Abstract
The Epstein-Barr virus (EBV) immediate-early (IE) protein BRLF1 (R) is a transcription factor that induces the lytic form of EBV infection. R activates certain early viral promoters through a direct binding mechanism but induces transcription of the other EBV IE gene, BZLF1 (Z), indirectly through cellular factors binding to a CRE motif in the Z promoter (Zp). Here we demonstrate that R activates expression of the fatty acid synthase (FAS) cellular gene through a p38 stress mitogen-activated protein kinase-dependent mechanism. B-cell receptor engagement of Akata cells also increases FAS expression. The FAS gene product is required for de novo synthesis of the palmitate fatty acid, and high-level FAS expression is normally limited to liver, brain, lung, and adipose tissue. We show that human epithelial tongue cells lytically infected with EBV (from oral hairy leukoplakia lesions) express much more FAS than uninfected cells. Two specific FAS inhibitors, cerulenin and C75, prevent R activation of IE (Z) and early (BMRF1) lytic EBV proteins in Jijoye cells. In addition, cerulenin and C75 dramatically attenuate IE and early lytic gene expression after B-cell receptor engagement in Akata cells and constitutive lytic viral gene expression in EBV-positive AGS cells. However, FAS inhibitors do not reduce lytic viral gene expression induced by a vector in which the Z gene product is driven by a strong heterologous promoter. In addition, FAS inhibitors do not reduce R activation of a naked DNA reporter gene construct driven by the Z promoter (Zp). These results suggest that cellular FAS activity is important for induction of Z transcription from the intact latent EBV genome, perhaps reflecting the involvement of lipid-derived signaling pathways or palmitoylated proteins. Furthermore, using FAS inhibitors may be a completely novel approach for blocking the lytic form of EBV replication.
Epstein-Barr virus (EBV) is a human gammaherpesvirus that causes infectious mononucleosis. EBV has also been found in association with a variety of cancers, including Burkitt's lymphoma, nasopharyngeal carcinoma, gastric carcinoma, B-cell lymphomas in immunosuppressed patients, and Hodgkin's disease (28, 46). EBV can infect cells in either a lytic or latent form. EBV infection of epithelial cells usually results in the lytic form of infection, whereas the virus usually remains latent in B cells (28, 46, 52). However, the virus periodically converts to the lytic form of infection in a small percentage of B cells. This viral reactivation is initiated by the two EBV immediate-early (IE) proteins, BZLF1 (Z) and BRLF1 (R) (8, 10, 18, 44, 47, 54, 64, 69), which are transcriptional activators (11, 16, 24, 25, 27, 32, 56).
Regardless of which EBV IE gene is initially transcribed, expression of either IE protein leads first to the expression of the other IE protein (1, 18, 69), and then the two IE proteins together induce full activation of the viral early genes. Z activates the R promoter by directly binding to Z-response elements in the R promoter (1, 33, 51). Although R activates certain early genes, such as BMRF1 and BHRF1, through a direct binding mechanism (22, 43), R activation of the IE Z promoter (Zp) is mediated through cellular transcription factors (including ATF-2 and c-Jun) that bind to a CRE site (ZII) in this promoter (3). R activates the stress mitogen-activated protein (MAP) kinases (p38 and c-Jun N-terminal kinase) (3), which phosphorylate and activate ATF-2 and c-Jun. In addition, R activates phosphatidylinositol 3 (PI3) kinase (13), and PI3 kinase activity is required for R-mediated induction of lytic viral gene transcription. However, at this time, it is not clear how the R protein, which is primarily nuclear, activates these various signal transduction pathways.
In this paper, we show that R induces expression of a cellular gene, the fatty acid synthase (FAS) gene, in host cells. The FAS enzyme is required for the production of palmitate, a saturated free 16-carbon fatty acid (50, 53, 60). FAS activity is important for the synthesis of cellular membranes as well as a variety of different lipid substrates. In addition, certain proteins are posttranslationally modified by palmitoylation of cysteine residues, and in some cases, palmitoylation helps localize proteins to lipid rafts (4, 15). Although high-level FAS expression is normally limited to liver, lung, brain, and intra-abdominal adipose tissue (50), a variety of cancers have also recently been shown to overexpress FAS (29, 39). Interestingly, a specific FAS inhibitor, C75, preferentially inhibits the growth of tumor cells versus primary cells (30, 42), suggesting that FAS activity may be important for the growth of certain tumors. In addition, the FAS inhibitor cerulenin inhibits the replication of several different viruses, including varicella-zoster virus, although the exact mechanisms for these effects have not been well defined (9, 23, 38).
