Abstract
Cell-based therapy of neurological disorders is hampered by poor survival of grafted neural progenitor cells (NPCs). We hypothesized that it is possible to enhance the survival of human NPCs (ReNcells) by co-transplantation of helper cells expressing basic fibroblast growth factor (bFGF) under control of doxycycline (Dox). 293 cells or C17.2 cells were transduced with a lentiviral vector encoding the fluorescent reporter mCherry and bFGF under tetracycline-regulated transgene expression (Tet-ON). The bFGF secretion level in the engineered helper cells was positively correlated with the dose of Dox(Pearson correlation test; r=0.95 and 0.99 for 293 and C17.2 cells, respectively). Using bioluminescence imaging (BLI) as readout for firefly luciferase-transduced NPC survival, the addition of both 293-bFGF and C17.2-bFGF helper cells was found to significantly improve cell survival up to 6-fold in vitro, while wild-type (WT, non-transduced) helper cells had no effect. Following co-transplantation of 293-bFGF or C17.2-bFGF cells in the striatum of Rag2−/− immunodeficient mice, in vivo human NPC survival could be significantly improved as compared to no helper cells or co-transplantation of WT cells for the first two days after co-transplantation. This enhancement of survival in C17.2-bFGF group was not achieved without Dox administration, indicating that the neuroprotective effect was specific for bFGF. The present results warrant further studies on the use of engineered helper cells, including those expressing other growth factors injected as mixed cell populations.
Keywords: Neural progenitor cells, transplantation, cell survival, bFGF, bioluminescent imaging
Introduction
One critical issue in stem cell transplantation for therapy of neurological disorders is the substantial loss of transplanted cells following transplantation, which, in many cases, can be as much as 90% of the total number of grafted cells. This dramatic cell loss has been reported for transplants into animal models of various neurological disorders, including stroke (Bacigaluppi, et al., 2008, Hicks, et al., 2009) and Parkinson’s disease (Politis and Lindvall, 2012, Sortwell, et al., 2000)
The survival of stem cells is highly dependent on the local microenviroment and presence of local growth factors. For neural progenitor cells (NPCs), basic fibrobast growth factor (bFGF) has been found to be the one of the most important growth factors both in vitro and in vivo (Kuhn, et al., 1997, Maric, et al., 2003, Nakatomi, et al., 2002, Zheng, et al., 2004), playing a major role in cell survival, self-renewal, and differentiation. Thus, it has been proposed to genetically manipulate neural progenitor cells (NPCs) for the production of bFGF. Indeed, bFGF overexpression in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex (Dayer, et al., 2007), and promotes perivascular cluster formation with a neurogenic potential (Jenny, et al., 2009). However, the risk associated with the direct genetic modification of NPCs is the random integration of the vector in the host genome, which can result in insertional mutagenesis and genotoxicity, potentially leading to aberrant differentiation and tumor formation (Baum, et al., 2011). A better strategy may be to add engineered cells (referred to here as helper cells) as a provider of growth factors in combination with unmodified NPCs. There have been many reports co-transplanting NPCs and other types of cells, such as chromaffin cells (Schumm, et al., 2004), olfactory ensheathing cells (Agrawal, et al., 2004), and wild-type (WT) or genetically engineered Schwann cells (Guo, et al., 2007, Niapour, et al., 2011). However, without genetic control, there is often not sufficient or too much production of these growth factors. Overproduction of bFGF is particularly unwarranted as overactivation of the bFGF signaling pathway is associated with tumorigenesis and malignancy (Wright and Huang, 1996).
We present here a novel strategy, where the helper cell production of bFGF can be switched on and off using the TetON (tetracycline-regulated transgene expression) system. We show a beneficial effect for two bFGF-engineered helper cell lines (293 and C17.2), which resulted in enhanced survival of xenografted human NPCs in vitro and in vivo following intrastriatal xenotransplantation.
Materials and methods
Construction of lentiviral vectors
Our overall strategy is shown in Figure 1. The bFGF gene NM_002006.4 was cloned from the lentivectorpWPI_SPbFGF (plasmid 25812, Addgene, Cambridge, MA) as previously described (Dayer, et al., 2007). FUW-M2rtTA was also obtained from Addgene with plasmid# 20342 (Hockemeyer, et al., 2008). TRE-CMV was first cloned from FUW-TetO-myc(Hockemeyer, et al., 2008) provided by Addgene (plasmid# 20723) into the modified lentivectorpSMPUW (Cell Biolabs, San Diego, CA), using EcoR1 and Fse1 to replace the EF1a promoter. bFGF without intron was then cloned into this vector using Fse1 and EcoRV. Finally, IRES (internal ribosomal entry site)-mCherry, courtesy of Dr. Roger Y. Tsien, was cloned into this vector by EcoRV and Pac1, to complete the final pSM-TRE-bFGF-IRES-cherry construct. Each step of manipulation on lentivectors was verified by digesting the corresponding restriction enzymes followed by sequencing. Lentivirus for both lentivectors (FUW-M2rtTA and pSM-TRE-bFGF-IRES-mCherry) was made and concentrated as described previously (Liang, et al., 2012).
Figure 1.
