Abstract
Mounting evidence suggests that white matter abnormalities and altered subcortical–cortical connectivity may be central to the pathology of schizophrenia (SZ). The anterior limb of the internal capsule (ALIC) is an important thalamo-frontal white-matter tract shown to have volume reductions in SZ and to a lesser degree in schizotypal personality disorder (SPD). While fractional anisotropy (FA) and connectivity abnormalities in the ALIC have been reported in SZ, they have not been examined in SPD. In the current study, magnetic resonance (MRI) and diffusion tensor imaging (DTI) were obtained in age- and sex-matched individuals with SPD (n=33) and healthy controls (HCs; n=38). The ALIC was traced bilaterally on five equally spaced dorsal-to-ventral axial slices from each participant’s MRI scan and co-registered to DTI for the calculation of FA. Tractography was used to examine tracts between the ALIC and two key Brodmann areas (BAs; BA10, BA45) within the dorsolateral prefrontal cortex (DLPFC). Compared with HCs, the SPD participants exhibited (a) smaller relative volume at the mid-ventral ALIC slice level but not the other levels; (b) normal FA within the ALIC; (c) fewer relative number of tracts between the most-dorsal ALIC levels and BA10 but not BA45 and (d) fewer dorsal ALIC–DLPFC tracts were associated with greater symptom severity in SPD. In contrast to prior SZ studies that report lower FA, individuals with SPD show sparing. Our findings are consistent with a pattern of milder thalamo-frontal dysconnectivity in SPD than schizophrenia.
Keywords: Schizotypal personality disorder, Diffusion tensor imaging, Tractography, Magnetic resonance imaging, Anisotropy, Internal capsule
1. Introduction
It has been widely hypothesized that schizophrenia-spectrum illnesses are characterized by abnormal connectivity between different brain regions (Carlsson, 1988; Buchsbaum, 1990a,b; Andreasen et al., 1998), including cortical–subcortical neuronal circuits. One particular white matter bundle that has been implicated in schizophrenia-spectrum pathophysiology is the anterior limb of the internal capsule (ALIC). The ALIC comprises myelinated axons reciprocally connecting the frontal cortex and thalamus (Melchitzky and Lewis, 2009). These fibers are directed horizontally, obliquely, laterally and upwards toward the frontal lobe and the ALIC is distinctly evident in horizontal sections making it suitable for reliable tracing on MRI scans (Carpenter & Sutin, 1993). This circuit and in particular, the dorsolateral prefrontal cortex (DLPFC) region of the frontal lobe are involved in attention, affect, working memory, and language, all of which are reported to be abnormal in schizophrenia, and to a lesser degree in schizotypal personality disorder (SPD) (Tekin and Cummings, 2002; Taber et al., 2004; Buchsbaum et al., 2006a,b,c). Consistent with this idea, Oh et al. (2009) used a diffusion tensor imaging (DTI) measure of fractional anisotropy (FA) and found abnormal projections from the ALIC to the DLPFC in chronic schizophrenia patients.
Volumetric studies have also implicated the ALIC in schizophrenia (reviewed by Di et al., 2009); however, the extent to which individuals with SPD may evince ALIC abnormalities of any type remains unclear. Studying SPD is important because these individuals exhibit schizophrenia-like symptoms including suspiciousness or paranoid ideation, ideas of reference, inappropriate or constricted affect, behavior or appearance that is odd, eccentric, or peculiar (American-Psychiatric-Association, 1994). Individuals with SPD lack frank psychosis, yet they share common genetic, phenomenologic, neurobiologic, outcome, and treatment response characteristics with schizophrenia (Siever et al., 2002). As discussed elsewhere (Buchsbaum et al., 2002; Siever and Davis, 2004; Hazlett et al., 2008a, 2012), neuroimaging data suggest that individuals with SPD exhibit greater frontal reserves which may protect them from the severe cognitive deterioration and social deficits associated with chronic schizophrenia. Thus, the degree to which ALIC abnormalities are found in SPD may help illuminate the pathophysiology of schizophrenia-spectrum disorders.
Considerable work investigating thalamo-frontal abnormalities in schizophrenia has employed conventional magnetic resonance imaging (MRI). We previously reported that schizophrenia patients with poor- but not good-outcome show reduced ALIC volume at the most dorsal levels compared with HCs (Brickman et al., 2006). Wobrock et al. (2008) reported reduced right ALIC volume in family members with schizophrenia, reduced left ALIC volume in family members without schizophrenia and bilateral reduction in maximal cross sectional area of the ALIC in both groups compared with HCs. Aside from the region-of-interest (ROI) approach, studies have also employed the voxel-based morphometry (VBM) method to examine thalamo-frontal abnormalities in first-episode schizophrenia patients prior to long-term medication use and the progressive effects of disease. Chua et al. (2007) reported significantly reduced volume in the right ALIC. Thus, the majority of these MRI studies support the concept of decreased thalamo-frontal connectivity in schizophrenia.
Diffusion tensor imaging (DTI) and fiber tractography studies also offer in-vivo anatomical evidence of disrupted white matter fiber tracts in schizophrenia. DTI provides quantitative measures of white matter integrity such as FA. In general, high FA values correspond to a high degree of axonal alignment (Basser et al., 1994; Pierpaoli et al., 1996). Most (Buchsbaum et al., 1998; Kubicki et al., 2005; Mamah et al., 2010; Mitelman et al., 2007; Skelly et al., 2008; Szeszko et al., 2005; Zou et al., 2008; Levitt et al., 2012) but not all (e.g., Spoletini et al., 2009; Levitt et al., 2010) DTI studies of schizophrenia and first-episode patients, including those using ROI methods and/or voxel-wise analyses, have reported decreased FA in the ALIC. Tract-tracing work has shown decreased FA in internal capsule projections to Brodmann areas (BAs) within the dorsolateral prefrontal cortex (DLPFC) (e.g., Oh et al., 2009) and thalamo-frontal fiber tracts that were followed for shorter distances in schizophrenia (Buchsbaum et al., 2006c). Thus, prior DTI research supports the concept of regional brain disconnection in schizophrenia and suggests that poor ALIC fiber integrity, in particular, may underlie some of the behavioral and cognitive symptoms observed in schizophrenia-spectrum disorders.
