Abstract
There is great interest in the development of cognitive markers that differentiate “normal” age-associated cognitive change from that of Alzheimer's disease (AD) in its prodromal (i.e., mild cognitive impairment; MCI) or even preclinical stages. Dual process models posit that recognition memory is supported by the dissociable processes of recollection and familiarity. Familiarity-based memory has generally been considered to be spared during normal aging, but it remains controversial whether this type of memory is impaired in early AD. Here, we describe findings of estimates of recollection and familiarity in young adults (YA), cognitively normal older adults (CN), and patients with amnestic-MCI (a-MCI). These measures in the CN and a-MCI patients were then related to a structural imaging biomarker of AD that has previously been demonstrated to be sensitive to preclinical and prodromal AD, the Cortical Signature of AD (ADsig). Consistent with much work in the literature, recollection, but not familiarity, was impaired in CN versus YA. Replicating our prior findings, a-MCI patients displayed impairment in both familiarity and recollection. Finally, the familiarity measure was correlated with the ADsig biomarker across the CN and a-MCI group, as well as within the CN adults alone. No other standard psychometric measure was as highly associated with the ADsig, suggesting that familiarity may be a sensitive biomarker of AD-specific brain changes in preclinical and prodromal AD and that it may offer a qualitatively distinct measure of early AD memory impairment relative to normal age-associated change.
Keywords: Memory, Recollection, Familiarity, Alzheimer's disease, Medial temporal lobe, Preclinical Alzheimer's disease, Mild cognitive impairment
1. Introduction
The ‘Dual Process Model’ proposes that recognition memory may be subserved by the dissociable processes of recollection and familiarity (Brown & Aggleton, 2001; Eichenbaum, Otto, & Cohen, 1994; Jacoby, 1991; Mandler, 1980; Yonelinas, 2002). Recollection is conceived as reflecting conscious, contextual retrieval of a prior episode or event, while familiarity is described as an acontextual sense of prior exposure. Data in support of this conceptualization include lesion and functional (e.g., single unit recordings, fMRI) studies in animals and humans that have suggested that these processes are differentially represented within medial temporal lobe (MTL) structures (Bowles et al., 2007; Brown, Warburton, & Aggleton, 2010; Diana, Yonelinas, & Ranganath, 2007; Fortin, Wright, & Eichenbaum, 2004; Wolk & Dickerson, 2011; Wolk, Dunfee, Dickerson, Aizenstein, & DeKosky, 2011; Yonelinas et al. 2002). Computational models have provided additional support for such dissociations (Norman, 2010; Norman & O'Reilly, 2003). Specifically, the hippocampus has been linked to recollection while surrounding anterior extrahippocampal MTL, particularly the perirhinal (PRC) and lateral entorhinal (ERC) cortices, are thought to support familiarity (Bowles et al., 2007; Ranganath, 2010; Wolk & Dickerson, 2011; Wolk et al., 2011; Yonelinas et al., 2007). However, the dual process model and these anatomic mappings are not without controversy (Wixted, Mickes, & Squire, 2010).
Alzheimer's disease (AD) is a progressive neurodegenerative condition usually characterized by an early amnestic syndrome and associated with deposition of fibrillar amyloid-β (Aβ) plaques and neurofibrillary tangles (NFT) composed of hyperphosphorylated tau protein. While Aβ deposition may represent an antecedent event in AD pathophysiology, NFT's are more tightly correlated with synapse and neuron loss, as well as clinical severity (Arriagada, Growdon, Hedley-Whyte, & Hyman, 1992; Gomez-Isla et al., 1997). This pathologic process is thought to precede clinical diagnosis of AD by years, if not decades. Based on the notion that disease modifying medicines are likely to be most effective and desirable before significant neurodegeneration, there has been increasing interest in diagnosis at the prodromal (i.e., mild cognitive impairment, MCI) or preclinical stages of disease (Albert et al., 2011; Sperling et al., 2011). The latter stage is an emerging construct in the field and is defined on the basis of biomarker evidence of AD pathology in cognitively normal individuals.
A major challenge to the field has been the development of cognitive measures sensitive and specific to the consequences of this early pathology in the context of ‘normal’ age-associated memory decline (Buckner, 2004; Park & Reuter-Lorenz, 2009). While quantitative differences between age-associated decline and early AD become apparent as the disease progresses, it is also possible that there are qualitative differences in the nature of memory loss. Prior work has supported the notion that recollection-based memory is impaired in normal aging, but that familiarity is spared (Davidson & Glisky, 2002; Howard, Bessette-Symons, Zhang, & Hoyer, 2006; Jacoby, 1999; Jennings & Jacoby, 1997; Light, Patterson, Chung, & Healy, 2004; Parkin & Walter, 1992; Yonelinas, 2002). Interestingly, the earliest regions of NFT burden in AD are the PRC followed by ERC, prior to direct involvement of the hippocampal formation (Braak & Braak, 1991; Delacourte et al., 1999). Based on the anatomic mappings of the dual memory processes described above, this would suggest that familiarity-based memory should be particularly sensitive to these early AD pathologic changes. Thus, measures of the integrity of familiarity offer a potentially sensitive and specific measure to early AD-related NFT pathology and may be useful marker in preclinical and prodromal phases.
