Abstract
Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) plays a critical role in transformation of primary B lymphocytes to continuously proliferating lymphoblastoid cell lines (LCLs). To identify cellular genes in B cells whose expression is regulated by EBNA-LP, we performed microarray expression profiling on an EBV-negative human B-cell line, BJAB cells, that were transduced by a retroviral vector expressing the EBV EBNA-LP (BJAB-LP cells) and on BJAB cells that were transduced with a control vector (BJAB-vec cells). Microarray analysis led to the identification of a cellular gene encoding the CC chemokine TARC as a novel target gene that was induced by EBNA-LP. The levels of TARC mRNA expression and TARC secretion were significantly up-regulated in BJAB-LP compared with BJAB-vec cells. Induction of TARC was also observed when a subline of BJAB cells was converted by a recombinant EBV. Among the EBV-infected B-cell lines with the latency III phenotype that were tested, the LCLs especially secreted significantly high levels of TARC. The level of TARC secretion appeared to correlate with the level of full-length EBNA-LP expression. These results indicate that EBV infection induces TARC expression in B cells and that EBNA-LP is one of the viral gene products responsible for the induction.
Epstein-Barr virus (EBV) is a causative agent of infectious mononucleosis and is associated with a variety of human malignancies, including endemic Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's diseases, gastric carcinoma, and lymphoproliferative diseases in immunosuppressed patients (38, 51). In vitro, EBV can readily infect human B cells and cause B-cell proliferation that continues for long periods (38, 51). The lymphoblastoid cell lines (LCLs) that arise after transformation by EBV express only a limited number of the more than 80 genes (38, 51) carried on the 172-kbp EBV genome. The expressed genes include those for the EBV nuclear antigens (EBNA) EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA leader protein (EBNA-LP); the latent membrane proteins (LMP) LMP-1, LMP-2A, and LMP-2B; two small RNAs (EBV-encoded small RNAs [EBERs]); and BamA right forward transcripts (38, 51). This type of latent infection in LCLs is designated latency III (38, 51). Among the viral proteins expressed in latency III, EBNA-1, EBNA-2, EBNA-3A, EBNA-3C, EBNA-LP, and LMP-1 are critical for the process that leads to efficient differentiation of EBV-infected resting B cells into proliferating B lymphoblasts, whereas EBNA-3B, LMP-2A, LMP-2B, EBERs, and BamA right forward transcripts are not (38).
EBNA-LP, an initial gene product that is expressed together with EBNA-2 upon EBV infection of B cells (2), consists of the W1W2 multiple-repeat domain and the unique Y1Y2 C-terminal domain (Fig. 1A) (54). EBNA-LP plays a critical role in EBV-induced B-cell transformation, based on observations that recombinant EBNA-LP mutants have severely impaired transforming activity (3, 20, 41). Although the mechanism by which EBNA-LP acts in EBV-induced B-cell transformation remains unclear, several lines of evidence, listed below, suggest biological roles for the protein in the transformation process.
FIG. 1.
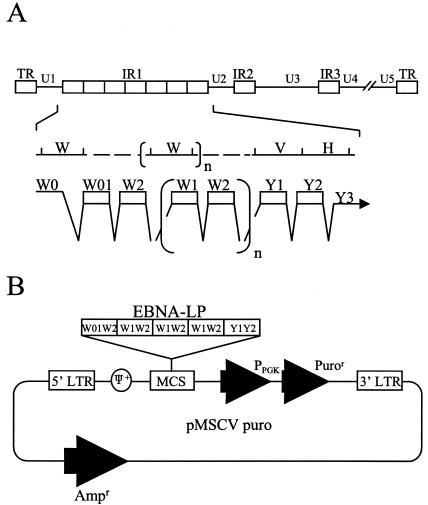
(A) Schematic diagram of the sequence of the EBV genome and location of the EBNA-LP gene. The top line is a linear representation of the EBV genome. The unique sequences are represented as U1 to U5. The terminal and internal repeats flanking the unique sequences are shown as open rectangles with their designations given above the rectangles. The middle line shows an expanded section of the domain encoding the EBNA-LP gene. The exons of EBNA-LP open reading frames are derived from the BamHI W and Y fragments. The bottom line shows the structures of the EBNA-LP transcript and coding regions. (B) Schematic diagram of the retroviral vector expressing EBNA-LP used for establishment of BJAB cells stably expressing EBNA-LP (BJAB-LP cells). LTR, long terminal repeat; MCS, multiple cloning site.
(i) The primary function of EBNA-LP is transcriptional coactivation with EBNA-2. EBNA-LP stimulates EBNA-2-mediated transcriptional activation of viral and cellular genes, such as those for LMP-1 and cyclin D2 (23, 46, 58). Recent studies reveal that cellular localization (44, 50, 73), phosphorylation on Ser-35 by both cellular and EBV-encoded protein kinases (including cdc2 and BGLF4) (33, 34, 74), and protein complex formation with either self (67) or cellular protein HA95 and protein kinase A (21, 22) are critical for the regulation of EBNA-LP coactivating functions.
(ii) EBNA-LP interacts with many cellular proteins. The cellular proteins include pRb, p53, the 70-kDa family of heat shock proteins (Hsp70), HS1-associated protein X1, α- and β-tubulins, Hsp27, HA95, protein kinase A, estrogen-related receptor 1, and bcl-2 (21, 22, 24, 36, 39, 42, 43, 64). In LCLs, EBNA-LP is localized to discrete nuclear foci called ND10, which also contain Hsp70, CBP/p300, and a distinct antigenic form of pRb (5, 31, 62, 63). Although the functional consequences of these potential interactions are unknown, the plethora of interactions implies that EBNA-LP is not only a coactivator of EBNA-2 but also a multifunctional protein that modulates various components of the cellular machinery and that the functions of EBNA-LP in EBV-induced B-cell transformation results from the sum of these interactions. Consistently, it has been recently proposed that EBNA-LP has the potential to inhibit pre-mRNA cleavage and polyadenylation (12).
The object of this report is to unveil any previously unreported, novel function(s), of EBNA-LP. The numerous interactions between EBNA-LP and various cellular components predict that additional biological activities of EBNA-LP remain unreported. Further understanding of EBNA-LP action in infected cells requires the identification of the activities. We used microarray expression profiling to identify cellular genes whose expression is regulated solely by EBNA-LP in B cells. The study results show that (i) the thymus- and activation-regulated chemokine (TARC) gene, which encodes a CC chemokine shown to selectively attract Th2-type T lymphocytes (29), is regulated; (ii) EBNA-LP up-regulates both expression of TARC mRNA and secretion of TARC protein in B cells; and (iii) EBV latency III infection induces TARC in B cells.
MATERIALS AND METHODS
Cells.
Ramos is an EBV-negative Burkitt's lymphoma (BL) cell line. BJAB is an EBV-negative non-BL-type human B-cell line. P3HR1 and Raji are EBV-positive BL lines. B95-8 is a marmoset cell line carrying infectious mononucleosis-derived EBV. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were isolated by Ficoll-Paque Plus (Amersham Pharmacia Biotech) density gradient centrifugation. CD19-positive B cells were isolated from PBMCs with M-450 anti-CD19 Dynabeads (Dynal, Great Neck, N.Y.) according to the manufacture's directions. All cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and antibiotics. The amphotropic retrovirus packaging cell line Bing (47) was kindly provided by W. Pear and was maintained in Dulbecco's modified Eagle's medium supplemented with 10% FCS and antibiotics.
