Abstract
Obese individuals have elevated platelet activation and arterial stiffness, but the strength and temporality of the relationship between these factors remain unclear. We aimed to determine the effect of increased arterial stiffness on circulating platelet activity in overweight/obese young adults. This analysis included 92 participants (mean age 40 years, 60 women) in the Slow Adverse Vascular Effects of excess weight (SAVE) trial, a clinical trial examining the effects of a lifestyle intervention with or without sodium restriction on vascular health in normotensive overweight/obese young adults. Carotid-femoral (cf), brachial-ankle (ba), and femoral-ankle (fa) pulse wave velocity (PWV) served as measures of arterial stiffness and were measured at baseline and 6, 12, and 24 months follow-up. Platelet activity was measured as plasma beta-thromboglobulin (β-TG) at 24 months. Higher plasma β-TG was correlated with greater exposure to elevated cfPWV (p=0.02) and baPWV (p=0.04) during the preceding two years. After adjustment for serum leptin, greater exposure to elevated baPWV remained significant (p=0.03) and exposure to elevated cfPWV marginally significant (p=0.054) in predicting greater plasma β-TG. Greater arterial stiffness, particularly central arterial stiffness, predicts greater platelet activation in overweight/obese individuals. This relationship might partly explain the association between increased arterial stiffness and incident atherothrombotic events.
Keywords: platelet activation, arterial stiffness, pulse wave velocity, obesity, weight loss
INTRODUCTION
Platelets play an important role not only in thrombotic vascular events but also in the initiation and progression of atherosclerosis (1). Platelets release inflammatory molecules and growth factors that contribute to endothelial activation as well as the migration and proliferation of vascular smooth muscle cells, all of which are key processes in atherosclerosis (1). In the reverse direction, platelet activation is triggered by endothelial cell erosion and ruptured atherosclerotic plaques (1) as well as by elevated shear stress (2). Arterial stiffness is an established marker of vascular health, and stiffer central arteries promote greater wall shear and tensile stresses, speed up the fatigue of arterial wall components, and promote the vulnerability of atherosclerotic plaques throughout the vasculature (3). Thus, it stands to reason that greater arterial stiffness may also promote a prothrombotic phenotype, including greater platelet activation, throughout the arterial tree. By measuring pulse wave velocity (PWV), arterial stiffness can be estimated in any region of the arterial tree (3). Previous small cross-sectional studies of apparently healthy adults observed associations between increased platelet activation and increased carotid-femoral pulse wave velocity (cfPWV) (4,5), a measure of aortic stiffness and independent predictor of cardiovascular events (6). Other studies of apparently healthy adults have found associations between several markers of in vivo platelet activation and brachial-ankle pulse wave velocity (baPWV), a measure of mixed central (aortic) and peripheral arterial stiffness (4,7). Such cross-sectional associations may be explained by either the influence of activated platelets on the vasculature or the effect of vascular damage and dysfunction on circulating platelets.
Overweight and obese individuals have increased arterial stiffness (8) as well as greater platelet reactivity as measured by agonist induced platelet aggregation (9) and greater circulating platelet activity as measured by urinary 11-dehyhdro-TxB2 (10), plasma sCD40L (11), soluble P-selectin levels (12), or mean platelet volume (MPV) (13). Several obesity related factors have been associated with increased circulating platelet activity, including elevated waist-hip ratio, body mass index (BMI), and circulating leptin, insulin, and C-reactive protein (CRP) (10). In addition, increased oxidative stress and endothelial dysfunction promote the activation of platelets in obese individuals (14).
Though obesity is linked to both increased platelet activation and increased arterial stiffness, no studies have examined the temporality of this relationship. The aim of this study was to determine the prospective associations between exposure to greater arterial stiffness, measured three times during the course of a one year lifestyle intervention and again one year post-intervention, and circulating platelet activity, as measured by plasma β-thromboglobulin (β-TG) (15,16) at the final study time point, in overweight and obese young adults. We hypothesized that, compared to baPWV and femoral-ankle (fa) PWV, cfPWV would be more strongly associated with platelet activity, due to the influence of aortic stiffness on wall stress, blood flow patterns, and atherosclerotic progression throughout the arterial tree (3). We also hypothesized that the association between exposure to higher cfPWV over time and higher platelet activity would be independent of the effects of exposure to other cardiovascular and metabolic risk factors during the two year study.
MATERIALS AND METHODS
Study Population
The Slow Adverse Vascular Effects of excess weight study (SAVE) is a randomized-controlled trial (NCT00366990) evaluating the effects of weight loss, increased physical activity, and reduced dietary sodium intake on vascular health. Participants were recruited from June 2007 through May 2009 using mass mailing.
Eligible participants were men and women 20–45 years of age who were overweight or obese (body mass index (BMI) 25–39.9 kg/m2) and physically inactive (<8 months of consistent physical activity (PA) during the past 12 months). Exclusions included 1) diabetes, 2) hypertension or average screening blood pressure ≥140/90 mmHg, 3) cholesterol lowering, anti-psychotic, or vasoactive medication use and 4) current pregnancy or lactation.
Participants who provided a blood sample for the measurement of β-TG at the final study visit were included in this analysis (n=92). Because this substudy was begun more than two years after the start of the parent clinical trial and because many subjects were excluded from this substudy due to their recent use of aspirin or NSAIDs, the sample size of this study was substantially smaller than the total number of trial participants (n=349). All subjects signed informed consent, and the study was approved by the institutional review board of the University of Pittsburgh (Pittsburgh, PA).
