as “skeleton photoperiods”, such as 30 min pulses of stroboscopic light at dawn and dusk in an otherwise normal light/dark cycle largely prevented the development of deprivation-induced myopia (Nickla, 1996; Kee et al., 2001).
There are several other rhythms that may be involved in ocular growth regulation: rhythms in intraocular pressure (IOP) (Nickla et al., 1998a; Papastergiou et al., 1998), retinal levels of the indoleamine hormone melatonin and its receptors (Schaeffel et al., 1995; Hoffmann and Schaeffel, 1996; Summers-Rada and Wiechmann, 2006), retinal dopamine, and scleral biosynthesis rates (Nickla et al., 1999), to name a few. Nearly 20 years have elapsed before the animal research on rhythms in ocular dimensions was tested and replicated in humans (Stone et al., 2004; Wilson et al., 2006; Brown et al., 2009), only made possible by new technologies that allowed precise non-invasive imaging of human axial length and choroidal thickness. This review will discuss the various lines of evidence linking ocular diurnal rhythms to the emmetropization process.
2. Ocular dimensions: choroidal thickness and axial length
In normal chicken eyes measured at 12 h intervals, eyes grew faster during the day than during the night, while eyes that were deprived of form vision by translucent diffusers grew rapidly during both dayand night (Weiss and Schaeffel, 1993), resulting in excessive overall elongation. At first glance, this data appeared to indicate a loss of rhythmicity, but subsequent work measuring eyes at 6 h intervals showed that the rhythm in axial length persisted in form deprived eyes (Nickla et al., 1998b); the apparent discrepancy between the two studies being due to the measurement times of 8 am and 8 pm in the Schaeffel experiment falling on the “mesor” of the sinusoidal rhythm, the times equidistant between the peak and trough. An important observation, however, that came out of the original study was that normal eyes appeared to shrink at night, which suggested to Weiss and Schaeffel the influence of a rhythm in choroidal thickness, because their ultrasound system defined the back of the eye at the retinal interface rather than the scleral one. As it had recently been demonstrated that the choroid changed its thickness in response to retinal defocus, and thus acted as a focusing mechanism (Wallman et al.,1992,1995), in concert with the changes in eye length, this observation was opportune. The inference was confirmed in two labs, using two different methodologies: high frequency (30 MHz) A-scan ultrasonography (Nickla et al., 1998b) and partial coherence interferometry (Papastergiou et al., 1998). Both of these methods have the ability to delineate the scleral interface, as well as the retinal one, and so distinguish between a rhythm in vitreous chamber depth that would be influenced by the choroidal rhythm, and a rhythm in actual eye length. We found that normal eyes grew during the day and slowed growth (or shrunk!) at night, as reported by Weiss and Schaeffel, but also that there was a rhythm in choroidal thickness with a peak during mid-night and trough during mid-day; the mean difference in phase between the two was about 9 h (Fig. 1A; Nickla et al., 1998b). Fast growing form deprived eyes showed similar rhythms, but in these the two rhythms were in exact anti-phase (mean of 12 h apart as opposed to 9 h). By contrast, in eyes growing more slowly than normal in response to myopic defocus (recovering eyes, or eyes wearing positive lenses) there was a phase delay in the axial length rhythm and a phase-advance in the choroidal one, that shifted the two rhythms into phase with one another (peaks at 8 pm; Fig. 1B). Furthermore, there is a significant positive correlation between the differences in phase and the ocular growth rates over the subsequent 24 h (r = 0.504; p < 0.05; Fig. 1C). While not definitive, this correlation supports a causative relationship between phase differences and ocular growth rates. Definitive evidence would entail, for example, causing a shift into phase in the two rhythms via a manipulation not directly linked to producing growth rate changes, and showing that it did not have any effect on growth.
Fig. 1.
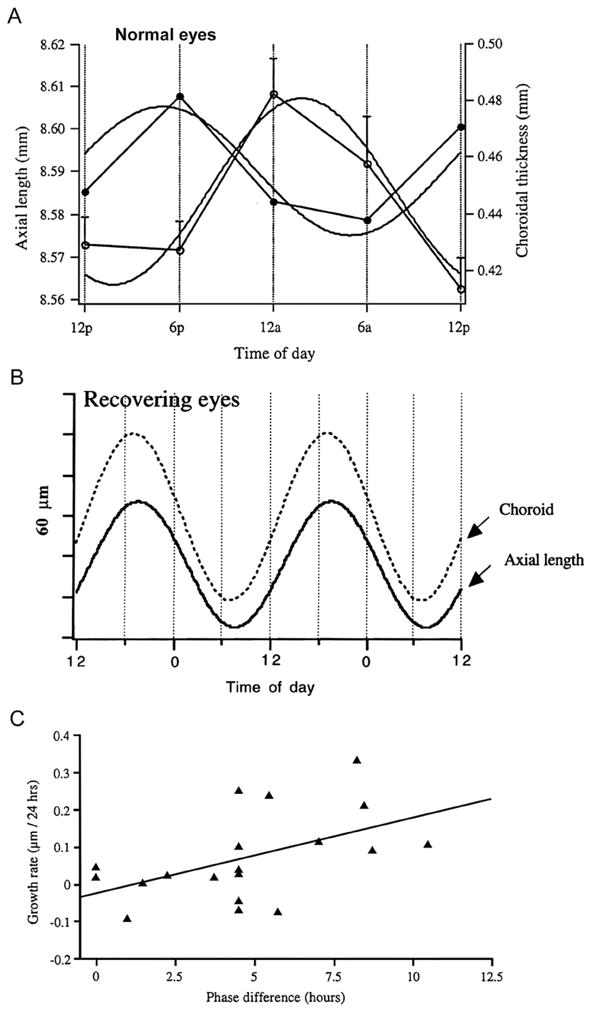
Rhythms in axial length and choroidal thickness in chicks. A. Mean axial length (black circles) and choroidal thickness (white circles) in normal eyes measured at 6 h intervals; the residuals (regression) were subtracted from the axial length data to yield the pure cyclic component (symbols). The curves are the sine waves with a fixed 24 h period fit to the data. From Nickla (2005). Used with permission from Nickla (2005)© Springer. B. Rhythms in axial length and choroidal thickness in eyes recovering from form deprivation myopia. Only the sine waves (24 h period) that were fit to the data are shown. From Nickla et al. (1998b). Used with permission from Nickla et al. (1998b)© Academic Press Limited. C. Growth rate per 24 h as a function of difference in phase (hours) between the rhythms in axial length and choroidal thickness in birds that were responding to positive or negative lenses, and normal eyes. The correlation is significant (r = 0.504; p < 0.05). Used with permission from Nickla (2005)© Springer.