Given these schizophrenia-related ALIC abnormalities, as well as work implicating this white matter tract in other psychiatric and neurological disorders (Duran et al., 2009; Kumar et al., 2009; Pavuluri et al., 2009), the current study sought to investigate the ALIC in SPD. To our knowledge, the only study that examined the ALIC in SPD reported decreased right ALIC volume compared with HCs (Suzuki et al., 2004). Another SPD study examining fronto-temporal disconnectivity reported reduced FA in the uncinate fasciculus which was also associated with greater symptom severity (Nakamura et al., 2005). We recently reported lower FA in white matter underlying left temporal lobe but not dorsolateral prefrontal regions in SPD (Hazlett et al., 2011). While this suggests altered fronto-temporal connectivity in SPD, we are unaware of DTI research examining the ALIC and thalamo-frontal connectivity in SPD.
Additionally, we expanded our DTI analysis of anisotropy beyond just FA to include counts of ALIC fiber tracts traveling from the ALIC to two key BAs (BA10 and BA45) within the DLPFC. Inclusion of these two BAs is based on prior neuroimaging work showing abnormalities in internal capsule projections to BA45 in schizophrenia (Oh et al., 2009) and research suggesting that BA10 may be a compensatory region in SPD that may protect against the frank psychosis observed in schizophrenia (e.g., Buchsbaum et al., 1997, 2002; Hazlett et al., 2012).
Given the SPD-related evidence for ALIC volume deficits (Suzuki et al., 2004) and fewer pixels in the region of the mediodorsal nucleus (Hazlett et al., 1999), we hypothesized that similar to schizophrenia, individuals with SPD would show ALIC abnormalities in volume, FA, and the relative number of ALIC–DLPFC fiber tracts. We further hypothesized that among the SPD group, these abnormalities would be associated with symptom severity. Consistent with prior work indicating sparing of frontal lobe FA abnormalities (e.g., Nakamura et al., 2005; Hazlett et al., 2011) in SPD compared with schizophrenia, we expected ALIC abnormalities in SPD to be less marked overall than those observed in schizophrenia.
2. Methods
2.1. Participants
The MRI sample comprised two age-, gender-, and education-matched groups: 33 individuals with SPD and 38 HCs for our volumetric analysis. DTI data were available for a subset of participants also demographically-matched: 29 SPD and 33 HCs. See Table 1 for diagnostic screening and demographic information. All participants provided written informed consent approved by the Mount Sinai IRB. Table 1 provides a description of the symptom-severity score.
Table 1.
Demographics for the healthy controls and individuals with schizotypal personality disorder (SPD).
| Characteristic | Healthy controls (n=38) |
SPD patients (n=33) |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t value | p value | |
| Age (years) | 34.05 | 10.8 | 22–58 | 35.67 | 10.62 | 20–54 | Age: t(69)=−0.63 | 0.53 |
| Educationa | 5.0 | 2.82 | 1–9 | 4.36 | 2.09 | 1–8 | Education: t(69)=1.07 | 0.29 |
| Sex | n | % | n | % | Sex: | |||
| Male | 16 | 42% | 18 | 55% | t(69)=−1.04 | 0.30 | ||
| Female | 22 | 58% | 15 | 45% | ||||
| Handedness | n | % | n | % | Handedness: | |||
| Right | 34 | 89% | 28 | 85% | t(69)=−0.48 | 0.63 | ||
| Left | 3 | 8% | 4 | 12% | ||||
| Both | 1 | 3% | 1 | 3% | ||||
| Symptom severityb | – | – | 7.21 | 1.15 | 5–10.5 | |||
| Past MDDc | n | % | n | % | ||||
| – | – | 7 | 21% | |||||
| Psychoactive meds | n | % | n | % | ||||
| Never medicated | – | – | 27 | 82% | ||||
| Previously medicated | – | – | 6d | 18% | ||||
All study participants were interviewed by a psychologist using the Structured Clinical Interview for DSM-IV Axis I disorders (First et al., 1996) and the Structured Interview for DSM-IV Personality Disorders (SIDP-IV) (Pfohl et al., 1997) followed by a consensus meeting. Exclusion criteria included a history of schizophrenia, a psychotic disorder, bipolar (type I), or current (in the last 6 months) major depressive disorder (MDD). The SPD participants were free of psychoactive medication >2 weeks (majority were never medicated; see table for details). Healthy controls with an Axis-I or II psychiatric illness or an Axis-I diagnosis in a first-degree relative were excluded from the study. Exclusion criteria for all participants included the following: severe medical illness, neurological illness, head injury, past substance dependence, as well as substance abuse or dependence in the prior six months. All participants had a negative urine toxicology screen at the time of the study. All of the healthy controls and the majority of individuals with SPD (90%) were recruited through advertisement in local newspapers. The remaining three individuals with SPD were recruited through referrals from the outpatient-psychiatry clinic at Mount Sinai (these participants did not differ in symptom severity from the 30 recruited from the community and only one of the three had previously received psychoactive medication).
Education=highest degree earned: 1=no high school diploma; 2=GED; 3=high school diploma; 4=technical training; 5=some college, no degree; 6=associate degree; 7 = bachelor's degree; 8 = master’s degree; 9 = MD/PhD/JD/PharmD.
