Abstract
Subgroup D adenovirus (Ad) types 8, 19, and 37 (Ad8, -19, and -37, respectively) are causative agents of epidemic keratoconjunctivitis and genital tract infections. Previous studies showed that Ad37 binds to a 50-kDa membrane glycoprotein expressed on human ocular (conjunctival) cells. To identify and characterize the role of the 50-kDa glycoprotein in Ad37 infection, we partially purified this molecule from solubilized Chang C conjunctival cell membranes by using lentil lectin chromatography and preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Liquid chromatography coupled to nano-electrospray ionization-tandem mass spectrometry was subsequently used to identify four Ad37 receptor candidates: CD46, CD87, CD98, and CD147. Immunodepletion analyses demonstrated that the 50-kDa protein is identical to CD46 (also known as membrane cofactor protein). The Ad37, but not Ad5, fiber knob bound to the extracellular domain of CD46, demonstrating a direct interaction of an Ad37 capsid protein with CD46. An antibody specific for the N-terminal 19 amino acids of CD46 also blocked Ad37 infection of human cervical carcinoma and conjunctival cells, indicating a requirement for CD46 in infection. Finally, expression of a 50-kDa isoform of human CD46 in a CD46-null cell line increased cell binding by wild-type Ad37 and gene delivery by an Ad vector pseudotyped with the Ad37 fiber, but not by a vector bearing the Ad5 fiber. Together, these studies demonstrate that CD46 serves as an attachment receptor for Ad37 and shed further light on the cell entry pathway of subgroup D Ads.
Adenoviruses (Ads) can infect many different human organs, including the upper respiratory system, the gastrointestinal tract, and the eye (for a review, see reference 32). The 51 known serotypes of human Ads are classified into six subgroups (A to F), defined by oncogenicity, erythrocyte hemagglutination patterns, and DNA homology (32). While subgroup B, C, D, and E Ads have been isolated from conjunctivitis (viral pink eye) patients, only certain subgroup D Ad serotypes (Ad8, Ad19, and Ad37) are associated with epidemic keratoconjunctivitis (EKC), a severe and highly contagious eye infection (17). EKC causes temporary blurred vision and irritation, with symptoms lasting weeks to months (42). In addition, Ad37 and the highly homologous Ad19 cause genital tract infections, such as cervicitis in women and urethritis in men, which are often accompanied by conjunctivitis (2, 21, 49). Currently, there are no effective treatments for these diseases. The receptor binding proteins (fibers) of Ad37 and Ad19a are identical (2, 40), suggesting that Ad37 and Ad19a tropism for the eye and genital tract is mediated by the expression of a common receptor.
Fibers of Ads from most subgroups bind to the coxsackievirus-Ad receptor (CAR) (8). Only subgroup B Ads (15, 19, 44, 46) and Ad37 (1, 52) have been definitively shown to use different cell attachment receptors. The fiber protein consists of three distinct domains: an N-terminal tail that attaches to the capsid, a central shaft that varies in length and flexibility, and a C-terminal knob that attaches to the receptor (for a review, see reference 13). The knobs of subgroup B Ads lack a binding site for CAR (9), but subgroup D Ads, including Ad37, have been shown to bind CAR immobilized on a solid support (44, 52), although with low affinity (28). The ability of Ad37 to bind CAR at the cell surface is further impaired by their relatively short (47) and rigid (12, 53) shaft domains, which prevent appropriate alignment with CAR on the cell plasma membrane. Thus, Ad37 likely binds to a different receptor on conjunctiva or the genital tract.
Ad37 has been reported to bind sialic acid carbohydrates presented on an unspecified glycoprotein (1) and/or unidentified 50- and 60-kDa glycoproteins expressed on conjunctival epithelial cells (52). On Chinese hamster ovary (CHO) cells, Ad37 recognizes α(2→3)-linked sialic acid displayed on a surface glycoprotein (1). A virus blot overlay protein blot assay (VOPBA) demonstrated calcium-dependent Ad37 binding to 50- and 60-kDa membrane glycoproteins expressed on permissive Chang C cells, a human conjunctival cell line (52). Although both glycoproteins are highly expressed on permissive Chang C cells, only the 60-kDa protein is expressed in less permissive A549 lung epithelial cells. Since the expression of the 50-kDa protein correlated with a substantial increase in Ad37 infection, we reasoned that this protein serves as the primary receptor for this virus type (52). In this study, we used a combination of biochemical techniques, immunological assays, and molecular biological approaches to identify the 50-kDa receptor and confirm its role in Ad37 infection. These studies increase our knowledge of virus tropism and lay the foundation for further studies to define the host cell factors that influence Ad infection of specific organ systems.
MATERIALS AND METHODS
Cells and wild-type viruses.
Human Chang C conjunctival cells, A549 lung epithelial cells, and HeLa cervical carcinoma cells were obtained from the American Type Culture Collection (ATCC; Rockville, Md.). Cells were maintained in complete Dulbecco's modified Eagle's Medium (DMEM; Invitrogen, Carlsbad, Calif.) with 10% fetal bovine serum (FBS). CHO cells that were stably transfected with an expression plasmid containing the C2 isoform of CD46 (CHO-C2 cells) (39) were maintained in complete DMEM-F-12 medium (Invitrogen) with 10% FBS and 0.5-mg/ml G418 sulfate (Invitrogen). CHO cells expressing human decay-accelerating factor (CHO-DAF) (33) were maintained in Ham's F-12 medium with 10% FBS. Wild-type Ad37 virus (ATCC) was propagated in A549 cells and purified by banding on cesium chloride (CsCl) gradients. Briefly, A549 cells were infected at 75 to 80% confluency with Ad37. Virions were purified 2 to 3 days postinfection by ultracentrifugation on 16 to 40% CsCl gradients and dialyzed into a mixture of 10 mM Tris (pH 8.1), 150 mM sodium chloride, and 10% glycerol. The viral protein concentration was determined by Bradford protein assay (Bio-Rad, Richmond, Calif.) and used to calculate the viral particle concentration (1 μg = 4 × 109 Ad2 virions).
Receptor purification and identification.
The details of the Ad37 receptor purification and identification by mass spectrometry (MS) are described elsewhere (S. A. Trauger, E. Wu, S. B. Bark, G. Nemerow, and G. Siuzdak, submitted for publication). Briefly, approximately 5 × 108 Chang conjunctival cells were grown in a Cell Factory (Nunc, Roskilde, Denmark). The cells were washed twice with phosphate-buffered saline (PBS [pH 7.4]) and detached with 5 mM EDTA in PBS for 5 to 10 min. Detached cells were lysed by Dounce homogenization and fractionated by low- and high-speed centrifugation (52). The membrane proteins from the membrane (high-speed pellet) fraction were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in the presence and absence of 0.2 M β-mercaptoethanol and analyzed by VOPBA as previously described (52).