Consistent with this notion, we and others have reported decrements in familiarity in MCI patients relative to agematched controls (Algarabel et al., 2009; Ally, Gold, & Budson, 2009; Embree, Budson, & Ally, 2012; Wolk, Signoff, & Dekosky, 2008); however, this has not been a universal finding (Hudon, Belleville, & Gauthier, 2009; Serra et al., 2010; Westerberg et al., 2006). Here, we investigate the sensitivity of this measure relative to recollection to the neurodegeneration (i.e., brain atrophy) associated with preclinical and prodromal AD. We first verify that recollection is relatively selectively impaired in normal aging, but that both recollection and familiarity are impaired in those with likely prodromal AD (i.e., MCI). Second, we determine whether either of these memory measures is correlated with AD-related neurodegeneration along the continuum from normal aging to MCI. In particular, we test the hypothesis that the familiarity measure is sensitive to evidence of preclinical AD in the cognitively normal group. For the latter analyses we will use a structural MRI biomarker, the “cortical signature of AD” (ADsig), which we have previously demonstrated to be sensitive to early AD and, in cognitive normal adults, to the presence of the molecular pathology of AD, either based on cerebrospinal fluid measures or amyloid imaging, and the likelihood of cognitive decline and/or progression to AD (Dickerson & Wolk, 2012; Dickerson et al., 2009, 2011). The ADsig is composed of a set of nine regions of interest (ROIs) that were defined in a data driven manner based on the analysis of several datasets, rather than traditional anatomic boundaries. These regions represent the areas with the largest effect size in comparison of patients with mild AD relative to cognitively normal adults (Dickerson et al., 2009). The mean of these regions constitutes a powerful summary measure that accounts for heterogeneity in distribution of disease pathology, individual differences in premorbid structure, and noise inherent in measuring individual ROIs and, thus, is an important approach to validating the sensitivity of the experimental measures to the presence of early AD neurodegeneration. Finally, we will compare these experimental measures to standard psychometric tests in their relationship to the AD signature biomarker.
2. Materials and methods
2.1. Participants
50 cognitively normal (CN) older adults and 32 patients with amnestic-MCI (a-MCI) were recruited from the Penn Memory Center/Alzheimer's Disease Center (PMC/ADC). Patients with a-MCI were diagnosed following the criteria described by Petersen (2004). All patients underwent extensive evaluation by a neurologist, geriatric psychiatrist, or gerontologist including physical and neurological exam, history from both patient and informant, and psychometric testing as described by the National Alzheimer's Coordinating Center (NACC) Uniform Dataset (UDS) (Davidson, Anaki, Saint-Cyr, Chow, & Moscovitch, 2006). Diagnoses were determined by a consensus conference whose members included experienced clinicians at the PMC/ADC. While formal cutoffs of psychometric testing were not applied, generally, patients were greater than 1.5 standard deviations below the norm on at least one standard memory measure to qualify for a-MCI status. Inclusion criteria included age between 55 and 85, >7 years education, and English speaking at an early age. Participants were excluded if they had a history of clinical stroke, traumatic brain injury, alcohol or drug abuse/dependence, prior electroconvulsive therapy, and any significant disease or medical/psychiatric condition that was felt to impact neuropsychological performance.
An additional 25 CN adults and 21 a-MCI patients recruited from the University of Pittsburgh Alzheimer's Disease Research Center (ADRC) and evaluated in a highly similar manner to the University of Pennsylvania cohort were included for behavioral analysis. Most of these patients were participants in a prior study using the same memory paradigm (Wolk et al., 2008). Finally, 18 young adults (YA) were recruited through advertisements on the University of Pennsylvania campus (data from 1 YA was not included due to very poor performance). The study was approved by the Institutional Review Board of the University of Pennsylvania.
2.2. Standard and experimental cognitive measures
The following standard psychometric measures were obtained and analyzed for the purpose of the present study: (1) Mini-Mental Status Exam [MMSE; (Folstein, Folstein, & McHugh, 1975)]; (2) consortium to establish a registry for Alzheimer's disease (CERAD) word list memory test (Morris et al. 1989); a 10-word memory test that includes 3 immediate memory trials, a delayed recall trial, and recognition memory; (3) category fluency [animals; (Spreen & Strauss, 1998)] (4) trail making test A and B (Reitan, 1958); (5) a 30-item version of the Boston naming test [BNT; (Kaplan, Goodglass, & Weintraub, 1983)]; and (6) digit span subtest of the Wechsler Adult Intelligence Scale III (Wechsler, 1997).
2.3. Experimental paradigm
All participants completed an experimental measure to estimate recollection and familiarity. This task is a variation of the process dissociation procedure and has been described in detail previously (Wolk et al., 2008). Briefly, at study, unrelated word pairs were presented and participants were asked to form a mental image of the referents of the two words and decide which is larger in size in a self-paced manner. At test they were shown intact pairs, rearranged pairs in which each word was previously studied but with a different associate, and novel pairs in which neither word was previously studied. They were instructed to make an “Old/New” decision, but to only endorse intact pairs as “Old.” The rearranged pairs produce a condition in which recollection opposed familiarity. As each word of the rearranged pair had been studied previously, these items would be associated with familiarity, driving the subject to incorrectly endorse the pair as “Old.” However, the contextual retrieval of recollection would allow the subject to recall that the words had a different associate at study and correctly endorse the pair as “New.”
Based on the rate of “Old” endorsements for these classes of items, estimates of recollection (R) and familiarity (F) can be calculated based on the following: R = probability(intact)–probability(rearranged); F = probability(rearranged)/(1-R). To account for differences in base rates of false alarms (“old” responses to novel pairs), familiarity was calculated using a measure of discrimination (d′) derived from signal detection theory (Davidson et al., 2006; Yonelinas, Regehr, & Jacoby, 1995).
2.4. MRI imaging and analysis
MRI scans were acquired on a 3T Siemens Trio™ scanner at the Hospital of the University of Pennsylvania using an 8-channel array coil in a subset of the above patients (34 CN, 29 MCI). For the cortical thickness analysis, the following sequence was acquired: a T1-weighted gradient echo MRI (MPRAGE) (TR/TE/TI = 1600/3.87/950 ms, 15° flip angle, 1.0 × 1.0 × 1.0 mm3 resolution, acquisition time 5:13 min). One CN participant's scan was discarded due to poor image quality.
T1 image volumes were examined quantitatively by a cortical surface-based reconstruction and analysis of cortical thickness. The general procedures for this processing method have been described in detail and applied and validated in a number of publications, and the technical details can be found in these manuscripts (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl et al. 2002; Fischl, Sereno, & Dale, 1999; Fischl, Sereno, Tootell, & Dale, 1999; Fischl et al. 2004). The “cortical signature of AD” is derived from a set of regions defined in a data driven manner based on analysis of several mild AD datasets (Dickerson et al., 2009), as opposed to being determined strictly by anatomic boundaries and include portions of the following regions: rostral medial temporal cortex, rostral inferior temporal gyrus, temporal pole/anterior temporal lobe, angular gyrus, supramarginal gyrus, superior parietal lobule, precuneus, superior frontal gyrus, and inferior frontal sulcus/caudal middle frontal gyrus. The mean thickness of these bilateral regions constitutes the “cortical signature of AD” biomarker (ADsig) that has previously demonstrated sensitivity to preclinical AD based on molecular measures for the presence of beta-amyloid (Aß) plaques, a hallmark pathologic feature of AD, or eventual development of clinical AD (Dickerson & Wolk, 2012; Dickerson et al., 2009, 2011).