Intervention
Three hundred and forty-nine participants received a 1-year lifestyle intervention consisting of diet and physical activity (PA). Participants were randomized to either 1) diet and PA alone (Control Na/lifestyle) or to 2) diet and PA plus reduced sodium intake (Low Na/lifestyle). The lifestyle intervention was delivered in group sessions that occurred weekly for months 1–4, biweekly for months 5–8, and monthly for months 9–12. The goal of the intervention was a 10% reduction in body weight over 6 months and continued maintenance of weight loss thereafter. The additional goal of the sodium reduction intervention (Low Na) was to gradually reduce daily sodium intake to approximately 1 mg Na+/1 kcal/day, an average reduction of about 50% from the participant’s usual diet (17).
Clinic Visits
Participants were to complete clinic visits at screening, baseline, and 6, 12, and 24 months following randomization. Self-reported demographic information, self- and interviewer-administered questionnaires, anthropometric measurements, fasting blood draw, 24-hour urine collection, and non-invasive tests of vascular structure and function were collected at these visits.
Demographic and Physical Measures
Age, race, and smoking status were self-reported. For this analysis, race was recoded as black vs. non-black. Ethnicity was coded as Hispanic or Non-Hispanic. Smoking status was assessed as ever vs. never. Weight was measured in kilograms using a balance scale. Height was measured in centimeters using a stadiometer. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured against the participant’s skin at the narrowest part of the torso between the ribs and the iliac crest. Blood Pressure (BP) was measured with a mercury sphygmomanometer after participants sat quietly for 5 minutes with feet flat on the floor. Final BP was the average of the last 2 of 3 readings taken 30 seconds apart.
Blood and Urine Assays
Blood analytes were measured at the Heinz Laboratory at the University of Pittsburgh’s Graduate School of Public Health using standard methods as previously described (18,19). Briefly, blood specimens were obtained between 0700 and 1130 h from upright subjects after a fasting period of at least 9 hours. The intra- and inter-assay CV% for insulin were 4.8% and 10.5% respectively. The CV% for the other assays were all <3%.
Twenty-four hour urine collections were performed within 2 weeks of the clinic visits at which all other measurements were determined. Collections considered valid had volume between 500 and 4000 mL, duration ≥22 and ≤26 hours, and creatinine within the expected range (20). Sodium, potassium, and creatinine were determined as previously described (18).
Platelet Activity
Circulating platelet activity was measured as plasma β-TG, a platelet-specific alpha granule protein released upon activation (15). Participants were eligible to provide a blood sample for the measurement of plasma β-TG if they had not taken aspirin in the preceding 14 days or any NSAID, antiplatelet, or anticoagulant medication in the preceding 10 days. At the 24 month visit, blood for the measurement of β-TG was drawn into a 4.5 mL vacutainer tube (Becton-Dickinson, Franklin Lakes, NJ) containing an anticoagulant/antiplatelet mixture of citric acid, theophylline, adenosine, and dipyridamole (Thermo Fischer Scientific, Pittsburgh, PA). The tube was chilled on ice for 15–60 minutes then centrifuged at 2000g for 30 minutes at 4°C, after which platelet-poor plasma was obtained from the upper portion of the supernatant and kept frozen at −70°C until assayed. Plasma β-TG was determined using an enzyme linked immunosorbent assay (Asserachrom, Diagnostica Stago, Parsippany, NJ). The intra- and inter-assay CV% were 3.8% and 13.2% respectively. Normal levels of plasma β-TG, according to the test instructions, are typically below 50 IU/mL.
Pulse Wave Velocity
Pulse wave velocity measures were generated using the VP2000 system (Omron Health Care Co., Kyoto, Japan), a noninvasive automated waveform analyzer. Aortic stiffness was assessed by cfPWV and peripheral arterial stiffness by faPWV; baPWV served as a mixture of central and peripheral arterial stiffness. Following ten minutes of rest in a supine position, the participant had occlusion and monitoring cuffs placed around both arms and ankles, ECG electrodes on both wrists and a phonocardiogram on the left edge of the sternum. Occlusion cuffs at the brachial and tibial arteries were connected to pressure sensors that measured blood pressure and pressure waveforms at these peripheral sites as previously described (21). Handheld tonometers were used to simultaneously obtain femoral and carotid pulse waveforms. PWV (in cm/sec) was calculated as the path length between arterial sites of interest divided by the time delay between the foot of the respective waveforms. For cfPWV path length, the distance between the carotid and femoral sites was measured (in cm) over the surface of the body with a tape measure. The path lengths for baPWV and faPWV were calculated using height-based formulas (21). For baPWV and faPWV, results for the right and left legs were averaged. For all PWV measures, data were collected twice for each participant, and the values were averaged. Participants with valid PWV measures (defined as between 300 m/s and 2500 m/s) were included in analyses. Intraclass correlation coefficients (ICC) for within technologist replicate measures were 0.76 (cfPWV), 0.97 (baPWV), and 0.96 (faPWV), and for between technologists replicates were 0.60 (cfPWV), 0.87 (baPWV), and 0.87(faPWV).
Statistical Methods
Descriptive statistics were calculated to summarize study variables at baseline and 6, 12, and 24 months follow-up and were presented as median/inter-quartile range (IQR) or mean (SD) for continuous variables and frequency and percentages for categorical variables. Whether the changes in body size, cardiovascular and metabolic risk factors, and PWV measures were statistically significantly different from zero at each follow-up visit was determined by testing the coefficient for time, as a nominal variable, in a linear mixed model with unstructured covariance. Non-normally distributed variables were transformed as necessary prior to modeling. Intervention arm was included as a covariate in every model for consistency with trial design. An interaction between intervention arm and time since baseline was used to test whether changes over time in study variables differed by intervention arm.