Membrane proteins, solubilized by the addition of CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}to 0.5%, were mixed with ∼600 μl of washed, settled Sepharose beads cross-linked to lectin from Lens culinaris (lentil lectin Sepharose; Sigma, St. Louis, Mo.) and rocked for 1 h at 4°C. The entire volume was transferred into a disposable column (Bio-Rad) and drained. The lentil lectin Sepharose was washed with 8 ml of 20 mM Tris-HCl (pH 8.1), 0.5 M NaCl, 0.5% CHAPS (wash buffer). Bound proteins were eluted with 1.5 ml of 0.2 M α-methylmannoside, 1 mM EDTA, 16 mM Tris-HCl (pH 8.1), 0.4% CHAPS, and 0.4 M NaCl. All remaining bound proteins were eluted with 1.5 ml of 40 mM Tris-HCl (pH 8.1), 1% SDS, 10% glycerol, and trace bromophenol blue (1× SDS buffer). The virus-binding proteins present in each fraction were detected by an Ad37 VOPBA as previously described (52).
Proteins eluted with SDS buffer were concentrated and then subjected to preparative SDS-gel electrophoresis in an 8% Tris-glycine polyacrylamide gel (Invitrogen) without boiling or addition of reducing agents. The gel was stained with Simply Blue (Invitrogen), and a diffuse band from 48 to 50 kDa was excised. The proteins in the gel slice were deglycosylated with PNGase-F, digested with trypsin, reduced, and alkylated, and the resultant peptides were extracted with organic solvent. This complex mixture was analyzed by reverse-phase high-performance liquid chromatography coupled to nano-electrospray tandem MS (nanoLC-MS/MS). Masses of peptides and their fragment ions were compared to predicted masses obtained from proteins in the National Center for Biotechnology Information mammalian protein database to identify the proteins present in the gel slice.
Immunodepletion analysis.
Two hundred microliters of soluble membrane proteins diluted with wash buffer was mixed with 20 μl of 0.5-mg/ml monoclonal antibody against CD46, CD87, CD98, or CD147 (BD Pharmingen, San Diego, Calif.) and rocked at 4°C for 2 h. Fifty microliters of protein A/G agarose (Pierce, Rockford, Ill.) was added to the mixtures, which were then rocked for another 2 h at 4°C. The agarose beads were gently pelleted, and the immunodepleted supernatant was removed and analyzed as described below. The beads were washed with 200 μl of wash buffer three times. Finally, 25 μl of 4× SDS buffer was added to the agarose bead pellets, and the beads were heated to 95°C for 5 min to elute the immunoprecipitated proteins.
Twenty microliters of each immunodepletion supernatant or 10 μl of the immunoprecipitation was separated by electrophoresis in an 8 to 16% Tris-glycine polyacrylamide gradient gel (Invitrogen). The proteins were then transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, Mass.). The membrane was blocked with 5% milk in a mixture of 10 mM Tris-HCl (pH 8.1), 150 mM NaCl, and 0.02% Tween 20 overnight at 4°C. Receptors were detected by an Ad37 VOPBA as previously described (52).
sCD46 and Ad fiber knob construction.
The Ad37 fiber knob domain containing a T7 tag was expressed in bacteria and purified on a T7 tag affinity column as previously described (52). The Ad5 fiber knob containing a hexahistidine tag was expressed in bacteria in a similar fashion as previously described (54) and purified by Ni-agarose affinity chromatography (Qiagen) as described by the manufacturer's instructions. The extracellular domain of the C isoform of CD46 was amplified by a PCR from a mammalian expression plasmid coding for the C2 isoform of CD46 (39) by using Expand DNA polymerase (Roche) and 250 μM 5′ primer SCR1f (5′-GCTAGCTTGTGAGGAGCCACCAACATTTGA-3′) and 3′ primer EXTr (5′-GCGGCCGCATCCAAACTGTCAAGTATTCCT-3′). The PCR product was gel purified and cloned into pCR2.1-TOPO (Invitrogen) according to the manufacturer's instructions. Plasmid DNA was amplified from the culture of a colony of transformed cells and purified by using a Qiagen plasmid Miniprep kit according to instructions. Purified plasmid and mammalian expression plasmid pCEP-Pu-CHis (29) (kindly provided by O. Pertz, The Scripps Research Institute, La Jolla, Calif.) were digested with 5 U of NheI and NotI (New England Biolabs, Beverly, Mass.) restriction enzymes. Digested vector and insert were gel purified and ligated with T4 DNA Ligase (Invitrogen), and the resultant plasmid was then transformed into TOP10 cells (Invitrogen), amplified, and purified with the Qiagen plasmid Maxiprep. 293EBNA cells were transfected with the plasmid by using the calcium phosphate transfection kit (Invitrogen/Gibco). Secreted soluble CD46-C (sCD46) was purified from the culture media 3 to 4 days posttransfection by Ni-agarose affinity chromatography. Purified sCD46 was dialyzed against PBS and analyzed by SDS-PAGE and Western blotting.
Ad37 knob ELISA for binding.
Wells of a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Immulon 4 HBX; Dynex Technologies, Chantilly, Va.) were coated overnight with 1 μg of bovine serum albumin (BSA), purified Ad37 knob (52), or purified Ad5 knob. Wells were briefly blocked with Superblock in PBS (Pierce) and incubated with various amounts of sCD46 in BLOTTO in PBS (Pierce) containing 1 mM calcium chloride for 1.5 h at room temperature. Knob-coated wells were washed with PBS-CaCl2, incubated with CD46 rabbit polyclonal antibody (Santa Cruz Biotechnology), washed, incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody, and washed again. The ELISA was developed with ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrates and analyzed by measuring A405.
Ad37 pseudotyping of an Ad5 vector by replacement of the fiber gene.
To generate an Ad37 pseudotype with a low ratio of particles per infectious unit, the Ad5 fiber was removed from the Ad5 vector genome and replaced with the Ad37 fiber (Ad5.GFP.37f). First, a derivative of pAdEasy (22) with a chimeric Ad5/Ad37 fiber was generated. The plasmid pDV153 (D.Von Seggern, unpublished observations) contains the rightmost part of an E3-deleted Ad5 genome and has a unique MfeI site immediately downstream of the fiber stop codon. The 3′ fragment (encoding the shaft and knob domains) of the fiber gene was removed by digestion of pDV153 with SphI and MfeI. The corresponding portion of the Ad37 fiber was amplified from viral genomic DNA by using the PCR primers 5′-TACCAATGGCATGCTATCCCTCAAGG-3′ and 5′-AAACACGGCAATTGGTCTTTCATTC-3′ and inserted into MfeI/SphI-digested pDV153. (The SphI and MfeI sites are underlined.) The resulting plasmid (pDV154) contains a fusion protein consisting of 59 amino acids of the Ad5 fiber (the tail domain) and 306 amino acids (the shaft and knob domains) of the Ad37 fiber protein. The SpeI/PacI fragment of pDV154, containing the rightmost 6.2 kb of the Ad5 genome with the modified fiber, was then used to replace the corresponding fragment of pAdEasy1, creating pDV158. Recombination of pDV158 with pAdTrack in Escherichia coli, as described previously (22), generated a plasmid with a full-length Ad5 genome containing the chimeric fiber protein and a cytomegalovirus (CMV)-driven enhanced green fluorescent protein (eGFP) reporter gene cassette. This plasmid was transfected into 293 cells for virus production.
