Abstract
During lung development, Fibroblast growth factor 10 (Fgf10), which is expressed in the distal mesenchyme and regulated by Wnt signaling, acts on the distal epithelial progenitors to maintain them and prevent them from differentiating into proximal (airway) epithelial cells. Fgf10-expressing cells in the distal mesenchyme are progenitors for parabronchial smooth muscle cells (PSMCs). After naphthalene, ozone or bleomycin-induced airway epithelial injury, surviving epithelial cells secrete Wnt7b which then activates the PSMC niche to induce Fgf10 expression. This Fgf10 secreted by the niche then acts on a subset of Clara stem cells to break quiescence, induce proliferation and initiate epithelial repair. Here we show that conditional deletion of the Wnt target gene c-Myc from the lung mesenchyme during development does not affect proper epithelial or mesenchymal differentiation. However, in the adult lung we show that after naphthalene-mediated airway epithelial injury c-Myc is important for the activation of the PSMC niche and as such induces proliferation and Fgf10 expression in PSMCs. Our data indicate that conditional deletion of c-Myc from PSMCs inhibits airway epithelial repair, whereas c-Myc ablation from Clara cells has no effect on airway epithelial regeneration. These findings may have important implications for understanding the misregulation of lung repair in asthma and COPD.
Introduction
A complex interplay between endodermal and mesodermal cell types defines early developmental competence and cell fate in the lung. As such, proximal-distal patterning of the lung is accompanied by the gradual restricted ability of developmental progenitors to generate the various epithelial lineages in the mature organ [1]. During lung development, Fgf10 (Fibroblast growth factor 10) is expressed in mesenchyme distal to the branching tips where it maintains the multipotent distal epithelial progenitors, but is suppressed proximally and at bifurcation points [2], [3], [4], [5], [6], [7]. We previously identified the Fgf10-expressing cells in the distal mesenchyme as parabronchial smooth muscle cell (PSMC) progenitors [3], [8]. Fgf10 expression as well as the amplification of these PSMC progenitors is regulated by Wnt signaling [3], [9], [10]. Suppression of Fgf10 expression around the developing airway is crucial to allow for proper maturation of the lung airway epithelium [11], [12], [13], [14], [15].
The adult lung is a vital and complex organ that normally turns over very slowly. The epithelial cells that line the airways are constantly exposed to potential toxic agents and pathogens in the environment, and they must therefore be able to respond quickly and effectively to both cellular damage and local production of immune cytokines. Adult stem cells are implicated in both homeostatic tissue maintenance and functional restoration after injury in organs such as skin and gut.
A widely used lung injury model involves the destruction of Clara cells by naphthalene. Only those Clara cells that express cytochrome P4502F2 (encoded by Cyp2f2) are able to convert naphthalene into toxic epoxides leading to cell death. Within a few hours after naphthalene administration nearly all Clara cells have died, except for the few less differentiated variant Clara stem cells that do not express Cyp2f2, making them therefore resistant against naphthalene [16], [17], [18], [19], [20]. Ciliated cells quickly spread out, or squamate, under the dying Clara cells in an attempt to cover the basal lamina and maintain the permeability barrier of the epithelium [21].
We have previously shown that surviving ciliated cells after naphthalene, ozone or bleomycin-mediated airway epithelial injury start to secrete Wnt7b, which then activates the PSMC niche to induce Fgf10 expression [22]. We found that Fgf10 secreted by the niche acts on surviving Clara stem cells to break quiescence, induce proliferation and initiate epithelial repair. Here we show that after naphthalene-mediated airway epithelial injury, the Wnt target c-Myc is important for the activation of the PSMC niche and as such induces proliferation and Fgf10 expression in PSMCs. Myc proteins coordinate many interdependent processes, including cell growth (increase in cell mass), cell proliferation (DNA replication and cell cycle progression), differentiation and apoptosis [23]. Using an allelic series of mice in which c-Myc expression was incrementally reduced to zero, Trumpp et al. showed that fibroblasts from these mice exhibit reduced proliferation and after complete loss of c-Myc function exit the cell cycle [24]. Our data indicate that conditional deletion of c-Myc from PSMCs prevents activation of the airway epithelial stem cell niche after airway epithelial injury resulting in deficient epithelial repair.