Similar descriptive statistics were presented for the area under the curve (AUC) of the four serial measurements of each factor of interest, and this served as a measure of average risk factor burden over the two year study period. To calculate the AUC for each risk factor for every study participant, linear mixed models (growth curves) with the serial risk factor measurements as the dependent variable were used. For these models, the intercept, linear time since baseline, and quadratic time since baseline effects had both fixed and random components. Higher-order random and fixed effects were not kept in the model if not significant at p<0.10. Intervention arm was again included as a covariate in each model for consistency with trial design. Time since baseline was centered at its mean value to minimize collinearity. The AUC was then calculated by integrating the individual’s estimated growth curve over his/her total follow-up period (22). Finally, this value was divided by the individual’s total follow-up time, since there was subtantial variability in the timing of the 24 months visit in order to maximize attendance.
Next, associations between β-TG and either risk factors at 24 months or average risk factor exposures over the course of the study were tested by examining Pearson correlation coefficients. Risk factors of interest were cardiometabolic and vascular factors known to be associated with CVD risk and included BMI, waist circumference, mean arterial pressure (MAP), pulse pressure (PP), LDL-C, HDL-C, triglycerides, insulin, HOMA-IR, CRP, leptin, adiponectin, aldosterone, 24-hr urinary sodium excretion, heart rate, and each PWV measure (cfPWV, baPWV, and faPWV). The available sample size provided 80% power to detect correlations of r=0.29. For each of those AUC PWV measures that showed statistically significant correlations with β-TG, separate multiple linear regression models for β-TG were examined. After centering covariates to reduce collinearity, stepwise selection of covariates (other than PWV) was used with entry and removal p-values of 0.15 and 0.10 respectively. Covariates considered for inclusion included all measures of average risk factor exposures that had shown statistically significant correlations with β-TG at p<0.10. In all of these analyses, non-normally distributed variables were transformed as necessary. To evaluate whether change in body weight explained the associations between PWV and β-TG, percent change in body weight during the two year study was added to the final multivariable models. Values of p<0.05 were considered statistically significant. Statistical analyses were performed using the statistical package SAS (Statistical Analysis Software release 9.3, Cary, NC, USA).
RESULTS
Plasma β-TG was measured in 92 individuals at the 24 month visit of the parent clinical trial. The sample had an average age of 40.2 years (SD 5.9) and consisted of 60 women, 13 African-Americans, and 8 current and 20 past smokers. Clinical characteristics of this sample over the course of the two year study are shown in Table 1. With the exception of MAP, which was slightly lower among individuals in whom platelet activity was measured, characteristics were similar between trial participants with and without platelet activity data. Median plasma β-TG was 25.8 IU/mL (IQR 18.6, 35.9), which was within the normal range and did not differ by race, sex, age, or smoking status (p>0.20 for all).
Table 1.
Clinical characteristics over the course of the study
| Characteristic | Baseline (N=92) |
6 Months (N=90) |
12 Months (N=90) |
24 Months (N=92) |
AUC (N=92) |
|---|---|---|---|---|---|
| BMI (kg/m2) | 32.4 (3.6) | 29.7 (4.0)* | 29.9 (4.2)* | 31.1 (4.2)* | 30.3 (3.7) |
| Waist Circumference (cm) | 101.6 (11.0) | 95.0 (11.7)* | 96.0 (12.2)* | 98.1 (12.6)* | 96.4 (10.6) |
| Mean Arterial Pressure (mmHg) | 86.4 (7.6) | 83.3 (7.2)* | 84.1 (7.6)* | 85.7 (7.8) | 84.6 (5.4) |
| Pulse Pressure (mm Hg) | 40.0 (7.6) | 39.3 (7.6) | 37.0 (7.1)* | 37.8 (7.5)* | 38.6 (4.8) |
| Glucose (mg/dL) | 98.8 (9.2) | 98.2 (9.2) | 98.9 (8.8) | 98.1 (10.4) | 98.5 (6.1) |
| Insulin (µU/mL)† | 12.5 (10.1, 16.2) | 11.3 (9.1, 14.9)* | 12.0 (9.3, 16.0) | 12.1 (9.6, 16.2) | 11.4 (9.7, 14.3) |
| HOMA-IR (mmol/L × µU/mL)† | 3.0 (2.5, 4.2) | 2.8 (2.2, 3.6)* | 2.9 (2.2, 4.0) | 3.1 (2.2, 4.0) | 2.8 (2.4, 3.5) |
| LDL-C (mg/dL) | 124.9 (34.3) | 120.6 (33.4) | 124.5 (33.5) | 123.0 (33.7) | 123.0 (29.2) |
| HDL-C (mg/dL) | 51.3 (14.6) | 52.5 (13.8) | 54.3 (13.8)* | 54.0 (14.2)* | 53.4 (13.1) |
| Triglycerides (mg/dL)† | 110.5 (74, 189) | 89 (64, 138)* | 99.5 (73, 135)* | 98 (77, 146)* | 94.9 (73.0, 137.9) |
| CRP (mg/L)† | 2.5 (1.3, 5.2) | 1.9 (0.85, 3.8)* | 2.0 (0.80, 3.7)* | 2.5 (0.83, 4.5)* | 1.8 (0.76, 2.7) |
| Leptin (ng/mL) | 22.7 (13.0) | 15.0 (10.6)* | 19.1 (14.2)* | 20.7 (13.3)* | 17.7 (10.1) |
| Adiponectin (µg/mL) | 11.2 (5.9) | 11.7 (5.9) | 11.2 (5.7) | 9.6 (5.5)* | 11.0 (5.2) |
| Aldosterone (pg/mL)† | 97.1 (78.5, 126.5) | 107 (79.3, 147) | 102 (83.9, 140) | 108.5 (88, 160)* | 108.0 (97.4, 131.8) |
| Sodium Excretion (mmol/24hr)# | 187.3 (64.3) | 156.2 (66.3)* | 154.0 (61.2)* | 154.0 (61.6)* | 157.4 (27.3) |
| Heart Rate (beats/min) | 62.7 (8.2) | 60.7 (7.5)* | 62.6 (8.8) | 61.9 (8.3) | 62.0 (5.2) |
| Carotid-femoral PWV (cm/s)† | 817 (700, 934.5) | 767.5 (683, 907.5)* | 785.5 (680.3, 907.5)* | 787.3 (690.3, 903) | 774.6 (717.0, 875.0) |
| Brachial-ankle PWV (cm/s) | 1218.7 (128.4) | 1196.5 (140.8) | 1201.2 (130.9) | 1205.3 (130.5) | 1207.9 (108.2) |
| Femoral-ankle PWV (cm/s) | 946.1 (102.9) | 945.0 (112.9) | 939.8 (105.5) | 941.0 (100.6) | 945.3 (78.0) |
Mean (SD) or median (interquartile range (IQR)) are shown.