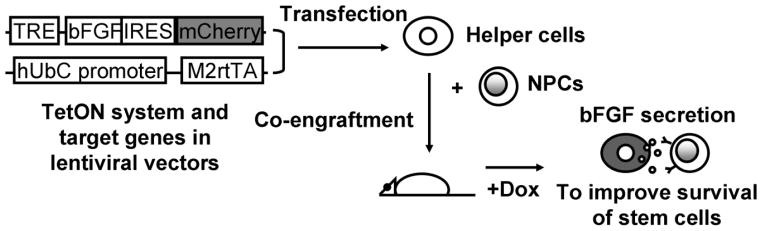
Schematic representation of strategy to improve survival of transplanted NPCs. A TetON system, consisting of M2rtTA driven by the human ubiquitin C (hUbC) promotor and the target genes (bFGF and mCherry) driven by a TRE promoter, are cloned into a lentivector for transduction of 293 and C17.2 helper cells. NPCs and helper cells are co-engrafted and at certain time points after transplantation, Dox is administered to induce target gene expression in helper cells. bFGF is then being produced, triggering activation of signaling pathways that enhance NPC survival.
Cell culture, transduction, and FACS sorting
C17.2 NPCs stably expressing LacZ (courtesy of Dr. Evan Y. Snyder) and 293 cells (Invitrogen, Carlsbad, CA) were cultured as described previously (Liang, et al., 2012). For transfection, cell lines were transduced with both lentiviral particles (FUW-M2rtTA and pSM-TRE-bFGF-IRES-cherry). Doxycycline (Dox, Sigma-Aldrich, St. Louis, MO) was added to transduced cells at doses of 20 ng/ml to 200 μg/ml to confirm successful transduction, as evidenced by expression of mCherry. ReNcell CX human NPCs (ReNeuron, Guilford, UK) were cultured in ReNcell maintenance media (Millipore, Billerica, MA) supplemented with 20 ng/ml bFGF (Invitrogen) and 20 ng/ml EGF (Invitrogen). ReNcells were transduced with lentivirus encoding FU-luc2-IRES-Venus (Liang, et al., 2012) and purified by flow cytometry (FACSAria cell sorter, Becton Dickinson, Bedford, MA), For sorting Dox-responsive cells, cells were incubated with 2 μg/ml Dox overnight, and sorted against the background of non-Dox treated cells.
Quantification of bFGF expression and helper cell numbers
To measure the bFGF production level of the two transduced helper cell lines, 2×104 293 or C17.2 cells were plated into 96-well plates. Cells were incubated with 20 ng/ml to 200 μg/ml Dox for 24 hours later, after which the supernatant was collected. The amount of secreted bFGF in the medium was measured using an ELISA kit (Pepro Tech, Rocky Hill, NJ). The total cell numbers in each well were measured with a CellTiter 96 assay (Promega, Madison, WI), using a calibration curve for each cell line. To this end, 1.0×105 cells were serially diluted in 96-well plates in triplicate. The proliferation rate was calculated as the ratio of post vs. pre-treatment cell number. The bFGF secretion per cell without and with varying Dox concentrations was calculated by dividing the bFGF concentration with the effective cell number in each well (the average of the number at the beginning and end of the experiment).
Bioluminescence of ReNcells in vitro
WT or transduced helper cells (293 and C17.2) were plated into non-coated 96 well plates at 2.5×104, 5.0×104, and 1.0×105 cells per well. 2.5×104ReNcells were added to the wells, and cultured overnight in maintenance media. For bioluminescence measurements, the medium was removed and 15 μg/ml luciferin in 10 mM phosphate buffered saline (PBS, pH=7.4 with calcium and magnesium) was added. The luminescence signal was measured using a multilabel plate reader (Perkin Elmer, Waltham, MA). The number of ReNcells was calculated from the BLI radiance values using a calibration curve.
Cell Transplantation
All animal procedures were approved and conducted in accordance with our institutional guidelines for the care of laboratory animals. Immunodeficient male Rag2−/− mice (8–12 weeks old, Taconic, Derwood, MD) were anesthetized with 2% isoflurane, shaved, and placed in a stereotaxic device (Stoelting, Wood Dale, IL). Helper cells and ReNcells were harvested, washed, and suspended in PBS at a density of 1×105 cells/μL. A 2 μl cell suspension (containing 1 μl helper cells and 1 μlReNcells) was then injected into the right striatum (AP=0; ML=2.0; DV=3.0) at a rate of 0.5 μl/min using a Hamilton 31G microinjection needle (Hamilton, Reno, NV). Five cohorts of animals (n=5 each) were studied: 293-WT +Dox, 293-bFGF +Dox, C17.2-bFGF −Dox, C17.2-bFGF +Dox, and ReNcells only −Dox. The needle was withdrawn slowly after the injection was complete (Liang, et al., 2012). Dox was administered subcutaneously (s.c.) at a dose of 100 mg/kg/day, starting two hours after cell transplantation.