2.5. Statistical analysis
Statistical analyses were performed in a standard fashion using SPSS 20.0 (Chicago, IL). Group differences in demographic data were determined by χ2 (for frequencies) and 2-sample t-tests. Comparisons of cortical thickness measures between groups employed ANCOVA with age and gender as covariates. Pearson correlation of recollection and familiarity measures with ADsig thickness were calculated, including age, gender and education as covariates. For comparison, additional correlations were calculated for standard psychometric measures and ADsig thickness.
3. Results
3.1. Demographic data
The cognitive analysis included 50 CN adults [mean 71.2 ± 9.0 (SD) years; mean education 16.2 ± 2.8; mean MMSE 29.4 ± 1.0 (SD)] and 32 patients with a-MCI [mean 72.0 ± 6.9 (SD) years; mean education 16.9 ± 2.4; mean MMSE 27.4 ± 1.4 (SD)] (see Table 1). The diagnostic groups did not differ by age [t(80) < 1.0, p > 0.1], education [t(80) = 1.2, p > 0.1], or gender [χ2 =0.4, p > 0.1]. Patients with a-MCI performed significantly more poorly on the MMSE than CN participants [t(80) = 7.4, p < 0.001]. By definition, the a-MCI group displayed significant impairment on memory measures [CERAD delayed recall: t(80) = 11.6, p< 0.001]. While non-memory domains tended to be less impaired (most within 1 standard deviation below the control mean), these measures were also generally associated with significantly poorer performance for the a-MCI group.
Table 1.
Demographic and cognitive data.
| CN (n=50) | a-MCI (n=32) | YC (n=17) | |
|---|---|---|---|
| Age | 71.2 (9.0) | 72.0 (6.9) | 23.9 (1.6) |
| Gender | 29 F:21 M | 17 F:15 M | 12 F: 5 M |
| Formal education (years) | 16.2 (2.8) | 16.9 (2.3) | 16.5 (1.1) |
| MMSE | 29.4 (1.0) | 27.4 (1.4)*** | |
| CERAD immediate total | 23.3 (4.1) | 17.1 (3.8)*** | |
| CERAD delayed recall | 8.2 (1.7) | 3.3 (2.0)*** | |
| CERAD delayed recognition | 9.8 (.52) | 8.9 (1.4)*** | |
| Digit span forward | 7.1 (1.0) | 6.4 (1.0)** | |
| Digit span backward | 5.2 (1.3) | 4.5 (0.8)** | |
| Trails A (s) | 31.8 (11.8) | 41.8 (24.0)* | |
| Trails B (s) | 73.7 (32.2) | 129.3 (75.5)*** | |
| Category fluency (animals) | 21.8 (5.6) | 16.2 (4.7)*** | |
| BNT | 28.6 (1.7) | 26.5 (3.2)*** | |
| Recollection total (proportion) | .36 (.17) | .21 (.12)*** | .60 (.13)*** |
| Familiarity (d′) total | 2.18 (0.56) | 1.29 (.51)*** | 1.97 (.52) |
| ADsig mean thickness (mm) | 2.77 (0.13) | 2.58 (0.17)*** | |
| PVC mean thickness (mm) | 1.57 (0.14) | 1.49 (0.13)* |
Note: Eight CN adults and two a-MCI patients did not complete the digit span test. Structural measures included 33 CN adults and 29 a-MCI patients.
p<0.05; all reported significance levels are in comparison to CN group.
p<0.01; all reported significance levels are in comparison to CN group.
p<0.001, all reported significance levels are in comparison to CN group.
3.2. Recollection and familiarity
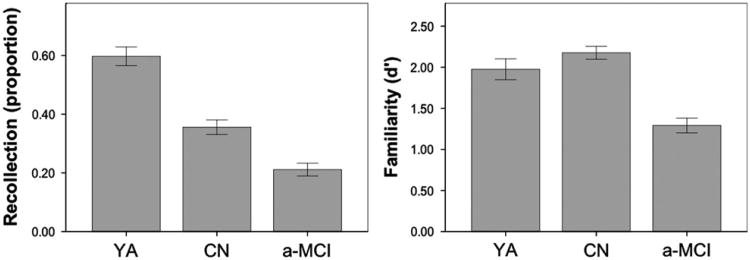
Consistent with our prior report, patients with a-MCI demonstrated significant impairments on both the experimental recollection [t(80) = 4.1, p < 0.001] and familiarity [t(80) = 7.3, p<0.001] measures (Fig. 1); however, the familiarity measure appeared to better discriminate between the two groups with a Cohen's d effect size of 1.66 for familiarity compared to 0.96 for recollection. Further, control-referenced z-scores were calculated to better equate the measures and revealed a significantly greater impairment in familiarity than recollection [familiary: −1.68, recollection: −0.79; t(31) = 57, p < 0.001] in the a-MCI group. Importantly, a-MCI patients were not at floor levels of performance with either measure.
Fig. 1.
Measures of recollection and familiarity in the young adult (YA), cognitively normal (CN), and amnestic-Mild cognitive impairment (a-MCI). Error bars represent 1 standard error of the means.
These findings are quite similar to our prior report using these measures in a different sample from the University of Pittsburgh (Wolk et al., 2008). When these two samples are combined, 75 CN adults [mean 71.4 ± 8.8 (SD) years; mean education 16.2 ± 2.9; mean MMSE 29.5 ± 0.9 (SD)] can be compared to 53 a-MCI patients [mean 71.6 ± 7.2 (SD) years; mean education 16.8 ± 2.5; mean MMSE 27.8 ± 1.6 (SD)]. Again, we found that both recollection [.35 vs. .21; t(126) = 4.9, p < 0.001] and familiarity [2.22 vs. 1.39, t(126) = 8.3, p < 0.001] were significantly impaired in a-MCI, but control-referenced z-scores supported a greater impairment in the familiarity measure [−78 vs. −1.50, recollection vs. familiarity; t(52) = 5.6, p < 0.001].