Construction of recombinant retroviruses and retrovirus-mediated gene transfer.
To construct the recombinant retroviral vector pMSCV-LP, EcoRI and PstI fragments of pGBT9-EBNA-LPR4 (36) were blunted and cloned into the HpaI site of pMSCV-puro (kindly provided by W. Pear). To produce retrovirus either with (MSCV-LP) or without (MSCV-vec) EBNA-LP, the pMSCV-LP or pMSCV plasmids were transfected into the Bing packaging cell line by calcium phosphate precipitation as described previously (48). At 48 h posttransfection, the supernatant containing amphotropic retrovirus was harvested, passed through a 0.45-μm-pore-size filter, and stored at −80°C. BJAB cells were infected with retrovirus-containing supernatant as described previously (48). At 72 h postinfection, cells were plated in flat-bottom 96-well microtiter plates in medium containing 0.4 μg/ml puromycin (Sigma). Cells were fed once a week with the same medium, and resistant cells appeared after approximately 2 weeks. Clones of resistant cells were obtained by limiting dilution on mouse primary thymocyte feeder cells.
Microarray analysis.
Synthetic polynucleotides (80-mers) representing 13,440 human genes (MicroDiagnostic, Tokyo, Japan) were arrayed with a custom-made arrayer. Poly(A)+ RNA was prepared from cells with TRIzol reagent (Invitrogen) and a Poly(A)Purist kit (Ambion). Two micrograms of poly(A)+ RNA was labeled with Cyanine 5-dUTP or Cyanine 3-dUTP. Hybridization and subsequent washes of arrays were performed with a labeling and hybridization kit (MicroDiagnostic). Hybridization signals were measured with a GenePix 400A scanner (Axon Instruments) and then processed into primary expression ratios of Cyanine 5-labeled to Cyanine 3-labeled samples by the GenePix Pro software (Axon Instruments). A secondary ratio of expression of each gene was calculated by averaging the primary expression ratio obtained from an experiment with Cyanine 5-labeled target and Cyanine 3-labeled control samples and the reciprocal of the primary expression ratio obtained from an experiment with Cyanine 5-labeled control and Cyanine 3-labeled target samples. The secondary expression ratios calculated from the pair of experiments were converted into log2 values as the final expression ratios.
Northern blot hybridization.
Total RNA from cultured cells was prepared by using ISOGEN reagent according to the instructions of the manufacturer (Nippongene). Total RNA samples were electrophoresed through 0.8% agarose gels containing 2.2 M formaldehyde and transferred to Hybond-N membranes (Amersham Pharmacia Biotech). The blots were first hybridized with the cDNA fragment probe, which encoded a part of TARC, labeled with [α-32P]dCTP by using a Rediprime II labeling kit (Amersham Pharmacia Biotech) as described previously (35). After hybridization with the TARC probe, the blot was stripped by boiling in 0.1% sodium dodecyl sulfate and then rehybridized with a 32P-labeled cDNA fragment encoding part of glyceraldehyde-3-phosphate dehydrogenase (G3PDH). Relative amounts of mRNA were quantified with an LAS-1000 image analyzer and the software Image Gauge (Fujifilm). The cDNAs used as probes for TARC and G3PDH were amplified by reverse transcription-PCR (RT-PCR) from RNA samples of both the BJAB cells that stably expressed EBNA-LP and the control BJAB cells. RT-PCR was performed with the Superscript one-step RT-PCR system according to the instructions of the manufacturer (Invitrogen) with appropriate primer pairs and under the amplification conditions shown in Table 1. The amplified DNA fragment encoding TARC (nucleotides [nt] 109 to 370) was purified, verified by sequencing, and used as the probe. For the G3PDH probe, the amplified DNA fragment was cloned into pGEM-T Easy vector to yield pGEM-G3PDH and sequenced. The EcoRI fragment of pGEM-G3PDH encoding G3PDH (nt 171 to 606) was used as the probe. RT-PCR was performed in a 50-μl reaction solution containing 0.1 μg of RNA, using 10 pmol of each primer.
TABLE 1.
Primer sequences used for the amplification of TARC, BamHI W, and GAPDH genes
| Amplified region (nt) | Forward primer | Reverse primer | Product size (bp) | Annealing temp (°C)a | No. of amplification cycles |
|---|---|---|---|---|---|
| TARC (109-370) | 5′-GCACATCCACGCAGCTCGAGGGAC-3′ | 5′-GGGAGACAGTCAGGAGTCTGGGGT-3′ | 262 | 60 | 40 |
| BamHI W (45484-45617) | 5′-CAAGAACCCAGACGAGTCCGTAGAA-3′ | 5′-AAGAAGCATGTATACTAAGCCTCCC-3′ | 134 | 58 | 30 |
| GAPDH (171-606) | 5′-CATTGACCTCAACTACATGG-3′ | 5′-AGTGATGGCATGGACTGTGG-3′ | 436 | 60 | 40 |
Denaturing was at 94°C for 60 s, annealing was at the indicated temperature for 60 s, and extension was at 72°C for 60 s.
Detection of EBV genome by PCR.
For detection of the EBV genome, the BamHI W region of EBV DNA was amplified by PCR from DNA isolated from EBV-infected cell cultures, and the specificity of amplification was verified by Southern blot hybridization with internal 32P-labeled oligonucleotide as described previously (65, 66). For DNA extraction from cultured cells, cell pellets were subjected to one cycle of freezing and thawing, followed by addition of 50 mM NaOH. After vigorous vortexing, the solution was boiled for 10 min and neutralized with 1 M Tris-HCl (pH 8.0).
Immunoblotting.
The electrophoretically separated proteins were transferred to nitrocellulose sheets and detected with antibodies as described previously (37).
Immunofluorescence.
Cells were harvested, washed once with phosphate-buffered saline (PBS), and smeared onto glass slides. The cells were fixed with acetone for 10 min, blocked with PBS containing 5% goat serum for 30 min, rinsed once in PBS, reacted for either for 1 h at room temperature or overnight at 4°C with a mouse monoclonal antibody to EBNA-LP (LP-4D3), rinsed three times with PBS, reacted for 1 h with goat anti-mouse immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (Sigma), rinsed with PBS, and mounted in PBS containing 90% glycerol. The slides were examined with a Zeiss LSM 510 laser scanning microscope.
Quantification of immunoreactive TARC.
A Quantikine kit (R&D Systems) for human TARC was used to quantify TARC protein secreted by cultured cells. Briefly, 105 cells were cultured in 48-well microplates for 3 days in RPMI medium without FCS. The supernatants were passed through 0.45-μm-pore-size filters, and TARC protein was quantified with an enzyme-linked immunosorbent assay (ELISA) system according to the instructions of the manufacturer (R&D Systems) with a microplate reader (Bio-Rad).
Virus, virus infection, and LCL assay.
