Abstract
Background
Several studies have investigated whether the polymorphisms in the prostaglandin endoperoxide synthase 1 (PTGS1) and PTGS2 genes and nonsteroidal anti-inflammatory drug (NSAID) use are associated with cancer risk; however, those studies have produced mixed results. Therefore, we performed a meta-analysis to evaluate the association between the PTGS1 and PTGS2 polymorphisms and the effect of NSAID use on the risk of developing cancer.
Methods
We conducted a comprehensive search in PubMed through March 2012. The odds ratios (ORs) with the corresponding 95% confidence intervals (CIs) were calculated using the fixed-effect model or the random-effect model.
Results
The database search generated 13 studies that met the inclusion criteria. For PTGS1 rs3842787, NSAID users homozygous for the major allele (CC) had a significantly decreased cancer risk compared with non-NSAID users (OR = 0.73, 95% CI = 0.59–0.89). For PTGS2 rs5275 and rs20417, there were no significant differences between the gene polymorphism and NSAID use on cancer risk among the 8 and 7 studies, respectively. However, in the stratified analysis by the type of cancer or ethnicity population, NSAID users homozygous for the major allele (TT) in rs5275 demonstrated significantly decreased cancer risk compared with non-NSAID users in cancer type not involving colorectal adenoma (OR = 0.70, 95% CI = 0.59–0.83) and among the USA population (OR = 0.67, 95% CI = 0.56–0.82). NSAID users homozygous for the major allele (GG) in rs20417 displayed a significantly decreased cancer risk than non-NSAID users among the US population (OR = 0.72, 95% CI = 0.58–0.88). For the PTGS2 rs689466 and rs2745557 SNPs, there were no significant differences.
Conclusion
This meta-analysis suggests that the associations between PTGS polymorphisms and NSAID use on cancer risk may differ with regard to the type of cancer and nationality.
Introduction
Prostaglandin endoperoxide synthase 1 (PTGS1) and PTGS2, known as cyclooxygenase 1 (COX1) and COX2, catalyze the oxidative conversion of arachidonic acid to prostaglandin (PG) H2, which is subsequently metabolized to various biologically active metabolites, such as prostacyclin and thromboxane A2 [1]. Although both PTGS1 and PTGS2 catalyze the same committed step in prostanoid biosynthesis with similar efficiencies, they are encoded by distinct genes located on different chromosomes, and they substantially differ in their expression pattern [1]. PTGS1 is constitutively expressed in most tissues and is responsible for the biosynthesis of PGs involved in various housekeeping functions, such as the regulation of renal, gastrointestinal, and platelet function [1]. PTGS2 is rapidly induced by growth factors, inflammatory cytokines, and tumor promoters [2], and it primarily catalyzes PG synthesis in cells involved in both local and systemic inflammatory responses [1].
Inflammation increases the risk of several types of cancer, including colon, prostate, and pancreatic cancer [2], [3]. Therefore, it is postulated that reducing inflammation might decrease the development of cancer. Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit PTGS-mediated PG synthesis and reduce inflammation. NSAIDs are popular medicines used worldwide for the prevention and/or treatment of various diseases. Several epidemiological studies have investigated whether NSAID use is correlated to a reduced risk of developing cancer; however, this is a debatable matter. Furthermore, it is suggested that genetic variation in PTGS1 and PTGS2 might be related to cancer risk and/or drug efficacy in humans. To date, several studies have investigated associations of the polymorphisms in the PTGS1 and PTGS2 genes and NSAID use on cancer risk; however, these studies have produced mixed results. Therefore, we performed a meta-analysis to determine the association between the polymorphisms in PTGS1 and PTGS2 and NSAID use on the risk of developing cancer.
Materials and Methods
Literature Search
We searched for publications in MEDLINE, EMBASE, Science Direct and the Cochrane Library by using the keywords and strategy terms “cyclooxygenase” or “COX” or “PTGS”, “NSAID”, “genotype” or “polymorphism”, and “cancer” or “carcinoma” (last search was in March 2012). Non-controlled trials were excluded. Randomized controlled trials with three or more groups were retained if at least two groups addressed an eligible comparison.
Inclusion Criteria
Studies were chosen if the following criteria were provided: (1) full-text articles were written in English; (2) controlled trials comparing PTGS polymorphisms and the risk of developing cancer, including NSAID use status; (3) sufficient published data for estimating an odds ratio (OR) or relative risk with 95% confidence interval (CI); and (4) the numbers of case, control, NSAID users, and non-NSAID-users by PTGS genotypes were clarified. The following information was not considered as selective criteria: (1) blindness of the trial; (2) type of cancer; (3) type of NSAID; and (4) NSAID dose method.
Data Extraction
Data extraction was performed independently by two authors (Nagao and Sato) by using a standard protocol according to the criteria. The following data were extracted: the name of the first author, year of publication, country of research institution, type of cancer, study design, age, gender, and the number of cases and controls with NSAID users or non-users by genotype.