If these rhythms were a peculiarity of chicken eyes, they would have remained of limited interest, but happily, this was not the case. We found that marmoset eyes showed rhythms in axial length and choroidal thickness similar to those of chick eyes: in young, rapidly growing eyes, the two rhythms were approximately out-of-phase, while in older, slowly growing eyes, they were in phase (Nickla et al., 2002; Fig. 2A), strengthening the notion of a mechanistic association between relative phase and ocular growth rate. Studies on humans awaited the development of a safe, precise imaging technology. The earliest such studies used partial coherence interferometry, and reported diurnal oscillations in both axial length (Stone et al., 2004; Wilson et al., 2006) and choroidal thickness (Brown et al., 2009), measured over the course of 2 nonconsecutive days, but the phase relationships were not consistent across, or within, subjects (Brown et al., 2009). The most recent study used a non-contact optical biometer (Lenstar LS 900), and collected data over 2 consecutive days in 15 emmetropes and 15 myopes. These authors found rhythms in axial length and choroidal thickness that were in approximate anti-phase to one another, with eyes being longest during the day and choroids thickest during the night in both groups (Chakraborty et al., 2011; Fig. 2B), similar to marmosets and chicks. In humans, the question of an association between phase relationships and growth rates awaits clinical studies of young children in whom changes in growth rates can be followed longitudinally.
Fig. 2.
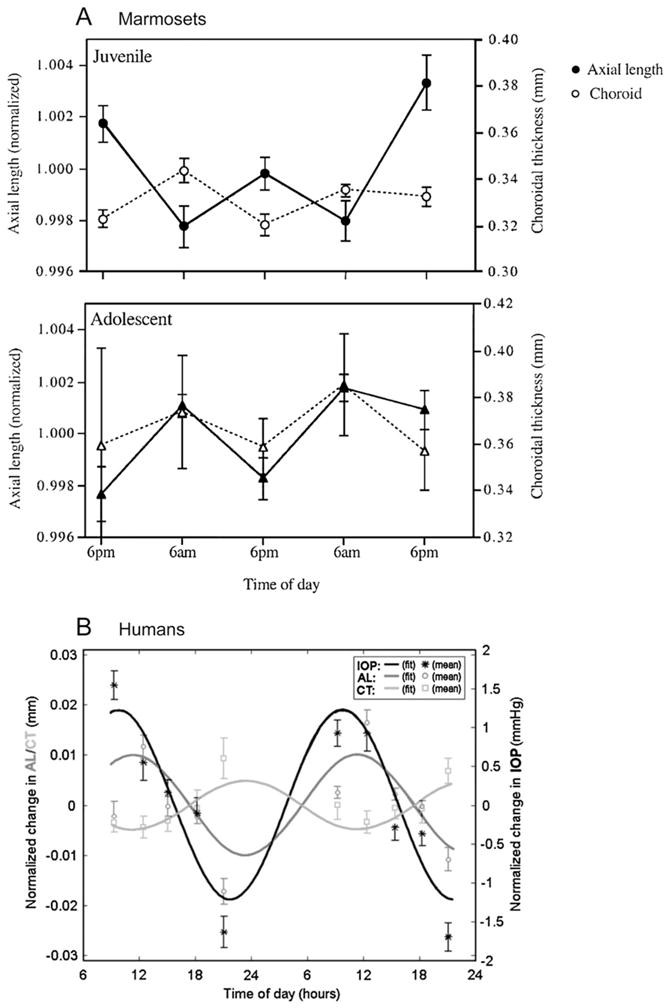
A. Rhythms in axial length (black circles) and choroidal thickness (white circles) in juvenile (top) and adolescent (bottom) marmosets measured at 12 h intervals. From Nickla et al. (2002). Used with permission from Nickla et al. (2002)© Association for Research in Vision and Ophthalmology. B. Rhythms in axial length, choroidal thickness and IOP in human subjects measured at 10 different times over the course of 2 days (symbols). The curves are the sine waves with a fixed 24 h period fit to the data. Note that the rhythms in length and choroidal thickness are in approximate anti-phase to one another. Used with permission from Chakraborty et al. (2011)© Association for Research in Vision and Ophthalmology.
In conclusion, there is a conservation across species in the existence of, and phase relationships between, these two rhythms, which suggests a functional role for them in some aspect of ocular physiology, perhaps emmetropization. The following section describes a rhythm at the molecular level that may be involved in the phase-related changes in ocular growth rate.
3. Scleral proteoglycan biosynthesis rates
The rate of growth of the chicken eye is largely determined by the rate of synthesis of the extracellular matrix proteoglycan aggrecan by chondrocytes (Rada et al., 1990, 1992, 1998; Rada and Matthews, 1994). Because ocular growth shows diurnal fluctuations, then scleral proteoglycan synthesis should as well, and their phases should be correlated. Punches of sclera dissected from normal or form deprived eyes in the morning, afternoon, or midnight, and cultured for 2 h in radiolabeled sulfur, showed that proteoglycan synthesis was highest during the morning and lowest during the mid-night (Nickla et al., 1999). Molecular weight characterization indicated that the molecule was aggrecan.
In an attempt to define the phase of the rhythm more precisely, “conditioned” medium from punches of sclera was collected in a perifusion system at 2 h intervals over 3 days (Nickla et al., 1999; Fig. 3, left panels). The secreted proteoglycans exhibited a circadian rhythm with its major frequency component at 1 per 24 h. There were no differences in phase or amplitude between scleras from normal versus deprived eyes (compare Fig. 3A and B). Surprisingly, however, the acrophase occurred 16 h from the start of the culture, regardless of the time of day the culture began, indicating that the rhythm was phase-shifted by the culture conditions. One plausible explanation is the existence of separate rhythms in proteoglycan synthesis by the scleral chondrocytes (present in both types of experiments) and that of its aggregation into the mature macro-molecular structure in the extracellular matrix (which would presumably not occur in the conditioned medium).
Fig. 3.