Symptom severity=sum of the severity ratings (see text for full description) for the DSM-IV SPD diagnostic criteria. As required for a DSM-IV diagnosis of SPD, individuals met at least five of the nine SPD criteria. To quantify the level of symptom severity for each patient, each of the DSM-IV criteria established during the SID-P interview was rated on a 4-point scale (0=absent, 0.5=somewhat present, 1.0=definitely present/prototypic, 2.0=severe, pervasive) and then summed using our previously published methods (e.g., Goldstein et al., 2009). Our intra-class correlation for the SID-P is 0.73 for the SPD diagnosis.
MDD=major depressive disorder; past MDD was defined as prior episode occurring >2 months from time of the MRI scan.
Of the 6 SPD patients who previously received psychoactive medications, 2 received an antipsychotic, 5 received an antidepressant, and 3 received a stimulant.
2.2. Image acquisition
All imaging was performed on a 3-T head-dedicated Allegra MRI scanner (Siemens, Ehrlangen, Germany). The following structural scans were acquired: Axial 3D-MP-Rage (repetition time (TR)= 2500 ms, echo time (TE)=4.4 ms, field of view=21 cm, matrix size=256×256, 208 slices with thickness=0.82 mm) and DTI using a pulsed-gradient spin-echo sequence with EPI-acquisition (TR= 4100 ms, TE=80 ms, FOV=21 cm, matrix=128×128, 28 slices, thickness=3 mm, skip=1 mm, b-factor=1250 s/mm2, 12-gradient directions, 5 averages).
2.3. Image processing
Structural MRI and raw DTI scans were transferred to an off-line workstation for post-processing. DICOM files were converted to Analyze 7.5 format using MRIConvert. It also extracted the b-values and gradient directions which are required for using the Functional Software Library package (FSLv.4.1.4; FMRIB, Oxford, UK). Eddy-current correction was done using FSL’s eddy correction tool. Next, a binary mask of the brain was created using the FSL Brain Extraction tool (BET). The FA, eigenvalues, and eigenvectors of the diffusion tensor were generated by FSL’s DTIFit tool. The FA and eigenvector images were coregistered to anterior–posterior commissure (ACPC)-positioned structural MRI with 12-parameter transformation using FSL’s FLIRT tool.
2.4. Tracing the anterior limb of the internal capsule
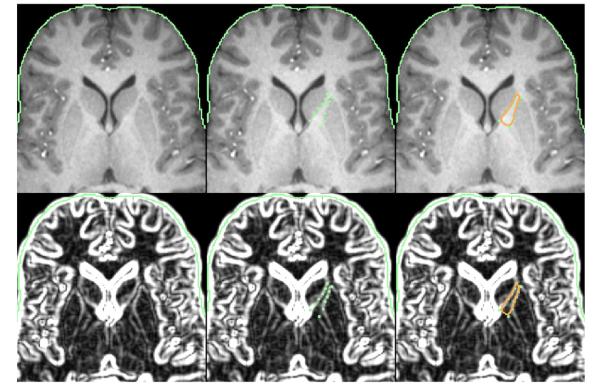
For each participant, the left and right limbs of the anterior internal capsule were manually traced in the axial plane on five dorsal-to-ventral slices using our standard in-house software for visualizing MRI scans (e.g., Buchsbaum et al., 2003; Mitelman et al., 2003) by a single tracer (T.C.) who was blind to diagnosis. We employed the same methods as in our earlier study of schizophrenia patients (scanned on a 1.5-Tesla MRI system; Brickman et al., 2006). These tracing methods are described in Figs. 1 and 2.
Fig. 1.
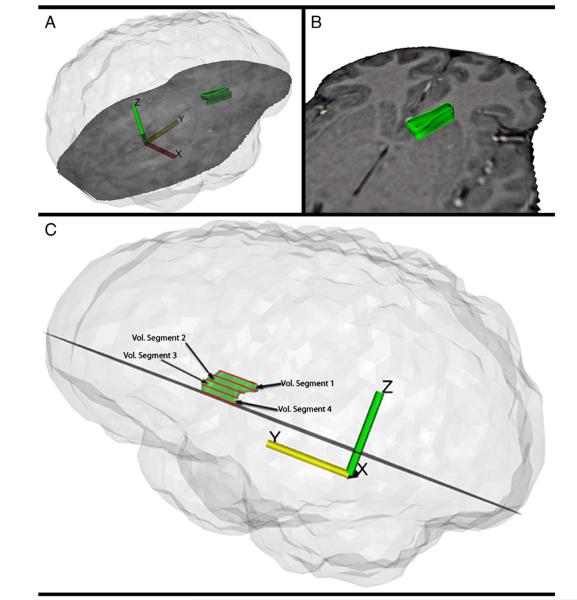
Example of the placement of landmarks for manual tracing of the anterior limb of the internal capsule. The top row depicts a typical axial MRI showing the traced anterior limb of the internal capsule (ALIC) region-of-interest. The bottom row depicts the same MRI image with the automatic boundary-finding method, based on a sobel-gradient filter which allows for maximization of gray/white matter contrast for accurate placement of landmarks on the borders between the striatum and internal capsule. As shown in the middle column, a series of landmark points along the parallel walls of the caudate and putamen were placed to create a polygon containing the fibers of the ALIC. The right column shows the automated spline curve that was used to connect the landmark points and define the ALIC area. As in our prior striatal work (e.g., Buchsbaum et al., 2003), in order to trace the ALIC, we selected five slices based on the anatomy of the striatum with the most dorsal slice defined as the first slice in which gray matter belonging to the putamen was visible. The most ventral slice was defined as the last slice in which the caudate and putamen remained unmerged. The number of slices between the most dorsal and most ventral slices was divided by six to yield an increment accurate to two decimal places. This increment was added to the most ventral slice number five times with the result rounded to remove any decimal. This yielded five proportionally and equally spaced slices for tracing lying between the most dorsal and ventral levels of the ALIC. As discussed in Brickman et al. (2006), this set of axial slices is well suited for the examination of the white-matter tracts within the internal capsule. The ALIC was defined as the region bound medially by the caudate and laterally by the putamen. A sobel-gradient filter and magnified MRI image were used during manual tracing to allow for maximum gray/white matter contrast and accurate placement of landmark points. The ALIC medial boundary was traced along the wall of the caudate between its most lateral anterior corner and its most posterior corner. The lateral boundary of the ALIC was traced along the caudate-parallel wall of the putamen between its most anterior corner and its most medial corner. An automated spline curve was used to connect the landmark points and define the area of the ALIC. ALIC volume was determined for the four contiguous segments lying between the five slices that were traced. ALIC areas obtained from the five slices were used to extrapolate the volume of each of the four segments by the following formula: Segment volume = (A + B + sqrt(AB)) * (H/3) where H=distance between the slices bounding the segment, A=area of the more dorsal-lying slice, and B=area of the more ventral-lying slice (Fig. 2). We obtained absolute ALIC volume (in mm3) which was divided by total brain volume and then multiplied by 100. While ratio (relative) measures may not adequately correct for normal variation in intracranial volume, they are more commonly reported (Hazlett et al., 2008a). To determine inter-rater reliability for tracing the ALIC, two independent tracers (T.C. and J.E.) each traced both the left and right ALIC on the five dorsal/ventral slice levels for a subset of the sample comprising 10 randomly chosen subjects (5 healthy controls and 5 SPD participants). Next, whole left and right volume for the ALIC was compared between the two tracers. The intra-class correlation was 0.95 which is consistent with and in some cases higher than values reported in other studies examining inter-tracer reliability of tracing small structures (Spinks et al., 2002; Levitt et al., 2012).