Statistical Analysis
All statistical analyses were performed using the rmeta package for R, version 2.14.2 (The R Foundation for Statistical Computing, Tsukuba, Japan; http://www.R-project.org). Two-sided probability (P) values of <0.05 were considered statistically significant. ORs with 95% CIs were calculated to assess the strength of the following associations: (1) between PTGS genotype with NSAID users and the risk of developing cancer, (2) between NSAID users homozygous for the major allele and the risk of developing cancer, (3) between PTGS genotype with non-NSAID users and the risk of developing cancer, and (4) between NSAID users with minor allele carriers and the risk of developing cancer.
All meta-analyses were appraised for inter-study heterogeneity by using χ2-based Q statistics for statistical significance of heterogeneity. If there was no heterogeneity based on a Q-test P value more than 0.05, a fixed-effect model using the Mantel-Haenszel (M-H) method was used. Otherwise, the random-effects model using the DerSimonian and Laird method was employed. Sensitivity analyses were performed to assess the stability of the results by sequential omission of individual studies. To evaluate the possible publication bias, Egger’s test (linear regression method) and Begg’s test (rank correlation method) were used, and P values of <0.05 were considered representative of significant statistical publication bias.
Results
Characteristics of the Studies in Our Meta-analysis
A total of 51 relevant reports were initially identified. Thirty-eight of the 51 studies were excluded because they did not meet our criteria. Among the 38 excluded studies, 28 studies did not perform the analysis for recurring SNPs, and 10 studies did not provide the number of subjects to calculate for OR. Therefore, 13 of the 51 studies were included in the meta-analysis (Fig. 1). All of the studies were published in English. The characteristics of the selected studies are summarized in Table 1 and Table S1. The 13 studies analyzed the following polymorphism: PTGS1 rs3842787 (n = 3) [4]–[6], PTGS2 rs5275 (n = 8) [5], [7]–[13], PTGS2 rs20417 (n = 7) [4], [8]–[10], [12], [14], [15], PTGS2 rs689466 (n = 3) [8], [11], [12], and rs2745557 (n = 3) [5], [9], [16].
Figure 1. The flow diagram of the literature search and the study selection.
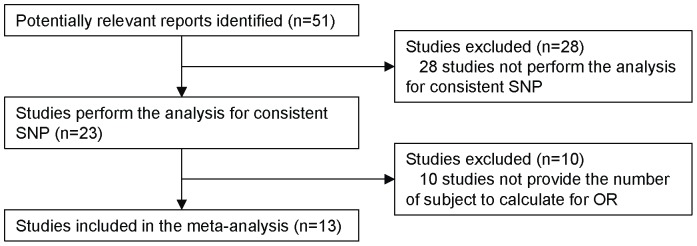
Table 1. Summary of articles included in the meta-analysis.
| Study | Country | Outcome | Study design | Age | Gender | case | control | |||||||||
| Males/Females | No | Yes | No | Yes | ||||||||||||
| PTGS1 rs3842787 | CT+TT/CC | CT+TT/CC | CT+TT/CC | CT+TT/CC | ||||||||||||
| Hubner et al, 2007 [4] | UK | CRA | cohort study | 57.3 ± 9.3 | 289/256 | 8/66 | 8/55 | 20/186 | 30/173 | |||||||
| Gallicchio et al, 2006 [5] | USA | BC | cohort study | 53.2 | 0/1467 (females only) | 10/55 | 2/13 | 136/770 | 51/305 | |||||||
| Ulrich et al, 2004 [6] | USA | CRA | case-control study | 30-74 | Without details | 41/287 | 29/190 | 56/288 | 38/273 | |||||||
| PTGS2 rs5275 | TC+CC/ TT | TC+CC/ TT | TC+CC/ TT | TC+CC/ TT | ||||||||||||