Results
c-Myc Expression in the Lung Mesenchyme is not Required for Normal Lung Development
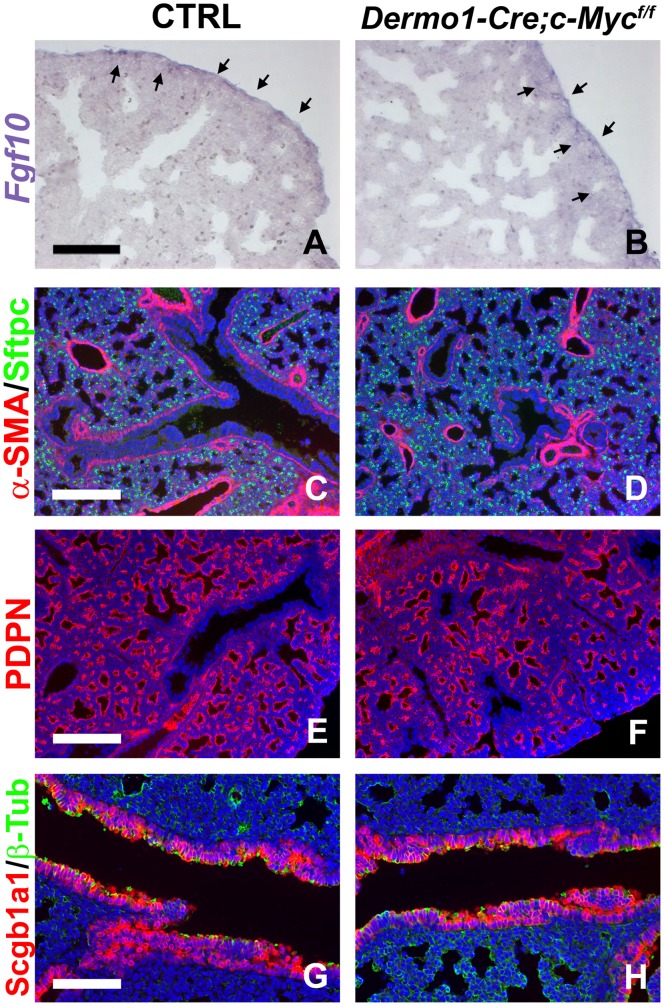
During lung development, Nmyc expression is normally restricted to a distal population of undifferentiated epithelial cells [25], whereas c-Myc is only expressed in the mesenchyme [3]. c-Myc expression is regulated by β-catenin signaling and is lost upon conditional deletion of β-catenin from the lung mesenchyme [3]. In some organs most of the effects of β-catenin signaling are primarily mediated by c-Myc [26]. To test whether during lung development the effects of mesenchymal β-catenin signaling are primarily mediated via c-Myc we conditionally deleted c-Myc from the lung mesenchyme using a Dermo1(Twist2)-Cre line [27]. Interestingly, while ablation of β-catenin from the lung mesenchyme resulted in major differentiation defects and reduced Fgf10 expression [3], [28], we found that conditional deletion of c-Myc from the lung mesenchyme has no significant effect on either (Fig. 1A–D). At E18.5, Dermo1-Cre;c-Mycf/f [24] conditional knock out lungs appear normal, with normal Fgf10 expression (Fig. 1A,B) and with proper differentiation of the airway and vascular smooth muscle cells (Fig. 1C,D), proper differentiation of the distal epithelium in ATII (Sftpc) and ATI (Pdpn) cells (Fig. 1C–F) and proper differentiation of the bronchial epithelium into Clara (Scgb1a1) and ciliated cells (ß-Tub) (Fig. 1G,H).
Figure 1. Mesenchyme-specific c-Myc ablation does not affect lung development.
(A,B) Fgf10 in situ hybridization on E18.5 ctrl (A) and Dermo1-Cre;c-Mycf/f (B) lungs showing that Fgf10 expression is not affected. (C–H) Immunostaining for α-SMA (smooth muscle cells) and Sftpc (ATII cells) (C,D), PDPN (ATI cells) (E,F), and Scgb1a1 (Clara cells) and β-tubulin (ciliated cells) (G,H) on E18.5 ctrl (C,E,G) and Dermo1-Cre;c-Mycf/f (D,F,H) lungs. n≥3. Scale bars: 100 µM (A,B and G,H); 200 µM (C–F).
c-Myc Regulates Activation of the Airway Epithelial Stem Cell Niche after Airway Epithelial Injury
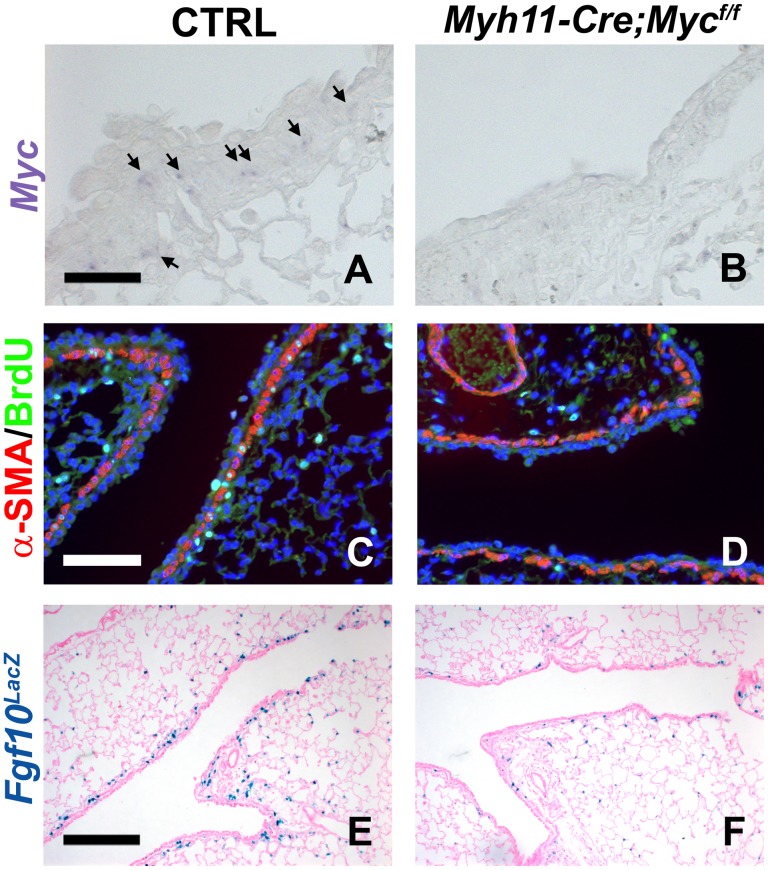
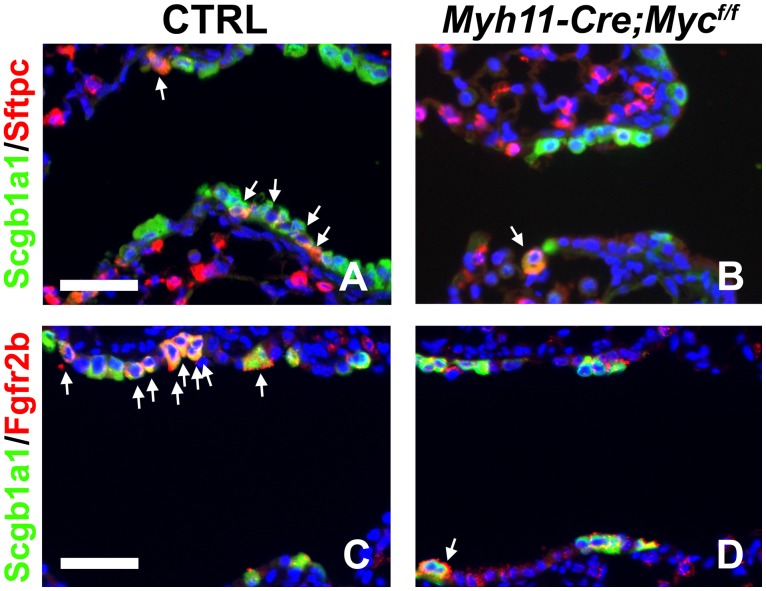