To establish EBV-converted cell lines, recombinant EBV (rEBV) that has the neomycin resistance gene as a selection marker inserted into BXLF1 (57) was used. rEBV was prepared from the rEBV producer cell line Akata/rEBV as described previously (25). A subline of BJAB cells, BJ-cl1R, which is more than 95% positive for CD21/CR2 (26), was converted in vitro by exposure to the rEBV, followed by selection in RPMI medium containing 0.8 mg of G418 (Sigma) per ml. To establish LCLs, EBV from culture supernatants of B95-8 cells was passed through a 0.45-μm-pore-size filter and stored at −80°C in small aliquots. PBMCs (5 × 104/well) were infected with the B95-8 supernatant in 96-well flat-bottom microtiter plates. After viral adsorption for 3 h at 37°C, the cells were cultured in fresh RPMI 1640 medium containing 8% FCS and 1 μg of cyclosporineA per ml, either with or without 500 ng of recombinant TARC (R&D Systems) per ml. Cultures without recombinant TARC were also incubated with either 1 μg of a mouse monoclonal antibody to TARC (54026.11; R&D Systems) per ml or 1 μg of a control purified IgG1 (R&D Systems) per ml. Cells were fed weekly with fresh medium for 4 weeks. The LCLs in the wells were counted and assayed for both proliferation and viability by using a Cell Counting Kit-8 (Dojindo) according to the manufacturer's instructions.
Antibodies.
The mouse monoclonal antibody to EBNA-LP (LP-4D3) was generated in our laboratory with purified glutathione S-transferase fused to EBNA-LP (36, 67) as the antigen by using the standard procedure (17). LP-4D3 showed characteristics similar to those of JF186 (14) in immunoblotting and immunofluorescence assays (G. Matsuda, R. Furuya, C. Kamagata, and Y. Kawaguchi, unpublished observation). EBV-seropositive reference human serum (anti-EBNA1 titer, 1,280; anti-EBNA2 titer, 160) was used to detect EBNA-1 and EBNA-2. CS1-4 mouse monoclonal antibody (Dako) was used to detect LMP-1. Mouse monoclonal antibody to TARC (54026.11) was purchased from R&D Systems and β-actin antibody (AC-15) was purchased from Sigma.
RESULTS
Establishment of BJAB cell lines stably expressing EBNA-LP.
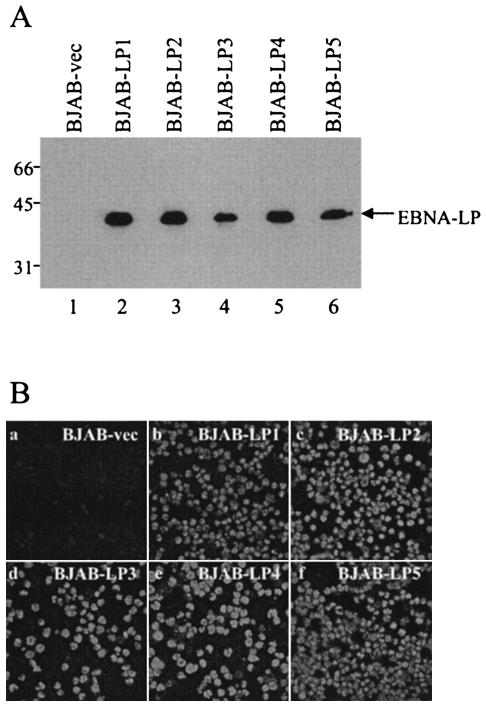
To examine the effect of EBNA-LP on cellular gene expression in B cells, we used the EBV-negative human B-cell line BJAB as a starting point for construction of a cell line that stably expressed EBNA-LP (BJAB-LP). A recombinant retrovirus encoding EBNA-LP (MSCV-LP) was generated by transfection of a recombinant retroviral vector containing EBNA-LP cDNA (pMSCV-LP) into the Bing packaging cell line. The BJAB cells were infected with MSCV-LP, and infected cells were selected with puromycin. The puromycin-resistant cells were cloned by limiting dilution, and five clones (BJAB-LP1 to BJAB-LP5) were selected. Puromycin-resistant BJAB cells infected with only MSCV-vec (BJAB-vec) were also generated as controls. Only cell clones derived from MSCV-LP infection expressed EBNA-LP, as determined by both immunoblotting (Fig. 2A) and immunofluorescence assays (Fig. 2B).
FIG. 2.
(A) Immunoblot of electrophoretically separated cell lysates from BJAB-vec (lane 1) and BJAB-LP1 to BJAB-LP5 (lanes 2 to 5) cells. The cells were harvested and immunoblotted with the mouse monoclonal antibody to EBNA-LP (LP-4D3). Molecular weights (in thousands) are shown on the left. (B) Digital images of BJAB-vec (a) and BJAB-LP1 to BJAB-LP5 (b to f) from immunofluorescence assays. The cells were smeared on glass slides, fixed, and reacted with the EBNA-LP antibody (LP-4D3) and anti-mouse IgG conjugated to fluorescein isothiocyanate.
Microarray analysis of BJAB cells stably expressing EBNA-LP.
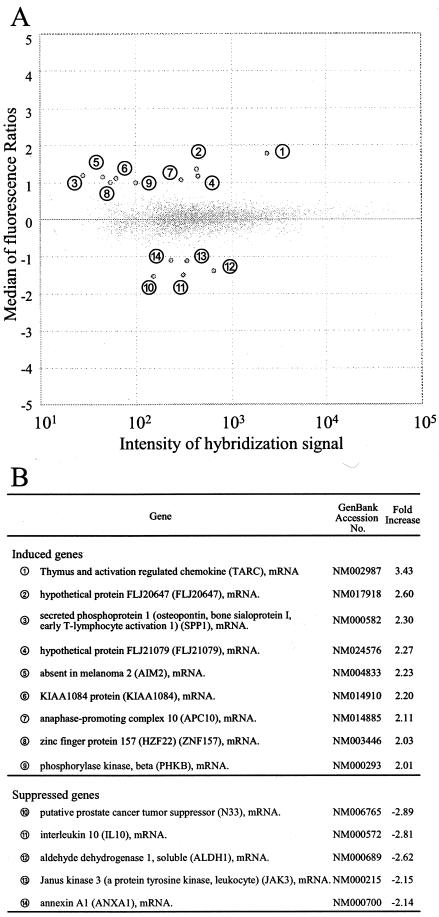
RNA was extracted from a mixture of the five independent clones of BJAB-LP cells and compared with RNA from BJAB-vec cells by using the microarray with synthetic polynucleotides representing 13,440 human genes. Fourteen genes were identified by the criterion of a more-than-twofold change in expression ratio (Fig. 3). The gene for the CC chemokine TARC was selected for further characterization, since the change in expression of TARC was the highest among the 14 genes and it has been reported that chemokines play various roles in herpesvirus infections (1).
FIG. 3.