| Lurie et al, 2010 [7] | USA | OC | case-control study | ≥18 | 0/2454 (females only) | 300/282 | 194/172 | 452/375 | 344/361 | |||||||
| Andersen et al, 2009 [8] | Denmark | CRC | cohort study | 50-64 | 619/505 | 151/94 | 61/53 | 306/222 | 144/93 | |||||||
| Barry et al, 2009 [9] | USA | CRA | cohort study | 57.6 ± 9.6 | 630/349 | 81/72 | 156/118 | 103/70 | 200/163 | |||||||
| Gong et al, 2009 [10] | USA | CRA | case-control study | 30-74 | 168/205 | 84/50 | 14/14 | 96/54 | 46/15 | |||||||
| Vogel et al, 2008 [11] | Denmark | LC | nested case-cohort study | 50-64 | 631/516 | 151/125 | 69/54 | 290/218 | 139/90 | |||||||
| Vogel et al, 2007 [12] | Denmark | BCC | nested case-cohort study | 50-64 | 293/326 | 131/92 | 49/29 | 120/97 | 49/46 | |||||||
| Vogel et al, 2006 [13] | Denmark | BC | nested case-cohort study | 50-64 | 0/712 (females only) | 83/73 | 108/92 | 84/50 | 119/103 | |||||||
| Gallicchio et al, 2006 [5] | USA | BC | cohort study | 53.2 | 0/1467 (females only) | 37/29 | 5/9 | 511/396 | 198/158 | |||||||
| PTGS2 rs20417 | GC+CC/GG | GC+CC/GG | GC+CC/GG | GC+CC/GG | ||||||||||||
| Daraei et al, 2012 [14] | Iran | CRC | case-control study | 58.2±14.8 | 117/113 | 64/31 | 8/7 | 47/44 | 19/10 | |||||||
| Andersen et al, 2009 [8] | Denmark | CRC | cohort study | 50–64 | 619/505 | 65/180 | 27/87 | 131/397 | 68/169 | |||||||
| Barry et al, 2009 [9] | USA | CRA | cohort study | 57.6±9.6 | 630/349 | 40/109 | 86/181 | 47/117 | 97/263 | |||||||
| Gong et al, 2009 [10] | USA | CRA | case-control study | 30–74 | 168/205 | 45/89 | 9/19 | 60/90 | 24/37 | |||||||
| Hubner et al, 2007 [4] | UK | CRA | cohort study | 57.3±9.3 | 289/256 | 19/55 | 19/44 | 49/157 | 49/154 | |||||||
| Vogel et al, 2007 [12] | Denmark | BCC | nested case-cohort study | 50–64 | 293/326 | 59/164 | 25/53 | 49/168 | 23/72 | |||||||
| Ulrich et al, 2005 [15] | USA | CRA | case-control study | 30–74 | Without details | 95/217 | 64/127 | 96/228 | 83/177 | |||||||
| PTGS2 rs689466 | AG+GG/AA | AG+GG/AA | AG+GG/AA | AG+GG/AA | ||||||||||||
| Andersen et al, 2009 [8] | Denmark | CRC | cohort study | 50–64 | 619/505 | 89/156 | 40/74 | 199/329 | 84/153 | |||||||
| Vogel et al, 2008 [11] | Denmark | LC | nested case-cohort study | 50–64 | 631/516 | 90/186 | 49/74 | 194/314 | 81/148 | |||||||
| Vogel et al, 2007 [12] | Denmark | BCC | nested case-cohort study | 50–64 | 293/326 | 79/144 | 25/53 | 91/126 | 42/53 | |||||||
| PTGS2 rs2745557 | GA+AA/GG | GA+AA/GG | GA+AA/GG | GA+AA/GG | ||||||||||||
| Barry et al, 2009 [9] | USA | CRA | cohort study | 57.6±9.6 | 630/349 | 50/105 | 89/187 | 59/113 | 114/255 | |||||||
| Cheng et al, 2007 [16] | USA | PC | case-control study | Without details | 1337/0 (males only) | 64/264 | 78/413 | 80/144 | 108/186 | |||||||
| Gallicchio et al, 2006 [5] | USA | BC | cohort study | 53.2 | 0/1467 (females only) | 19/50 | 8/10 | 306/631 | 123/239 | |||||||
Abbreviations: No, non-NSAID users; Yes, NSAID users; PC, prostate cancer; CRC, colorectal cancer; OC, ovarian cancer; CRA, colorectal adenoma; LC, lung cancer; BCC, basal cell carcinoma; BC, breast cancer.
The Hardy-Weinberg equilibrium could not be estimated because the allele frequencies were not clarified in the literature.