We recently showed that after airway epithelial injury, surviving epithelial cells secrete Wnt7b, which then activates PSMCs (which constitute a niche for airway epithelial stem cells) to induce proliferation and Fgf10 expression [22]. This Fgf10 secreted by the PSMC niche then acts on a subset of Clara stem cells to break quiescence, induce proliferation and initiate epithelial repair [22]. To investigate the requirement of c-Myc in the activation of the PSMC niche and the induction of Fgf10 expression in the adult lung after airway epithelial injury we generated Myh11-Cre;c-Mycf/f mice (Myh11: smooth muscle myosin heavy chain) [29], in which we conditionally deleted c-Myc from the PSMCs, shown by in situ hybridization in Fig. 2A,B. Interestingly, we found that the PSMC niche in Myh11-Cre;c-Mycf/f lungs does not get activated after naphthalene-mediated airway epithelial injury. This is manifested by reduced proliferation of the PSMCs, as 9.2% ±1% of PSMCs were BrdU positive in control lungs vs. 2.6% ±0.23% of PSMCs in Myh11-Cre;c-Mycf/f lungs (P = 0.000005, n≥4) (Fig. 2C,D) [22]. To investigate whether induction of Fgf10 expression is also regulated by c-Myc we crossed Myh11-Cre;c-Mycf/f mice with an Fgf10LacZ reporter line [3], [7], [8], [22], [30]. In contrast to our observations during lung development we found that in the adult lung, 3 days after naphthalene-mediated airway epithelial injury, Fgf10 expression in the PSMC niche is regulated by c-Myc, as demonstrated by the lack of induction of Fgf10 expression in Myh11-Cre;c-Mycf/f;Fgf10LacZ mice (Fig. 2F) compared to control littermates (Fig. 2E). We previously reported a similar drastic reduction in Fgf10 expression and proliferation in PSMCs, after naphthalene-mediated airway epithelial injury, in mice overexpressing Dkk1, a secreted inhibitor of Wnt signaling [22]. To investigate whether epithelial Fgf10 signaling is also reduced we checked for Scgb1a1+Sftcp+ [22], [31] and Scgb1a1+Fgfr2b+ [22] double positive Clara stem cells in the regenerating distal airways near bronchoalveolar duct junctions (BADJs). In accordance with our previously reported results showing that Sftpc and Fgfr2b expression in Clara stem cells is at least in part regulated by Fgf10 [22], we found a reduction in Scgb1a1+Sftcp+ and Scgb1a1+Fgfr2b+ double positive distal airway Clara stem cells at the BADJs in Myh11-Cre;c-Mycf/f mice 7 days after naphthalene injury compared to control littermates (Fig. 3A–D) [22], [31], [32].
Figure 2. c-Myc regulates activation of the airway epithelial stem cell niche after airway epithelial injury.