Infection assay.
Human Chang C cells were detached with 5 mM EDTA in DMEM-FBS and split into aliquots of 40,000 cells per well in a 24-well plate. After culture at 37°C, cells were washed twice with sterile PBS to remove serum, and 125 μl of 2× medium 199 (Invitrogen) and 25 μl of 1 M HEPES (pH 7) were added to each well. Polyclonal antibodies raised against N-terminal peptides of CD46 and CD55 (Santa Cruz Biotechnology) were first dialyzed against PBS. Various amounts of antibodies were diluted to 80 μl in sterile PBS and added to cells for 1 h at room temperature with gentle rocking. Twenty microliters of 2.0 × 106 particles of Ad5.GFP.37f per μl was added to each well, and the wells were rocked at room temperature for 2 h. Virus was removed, and cells were cultured overnight in DMEM-FBS. HeLa cells were also preincubated with antibody or 1 mU of neuraminidase from Vibrio cholerae (Sigma) for 30 min at 37°C to increase enzyme activity. A total of 40,000 CHO-DAF and CHO-C2 cells were infected with 1,000 Ad5.GFP or Ad5.GFP.37f particles per cell for 1 h at 37°C in 250 μl of 1:1 complete F-12 medium-PBS mixture with or without 25 μg of anti-CD46 antibody. The CHO cells were washed twice with complete F-12 medium and cultured overnight. The next day, human or CHO cells were detached with trypsin or 5 mM EDTA in complete medium and analyzed for GFP by flow cytometry (FACScan flow cytometer; Becton Dickinson, Franklin Lakes, N.J.).
Cell binding assay.
To quantitate Ad binding, CHO-DAF and CHO-C2 cells were detached with 5 mM EDTA and washed twice with serum-free F-12 medium (SFF) to remove trace EDTA. A total of 2.5 × 105 cells were distributed to microcentrifuge tubes and pelleted by centrifugation. Some pellets were resuspended in 100 μl of SFF with 10 mU of neuraminidase (from V. cholerae; Roche). These cells were incubated at 37°C for 1 h and washed twice with SFF. A total of 109 wild-type Ad37 virions (4,000 particles per cell) were added to all samples in 100 μl of PBS in the presence or absence of 5 mM EDTA. Cells were rocked at 4°C for 1 h with virus. Cells were pelleted, washed twice with 200 μl of serum-free DMEM, and then resuspended in 200 μl of PBS. Total DNA (viral and genomic) was purified with the QiaAmp DNA Mini kit (Qiagen, Valencia, Calif.) according to the manufacturer's instructions.
Ten microliters of purified total DNA was mixed with 25 μl of TaqMan PCR master mix, rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control reagents, 1.5 μl of 10 mM AQ1-D (5′-GCCACCGTGGGGTTCCTAAACTT-3′) and AQ2-D (5′-GCCGCAGTGGTCTTACATGCACATC-3′) primers (0.3 mM final), and 0.1 μl of 100 mM Adenoprobe (5′-6FAM-TGCACCAGACCCGGGCTCAGGTACTCCGA-TAMRA-3′; primers and reagents from Applied Biosystems, Foster City, Calif.). This mixture was then diluted to a final volume of 50 μl. These primers and Adenoprobe were designed to recognize the hexon gene of subgroup D Ads (23). Rodent GAPDH control reagents were designed to amplify a segment of the host cell genomic GAPDH gene. After initial denaturation and activation of the AmpliTaq Gold DNA polymerase by heating to 50°C for 2 min and then 95°C for 10 min, the amplicons were amplified with 40 cycles of 15 s at 95°C followed by 1 min at 60°C. Fluorescence of reporter dyes FAM and VIC was measured during each cycle in an ABI Prism 7900 sequence detection system (Applied Biosystems). Known amounts of wild-type Ad37 and purified cellular DNA were used as standards to determine the number of copies of Ad genomes and cell number in each sample.
Flow cytometry.
CHO-DAF and CHO-C2 cells were detached by 5 mM EDTA in complete F-12 medium. Cells were incubated with medium (control) or a 1:200 dilution of mouse monoclonal antibodies against CD46 or DAF/CD55 (BD Pharmingen) for 1 h on ice, washed with medium, incubated with a 1:500 dilution of Alexa 488-conjugated antimouse antibody for 30 min on ice, and washed again. Cells were resuspended in PBS and analyzed for green fluorescence in a FACScan flow cytometer.
Statistical analyses.
The effects of antibody treatment and concentration on infection of Chang C cells were separately analyzed by a two-way analysis of variance (ANOVA). sCD46 binding to BSA and Ad5 and Ad37 knobs was compared by general linear model repeated-measure analyses of all three data sets and each pair of data sets. The effects of treatments on Ad37 binding to CHO-C2 cells were analyzed by an ANOVA and separate t tests. Statistical analyses were performed by using SPSS 11.0 software (Chicago, Ill.).
RESULTS
Purification and identification of an Ad37 receptor.
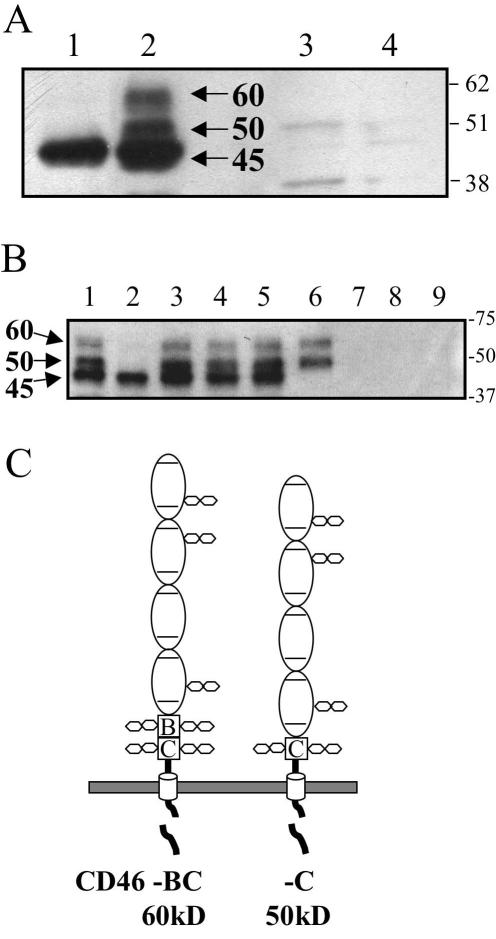
Chang C conjunctival cells, which express ∼24,000 Ad37 binding sites per cell (24), were chosen as the source of the 50- and 60-kDa receptors. Approximately 109 Chang cells, carrying 2.4 × 1013 molecules (or 40 pmol) of receptor, were gently lysed by Dounce homogenization and fractionated by low- and high-speed centrifugation. The high-speed pellet was resuspended and then analyzed for the presence of virus binding proteins by using the VOPBA (Fig. 1A). As described previously, binding of Ad37 to the 50- and 60-kDa proteins was sensitive to chelation of calcium by EDTA (52). In addition, all three membrane proteins failed to bind Ad37 when treated with a reducing agent, suggesting the importance of inter- or intrachain disulfide bonds in receptor structure or function.