Meta-analysis of the PTGS1 Polymorphisms and NSAID Use on the Risk of Developing Cancer
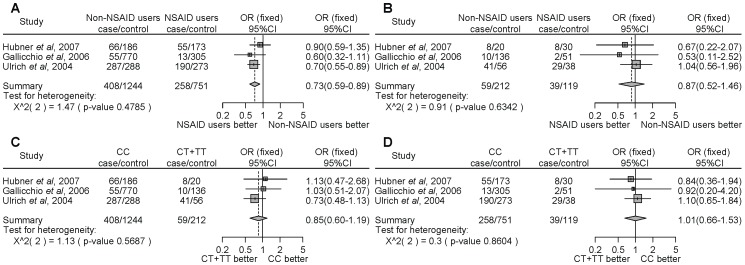
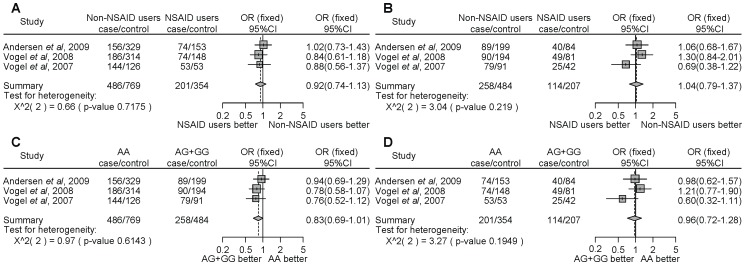
For PTGS1 rs3842787, NSAID users homozygous for the major allele (CC) demonstrated a significantly decreased cancer risk compared with non-NSAID users (Fig. 2A, OR = 0.73, 95% CI = 0.59–0.89). However, there were no significant differences in the risk of developing cancer between NSAID users and non-NSAID users with minor allele carriers (CT+TT) (Fig. 2B, OR = 0.87, 95% CI = 0.52–1.46). There was no significant difference between homozygous for the major allele or carriers of the minor allele among non-NSAID (Fig. 2C, OR = 0.85, 95% CI = 0.60–1.19) or NSAID (Fig. 2D, OR = 1.01, 95% CI = 0.66–1.53) users. We did not detect any significant heterogeneity.
Figure 2. Forest plot of the association between the PTGS1 rs3842787 polymorphism and NSAID use on cancer risk.
The difference in the development of cancer between NSAID use and non-NSAID use from individuals homozygous for the major allele (a), between NSAID use and non-NSAID use from individuals with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
Meta-analysis of the PTGS2 Polymorphisms and NSAID Use on the Risk of Developing Cancer
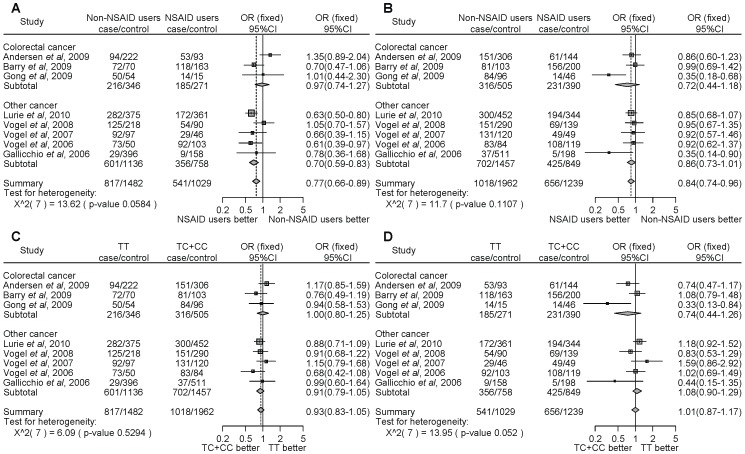
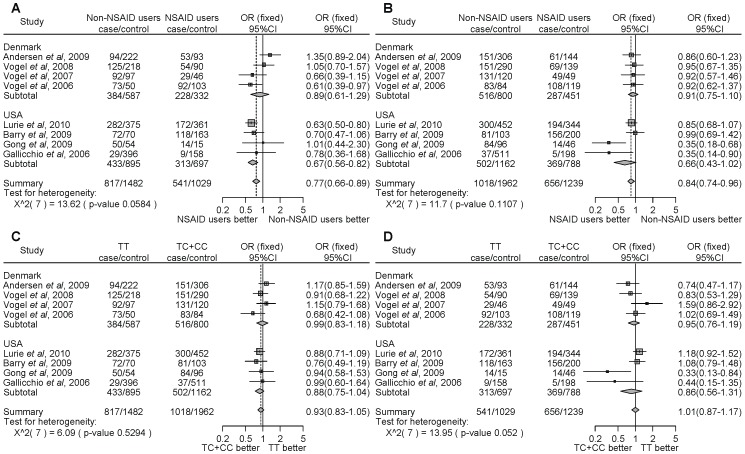
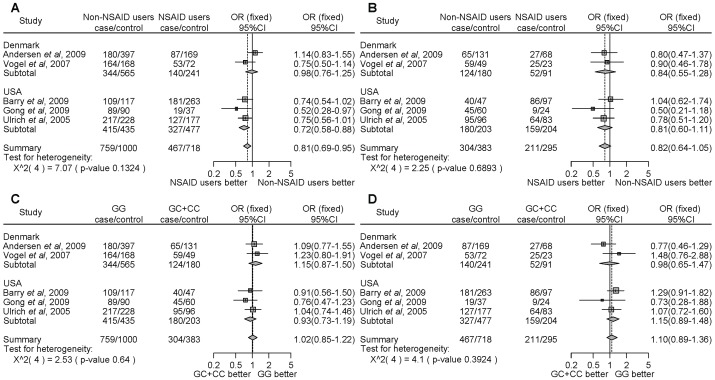
For PTGS2 rs5275, NSAID users significantly decreased the cancer risk compared with non-NSAID users homozygous for the major allele (TT) (Fig. 3A, OR = 0.77, 95% CI = 0.66–0.89). Similarly, NSAID users significantly decreased the cancer risk compared with non-NSAID users with the minor allele carriers (TC+CC) (Fig. 3B, OR = 0.84, 95% CI = 0.74–0.96). However, there were no associations with the PTGS2 rs5275 polymorphism and NSAID use on the risk of developing cancer (Fig. 3C, D). Thus, the results of the meta-analysis among the 8 studies indicate that NSAID use significantly decreased cancer risk compared with non-NSAID use, despite the PTGS2 polymorphism. In the stratified analysis by the type of cancer, there were no associations with colon cancer (Fig. 3A–D). However, NSAID users, in contrast to non-NSAID users, homozygous for the major allele, demonstrated a statistically significant decrease of cancers other than colon cancer (Fig. 3A, OR = 0.70, 95% CI = 0.59–0.83). In the subgroup analysis by locality, there were no associations among people of Denmark (Fig. 4A–D). In the USA, NSAID users, in contrast to non-NSAID users, homozygous for the major allele, demonstrated a statistically significant decrease of cancer. (Fig. 4A, OR = 0.67, 95% CI = 0.56–0.82). We did not detect any significant heterogeneity.