Bioluminescence imaging (BLI) of transplanted cell survival
BLI was performed on all animals using an optical IVIS 200 system (Caliper Life Sciences, Hopkinton, MA) at 0–5 days after transplantation as described previously (Liang, et al., 2012). Briefly, mice were intraperitoneally injected with 150 mg/kg of luciferin (Caliper Life Sciences) to detect firefly luciferase activity. Mice were anesthetized with 1–2% isoflurane and imaged at 10 min, 15 min, and 20 min after luciferin injection, with a one-minute exposure time. Peak emission values through the observation window were used for quantification. Images were acquired and processed using LIVINGIMAGE® software (version 2.50, Caliper Life Sciences). For quantification, the photon signal in units of maximum photons per second per cm square per steradian (photons/sec/cm2/sr, abbreviated as p/s), was measured from a region of interest, which was kept constant in area and positioning for all experiments.
Immunofluoresence staining
4% PFA-fixed tissues were cryprotected and cut into 40 μm serial sections. Every fifth section of the transplanted cell area was collected for counting of Venus GFP-transfected ReNcells (green). For immunostaining, sections were first rinsed with PBS, blocked with 10% goat serum, and the incubated overnight at 4°C with anti-β galactosidase antibody (Cappel, Durham, NC) as primary antibody. Sections were rinsed with PBS and incubated with anti-Rabbit Alexafluor 594 (Invitrogen) as the secondary antibody was. Histochemical and immunofluorescent images were obtained using a Olympus BX51 microscope equipped with an Olympus DP70 camera. Fluorescent images were analyzed with ImageJ for semi-quantification of fluorescent intensity.
Statistical analysis
All data were analyzed using prism 4.03 software (GraphPad, La Jolla, CA) with the data expressed as mean ± SEM. Using linear regression analysis, the Pearson’s correlation coefficient was determined for the bFGF ELISA studies. For comparison of the two groups of data in cell culture experiments, a two-tailed standard Student’s t test was used. For comparison of more than 2 groups of data (i.e., survival ratio in culture and in vivo BLI data), a one-way analysis of variance (ANOVA) test was used with a post hocNeuman-Keuls’ test. In all cases, p< 0.05 was considered statistically significant.
Results
Helper cells exhibit functional transgene expression in vitro
We first evaluated the expression of mCherry in helper cells induced by Dox at various concentrations. Increasing the dose of Dox from 20 ng/ml to 200 μg/ml resulted in an increased expression of mCherry in both 293 and C17.2 helper cells (Fig. 2A). Quantification of the relative fluorescent intensity revealed a positive correlation between the dose of Dox and fluorescent intensity with correlation coefficients 0.49 and 0.55 for 293 and C17.2 cells, respectively (Fig. 2B). bFGF-engineered 293 cells expressed bFGF at much higher baseline levels compared to WT 293 cells (64.43±5.62 vs. 7.10±1.96 fg/ml/cell, p<0.01). C17.2 cells exhibited low baseline bFGF secretion levels, with no statistical difference between bFGF-C17.2 and WT C17.2 cells in the absence of Dox. Dox treatment significantly increased secretion in 293 and C17.2 cells in a dose-dependent manner (Fig. 2C), with correlation coefficients of 0.95 and 0.99, respectively. As compared to non-transfected WT cells in the absence of Dox, a higher proliferation rate was seen in 293-bFGF cells (1.97±0.13 vs 1.3±0.06, p<0.01), but not in engineered C17.2 cells, compared to WT cells (Fig. 2D). Dox increased the proliferation of C17.2 cells in a dose-dependent manner, but it had the opposite effect on 293 cells. At 200 μg/ml, Dox significantly reduced the cell proliferation in 293-bFGF cells as compared to a dose of 20 μg/ml (0.91±0.05 vs. 2.1±0.01, p<0.01). C17.2 cells displayed inhibition of proliferation already when the dose was increased from 2 μg/ml to 20 μg/ml (3.2±0.20 vs. 2.1±0.01, p<0.01), with a significant further reduction at a dose of 200 μg/ml (p<0.01) (Fig. 2D). The reduction in proliferation rate of helper cells exposed to high concentration of Dox is likely due a toxic effect of Dox itself rather then the secreted bFGF, since we also found a reduction in the proliferation rate of WT helper cells in the presence of increasing doses of Dox (data not shown). Taken together, these experiments demonstrate that genetically engineered helper cells are capable of responding to Dox by secreting bFGF in a dose-dependent manner.
Figure 2.