FIG. 1.
Identification of the Ad37 receptor. (A) Membrane proteins from Chang C cells were probed on an Ad37-specific VOPBA in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM EDTA as described in Materials and Methods. Proteins in lanes 3 and 4 were reduced with β-mercaptoethanol prior to SDS-PAGE, while proteins in lanes 1 and 2 were not. The 45-, 50-, and 60-kDa proteins that bind Ad37 are indicated by arrows. (B) Soluble Chang membrane proteins (lane 1); proteins immunodepleted with antibodies directed against CD46, CD87, CD98, or CD147 (lanes 2 to 5, respectively); or proteins recovered in the immunoprecipitate (CD46, lane 6; CD87, lane 7; CD98, lane 8; CD147, lane 9) were probed with the Ad37 VOPBA. (C) The domain structure of CD46 is presented schematically. Horizontal lines in each SCR domain represent pairs of conserved disulfide bonds in the CD46 structure. Hexagonal chains represent locations of glycosylation. Four major isoforms (BC1, BC2, C1, and C2) are generated by alternative splicing of coding regions for serine/threonine/proline (STP)-rich domains (squares) and two possible cytoplasmic tails.
The diffuse pattern of the 50- and 60-kDa proteins suggested that these candidate receptors were glycosylated. Thus, we next purified the putative receptors further by using lentil lectin chromatography from detergent-solubilized Chang membrane proteins. This procedure separated these target molecules from >99% of the remaining membrane protein mass. The receptor fraction was concentrated and further separated by semi-native SDS-PAGE. Based on the fact that Ad37, but not Ad19p, recognizes the 50-kDa protein on Chang C cells, but not on less-susceptible A549 cells (52), the region of the gel corresponding to molecular masses of 48 to 50 kDa was excised and subjected to deglycosylation, reduction, and alkylation to improve peptide identification during MS analyses. Proteins were digested by trypsin, and subsequent analysis by reverse-phase liquid chromatography coupled to nanoLC-MS/MS matched peptides to over 50 known proteins, including endoplasmic reticulum and mitochondrial membrane proteins, actin, and keratins. However, only four plasma membrane proteins (CD46, CD87, CD98, and CD147) were detected (Table 1). These analyses are described in further detail elsewhere (S. A. Trauger et al., submitted for publication).
TABLE 1.
Peptides identified by nanoLC-MS/MS analysis of the 50-kDa gel fragment
| Putative receptor | No. of peptides observed | Sequence coverage (%) | Sequences of peptides identified |
|---|---|---|---|
| CD46 | 7 | 27 | CEEPPTFEAMELIGK |
| NHTWLPVSDDACYR | |||
| GSVAIWSGKPPICEK | |||
| VLCTPPPK | |||
| GFYLDGSDTIVCDSNSTWDPPVPK | |||
| GTYLTDETHRa | |||
| ADGGAEYATYQTKa | |||
| CD87 | 5 | 22 | ITSLTEVVCGLDLCNQGNSGR |
| SGCNHPDLDVQYR | |||
| GPMNQCLVATGTHEPK | |||
| LWEEGEELELVEK | |||
| VEECALGQDLCR | |||
| CD98 | 5 | 8 | APAAEKEEAR |
| SADGSAPAGEGEGV TLQR | |||
| AAPAAEEKEEAR | |||
| ALAAPAAEEKEEAR | |||
| VQDAFAAAK | |||
| CD147 | 7 | 36 | AAGTVFTTVEDLGSK |
| GGVVLKEDALPGQK | |||
| FFVSSSQGR | |||
| KPEDVLDDDDAGSAPLK | |||
| RKPEDVLDDDDAGSAPLK | |||
| SELHIENLNMEADPGQYR | |||
| PPVTDWAWYK |
From two cytoplasmic tails of CD46.
Immunodepletion analyses of candidate receptors.
To determine if any of the four membrane proteins corresponded to the 50-kDa protein, antibodies specific for CD46, CD87, CD98, and CD147 were used to immunodeplete the 50-kDa protein in the Ad37 VOPBA (Fig. 1B). Antibodies against CD87, CD98, and CD147 failed to immunodeplete the 50- or 60-kDa virus-binding proteins (compare lanes 3, 4, and 5 with lane 1). In contrast, both the 50- and 60-kDa proteins were immunodepleted by the anti-CD46 antibody (lane 2). Moreover, the 50- and 60-kDa virus-binding molecules were present in the anti-CD46 immunoprecipitate (lane 6). The Ad37-binding 45-kDa protein (Fig. 1A), which served as an internal control, was identified as CAR by using a similar immunodepletion assay (data not shown). These data indicate that, of the four plasma membrane proteins identified by MS analysis, only CD46 directly interacts with Ad37.
CD46, also known as membrane cofactor protein (MCP), is an important regulator of complement activity (for a review, see reference 31). It protects host cells from destruction by the complement system by serving as a cofactor for the enzymatic degradation of C3b and C4b by serum factor I. CD46 also plays an important role in reproduction. The protein consists of a membrane signal peptide; four short consensus repeat (SCR) domains; one (C) or two (BC) regions that are rich in serine, threonine, and proline (STP-B and -C domains); a short sequence of unknown function; a transmembrane domain; and one of two cytoplasmic C-terminal tails. Four main isoforms (BC1, BC2, C1, and C2) are generated by alternative splicing (Fig. 1C). SDS-PAGE of CD46 (data not shown; (4) showed that the C and BC isoforms migrate to the same molecular masses as the candidate 50- and 60-kDa receptors for Ad37 (52).
Recombinant Ad37 fiber knob binds the extracellular domain of CD46.