Fig. 2.
Three-dimensional reconstruction of the anterior limb of the internal capsule. The anterior limb of the internal capsule (ALIC) was traced on five proportionally spaced dorsal-to-ventral slices, yielding ALIC area at the five slice levels. ALIC volume was calculated for four dorsal-to-ventral segments of the ALIC based the areas of consecutive slices and distances between them. Panels A and B show the three-dimensional reconstruction which represent ALIC volume between and including the most dorsal and most ventral slices. Panel C shows the five manually-traced slices in red and the four dorsal to ventral contiguous volume segments. In order to examine group differences in the number of fiber tracts that extend from the ALIC to specific DLPFC Brodmann areas (BAs; BA10 and BA45), we used our cortical Brodmann-area program based on a coronal atlas composed of 33 axial maps of BAs from a microscopic examination of the entire hemisphere of a post-mortem brain. Our earlier use of the Perry atlas (Perry et al., 1991) including published illustrations of the maps has been described in detail elsewhere (e.g., Mitelman et al., 2005). This enabled us to count the number of fiber tracts originating from the five slice levels of the ALIC and extending to the two BA ROIs within the DLPFC. Briefly, we followed the atlas of Mori et al. (2005) and our published fiber-tractography methods which use a multiple-ROI selection technique (Buchsbaum et al., 2006c) beginning with every pixel in the internal capsule at each slice level. As in our prior study, the criterion for continuing the path to the respective BAs was an absolute anisoptropy value of 0.2 (this value is similar to that used in a recent tractography study by Oh et al., 2009), interpolated steps of 0.3 mm and a change of less than 30° from voxel to voxel.
2.5. Fractional anisotropy (FA) and fiber-tract counting
Mean FA within the ALIC was obtained for each of the five slice levels per hemisphere by averaging voxels within each hand-traced ROI. Fiber-tract counting methods are described in Fig. 2.
2.6. Statistical analysis
For all multivariate analysis of variance (MANOVA) analyses, we report the multivariate (Wilks Lambda) F from Statistica (StatSoft Inc, 2010) to adjust probabilities for repeated-measure effects with more than two levels. Fisher’s least significant difference (LSD) post-hoc tests were used to follow-up significant interaction effects with diagnostic group.
To examine group differences in regional ALIC size, dependent variables were the volume values from four dorsal-to-ventral segments of the ALIC in each hemisphere. We conducted MANOVAs on the relative volume variables with diagnostic-group (HCs-vs.-SPD) as the between-group factor and dorsal/ventral segment (1 to 4: dorsal, mid-dorsal, mid-ventral, and ventral) and hemisphere (left, right) as repeated-measures factors. Only relative volume was analyzed because (a) it is the standard; (b) reporting both absolute and relative values doubles the number of comparisons; and (c) analysis of relative and absolute volume values is generally consistent.
For the FA analysis, the dependent variables were the FA values within the ALIC traced on each of the dorsal-to-ventral slices in each hemisphere. The statistical design for examining FA was the same as described above for volume, except with 5 segments (dorsal, mid-dorsal, middle, mid-ventral, and ventral).
To examine between-group differences in thalamo-frontal connectivity, we used tractography to examine the absolute and relative number of fiber tracts between each of the five dorsal/ventral levels of the ALIC as our “seed” and two key DLPFC BAs as our “targets”. The relative number of fiber tracts “terminating” in each DLPFC BA was expressed as a percentage of the total number of fibers counted in the ALIC. We performed a diagnostic-group×dorsal/ventral ALIC slice level (1 to 5:dorsal, mid-dorsal, middle, mid-ventral, ventral)×DLPFC Brodmann area (1 to 2: BA10, 45)×hemisphere (left, right) MANOVA with slice level, DLPFC Brodmann area, and hemisphere as repeated-measures factors.
We conducted exploratory Pearson correlations between overall SPD symptom severity and only the dependent variables that showed significant between-group differences in ALIC volume, FA and/or fiber tracts between DLPFC regions.