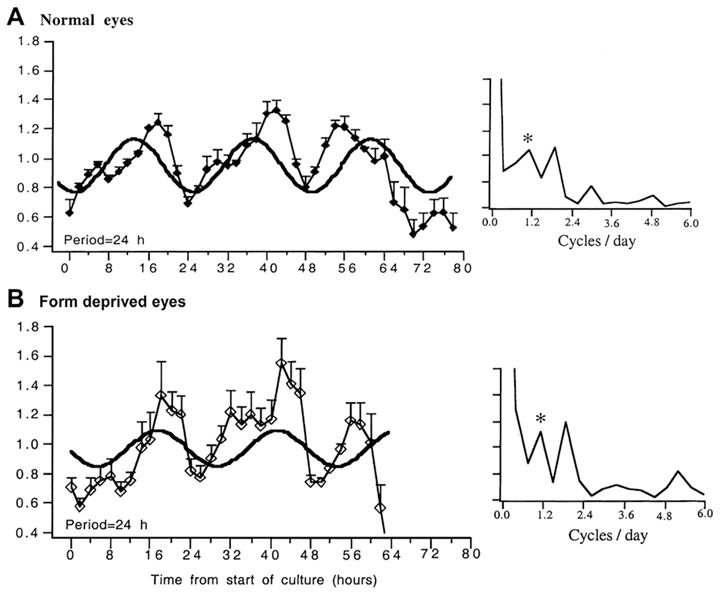
Rhythms in scleral glycosaminoglycan synthesis in the medium from isolated pieces of sclera measured at 2 h intervals over about 72 h; scleras from normal eyes (top) and form deprived eyes (bottom). The data are expressed as counts of sulfur-35 normalized to the mean for each experiment; bars are standard errors. Time 0 is the start of culture (x axis). The curves are the sine waves fit to the data, with the period indicated on bottom left of the graphs. On the right are spectral frequency (Fourier) analyses of the mean data, with the diurnal (1 per 24 h) frequency indicated by the asterisk. Note that both have a frequency component at 1.875 cycles per 24 h. Used with permission from Nickla et al. (1999)© Springer.
An interesting observation from this study was the existence of a higher-frequency component, occurring at 1.875 cycles per day (Fig. 3, right panels). While this was puzzling, it was not unlikely that these reflected true ultradian rhythms in axial length, because the 6 h interval measurements in intact birds is below the frequency required to detect rhythms with higher frequencies than 1 per 24 h, as the Nyquist frequency for circadian rhythms is 12 h. Ultradian rhythms in axial length were verified in a recent study that used measurement intervals of 4 h, and found that a majority (15/18) of chick eyes showed rhythms in optical length (sclera to retina) with periods of around 12 h (Campbell et al., 2012).
In conclusion, these results strongly support the notion that diurnal rhythms in scleral extracellular matrix synthesis underlie the diurnal (and ultradian) rhythms in axial length in chicks. Furthermore, they indicate that the rhythm in axial length is endogenous, as opposed to being light-driven; this was subsequently verified by the finding that axial length continued to oscillate with a 24 h period in constant darkness (Nickla et al., 2001). Finally, the finding that isolated pieces of sclera continued to oscillate for 3 cycles demonstrated the existence of an endogenous clock in scleral chondrocytes, the first report of clocks in non-neuronal tissues in vertebrates.
4. Intraocular pressure
IOP shows a diurnal rhythm in all species studied (rats: Krishna et al., 1995; rabbits: Rowland et al., 1981; Liu and Dacus, 1991; Schnell et al., 1996; chicks: Nickla et al., 1998a; Papastergiou et al., 1998; humans: Drance, 1960; Henkind et al., 1973; Frampton et al., 1987) and could provide a mechanism for the diurnal oscillations in axial length (Weiss and Schaeffel, 1993; Nickla et al.,1998b, 2002; Papastergiou et al.,1998; Stone et al., 2004; Chakraborty et al., 2011). In chickens, IOP is high during the day and low at night, with a similar phase as that of the rhythm in axial length (Fig. 4). The rhythm persists in constant darkness, but with a smaller amplitude, suggesting that light per se influences IOP levels (Nickla et al., 1998a). Measurements of ocular compliance showed proportional changes in axial length as a function of changes in IOP, with a mean of 8 μm/mm Hg, not inconsistent with the rhythm in IOP having a direct influence on the rhythm in eye length. However, several lines of evidence argue that this is not so. First, the IOP of individual eyes exhibit a variability in phase, furthermore, its mean acrophase tends to lead that of the rhythm in axial length by several hours (Fig. 4). Second, the existence of a circadian rhythm in scleral proteoglycan synthesis (Nickla et al., 1999) supports a role for this rhythm in the fluctuations in eye length. Finally, form deprivation desynchronizes the phase of the rhythm in IOP from the light/dark cycle, but has no effect on the rhythm in axial length (Nickla et al., 1998a). We speculate that IOP does play a role, but not a direct mechanical one: perhaps the efficacy of its force is dependent on the phase of a putative rhythm in scleral compliance, which may in turn be influenced by the phase of the rhythm in scleral proteoglycan synthesis. It is likely that IOP plays a greater role in the smaller-amplitude oscillations in axial length found in year-old chickens, in whom eye growth has almost ceased (Papastergiou et al., 1998). This may be true too, in older marmosets, in whom the peak in the IOP rhythm is approximately coincident with that of eye length (Nickla et al., 2002). In humans as well, a similar conclusion was drawn, that the rhythms in axial length were not a result of “passive expansion and contraction” in response to changing IOP (Wilson et al., 2006; Chakraborty et al., 2011). In 10 people measured at 3 h intervals (Wilson et al., 2006), while the mean peaks in IOP and eye length occurred in the afternoon (Fig. 2B), there was variability between subjects, and between days, with no correlation between respective peaks measured on the same day.
Fig. 4.
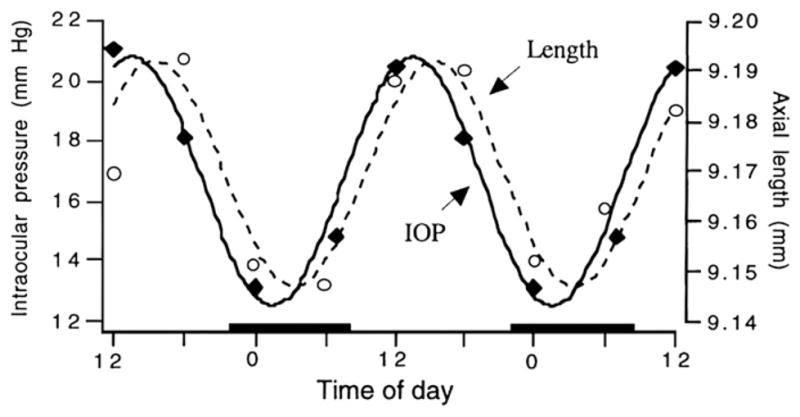
The rhythms in IOP (black symbols) and axial length (open symbols) and the sine waves with a fixed 24 h period, fit to the data. The data for the two parameters are from different sets of birds (n = 10 in each group). Black bars on x axis denote night. Used with permission from Nickla et al. (1998a)© Academic Press Limited.