To provide context for the general age-related modulation of these memory measures, we also compared the CN older adults to the YA group (Fig. 1). Consistent with most prior reports, the CN older adult group performed significantly more poorly on the measure of recollection [t(65) = 5.2, p < 0.001] than the YA group. Also consistent with the majority of the literature, there was no difference in familiarity between young and older adults [t(65) = 1.3, p > 0.1]. Note that while there was no statistical difference in familiarity, the CN older adults appeared to trend somewhat higher than the YA. Prior work has suggested that when recollection is high (> 0.6), familiarity assessments may be somewhat depressed (Yonelinas, 2002). To reduce this potential confound (the YA group had a mean recollection of 0.60), we repeated the familiarity comparison including only those individuals who had recollection scores below 0.6. This restriction resulted in no change in the above group comparisons [recollection: t(50)=3.7, p = 0.001; familiarity: t(50) < 1, p > 0.1]. Altogether, these results support the notion that aging is associated with a relatively selective impairment in recollection while familiarity decline is more specific to early AD.
3.3. Relationship of recollection and familiarity to ADsig
ADsig cortical thickness was compared in the 33 CN adults and 29 a-MCI patients with available MRI scans. Consistent with the sensitivity of the ADsig cortical thickness measure to early AD neurodegeneration, the a-MCI group displayed significant thin-ning, controlled for age and gender [F(1,58) = 24.0, p < 0.001], with a Cohen's d effect size of 1.29 (Table 1). Note that group differences and absolute estimates of recollection and familiarity were highly similar in this subset of patients with MRI available relative to the data for the entire cohort.
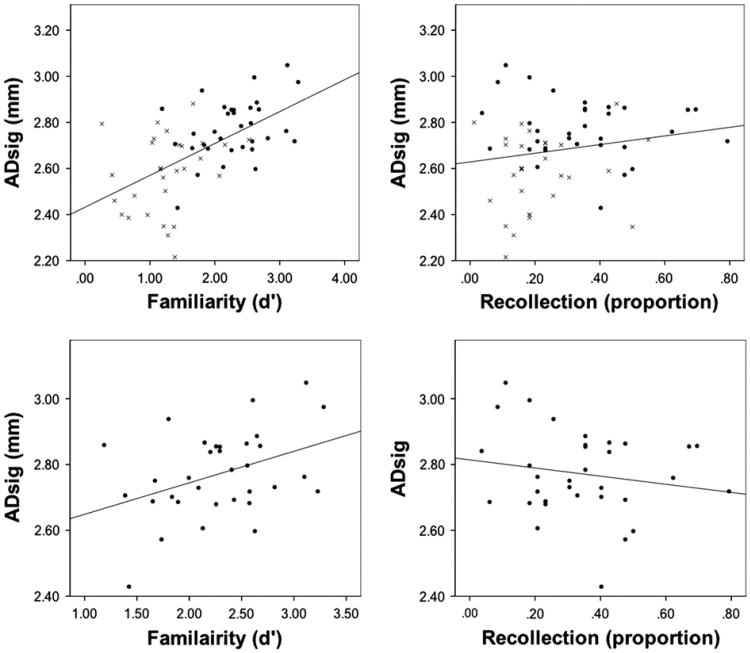
Given that familiarity appears to be more sensitive and specific to early AD based on the above analysis, we hypothesized that familiarity would be more highly related to the AD-specific brain changes measured by the ADsig than recollection across the spectrum from CN adults to MCI. To examine this, we calculated correlations of both of these measures with the ADsig, controlling for age, education, and gender, across both groups. As predicted, familiarity strongly correlated with ADsig thickness (r = .58, r < 0.001) while recollection did not (r = .10, p > 0.1) (Table 2 and Fig. 2). To assess the specificity of these effects to AD-related neurodegeneration, we also correlated these measures to cortical thickness in a “control” region, the primary visual cortex (PVC), which is expected to be less affected by the AD pathologic process. While the familiarity measure correlated much more strongly with ADsig thickness, both measures correlated with PVC thickness (recollection: r = .23, p < 0.09; familiarity: r = .29, p < 0.05). Thus, across the continuum from normal aging to a-MCI, familiarity, but not recollection, tracks with a sensitive measure of AD-related atrophy, while both measures are less strongly associated with a non-specific measure of grey matter loss.
Table 2.
Correlation of experimental and standard cognitive measures with AD signature.
| CN/MCI (n=62) | CN/MCI controlled for group (n=62) | CN (n= 33) | ||||
|---|---|---|---|---|---|---|
| Adsig | PVC | ADsig | PVC | ADsig | PVC | |
| Recollection | .10 | .23 | −.17 | .14 | −.25 | .35# |
| Familiarity | .58*** | .29* | .29* | .16 | .39* | .22 |
| MMSE | .48*** | .13 | .19 | −.05 | .19 | −.07 |
| CERAD immediate total | .41** | .23 | −.02 | .06 | .06 | .08 |
| CERAD delayed recall | .56*** | .35** | .17 | .26+ | .24 | .26 |
| CERAD delayed recognition | .48*** | .27* | .30* | .17 | −.01 | −.28 |
| Digit span forward | .27* | .16 | .14 | .01 | −.02 | −.05 |
| Digit span backward | .19 | .18 | .03 | .12 | .05 | .20 |
| Trails A (s) | −.07 | −.14 | .15 | −.06 | −.14 | −.22 |
| Trails B (s) | −.13 | −.12 | .16 | −.01 | .13 | −.13 |
| Category fluency (animals) | .43** | .29* | .17 | .19 | .27 | .24 |
| BNT | .43** | .08 | .25# | — .04 | .19 | .08 |
Note: One a-MCI patient in this analysis did not complete digit span. Correlations with Trails A did not include one clear a-MCI outlier over 4 SD's from the rest of the group.
p=0.06;
p=0.05;
p < 0.05;
p < 0.01;
p < 0.001.