Effect of EBNA-LP on cellular gene expression. (A) Microarray analysis. Poly(A)+ RNA was isolated from BJAB-LP or BJAB-vec cells, labeled with different fluorescent dyes, and hybridized with a microarray that had synthetic polynucleotides representing 13,440 human genes. Hybridization signals were then analyzed. One dot corresponds to one cellular gene. The fluorescence ratio was represented as log2 (BJAB-LP/BJAB-vec). Fourteen genes whose fluorescence ratios were greater than 1 are numbered. (B) List of cellular genes whose expression was changed twofold or more by EBNA-LP protein.
EBNA-LP increases the level of TARC in B cells.
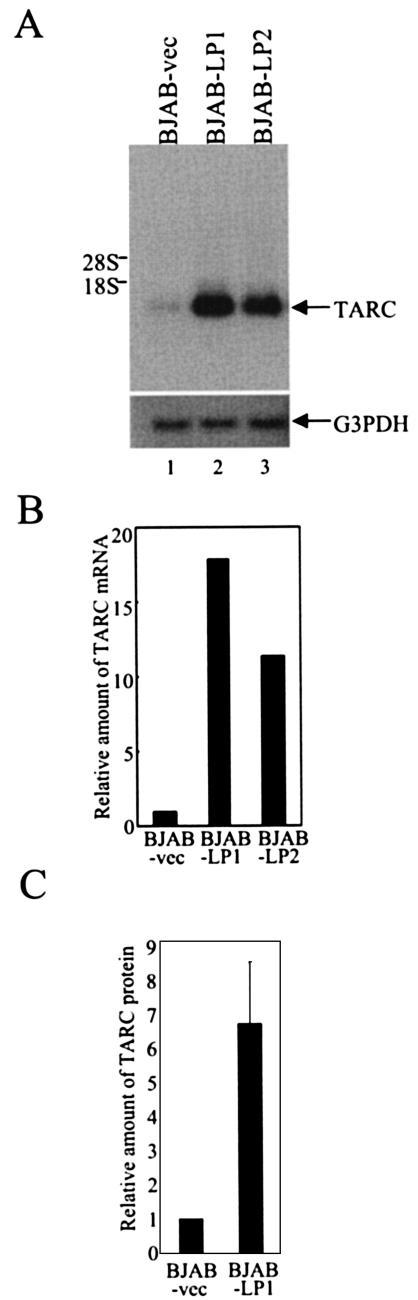
To validate the results from microarrays showing that TARC is induced by expression of EBNA-LP in B cells, we first compared the steady-state levels of TARC mRNA in BJAB-LP cells expressing EBNA-LP and control BJAB-vec cells by Northern blot analysis. The steady-state levels of TARC mRNA in both BJAB-LP1 and BJAB-LP2 cells were increased by more than 10-fold compared with TARC mRNA levels in BJAB-vec cells (Fig. 4A and B). Similar results were obtained with BJAB-LP3 to -LP5 (data not shown). In contrast, the G3PDH mRNA levels in all of the cell lines remained unchanged (Fig. 4A and data not shown). The levels of TARC protein in the supernatants of BJAB-LP1 cells were 6.6-fold higher than those in supernatants from BJAB-vec cells (Fig. 4C). These results show that EBNA-LP up-regulates the level of TARC expression in B cells.
FIG. 4.
Effect of EBNA-LP on TARC expression. (A) Steady-state levels of TARC or G3PDH mRNA in BJAB-LP and BJAB-vec cells. Total RNAs isolated from BJAB-vec (lane 1), BJAB-LP1 (lane 2), or BJAB-LP2 (lane 3) cells were fractionated by electrophoresis on an agarose-formaldehyde gel and analyzed by Northern blot analysis with 32P-labeled probe DNAs. Locations of the 28S and 18S rRNAs are indicated on the left. (B) Quantification of relative amounts of TARC mRNA in the indicated cell lines. The relative amounts of TARC mRNA were normalized to those of G3PDH, and the results are shown as values relative to that for BJAB-vec. (C) Quantification of relative amounts of TARC protein secreted in supernatants of the indicated cell lines. The amounts of TARC protein in the supernatants were measured by ELISA, and the results are shown as a value relative to that for BJAB-vec. The results represent averages from five independent experiments, and the standard deviation is presented.
EBV latency III infection also increases the level of TARC in B cells.
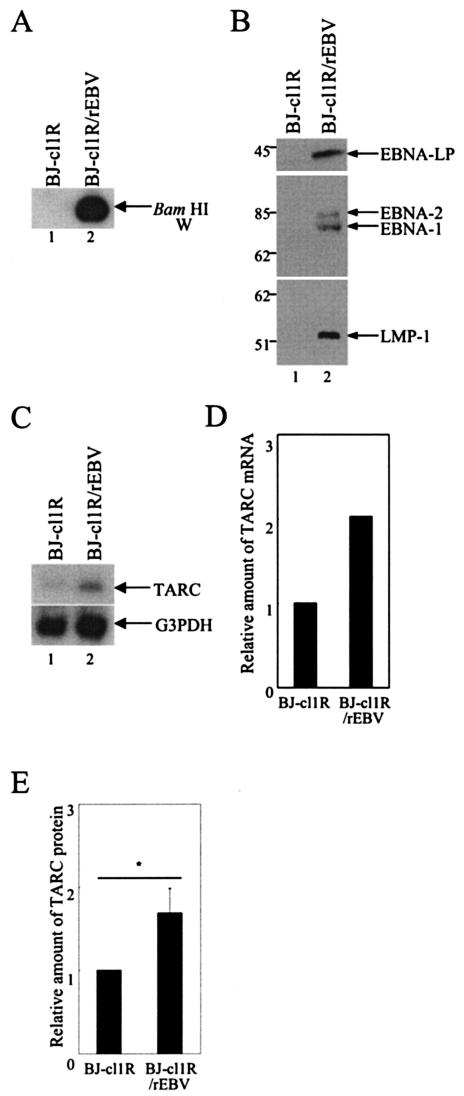
We tested whether TARC was induced not only by constructed EBNA-LP overexpression but also by EBNA-LP expression in EBV infection in B cells. The BJ-cl1R subclone of BJAB cells was infected with rEBV carrying a neomycin resistance gene to allow selection. The neomycin-resistant BJ-cl1R/rEBV cells represented type III EBV infection, since the presence of the EBV genome was detected by PCR and Southern blot analysis (Fig. 5A) and expression of EBNA-1, EBNA-2, EBNA-LP, and LMP1 in BJ-cl1R/rEBV cells was detected by immunoblotting (Fig. 5B). The expression of TARC in BJ-cl1R/rEBV cells was compared with expression in the parental BJ-cl1R cells by Northern blot analysis, and the relative amounts of TARC mRNA were normalized to those of G3PDH mRNA. The steady-state level of TARC mRNA in BJ-cl1R/rEBV cells was greater than twofold higher than that in BJ-cl1R cells (Fig. 5C and D). The TARC secretion in the supernatant of BJ-cl1R/rEBV cells, detected by ELISA, was consistently increased compared with that in the supernatant of BJ-cl1R cells (Fig. 5E). These results indicate that EBV infection induces TARC expression in B cells.
FIG. 5.