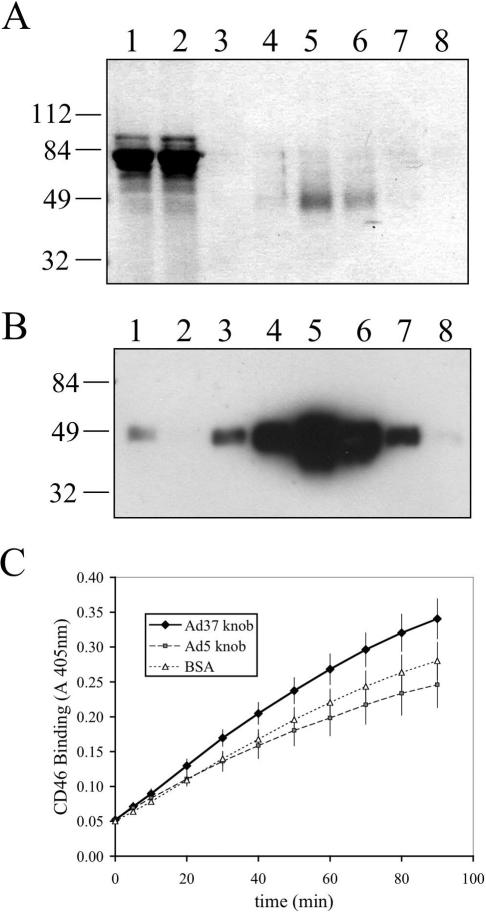
Attachment to cells by most Ads is mediated by the fiber protein (13, 38). Moreover, the cell receptor binding sites of several Ad serotypes have been located in the fiber knob domain (9, 18, 43, 44, 48). To investigate whether the Ad37 fiber knob is capable of direct binding to CD46, we produced soluble, recombinant CD46 (sCD46) containing the complete extracellular domain of the C isoform (Fig. 2A and B). The purified receptor was analyzed for direct binding to an Ad37 knob in an ELISA (Fig. 2C). Soluble CD46 was capable of binding to the Ad37 fiber knob (P > 0.05) but failed to recognize the Ad5 fiber knob (P = 0.30), as determined by comparison to nonspecific binding with BSA. These experiments confirm that Ad37 specifically associates with the extracellular domain of CD46.
FIG. 2.
Purification and analysis of sCD46 binding to Ad fiber knobs. The extracellular domain of the C isoform of CD46 with a C-terminal hexahistidine tag was purified from 293EBNA cell culture medium by Ni-agarose affinity chromatography. Culture medium (lane 1), column flowthrough (lane 2), wash fraction (lane 3), and elution fractions 1 to 5 (lanes 4 to 8, respectively) were analyzed by SDS-PAGE (A) and anti-CD46 Western blotting (B). Binding of purified sCD46 to immoblized BSA or Ad37 or Ad5 fiber knobs in an ELISA (C). Data represent the averages and standard deviations of quadruplicate determinations.
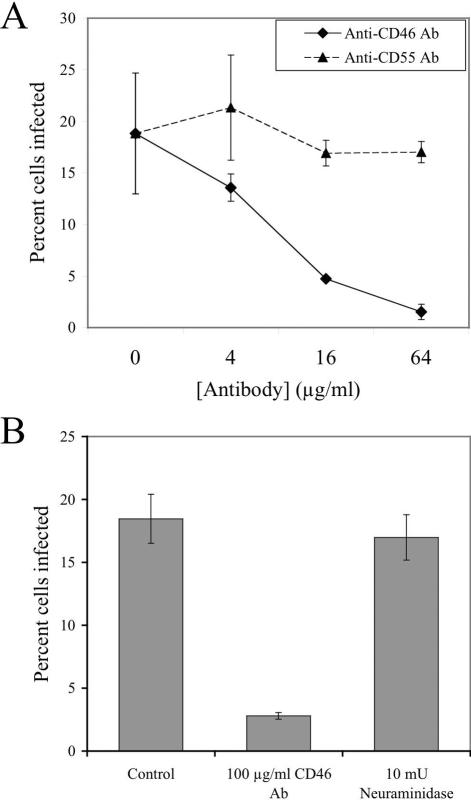
Antibody to CD46 blocks Ad37 infection of Chang conjunctival and HeLa cervical carcinoma cells.
To investigate whether CD46 is actually required for Ad37 infection of host cells, we analyzed virus infection in the presence of a CD46-specific antibody. Preincubation of Chang cells with a monospecific antibody directed against the N-terminal 19 amino acids of CD46 blocked Ad37 infection (Fig. 3A; P < 0.001) in a concentration-dependent manner (two-way ANOVA, P < 0.001). A control antibody specific for the N terminus of CD55 (DAF), another member of the complement control protein family (31), had no effect. Infection of HeLa cervical carcinoma cells, which also express the 50-kDa isoform of CD46 (39), was also inhibited by the anti-CD46 antibody (Fig. 3B). Although Arnberg et al. (3) previously reported that Ad37 uses sialic acid as a receptor on HeLa cells, we failed to detect an effect on Ad37 infection by treatment of these CD46-expressing cells with neuraminidase (Fig. 3B).
FIG. 3.
Antibody specific for CD46 blocks Ad37 infection of Chang conjunctival and HeLa cervical epithelial cells. Chang conjunctival cells were preincubated with various concentrations of an antibody (Ab) specific for the N-terminal 19 amino acids of CD46 or CD55 (DAF) and then infected with 1,000 particles per cell of Ad vector pseudotyped with the Ad37 fiber (A). HeLa cells were preincubated with 100-μg/ml CD46 antibody or 1 mM V. cholerae neuraminidase prior to infection with Ad37 vector (B). Infection was measured as expression of GFP transgene by flow cytometry. Data represent the averages and standard deviations of triplicates.
Expression of CD46 in CHO cells promotes Ad37 infection and cell binding.
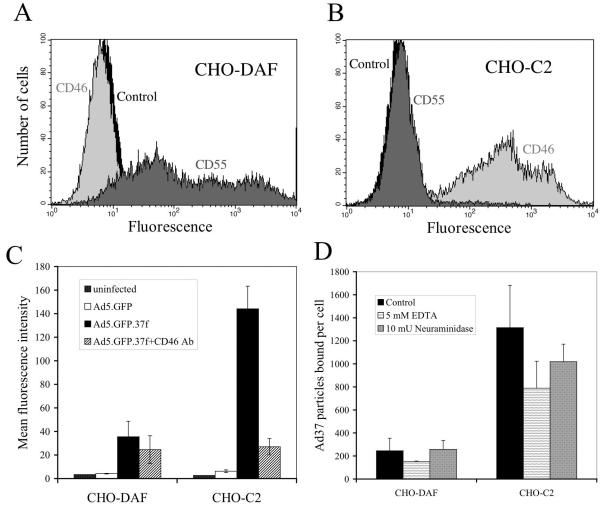
To further explore the function of CD46, we assessed infection of CHO cells expressing human DAF/CD55 (CHO-DAF; Fig. 4A) or the C2 isoform (50 kDa) of CD46 (CHO-C2; Fig. 4B) by an Ad vector pseudotyped with the Ad37 fiber. The extracellular domains of CD46 and CD55/DAF each contain four SCR domains and an STP-rich region. Expression of CD46 resulted in a four- to fivefold enhancement of Ad37 fiber-mediated gene delivery over expression of DAF/CD55 (Fig. 4C). The relatively low level of Ad infection of CHO-DAF cells conferred by the Ad37 fiber was similar to the level observed with receptor-null CHO cells (data not shown) and consistent with previous reports (1). In contrast to Ad37, CD46 expression did not improve Ad5-mediated gene delivery. Antibody directed against CD46 also abolished Ad37 infection of CD46-expressing cells, confirming the requirement for this receptor in Ad37 infection. These data indicate that CD46 specifically promotes Ad infection via the Ad37 fiber, but not the Ad5 fiber.
FIG. 4.