(A,B) c-Myc in situ hybridization on ctrl (A) and Myh11-cre;c-Mycf/f (B) lungs 7 days after naphthalene injury, showing that c-Myc is expressed by airway smooth muscle cells after airway epithelial injury and the absence of c-Myc expression in Myh11-cre;c-Mycf/f lungs. (C,D) Immunostaining for BrdU (proliferation marker) and α-SMA (airway smooth muscle cells) on ctrl (C) and Myh11-cre;c-Mycf/f (D) lungs 3 days after naphthalene treatment. (E,F) β-gal staining on Fgf10LacZ ctrl (E) and Myh11-cre;c-Mycf/f;Fgf10LacZ (F) lungs 3 days after naphthalene injury. n≥3. Scale bars: 50 µM (A,B); 100 µM (C,D); 200 µM (E,F).
Figure 3. Conditional c-Myc deletion from PSMCs reduces Fgf10 signaling in Clara stem cells after naphthalene-mediated injury.
(A–D) Immunostaining for Scgb1a1 and Sftpc (A,B), Scgb1a1 and Fgfr2b (C,D) on ctrl (A,C) and Myh11-cre;c-Mycf/f (B,D) lungs 7 days after naphthalene. n≥3. Scale bars: 50 µM (A–D).
Conditional Deletion of c-Myc from Airway Smooth Muscle Severely Impairs Airway Epithelial Regeneration After Injury
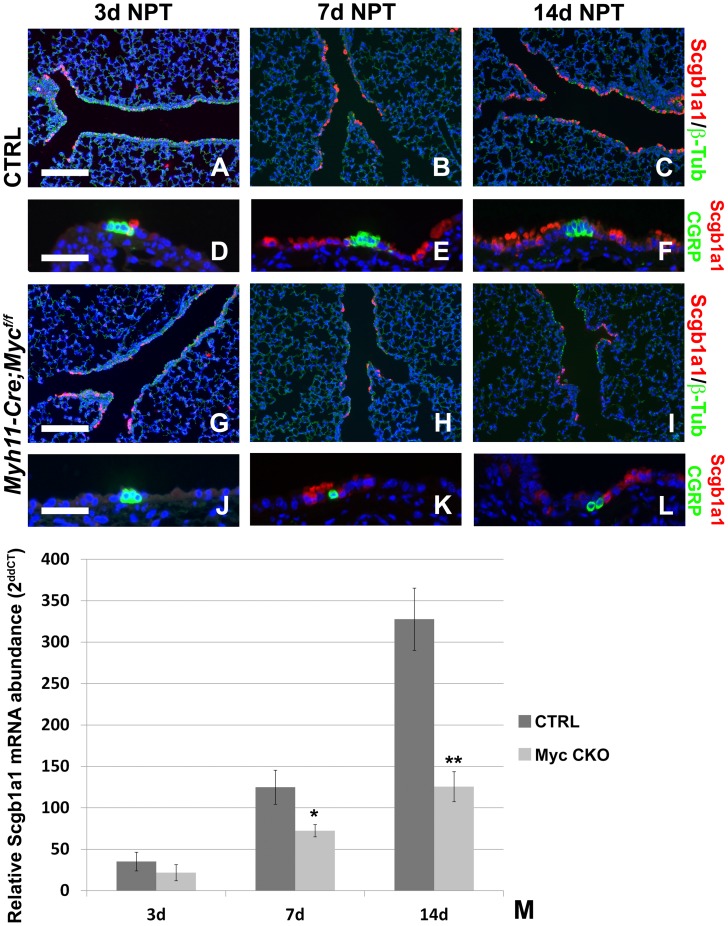
Fgf10 secreted by the PSMC niche after airway epithelial injury is critical for proper regeneration of the airway epithelium [22]. We next investigated how airway epithelial regeneration is affected in Myh11-Cre;c-Mycf/f mice with a conditional inactivation of c-Myc from the PSMC niche. Myh11-Cre;c-Mycf/f and control littermates were injured with naphthalene resulting in a >95% decrease in Scgb1a1 expression, as a measure of Clara cell loss, by 3 days after injury and airway epithelial regeneration was monitored over time. At 3 days post injury both control and Myh11-Cre;c-Mycf/f mice show similar levels of injury demonstrated by low levels of Scgb1a1 mRNA expression (a Clara stem cell-specific marker) and the presence of limited Scgb1a1 positive Clara stem cells at BADJs and near CGRP-expressing neuroendocrine bodies, while most of the airway is lined with ciliated cells (β-tubulin) (Fig. 4A,D,G,J,M). At 7 days after injury, Myh11-Cre;c-Mycf/f mice show a 40% decrease in airway epithelial regeneration (Fig. 4H,K,M) compared to control mice (Fig. 4B,E,M). This decrease in regeneration is even more evident at 14 days post injury, with Myh11-Cre;c-Mycf/f mice showing an almost 3 fold decrease in regeneration (Fig. 4I,L,M) compared to control mice (Fig. 4C,F,M).
Figure 4. Conditional c-Myc deletion from the epithelial stem cell niche impairs epithelial regeneration after injury.