3. Results
3.1. Internal capsule and whole brain volume
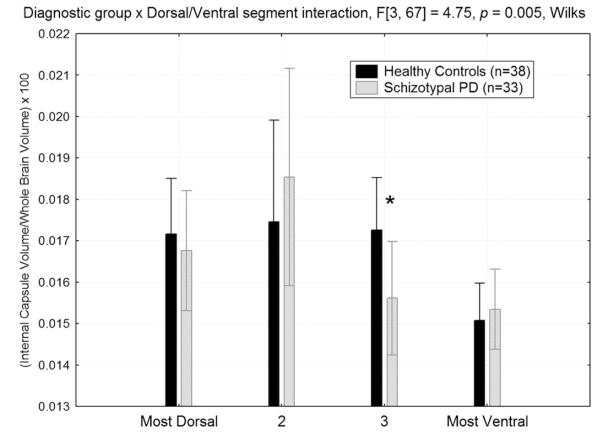
Compared with the HC group, individuals with SPD had significantly smaller relative ALIC volume at the more ventral slice level (slice-3 in Fig. 3), averaged across hemisphere (p<0.05, Fisher’s LSD test) but the groups looked more similar at the most dorsal (slice-1), more dorsal (slice-2) and ventral levels giving rise to a diagnostic-group× dorsal–ventral slice interaction (F[3,67]=4.75, p=0.005, Wilks; Fig. 3). Only the slice-3 follow-up test reached significance.
Fig. 3.
Relative volume of the anterior limb of the internal capsule (ALIC) from the dorsal to ventral slice level in the healthy control and schizotypal personality disorder groups. Compared with healthy controls, the SPD patients had smaller relative ALIC volume at the mid-ventral slice level (labeled slice “3” above), group×dorsal/ventral segment interaction, F[3,67]=4.75, p=0.005, Wilks. The asterisk denotes HC>SPD, Fisher’s LSD post-hoc test, p=0.049. For absolute volume of the ALIC (not shown), we also observed a significant diagnostic group×dorsal–ventral slice interaction (F[3,67]=4.61, p=0.005, Wilks) and the pattern of findings looked nearly identical to that observed for relative volume. The between-group difference for the mid-ventral level of the ALIC was also significant for absolute volume (p=0.02, Fisher’s LSD test).
Overall, relative volume of the ALIC (averaged across both slice level and hemisphere) did not significantly differ between groups (p-values>0.44 for main effect of diagnostic group) and none of the other interactions involving diagnostic group reached significance (p-values>0.20). Of note, whole brain volume (calculated by adding gray and white matter volume for all 39 BAs; see Mitelman et al., 2003) did not differ between groups (p=0.60; HC mean=1,215,492 mm3, SD=122,730; SPD mean=1,200,745 mm3, SD=113,807).
3.2. Fractional anisotropy
There were no significant between-group differences for FA in the ALIC (main effect of Group did show a non-significant trend for being higher in SPD (p=0.09; HC mean=0.32, SE=0.10; SPD mean=0.35, SE=0.11; interactions with group: p-values>0.26).
3.3. Fiber-tract counting from ALIC to prefrontal cortex
As shown in Fig. 4, individuals with SPD had fewer fiber tracts between the more dorsal slice levels of the ALIC and BA10 but not BA45 of the DLPFC, consistent with a regionally-specific deficit (diagnostic-group×DLPFC BA×dorsal/ventral slice interaction, F[4,57]=2.84, p=0.032, Wilks). Despite the significant 3-way interaction, none of the HC-vs.-SPD Fisher’s LSD tests reached significance (p-values>0.16). The groups did not differ in the overall number of relative fiber tracts between the ALIC and the DLPFC (p=0.53).
Fig. 4.
Mean percentage of fiber tracts at five dorsal-to-ventral slice levels of the anterior limb of the internal capsule extending to dorsolateral prefrontal cortex (DLPFC) Brodmann areas (BAs 10 and 45) for healthy control and SPD groups. The SPD group showed fewer relative fiber tracts from the more dorsal (labeled “D” and “2” in the figure) but not ventral (labeled “V” in the figure) part of the ALIC extending to BA10 of the DLPFC. In contrast, the groups did not differ in the number of ALIC fiber tracts extending to BA45 of the DLPFC, diagnostic-group×DLPFC Brodmann area×dorso-ventral slice interaction (F[4,57]=2.84, p=0.032, Wilks.
3.4. Medication
In order to assess for a potential medication confound, the analyses that were statistically significant above were rerun without the six individuals with SPD that previously received psychoactive medication (described in Table 1). The findings from the medication-naïve sample either remained significant or showed trend-level significance.
3.5. Clinical correlates of ALIC relative volume and tract counting
Correlations for relative ALIC volume of the middle-ventral segment (slice-3 in Fig. 3) and relative number of white matter tracts between ALIC (at the dorsal, 2, 3, and 4 slices) and BA10 of the DLPFC (a total of 5 correlations) and overall SPD symptom severity was conducted. Greater symptom severity was associated with fewer number of white matter tracts between dorsal ALIC and BA10, r=−0.39, p<0.04. None of the other correlations reached significance.
4. Discussion
This is the first multi-modal study to examine volume, FA, and tractography of the ALIC in unmedicated individuals with SPD. The study’s main findings demonstrate that SPD differs from normal by showing a pattern of smaller ALIC volume (averaged across hemisphere) at the mid-ventral level and fewer fiber tracts between the ALIC and BA10 but not BA45. Additionally, greater DSM-IV SPD symptom severity is associated with reduced dorsal ALIC–DLPFC fiber tracts.
To our knowledge, only one study has examined ALIC volume in SPD (Suzuki et al., 2004) and the findings indicated smaller absolute ALIC volume in the right hemisphere. Our volume findings are somewhat consistent because we observed smaller-than-normal ALIC volume in SPD, although it was confined to the more-ventral ALIC level. A likely reason for our SPD-related volume deficit being less marked than those reported by Suzuki and colleagues is differing patient characteristics. SPD patients in the present study were unmedicated at the time of their scan and primarily antipsychotic naïve (31 of 33; 94%). In contrast, only 2 of the 24 SPD patients in Suzuki et al. were antipsychotic naïve (8%), suggesting that greater disease severity and/or medication may be an important factor in observing more widespread volumetric differences in the ALIC.