EBV infection induces TARC expression in B cells. (A) The EBV genome in BJ-cl1R (lane 1) or BJ-cl1R/rEBV (lane 2) cells was detected by PCR followed by Southern blot analysis. (B) Expression of EBNA-1, EBNA-2, EBNA-LP, and LMP-1 in BJ-cl1R (lane 1) and BJ-cl1R/rEBV (lane 2) cells was examined by immunoblotting. Molecular weights (in thousands) are shown on the left. (C) Steady-state levels of TARC or G3PDH mRNA in BJ-cl1R (lane 1) and BJ-cl1R/rEBV (lane 2) cells were analyzed by Northern blot analysis. Experiments were done exactly as described in the legend for Fig. 4A. (D) Quantification of the relative amounts of TARC mRNA in the indicated cell lines. The relative amounts of TARC mRNA were normalized to those of G3PDH, and the results are shown as a value relative to that with BJ-cl1R cells. (E) Quantification of the relative amounts of TARC protein secreted in the supernatants of the indi-cated cell lines. Experiments were done exactly as described in the legend for Fig. 4C. The results are shown as a relative value to that with BJ-cl1R. The results represent averages from four independent experiments, and the standard deviations are presented. *, P < 0.05.
LCLs secrete high levels of TARC.
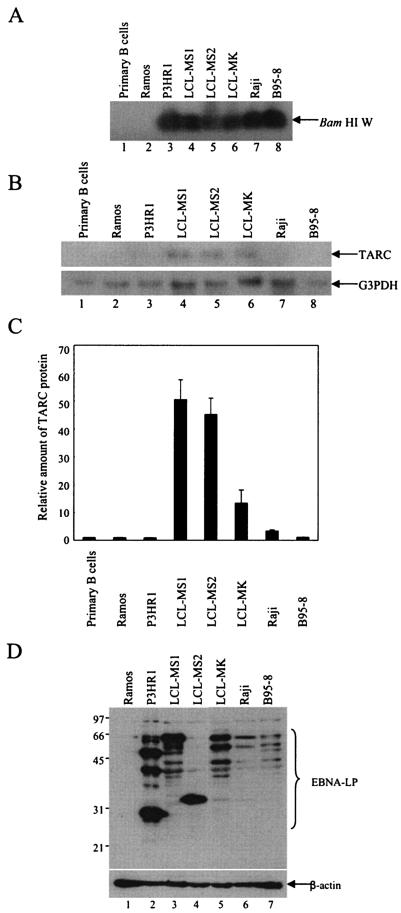
To pursue the correlation between TARC induction and EBNA-LP expression in B cells with the latency III phenotype, we examined expression of TARC in primary B cells, Ramos cells, P3HR1 cells, Raji cells, B95-8 cells, and LCLs. EBV infection was confirmed by the detection of the EBV genome (Fig. 6A) and expression of EBNA-LP (Fig. 6D). As reported previously (14), EBNA-LP was detected in immunoblots as multiple protein species, depending upon the cell line, since EBNA-LP has the W1W2 multirepeat domain (Fig. 6D). The expression level of EBNA-LP in each cell line was variable. LCLs and P3HR1 cells expressed relatively abundant EBNA-LP, while Raji and B95-8 cells showed much lower levels of expression (Fig. 6D). Northern blot analysis revealed that the mRNA levels of TARC in the EBV-infected cells were variable (Fig. 6B). TARC expression was barely detectable in P3HR1, Raji, B95-8, and control Ramos and primary B cells (Fig. 6B). In contrast, the mRNA levels of TARC in LCLs were significantly higher than those in the other cell lines. Consistently, LCLs secreted a significantly higher level of TARC protein than primary B cells and the Ramos, P3HR1, Raji, and B95-8 cell lines (Fig. 6C). TARC secretion appeared to correlate with expression of EBNA-LP (Fig. 6C and D). P3HR1 cells were the exception, since levels of TARC secretion were barely detectable even though the cells expressed relatively abundant EBNA-LP. However, the genome of EBV in P3HR1 has a deletion in the region encoding EBNA-2, as well as a deletion of the Y1Y2 domain of EBNA-LP. Therefore, the cells express defective EBNA-LP that contains only W repeat domains (30).
FIG. 6.
TARC expression in EBV-negative and -positive B-cell lines. (A) EBV genomes in the indicated cells and cell lines were analyzed by PCR followed by Southern blot analysis. (B) Steady-state levels of TARC or G3PDH mRNA in the indicated cells and cell lines were examined by Northern blot analysis. (C) Quantification of the relative amounts of TARC protein secreted in the supernatants of the indicated cells and cell lines. Experiments were done exactly as described in the legend for Fig. 4C. The results are shown as values relative to those for Ramos cells. The results represent averages from three independent experiments, and standard deviations are presented. (D) Expression of EBNA-LP or β-actin protein in the indicated cell lines was analyzed by use of immunoblots probed with the EBNA-LP antibody (LP-4D3). Molecular weights (in thousands) are shown on the left.
Lack of effect of TARC on EBV-induced B-cell transformation.
The effect of TARC on EBV-induced B-cell transformation was examined. Normal human PBMCs were infected with EBV, either with or without TARC. The cultures were also incubated with either TARC-neutralizing antibody (54025.11) or a control purified IgG1. The efficiency with which LCLs were established was not influenced by the presence of either TARC or TARC antibody (data not shown). These results indicate that TARC is not directly involved in EBV-induced B-cell transformation.
DISCUSSION
The key finding of our study is that the EBNA-LP is able to stimulate expression of a T-cell chemoattractant, TARC, in the absence of other EBV proteins. To our knowledge, this is the first example of a cellular gene whose expression is stimulated by EBNA-LP alone. The important aspects of this study are as follows.
(i) Microarray analysis identified TARC as a novel target of EBNA-LP. In microarray analysis, we identified several candidate genes that are regulated in response of EBNA-LP expression in B cells. We focused on the TARC gene, since the change in expression of TARC that was mediated by EBNA-LP was the highest among the candidate genes. The up-regulation of TARC mRNA, mediated by EBNA-LP, was confirmed by Northern blot analysis, and the up-regulation of TARC protein levels was verified by ELISA. These results reinforced the evidence obtained by microarray analysis that TARC expression in B cells is stimulated by EBNA-LP.
(ii) EBV latency III infection induces TARC expression in B cells. EBV-negative BJAB cells that were converted to EBV positivity by rEBV infection expressed more TARC mRNA and secreted higher levels of TARC protein than parental cells. These data provide additional support for the conclusion that EBV infection stimulates TARC expression in B cells. These results also eliminate the possibility that induction of TARC in B cells by EBNA-LP was a consequence of genetically engineered overexpression of the protein by a retrovirus vector. Furthermore, we found a potential correlation between TARC secretion and EBNA-LP expression in EBV-infected cell lines with a latency III phenotype. LCLs expressed abundant EBNA-LP and secreted a significantly high level of TARC protein, while Raji and B95-8 cells exhibited low levels of expression of both EBNA-LP and TARC. P3HR1 was exceptional in that it showed high levels of EBNA-LP expression but no TARC secretion. This is probably because P3HR1 has a deletion in the carboxyl-terminal domain of EBNA-LP as well as the whole region encoding EBNA-2. These results suggest that functional EBNA-LP expression is required for TARC induction. As described in the introduction, EBNA-LP is a coactivator of EBNA-2 (23, 46, 58). We also performed similar microarray analysis with BJAB cells stably expressing EBNA-2. The results were that EBNA-2 was not able to induce TARC in B cells, suggesting that EBNA-2 may not be involved in the induction of TARC in EBV-infected B cells (data not shown).