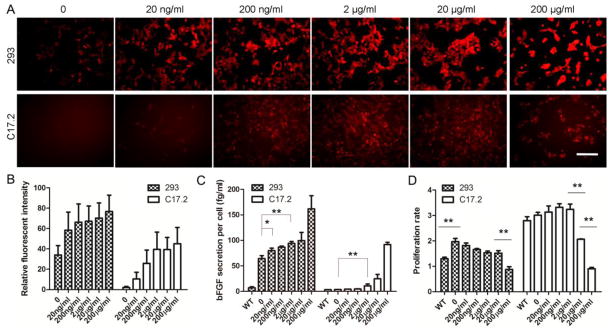
Dox-induced transgene expression and survival of helper cells. (A) Genetically engineered 293 and C17.2 cells were exposed to different concentrations of Dox for 24 hours. The expression of mCherry increases with increasing Dox concentration. Images were acquired at the same exposure time for each group of cells. Scale=200 μm. (B) The relative fluorescent intensity against the background level was semi-quantified using ImageJ software. (C) Measurements of bFGF secretion levels in helper cell culture supernatant show a significant difference between 293-bFGF and 293-WT cells (p<0.01, n=4), but not for C17.2 cells. Addition of Dox at a concentration of ≥20ng/ml increased bFGF secretion level in genetically engineered 293 cells (p<0.05, n=4). A significant increase of bFGF secretion (p<0.01, n=4) was also observed for C7.2 cells at a Dox concentration of 2 μg/ml or higher. (D) A significant difference exists between the proliferation rate of 293-bFGF and 293-WT cells, even without the addition of Dox (p<0.01, n=4). The addition of Dox at 200 μg/ml significantly reduced the proliferation rate of both 293-bFGF and C17.2-bFGF helper cells (p<0.01, n=4) while for C17.2 cells,Dox at 20 μg/ml also reduced the proliferation rate (p<0.01, n=4). (*, p<0.05; **, p<0.01; ***, p<0.001)
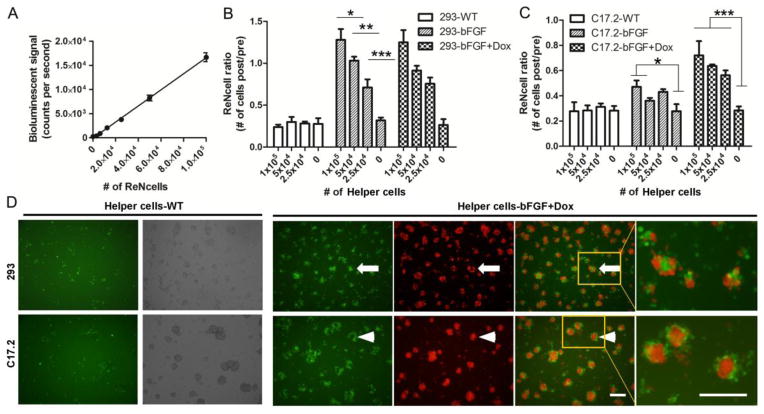
Co-culture of helper cells enhances survival of ReNcells in vitro
ReNcells, a human NPC cell line, were used to assess the potential beneficial effects of adding helper cells. To this end, cells were cultured on non-coated 96-wells in order to mimic non-adhesive conditions occurring in vivo following transplantation, promoting cell death. A standard curve of BLI signal and the number of live cells was first created to verify its linearity (R2=0.987, n=10) (Fig. 3A). In control groups without helper cells, only 28±4% of the initial number of ReNcells survived after overnight incubation (Figs. 3B,C). Co-culture with either 293 or C17.2 WT helper cells did not affect the survival rate of ReNcells, although the WT helper cells formed similar colonies as those engineered with bFGF (Fig. 3D). In contrast, a significantly higher number of ReNcells survived in the presence of co-cultured 293 bFGF helper cells, with or without adding Dox, in a dose dependent manner (p<0.05 for comparison of each pair of groups) The protective effect of C17.2-bFGF helper cells was smaller, and only occurred in the presence of Dox. These results suggest that, as compared to C17.2-bFGF, 293-bFGF helper cells produce higher baseline levels of bFGF without Dox induction.
Figure 3.
Helper cells enhance the survival of ReNcellsin vitro. (A) BLI calibration curve shows a linear correlation of signal with increasing cell concentration (R2=0.987, n=10). 293 (B) and C17.2 (C) helper cells were co-cultured overnight with luciferase-expressing ReNcells at different cell densities (2.5×104, 5×104, and 1×105 helper cells per 96-well; Dox was given at 2 μg/ml). The ReNcell ratio was calculated by dividing the cell number of pre-treatment by post-treatment, using the standard curve in (A). Data are expressed as mean±S.E.M. (n=4). For 293-bFGF cells (B), a significant difference in BLI signal exists between groups with different density of engineered 293 helper cells, whether or not Doxis.added. For C17.2-bFGF cells (C), all three helper cell densities were found to improve the survival of ReNcells in the presence of Dox (p<0.001). Without Dox, only the highest cell density was benificial (p<0.05). (D) Representative microscopic images acquired at a density of 5×104 helper cells showing the morphology of ReNcells (Venus GFP, green) and helper cells (mCherry, red). ReNcells co-cultured with 293-WT or C17.2-WT cells are loosely scattered after overnight incubation on a non-coated surface (first column). Bright field images show clusters of helper cells (second column). Helper cells expressing bFGF and mCherry interacted with ReNcells in a distinct pattern (ReNcells/green channel=third column, helper cells/red channel= fourth column, overlay=fifth column with sixth column as higher magnification inset). Scale bar=200 μm. (*, p<0.05; **, p<0.01; ***, p<0.001)
In co-cultures of ReNcells and WT helper cells, ReNcells were loosely attached to the plate surface at low densities (Fig 3D, helper cells-WT). In contrast, when ReNcells were co-cultured with 293-bFGF or C17.2-bFGF helper cells in the presence of Dox, they grew into distinct clusters (Fig. 3D, helper cells-bFGF+Dox). ReNcells appeared to interact differently with the two types of helper cells: ReNcells grew within the center of 293-bFGF cells (arrows in Fig. 3D), while they formed rings surrounding the C17.2-bFGF cells (arrowheads in Fig. 3D).