5. Melatonin and dopamine
Ocular light plays a role in human physiology by transmitting time of day information to the suprachiasmatic nucleus, the master clock. Melatonin is a major output, or hand, of the clock, and is a key hormone that mediates the effects of “night” on various physiological processes (review: Herzog and Block, 1999), including rod disk shedding (Besharse and Dunis, 1983), retinomotor movements (Pierce and Besharse, 1985), and RPE pigment aggregation. Melatonin is synthesized by retinal photoreceptors (Bubenik et al., 1976; Wiechmann and Craft, 1993) and pinealocytes (review: Underwood and Siopes, 1985), and its levels are high during the night and low during the day. The rhythm persists in isolated photoreceptors in vitro, and can be phase-shifted by light, therefore photoreceptors contain an endogenous clock (Underwood et al., 1990; Terman et al., 1991; Cahill and Besharse, 1993, 1995). The reciprocal hormone for melatonin is dopamine, which is synthesized by amacrine cells; dopamine levels are high during the day and low at night, and the two molecules form a mutually-inhibitory feedback system (Dubocovich, 1983; Iuvone and Besharse, 1986). There is evidence that both of these molecules may be involved in the visual regulation of ocular growth, with dopamine having the more compelling evidence of the two.
Retinal levels of dopamine were decreased in form deprived eyes of chicks (Stone et al., 1989) and monkeys (Iuvone et al., 1989), but only during the daytime, when levels are normally high (Fig. 5), hinting at a circadian influence. Intravitreal injections of dopamine agonists inhibited the development of form deprivation (Stone et al., 1989; Iuvone et al., 1991; Rohrer et al., 1993), and negative lens-induced myopia (Schmid and Wildsoet, 2004) by inhibiting ocular growth rate, and dopamine antagonists prevented the ameliorative effects of brief daily vision when given prior to removing the diffusers (McCarthy et al., 2007). The D2 receptor antagonist sulpiride also enhanced myopia in form deprived eyes (Schaeffel et al., 1995). It was proposed that dopamine mediates its inhibitory effects on eye growth by acting as a signal for “light” or “vision” (McCarthy et al., 2007), however, this notion is complicated by the finding that the D2 antagonist spiperone does not have the same “vision-blocking” effect in negative lens-wearing eyes (Nickla and Totonelly, 2011), suggesting the involvement of different receptors in the two myopiagenic paradigms. Regardless, dopamine is currently a “hot” molecule due to the recent interest caused by the finding of Rose et al. that time spent outdoors has a protective effect against myopia in Australian schoolchildren (2008). Of the various “outdoor” factors that might mediate this effect, one is exposure to sunlight, or maybe a specific range of the spectrum (UV exposure?; Sherwin et al., 2012), and because light evokes the release of dopamine (Godley and Wurtman, 1988; Dearry and Burnside, 1989; Boatright et al., 1994), dopamine is once again in the limelight. In chickens, bright light inhibited the development of deprivation-induced myopia (Ashby et al., 2009), and spiperone prevented this inhibition, in support of a dopaminergic mechanism. However, the same inhibitory effect of light was not found in eyes wearing negative lenses (Ashby and Schaeffel, 2010; Smith et al., 2012b) (but see Siegwart et al., 2012), and spiperone has not been tested in this paradigm, thus interpretation of the possible influences of light and dopamine remains controversial.
Fig. 5.
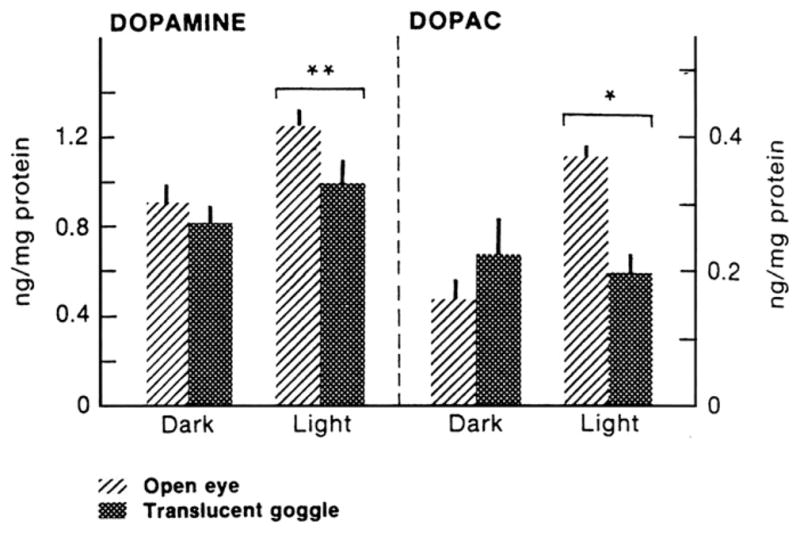
The effect of 2 weeks of form deprivation on the light-induced rise in retinal dopamine and DOPAC (dopamine metabolite). Birds were sacrificed 2 h before the end of the light cycle, or 2 h into the dark period. In normal eyes, dopamine and DOPAC are higher during the light; form deprivation inhibits the light-induced rise in both molecules. From Stone et al. (1989); used with permission from Dr. Richard Stone.
A role for melatonin in eye growth regulation is more speculative. In chicks, there are melatonin receptors in the cornea, retina, choroid and sclera (Wiechmann and Rada, 2003), the proteins for which all exhibit circadian rhythmicity (Summers-Rada and Wiechmann, 2006; Wiechmann and Summers-Rada, 2008). Form deprivation does not appear to affect either the retinal levels of melatonin or its circadian rhythm. However, intravitreal injections of high doses of melatonin enhanced ocular growth and myopia in form deprived eyes (Hoffmann and Schaeffel, 1996). Similarly, systemic administration of melatonin caused an increase in eye length in both deprived and contralateral control eyes (Summers-Rada and Wiechmann, 2006).
In conclusion, both dopamine and melatonin, molecules intimately involved in ocular circadian physiology, may play pivotal roles in the control of ocular growth, but the evidence is incomplete. One caveat that must be taken into account is that different mechanisms may underlie form deprivation, the paradigm for the majority of the above-cited studies, and negative lens-wear (Schmid and Wildsoet, 2004; Nickla and Totonelly, 2011; Nickla and Schroedl, 2012), which would complicate the extrapolation of the results from the form deprivation models into meaningful conclusions for humans. Furthermore, we cannot rule out the possibility that other molecules that show promise in animal models of myopia, such as muscarinic agents or nitric oxide, also exhibit as-yet-untested diurnal oscillations that might be important in this context.
6. Time of day, light cycles, and light intensity
The 24-h diurnal cycle of light and dark is the time-giver that entrains all circadian rhythms, including ocular ones, without which rhythms would free-run at their own endogenous periods. The work of Lauber and colleagues was the starting point for decades of research studying the influence(s) of light cycles and other lighting parameters on the emmetropization system.