Our volume findings help distinguish SPD from schizophrenia as schizophrenia patients show bilateral ALIC volume reduction (Suzuki et al., 2002; Zhou et al., 2003; Lang et al., 2006) whereas our results indicate less marked and more regionally restricted rather than overall ALIC volume deficits in SPD. This may be an anatomical difference related to better functional outcomes in SPD compared with schizophrenia. Our findings parallel a prior study from our group that examined ALIC volume in good- and poor-outcome schizophrenia (Brickman et al., 2006). Poor-outcome patients had decreased ALIC volume at dorsal levels compared with HCs while ALIC volume in good-outcome patients did not differ from normal. Thus, our SPD findings suggest that the neuropathology of SPD is more consistent with good- than poor-outcome schizophrenia. As such, a spectrum of ALIC volume abnormality may relate to functional capacity and symptom severity. Among the SPD group, those with more severe symptom severity showed greater reduction in ALIC–DLPFC fiber tracts. This supports the concept that dysconnectivity in the ALIC affects symptom severity and may contribute to genetic vulnerability to schizophrenia (Wobrock et al., 2008).
There were no significant between-group FA differences in the ALIC. We are unaware of another study looking specifically at ALIC anisotropy in SPD. Nakamura et al. (2005) reported bilaterally-reduced FA in the uncinate fasciculus, but no significant reduction of FA in the cingulum bundle. We recently reported significantly lower FA in the left temporal lobe but not prefrontal regions in SPD. In the cingulum, FA was lower in the SPD group in the posterior regions (BAs 31 and 23) and higher in the anterior (BA 25) regions (Hazlett et al., 2011). Thus, our finding of normal FA in the ALIC is consistent with the concept that SPD is characterized by sparing of the severe frontal-lobe abnormalities observed in schizophrenia (Siever and Davis, 2004; Hazlett et al., 2008a,b).
Yet another possibility is related to our finding that pulvinar volume is decreased in both patients with schizophrenia and SPD, whereas, MDN volume loss was seen only in schizophrenia (Byne et al., 2001). The pulvinar, particularly its medial division, projects extensively to the PFC (reviewed in Byne et al., 2009). While its projections contribute to the ALIC (Leh et al., 2008), their relative contribution to the volume of the ALIC is not known; nor is it known how the trajectories of these projections might differ from those of other prefrontal projections. It is, therefore, feasible that a loss of pulvinar projections in SPD could contribute to our findings of regionally selective volume deficits within the ALIC.
Our finding of fewer fiber tracts between dorsal ALIC and BA10 in SPD is consistent with a recent study that used a similar methodology to examine ALIC white-matter tracts terminating in the DLPFC. Oh et al. (2009) reported decreased thalamo-frontal tracts in schizophrenia patients including projections from the internal capsule to several DLPFC regions, particularly BA10. In brief, areas 10 and 45 receive their major thalamic inputs from different subdivisions of the MDN, with the projections to BA45 originating more ventrally (Giguere and Goldman-Rakic, 1988; Contini et al., 2010). The reduced number of fiber tracts between the more dorsal levels of the ALIC and BA10 but not BA45 may, therefore, reflect this topography and suggest a deficit of fibers arising from the more anterior portion of the MDN. Of note, fiber tracts traced to the DLPFC are concentrated at the dorsal/superior levels of the ALIC which is perhaps an anatomical function of fiber tracts fanning out to the frontal cortex as they approach the corona radiata (see discussion in Sullivan et al., 2010). While both the MDN and pulvinar project to BA10 (Rotshtein et al., 2011) and BA45 (Contini et al., 2010), fMRI studies suggest that, compared with the pulvinar, the MDN has stronger connectivity with BA45 (Buchsbaum et al., 2006a). Thus, a ALIC-BA10 but not BA45 white-matter tract deficit is consistent with our previous report of a pulvinar but not MDN deficit in SPD (Byne et al., 2001). Finally, the finding of fewer ALIC fiber tracts going to BA10 in SPD is interesting given our prior work showing that SPD patients exhibit higher-than-normal glucose metabolism rates in BA10 (Buchsbaum et al., 2002). Future SPD work is needed to directly test the hypothesis that hyperactivation of BA10 during cognitive processing is, in part, a compensatory mechanism for having fewer thalamo-frontal fiber tract connections as suggested by the current study.
Although we cannot draw functional conclusions from our measure of thalamo-frontal connectivity, our findings are related to prior activation studies in the spectrum. For example, we showed higher relative glucose metabolic rates in BA10 during a verbal-learning task in SPD compared with HCs and schizophrenia patients (Buchsbaum et al., 2002). Also, greater-than-normal activation of frontal–striatal–thalamic circuitry in SPD while trying to ignore a stimulus designed to distract has been reported (Hazlett et al., 2008b). Other studies have also reported greater activation of DLPFC regions, including BA10 during DLPFC-mediated tasks both in individuals with SPD and non-psychotic siblings of schizophrenia patients (e.g., Buchsbaum et al., 1997; Delawalla et al., 2008). A working-memory fMRI study showed that schizophrenia patients who performed poorly exhibit DLPFC hypoactivation, while better-performing patients showed hyperactivation. The authors concluded that although increased activation of the DLPFC was associated with better working memory in patients, compared with HCs, it is inefficient (Callicott et al., 2003). Further, our prior morphometric study showed that compared with HCs, BA10 is larger in SPD and smaller in schizophrenia (Hazlett et al., 2008a). Taken together, it seems reasonable to speculate that individuals with SPD may compensate for fewer ALIC-BA10 white-matter connections by activating BA10 to a greater degree during cognitive tasks and having larger BA10 volume compared with HCs. Future work combining DTI and fMRI is needed to better understand the relationship between connectivity and function.