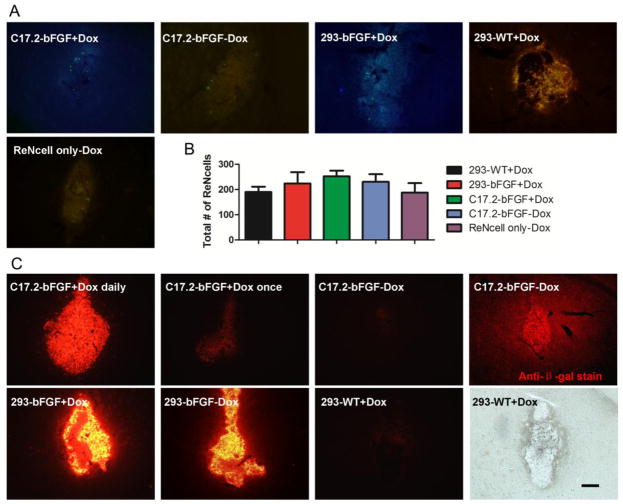
Co-transplantation of helper cells enhances survival of ReNcells in vivo
To further assess the pro-survival effect of engineered helper cells, ReNcells were co-transplanted with 293 or C17.2 cells in the striatum of immunodeficient (Rag2−/−) mice. The survival of implanted ReNcells was monitored with BLI serially over time. Substantial cell death occurred in all transplanted cell groups (Figs. 4A). By five days after transplantation, the BLI signal went to background levels for all transplanted cohorts (Fig. 4B). However, normalization of BLI data revealed a short but significant improvement of ReNcell survival for the group with co-transplanted of C17.2-bFGF +Dox lasting for two days after cell transplantation (Fig. 4B and 4C). On day 1, the BLI signal in this cohort was 2.18±1.26 fold higher than the initial signal immediately after transplantation, which is in sharp contrast to the other cohorts where the signal was below 50% of the starting value (p<0.001) as is shown in Fig. 4C. A non-normalized plotting of BLI signal (Fig. 4B) also revealed the initial increase in the C17.2-bFGF +Dox group one day after transplantation (C17.2-bFGF+Dox group vs. each of the other groups, p<0.001). These data indicate that ReNcells proliferated only in the C17.2-bFGF +Dox group and only for about 24 hours. On day 2, the BLI signal from this helper group went down to 0.49±0.29 of the starting BLI signal (Fig. 4C), which was still significantly higher than all the other groups (p<0.001). Instead, co-transplantation of WT 293 helper cells did not improve the survival of ReNcells at any time point.
Figure 4.
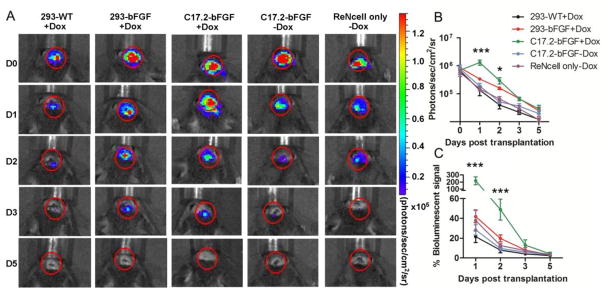
In vivo survival of ReNcells after transplantation into Rag2−/−immunodeficient mice. (A). BL images of representative animals transplanted with 1×105ReNcells with or without helper cells show strong BLI signal on the same day after transplantation, which decreases progressively over the following days. (B) Quantification of BLI signal for each group (n=5 each), with the intensity expressed as photon/sec/cm2/sr. (C) Estimation of percent donor cell survival plotted as % signal activity (normalized to day 0) over the 5-day period following transplantation. In (B), on day 1, there was a significant difference between the BLI signal from the C17.2-bFGF+Dox group and the other four groups (p<0.001). On day 2, p<0.05 between C17.2-bFGF+Dox and 293-bFGF+Dox, and p<0.001 for C17.2-bFGF+Dox vs. each of the rest three groups. In (C), on days 1 and 2, there was a significant difference between the BLI signal from the C17.2-bFGF+Dox group and the other four groups (p<0.001). (*, p<0.05; **, p<0.01; ***, p<0.001)
Histological analysis of cell transplants
Following the last BLI time point, mice were sacrificed and examined for immunofluorescence. Consistent with BLI data, there were few surviving ReNcells at 5 days after implantation in all the 5 transplantation cohorts (Fig. 5A), with no significant differences between each pair of groups (Fig. 5B). A qualitative comparison of the mCherry fluorescence showed that the transgene expression in C17.2-bFGF cells could be controlled well by Dox administration (Fig. 5C). Daily s.c.administration of Dox resulted in a robust expression of mCherry, while a single injection of Dox lead to a much weaker expression of mCherry. No expression could be detected when Dox was not administered. In contrast, 293-bFGF cells did not display a Dox-dependent expression of mCherry, which was maintained at a relatively high level compared to that seen in C17.2-bFGF +Dox cells. It is apparent that both helper cells survived well after transplantation despite the fact that very few ReNcells were viable. This validates the use of Rag2−/− mice as a suitable recipient for sustaining of allografts (mouse C17.2 cells) and xenografts (human HEK cells), despite the poor survival of xenografted human NPCs.