Figure 3. Forest plot of the association between the PTGS2 rs5275 polymorphism and NSAID use on cancer risk stratified by the type of cancer and overall incidence of cancer.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
Figure 4. Forest plot of the association between the PTGS2 rs5275 polymorphism and NSAID use on cancer risk stratified by ethnicity.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
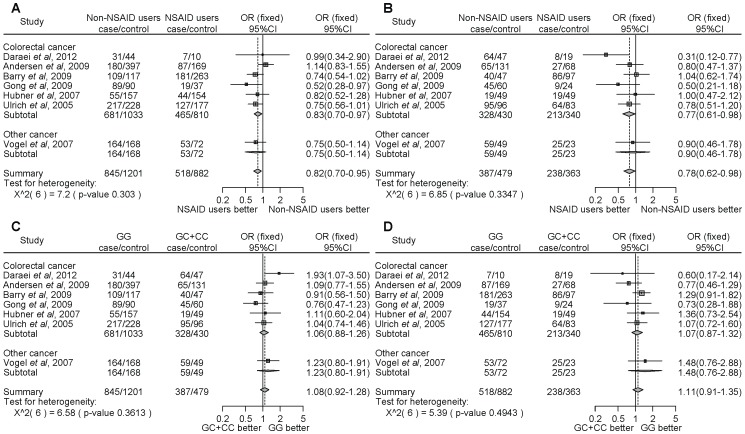
For PTGS2 rs20417, NSAID use significantly decreased cancer risk compared with non-NSAID use in individuals homozygous for the major allele (GG) (Fig. 5A, OR = 0.82, 95% CI = 0.70–0.95). Similarly, NSAID use significantly decreased cancer risk compared with non-NSAID use in individuals with the minor allele carriers (GC+CC) (Fig. 5B, OR = 0.78, 95% CI = 0.62–0.98). However, there were no associations with the risk of developing cancer with NSAID use and the PTGS2 rs20417 polymorphism (Fig. 5C, D). Thus, the results of the meta-analysis among the 7 studies also indicate that NSAID use significantly decreased cancer risk compared with non-NSAID use, regardless of the PTGS2 polymorphism. In the stratified analysis by the type of cancer, NSAID users, in contrast to non-NSAID users, homozygous for the major allele or carriers of the minor allele, demonstrated a statistically significantly decrease in colon cancer risk (Fig. 5A, OR = 0.83, 95% CI = 0.70–0.97; Fig. 5B, OR = 0.77, 95% CI = 0.61–0.98, respectively). In the subgroup analysis by locality, there were no associations among people from Denmark (Fig. 6A–D). In the USA, NSAID users, in contrast to non-NSAID users, homozygous for the major allele demonstrated a statistically significant decrease of cancer (Fig. 6A, OR = 0.72, 95% CI = 0.58–0.88).
Figure 5. Forest plot of the association between the PTGS2 rs20417 polymorphism and NSAID use on cancer risk stratified by the type of cancer and overall incidence of cancer.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
Figure 6. Forest plot of the association between the PTGS2 rs20417 polymorphism and NSAID use on cancer risk stratified by ethnicity.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
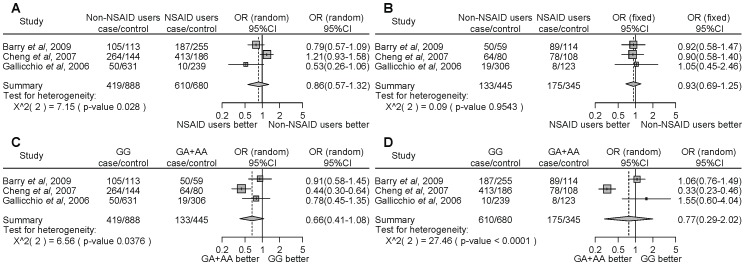
For PTGS2 rs689466 and rs2745557, we found that there were no associations between the risk of developing cancer and NSAID use and polymorphisms (Fig. 7A–D and Fig. 8A–D).