P<0.05 versus baseline in a linear mixed model with time since baseline as a nominal variable and with adjustment for intervention arm. LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, HOMA-IR= homeostasis model assessment of insulin resistance, CRP=C-reactive protein, PWV=Pulse Wave Velocity, AUC=area under the curve divided by total follow-up time in years.
Log transformed for mixed modeling and AUC calculation; median and IQR AUC values shown were back transformed.
Baseline N=71, 6 Months N=68, 12 Months N=68, 24 Months N=60.
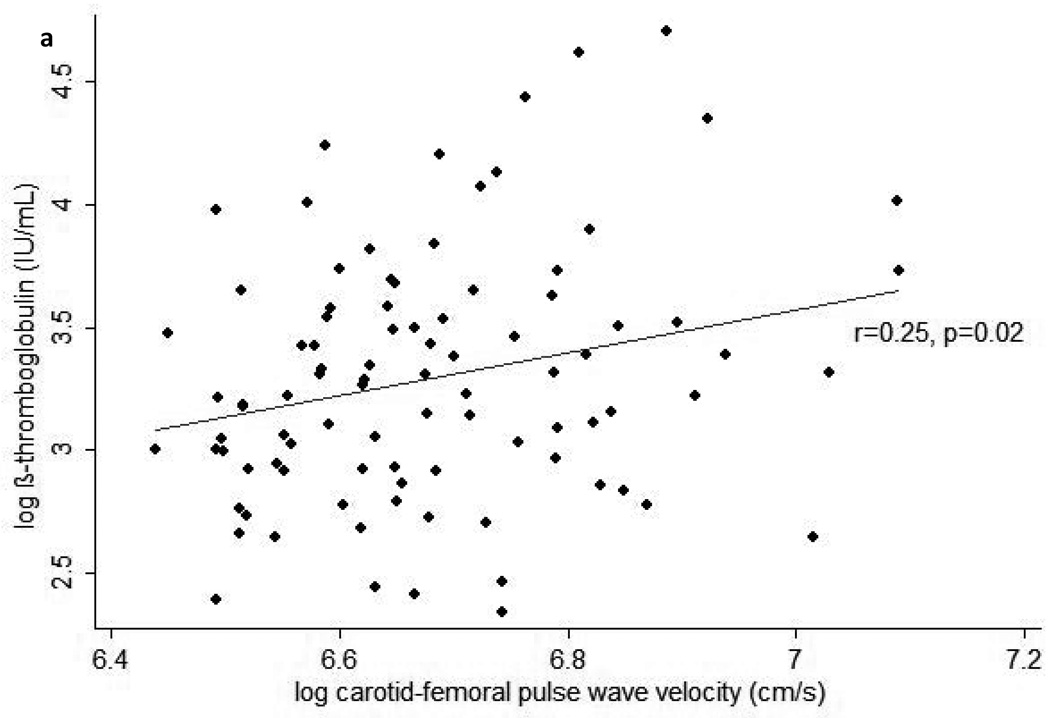
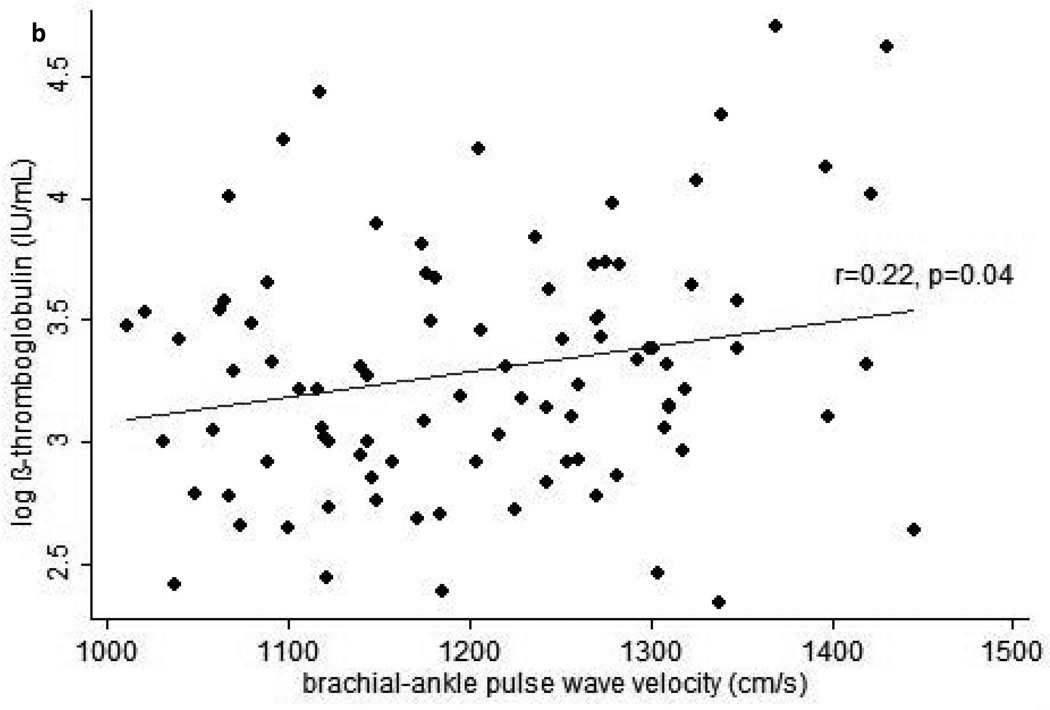
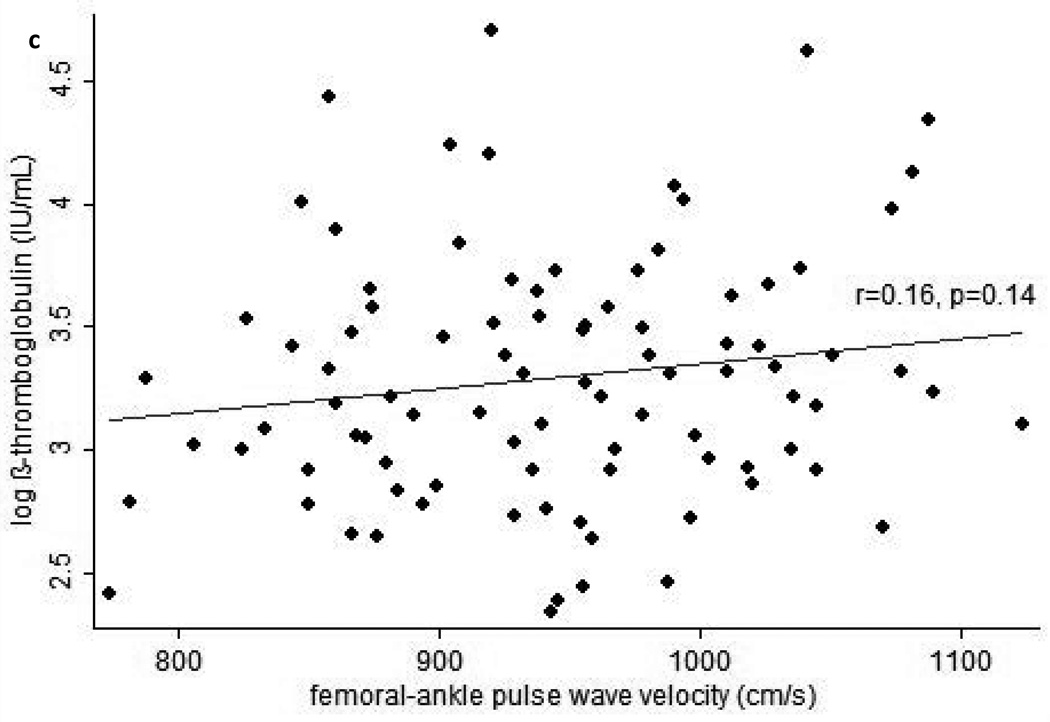
In cross-sectional analyses, higher plasma β-TG was statistically significantly correlated with higher BMI (r=0.25, p=0.02), leptin (r=0.21, p=0.049), and baPWV (r=0.22, p=0.04), and marginally significantly correlated with higher cfPWV (r=0.19, p=0.07) and faPWV (r=0.20, p=0.06). Similarly, higher plasma β-TG was correlated with higher BMI and leptin when these factors were assessed as AUC from baseline to the 24 month study visit (Table 2). In addition, higher plasma β-TG was statistically significantly