Figure 5.
Immunohistological analysis at Day 5 after transplantation. (A) Mice were sacrificed on day 5 after BLI for histological analysis of living ReNcells (green). (B) Stereological counting of ReNcells. Data are expressed as mean±S.E.M. (n=5). (C) Red channel (mCherry) fluorescent images showing the relative expression level of the transfected genes in each helper cell group. All images were acquired using the same exposure time (1/10 sec). C17.2-bFGF with daily administration of Dox showed the strongest gene expression, whereas the absence of Dox resulted in negligible expression of mCherry. A single Dox injection resulted in a discernable expression of mCherry, although it was weak compared to the daily Dox injection. Further graft morphology was examined by anti-β galactosidaseimmunostaining as marker for C17.2 cells. 293 cells expressed mCherry with or without Dox administration. 293-WT cells are shown as control lacking transgene expression. Scale bar=200 μm.
Discussion
Limited cell survival following intracerebral transplantation remains one of the critical issues in cell-based therapy. In this study, we have developed a novel system that allows the controllable expression of bFGF in helper cells, and investigated their survival-enhancing effects on NPCs in vitro and in vivo. We provide here, for the first time, direct evidence that co-transplantation of helper cells exhibiting tunable bFGF expression are beneficial for xenografted human NPCs.
bFGF binds to the high-affinity transmembrane FGF receptors (FGFR1–4). Upon binding, it triggers the activation of cytoplasmic signal transduction pathways, such as the Ras/ERK pathway (responsible for proliferation and differentiation), the Akt pathway (associated with cell survival), or the protein kinase C pathway that is associated with migration (Dorey and Amaya, 2010). For each specific cell type, the subtype of FGFR determines which pathway is to be activated. For many lineages of stem cells, including NPCs, bFGF has been shown to be a potent mitogenic cytokine. Thus, bFGF has been used extensively to propagate neuroepithelial cells in vitro (Rao, 1999).
We have chosen ReNcells as our transplant paradigm, a human neural progenitor cell line derived from the cortical region of human fetal brain, immortalized by retroviral transduction with the c-myc oncogene. It is able to differentiate into neurons and glial cells (Donato, et al., 2007), with a stringent dependence on bFGF and EGF for propagation in cell culture. Due to the lack of major components in the immune response (Shinkai, et al., 1992), Rag2−/− mice have been widely used as a recipient mouse strain for cell xenotransplantation studies (Berman, et al., 2011, Ito, et al., 2012). In our initial in vivo transplantation studies without helper cells, we found poor survival of ReNcells (>90% cell loss) during the first few days after transplantation into Rag2−/− mice, though C17.2 cells or 293 cells could readily survive in the same setting (Fig. 5C). Other studies have also found, using BLI as readout, a rapid reduction of viability of grafted NPCs during the first few days after transplantation (Okada, et al., 2005, Sher, et al., 2009).Our histology confirmed the poor survival of ReNcells, which lies in the lower range of survival rate from compared to transplantation studies using NPCs (Barker, et al., 1996, Kallur, et al., 2006, Sortwell, et al., 2000). CTX0E03, another NPC cell line that is also commercially available from the same company, has been reported to exert protective effects in an experimental model of stroke (Pollock, et al., 2006, Stroemer, et al., 2009), though surviving cells were only found in 37% of all recipient animals (Stroemer, et al., 2009).
While the BLI signal is commonly used as a measure to probe in vivo cell proliferation, the emitted signal may exhibit variability between identically transplanted animals. The underlying basis for these variations may be derived from small differences in the stereotaxic coordinates of transplantation, the presence of post-surgical blood products, and the detection sensitivity of BLI for each specific setting (Bradbury, et al., 2007). However, comparing the surviving cell number in a within-subjects manner (corrected for baseline values) provides a more reliable means of determining the number of transplanted progenitors that are surviving. In our experimental setup, cells were transplanted into striatum at a depth close to the range of the detection limit of optical imaging (2–3 mm), resulting in less sensitivity compared to cell transplantation into more superficial places such as the subcutaneous space. This may explain the discrepancy at the end timepoint between the in vivo BLI data and the histological counting of a few hundred surviving cells. With the BLI signal being near the level of the background noise, quantification of those low cell numbers is not possible using BLI.
It is known that the in vitro survival of NPCs, be it primary cells or cells derived from embryonic and induced pluripotent stem cells, is highly dependent on the presence of specific growth factors, requiring stringent protocols for differentiation and maintenance in vitro (Cai and Grabel, 2007, Osakada and Takahashi, 2011). Therefore, the substantial loss of live implanted NPCs during the early days of transplantation may result from a lack of sufficient growth factors in the host environment as compared to the preceding conditions in cell culture. In addition, lack of adhesion may result in anoikis-induced cell death, i.e., a form of programmed cell death that is induced by anchorage-dependent cells detaching from the surrounding extracellular matrix. To simulate the context of stem cell transplantation, we developed a model of stem cell death in culture by deprivation of cell adhesion and growth factors. Under these conditions, ReNcells underwent substantial cell death (around 70% of total population) after overnight incubation. This model could further serve as an in vitro assay for screening cells and agents with pro-survival property.