Figure 7. Forest plot of the association between the PTGS2 rs689466 polymorphism and NSAID use on cancer risk.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
Figure 8. Forest plot of the association between the PTGS2 rs2745557 polymorphism and NSAID use on cancer risk.
The difference in the development of cancer between NSAID users and non-NSAID users homozygous for the major allele (a), between NSAID users and non-NSAID users with minor allele carriers (b), between the non-NSAID users homozygous for the major allele and the minor allele carriers (c), and between the NSAID users homozygous for the major allele and the minor allele carriers (d). Squares represent study-specific ORs; horizontal lines represent 95% CIs; size of square reflects study-specific statistical weight (inverse of the variance); diamonds represent summary OR and 95% CI.
Sensitivity Analyses
For PTGS1 rs3842787, sensitivity analyses indicated that the results of one independent study by Ulrich et al. [6] affected our original results considerably, and inclusion of this study was primarily responsible for the significant difference observed in the risk of cancer development between NSAID users and non-NSAID users homozygous for the major allele. For PTGS2 rs5275, sensitivity analyses indicated that inclusion of the independent study by Lurie et al. [7] was primarily responsible for the significant difference observed in the risk of cancer development between NSAID users and non-NSAID users homozygous for the major allele in the overall group, cancer subgroups other than colon cancer, and the USA subgroup. Similarly, inclusion of the independent study by Barry et al. [9] was mainly responsible for our original results in which no associations were observed between gene polymorphism and the risk of cancer development among NSAID users in the colon cancer subgroup. For PTGS2 rs20417, sensitivity analyses indicated that inclusion of the independent studies by Barry et al. [9], Gong et al. [10], and Ulrich et al. [15] was responsible for the significant difference observed in the risk of cancer development between NSAID users and non-NSAID users homozygous for the major allele in the colon cancer subgroup. In addition, inclusion of independent studies by Daraei et al. [14], Gong et al. [10], and Ulrich et al. [15] was found to be primarily responsible for the significant difference in the risk of cancer development between NSAID users and non-NSAID users with minor allele carriers in the overall group and the colon cancer subgroup. For PTGS2 rs689466, sensitivity analyses indicated that inclusion of the independent study by Andersen et al. [8] was mainly responsible for our original results in which no associations were observed between gene polymorphism and the risk of cancer development among non-NSAID users. For PTGS2 rs2745557, sensitivity analyses indicated that the results of one independent study by Cheng et al. [16] were primarily responsible for no significant difference being observed in the risk of cancer development between NSAID users and non-NSAID users homozygous for the major allele. These results suggest that a limited number of studies could substantially influence the ORs.
Publication Bias
Begg’s test and Egger’s test were performed to estimate the publication bias of the literature (Table 2). Egger’s test did not indicate any evidence of potential publication bias; Begg’s test indicated that publication biases generally have no significant effect on the results of overall analysis, except for the association between the PTGS2 rs5275 polymorphism and NSAID users (P = 0.026), which was most likely due to the limited number of studies on PTGS2 rs5275 polymorphism.
Table 2. Egger’s and Begg’s test to measure the funnel plot asymmetric.
| Polymorphisms | ||||
| PTGS1 rs3842787 | No vs. Yes (CC) | No vs. Yes (CT+TT) | CC vs. CT+TT (No) | CC vs. CT+TT (Yes) |
| PE | 0.987 | 0.075 | 0.101 | 0.527 |
| PB | 0.602 | 0.117 | 0.117 | 0.602 |
| PTGS2 rs5275 | No vs. Yes (TT) | No vs. Yes (TC+CC) | TT vs. TC+CC (No) | TT vs. TC+CC (Yes) |
| PE | 0.415 | 0.071 | 0.844 | 0.066 |
| PB | 0.458 | 0.322 | 1.000 | 0.026 |
| PTGS2 rs20417 | No vs. Yes (GG) | No vs. Yes (GC+CC) | GG vs. GC+CC (No) | GG vs. GC+CC (Yes) |
| PE | 0.622 | 0.183 | 0.604 | 0.313 |
| PB | 0.881 | 0.293 | 0.652 | 0.293 |
| PTGS2 rs689466 | No vs. Yes (AA) | No vs. Yes (AG+GG) | AA vs. AG+GG (No) | AA vs. AG+GG (Yes) |
| PE | 0.847 | 0.150 | 0.680 | 0.155 |
| PB | 0.602 | 0.117 | 0.602 | 0.117 |
| PTGS2 rs2745557 | No vs. Yes (GG) | No vs. Yes (GA+AA) | GG vs. GA+AA (No) | GG vs. GA+AA (Yes) |
| PE | 0.379 | 0.065 | 0.431 | 0.768 |
| PB | 0.117 | 0.117 | 0.602 | 0.602 |
Abbreviations: No, non-NSAID users; Yes, NSAID users; PE: P for Egger’s test, PB; P for Begg’s test.