(A–L) Immunostaining for Scgb1a1 (Clara stem cells) and β-tubulin (ciliated cells) (A–C,G–I) or Scgb1a1 (Clara stem cells) and CGRP (neuroendocrine bodies) (D–F,J–L) on ctrl (A–F) and Myh11-cre;c-Mycf/f (G–L) lungs 3 days (A,D,G,J), 7 days (B,E,H,K) and 14 days (C,F,I,L) after naphthalene injury. (M) qPCR analysis of relative Scgb1a1 mRNA abundance in lungs from ctrl and Myh11-cre;c-Mycf/f mice 3, 7 and 14 days after naphthalene treatment. **P<0.01, *P<0.05 vs. respective control. n≥3. Scale bars: 200 µM (A–C and G–I); 50 µM (D–F and J–L).
Conditional Deletion of c-Myc from Clara Stem Cells does not Affect Airway Epithelial Regeneration after Injury
We have previously shown that after naphthalene injury a subset of Clara cells undergo a transient epithelial to mesenchymal transition (EMT) to acquire stem cell-like properties and as such are able to transiently induce the expression of Myh11 [22]. To investigate whether the decrease in airway epithelial regeneration in Myh11-Cre;c-Mycf/f mice is not due to deletion of c-Myc from these Clara cells, transiently expressing Myh11-Cre, we generated Scgb1a1-Cre;c-Mycf/f [33], [34] mice in which the c-Myc gene is deleted specifically from all Clara cells. We found that airway epithelial regeneration after naphthalene injury is not affected in Scgb1a1-Cre;c-Mycf/f mice compared to control littermates (Fig. 5A–E), indicating that epithelial c-Myc does not play an important role in airway epithelial regeneration and that the defect in regeneration observed in Myh11-Cre;c-Mycf/f mice can be attributed solely to the loss of c-Myc from the PSMC niche. This is consistent with the fact that during lung development c-Myc expression is restricted to the mesenchyme, whereas Nmyc is expressed solely in the epithelium [3], [25].
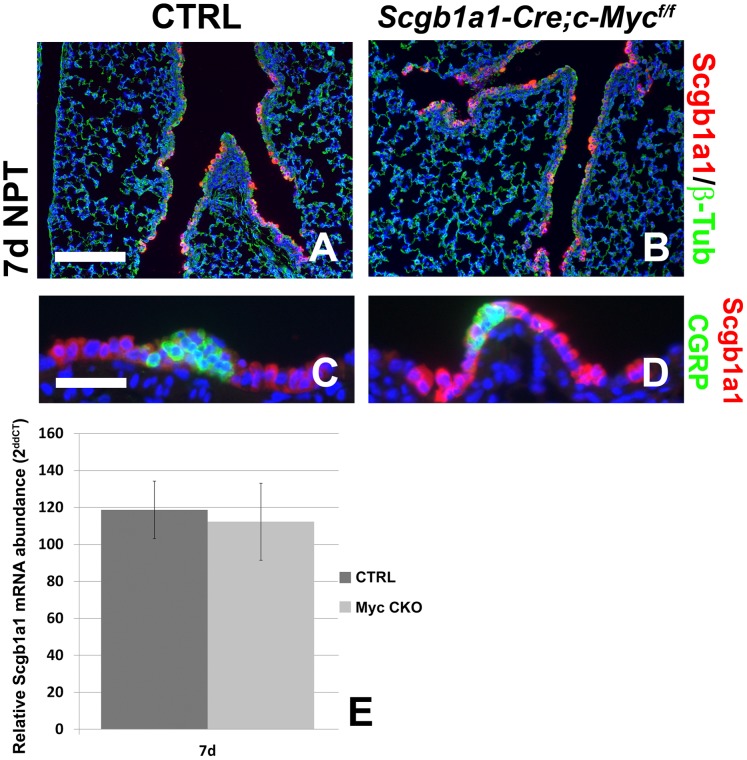
Figure 5. Epithelial c-Myc deletion does not affect airway epithelial regeneration after injury.
(A–D) Immunostaining for Scgb1a1 (Clara stem cells) and β-tubulin (ciliated cells) (A,B) or Scgb1a1 (Clara stem cells) and CGRP (neuroendocrine bodies) (C,D) on ctrl (A,C) and Scgb1a1-Cre;c-Mycf/f (B,D) lungs 7 days after naphthalene injury. (E) qPCR analysis of relative Scgb1a1 mRNA abundance in lungs from ctrl and Scgb1a1-Cre;c-Mycf/f mice 7 days after naphthalene treatment. P>0.05, n≥3. Scale bars: 200 µM (A,B); 50 µM (C,D).
Discussion
The lung has a complex three-dimensional structure that features major differences along its proximal-distal axis in terms of the composition of the endoderm-derived epithelium. The trachea and primary lung buds arise by different morphogenetic processes from contiguous regions of the embryonic foregut [35]. A distinguishing feature of the adult mouse cartilaginous airways (i.e. trachea and primary bronchi) is that Fgf10 is expressed in the mesenchyme between the cartilage rings [36], [37] and that they contain a discontinuous population of basal stem cells that express p63 and specific keratins (K14 and K5). In addition to basal cells, the luminal epithelium in cartilaginous airways consists of two main columnar epithelial cell types: ciliated cells and Clara cells with a limited number of Clara cell-derived goblet cells. Ciliated cells contain cilia which are involved in the clearance of mucus produced by goblet cells, whereas Clara cells produce secretoglobins, the most abundant of which is Scgb1a1 (also known as CCSP, CC10 and CCA) [38], [39], [40].