Our data support our hypothesis that EBV infection induces TARC expression in B cells and that the EBNA-LP is the viral gene product responsible for the induction. The relevant issues are as follows.
(i) Chemokines, a family of low-molecular-weight proteins, play an essential role in providing directional cues for the trafficking of leukocytes to sites of inflammation (72). A growing body of evidence suggests that their function is not restricted to chemotaxis, since they have been implicated in cell proliferation (7, 19), cell adhesion (40, 68), angiogenesis (61), and apoptosis (69). Conserved cysteine residues that are appropriately spaced are the hallmark of the two major subfamilies of chemokines, designated CXC and CC (9). TARC is the first CC chemokine shown to selectively attract T lymphocytes (29). TARC is a functional ligand for CC chemokine receptor 4, which is selectively expressed on Th2 cells (27) and induces chemotaxis of Th2-type CD4+ T lymphocytes in vitro (28). Consistent with the in vitro data, it has been reported that TARC is associated with Th2-type diseases, such as atopic dermatitis and bronchial asthma (18, 32, 55, 56, 71). TARC, therefore, plays a key role in regulating the trafficking and effector functions of Th2 cells. These features of TARC render it an ideal target for a viral protein such as EBNA-LP, since it is well known that some viruses, including EBV, have evolved mechanisms to evade detection and ultimately deregulate the host immune response.
(ii) A role for chemokines in virally encoded functions is shared by herpesviruses. Both beta- and gammaherpesviruses have the ability to modify the cellular chemokine environment, by encoding either chemokines, chemokine homologues, or chemokine receptors (1). The physiological roles of the virally encoded, chemokine-related activities are unknown. However, accumulating evidence suggests that they play roles in viral dissemination (15, 49, 53, 75), viral pathogenesis (6), and the immune response (10, 13, 59, 60). Since some herpesviruses encode homologues in their genome, whereas others encode viral proteins that modulate the level of chemokines, it is conceivable that herpesviruses employ different mechanisms for affecting chemokine activity.
(iii) The biological significance of the TARC induction mediated by EBNA-LP is unclear. One hypothesis is that TARC induction is beneficial to EBV-induced B-cell transformation and survival of infected cells. TARC is a chemoattractant for Th2-type CD4+ T cells (28), which express both CD40 ligand and Th2 cytokines such as interleukin-4 that induce B-cell activation (4). Th2-type CD4+ T cells may be attracted to EBV-infected B cells by EBNA-LP-induced TARC and stimulate the infected B cells by the effects of CD40 ligand and interleukin-4. Earlier reports that the development of EBV-driven human B-cell lymphoproliferative disorders and tumors in SCID/hu mice is dependent on the presence of T cells, especially CD4+ T cells (8, 70), support this possibility. In addition, EBV-specific CD4+ T cells mediate activation of resting B cells and induction of expression of viral BZLF1, a viral lytic cycle transactivator, in latently infected B cells via the CD40 ligand- and CD40-dependent pathway (16). Furthermore, it is well established that Th2 cytokines down-regulate the Th1 immune response (11, 52). As suggested from the model of Kaposi's sarcoma-related herpesvirus-encoded chemokines (10, 13, 59, 60), TARC induced by EBNA-LP may drive the immune response from a Th1- towards a Th2-type by recruitment of Th2-type T cells. This immunomodulatory action may be involved in immune evasion of EBV-infected B cells.
An alternative hypothesis is that the TARC induced by EBNA-LP functions as an autocrine factor for activation of chemokine receptors, which is known to modulate pathways typical of those attributed to growth factor-mediated cell activation and induction of cell proliferation (72). However, this possibility is made less likely by the previous report that peripheral blood resting B cells, EBV-immortalized B cells, and the EBV-positive BL lines (Akata, Daudi, Raji, Jijoye, and AG876) do not express CC chemokine receptor 4 (45). Consistently, the presence of excess amounts of TARC or the neutralizing antibody to TARC had no effect on EBV-induced B-cell transformation (data not shown). Furthermore, the Janus-associated kinase-STAT, mitogen-activated protein kinase pathway, and focal adhesion kinase activation, all of which can be engaged following chemokine receptor activation (72), remained unchanged when EBNA-LP was overexpressed in B cells (data not shown).
In conclusion, the EBV regulatory protein EBNA-LP is able to induce TARC expression in B cells. The biological significance of the TARC induction mediated by EBNA-LP is unknown. Further experimentation is clearly important to determine the full range of biological activities of TARC, as well as to investigate a possible role in immune evasion during EBV infection.
Acknowledgments
We thank E. Kieff for EBNA-LP cDNA and E. Pear for pMSCV-puro and Bing. We thank E. Iwata, T. Tsuruguchi, and H. Noma for technical assistance. We also thank all of the members of our laboratory for helpful discussions.