The bold value indicates a potential publication bias.
Discussion
In the current study, we searched the literature to determine the association between PTGS1 or PTGS2 polymorphisms and NSAID use on the risk of developing cancer. Although many SNPs located in the region of PTGS1 are known, 1 polymorphism (rs3842787) was analyzed by 3 independent researchers to determine whether the gene polymorphism and NSAID use is associated with cancer risk. Ulrich et al. [6] reported that NSAID use by individuals with the wild type polymorphism of PTGS1 rs3842787 had a significantly reduced (Fig. 2A, OR = 0.70, 95% CI = 0.55–0.89) adenoma risk compared with non-NSAID users. However, Gallicchio et al. [5] and Hubner et al. [4] reported that there was no association between the PTGS1 rs3842787 polymorphism and NSAID use on the development of cancer. Our meta-analysis showed that the NSAID users had a lower risk of developing cancer compared with the non-NSAID users among individuals homozygous for the major allele of PTGS1 rs3842787. The rs3842787 SNP is located in exon 2 of PTGS1, and causes the substitution of a leucine for a proline at codon 17 (P17L). These results suggest that the PTGS1 rs3842787 non-synonymous polymorphism may be an important pharmacogenomic biomarker.
For PTGS2, there have been studies of 4 SNPs (rs5275, rs20417, rs689466, and rs2745557), which were analyzed for an association with cancer risk and NSAID use; however, the studies have produced mixed results. The rs5275 SNP is located in exon 10 (3′-untranslated region: 3′-UTR) of the PTGS2 gene, which is downstream of the stop codon, and the C allele has been associated with lower steady-state PTGS2 mRNA levels [7]. The rs20417 SNP is located in the promoter region of the PTGS2 gene. The C variant allele of the rs20417 has significantly lower promoter activity than the G allele [10]. In a recent meta-analysis study, the rs20417 emerged to be an influential SNP on colorectal cancer risk in the Asian population [17]. The rs689466 SNP is also located in the promoter region of the PTGS2 gene. The A allele of the rs689466 has been associated with strikingly higher promoter activity [18]. Dong et al. [19] reported that the A allele of rs689466 was significantly associated with increased risk of digestive system cancers. The location of these polymorphisms on the gene promoter region would directly influence the regulation of gene expression and the rate of enzyme production [14]. Therefore, it is considered that these polymorphisms, in conjunction with NSAID use, have an influence on cancer risk; however, our meta-analysis did not detect associations in any group. On the other hand, we found that the associations between PTGS2 polymorphisms and NSAID use on cancer risk differ by the type of cancer and ethnicity. Because PTGS2 is not constitutively expressed in tissues but is induced by growth factors, inflammatory cytokines, and tumor promoters, the effect of NSAIDs on PTGS2 may differ by tissues. Furthermore, Zhang et al. [20] found that the haplotype of PTGS2 including rs20417 and rs689466 SNP was associated with gastric cancer in Chinese populations, which indicates the necessity to study haplotypes.
In these studies, the types of NSAIDs (e.g., aspirin, ibuprofen, and other NSAIDs), dose methods (e.g., dosage and duration), study design (e.g., case control study or cohort study), population (e.g., age, gender, type of cancer, and ethnic), and study power are different. In addition, there was the lack of specificity for cancer type in our analysis because few studies have investigated the effect of associations between polymorphisms in PTGS1 and PTGS2 genes and NSAID use on cancer risk. Thus, it is difficult to draw any conclusion about the relationship between PTGS genotype and NSAID use on the risk of developing cancer. Nonetheless, our results provide limited evidence. Drug response is a complex phenomenon dependent on inherited and environmental factors. To carry more credibility, further analyses with study design formulation are required in several countries.
Supporting Information
Characteristics of studies included in the meta-analysis.