The more distal airways (small bronchi and bronchioles) have a columnar epithelium surrounded by airway smooth muscle which does not express Fgf10 during normal homeostasis [8]. Clara stem cells predominate over ciliated cells and there are more neuroendocrine cells than in the trachea. More importantly, there is no evidence of basal cells in smaller airways in the mouse during normal homeostasis [41].
In the cartilaginous airways basal cells are considered to be on top of the stem cell hierarchy and are able to self renew and give rise to both Clara cells, goblet cells and ciliated cells [42]. Clara cells themselves are also considered stem cells and during normal homeostasis can give rise to new Clara cells and terminally differentiated ciliated cells [43], [44]. Cellular plasticity (including but not limited to differentiation, dedifferentiation, and transdifferentiation) is a frequently encountered cell behavior during injury repair [45], [46], [47], [48], [49], [50], [51]. Interestingly, p63 is a master regulator required for the development of basal cells [52] and induces a basal cell phenotype and squamous metaplasia when ectopically expressed in Clara cells [53]. This form of Clara cell reprogramming may happen to some extent after airway epithelial injury, as under such conditions Clara cells have been shown to be able to give rise to basal cells [44].
Interestingly, our unpublished data suggest that Fgf10 plays a role in the differentiation of airway epithelial cells into basal stem cells during lung development (Volckaert et al., manuscript submitted).
Our data presented here indicate that c-Myc plays an important role in regulating the activity of the PSMC niche in the adult lung. We found a role for c-Myc in regulating proliferation of PSMCs as well as the induction of Fgf10 expression within PSMCs cells after airway epithelial injury. Interestingly, we found no important role for c-Myc in the mesenchyme during lung development indicating that the function of c-Myc during lung development is redundant and that other not yet identified factors may compensate for the loss of c-Myc during lung development. The lack of defective smooth muscle cell differentiation or maintenance in Myh-Cre;c-Mycf/f lungs suggests that c-Myc may play a specific role in activation of the PSMC niche after injury. Together with the finding that epithelial c-Myc does not play an important role in lung epithelial homeostasis or repair after injury we conclude that targeting c-Myc may be a great way to treat lung diseases characterized by abnormal proliferation of smooth muscle cells, such as asthma and pulmonary arterial hypertension in which Wnt signaling plays a role [54]. In addition, we have previously shown that Fgf10 secreted by the PSMCs modulates the differentiation of Clara cells into goblet cells [22], which is a hallmark of the asthmatic airway. Future experiments will be needed to determine if loss of mesenchymal c-Myc may also reduce proliferation of (myo)fibroblasts in the bleomycin model of pulmonary fibrosis, in which Wnt signaling plays an important role [55], [56], [57], [58], [59], [60], [61]. If so, targeting c-Myc might be an effective and selective way to treat fibroproliferative lung diseases in general.
Materials and Methods
Study Approval
All experiments were conducted in strict accordance with the recommendations in the guide for the care and use of laboratory animals. The protocol was approved by the National Jewish Health institutional animal care and use committee #AS2774.
Mouse Strains
Myh11-Cre [Tg(Myh11-cre,-EGFP)2Mik/J] mice were obtained from Jackson Laboratories. Dermo1-Cre mice were a kind gift from Dr. David Ornitz [27]. Scgb1a1-Cre were a kind gift from Dr. Thomas Mariani [33], [34]. c-Mycf/f mice were a kind gift from Dr. Andreas Trumpp [24]. Adult mice were 8 weeks old at time of naphthalene administration. Animals were maintained in a pathogen-free environment.
β-gal Staining
Tissues containing Fgf10LacZ allele were dissected, and β-gal staining was performed at 3 days after naphthalene injury. Lungs were dissected and fixed in 4% PFA in PBS at room temperature for 5 minutes, rinsed in PBS, injected with freshly prepared X-gal solution, transferred into a vial of X-gal solution, and stained at 37°C overnight. After rinsing with PBS, lungs were postfixed in 4% PFA in PBS at room temperature overnight. For microtome sections, after 4% PFA fixation, lungs were washed in PBS, dehydrated, and paraffin embedded.