CD46 expression selectively promotes Ad37 infection. CHO cells expressing the human DAF (CHO-DAF) (A) or the C2 isoform of CD46 (CHO-C2) (B) were analyzed by flow cytometry for CD46 (dark gray) and CD55 (light gray) expression with monoclonal antibodies. CHO-C2 cells or CHO-DAF cells were infected with Ad vectors carrying the fibers of Ad5 or Ad37 in the absence or presence of 100 μg of anti-CD46 antibody (Ab) per ml (C). GFP transgene expression was measured 24 h postinfection by flow cytometry. Data represent the averages and standard deviations of quadruplicates. CHO-DAF and CHO-C2 cells were incubated with wild-type Ad37 virus particles at 4°C for 1 h to measure virus binding to cells (D). Bound virus particles and cell counts were determined by quantitative PCR. Data represent the averages and standard deviations of triplicate or quadruplicate determinations of virus counts divided by cell counts (number of particles bound per cell).
Ads use separate receptors for attachment (8) and internalization (51). Cell binding is primarily mediated by the fiber protein, while internalization is enhanced by the interaction between the Ad penton and αv integrins for Ad serotypes in subgroups A to E (35, 51). Monoclonal antibodies against αvβ3 and αvβ5 also blocked pseudotyped Ad37 infection (12), suggesting that the interaction between the Ad37 fiber and its receptor mediates virus attachment, not internalization. To determine if CD46 serves as an attachment receptor, we measured Ad37 binding to receptor-null CHO-DAF and receptor-expressing CHO-C2 cells. The expression of CD46 increased Ad37 binding to CHO cells approximately fivefold in comparison to CHO-DAF cells (Fig. 4D). The increased binding correlates well with the four- to fivefold increase in gene delivery due to CD46 expression (Fig. 4C), indicating that the susceptibility conferred by CD46 expression is mainly due to Ad37 binding. Removal of cell surface sialic acid with neuraminidase had little effect on Ad37 binding to CHO cells expressing DAF or CD46 (P = 0.20), indicating that sialic acid is not required for binding to these cell types. In agreement with previous studies (24), we found that chelation of divalent cations led to a decrease in virus binding (P = 0.07), suggesting a role for divalent cations in Ad37 adhesion to the cell. Together, these data demonstrate that CD46 functions as an attachment receptor for Ad37.
DISCUSSION
Ad37 is a causative agent for EKC and genital tract infections. In an effort to understand Ad37 entry and pathogenesis, we applied improvements in MS and bioinformatics to identify a 50-kDa Ad37 binding protein as CD46. In contrast to previous receptor identification efforts, this approach did not require pure protein. This technique allowed the identification of proteins in a complex sample and substantially reduced the number of possible receptors from thousands of molecules in the human genome to a manageable four candidates. Immunodepletion of each of these four candidates indicated that the Ad37 capsid selectively binds to CD46. We showed that the Ad37 fiber knob directly binds to the extracellular domain of CD46 and that this interaction requires no other viral or cellular proteins. Chelation of divalent cations also had a negative effect on virus adhesion to the cell surface of CD46-expressing CHO cells, which is consistent with the calcium dependence of Ad37 adhesion to human cells (52). The crystal structure of CD46 revealed the presence of a calcium ion located between SCR1 and SCR2, which was important for crystallization (11). This calcium ion may help stabilize CD46 in an orientation that allows efficient Ad37 association. Ablation of Ad37 binding to CD46 upon reduction (Fig. 1A) can be attributed to the eight disulfide bonds that stabilize the four SCRs of CD46 (Fig. 1C). This finding suggests that the Ad37 binding site is located in the SCR domains of CD46. In support of this possibility, Ad37 binding and infection of cells were significantly reduced by an antibody raised against the N-terminal 19 residues of CD46 in SCR1. Importantly, expression of CD46 in CHO cells promotes Ad37, but not Ad5, infection. Together, these studies indicate that CD46 serves as an attachment receptor for Ad37.
CD46 was also recently identified as the receptor for subgroup B Ads, Ad11 and Ad35 (19, 46). In contrast to our studies, Segerman et al. (46) found that an antibody specific for SCR3 and/or -4, but not an antibody specific for SCR1, blocked Ad11 binding to CHO cells expressing the BC1 isoform of CD46 and partially inhibited Ad11 binding to A549 cells (46), suggesting that Ad11 binds an epitope in SCR3 or -4. Unlike Ad37 adhesion to cells (52), Ad11 association with CD46 did not require divalent cations (46). Moreover, expression of the C2 isoform of CD46 on CHO cells had little, if any, effect on gene delivery by Ad9, another subgroup D member (19). In conjunction with the identification of the Ad11 and Ad35 receptor, our experiments indicate that members of the same virus family use distinct modes of interaction to bind to the same receptor and that members of the same Ad subgroup can use different receptors. Further studies will be necessary to more precisely map the sites of Ad37, Ad35, and Ad11 binding to CD46.
In addition to its role as a receptor for Ad37, Ad35, and Ad11, CD46 is also a receptor for several other microbial pathogens, including Neisseria gonorrhoeae (27), human herpesvirus 6 (HHV-6) (45), and measles virus (16, 36). N. gonorrhoeae and measles virus are also associated with conjunctivitis (20, 37), and HHV-6 causes corneal infections and retinitis in AIDS patients (41). In addition, both Ad37 and N. gonorrhoeae are causative agents for genital tract infections. An interesting link between these ocular and genital tract infections is the common receptor usurped by diverse microbes. Moreover, CD46 ligation has been shown to downregulate certain cellular immune responses (34), some of which arise following signal transduction via the receptor cytoplasmic domain (50). Obviously, these receptor-mediated events could influence host responses to these pathogens and thereby impact disease progression. The use of an immunologically important molecule by a growing number of microbial agents therefore may not be coincidental but rather may have evolutionary benefits.
The expression of the C isoforms of CD46 in conjunctival (Chang C) and cervical (HeLa) cells (39) explains the in vitro tropism of Ad37 for these cell types. CD46 is selectively expressed in both the anterior (corneal epithelium) and posterior (retina) chambers of the eye (10) and has also been found in ocular fluids (14). It is likely that the presence of CD46 in the eye contributes to Ad37 tropism for ocular tissue. The identification of CD46 as an Ad37 receptor does not completely explain the limited tropism of this virus, however, because this receptor is broadly distributed on many cell types. One possible explanation is that certain CD46 isoforms (i.e., C versus BC) abundantly expressed in the eye or genital tract may be preferentially used by Ad8, Ad19, and Ad37. In other words, the selective expression of particular CD46 isoforms, which has been shown in the brain and central nervous system (26), may further limit Ad37 tropism in vivo. Ad37 binds well to Chang conjunctival cells, which express both BC and C isoforms of CD46, but poorly to A549 lung epithelials cells (24), which only express the BC isoform (data not shown). This directly correlates with earlier observations that Ad37 only bound the 60-kDa membrane protein on A549 cells, but bound both 50- and 60-kDa proteins on Chang cells (52). Only the expression of the 50-kDa isoform correlates with Ad37 binding and infection, suggesting that Ad37 preferentially associates with the C isoforms of CD46 on cell surfaces. In addition, expression of the BC1 and BC2 isoforms of CD46 on CHO cells resulted in only a small (20 to 30%) increase in Ad37 binding compared to binding of CHO cells (46), indicating that the BC isoforms mediate Ad37 binding poorly. In contrast, we show that expression of the C2 isoform of CD46 on CHO cells increases Ad37 binding and infection of CHO cells by over 300% (Fig. 4), indicating that Ad37 can use the C2 isoform of CD46 as a cell receptor. The contributions of the cytoplasmic tails (CYT1 and CYT2) to Ad37 infection are unknown.