(XLSX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Smith WL, Garavito RM, DeWitt DL (1996) Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160. [DOI] [PubMed] [Google Scholar]
- 2. Prescott SM, Fitzpatrick FA (2000) Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta 1470: 69–78. [DOI] [PubMed] [Google Scholar]
- 3. Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454: 436–444. [DOI] [PubMed] [Google Scholar]
- 4. Hubner RA, Muir KR, Liu JF, Logan RF, Grainge MJ, et al. (2007) Polymorphisms in PTGS1, PTGS2 and IL-10 do not influence colorectal adenoma recurrence in the context of a randomized aspirin intervention trial. Int J Cancer 121: 2001–2004. [DOI] [PubMed] [Google Scholar]
- 5. Gallicchio L, McSorley MA, Newschaffer CJ, Thuita LW, Huang HY, et al. (2006) Nonsteroidal antiinflammatory drugs, cyclooxygenase polymorphisms, and the risk of developing breast carcinoma among women with benign breast disease. Cancer 106: 1443–1452. [DOI] [PubMed] [Google Scholar]
- 6. Ulrich CM, Bigler J, Sparks R, Whitton J, Sibert JG, et al. (2004) Polymorphisms in PTGS1 ( = COX-1) and risk of colorectal polyps. Cancer Epidemiol Biomarkers Prev 13: 889–893. [PubMed] [Google Scholar]
- 7. Lurie G, Terry KL, Wilkens LR, Thompson PJ, McDuffie KE, et al. (2010) Pooled analysis of the association of PTGS2 rs5275 polymorphism and NSAID use with invasive ovarian carcinoma risk. Cancer Causes Control 21: 1731–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen V, Ostergaard M, Christensen J, Overvad K, Tjønneland A, et al. (2009) Polymorphisms in the xenobiotic transporter Multidrug Resistance 1 (MDR1) and interaction with meat intake in relation to risk of colorectal cancer in a Danish prospective case-cohort study. BMC Cancer 9: 407. Available: http://www.biomedcentral.com/1471-2407/9/407. Accessed 8 September 2011. [DOI] [PMC free article] [PubMed]
- 9. Barry EL, Sansbury LB, Grau MV, Ali IU, Tsang S, et al. (2009) Cyclooxygenase-2 polymorphisms, aspirin treatment, and risk for colorectal adenoma recurrence–data from a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 18: 2726–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong Z, Bostick RM, Xie D, Hurley TG, Deng Z, et al. (2009) Genetic polymorphisms in the cyclooxygenase-1 and cyclooxygenase-2 genes and risk of colorectal adenoma. Int J Colorectal Dis 24: 647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogel U, Christensen J, Wallin H, Friis S, Nexø BA, et al. (2008) Polymorphisms in genes involved in the inflammatory response and interaction with NSAID use or smoking in relation to lung cancer risk in a prospective study. Mutat Res 639: 89–100. [DOI] [PubMed] [Google Scholar]
- 12. Vogel U, Christensen J, Wallin H, Friis S, Nexø BA, et al. (2007) Polymorphisms in COX-2, NSAID use and risk of basal cell carcinoma in a prospective study of Danes. Mutat Res 617: 138–146. [DOI] [PubMed] [Google Scholar]
- 13. Vogel U, Christensen J, Nexø BA, Wallin H, Friis S, et al. (2006) Peroxisome proliferator-activated receptor-gamma2 Pro12Ala, interaction with alcohol intake and NSAID use, in relation to risk of breast cancer in a prospective study of Danes. Carcinogenesis 28: 427–434. [DOI] [PubMed] [Google Scholar]
- 14. Daraei A, Salehi R, Mohamadhashem F (2012) PTGS2 (COX2) -765G>C gene polymorphism and risk of sporadic colorectal cancer in Iranian population. Mol Biol Rep 39: 5219–5224. [DOI] [PubMed] [Google Scholar]
- 15. Ulrich CM, Whitton J, Yu JH, Sibert J, Sparks R, et al. (2005) PTGS2 (COX-2) -765G>C promoter variant reduces risk of colorectal adenoma among nonusers of nonsteroidal anti-inflammatory drugs. Cancer Epidemiol Biomarkers Prev 14: 616–619. [DOI] [PubMed] [Google Scholar]
- 16. Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, et al. (2007) COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer 97: 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao H, Xu Z, Long H, Li XQ, Li SL (2010) The -765C allele of the cyclooxygenase-2 gene as a potential risk factor of colorectal cancer: a meta-analysis. Tohoku J Exp Med 222: 15–21. [DOI] [PubMed] [Google Scholar]
- 18. Zhang X, Miao X, Tan W, Ning B, Liu Z, et al. (2005) Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology 129: 565–576. [DOI] [PubMed] [Google Scholar]
- 19. Dong J, Dai J, Zhang M, Hu Z, Shen H (2010) Potentially functional COX-2–1195G>A polymorphism increases the risk of digestive system cancers: a meta-analysis. J Gastroenterol Hepatol 25: 1042–1050. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XM, Zhong R, Liu L, Wang Y, Yuan JX, et al. (2011) Smoking and COX-2 functional polymorphisms interact to increase the risk of gastric cardia adenocarcinoma in Chinese population. PLoS One 6: e21894. Available: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0021894. Accessed 24 May 2013. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of studies included in the meta-analysis.
(XLSX)