Immunofluorescence
All staining was done on paraffin sections of formalin-fixed lungs. Immunofluorescent staining was performed with the following primary antibodies: mouse anti–β-tubulin (3F3-G2; Seven Hills Bioreagents), goat anti-Scgb1a1 (T-18; Santa Cruz Biotechnology Inc.), rabbit anti-Scgb1a1 (Seven Hills Bioreagents), rabbit anti-CGRP (Sigma-Aldrich), rabbit anti-Fgfr2 (Bek) (C-17; Santa Cruz Biotechnology Inc.), mouse anti–α-SMA cy3 conjugate and unconjugated (14A; Sigma-Aldrich), rabbit anti-Sftpc (Seven Hills Bioreagents), mouse anti-PDPN (Iowa hybridoma bank). All fluorescent staining was performed with secondary antibodies from Jackson Immunoresearch (except the Cy3-conjugated α-SMA) and mounted using Vectashield with DAPI (Vector Labs). Photographs were taken with a Zeiss AxioImager and Axiovision software.
qPCR
RNA was isolated from lung accessory lobes using RNALater (Ambion) and Total RNA Kit I (Omega Biotek) according to the manufacturer’s instructions. RNA concentration was determined by spectrophotometry. cDNA was generated using SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s instructions. Comparative real-time PCR was performed for β-glucuronidase (Mm00446953_m1) and Scgb1a1 (Mm00442046_m1) Taqman Gene Expression Assays (Applied Biosystems) using a StepOne Plus system (Applied Biosystems). β-glucuronidase was used as a reference control to normalize equal loading of template cDNA.
Naphthalene Treatment
Naphthalene (Sigma-Aldrich) was dissolved in corn oil at 30 mg/ml and administered intraperitoneally at 8 weeks of age, with doses adjusted according to strain to achieve a 95% decrease in the abundance of Scgb1a1 mRNA in total lung RNA of WT mice at 3 days after injection. Control mice for regeneration studies were WT littermates.
Proliferation
Mice were given intraperitoneal injections of 10 µl BrdU (GE Healthcare) per gram body weight 4 hours before sacrifice. Lungs were fixed in 4% paraformaldehyde, dehydrated, and paraffin embedded. Sections were treated with monoclonal anti-BrdU (clone BU-1; GE Healthcare) according to the manufacturer’s instructions. FITC-labeled anti-mouse secondary antibodies were used (Jackson Immunoresearch). All slides were mounted using Vectashield with DAPI.
In situ Hybridization
In situ hybridization on paraffin sections of formalin-fixed lungs was performed as previously described [3]. A 584-bp Fgf10 mouse cDNA [2] and 201-bp fragment of c-Myc [3] mouse cDNA were used as templates for the synthesis of digoxigenin-labeled antisense riboprobes.
Statistics
For BrdU labeling and qPCR analysis, each experiment was repeated with samples obtained from at least 3 different lungs preparations. All results are expressed as mean ± SEM. The significance of differences between 2 sample means was determined by the Student’s t test. P values less than 0.05 were considered statistically significant.
Funding Statement
These studies were supported by funding from the US National Institutes of Health (NIH) R01 HL092967 (to SDL, www.nih.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rawlins EL, Clark CP, Xue Y, Hogan BL (2009) The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 136: 3741–3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL (1997) Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124: 4867. [DOI] [PubMed] [Google Scholar]
- 3. De Langhe SP, Carraro G, Tefft D, Li C, Xu X, et al. (2008) Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One 3: e1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Langhe SP, Carraro G, Warburton D, Hajihosseini MK, Bellusci S (2006) Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Dev Biol 299: 52–62. [DOI] [PubMed] [Google Scholar]
- 5.Goss AM, Tian Y, Cheng L, Yang J, Zhou D, et al.. (2011) Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. [DOI] [PMC free article] [PubMed]
- 6. Nyeng P, Norgaard GA, Kobberup S, Jensen J (2008) FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, et al. (2007) Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 307: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mailleux AA, Kelly R, Veltmaat JM, De Langhe SP, Zaffran S, et al. (2005) Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development 132: 2157–2166. [DOI] [PubMed] [Google Scholar]
- 9. Chen F, Cao Y, Qian J, Shao F, Niederreither K, et al. (2010) A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest 120: 2040–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, et al. (2009) Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell 17: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sedita J, Izvolsky K, Cardoso WV (2004) Differential expression of heparan sulfate 6-O-sulfotransferase isoforms in the mouse embryo suggests distinctive roles during organogenesis. Dev Dyn 231: 782–794. [DOI] [PubMed] [Google Scholar]
- 12. Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, et al. (2003) Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol 258: 185–200. [DOI] [PubMed] [Google Scholar]
- 13. McKeehan WL, Wang F, Kan M (1998) The heparan sulfate-fibroblast growth factor family: diversity of structure and function. Prog Nucleic Acid Res Mol Biol 59 135–76: 135. [DOI] [PubMed] [Google Scholar]
- 14. Izvolsky KI, Zhong L, Wei L, Yu Q, Nugent MA, et al. (2003) Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol 285: L838–846. [DOI] [PubMed] [Google Scholar]
- 15. Shimokawa K, Kimura-Yoshida C, Nagai N, Mukai K, Matsubara K, et al. (2011) Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev Cell 21: 257–272. [DOI] [PubMed] [Google Scholar]
- 16. Giangreco A, Reynolds SD, Stripp BR (2002) Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161: 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR (2001) Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 24: 671–681. [DOI] [PubMed] [Google Scholar]
- 18. Plopper CG, Macklin J, Nishio SJ, Hyde DM, Buckpitt AR (1992) Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest 67: 553–565. [PubMed] [Google Scholar]
- 19. Reynolds SD, Giangreco A, Power JH, Stripp BR (2000) Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 156: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stripp BR, Maxson K, Mera R, Singh G (1995) Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 269: L791–799. [DOI] [PubMed] [Google Scholar]