Fig. 2.
Relationship between AD signature (ADsig) cortical thickness recollection and familiarity measures. The top plots include both cognitively normal adults (black circles) and amnestic mild cognitive impairment patients (grey x's). The second row includes just the cognitively normal group. Best linear fit lines are presented.
Finally, to mitigate against the potential role of group effects in these correlations, we repeated these analyses, but included group as a covariate as well. Here too, familiarity significantly correlated with ADsig (r = .29, p < 0.05) while recollection, again, did not (r = − .17, p > 0.1). When controlling for group, neither of these measures correlated with PVC (recollection: r = .14, p > 0.1; familiarity: r = .16, p < 0.1). However, when examining the MCI group alone, neither the recollection (r = −0.09, p > 0.1) nor familiarity (r = .18, p > 0.1) measure significantly correlated with ADsig. It is worth noting that removal of a potential outlier (lowest familiarity, 3rd highest cortical thickness), increased the positive correlation with familiarity, but still did not reach statistical significance (r = .33, p = .11).
Most importantly, in addition to examining the sensitivity to ADsig thinning across the continuum from controls to prodromal AD (i.e., MCI), we were specifically interested in whether familiarity would be sensitive to potential preclinical AD neurodegenerative change. Therefore, we repeated the above analyses within the CN group alone (Table 2 and Fig. 2). Consistent with our hypothesis, the familiarity measure correlated with ADsig (r = .39, p < 0.05) while recollection did not (r = − .18, p > 0.1). As noted earlier, high recollection scores (> 0.60) tend to distort familiarity estimates with the Process Dissociation Procedure and removal of the 4 CN adults with high recollection further strengthened the correlation (r = .44, p < 0.05). Interestingly, the recollection measure again approached significance in correlation with PVC (r = .35, p = 0.06) in the CN sample, while the familiarity measure did not (r = .22, p > 0.1).
3.4. Comparison with other standard psychometric measures
We also wanted to compare the experimental recollection and familiarity measures in their sensitivity to AD-related neurodegeneration, as measured by the ADsig, with standard psychometric measures. To do so, we examined the same correlations as performed above, controlling for age, education, and gender (Table 2). First, we included the entire cohort. While the familiarity measure was the most strongly associated with ADsig thickness, delayed recall on the CERAD 10-item word-list was similar in correlation strength (r=.56, p < 0.001). Further, a number of other measures also produced significant, but modestly weaker, correlations, including the MMSE (r=.48, p < 0.001), immediate (r=.41, p < 0.01) and recognition memory (r=.48, p < 0.001) on the CERAD word-list memory test, category fluency (r=.43, p < 0.01), and BNT (r=.43, p < 0.01). Interestingly delayed recall on the CERAD 10-item word-list also correlated most strongly with the PVC thickness (r=.35, p < 0.01), suggesting that while sensitive to AD-related change, it may be a fairly non-specific measure.
When group was included as a covariate, delayed recall no longer significantly correlated with the ADsig measure. Notably, recognition memory on the CERAD was the only standard psychometric memory measure that correlated with ADsig when controlling for group and to a similar degree as the experimental familiarity measure, perhaps due to the potential dependence on familiarity for item recognition memory. 1 Finally, and most importantly, when these correlations were performed only with the CN group, no standard psychometric measure was significantly associated with ADsig. Thus, only the familiarity measure appeared sensitive to AD-related neurodegenerative change in those with normal cognition.
3.5. Exploration of familiarity measure with component ROIs of ADsig
While not a central focus of the current analysis, we also explored the relationship of the familiarity measure with the individual ROIs that constitute the ADsig. Again, to reduce the potential distortion of the familiarity measure due to high recollection (40.6), we excluded four CN adults from the analysis. Not surprisingly, the familiarity measure positively correlated with all of the ROIs although did not quite reach significance with a region in the inferior temporal (r=.25, p=0.07) and superior frontal gyrus (r=.23, p < 0.1). Also expected, the rostral medial temporal lobe ROI, which includes a portion of the ERC and PRC, displayed the strongest correlation with familiarity (r=.49, p < 0.001), but several regions were similarly strongly correlated, including anterior temporal lobe/temporal pole, superior parietal lobule, caudal middle frontal gyrus, and supramarginal gyrus (r's=.44 to.46, p's < 0.01). None of these individual ROIs were as strongly correlated as the full ADsig summary measure (r=.58, p < 0.001). To further explore the relative contribution of these regions to prediction of familiarity, a heirarchical regression model was developed in which age, education, and gender were entered as covariates first and then each of the 9 ROIs were entered in a stepwise fashion. The best model [F(3,54=6.2, p < 0.001] included the medial temporal lobe (β=.43, p=0.001) and the supramarginal gyrus ROI (β=.39, p=0.002), suggesting that regions beyond the medial temporal lobe may contribute to driving the overall relationship with the ADsig. Nonetheless, some of this effect may be driven by the relative sensitivity of these regions to the presence of AD pathology rather than those regions contribution to familiarity per se.
4. Discussion
In this study, we further investigated whether familiarity-based memory is sensitive to AD in pre-dementia stages of disease. Consistent with our prior work (Wolk et al., 2008), we found that both recollection and familiarity were impaired in a-MCI. Also consistent with the majority of the literature, weaker recollection, but not familiarity, was associated with normal aging (Davidson & Glisky, 2002; Howard et al., 2006; Jacoby, 1999; Jennings & Jacoby, 1997; Light et al., 2004; Parkin & Walter, 1992; Yonelinas, 2002). These findings suggest that alterations of familiarity may be specific for early AD and a potentially useful tool in discrimination of disease from age effects. Indeed, our measure of familiarity was strongly associated with atrophy in “AD-signature” regions of the cerebral cortex, a well-established measure of cortical thickness that is sensitive to AD-associated atrophy spanning the spectrum from preclinical to mild AD (Dickerson & Wolk, 2012; Dickerson et al., 2009, 2011; Quiroz, Stern, & Reiman, 2012). In particular, we found that familiarity-based memory was associated with this measure in cognitively normal older adults, suggesting that decrements in familiarity may be sensitive to preclinical AD neurodegeneration. We will discuss each of these points in more detail below.