correlated with greater cfPWV and baPWV when the latter were assessed as AUC (Figure 1). When interactions between each independent variable and treatment arm in the parent clinical trial were examined, none were found to be significant in any model for plasma β-TG.
Table 2.
Pearson correlations between β-thromboglobulin at 24-months follow-up and average exposures to cardiometabolic and vascular risk factors over the preceding two years
| Variable | r | P value |
|---|---|---|
| BMI | 0.28 | 0.007 |
| Waist Circumference | 0.07 | 0.48 |
| Mean Arterial Pressure | 0.05 | 0.63 |
| Pulse Pressure | 0.14 | 0.17 |
| LDL-C | 0.07 | 0.52 |
| HDL-C | 0.06 | 0.58 |
| Triglycerides | 0.09 | 0.39 |
| Insulin | 0.14 | 0.17 |
| HOMA-IR | 0.15 | 0.16 |
| CRP | 0.19 | 0.08 |
| Leptin | 0.25 | 0.01 |
| Adiponectin | −0.07 | 0.48 |
| Heart Rate | −0.04 | 0.71 |
| 24-hr Sodium Excretion | 0.02 | 0.83 |
Plasma β-TG, triglycerides, insulin, HOMA-IR, and CRP were log transformed. BMI=body mass index; SBP=systolic blood pressure; DBP=diastolic blood pressure; LDL-C=low density lipoprotein cholesterol; HDL-C=high density lipoprotein cholesterol; CRP=C-reactive protein. N=92.