This study was supported in part by Grants-in-Aid for Scientific Research (to Y.K. and Y.N.) and Grants-in-Aid for Scientific Research in Priority Areas (to Y.K. and Y.N.) from the Ministry of Education, Culture, Science, Sports and Technology (MEXT) of Japan and the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 2.Alfieri, C., M. Birkenbach, and E. Kieff. 1991. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology 181:595-608. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. J., G. J. Inman, B. D. Parker, D. T. Rowe, and P. J. Farrell. 1992. Cell growth effects of Epstein-Barr virus leader protein. J. Gen. Virol. 73:1547-1551. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., P. de Paoli, A. Valle, E. Garcia, and F. Rousset. 1991. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science 251:70-72. [DOI] [PubMed] [Google Scholar]
- 5.Bandobashi, K., A. Maeda, N. Teramoto, N. Nagy, L. Szekely, H. Taguchi, I. Miyoshi, G. Klein, and E. Klein. 2001. Intranuclear localization of the transcription coadaptor CBP/p300 and the transcription factor RBP-Jk in relation to EBNA-2 and -5 in B lymphocytes. Virology 288:275-282. [DOI] [PubMed] [Google Scholar]
- 6.Boshoff, C., Y. Endo, P. D. Collins, Y. Takeuchi, J. D. Reeves, V. L. Schweickart, M. A. Siani, T. Sasaki, T. J. Williams, P. W. Gray, P. S. Moore, Y. Chang, and R. A. Weiss. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278:290-294. [DOI] [PubMed] [Google Scholar]
- 7.Broxmeyer, H. E., B. Sherry, L. Lu, S. Cooper, C. Carow, S. D. Wolpe, and A. Cerami. 1989. Myelopoietic enhancing effects of murine macrophage inflammatory proteins 1 and 2 on colony formation in vitro by murine and human bone marrow granulocyte/macrophage progenitor cells. J. Exp. Med. 170:1583-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coles, R. E., T. J. Boyle, J. M. DiMaio, K. R. Berend, D. F. Via, and H. K. Lyerly. 1994. T cells or active Epstein-Barr virus infection in the development of lymphoproliferative disease in human B cell-injected severe combined immunodeficient mice. Ann. Surg. Oncol. 1:405-410. [DOI] [PubMed] [Google Scholar]
- 9.Curnock, A. P., M. K. Logan, and S. G. Ward. 2002. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology 105:125-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dairaghi, D. J., R. A. Fan, B. E. McMaster, M. R. Hanley, and T. J. Schall. 1999. HHV8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J. Biol. Chem. 274:21569-21574. [DOI] [PubMed] [Google Scholar]
- 11.Del Prete, G. 1998. The concept of type-1 and type-2 helper T cells and their cytokines in humans. Int. Rev. Immunol. 16:427-455. [DOI] [PubMed] [Google Scholar]
- 12.Dufva, M., J. Flodin, A. Nerstedt, U. Ruetschi, and L. Rymo. 2002. Epstein-Barr virus nuclear antigen 5 inhibits pre-mRNA cleavage and polyadenylation. Nucleic Acids Res. 30:2131-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endres, M. J., C. G. Garlisi, H. Xiao, L. Shan, and J. A. Hedrick. 1999. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J. Exp. Med. 189:1993-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke, J., M. Rowe, B. Kallin, I. Ernberg, A. Rosen, J. Dillner, and G. Klein. 1987. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J. Virol. 61:3870-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming, P., N. Davis-Poynter, M. Degli-Esposti, E. Densley, J. Papadimitriou, G. Shellam, and H. Farrell. 1999. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 73:6800-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu, Z., and M. J. Cannon. 2000. Functional analysis of the CD4+ T-cell response to Epstein-Barr virus: T-cell-mediated activation of resting B cells and induction of viral BZLF1 expression. J. Virol. 74:6675-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galfre, G., and C. Milstein. 1981. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 73:3-46. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalo, J. A., Y. Pan, C. M. Lloyd, G. Q. Jia, G. Yu, B. Dussault, C. A. Powers, A. E. Proudfoot, A. J. Coyle, D. Gearing, and J. C. Gutierrez-Ramos. 1999. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J. Immunol. 163:403-411. [PubMed] [Google Scholar]
- 19.Graham, G. J., L. Zhou, J. A. Weatherbee, M. L. Tsang, M. Napolitano, W. J. Leonard, and I. B. Pragnell. 1993. Characterization of a receptor for macrophage inflammatory protein 1 alpha and related proteins on human and murine cells. Cell Growth Differ. 4:137-146. [PubMed] [Google Scholar]
- 20.Hammerschmidt, W., and B. Sugden. 1989. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature 340:393-397. [DOI] [PubMed] [Google Scholar]
- 21.Han, I., S. Harada, D. Weaver, Y. Xue, W. Lane, S. Orstavik, B. Skalhegg, and E. Kieff. 2001. EBNA-LP associates with cellular proteins including DNA-PK and HA95. J. Virol. 75:2475-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, I., Y. Xue, S. Harada, S. Orstavik, B. Skalhegg, and E. Kieff. 2002. Protein kinase A associates with HA95 and affects transcriptional coactivation by Epstein-Barr virus nuclear proteins. Mol. Cell. Biol. 22:2136-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi, M., Y. Kawaguchi, K. Hirai, and F. Mizuno. 2003. Physical interaction of Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) with human oestrogen-related receptor 1 (hERR1): hERR1 interacts with a conserved domain of EBNA-LP that is critical for EBV-induced B-cell immortalization. J. Gen. Virol. 84:319-327. [DOI] [PubMed] [Google Scholar]
- 25.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai, S., M. Sugiura, O. Oikawa, S. Koizumi, M. Hirao, H. Kimura, H. Hayashibara, N. Terai, H. Tsutsumi, T. Oda, S. Chiba, and T. Osato. 1996. Epstein-Barr virus (EBV)-carrying and -expressing T-cell lines established from severe chronic active EBV infection. Blood 87:1446-1457. [PubMed] [Google Scholar]
- 27.Imai, T., M. Baba, M. Nishimura, M. Kakizaki, S. Takagi, and O. Yoshie. 1997. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J. Biol. Chem. 272:15036-15042. [DOI] [PubMed] [Google Scholar]
- 28.Imai, T., M. Nagira, S. Takagi, M. Kakizaki, M. Nishimura, J. Wang, P. W. Gray, K. Matsushima, and O. Yoshie. 1999. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int. Immunol. 11:81-88. [DOI] [PubMed] [Google Scholar]
- 29.Imai, T., T. Yoshida, M. Baba, M. Nishimura, M. Kakizaki, and O. Yoshie. 1996. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J. Biol. Chem. 271:21514-21521. [DOI] [PubMed] [Google Scholar]
- 30.Jeang, K. T., and S. D. Hayward. 1983. Organization of the Epstein-Barr virus DNA molecule. III. Location of the P3HR-1 deletion junction and characterization of the NotI repeat units that form part of the template for an abundant 12-O-tetradecanoylphorbol-13-acetate-induced mRNA transcript. J. Virol. 48:135-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang, W. Q., L. Szekely, V. Wendel-Hansen, N. Ringertz, G. Klein, and A. Rosen. 1991. Co-localization of the retinoblastoma protein and the Epstein-Barr virus-encoded nuclear antigen EBNA-5. Exp. Cell Res. 197:314-318. [DOI] [PubMed] [Google Scholar]
- 32.Kakinuma, T., K. Nakamura, M. Wakugawa, H. Mitsui, Y. Tada, H. Saeki, H. Torii, A. Asahina, N. Onai, K. Matsushima, and K. Tamaki. 2001. Thymus and activation-regulated chemokine in atopic dermatitis: serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 107:535-541. [DOI] [PubMed] [Google Scholar]
- 33.Kato, K. Y., A. Tohya, Y. Akashi, H. Nishiyama, and Y. Kawaguchi. 2003.. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine 35 that regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi, Y., K. Maeda, T. Miyazawa, M. Ono, C. Kai, and T. Mikami. 1994. Nucleotide sequence and characterization of the feline herpesvirus type 1 immediate early gene. Virology 204:430-435. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi, Y., K. Nakajima, M. Igarashi, T. Morita, M. Tanaka, M. Suzuki, A. Yokoyama, G. Matsuda, K. Kato, M. Kanamori, and K. Hirai. 2000. Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J. Virol. 74:10104-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams & Wilkins, Philadelphia, Pa.