Confirming our prior findings, which utilized the current and two other experimental tasks (Wolk et al., 2008), we found that familiarity-based memory is significantly impaired in a-MCI. In fact, as reported before, familiarity appeared to better discriminate groups than our recollection measure. However, it should be at least noted that when one considers the additive effects of both aging and a-MCI status, that impairment of recollection may be the most salient feature of a-MCI patients relative to “normal” memory in young adults. While there has been universal agreement that associative, or recollection-based memory (Algarabel et al., 2012; Ally et al., 2009; Embree et al., 2012; Serra et al., 2010; Westerberg et al., 2006; Wolk et al., 2008), is impaired in early AD, there has been considerable disagreement about whether familiarity is also involved in prodromal stages of disease. Indeed, studies of MCI have reported findings of both sparing (Hudon et al., 2009; Serra et al., 2010; Westerberg et al., 2006) and involvement (Algarabel et al., 2009; Ally et al., 2009; Embree et al., 2012; Wolk et al., 2008) of this memory process.
A number of factors have been postulated to potentially explain the conflicting results in the literature. One of the most important is related to subject characteristics and the relatively small sample sizes of prior studies. This is particularly an issue in studies of a-MCI in which only a portion of the patients likely have underlying AD as the etiology for their cognitive decline (Petersen et al., 2009). Progression to clinical AD after 5 years in this population ranges from 50 to 75% in specialty centers such as our own and rates of “positive” amyloid PET scans, which are sensitive to fibrillar amyloid plaques that are a hallmark of AD, have similarly been around 45–70% (Fleisher, Chen, & Liu, 2011; Jagust et al., 2010; Wolk et al., 2009). Thus, a-MCI is clearly a heterogeneous construct with different populations varying in their relative enrichment of patients with prodromal AD. This can have significant implications for results in small cohorts. For example, some proportion of a-MCI patients may just be exhibiting greater age-associated decline, which may influence the qualitative pattern of memory impairment when over-represented in any particular study. Furthermore, about 1/3 of cognitively normal older adults have underlying AD pathology (Morris et al., 2011; Wolk & Klunk, 2009). Thus, the relative proportion of preclinical AD patients in the control group also may influence findings.
When combining patients from the current sample with previously collected data, the current dataset represents by far the largest sample to date addressing this issue (75 CN adults, 53 a-MCI patients). Even taken alone, data from the newly collected cohort is still larger than most prior studies and replicated our previously reported findings (Wolk et al., 2008). In addition, this is the first study to examine these measures in the context of another AD biomarker, the AD signature MRI marker of cortical neurodegeneration. The finding that this structural measure strongly discriminated our a-MCI group from CN adults suggests that our a-MCI cohort was indeed enriched in patients with prodromal AD.
In addition to heterogeneity of etiology, a-MCI patients can differ in severity within this category, and it is worth noting that the mean MMSE of this sample is slightly higher than a-MCI patients in the Alzheimer's Diseases Neuroimaging Initiative (ADNI) (27.4 vs. 27.0, respectively), a cohort of ∼400 individuals that has served as a benchmark for the field of ‘typical’ a-MCI (Petersen et al., 2010). Thus, it is unlikely that the involvement of familiarity-based memory in this study relative to others is due to our cohort's being particularly more impaired.
Another potential source of inconsistency across studies is the diagnostic criteria for a-MCI. While the current study included patients with both single- and multiple-domain a-MCI (i.e., patients who have memory plus at least one other cognitive domain in the impaired range), other studies have exclusively enrolled patients with single-domain a-MCI. Indeed, some investigators have argued that this may explain the relative sparing of familiarity or item memory in their cohorts relative to other groups (Serra et al., 2010; Troyer et al., 2012). This difference in recruitment may indeed account for some of the discrepancies across studies. However, it is important to note that while the construct of single-domain a-MCI was hypothesized to reflect a more “pure” prodromal AD group relative to multiple-domain a-MCI (Petersen, 2004), which was conceptualized as being more heterogeneous, most data have not supported this contention. A number of studies have found higher rates of conversion to AD, less reversion to normal, and greater rates of evidence for molecular markers of AD in multiple-domain a-MCI patients (Aretouli, Okonkwo, Samek, & Brandt, 2011; Han et al., 2012; Nordlund et al., 2012; Wolk et al., 2009). Thus, multiple-domain a-MCI may actually be more enriched with prodromal AD patients than the single-domain form.
A final potential source of disagreement between studies is the type of process estimation method utilized. All of these approaches are associated with a variety of assumptions and are variably “process pure” in their estimates. The current study used a modification of the process dissociation procedure, which is subject to this issue as much as other commonly used assessments. It will be important for multiple approaches to be used in the same cohorts to assess more rigorously whether differences reported are related to the paradigms and their interpretation or subject characteristics.
It is also important to note that the presence of familiarity impairment in prodromal AD or MCI is quite consistent with the MTL amnesic literature. A number of studies, using a variety of estimation methods, have reported impairment of recollection, but sparing of familiarity, in patients with selective hippocampal lesions (Aggleton et al., 2005; Mayes et al., 2004; Yonelinas et al., 2002). However, when lesions include extra-hippocampal cortical MTL structures, additional impairment of familiarity is reported (Bowles et al., 2007; Stark & Squire, 2000; Yonelinas, 2002; Yonelinas et al., 2002). As already described, AD NFT pathology begins in extrahippocampal MTL regions (PRC/ERC) and by the time patients develop the clinical symptoms of MCI, autopsy studies have suggested that most are at least at Braak Stage II or III (Guillozet, Weintraub, Mash, & Mesulam, 2003; Petersen et al.,, 2006) in which there is, if anything, greater involvement of these extrahippocampal MTL structures, but frequently hippocampal pathology as well. Indeed, the greater involvement of these extrahippocampal regions in this stage of disease is consistent with the more significant impairment of familiarity relative to recollection observed. Nonetheless, as the hippocampus is also likely involved in most patients with prodromal AD, one would predict that patients with MCI would qualitatively display a memory profile similar to that of amnesic patients with more global MTL involvement, as was observed here.