Figure 1.
Scatterplots of average pulse wave velocity (PWV) over 24-months vs. beta-thromboglobulin at the end of 24-months follow-up for (a) carotid-femoral PWV, (b) brachial-ankle PWV, and (c) femoral-ankle PWV. Average PWV values were calculated by integrating each subject’s estimated growth curve (derived from linear mixed models for the serial PWV measurements) over his or her total follow-up period, then dividing this value by the subject’s total follow-up time.
In multiple linear regression models derived from the stepwise selection of AUC exposures showing correlations with plasma β-TG at p<0.10, greater average exposures to elevated baPWV and leptin over the two year study were significant predictors and greater average exposure to elevated cfPWV during the two year study was a marginally significant predictor of greater plasma β-TG at the end of the study (Table 3). Adding BMI AUC to these models resulted in no predictor being statistically significant in either model for plasma β-TG, but in these models cfPWV AUC (p=0.15) and baPWV AUC (p=0.10) did come the closest to achieving statistical significance of all included predictors. Adding instead the percentage change in body weight at 24 months resulted in no predictor achieving statistical significance in either model for plasma β-TG, but in these models cfPWV AUC (p=0.09) and baPWV AUC (p=0.05) were marginally significant. When percentage body weight reduction during the two year study was evaluated alone, greater weight loss was significantly associated with lower log β-TG at the end of the study (β(SE)=1.57(0.75), p=0.03), but this association was not maintained (p>0.30) after adjusting for leptin AUC and either cfPWV AUC or baPWV AUC.
Table 3.
Multiple linear regression models for log β-thromboglobulin: associations with average pulse wave velocity exposures during the 24-month study
| Variable | Parameter Estimate (SE) |
P value |
|---|---|---|
|
Model including Carotid-femoral PWV | ||
| Carotid-femoral PWV (cm/s) | 0.71 (0.36) | 0.054 |
| Leptin (ng/mL) | 0.011 (0.005) | 0.04 |
|
Model including Brachial-ankle PWV | ||
| Brachial-ankle PWV (cm/s) | 0.0010 (0.0005) | 0.03 |
| Leptin (ng/mL) | 0.013 (0.005) | 0.01 |
All independent variables are average risk factor exposures over the course of the two year study (area under the curve divided by follow-up time for each subject). β-thromboglobulin and carotid-femoral PWV were log transformed. PWV=Pulse Wave Velocity. The variables considered for inclusion but removed by the stepwise procedure were plasma C-reactive protein and body mass index.
DISCUSSION
The main finding of this study was that, in overweight and obese but otherwise healthy young adults, greater circulating platelet activity, as measured by plasma β-TG, was predicted by greater exposure to stiffer arteries during the preceding two years. However, greater exposure to excess weight and serum leptin over the same time period, when examined together, removed the statistical significance of this association. Our findings suggest that elevated arterial stiffness, particularly central arterial stiffness, might be one of the mechanisms by which platelet activation is increased in overweight and obese individuals. Because of the key roles that platelets play in thrombotic events and the initiation and progression of atherosclerosis (1), these findings might also partly explain the association between arterial stiffness and incident cardiovascular events (6).
Arterial stiffness is an established marker of vascular health, and stiffer central arteries promote greater wall shear and tensile stresses, speed up the fatigue of arterial wall components, and promote endothelial damage and atherosclerotic plaque vulnerability throughout the arterial tree (3). The present findings agree with those from at least three cross-sectional studies of individuals at low to moderate CVD risk, in which associations were found between several markers of in vivo platelet activation and both cfPWV(5) and baPWV(4,7). Though the present observational study cannot establish that arterial stiffening causes increased platelet activation, there are several potential mechanisms that may explain this prospective association. First, platelet activation is triggered by endothelial damage (1), and several studies have found evidence of endothelial dysfunction, as measured by flow-mediated dilation (FMD), in individuals with elevated arterial stiffness, as measured by aortic PWV (23), cfPWV (23) or baPWV (24). Circulating levels of endothelial microparticles, which are released upon endothelial cell activation and closely related to reduced endothelial structural and functional integrity, are positively associated with baPWV in type 2 diabetics and healthy adults (25). Second, shear stress plays an important role in platelet activation and is influenced by arterial stiffness. Pathologically high shear stress activates platelets and is an important driver of thrombus formation in occluded vessels (2). However, even in individuals with minimal atherosclerosis, as central arteries stiffen blood flow velocity increases, thereby increasing shear stress and creating a steep systolic pressure waveform that enhances the pulsatility of shear stresses in peripheral vessels (3). Such oscillatory shear stress can induce a prothrombotic, prooxidant, and proinflammatory state in vascular endothelial cells, particularly in less compliant vessels. In addition, flow reversal may occur during diastole in peripheral vessels as the central arteries stiffen, which may trigger pathological changes in the endothelium (26). Finally, cyclic strain of the vascular wall induces endothelial cell expression of adhesion molecules and vascular smooth muscle cell migration and proliferation (27). Altogether, these changes promote platelet activation and atherosclerosis (1). It is therefore evident that the close relationship between arterial stiffness, shear stress, and endothelial damage might explain the association between greater arterial stiffness and greater circulating platelet activity.