Somewhat surprisingly, we demonstrate here that cellular FAS activity appears to be essential for lytic EBV gene induction in latently infected cells. Using two different specific FAS inhibitors, cerulenin and C75, we show that FAS activity is required for R-mediated induction of IE (Z) and early (BMRF1) lytic genes in Jijoye cells, for the induction of Z, R, and BMRF1 transcription after cross-linking of the B-cell receptor in Akata cells, and for constitutively lytic gene expression in EBV-infected gastric carcinoma (AGS) cells. In contrast, FAS inhibitors do not prevent a plasmid that contains the Z gene product under the control of a strong heterologous promoter from inducing lytic gene expression in Jijoye cells. In addition, FAS is not required for R activation of the Z promoter in the context of a naked DNA reporter gene construct.
These results suggest that cellular FAS activity is important for efficient activation of Z transcription from the intact latent EBV genome. Although the precise mechanism by which FAS activity contributes to Z transcription is not yet known, we speculate that lipid-derived signal transduction pathways or palmitoylated proteins may be involved in the activation of Z transcription in latently infected cells. In any event, the ability of R, as well as B-cell receptor engagement, to activate FAS expression in host cells that would otherwise express minimal FAS likely plays a key role in enhancing the efficiency of lytic viral gene expression. Conversely, agents that inhibit FAS activity may prove useful for preventing the lytic form of EBV infection in patients.
MATERIALS AND METHODS
Cell lines.
HeLa is a cervical carcinoma cell line and was maintained in Dulbecco's modified Eagle's medium H supplemented with 10% fetal calf serum. Telomerase-immortalized human keratinocytes (TIK) were a gift from A. J. Klingelhutz at the University of Iowa and originally derived from human neonatal foreskin as previously described (17). TIK cells were maintained in keratinocyte-SFM medium (Gibco-BRL) with epidermal growth factor and bovine pituitary extract added. Akata and Jijoye cells are EBV-positive Burkitt's lymphoma cell lines and were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum. The EBV-positive AGS (gastric carcinoma) cell line was established by infecting gastric carcinoma cells with an EBV containing a green fluorescent protein (GFP)-hygromycin cassette as previously described (18) (a gift from H.-J. Delecluse at the University of Birmingham, Birmingham, United Kingdom). EBV-positive AGS cells were maintained in F12 nutrient mixture (Gibco-BRL) with hygromycin selection. All cell lines were cultured at 37°C in a 5% CO2 incubator.
Adenovirus construction and infection.
The EBV IE gene, BRLF1, and the control lacZ gene, under control of the IE cytomegalovirus promoter, were inserted via cre-loxP-mediated recombination into an adenovirus type 5 derivative lacking the E1 and E3 genes to create adenovirus-BRLF1 (AdR) and adenovirus-LacZ (AdLacZ), as previously described (64). Virus stocks were grown in 293 cells, purified by cesium chloride gradient treatment twice, followed by dialysis. HeLa and TIK cells were plated at a density of 1.0 × 106 and 3 × 106 cells per 150-mm-diameter plate, respectively, 24 h prior to infection. Cells were infected with no adenovirus (mock infection), AdLacZ, or AdR at a multiplicity of infection (MOI) of 20. Cells were harvested 24 h postinfection for RNA preparation.
Plasmids.
The R and Z expression vectors contain the R and Z genomic sequences, respectively, inserted in the pSG5 expression vector (Stratagene) under the control of the simian virus 40 promoter (a gift from Diane Hayward) (48). The Zp-CAT construct contains the Z promoter (Zp) sequences from positions −221 to +12 linked to the chloramphenicol acetyltransferase (CAT) gene (a gift from Erik Flemington) as previously described (19). Appropriate vector controls were included in each experiment.
Transfection.