The dual process model espoused here has not been without controversy and some have questioned whether various methods that estimate recollection and familiarity conflate these measures with ‘strong’ and ‘weak’ memories, respectively (Squire, Wixted, & Clark, 2007; Wixted et al., 2010). From this perspective, dissociations of ‘familiarity’ impairment in patients with selective hippocampal lesions versus more global MTL involvement are suggested to be on the basis of the capacity of extrahippocampal structures to support such ‘weak’ memories, as opposed to ‘strong’ memories dependent on the hippocampus. While the current findings do not clearly adjudicate between this perspective and the dual process model, the pattern of memory loss in a-MCI is, again, in keeping with that of non-neurodegenerative patients with combined cortical and hippocampal MTL lesions. That is, even if the impairment of familiarity really is just a reflection of a reduced capacity for supporting weak memories in a-MCI, the current finding would be congruent with the diminishment of both weak and strong memories in amnesics with global MTL lesions.
Irrespective of the theoretical model, our familiarity measure demonstrates sensitivity to brain changes suggestive of preclinical AD in the CN group in contrast to recollection or any other standard psychometric measure. This finding adds to an emerging literature that subtle cognitive changes may be evident at this stage of disease (Hedden et al., 2012; Mielke et al., 2012; Parra et al., 2010; Rentz et al., 2011). Given the movement of the AD field towards therapeutic trials in preclinical populations, cognitive tests that are sensitive to early ‘asymptomatic’ brain changes will become increasingly important as measures of treatment effect. One strategy is to use difficult memory tests with the expectation that they will be particularly challenging to individuals with preclinical AD. For example, Rentz et al. (2011) have found that a task that requires the binding of names to faces, which declines with normal aging, is sensitive to evidence of preclinical AD measured by amyloid imaging . Alternatively, familiarity impairment may reflect a qualitative, rather than quantitative, difference with normal age-associated decline, as this form of memory appears relatively inert to normal aging in this and other reports (Davidson & Glisky, 2002; Howard et al., 2006; Jacoby 1999; Jennings & Jacoby, 1997; Light et al., 2004; Parkin & Walter, 1992; Yonelinas, 2002). Thus, it may be a particularly specific measure to the early neurodegenerative effects of preclinical disease. It is, perhaps, the case that several different measures, varying in their sensitivity and specificity, will ultimately produce an optimal cognitive battery for screening and monitoring of preclinical AD.
It should also be noted that the paradigm used here may gain additional specificity in differentiating preclinical and prodromal AD from normal age-related changes based on several additional properties of the testing format. Age-associated changes in episodic memory are generally thought secondary to decrements in controlled and effortful strategic processing supported by frontal-subcortical networks (Buckner, 2004; Moscovitch & Winocur, 1995; Park & Reuter-Lorenz, 2009). Older adults tend to perform better on memory tasks that provide some degree of ‘environmental support’ at encoding and retrieval, which mitigates against their decline in strategic processing (Logan, Sanders, Snyder, Morris, & Buckner, 2002; Naveh-Benjamin, Brav, & Levy, 2007). The current paradigm required semantic elaboration as part of the incidental encoding procedure and used a recognition memory format. Both of these properties may have reduced the influence of normal age-associated effects on memory performance. Further, familiarity-based memory itself is thought to be a relatively ‘automatic’ process and is therefore less susceptible to these age-related changes in controlled processing.
There are several limitations to the current work. When just considering the CN group with available MRI data (n=34), the sample size is still relatively small for a study of preclinical disease. Most work suggest that ∼1/4 to 1/3 of older adults display evidence of preclinical disease and thus we would expect only 10–15 individuals in the CN group to be in this early AD stage (Morris et al., 2011; Wolk & Klunk, 2009). Given the likely subtlety of cognitive deficits in this population, much larger studies will be needed to confirm the sensitivity of this and other promising novel measures of preclinical AD. In addition, even when these measures are linked to an association with an AD biomarker (e.g., amyloid imaging, ADsig), longitudinal data will be important to determine whether they both predict and track cognitive deterioration and eventual development of clinical AD.
Another potential issue with the current work is that we used a structural imaging marker of preclinical AD rather than a molecular one. The proposed research criteria for preclinical AD requires the presence of ‘cerebral amyloidosis’, which can be detected with amyloid imaging or cerebrospinal fluid Aβ (Sperling et al., 2011). In the present data, we do not know whether or not those with greater AD signature thinning truly have cerebral amyloidosis. However, our prior work has demonstrated that cortical thinning of the AD signature is associated with a high rate of an AD-like CSF profile, ‘positive’ amyloid scans, and progression to AD (Dickerson & Wolk, 2012; Dickerson et al., 2009, 2011). Further, there may be advantages to using a marker of downstream neurodegeneration in preclinical populations when trying to relate to sensitive cognitive measures. Preclinical AD has been conceptualized as including a stage in which there is development of cerebral amyloidosis without neurodegenerative change (stage 1). This is thought to be followed by development of synaptic and neuronal injury/loss (stage 2 and 3), which may be evident with structural imaging (Sperling et al., 2011). As we also expect changes in cognition to be a reflection of the down-stream neurodegenerative changes of AD molecular pathology, inclusion of all preclinical patients defined solely by amyloid status (stages 1–3) may obscure the relationship of a cognitive measure with preclinical AD. Specifically, we would not expect those with stage 1 disease to display cognitive change relative to those without evidence of cerebral amyloidosis. Future studies will need to include both molecular and neurodegenerative markers to better characterize these patients and any relationship with subtle cognitive change.
Despite these limitations, the current work provides promising data that familiarity-based memory may be influenced by the presence of preclinical AD, but not normal, age-associated decline consistent with the early involvement of extra-hippocampal MTL structures in the AD pathologic process. Such cognitive measures, if reliable, are potentially of considerable value as an inexpensive screening tool to determine who should go on to obtain more specific molecular or structural measures. Additionally, given the growing interest in preclinical intervention studies, measures sensitive to subtle cognitive change may become important outcome measures with, perhaps, greater face-validity than other biomarker outcomes (e.g., structural change (Sperling, Jack, & Aisen, 2012)). Certainly more work assessing this and other psychometric measures informed by the cognitive neuroscience literature and the anatomical distribution of AD pathology is merited.