Importantly, however, in this study the associations between plasma β-TG and both cfPWV and baPWV appeared to be explained by excess weight and serum leptin, when considered together. Several studies have shown positive associations between platelet activation and excess body weight (10,11,13) or reduced platelet activation with weight loss (10,11). One possible mechanism for these relationships is that obese individuals have larger platelets than normal weight individuals (13). These larger platelets have greater metabolic and enzymatic activity as well as thrombotic potential (28). In addition, platelets exhibit membrane receptors for both insulin and leptin, and insulin resistance and elevated circulating leptin promote platelet activation and aggregation (9,14). The correlation in this study between plasma β-TG and serum leptin, measured either concurrently or as the average exposure over the previous two years, suggests that leptin may play a role in platelet activation in overweight and obese adults.
The lack of correlation between plasma β-TG and either MAP or PP in this study was somewhat surprising in light of the statistically significant correlations detected between β-TG and measures of arterial stiffness. Cross-sectional studies in obese and normal weight adults have found significant correlations between markers of in vivo platelet activation and both SBP and DBP (12,29). One cross-sectional study of hypertensives also reported a correlation between pulse pressure and mean platelet volume (30). Furthermore, platelet activity is higher in hypertensives than normotensives (31). It may be that the inclusion of only normotensives prevented the present study from detecting associations between plasma β-TG and blood pressure. Additionally, PP was measured at the brachial artery. Central PP is more closely associated with aortic stiffness and increases more with age and other cardiovascular risk factors (32). Furthermore, central PP has a marginally better predictive ability for incident vascular events and is more closely associated with end-organ damage than brachial PP (32,33). Thus, had central PP been measured, it may have shown a correlation with platelet activity similar to that of cfPWV.
There were several important limitations to this study. First, the small sample size limited the statistical power to detect associations of interest. Second, despite the prospective design of this study, reverse causation could exist due to the tracking of plasma β-TG levels over time. It is possible that activated platelets may influence arterial stiffness, perhaps through their release of vascular smooth muscle cell growth factors and extracellular matrix modulators (34). Third, it would have been informative to evaluate several measures of platelet activity. The measurement of other platelet specific proteins in plasma or urine or the detection of platelet surface proteins using flow cytometry might have improved the accuracy of platelet activity assessment. However, plasma β-TG is a more sensitive marker of circulating platelet activity than flow cytometric measures, though it requires very careful sample collection to avoid ex vivo artifacts (16). We attempted to minimize ex vivo activation by avoiding trauma during blood draws, drawing blood samples used for β-TG measurement as the last of three samples, and keeping the samples on ice prior to centrifugation. A notable strength of this study is that all participants were normotensive and not on antihypertensive, lipid lowering, or vasoactive medications, which enabled us to evaluate associations of interest independent of potentially confounding treatment effects.
In conclusion, in overweight and obese but otherwise healthy young adults, greater exposure to arterial stiffness over a two year period is a predictor of greater circulating platelet activity, as measured by plasma β-TG. Greater exposure to excess weight and serum leptin are also associated with greater platelet activity. These findings suggest that elevated arterial stiffness, particularly central arterial stiffness, might be one of the mechanisms by which platelet activation is increased in overweight and obese individuals. Future studies of lifestyle modification and other arterial “de-stiffening” strategies in overweight and obese adults should examine whether sustained reductions in arterial stiffness can reduce the risk of thrombotic events.
ACKNOWLEDGEMENTS
This work was supported by grants R01 HL077525 and F31 HL106986 from the National Heart, Lung, and Blood Institute of the National Institutes of Health. We thank Beth Hauth (Department of Epidemiology, University of Pittsburgh) for expert assistance with the laboratory analyses. We greatly appreciate the efforts of the SAVE trial volunteers and the study and clinic coordinators (Laura Kinzel and Eileen Cole, Department of Epidemiology, University of Pittsburgh).
Footnotes
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References
- 1.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102(2):248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–3464. [PubMed] [Google Scholar]
- 3.Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci (Lond) 2007;113(4):157–170. doi: 10.1042/CS20070080. [DOI] [PubMed] [Google Scholar]
- 4.Yamasaki F, Furuno T, Sato K, Zhang D, Nishinaga M, Sato T, Doi Y, Sugiura T. Association between arterial stiffness and platelet activation. J Hum Hypertens. 2005;19(7):527–533. doi: 10.1038/sj.jhh.1001861. [DOI] [PubMed] [Google Scholar]
- 5.Dotsenko O, Chaturvedi N, Thom SA, Wright AR, Mayet J, Shore A, Schalkwijk C, Hughes AD. Platelet and leukocyte activation, atherosclerosis and inflammation in European and South Asian men. J Thromb Haemost. 2007;5(10):2036–2042. doi: 10.1111/j.1538-7836.2007.02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Wang RT, Li Y, Zhu XY, Zhang YN. Increased mean platelet volume is associated with arterial stiffness. Platelets. 2011 doi: 10.3109/09537104.2011.565431. [DOI] [PubMed] [Google Scholar]