Jijoye cells were transfected with 10 μg of plasmid DNA by electroporation with 1,500 V from a Zapper electroporation unit (Medical Electronics Shop, University of Wisconsin).
FAS inhibitor studies.
In Jijoye cells, the FAS inhibitor cerulenin (Sigma) (10 μg/ml) or C75 (Alexis Biochemicals) (10 μg/ml) was added to media immediately after electroporation of Z or R vector. In Akata cells, FAS inhibitors (cerulenin [5 μg/ml] or C75 [5 μg/ml]) were added to the media 1 h prior to treatment with anti-human immunoglobulin G (IgG) (Sigma) (0.1 mg/ml). Confluent EBV-positive AGS cells were treated with FAS inhibitors (5 μg/ml) for 48 h prior to immunoblot analysis. The dimethyl sulfoxide (DMSO) vehicle was used as a control in FAS inhibitor studies.
Reporter gene assays.
Jijoye cells were cotransfected with the Zp-CAT construct (1 μg) and a control vector (SG5) (4 μg) or the Zp-CAT construct (1 μg) and the SG5-R expression vector (4 μg). The C75 FAS inhibitor was added to some conditions immediately after electroporation. Cell extracts were prepared 72 h posttransfection and incubated at 37°C with [14C]chloramphenicol in the presence of acetyl coenzyme A as described previously (20). The percent acetylation of chloramphenicol was quantified by thin-layer chromatography followed by PhosphorImager screening (Molecular Dynamics).
Northern blot analysis.
Ten micrograms of total RNA, purified using the Trizol reagent (Invitrogen) as specified by the manufacturer, was separated on a 1% formaldehyde gel and transferred to nylon membranes (Schleicher & Schuell). Membranes were prehybridized for 25 min at 68°C in QuikHyb hybridization buffer (Stratagene) and then hybridized with randomly 32P-radiolabeled probes for 1 h at 68°C. After hybridization, membranes were washed twice in a solution consisting of 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% sodium dodecyl sulfate (SDS) at room temperature. The membranes were then washed in 0.2× SSC-0.1% SDS for 5 to 10 min at 60°C. The FAS probes were generated by reverse transcriptase PCR (RT-PCR) of 1 μg of total cellular RNA using the following primers: FAS sense 5′-TTTCCCGATATTCGGGCAGC-3′ and FAS antisense 5′-GTACATGAGCAGGCTCAGCC-3′. The PCR product was then randomly labeled. A randomly labeled (oligolabeling kit; Amersham) mouse glyceraldehyde phosphate dehydrogenase probe (Ambion) was used as a control.
RT-PCR.
EBV-negative and EBV-positive Akata cells were treated with or without anti-human IgG (Sigma; 0.1 mg/ml) for 24 h, and total RNA was isolated. RT-PCR was performed using a kit according to the instructions of the manufacturer (Promega). The FAS primers (sense, 5′-TTTCCCGATATTCGGGCAGC-3′; antisense, 5′-GTACATGAGCAGGCTCAGCC-3′) were used to synthesize the PCR products. PCR was run for 35 cycles, with 1 cycle consisting of 30 s at 95°C, 30 s at 56°C, and 30 s at 72°C.
Immunoblot analysis.
Immunoblot analysis of total cell extracts was performed as previously described (2) with the following antibodies: anti-β-actin mouse monoclonal antibody (Sigma) (diluted 1:5,000), anti-EBV BZLF1 (Argene) (diluted 1:100), anti-EBV BRLF1 (Argene) (diluted 1:100), and anti-EBV-BMRF1 (Capricorn Products, Inc.) (diluted 1:100).
Patients and tissues.
Oral hairy leukoplakia (OHL) biopsy specimens were obtained from human immunodeficiency virus (HIV)-seropositive patients identified at the University of North Carolina (UNC) Hospitals. Biopsy specimens of tissue from the lateral tongue border of healthy, HIV-negative subjects without leukoplakia were used as controls. Specimens were immediately frozen and stored at −80°C. A portion of each biopsy specimen was fixed in formalin and sent to UNC Hospitals Department of Pathology for histopathologic diagnosis. The presence of EBV in the OHL biopsy specimens was confirmed by detection of the terminal restriction enzyme fragments on Southern blots (62).
Immunofluorescence.