5. Limitations
A limitation of our study is that we did not include schizophrenia patients which would allow for direct schizophrenia-spectrum comparisons. Additionally, we did not employ multi-modal measures along with MRI and DTI such as white matter genetic variation and neurocognition. Studies have shown that genetic variation in the NRG1-ErbB4 signaling pathway is important for the development of normal white matter and that disruptions in this process may have consequences for white matter integrity within the ALIC, cognitive processing and perhaps, risk of psychosis (e.g., McIntosh et al., 2008; Zuliani et al., 2011).
6. Conclusions
Our findings of abnormalities in ALIC volume and fiber-tract connections support the hypothesis of structural dysconnectivity in SPD. It is interesting to note that unlike prior schizophrenia work showing lower FA in the ALIC (e.g., Levitt et al., 2012), the SPD group showed normal FA consistent with the concept of sparing of frontal–thalamic abnormalities (Siever and Davis, 2004; Hazlett et al., 2008a, 2012). Future multi-modal studies examining matched groups of SPD and schizophrenia patients will be needed to fully understand white-matter abnormalities and the behavioral and genetic correlates across the spectrum.
Acknowledgment
Role of funding Source Funding for this study was provided by NIMH grant 1R01MH073911 to Dr. Erin Hazlett. Partial support was also provided by the Mental Illness Research Education and Clinical Center, VISN3 Veterans Health Administration, and grant UL1RR029887 from the National Center for Research Resources, National Institutes of Health. The funding sources had no role in the study design, collection, analysis, interpretation of data, writing of the manuscript, or in the decision to submit the paper for publication.
None.
Footnotes
Contributors Dr. Hazlett wrote the grant application that secured funding, designed the study, conducted the statistical analyses, and wrote portions of the paper. Mr. Collazo, a medical student, worked on the project under Dr. Hazlett’s mentorship, traced the regions of interest blind to diagnosis, drafted portions of the paper and created the figures. Ms. Zelmanova, Mr. Entis, Dr. Chu, Ms. Goldstein, and Mr. Weiner helped collect the data and provided MRI processing support. Drs. Byne, Roussos, Buchsbaum, and Siever and Mr. Hershowitz provided feedback on the paper. Drs. Koenigsberg and New helped with participant recruitment and diagnosis. All authors have approved the final manuscript.
Conflict of interest All authors declare that they have no conflicts of interest.
References
- American-Psychiatric-Association . DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th ed APA; Washington, D.C.: 1994. [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical–subcortical–cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Buchsbaum MS, Ivanov Z, Borod JC, Foldi NS, Hahn E, et al. Internal capsule size in good and poor outcome schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2006;18:364–376. doi: 10.1176/jnp.2006.18.3.364. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS. The frontal lobes, basal ganglia, and temporal lobes as sites for schizophrenia. Schizophr. Bull. 1990a;16:379–389. doi: 10.1093/schbul/16.3.379. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS. Frontal lobes, basal ganglia, temporal lobes—three sites for schizophrenia? Schizophr. Bull. 1990b;16:377–378. doi: 10.1093/schbul/16.3.377. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Trestman RL, Hazlett E, Siegel BV, Jr., Schaefer CH, Luu-Hsia C, et al. Regional cerebral blood flow during the Wisconsin Card Sort Test in schizotypal personality disorder. Schizophr. Res. 1997;27:21–28. doi: 10.1016/S0920-9964(97)00081-9. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Tang CY, Peled S, Gudbjartsson H, Lu D, Hazlett EA, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. NeuroReport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nenadic I, Hazlett EA, Spiegel-Cohen J, Fleischman MB, Akhavan A, et al. Differential metabolic rates in prefrontal and temporal Brodmann areas in schizophrenia and schizotypal personality disorder. Schizophr. Res. 2002;54:141–150. doi: 10.1016/s0920-9964(01)00361-9. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, et al. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr. Res. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Buchsbaum BR, Chokron S, Tang C, Wei TC, Byne W. Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neurosci. Lett. 2006a;404:282–287. doi: 10.1016/j.neulet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, et al. Diffusion tensor imaging in schizophrenia. Biol. Psychiatry. 2006b;60:1181–1187. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Schoenknecht P, Torosjan Y, Newmark R, Chu KW, Mitelman S, et al. Diffusion tensor imaging of frontal lobe white matter tracts in schizophrenia. Ann. Gen. Psychiatry. 2006c;5:19. doi: 10.1186/1744-859X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitroupoulou V, et al. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch. Gen. Psychiatry. 2001;58:133–140. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am. J. Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Sutin J. Human Neuroanatomy. eighth ed Williams and Wilkins; Baltimore: 1993. [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JTK, Chen EYH, Wong JCH, et al. Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophr. Res. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Contini M, Baccarini M, Borra E, Gerbella M, Rozzi S, Luppino G. Thalamic projections to the macaque caudal ventrolateral prefrontal areas 45A and 45B. Eur. J. Neurosci. 2010;32:1337–1353. doi: 10.1111/j.1460-9568.2010.07390.x. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Csernansky JG, Barch DM. Prefrontal cortex function in non-psychotic siblings of individuals with schizophrenia. Biol. Psychiatry. 2008;63:490–497. doi: 10.1016/j.biopsych.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Chan RC, Gong QY. White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1390–1394. doi: 10.1016/j.pnpbp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Duran FL, Hoexter MQ, Velente AA, Miguel EC, Busatto GF. Association between symptom severity and internal capsule volume in obsessive compulsive disorder. Neurosci. Lett. 2009;452:68–71. doi: 10.1016/j.neulet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer R, Gibbon M, Williams J. Biometrics Research. New York State Psychiatric Institute; New York: 1996. Structured Clinical Interview for Axis I Disorders—Patient Edition. [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J. Comp. Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Goldstein KE, Hazlett EA, New AS, Haznedar MM, Newmark RE, Zelmanova Y, et al. Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophr. Res. 2009;112:14–23. doi: 10.1016/j.schres.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Byne W, Wei TC, Spiegel-Cohen J, Geneve C, et al. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am. J. Psychiatry. 1999;145:1190–1199. doi: 10.1176/ajp.156.8.1190. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Haznedar MM, Newmark R, Goldstein KE, Zelmanova Y, et al. Cortical gray and white matter volume in unmedicated schizotypal and schizophrenia patients. Schizophr. Res. 2008a;101:111–123. doi: 10.1016/j.schres.2007.12.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, et al. Frontal–striatal–thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008b;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Goldstein KE, Tajima-Pozo K, Speidel ER, Zelmanova Y, Entis JJ, et al. Cingulate and temporal lobe fractional anisotropy in schizotypal personality disorder. Neuroimage. 2011;55:900–908. doi: 10.1016/j.neuroimage.2010.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett EA, Goldstein KE, Kolaitis JC. A review of structural MRI and diffusion tensor imaging in schizotypal personality disorder. Curr. Psychiatry Rep. 2012;14:70–78. doi: 10.1007/s11920-011-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J. Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Khorram B, Goghari VM, Kopala LC, Vandorpe RA, Rui Q, et al. Reduced anterior internal capsule and thalamic volumes in first-episode psychosis. Schizophr. Res. 2006;87:89–99. doi: 10.1016/j.schres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Leh SE, Chakravarty MM, Ptito A. The connectivity of the human pulvinar: a diffusion tensor imaging tractography study. Int. J. Biomed. Imaging. 2008;789539 doi: 10.1155/2008/789539. (5 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Kubicki M, Nestor PG, Ersner-Hershfield H, Westin CF, Alvarado JL, et al. A diffusion tensor imaging study of the anterior limb of the internal capsule in schizophrenia. Psychiatry Res. 2010;184:143–150. doi: 10.1016/j.pscychresns.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JJ, Alvarado JL, Nestor PG, Rosow L, Pelavin PE, McCarley RW, et al. Fractional anisotropy and radial diffusivity: diffusion measures of white matter abnormalities in the anterior limb of the internal capsule in schizophrenia. Schizophr. Res. 2012;136:55–62. doi: 10.1016/j.schres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010;183:144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol. Psychiatry. 2008;13:1054–1059. doi: 10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Functional neuroanatomy. In: Sadock BJ, Sacock VA, Ruiz P, editors. Kaplan and Sadocks’ Comprehensive Textbook of Psychiatry. ninth ed Wolters Kluwer Health/Lippincott Williams and Wilkins; Philadelphia: 2009. pp. 5–41. [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann areas of the cortex in patients with schizophrenia with good and poor outcomes. Am. J. Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–770. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Torosjan Y, Newmark RE, Schneiderman JS, Chu KW, Brickman AM, et al. Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: a diffusion tensor imaging survey. Schizophr. Res. 2007;92:211–224. doi: 10.1016/j.schres.2006.12.029. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PC. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2005. [Google Scholar]
- Nakamura M, McCarley RW, Kubicki M, Dickey CC, Niznikiewicz MA, Voglmaier MM, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol. Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum. Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral EM, et al. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R, Oakley A, Perry E. Coronal brain map and dissection guide: Localization of Brodmann areas in coronal sections. 1991. Unpublished.
- Pfohl B, Blum N, Zimmerman M. Structured Clinical Interview for DSM-IV Personality (SIDP-IV) American Psychiatric Press; Washington, D.C.: 1997. [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, DiChiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Soto D, Grecucci A, Geng JJ, Humphreys GW. The role of the pulvinar in resolving competition between memory and visual selection: a functional connectivity study. Neuropsychologia. 2011;49:1544–1552. doi: 10.1016/j.neuropsychologia.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Davis KL. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am. J. Psychiatry. 2004;161:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, et al. Cognitive and brain function in schizotypal personality disorder. Schizophr. Res. 2002;54:157–167. doi: 10.1016/s0920-9964(01)00363-2. [DOI] [PubMed] [Google Scholar]
- Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophr. Res. 2008;98:157–162. doi: 10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks R, Magnotta VA, Andreasen NC, Albright KC, Ziebell S, Nopoulos P, et al. Manual and automated measurement of the whole thalamus and mediodorsal nucleus using magnetic resonance imaging. Neuroimage. 2002;17:631–642. [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Di Paola M, Banfi G, Rusch N, Martinotti G, et al. Reduced fronto-temporal connectivity is associated with frontal gray matter density reduction and neuropsychological deficit in schizophrenia. Schizophr. Res. 2009;108:57–68. doi: 10.1016/j.schres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- StatSoft Inc Statistica, (data analysis software system) (version 9.1) 2010 Tulsa, OK. www.statsoft.com.
- Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–4163. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, et al. Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr. Res. 2002;55:41–54. doi: 10.1016/s0920-9964(01)00224-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Zhou SY, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Volume reduction of the right anterior limb of the internal capsule in patients with schizotypal disorder. Psychiatry Res. 2004;130:213–225. doi: 10.1016/j.pscychresns.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am. J. Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- Taber KH, Wen C, Khan A, Hurley RA. The limbic thalamus. J. Neuropsychiatry Clin. Neurosci. 2004;16:127–132. doi: 10.1176/jnp.16.2.127. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings J. Frontal-subcortical neuronal circuits and clinical neuropsychiatry. An update. J. Psychosom. Res. 2002;53:647. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, et al. Reduction of the internal capsule in families affected with schizophrenia. Biol. Psychiatry. 2008;63:65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol. Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Zou LQ, Xie JX, Yuan HS, Pei XL, Dong WT, Liu PC. Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Acad. Radiol. 2008;15:285–289. doi: 10.1016/j.acra.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Zuliani R, Moorhead TW, Bastin ME, Johnstone EC, Lawrie SM, Brambilla P, et al. Genetic variants in the ErbB4 gene are associated with white matter integrity. Psychiatry Res. 2011;191:133–137. doi: 10.1016/j.pscychresns.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]