- 8.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42(4):468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 9.Dellas C, Schafer K, Rohm I, Lankeit M, Ellrott T, Faustin V, Riggert J, Hasenfuss G, Konstantinides S. Absence of leptin resistance in platelets from morbidly obese individuals may contribute to the increased thrombosis risk in obesity. Thromb Haemost. 2008;100(6):1123–1129. [PubMed] [Google Scholar]
- 10.Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 11.Desideri G, Ferri C. Effects of obesity and weight loss on soluble CD40L levels. JAMA. 2003;289(14):1781–1782. doi: 10.1001/jama.289.14.1781. [DOI] [PubMed] [Google Scholar]
- 12.De Pergola G, Pannacciulli N, Coviello M, Scarangella A, Di Roma P, Caringella M, Venneri MT, Quaranta M, Giorgino R. sP-selectin plasma levels in obesity: association with insulin resistance and related metabolic and prothrombotic factors. Nutr Metab Cardiovasc Dis. 2008;18(3):227–232. doi: 10.1016/j.numecd.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59(8):981–982. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 14.Santilli F, Vazzana N, Liani R, Guagnano MT, Davi G. Platelet activation in obesity and metabolic syndrome. Obes Rev. 2012;13(1):27–42. doi: 10.1111/j.1467-789X.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan KL, Owen J. Plasma levels of beta-thromboglobulin and platelet factor 4 as indices of platelet activation in vivo. Blood. 1981;57(2):199–202. [PubMed] [Google Scholar]
- 16.Matzdorff AC, Kemkes-Matthes B, Voss R, Pralle H. Comparison of beta-thromboglobulin, flow cytometry, and platelet aggregometry to study platelet activation. Haemostasis. 1996;26(2):98–106. doi: 10.1159/000217194. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr. 2010;92(5):1172–1180. doi: 10.3945/ajcn.2010.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper JN, Tepper P, Barinas-Mitchell E, Woodard GA, Sutton-Tyrrell K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin Exp Hypertens. 2012;34(1):63–70. doi: 10.3109/10641963.2011.618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Njoroge JN, El Khoudary SR, Fried LF, Barinas-Mitchell E, Sutton-Tyrrell K. High urinary sodium is associated with increased carotid intima-media thickness in normotensive overweight and obese adults. Am J Hypertens. 2011;24(1):70–76. doi: 10.1038/ajh.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis D, Lloyd C, Becker DJ, Forrest KYZ, Orchard TJ. The changing course of diabetic nephropathy: Low-density lipoprotein cholesterol and blood pressure correlate with regression of proteinuria. Am J Kidney Dis. 1996;27:809–818. doi: 10.1016/s0272-6386(96)90518-1. [DOI] [PubMed] [Google Scholar]
- 21.Kimoto E, Shoji T, Shinohara K, Hatsuda S, Mori K, Fukumoto S, Koyama H, Emoto M, Okuno Y, Nishizawa Y. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol. 2006;17(8):2245–2252. doi: 10.1681/ASN.2005101038. [DOI] [PubMed] [Google Scholar]
- 22.Shetty V, Morrell CH, Najjar SS. Modeling a Cross-Sectional Response Variable with Longitudinal Predictors: An Example of Pulse Pressure and Pulse Wave Velocity. J Appl Stat. 2009;36(6):611–619. doi: 10.1080/02664760802478208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51(6):1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Akishita M, Yu W, Hashimoto M, Ohni M, Toba K. Interrelationship between non-invasive measurements of atherosclerosis: flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis. 2004;173(1):13–18. doi: 10.1016/j.atherosclerosis.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Feng D, Lindpaintner K, Larson MG, O'Donnell CJ, Lipinska I, Sutherland PA, Mittleman M, Muller JE, D'Agostino RB, Levy D, Tofler GH. Platelet glycoprotein IIIa Pl(a) polymorphism, fibrinogen, and platelet aggregability: The Framingham Heart Study. Circulation. 2001;104(2):140–144. doi: 10.1161/01.cir.104.2.140. [DOI] [PubMed] [Google Scholar]
- 26.Vita JA, Mitchell GF. Effects of shear stress and flow pulsatility on endothelial function: insights gleaned from external counterpulsation therapy. J Am Coll Cardiol. 2003;42(12):2096–2098. doi: 10.1016/j.jacc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis. 2011;218(2):263–271. doi: 10.1016/j.atherosclerosis.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Jakubowski JA, Adler B, Thompson CB, Valeri CR, Deykin D. Influence of platelet volume on the ability of prostacyclin to inhibit platelet aggregation and the release reaction. J Lab Clin Med. 1985;105(2):271–276. [PubMed] [Google Scholar]
- 29.Csongradi E, Nagy B, Jr, Fulop T, Varga Z, Karanyi Z, Magyar MT, Olah L, Papp M, Facsko A, Kappelmayer J, Paragh G, Kaplar M. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb Haemost. 2011;106(4):683–692. doi: 10.1160/TH11-01-0030. [DOI] [PubMed] [Google Scholar]
- 30.Ordu S, Ozhan H, Caglar O, Alemdar R, Basar C, Yazici M, Erden I. Mean platelet volume in patients with dipper and non-dipper hypertension. Blood Press. 2010;19(1):26–30. doi: 10.3109/08037050903416402. [DOI] [PubMed] [Google Scholar]
- 31.Blann AD, Lip GY, Islim IF, Beevers DG. Evidence of platelet activation in hypertension. J Hum Hypertens. 1997;11(9):607–609. doi: 10.1038/sj.jhh.1000505. [DOI] [PubMed] [Google Scholar]
- 32.Protogerou AD, Papaioannou TG, Blacher J, Papamichael CM, Lekakis JP, Safar ME. Central blood pressures: do we need them in the management of cardiovascular disease? Is it a feasible therapeutic target? J Hypertens. 2007;25(2):265–272. doi: 10.1097/HJH.0b013e3280114f23. [DOI] [PubMed] [Google Scholar]
- 33.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 34.Weber C. Platelets and chemokines in atherosclerosis: partners in crime. Circ Res. 2005;96(6):612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]