Frozen tissue sections were cut to a thickness of 5 μm and placed on poly-l-lysine-coated slides. Tissue sections and cell lines were fixed in a chilled 1:1 mixture of methanol and acetone, blocked with 20% normal goat serum, stained with a primary antibody for FAS (BD Biosciences) (diluted 1:20), Z (Argene) (diluted 1:40), or R (Argene) (diluted 1:20) or with a isotype control antibody, and then stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (63). Slides were mounted with coverslips using Vectashield (Vector Laboratories) and then examined by confocal laser-scanning microscopy.
Affymetrix GeneChip analysis.
TIK cells were plated at a cell density of 2 × 107 per 150-mm-diameter dish and then either mock infected or infected with adenovirus expressing LacZ or R at an MOI of 17. The cells were harvested 48 h later, and total RNA was obtained using the RNAeasy kit (Qiagen). From each condition, cDNA was then synthesized using a T7-(dT)24 primer (cDNA kit from Life Technologies). Biotinylated cRNA was then generated from the cDNA reaction mixture using the BioArray high-yield RNA transcript kit. The cRNA was then fragmented in fragmentation buffer (5× fragmentation buffer consists of 200 mM Tris-acetate [pH 8.1], 500 mM potassium acetate, and 150 mM magnesium acetate) at 94°C for 35 min before the chip hybridization. Fifteen micrograms of fragmented cRNA was then added to a hybridization cocktail. The hybridization cocktail contains the following ingredients: 0.05 μg of fragmented cRNA per μl; 50 pM control oligonucleotide B2; BioB, BioC, BioD, and cre hybridization controls; 0.1 mg of herring sperm DNA per ml; 0.5 mg of acetylated bovine serum albumin per ml; 100 mM morpholineethanesulfonic acid (MES); 1 M Na+; 20 mM EDTA; 0.01% Tween 20; and the GeneChip HuGeneFL array, which provides gene expression data for approximately 5,000 full-length human sequences. Arrays were hybridized for 16 h in the GeneChip fluidics station 400 and were washed and scanned with the Hewlett-Packard GeneArray scanner. During the washing, the cRNA probe was labeled with R-phycoerythrin streptavidin. Affymetrix GeneChip Microarray Suite 4.0 software was used for washing, scanning, and basic analysis. Sample quality was assessed by examination of the 3′ to 5′ intensity ratios of certain genes.
RESULTS
R activates FAS expression.
To examine the effect of R on cellular gene expression, we performed microarray analyses using an Affymetrix chip containing 5,000 cellular genes, with RNA isolated from telomerase-immortalized human primary keratinocytes (TIK) which were mock infected, infected with an adenovirus vector expressing LacZ (AdLacZ) or infected with an adenovirus vector expressing R (AdR). The microarray analysis results indicated that cells infected with AdR expressed the cellular FAS gene at a level seven times higher than that of the mock-infected cells or AdLacZ-infected cells (data not shown).
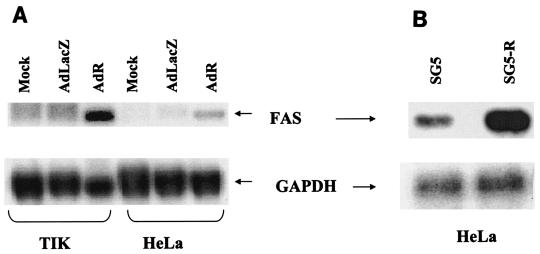
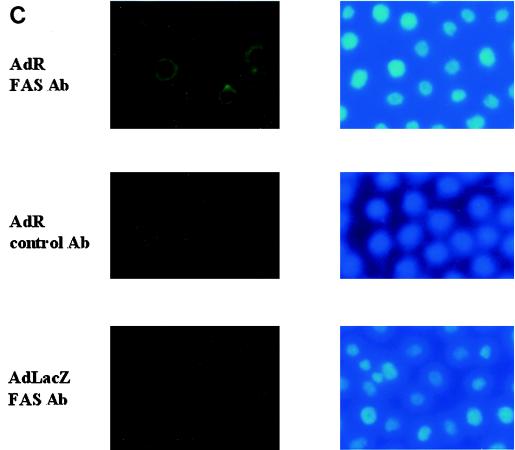
To confirm that R activates FAS expression, we performed Northern blot analysis of the RNA obtained from TIK and HeLa cells which were mock infected or infected with the AdLacZ or AdR vector. Compared to the mock-infected and AdLacZ-infected cells, both TIK and HeLa cells infected with the AdR vector had significantly increased levels of FAS mRNA (Fig. 1A). In addition, HeLa cells transfected with an R expression vector produced considerably more FAS than cells transfected with a control plasmid (Fig. 1B). These results indicate that R activates FAS gene expression in both HeLa and TIK cells and that this effect is not dependent upon the adenovirus vector. To confirm that R expression also induces increased FAS protein, immunofluorescence analysis of AdLacZ- or AdR-infected TIK cells was performed (Fig. 1C). AdR infection, as expected, significantly increased the level of FAS protein in TIK cells.