Acknowledgments
This work was supported by grants from NIH, including K23-AG028018, P30AG010124, R01AG29411, R21AG29840, P50-AG005134, and the Alzheimer's Association (NIRG-12-242765).
Footnotes
Oddly, trails A, a test of visuomotor processing speed, also correlated with the ADsig, but in the unexpected direction (faster performance associated with more thinning; r=.33, p < 0.05). However, this was clearly driven by an MCI ‘outlier’ who performed greater than 4 SD's worse than the rest of the group, and removal eliminated this effect.
References
- Aggleton JP, Vann SD, Denby C, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algarabel S, Escudero J, Mazon JF, et al. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired and Alzheimer's patients. Neuropsychologia. 2009;47(10):2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Algarabel S, Fuentes M, Escudero J, et al. Recognition memory deficits in mild cognitive impairment. Neuropsychology Development and Cognition Section B: Aging Neuropsychology and Cognition. 2012;19(5):608–619. doi: 10.1080/13825585.2011.640657. [DOI] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain and Cognition. 2009;69(3):504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of the 0.5 s: predictors of 2-year outcome in mild cognitive impairment. Journal of the International Neuropsychological Society. 2011;17(2):277–288. doi: 10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown CM, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Warburton EC, Aggleton JP. Recognition memory: material, processes, and substrates. Hippocampus. 2010;20(11):1228–1244. doi: 10.1002/hipo.20858. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Exploring the recognition memory deficit in Parkinson's disease: estimates of recollection versus familiarity. Brain. 2006;129(Pt 7):1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52(6):1158–1165. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Wolk DA. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78(2):84–90. doi: 10.1212/WNL.0b013e31823efc6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen N. Two functional components of the hippocampal memory system. Behavioral Brain Science. 1994;17:449–517. [Google Scholar]
- Embree LM, Budson AE, Ally BA. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia. 2012;50(9):2333–2340. doi: 10.1016/j.neuropsychologia.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Chen K, Liu X, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Archives of Neurology. 2011;68(11):1404–1411. doi: 10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Annals of Neurology. 1997;41(1):17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Archives of Neurology. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- Han JW, Kim TH, Lee SB, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimers Dementia. 2012;8(6):553–559. doi: 10.1016/j.jalz.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Hedden T, Mormino EC, Amariglio RE, et al. Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. Journal of Neuroscience. 2012;32(46):16233–16242. doi: 10.1523/JNEUROSCI.2462-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modeling and receiver operating characteristic curves. Psychology and Aging. 2006;21(1):96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the remember/know procedure in amnestic mild cognitive impairment and probable Alzheimer's disease. Brain and Cognition. 2009;70(1):171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process-dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- Jacoby LL. Ironic effects of repetition: measuring age-related differences in memory. Journal of Experimental Psychology: Learning Memory and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's disease neuroimaging initiative positron emission tomography core. Alzheimers Dementia. 2010;6(3):221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting agerelated deficits in recollection: telling effects of repetition. Psychology and Aging. 1997;12(2):352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test(Lea and Feibiger Philadelphia) The Boston naming test 1983 [Google Scholar]
- Light LL, Patterson MM, Chung C, Healy MR. Effects of repetition and response deadline on associative recognition in young and older adults. Memory and Cognition. 2004;32(7):1182–1193. doi: 10.3758/bf03196891. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: the judgement of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14(6):763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79(15):1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of Neurology. 2011;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. Journal of Neurology, Neurosurgery & Psychiatry. 2012;81(5):541–546. doi: 10.1136/jnnp.2008.171066. [DOI] [PubMed] [Google Scholar]
- Norman KA. How hippocampus and cortex contribute to recognition memory: revisiting the complementary learning systems model. Hippocampus. 2010;20(11):1217–1227. doi: 10.1002/hipo.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary learning systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocog-nitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Mendez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer's disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Archives of Neurology. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's disease neuroimaging initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz YT, Stern CE, Reiman EM, et al. Cortical atrophy in presymptomatic Alzheimer's disease presenilin 1 mutation carriers. Journal of Neurology, Neurosurgery & Psychiatry. 2012 doi: 10.1136/jnnp-2012-303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Reitan R. Validity of the trail making test as an indicator of organic brain disease. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, et al. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L, Bozzali M, Cercignani M, et al. Recollection and familiarity in amnesic mild cognitive impairment. Neuropsychology. 2010;24(3):316–326. doi: 10.1037/a0017654. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Science Translational Medicine. 2012;31(111) doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration norms and commentatory. 2nd. A compendium of neuropsychological tests: Administration, norms, and commentatory Oxford University Press; New York: 1998. [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Recognition memory and familiarity judgments in severe amnesia: no evidence for a contribution of repetition priming. Behavioral Neuroscience. 2000;114:459–467. doi: 10.1037//0735-7044.114.3.459. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, et al. Associative recognition in mild cognitive impairment: relationship to hippocampal volume and apolipo-protein E. Neuropsychologia. 2012;50(14):3721–3728. doi: 10.1016/j.neuropsychologia.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale WAIS-III Administration and scoring manual. 3rd. Wechsler Adult Intelligence Scale WAIS-III Administration and scoring manual. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, et al. When memory does not fail: familiarity-based recognition in mild cognitive impairment and Alzhei-mer's disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Mickes L, Squire LR. Measuring recollection and familiarity in the medial temporal lobe. Hippocampus. 2010;20(11):1195–1205. doi: 10.1002/hipo.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage. 2011;54(2):1530–1539. doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Klunk W. Update on amyloid imaging: from healthy aging to Alzheimer's disease. Current Neurology and Neuroscience Reports. 2009;9(5):345–352. doi: 10.1007/s11910-009-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia. 2008;46(7):1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Annals of Neurology. 2009;65(5):557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. A medial temporal lobe division of labor: insights from memory in aging and early Alzheimer disease. Hippocampus. 2011;21(5):461–466. doi: 10.1002/hipo.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Regehr G, Jacoby LL. Incorporating response bias in a dual-process theory of memory. Journal of Memory and Language. 1995;34(6):821–835. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5(11):1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]