Several reports have shown a beneficial effect of growth factor production from genetically engineered NPCs in improving the survival of implanted cells (Dayer, et al., 2007, Park, et al., 2006) as well in improving the behavioral outcome after cell therapy (Bakshi, et al., 2006, Jenny, et al., 2009, Suzuki, et al., 2007). In our in vitro PTSCD model, we found that co-culture of bFGF-expressing helper cells in the presence of Dox not only improved the survival of ReNcells, but they also increased the total number of ReNcells as compared to the initial numbers (1×105 cell group, Fig. 3). In vivo, we observed a protective effect when ReNcells were co-grafted with bFGF-expressing cells into the brains of immunodeficient mice (Fig. 4). The fact that the protective effect observed in vivo was not as robust as seen in vitro may be explained by the less favorable host microenvironment surrounding grafted cells, i.e. presence of hostile factors, such as limited nutrients and oxygen (due to the absence of vasculature), and inflammation-related factors including free radicals, cytokines, and natural killer cells (Laflamme, et al., 2007). This discrepancy between in vitro and in vivo protection was also reported for grafted dopamine neurons (Marchionini, et al., 2003). In addition to bFGF, other neurotrophic factors, such as GDNF, BDNF, NT3, and NT4 may therefore be needed for further improvement of cell survival.
We found that 293-bFGF helper cells produced more bFGF as compared to C17.2 (Fig. 2). The baseline, non-Dox induced bFGF secretion was also higher. Thus, in vitroReNcells were better protected in all 293-bFGF groups (Fig. 3). It is unclear why 293-bFGF helper cells then did not provide a better protective effect in vivo. Assuming that bFGF alone is not sufficient to provide complete protection, it could be that C17.2, being a neural stem cell line, may secrete other growth factors, such as such as GDNF, BDNF, and NGF (Lu, et al., 2003, Niles, et al., 2004, Yan, et al., 2004) that are critical under in vivo conditions.
Finally, we observed a distinct morphologic pattern of the interaction of ReNcells with helper cells. We hypothesize that C17.2 cells, being from neural origin, secrets additional factors that promotes the spread of ReNcells, reflecting favorable culture conditions in non-coated plates. The choice of 293 and C17.2 cells as helper cells was designed to provide a proof-of-principle evidence that our strategy is viable for both a neural and non-neural cell line. In summary, we have shown that bFGF-engineered helper cells can improve survival of primary cells used in cell transplantation therapy.
Highlights.
Helper cells produce bFGF upon addition of Dox in a dose-dependent manner
bFGF helper cells significantly improve survival of human NPCs cultured in vitro
Co-transplantation of bFGF helper cells prolongs survival of xenografted human NPCs
Acknowledgments
This work was supported by 2RO1 NS045062. For the plasmids used in this study, we are grateful to Dr. Roger Y. Tsien for providing IRES and mCherry, Dr. JozsefKiss for providing pWPI_SPbFGF, and Dr. Rudolf Jaenisch for providing FUW-M2rtTA and FUW-tetO-hMYC. We thank Mary McAllister for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal AK, Shukla S, Chaturvedi RK, Seth K, Srivastava N, Ahmad A, Seth PK. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: a cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol Dis. 2004;16:516–526. doi: 10.1016/j.nbd.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Bakshi A, Shimizu S, Keck CA, Cho S, LeBold DG, Morales D, Arenas E, Snyder EY, Watson DJ, McIntosh TK. Neural progenitor cells engineered to secrete GDNF show enhanced survival, neuronal differentiation and improve cognitive function following traumatic brain injury. Eur J Neurosci. 2006;23:2119–2134. doi: 10.1111/j.1460-9568.2006.04743.x. [DOI] [PubMed] [Google Scholar]
- 4.Barker RA, Dunnett SB, Faissner A, Fawcett JW. The time course of loss of dopaminergic neurons and the gliotic reaction surrounding grafts of embryonic mesencephalon to the striatum. Exp Neurol. 1996;141:79–93. doi: 10.1006/exnr.1996.0141. [DOI] [PubMed] [Google Scholar]
- 5.Baum C, Modlich U, Gohring G, Schlegelberger B. Concise review: managing genotoxicity in the therapeutic modification of stem cells. Stem Cells. 2011;29:1479–1484. doi: 10.1002/stem.716. [DOI] [PubMed] [Google Scholar]
- 6.Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn Reson Med. 2011;65:564–574. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury MS, Panagiotakos G, Chan BK, Tomishima M, Zanzonico P, Vider J, Ponomarev V, Studer L, Tabar V. Optical bioluminescence imaging of human ES cell progeny in the rodent CNS. J Neurochem. 2007;102:2029–2039. doi: 10.1111/j.1471-4159.2007.04681.x. [DOI] [PubMed] [Google Scholar]
- 8.Cai C, Grabel L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev Dyn. 2007;236:3255–3266. doi: 10.1002/dvdy.21306. [DOI] [PubMed] [Google Scholar]