FIG. 1.
R induces FAS expression. (A) Telomerase-immortalized primary keratinocytes (TIK) and HeLa cells were either mock infected or infected with adenovirus vectors encoding β-galactosidase (AdLacZ) or R (AdR) at an MOI of 20. Twenty-four hours postinfection, total RNA was isolated, and Northern blot analysis was performed to detect FAS expression. (B) HeLa cells were transfected with the control vector, SG5, or an SG5 vector expressing R (SG5-R). Total RNA was isolated 24 h after transfection, and Northern blot analysis was performed. In panels A and B, the positions of FAS and glyceraldehyde phosphate dehydrogenase (GAPDH) are indicated by the arrows. (C) TIK cells were infected with adenovirus vectors encoding β-galactosidase (AdLacZ) or R (AdR) at an MOI of 20. Cells were analyzed by immunofluorescence microscopy 24 h later using FAS antibody (Ab) or a control antibody (left panels) or stained with DAPI (right panels).
FAS is overexpressed in epithelial tongue cells derived from OHL.
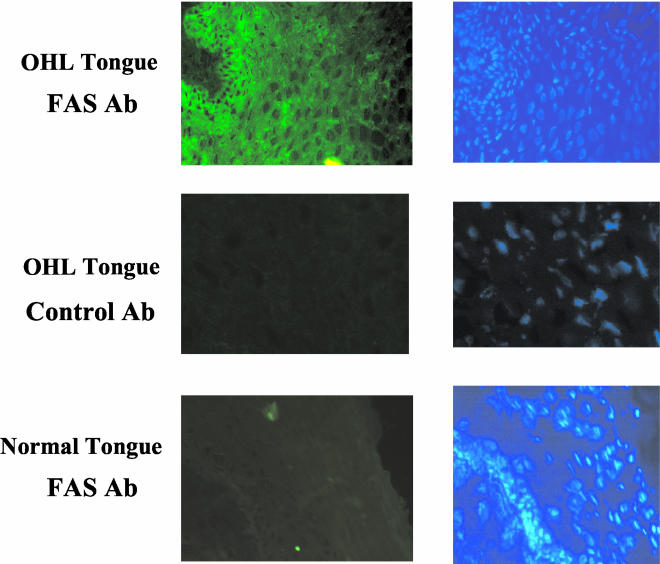
To determine whether FAS expression is increased by lytic EBV infection, we examined FAS expression in epithelial cells in the lateral tongue using biopsy specimens obtained from healthy individuals versus tongue biopsy specimens from patients with OHL. OHL is a lesion induced by lytic EBV infection of the tongue in immunosuppressed patients (21, 68). As expected, Z expression was observed in the condensed nuclei of koilocyte cells in the mid to upper strata in biopsy specimens of OHL lesions (data not shown), confirming that these cells contained the lytic form of EBV infection. Cytoplasmic FAS expression was observed in OHL tissue in the mid to upper strata within the stratum spinosum and stratum granulosum layer, but not in the control tongue tissue (Fig. 2). These results indicate that FAS protein expression is induced by lytic EBV infection in tongue epithelial cells.
FIG. 2.
FAS is overexpressed in OHL. Tongue biopsy specimens from a patient with OHL or a healthy individual were stained with either an anti-FAS antibody (Ab) or a control primary antibody, followed by a FITC-conjugated second antibody (left panels). DAPI staining was also performed (right panels). FAS protein expression was then examined using confocal fluorescence microscopy.