- 21. Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, et al. (2006) Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 34: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, et al. (2011) Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest 121: 4409–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dang CV (2012) MYC on the path to cancer. Cell 149: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trumpp A, Refaeli Y, Oskarsson T, Gasser S, Murphy M, et al. (2001) c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414: 768–773. [DOI] [PubMed] [Google Scholar]
- 25. Okubo T, Knoepfler PS, Eisenman RN, Hogan BL (2005) Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development 132: 1363–1374. [DOI] [PubMed] [Google Scholar]
- 26. Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, et al. (2007) Myc deletion rescues Apc deficiency in the small intestine. Nature 446: 676–679. [DOI] [PubMed] [Google Scholar]
- 27. Yu K, Xu J, Liu Z, Sosic D, Shao J, et al. (2003) Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130: 3063–3074. [DOI] [PubMed] [Google Scholar]
- 28. Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, et al. (2008) An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol 319: 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xin HB, Deng KY, Rishniw M, Ji G, Kotlikoff MI (2002) Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol Genomics 10: 211–215. [DOI] [PubMed] [Google Scholar]
- 30. Kelly RG, Brown NA, Buckingham ME (2001) The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell 1: 435–440. [DOI] [PubMed] [Google Scholar]
- 31. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, et al. (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835. [DOI] [PubMed] [Google Scholar]
- 32. Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, et al. (2012) Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30: 1948–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, et al. (2006) K-ras activation generates an inflammatory response in lung tumors. Oncogene 25: 2105–2112. [DOI] [PubMed] [Google Scholar]
- 34. Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Andalcio T, et al. (2006) Epithelial cell PPARgamma is an endogenous regulator of normal lung maturation and maintenance. Proc Am Thorac Soc 3: 510–511. [DOI] [PubMed] [Google Scholar]
- 35. Cardoso WV, Lu J (2006) Regulation of early lung morphogenesis: questions, facts and controversies. Development 133: 1611–1624. [DOI] [PubMed] [Google Scholar]
- 36. Sala FG, Del Moral PM, Tiozzo C, Alam DA, Warburton D, et al. (2011) FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development 138: 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tiozzo C, De Langhe S, Carraro G, Alam DA, Nagy A, et al. (2009) Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of Apert syndrome. Pediatr Res 66: 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rawlins EL, Hogan BL (2006) Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133: 2455–2465. [DOI] [PubMed] [Google Scholar]
- 39. Rock JR, Hogan BL (2011) Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 27: 493–512. [DOI] [PubMed] [Google Scholar]
- 40. Rock JR, Randell SH, Hogan BL (2010) Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 3: 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pack RJ, Al-Ugaily LH, Morris G (1981) The cells of the tracheobronchial epithelium of the mouse: a quantitative light and electron microscope study. J Anat 132: 71–84. [PMC free article] [PubMed] [Google Scholar]
- 42. Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, et al. (2009) Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106: 12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans MJ, Johnson LV, Stephens RJ, Freeman G (1976) Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 35: 246–257. [PubMed] [Google Scholar]
- 44. Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, et al. (2009) The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, et al. (2009) Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol 11: 190–196. [DOI] [PubMed] [Google Scholar]
- 46. Bedada FB, Gunther S, Kubin T, Braun T (2006) Differentiation versus plasticity: fixing the fate of undetermined adult stem cells. Cell Cycle 5: 223–226. [DOI] [PubMed] [Google Scholar]
- 47. Cobaleda C, Busslinger M (2008) Developmental plasticity of lymphocytes. Curr Opin Immunol 20: 139–148. [DOI] [PubMed] [Google Scholar]
- 48. Kai T, Spradling A (2004) Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature 428: 564–569. [DOI] [PubMed] [Google Scholar]
- 49.Stocum DL (2004) Tissue restoration through regenerative biology and medicine. Adv Anat Embryol Cell Biol 176: III–VIII, 1–101, back cover. [DOI] [PubMed]
- 50. Stocum DL (2004) Amphibian regeneration and stem cells. Curr Top Microbiol Immunol 280: 1–70. [DOI] [PubMed] [Google Scholar]
- 51. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA (2008) In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, et al. (2004) Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol 287: C171–181. [DOI] [PubMed] [Google Scholar]
- 53. Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR (2004) p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 18: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, et al. (2009) Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 119: 2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, et al. (2008) Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One 3: e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Konigshoff M, Eickelberg O (2009) WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 42: 21–31. [DOI] [PubMed] [Google Scholar]
- 57. Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, et al. (2009) WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xi Y, Wei Y, Sennino B, Ulsamer A, Kwan I, et al. Identification of pY654-beta-catenin as a critical co-factor in hypoxia-inducible factor-1alpha signaling and tumor responses to hypoxia. Oncogene. [DOI] [PMC free article] [PubMed]
- 59. Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, et al. Axin pathway activity regulates in vivo pY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem 287: 5164–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim Y, Kugler MC, Wei Y, Kim KK, Li X, et al. (2009) Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol 184: 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, et al. (2009) Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J Clin Invest 119: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]