- 39.Kitay, M. K., and D. T. Rowe. 1996. Protein-protein interactions between Epstein-Barr virus nuclear antigen-LP and cellular gene products: binding of 70-kilodalton heat shock proteins. Virology 220:91-99. [DOI] [PubMed] [Google Scholar]
- 40.Lloyd, A. R., J. J. Oppenheim, D. J. Kelvin, and D. D. Taub. 1996. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J. Immunol. 156:932-938. [PubMed] [Google Scholar]
- 41.Mannick, J. B., J. I. Cohen, M. Birkenbach, A. Marchini, and E. Kieff. 1991. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J. Virol. 65:6826-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mannick, J. B., X. Tong, A. Hemnes, and E. Kieff. 1995. The Epstein-Barr virus nuclear antigen leader protein associates with hsp72/hsc73. J. Virol. 69:8169-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda, G., K. Nakajima, Y. Kawaguchi, Y. Yamanashi, and K. Hirai. 2003. Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) forms complexes with a cellular anti-apoptosis protein Bcl-2 or its EBV counterpart BHRF1 through HS1-associated protein X-1. Microbiol. Immunol. 47:91-99. [DOI] [PubMed] [Google Scholar]
- 44.McCann, E. M., G. L. Kelly, A. B. Rickinson, and A. I. Bell. 2001. Genetic analysis of the Epstein-Barr virus-coded leader protein EBNA-LP as a co-activator of EBNA2 function. J. Gen. Virol. 82:3067-3079. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama, T., R. Fujisawa, D. Izawa, K. Hieshima, K. Takada, and O. Yoshie. 2002. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J. Virol. 76:3072-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nitsche, F., A. Bell, and A. Rickinson. 1997. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J. Virol. 71:6619-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pear, W. S., M. Scott, and G. P. Nolan. 1997. Generation of high titer, helper-free retroviruses by transient transfection. Methods Mol. Med. 7:41-57. [DOI] [PubMed] [Google Scholar]
- 49.Penfold, M. E., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. J. Schall. 1999. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA 96:9839-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng, R., A. V. Gordadze, E. M. Fuentes Panana, F. Wang, J. Zong, G. S. Hayward, J. Tan, and P. D. Ling. 2000. Sequence and functional analysis of EBNA-LP and EBNA2 proteins from nonhuman primate lymphocryptoviruses. J. Virol. 74:379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Williams & Wilkins, Philadelphia, Pa.
- 52.Romagnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227-257. [DOI] [PubMed] [Google Scholar]
- 53.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sample, J., M. Hummel, D. Braun, M. Birkenbach, and E. Kieff. 1986. Nucleotide sequences of mRNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc. Natl. Acad. Sci. USA 83:5096-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekiya, T., M. Miyamasu, M. Imanishi, H. Yamada, T. Nakajima, M. Yamaguchi, T. Fujisawa, R. Pawankar, Y. Sano, K. Ohta, A. Ishii, Y. Morita, K. Yamamoto, K. Matsushima, O. Yoshie, and K. Hirai. 2000. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 165:2205-2213. [DOI] [PubMed] [Google Scholar]
- 56.Sekiya, T., H. Yamada, M. Yamaguchi, K. Yamamoto, A. Ishii, O. Yoshie, Y. Sano, A. Morita, K. Matsushima, and K. Hirai. 2002. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy 57:173-177. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu, N., H. Yoshiyama, and K. Takada. 1996. Clonal propagation of Epstein-Barr virus (EBV) recombinants in EBV-negative Akata cells. J. Virol. 70:7260-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sinclair, A. J., I. Palmero, G. Peters, and P. J. Farrell. 1994. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 13:3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sozzani, S., W. Luini, G. Bianchi, P. Allavena, T. N. Wells, M. Napolitano, G. Bernardini, A. Vecchi, D. D'Ambrosio, D. Mazzeo, F. Sinigaglia, A. Santoni, E. Maggi, S. Romagnani, and A. Mantovani. 1998. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood 92:4036-4039. [PubMed] [Google Scholar]
- 60.Stine, J. T., C. Wood, M. Hill, A. Epp, C. J. Raport, V. L. Schweickart, Y. Endo, T. Sasaki, G. Simmons, C. Boshoff, P. Clapham, Y. Chang, P. Moore, P. W. Gray, and D. Chantry. 2000. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood 95:1151-1157. [PubMed] [Google Scholar]
- 61.Strieter, R. M., P. J. Polverini, D. A. Arenberg, and S. L. Kunkel. 1995. The role of CXC chemokines as regulators of angiogenesis. Shock 4:155-160. [DOI] [PubMed] [Google Scholar]
- 62.Szekely, L., W. Q. Jiang, K. Pokrovskaja, K. G. Wiman, G. Klein, and N. Ringertz. 1995. Reversible nucleolar translocation of Epstein-Barr virus-encoded EBNA-5 and hsp70 proteins after exposure to heat shock or cell density congestion. J. Gen. Virol. 76:2423-2432. [DOI] [PubMed] [Google Scholar]
- 63.Szekely, L., K. Pokrovskaja, W. Q. Jiang, H. de The, N. Ringertz, and G. Klein. 1996. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J. Virol. 70:2562-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szekely, L., G. Selivanova, K. P. Magnusson, G. Klein, and K. G. Wiman. 1993. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc. Natl. Acad. Sci. USA 90:5455-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka, M., Y. Kawaguchi, J. Yokofujita, M. Takagi, Y. Eishi, and K. Hirai. 1999. Sequence variations of Epstein-Barr virus LMP2A gene in gastric carcinoma in Japan. Virus Genes 19:103-111. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka, M., A. Yokoyama, M. Igarashi, G. Matsuda, K. Kato, M. Kanamori, K. Hirai, Y. Kawaguchi, and Y. Yamanashi. 2002. Conserved region CR2 of Epstein-Barr virus nuclear antigen leader protein is a multifunctional domain that mediates self-association as well as nuclear localization and nuclear matrix association. J. Virol. 76:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanaka, Y., D. H. Adams, S. Hubscher, H. Hirano, U. Siebenlist, and S. Shaw. 1993. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature 361:79-82. [DOI] [PubMed] [Google Scholar]
- 69.Van Snick, J., F. Houssiau, P. Proost, J. Van Damme, and J. C. Renauld. 1996. I-309/T cell activation gene-3 chemokine protects murine T cell lymphomas against dexamethasone-induced apoptosis. J. Immunol. 157:2570-2576. [PubMed] [Google Scholar]
- 70.Veronese, M. L., A. Veronesi, E. D'Andrea, A. Del Mistro, S. Indraccolo, M. R. Mazza, M. Mion, R. Zamarchi, C. Menin, M. Panozzo, et al. 1992. Lymphoproliferative disease in human peripheral blood mononuclear cell-injected SCID mice. I. T lymphocyte requirement for B cell tumor generation. J. Exp. Med. 176:1763-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vestergaard, C., H. Yoneyama, M. Murai, K. Nakamura, K. Tamaki, Y. Terashima, T. Imai, O. Yoshie, T. Irimura, H. Mizutani, and K. Matsushima. 1999. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J. Clin. Investig. 104:1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong, M. M., and E. N. Fish. 2003. Chemokines: attractive mediators of the immune response. Semin. Immunol. 15:5-14. [DOI] [PubMed] [Google Scholar]
- 73.Yokoyama, A., Y. Kawaguchi, I. Kitabayashi, M. Ohki, and K. Hirai. 2001. The conserved domain CR2 of Epstein-Barr virus nuclear antigen leader protein is responsible not only for nuclear matrix association but also for nuclear localization. Virology 279:401-413. [DOI] [PubMed] [Google Scholar]
- 74.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zou, P., Y. Isegawa, K. Nakano, M. Haque, Y. Horiguchi, and K. Yamanishi. 1999. Human herpesvirus 6 open reading frame U83 encodes a functional chemokine. J. Virol. 73:5926-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]