6.1. Time of day
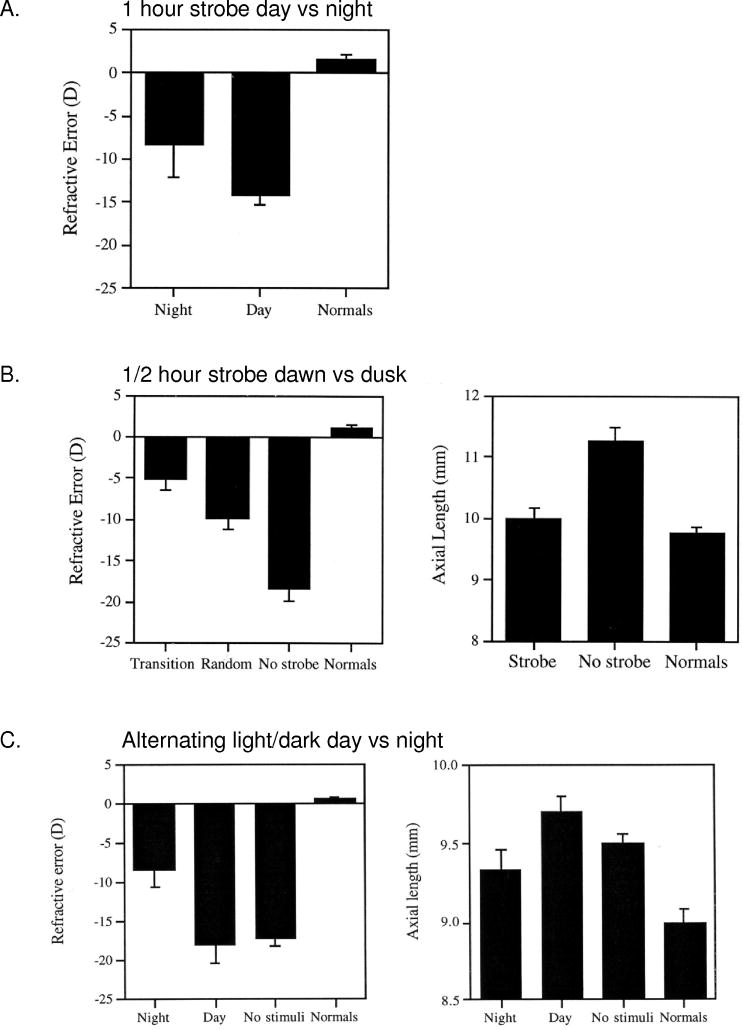
Circadian rhythms are synchronized to the external light/dark cycle by the phase-shifting effects of light at different times, defined by the phase–response curve. All rhythms are more sensitive to relevant stimuli during certain times, usually coinciding with the transition times of dawn and dusk (Pittendrigh, 1981), and light during the dark phase is likely to have disruptive effects on the synchronization of ocular rhythms. One interesting and controversial report in humans in support of this notion was an association between ambient nighttime light exposure during infancy and the subsequent development of myopia (Quinn et al., 1999). Later reports either supported this association (Chapell et al., 2001; Loman et al., 2002; Vannas et al., 2003; Czepita et al., 2004), or refuted it (Gwiazda et al., 2000; Zadnik et al., 2000; Saw et al., 2001; Guggenheim et al., 2003), and it remains an active area of debate to this day. One hypothesis held that ambient night lighting affected the phase of certain rhythms, in a manner similar to that of constant light in animal models. In animal models, various visual manipulations that inhibit the development of myopia are most effective when given at the transition times of dawn and dusk, or during the middle of the night, evincing a phase-dependent effect on the eye growth controller. First, the time at which brief daily periods of vision (in normal lab illumination) are effective at preventing the development of deprivation myopia (Napper et al., 1997) is phase-dependent: The efficacy of one 20-min period of vision is significantly greater during mid-night than during mid-day (Nickla, 1996). Second, the effectiveness of brief periods of stroboscopic stimulation (15 Hz) at preventing the development of myopia (Brennan et al., 1993; Kee et al., 2001) is also phase-dependent: One hour of strobe during mid-night is more effective than 1 h during mid-day (Fig. 6A) (Nickla, 1996). The most effective stimulus however, was half-hour pulses of strobe at “dawn” and “dusk” versus half-hour trains at randomized other times (Fig. 6B; Nickla, 1996; Kee et al., 2001). Finally, if night is “interrupted” by 5 min of light every 20 min, the amount of deprivation-induced myopia is inhibited, whereas interrupting the day with periods of darkness (alternating 15 min) is not effective (Fig. 6C; Nickla, 1996). One possible explanation for why visual stimulation is most effective at “transition times” in form deprived eyes is that it synchronizes various ocular rhythms whose phases were de-synchoronized by the deprivation (a “constant condition”?), and that this led to ocular growth inhibition.
Fig. 6.
A. The effect of 1 h of 15 Hz strobe given during mid-day (2 pm) or mid-night (2 am) on refractive error in form deprived eyes. The difference is significant at p < 0.05. B. Left. The effect of 15 Hz strobe stimulation for 30 min at “dawn” or “dusk” (8 am and 9:30 pm, respectively), and at two random 30 min times (“random”), in form deprived eyes and untreated controls (no strobe). Left: refractive error. Right: axial length for strobe versus no strobe birds. C. The effects of light/dark transitions given during the day (alternating 15 min of light and dark) versus night (5 min of light every 20 min) in form deprived eyes; the total amount of “light” in each was similar (14 and 17 h). “No stimuli” are untreated deprived eyes, “normals” are contralateral control eyes. Left, refractive error. Right, final axial length. Bars are standard errors in all graphs. From Nickla, 1996 (Ph.D. Dissertation).
The notion that circadian time is important in eye growth control may offer an explanation for the observations of in-phase rhythms in axial length and choroidal thickness in slow-growing eyes, and out-of-phase rhythms in fast growing eyes (Nickla et al., 1998b, 2002; Papastergiou et al., 1998). One can imagine that if the thickness of the choroid is directly correlated with the production of a growth factor, either stimulatory or inhibitory, then the timing of its acrophase in relation to that of another rhythm (proteoglycan synthesis or ocular compliance?) might have varying effects on the molecular events underlying ocular growth. In fact, the time of day dependent efficacy of brief periods of “vision” in form deprived eyes, which would in fact constitute periods of a small degree of myopic defocus, might be the result of a phase-dependent influence of thicker choroids on scleral growth, because these brief episodes cause transient choroidal thickening (Nickla, 2007).