R activation of FAS requires p38 stress MAP kinase.
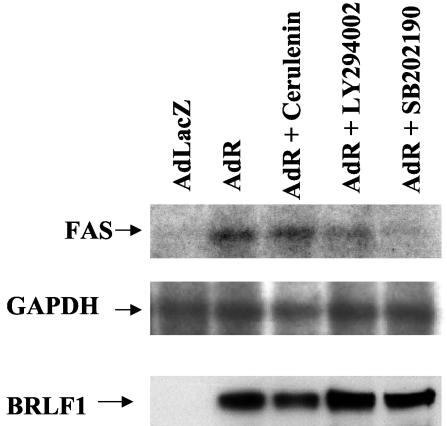
FAS expression is primarily induced in normal cells in response to low sterol levels. This effect is mediated by the activated, cleaved form of the sterol regulatory element binding proteins (SREBPs), which are transported into the nucleus and bind directly to the FAS promoter (35, 50). However, overexpression of FAS in cancer cell lines has recently been attributed to PI3 kinase or MAP kinase activation (57, 67). To determine whether R activates FAS expression through a PI3 kinase or p38 stress MAP kinase-dependent mechanism, we infected TIK cells with the control adenovirus vector (AdLacZ) or the R adenovirus vector in the presence or absence of the PI3 kinase inhibitor, LY294002, or the p38 kinase inhibitor (SB202190). In addition, the effect of a FAS inhibitor, cerulenin, was also examined. Total RNA was isolated 24 h postinfection, and Northern blot analysis was performed to detect FAS mRNA expression. Interestingly, the p38 kinase inhibitor completely prevented AdR-induced FAS activation in TIK cells, while the PI3 kinase inhibitor had less effect (Fig. 3, top panel). The FAS inhibitor did not affect R activation of the FAS gene. The levels of R protein were similar in all conditions (Fig. 3, bottom panel). These results suggest that p38 kinase activity is required for R-induced FAS activation.
FIG. 3.
R activation of FAS requires p38 kinase. TIK cells were treated with a p38 kinase inhibitor (SB202190 [20 μM]), a PI3 kinase inhibitor (LY294002 [15 μM)], or a FAS inhibitor (cerulenin [5 μg/ml]) for 1 h and then infected with AdLacZ or AdR at an MOI of 50. Total RNA was isolated 24 h postinfection, and Northern blot analysis was performed to detect FAS and glyceraldehyde phosphate dehydrogenase (GAPDH) expression. Immunoblot analysis was performed in the same experiment to detect BRLF1 protein expression.
FAS inhibitors prevent R-mediated lytic viral gene induction.
The previous results indicate that R activates FAS expression in host cells during lytic EBV infection. To determine whether cellular FAS activity is important for some aspect of lytic EBV infection, we examined the effects of two different specific FAS inhibitors, cerulenin and C75. Cerulenin is a natural product produced by certain fungi that specifically inhibits FAS activity by covalently binding to the beta keto acyl synthase moiety, one of the seven functional domains of FAS (12, 37, 40). C75 is a synthetic compound that also specifically inhibits FAS activity (30).
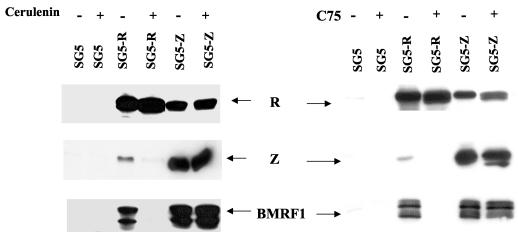
To determine whether the ability of R to activate lytic EBV gene expression requires FAS activity, Jijoye cells were electroporated with the SG5 control vector or SG5 vectors that express R (SG5-R) or Z (SG5-Z). After electroporation, cells were treated with a FAS inhibitor (cerulenin or C75) or vehicle (DMSO) and then harvested for immunoblot analysis of R, Z, and BMRF1 expression 48 h posttransfection. The ability of transfected R to induce Z or BMRF1 expression in Jijoye cells was almost completely eliminated by both the cerulenin and C75 FAS inhibitors (Fig. 4), although these inhibitors did not reduce R expression derived from the SG5-R vector. Thus, cellular FAS activity unexpectedly appears to be critical for the ability of R to activate Z expression from the latent EBV genome. Since both Z and R are required for efficient expression of the BMRF1 early gene from the intact viral genome (1, 18), the failure of R to activate BMRF1 expression in the presence of the FAS inhibitors may reflect its inability to activate Z.