The extracellular domain of the C isoforms differ from the BC isoforms only in the absence of the 15-residue STP-B domain, which has been shown to be heavily O-glycosylated and sialylated. Desialylation with neuraminidase decreased the apparent molecular mass of the BC isoform by ∼8 kDa and changed its pI from 5 to 7 (5). The effect of STP-B on CD46 structure and function is unclear, although it has been shown that the BC isoforms bind C4b and protect cells against complement cytotoxicity better than C isoforms (30). Cofactor activity for C4b cleavage, which maps to SCR2 and SCR3 (25), is apparently affected by STP-B, despite there being no known direct interaction between C4b and STP-B. N. gonorrhoeae, which appears to bind SCR3 of CD46 (27), also preferentially binds to the BC isoforms of CD46 (27). Thus, Ad37, like C4b and N. gonorrhoeae, may recognize a specific CD46 isoform, but in this case prefers the C isoforms of CD46. Ad37 attachment to cells has been shown to be sensitive to the orientation of molecules at the cell surface due to its short and rigid Ad37 fiber protein (53). One possibility is that the heavily sialylated STP-B domain could alter the orientation of CD46 with respect to the cell surface and negatively affect the presentation of the Ad37 binding site at the cell surface.
We observed that Ad37 infected receptor-null CHO cells better than Ad5 (Fig. 4C), a finding previously attributed to sialic acid binding by Ad37 (1). However, the typically weak nature of protein-carbohydrate interactions is inconsistent with the nanomolar dissociation constants (KD) of Ad37 binding to human cells: KD = 3.5 nM for fiber (24) and 0.35 nM for virions (3). It is possible that sialic acid carbohydrates serve as low-affinity virus binding sites on some rodent cells, although rodents are not known to be vectors for Ad37. However, we did not observe a specific requirement for sialic acid for Ad37 binding of CHO cells (Fig. 4D) or Ad37 infection of Chang conjunctival cells (52) or HeLa cells (Fig. 3B). Ad37 VOPBA of neuraminidase-treated Chang conjunctival cells showed that Ad37 no longer bound desialylated 60-kDa protein but still bound the 50-kDa protein (52). Upon further inspection of the VOPBA after identifying the Ad37 receptor as CD46, we found that the 60-kDa protein (CD46-BC) had instead decreased in apparent molecular mass by ∼8 kDa to slightly above that of the 50-kDa protein (CD46-C). The 50-kDa band also decreased in molecular mass by ∼3 kDa, similar to previous analyses of MCP with glycosidases (5), and was hidden by merging with the 45-kDa CAR band. This result clearly demonstrated that wild-type Ad37 virions retained the ability to bind desialylated CD46.
Another possibility is that sialic acid on molecules other than CD46 serves as a coreceptor to strengthen Ad37 interaction with cells. Strengthening of virus-receptor interaction via the use of sialic acid has been reported for reovirus serotype 3 (T3) adhesion to the reovirus receptor (7). Binding of sialic acid by reovirus T3 affected the kinetics of adhesion to HeLa cells but did not change the affinity. Reovirus, which attaches to cells via a fiber-like protein called σ1, still requires the presence of its protein receptor, junction adhesion molecule, for efficient binding to cell surfaces (6). Reovirus binding to sialic acid induces apoptosis in the infected cell. It is unknown whether Ad37 binding to sialic acid has a similar effect or contributes to disease symptoms, but sialic acid binding does not appear to be essential for virus entry into cells expressing CD46.
The identification of the receptor for Ad37 sheds further light on the host cell factors that facilitate Ad cell attachment and entry. MCP/CD46-fiber interaction may also represent a potential new target for treatment of Ad-associated EKC and genital tract infections. Antiviral agents that interfere with the Ad37 fiber-CD46 association may delay or prevent the dissemination of this highly infectious eye disease in hospitals and eye clinics.
Acknowledgments
This work was supported by NIH grants HL54352 and EY11431 to G. R. Nemerow and R01GM5-71094 to G. Siuzdak and a grant from the U.S. Army Breast Cancer Research Program (DAMD17-01-1-0391) for D. J. Von Seggern. E. Wu was supported by the LJIS Interdisciplinary Training Program and The Burroughs Wellcome Fund.
We thank Joan Gausepohl and Kelly White for preparation of the manuscript, Phyllis Frosst (The Scripps Research Institute) for constructing fiber-substituted Ad37, Carrie Wu (University of California, Irvine) for help with statistical analysis, and Marianne Manchester (The Scripps Research Institute) and Douglas Lublin, M. Kathryn Liszewski, and John P. Atkinson (Washington University, Saint Louis, Mo.) for their gift of CD46- and DAF-expressing CHO cells. We also thank Steve Bark for help in setting up the preliminary MS experiments.
Footnotes
This is manuscript no. 15965 from The Scripps Research Institute.