6.2. Light cycles and intensity
In chickens, both constant light (Jensen and Matson, 1957; Lauber and McGinnis, 1966) and constant darkness (Lauber and Kinnear, 1979; Gottlieb et al., 1987) result in excessive eye growth and corneal flattening (Osol et al., 1986). Constant light also reduces the compensation to both positive and negative lenses in chickens, but does not abolish it (Guo et al., 1996). In monkeys, constant light causes small amounts of myopia in some animals (Smith et al., 2001). Two questions arising from these studies are (1) what proportion of light to darkness are required for emmetropization, and (2) how “dark” does “night” have to be, for emmetropization? In chickens reared on L/D cycles varying from 0L/24D (constant darkness) to 14L/10D, only eyes exposed to very short “days” of 1L/23D and 3L/21D were significantly larger than eyes in a normal cycle (14L/10D) (Osol et al., 1986), so a minimum of 4 h of light seems sufficient for emmetropia. This was corroborated by the reverse study asking how much darkness is required: Li et al. (2000) found that a minimum of 4 h of darkness offset the effects of constant light. The second question, addressing the putative deleterious effects of ambient light at night, was answered by Liu et al. (2004) who reared chickens for 3 weeks in diurnal cycles with six different light intensities at night, from dim to bright. Only the highest night light intensity (500 μW/cm2) had any effect on growth, merely producing shallower anterior chambers. In conclusion, a minimum of about 4 h out of 24 h of both light and darkness is required for emmetropization, and light at night does not seem to predispose toward myopia. Thus the notion that ambient light at night during childhood is a risk factor for myopia development is not supported.
Finally, does daytime light intensity have any effect on eye growth? The findings of Rose et al. (2008) that length of time outdoors is a preventative factor in myopia development led to a series of studies testing the hypothesis that light intensity could be a crucial factor. To study this, Cohen et al. (2011) reared chickens for 90 days under three different intensities (50 lux, 500 lux or 10,000 lux) during the day. The “low” day group reached emmetropia by 30 days, and became myopic by 90 days; the “medium” day group reached emmetropia by 55 days and remained emmetropic; the “high” intensity day group remained hyperopic. Thus dim “days” makes the emmetropization system less accurate. In other studies, increasing the intensity of light inhibited the amount of myopia in form deprived chick eyes in two paradigms, one in which the diffusers were removed for 15 min per day in high light, the second in which deprived eyes were exposed to different intensities for 6 h a day with the diffusers left on (Ashby et al., 2009). A similar study in Rhesus monkeys found that high light levels reduced the development of form deprivation myopia, and shifted the refractions of untreated control eyes in the hyperopic direction (Smith et al., 2012a). These animal studies support a role for light intensity as a factor in myopia inhibition, but the question crucial for translating these results to humans is whether similar effects would be found in eyes wearing negative lenses. So far, the answer is no: In chickens, bright light slowed the compensation to negative lenses, but eyes became as myopic as those reared under “normal” light by 6 days (Ashby and Schaeffel, 2010). In infant monkeys, there was no difference in the amount of myopia induced by −3 D lenses in animals exposed to high ambient light and those exposed to normal light levels, although the development of myopia may have been slowed by high light (Smith et al., 2012b). While these results do not preclude light intensity being meaningful in terms of possible treatment therapies, they weaken its promise.
7. Expression of clock genes
A logical next step to elucidate the underlying molecular mechanisms involved in the effect(s) of ocular clocks on eye growth regulation was to look at gene expression. Stone and colleagues (2011) used microchip technology to look at differential gene expression in chicken eyes wearing +15 D or −15 D lenses for hours or days. Few differentially expressed transcripts were found in eyes wearing positive lenses, but approximately 1500 transcripts were differentially expressed in eyes wearing negative lenses for 6 h. Not surprisingly, several of these were intrinsic clock genes, such as PER3 (Period homolog 3), and others related to rhythmic phenomena, such as melanopsin and a melatonin receptor. The authors point out that dopamine regulates melanopsin mRNA expression, which links these findings to the diurnal dopaminergic system. As an interesting aside, form deprived chicken eyes showed very different results from those wearing negative lenses: only two genes were differentially expressed after 6 h of deprivation, and six after 3 days, none of which were related to rhythmic phenomena (McGlinn et al., 2007). This supplies one more piece of evidence for the caveat raised above regarding the need for caution in interpreting results from only one of the myopiagenic paradigms in animal studies.
8. Conclusions
This is a synopsis of the evidence that has accrued over 20 years associating circadian ocular rhythms with ocular growth regulation. Most of the seminal work was done in chickens, and it is heartening to those of us working on animal models that most, if not all of these findings have been corroborated in primates, and some in humans as well. While we may still be far from answers to the questions of how these various rhythms in molecular signals like dopamine and melatonin, mechanical rhythms in IOP, and scleral rhythms in synthesis rates interact to influence the growth of the eye, we can be guardedly optimistic that we are on the right path in continuing to study them to ascertain whether and how they are causally related to one another. The existence of out-of-phase rhythms in eye length and choroidal thickness in human eyes underscores the potential fundamental significance of the phase relationships between these rhythms: with the new technology the next question to ask is whether these phases are altered in the eyes of children who are in the process of developing myopia. Other important considerations are whether pharmacological or environmental manipulations of choroidal thickness, or its phase, can be of potential therapeutic value in inhibiting ocular growth in children. In conclusion, many new and disparate avenues of research have evolved from the initial findings of Lauber and colleagues 50 years ago, and we have hope that they will converge into a meaningful approach to stem the ever-increasing prevalence of myopia.
Acknowledgments
My debt of gratitude to Josh Wallman for his enthusiasm for this area of research, and support of my work, is immeasurable. Supported by NIH-EY-013636.