FIG. 4.
FAS inhibitors (cerulenin and C75) prevent R-mediated, but not Z-mediated, lytic EBV induction. Jijoye cells were electroporated with 10 μg of a control vector (SG5), an R expression vector (SG5-R), or a Z expression vector (SG5-Z), in the presence (+) or absence (−) of two different FAS inhibitors, cerulenin (10 μg/ml) or C75 (10 μg/ml). Cell extracts were prepared 48 h posttransfection, and immunoblot analysis was performed to detect IE (R and Z) and early (BMRF1) lytic EBV protein expression.
Assuming that FAS activity is primarily important for R activation of Z transcription, then a vector which expresses the Z gene product under the control of a constitutively active heterologous promoter should be able to induce lytic EBV gene expression even in the presence of the FAS inhibitors. As shown in Fig. 4, this did indeed prove to be the case. Neither cerulenin nor C75 significantly inhibited the ability of the SG5-Z expression vector (in which Z is driven by the simian virus 40 early promoter) to induce R or BMRF1 expression from the endogenous viral genome in Jijoye cells. These results suggest that FAS activity plays an important role in R-mediated lytic viral induction and more specifically that FAS activity is required for R activation of Z transcription in the context of the intact latent viral genome.
FAS inhibitors prevent lytic EBV induction after cross-linking of the B-cell receptor.
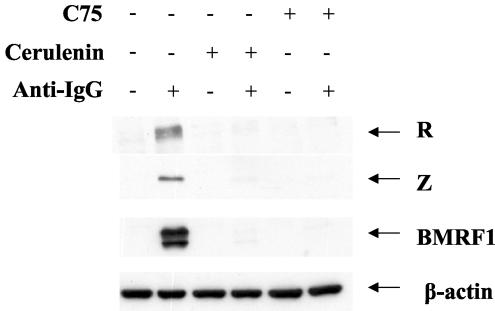
We have previously shown that certain cellular kinases which are required for R activation of Z transcription, including PI3 kinase and the stress MAP kinase p38, are likewise required for the induction of Z transcription after B-cell receptor stimulation in Akata cells (3, 13). Therefore, we examined whether cellular FAS activity is also required for activation of lytic EBV gene transcription induced by cross-linking of the B-cell receptor, since this method may be the most physiological model of activating lytic EBV infection in B cells in vitro (55). Akata cells were treated with cerulenin, C75, or DMSO for 1 h, followed by the addition of anti-human IgG to the medium for 24 h. Cell extracts were then prepared for immunoblot analysis of R, Z, and BMRF1 expression. As shown in Fig. 5, cross-linking of the B-cell receptor, as expected, efficiently induced R, Z, and BMRF1 expression in the absence of the FAS inhibitors. However, both cerulenin and C75 dramatically decreased the ability of B-cell receptor engagement to induce R, Z, and BMRF1 expression in Akata cells (Fig. 5). Akata cell viability in the presence of the FAS inhibitors was greater than 90% (data not shown). These results suggest that FAS activity is also required for the lytic viral gene expression that normally follows B-cell receptor stimulation.
FIG. 5.
FAS activity is required for lytic EBV induction after B-cell receptor engagement in Akata cells. EBV-positive Akata cells (a Burkitt's lymphoma line) were treated with anti-IgG (+) to induce lytic EBV expression in the presence (+) or absence (−) of FAS inhibitor cerulenin (5 μg/ml) or C75 (5 μg/ml). Cell extracts were prepared 24 h after treatment, and immunoblot analysis was performed to detect R, Z, and BMRF1 protein expression.
FAS inhibitors prevent constitutively lytic EBV gene expression in AGS cells.