- 9.Dayer AG, Jenny B, Sauvain MO, Potter G, Salmon P, Zgraggen E, Kanemitsu M, Gascon E, Sizonenko S, Trono D, Kiss JZ. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain. 2007;130:2962–2976. doi: 10.1093/brain/awm200. [DOI] [PubMed] [Google Scholar]
- 10.Donato R, Miljan EA, Hines SJ, Aouabdi S, Pollock K, Patel S, Edwards FA, Sinden JD. Differential development of neuronal physiological responsiveness in two human neural stem cell lines. BMC Neurosci. 2007;8:36. doi: 10.1186/1471-2202-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo JS, Zeng YS, Li HB, Huang WL, Liu RY, Li XB, Ding Y, Wu LZ, Cai DZ. Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord. 2007;45:15–24. doi: 10.1038/sj.sc.3101943. [DOI] [PubMed] [Google Scholar]
- 13.Hicks AU, Lappalainen RS, Narkilahti S, Suuronen R, Corbett D, Sivenius J, Hovatta O, Jolkkonen J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29:562–574. doi: 10.1111/j.1460-9568.2008.06599.x. [DOI] [PubMed] [Google Scholar]
- 14.Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenny B, Kanemitsu M, Tsupykov O, Potter G, Salmon P, Zgraggen E, Gascon E, Skibo G, Dayer AG, Kiss JZ. Fibroblast growth factor-2 overexpression in transplanted neural progenitors promotes perivascular cluster formation with a neurogenic potential. Stem Cells. 2009;27:1309–1317. doi: 10.1002/stem.46. [DOI] [PubMed] [Google Scholar]
- 17.Kallur T, Darsalia V, Lindvall O, Kokaia Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res. 2006;84:1630–1644. doi: 10.1002/jnr.21066. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 20.Liang Y, Walczak P, Bulte JW. Comparison of red-shifted firefly luciferase Ppy RE9 and conventional Luc2 as bioluminescence imaging reporter genes for in vivo imaging of stem cells. J Biomed Opt. 2012;17:016004. doi: 10.1117/1.JBO.17.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 22.Marchionini DM, Collier TJ, Camargo M, McGuire S, Pitzer M, Sortwell CE. Interference with anoikis-induced cell death of dopamine neurons: implications for augmenting embryonic graft survival in a rat model of Parkinson’s disease. J Comp Neurol. 2003;464:172–179. doi: 10.1002/cne.10785. [DOI] [PubMed] [Google Scholar]
- 23.Maric D, Maric I, Chang YH, Barker JL. Prospective cell sorting of embryonic rat neural stem cells and neuronal and glial progenitors reveals selective effects of basic fibroblast growth factor and epidermal growth factor on self-renewal and differentiation. J Neurosci. 2003;23:240–251. doi: 10.1523/JNEUROSCI.23-01-00240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 25.Niapour A, Karamali F, Nemati S, Taghipour Z, Mardani M, Nasr-Esfahani MH, Baharvand H. Co-transplantation of Human Embryonic Stem Cell-derived Neural Progenitors and Schwann Cells in a Rat Spinal Cord Contusion Injury Model Elicits a Distinct Neurogenesis and Functional Recovery. Cell Transplant. 2011 doi: 10.3727/096368911X593163. [DOI] [PubMed] [Google Scholar]
- 26.Niles LP, Armstrong KJ, Rincon Castro LM, Dao CV, Sharma R, McMillan CR, Doering LC, Kirkham DL. Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004;5:41. doi: 10.1186/1471-2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada S, Ishii K, Yamane J, Iwanami A, Ikegami T, Katoh H, Iwamoto Y, Nakamura M, Miyoshi H, Okano HJ, Contag CH, Toyama Y, Okano H. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J. 2005;19:1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- 28.Osakada F, Takahashi M. Neural induction and patterning in Mammalian pluripotent stem cells. CNS Neurol Disord Drug Targets. 2011;10:419–432. doi: 10.2174/187152711795563958. [DOI] [PubMed] [Google Scholar]
- 29.Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: Evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006;199:179–190. doi: 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012;10:1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollock K, Stroemer P, Patel S, Stevanato L, Hope A, Miljan E, Dong Z, Hodges H, Price J, Sinden JD. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199:143–155. doi: 10.1016/j.expneurol.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 33.Schumm MA, Castellanos DA, Frydel BR, Sagen J. Improved neural progenitor cell survival when cografted with chromaffin cells in the rat striatum. Exp Neurol. 2004;185:133–142. doi: 10.1016/j.expneurol.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Sher F, van Dam G, Boddeke E, Copray S. Bioluminescence imaging of Olig2-neural stem cells reveals improved engraftment in a demyelination mouse model. Stem Cells. 2009;27:1582–1591. doi: 10.1002/stem.76. [DOI] [PubMed] [Google Scholar]
- 35.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 36.Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165:268–277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- 37.Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JA, Huang A. Growth factors in mechanisms of malignancy: roles for TGF-beta and FGF. Histol Histopathol. 1996;11:521–536. [PubMed] [Google Scholar]
- 40.Yan J, Welsh AM, Bora SH, Snyder EY, Koliatsos VE. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J Comp Neurol. 2004;480:101–114. doi: 10.1002/cne.20344. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]