References
- Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- Ashby R, Schaeffel F. The effect of bright light on lens-compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Brennan NA, Squires MA, Napper GA, Vingrys AJ. Stroboscopic light acts to restrict occlusion induced myopia locally in the chick retina. Invest Ophthalmol Vis Sci. 1993;34 (Suppl):1208. [Google Scholar]
- Brown JS, Flitcroft DI, Ying G, Francis EL, Schmid GF, Quinn GE, Stone RD. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009;50:5–12. doi: 10.1167/iovs.08-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik GA, Brown GM, Grota LJ. Differential localization of N-acetyltransferase indoleaalkylamines in CNS and the Harderian gland using immunohistology. Brain Res. 1976;118:417–427. doi: 10.1016/0006-8993(76)90309-7. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Retin Res. 1995;14:267–291. [Google Scholar]
- Campbell M, Bunghardt K, Kisilak M, Irving E. Diurnal rhythms of spherical refractive error, optical axial length and power in the chick. Invest Ophthalmol Vis Sci. 2012;53:6245–6253. doi: 10.1167/iovs.11-8844. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- Chapell M, Sullivan G, Sardakis S, Costello L, Mazgajiewski N, McGinley J, McGlone J, Andris C, Pasquarella A. Myopia and night time lighting during sleep in children and adults. Percept Mot Skills. 2001;92:640–642. doi: 10.2466/pms.2001.92.3.640. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon A, Polat U. Dependency between light intensity and refractive development under light-dark cycles. Exp Eye Res. 2011;92:40–46. doi: 10.1016/j.exer.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Czepita D, Goslawski W, Mojsa A, Muszynska-Lachota I. Role of light emitted by incandescent or fluorescent lamps in the development of myopia and astigmatism. Med Sci Monit. 2004;10:168–171. [PubMed] [Google Scholar]
- Dearry A, Burnside B. Light-induced dopamine release from teleost retinas acts as a light-adaptive signal to the retinal pigment epithelium. J Neurochem. 1989;53:870–878. doi: 10.1111/j.1471-4159.1989.tb11785.x. [DOI] [PubMed] [Google Scholar]
- Drance SM. The significance of the diurnal tension variations in normal and glaucamatus eyes. Arch Ophthalmol. 1960;64:494–501. doi: 10.1001/archopht.1960.01840010496004. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Frampton P, Rin DD, Brown B. Diurnal variation of intraocular pressure and the overriding effects of sleep. Am J Optom Physiol Opt. 1987;64:54–61. doi: 10.1097/00006324-198701000-00010. [DOI] [PubMed] [Google Scholar]
- Godley BF, Wurtman RJ. Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res. 1988;452:393–395. doi: 10.1016/0006-8993(88)90046-7. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Wentzek LA, Wallman J. Different visual restrictions produce different ametropias and different eye shapes. Invest Ophthalmol Vis Sci. 1987;28:1225–1235. [PubMed] [Google Scholar]
- Guggenheim J, Hill C, Yam TF. Myopia, genetics and ambient lighting at night in a UK sample. Br J Ophthalmol. 2003;87:580–582. doi: 10.1136/bjo.87.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Sivak J, Callender M, Herbert K. Effects of continuous light on experimental refractiveerrors in chicks. Ophthalmic PhysiolOpt. 1996;16:486–490. [PubMed] [Google Scholar]
- Gwiazda J, Ong E, Held R, Thorn F. Myopia and ambient night-time lighting. Nature. 2000;404:144. doi: 10.1038/35004663. [DOI] [PubMed] [Google Scholar]
- Henkind P, Leitman M, Weitzman E. The diurnal curve in man: new observations. Invest Ophthalmol Vis Sci. 1973;12:705–707. [PubMed] [Google Scholar]
- Herzog E, Block G. Keeping an eye on retinal clocks. Chronobiol Int. 1999;16:229–247. doi: 10.3109/07420529909116855. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Schaeffel F. Melatonin and deprivation myopia in chickens. Neurochem Int. 1996;28:95–107. doi: 10.1016/0197-0186(95)00050-i. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Besharse JC. Dopamine receptor-mediated inhibition of serotonin N-acetyltransferase activity in retina. Brain Res. 1986;369:168–176. doi: 10.1016/0006-8993(86)90525-1. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in the rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Stone RA, Lambert S, Laties AM. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci. 1991;32:1674–1677. [PubMed] [Google Scholar]
- Jensen L, Matson W. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125:741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- Kee C-s, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- Krishna R, Mermoud A, Baerveldt G, Minckler DS. Circadian rhythm of intraocular pressure: a rat model. Ophthalmic Res. 1995;27:163–167. doi: 10.1159/000267660. [DOI] [PubMed] [Google Scholar]
- Lauber JK, Kinnear A. Eye enlargement in birds induced by dim light. Can J Ophthalmol. 1979;14:265–269. [PubMed] [Google Scholar]
- Lauber JK, Kivett VK. Environmental control of the rearing conditions and early preglucomatous lesions in chicks. Exp Eye Res. 1981;32:501–509. doi: 10.1016/s0014-4835(81)80029-2. [DOI] [PubMed] [Google Scholar]
- Lauber JK, McGinnis J. Eye lesions in domestic fowl reared under continuous light. Vis Res. 1966;6:619–626. doi: 10.1016/0042-6989(66)90073-3. [DOI] [PubMed] [Google Scholar]
- Lauber JK, Shutze JV, McGinnis J. Effects of exposure to continuous light on the eye of the growing chick. Proc Soc Exp Biol Med. 1961;106 (4):871–872. doi: 10.3181/00379727-106-26505. [DOI] [PubMed] [Google Scholar]
- Li T, Howland HC, Troilo D. Diurnal illumination patterns affect the development of the chick eye. Vis Res. 2000;40:2387–2393. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Pendrak K, Capehart C, Sugimoto R, Schmid G, Stone RA. Emmetropization under continuous but non-constant light in chicks. Exp Eye Res. 2004;79:719–728. doi: 10.1016/j.exer.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Liu JH, Dacus AC. Aqueous humor cyclic AMP and circadian elevation of intraocular pressure in rabbits. Curr Eye Res. 1991;10:1175–1177. doi: 10.3109/02713689109024135. [DOI] [PubMed] [Google Scholar]
- Loman J, Quinn G, Kamoun L, Ying G, Maguire M, Hudesman D, Stone RA. Darkness and near work: myopia and its progression in third-year law students. Ophthalmology. 2002;109:1031–1038. doi: 10.1016/s0161-6420(02)01012-6. [DOI] [PubMed] [Google Scholar]
- McCarthy CS, Megaw P, Devadas M, Morgan I. Dopaminergic agents affect the ability of brief periods of normal vision to prevent form-deprivation myopia. Exp Eye Res. 2007;84:100–107. doi: 10.1016/j.exer.2006.09.018. [DOI] [PubMed] [Google Scholar]
- McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA. Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci. 2007;48:3430–3436. doi: 10.1167/iovs.06-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper G, Brennan N, Barrington M, Squires M, Vessey G, Vingrys A. The effect of an interrupted daily period of normal visual stimulation on form deprivation myopia in chicks. Vis Res. 1997;37:1557–1564. doi: 10.1016/s0042-6989(96)00269-6. [DOI] [PubMed] [Google Scholar]
- Nickla D. The phase relationships between the diurnal rhythm in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A. 2005;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: Implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001;42:584–588. [PubMed] [Google Scholar]