REFERENCES
- 1.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 74:42-48. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnberg, N., Y.-F. Mei, and G. Wadell. 1997. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology 227:239-244. [DOI] [PubMed] [Google Scholar]
- 3.Arnberg, N., P. Pring-Åkerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76:8834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard, L., T. Seya, J. Teckman, D. M. Lublin, and J. P. Atkinson. 1987. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J. Immunol. 138:3850-3855. [PubMed] [Google Scholar]
- 5.Ballard, L. L., N. S. Bora, G. H. Yu, and J. P. Atkinson. 1988. Biochemical characterization of membrane cofactor protein of the complement system. J. Immunol. 141:3923-3929. [PubMed] [Google Scholar]
- 6.Barton, E. S., J. D. Chappell, J. L. Connolly, J. C. Forest, and T. S. Dermody. 2001. Reovirus receptors and apoptosis. Virology 290:173-180. [DOI] [PubMed] [Google Scholar]
- 7.Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. [DOI] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 9.Bewley, M. C., K. Springer, and Y.-B. Zhang. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 10.Bora, N. S., C. L. Gobleman, J. P. Atkinson, J. S. Pepose, and H. J. Kaplan. 1993. Differential expression of the complement regulatory proteins in the human eye. Investig. Ophthalmol. Vis. Sci. 34:3579-3584. [PubMed] [Google Scholar]
- 11.Casasnovas, J. M., M. Larvie, and T. Stehle. 1999. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 18:2911-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu, C. Y., E. Wu, S. L. Brown, D. J. Von Seggern, G. R. Nemerow, and P. L. Stewart. 2001. Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J. Virol. 75:5375-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chroboczek, J., R. W. H. Ruigrok, and S. Cusack. 1995. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199:163-200. [DOI] [PubMed] [Google Scholar]
- 14.Cocuzzi, E., L. B. Szczotka, W. G. Brodbeck, D. S. Bardenstein, T. Wei, and M. E. Medof. 2001. Tears contain the complement regulator CD59 as well as decay-accelerating factor (DAF). Clin. Exp. Immunol. 123:188-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Defer, C., M.-T. Belin, M.-L. Caillet-Boudin, and P. Boulanger. 1990. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J. Virol. 64:3661-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dörig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 17.Ford, E., K. E. Nelson, and D. Warren. 1987. Epidemiology of epidemic keratoconjunctivitis. Epidemiol. Rev. 9:244-261. [DOI] [PubMed] [Google Scholar]
- 18.Freimuth, P., K. Springer, C. Berard, J. Hainfeld, M. Bewley, and J. Flanagan. 1999. Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J. Virol. 73:1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaggar, A., D. M. Shayakhvetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 20.Handsfield, H. H., W. A. Hodson, and K. K. Holms. 1973. Neonatal gonococcal infection. JAMA 225:697-701. [DOI] [PubMed] [Google Scholar]
- 21.Harnett, G. B., and W. A. Newnham. 1981. Isolation of adenovirus type 19 from the male and female genital tracts. Br. J. Vener. Dis. 57:55-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He, T.-C., S. Zhou, L. T. Da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heim, A., C. Ebnet, P. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real time PCR. J. Med. Virol. 70:228-239. [DOI] [PubMed] [Google Scholar]
- 24.Huang, S., V. Reddy, N. Dasgupta, and G. R. Nemerow. 1999. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J. Virol. 73:2798-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata, K., T. Seya, Y. Yanagi, J. M. Pesando, P. M. Johnson, M. Okabe, S. Ueda, H. Ariga, and S. Nagasawa. 1995. Diversity of site for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J. Biol. Chem. 270:15148-15152. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone, R. W., S. M. Russell, B. E. Loveland, and I. F. McKenzie. 1993. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol. Immunol. 30:1231-1241. [DOI] [PubMed] [Google Scholar]
- 27.Kãllstrõm, H., M. K. Liszewski, J. P. Atkinson, and A.-B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 28.Kirby, I., R. Lord, E. Davison, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 2001. Adenovirus type 9 fiber knob binds to the coxsackie B virus-adenovirus receptor (CAR) with lower affinity than fiber knobs of other CAR-binding adenovirus serotypes. J. Virol. 75:7210-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohfeldt, E., P. Maurer, C. Vannahme, and R. Timple. 1997. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 414:557-561. [DOI] [PubMed] [Google Scholar]
- 30.Liszewski, M. K., and J. P. Atkinson. 1996. Membrane cofactor protein (MCP; CD46) isoforms differ in protection against the classical pathway of complement. J. Immunol. 156:4415-4421. [PubMed] [Google Scholar]
- 31.Liszewski, M. K., T. C. Farries, D. M. Lublin, I. A. Rooney, and J. P. Atkinson. 1996. Control of the complement system. Adv. Immunol. 61:201-283. [DOI] [PubMed] [Google Scholar]
- 32.Lukashok, S. A., and M. S. Horwitz. 1998. New perspectives in adenovirus. Curr. Clin. Top. Infect. Dis. 18:286-304. [PubMed] [Google Scholar]
- 33.Manchester, M., A. Valsamakis, R. Kaufman, M. K. Liszewski, J. Alvarez, J. P. Atkinson, D. M. Lublin, and M. B. Oldstone. 1995. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46). Proc. Natl. Acad. Sci. USA 92:2303-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marie, J. C., J. Kehren, M.-C. Trescol-Biémont, A. Evlashev, H. Valentin, T. Walzer, R. Tedone, B. Loveland, J.-F. Nicolas, C. Rabourdin-Combe, and B. Horvat. 2001. Mechanism of measles-virus induced suppression of inflammatory immune responses. Immunity 14:69-79. [DOI] [PubMed] [Google Scholar]
- 35.Mathias, P., M. Galleno, and G. R. Nemerow. 1998. Interactions of soluble recombinant integrin αvβ5 with human adenoviruses. J. Virol. 72:8669-8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oldstone, M. B. A., D. Homann, H. Lewicki, and D. Stevensen. 2002. One, two, or three step: measles virus receptor dance. Virology 299:162-163. [DOI] [PubMed] [Google Scholar]
- 38.Philipson, L., K. Lonberg-Holm, and U. Pettersson. 1968. Virus-receptor interaction in an adenovirus system. J. Virol. 2:1064-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Post, T. W., M. K. Liszewski, E. M. Adams, I. Tedja, E. A. Miller, and J. P. Atkinson. 1991. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J. Exp. Med. 174:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pring-Åkerblom, P., A. Heim, and E. J. Trijssenaar. 1997. Conserved sequences in the fibers of epidemic keratoconjunctivitis associated human adenoviruses. Arch. Virol. 142:205-211. [DOI] [PubMed] [Google Scholar]
- 41.Qavi, H. B., M. T. Green, G. K. SeGall, D. E. Lewis, and F. B. Hollinger. 1992. Frequency of dual infections of corneas with HIV-1 and HHV-6. Curr. Eye Res. 11:315-323. [DOI] [PubMed] [Google Scholar]
- 42.Ritterband, D. C., and D. N. Friedberg. 1998. Virus infections of the eye. Rev. Med. Virol. 8:187-201. [DOI] [PubMed] [Google Scholar]
- 43.Roelvink, P. W., G. M. Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 44.Roelvink, P. W., A. Lizonova, J. G. M. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 46.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shayakhmetov, D. M., and A. Lieber. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274-10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson, S. C., M. Rollence, B. White, L. Weaver, and A. McClelland. 1995. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J. Virol. 69:2850-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swenson, P. D., M. S. Lowens, C. L. Celum, and J. C. Hierholzer. 2003. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J. Clin. Microbiol. 33:2728-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, G., M. K. Liszewski, A. C. Chan, and J. P. Atkinson. 2000. Membrane cofactor protein (MCP; CD46): isoform-specific tyrosine phosphorylation. J. Immunol. 164:1839-1846. [DOI] [PubMed] [Google Scholar]
- 51.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 52.Wu, E., J. Fernandez, S. K. Fleck, D. J. Von Seggern, S. Huang, and G. R. Nemerow. 2001. A 50 kDa membrane protein mediates sialic acid-independent binding and infection of conjunctival cells by adenovirus type 37. Virology 279:78-89. [DOI] [PubMed] [Google Scholar]
- 53.Wu, E., L. Pache, D. J. Von Seggern, T.-M. Mullen, Y. Mikyas, P. L. Stewart, and G. R. Nemerow. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 77:7225-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia, D., L. J. Henry, R. D. Gerard, and J. Deisenhofer. 1994. Crystal structure of the receptor-binding domain of adenovirus type 5 fiber protein at 1.7 Å resolution. Structure 2:1259-1270. [DOI] [PubMed] [Google Scholar]