- Nickla D, Wildsoet C, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- Nickla DL. Biology. City University of New York; New York: 1996. Diurnal Rhythms and Eye Growth in Chicks; p. 222. [Google Scholar]
- Nickla DL. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84:951–959. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Rada JA, Wallman J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J Comp Physiol A. 1999;185:81–90. doi: 10.1007/s003590050368. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation vs negative lens-induced myopia. Exp Eye Res. 2012;102:93–103. doi: 10.1016/j.exer.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Totonelly K. Dopamine antagonists and brief vision distinguish lens-induced- and form-deprivation-induced myopia. Exp Eye Res. 2011;93:782–785. doi: 10.1016/j.exer.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998a;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998b;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Osol G, Schwartz B, Foss D. The effects of photoperiod and lid suture on eye growth in chickens. Invest Ophthalmol Vis Sci. 1986;27:255–260. [PubMed] [Google Scholar]
- Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements: interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. Circadian systems: entrainment. In: Aschoff J, editor. Biological Rhythms. Plenum Press; New York: 1981. pp. 796–803. [Google Scholar]
- Quinn GE, Shin CH, Maguire MG, Stone RA. Myopia and ambient lighting at night. Nature. 1999;399:113–114. doi: 10.1038/20094. [DOI] [PubMed] [Google Scholar]
- Rada JA, Achen VR, Rada KG. Proteoglycan turnover in the sclera of normal and experimentally myopic chick eyes. Invest Ophthalmol Vis Sci. 1998;39:1990–2002. [PubMed] [Google Scholar]
- Rada JA, Matthews AL. Visual deprivation upregulates extracellular matrix synthesis by chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1994;35:2436–2447. [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- Rada JA, Thoft RA, Hassell JR. Extracellular matrix changes in the sclera of chickens with experimental myopia. Invest Ophthalmol (ARVO Suppl) 1990;31:253. [Google Scholar]
- Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- Rose K, Morgan I, Ip J, Kifley A, Huynh S, Smith W, Mitchell P. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;116:1229–1230. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- Saw SM, Wu HM, Hong CY, Chua WH, Chia KS, Tan D. Myopia and night lighting in Singapore. Br J Ophthalmol. 2001;85:527–528. doi: 10.1136/bjo.85.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Bartmann M, Hagel G, Zrenner E. Studies on the role of the retinal Dopamine/Melatonin system in experimental refractive errors in chickens. Vis Res. 1995;35:1247–1264. doi: 10.1016/0042-6989(94)00221-7. [DOI] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet C. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Schnell CR, Debon C, Percicot CL. Measurement of intraocular pressure by telemetry in conscious, unrestrained rabbits. Invest Ophthalmol Vis Sci. 1996;37:958–965. [PubMed] [Google Scholar]
- Sherwin J, Hewitt A, Coroneo M, Kearns L, Griffiths L, Mackey D. The association between time spent outdoors and myopia using a novel biomarker of outdoor light exposure. Invest Ophthalmol Vis Sci. 2012;53:4363–4370. doi: 10.1167/iovs.11-8677. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Ward A, Norton T. ARVO. 2012. Moderately Elevated Fluorescent Light Levels Slow Form Deprivation and Minus Lens-induced Myopia Development in Tree Shrews; p. E-Abstract #3457. [Google Scholar]
- Smith EL, Bradley D, Fernandes A, Hung LF, Booth R. Continuous ambient lighting and eye growth in primates. Invest Ophthalmol Vis Sci. 2001;42:1146–1152. [PubMed] [Google Scholar]
- Smith ELI, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012a;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ELI, Hung LF, Huang J. Effects of High Ambient Lighting on Negative Lens Compensation in Infant Monkeys. ARVO. 2012b:E-Abstract #4659. [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, McGlinn AM, Baldwin DA, Tobias J, Iuvone P, Khurana T. Image defocus and altered retinal gene expression in chick: clues to the pathogenesis of ametropias. Invest Ophthalmol Vis Sci. 2011;52:5765–5777. doi: 10.1167/iovs.10-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RD, Quinn GE, Francis EL, Ying G, Flitcroft DI, Parekh P, Brown J, Orlow J, Schmid G. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- Summers-Rada J, Wiechmann A. Melatonin receptors in chick ocular tissues: implications for a role of melatonin in ocular growth regulation. Invest Ophthalmol Vis Sci. 2006;47:25–33. doi: 10.1167/iovs.05-0195. [DOI] [PubMed] [Google Scholar]
- Terman M, Reme CE, Wirz JA. The visual input stage of the mammalian circadian pacemaking system: II. The effect of light and drugs on retinal function. J Biol Rhythms. 1991;6:31–48. doi: 10.1177/074873049100600105. [DOI] [PubMed] [Google Scholar]
- Underwood H, Barrett KR, Siopes T. The quail’s eye: a biological clock. J Biol Rhythms. 1990;5:257–265. doi: 10.1177/074873049000500307. [DOI] [PubMed] [Google Scholar]
- Underwood H, Siopes T. Melatonin rhythms in quail: regulation by photo-period and circadian pacemakers. J Pineal Res. 1985;2:133–143. doi: 10.1111/j.1600-079x.1985.tb00634.x. [DOI] [PubMed] [Google Scholar]
- Vannas A, Ying G, Stone RA, Maguire M, Jormanainen V, Tervo T. Myopia and natural lighting extremes: risk factors in Finnish army conscripts. Acta Ophthalmol Scand. 2003;81:588–595. doi: 10.1046/j.1395-3907.2003.0151.x. [DOI] [PubMed] [Google Scholar]
- Wallman J, Xu C, Wildsoet C, Krebs W, Gottlieb M, Marran L, Nickla D. Moving the retina: a third mechanism of focusing the eye. Invest Ophthalmol Vis Sci. 1992;33:1053. [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb M, Nickla D, Marran L, Krebs W, Christensen A. Moving the retina: choroidal modulation of refractive state. Vis Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Weiss S, Schaeffel F. Diurnal growth rhythms in the chicken eye: relation to myopia development and retinal dopamine levels. J Comp Physiol A. 1993;172:263–270. doi: 10.1007/BF00216608. [DOI] [PubMed] [Google Scholar]
- Wiechmann A, Rada J. Melatonin receptor expression in the cornea and sclera. Exp Eye Res. 2003;77:219–225. doi: 10.1016/s0014-4835(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Wiechmann A, Summers-Rada J. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Craft CM. Localization of mRNA encoding the indoleamine synthesizing enzyme, hydroxyindole-O-methyltransferase, in chicken pineal gland and retina by in situ hybridization. Neurosci Lett. 1993;150:207–211. doi: 10.1016/0304-3940(93)90537-u. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Quinn GE, Ying G, Francis EL, Schmid G, Lam A, Orlow J, Stone RA. The relation of axial length and intraocular pressure fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1778–1784. doi: 10.1167/iovs.05-0869. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Jones LA, Irvin BC, Kleinstein RN, Manny RE, Shin JA, Mutti DO. Myopia and ambient night-time lighting. Nature. 2000;404:143–144. doi: 10.1038/35004661. [DOI] [PubMed] [Google Scholar]