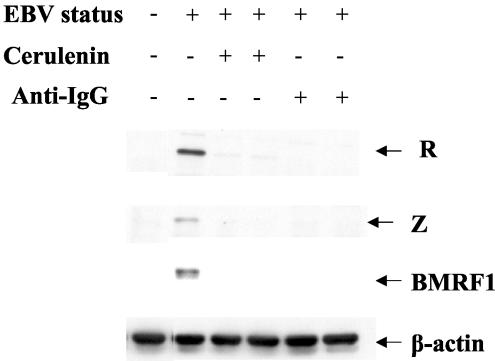
We have previously shown that AGS (gastric carcinoma) cells stably infected with EBV have a relatively high proportion of cells (3 to 5%) which spontaneously convert to the lytic form of EBV infection in the absence of any particular inducing stimuli (36). Although the cellular factors that induce lytic EBV infection in AGS cells are not well understood, this model system is potentially more relevant for examining the constitutively lytic EBV infection which occurs in epithelial cells (such as OHL lesions) than are studies performed in Jijoye and Akata cells. To determine the importance of FAS activity for spontaneously lytic EBV infection in AGS cells, confluent EBV-positive AGS cells were treated with DMSO, cerulenin (5 μg/ml,) or C75 (5 μg/ml), and cells were harvested 48 h later for immunoblot analysis of R, Z, and BMRF1 expression. The R, Z, and BMRF1 proteins were all expressed in EBV-positive AGS cells in the absence of FAS inhibitors, but both cerulenin and C75 reduced expression of all three lytic viral proteins (Fig. 6). Cell viability of confluent EBV-positive AGS cells in the presence of the FAS inhibitors was greater than 90% (data not shown). These results suggest that cellular FAS activity is also required for the spontaneous lytic EBV infection that normally occurs in AGS cells.
FIG. 6.
FAS activity is required for constitutive lytic viral gene expression in EBV-positive AGS (gastric carcinoma) cells. EBV-positive AGS cells were treated with (+) or without (−) cerulenin (5 μg/ml) or C75 (5 μg/ml) for 48 h, and cells were harvested for immunoblot analysis. Extracts from EBV-negative (−) AGS cells (leftmost lane) were used as a negative control.
FAS inhibitors do not prevent R activation of Zp in a naked DNA reporter gene assay.
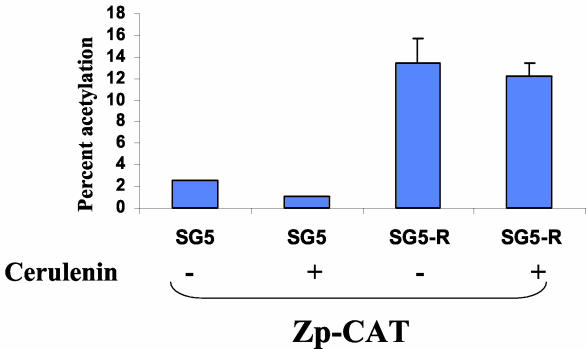
The previous results clearly indicate that FAS activity is required for the ability of R to activate Z transcription from the intact latent viral genome. To determine whether the FAS effect could be further dissected using reporter gene assays, Jijoye cells were transfected with a reporter gene construct containing the CAT gene driven by the Z promoter (Zp-CAT) in the presence or absence of the R expression vector, and the ability of the FAS inhibitors (C75 and cerulenin) to prevent R-induced activation of the Zp-CAT vector was examined. As shown in Fig. 7, the C75 inhibitor did not significantly affect the ability of R to activate the Zp-CAT construct, even though an immunoblot analysis of the same extracts used in the CAT assay indicated that C75 inhibited the ability of R to activate Z transcription from the endogenous viral genome (data not shown). Likewise, cerulenin did not significantly inhibit the ability of R to activate the Zp-CAT construct (data not shown). These results indicate that FAS activity may help to induce Z transcription in the context of the intact virus by reversing an epigenetic modification (such as an inhibitory chromatin structure or viral genome methylation) that cannot be modeled using naked Zp DNA. Alternatively, it is possible that a FAS-responsive region of the Zp lies outside the sequences present in our Zp-CAT construct (positions −221 to +12).
FIG. 7.
FAS activity is not required for R activation of Zp in a reporter gene assay. Jijoye cells were transfected with the Zp-CAT construct (in which the CAT gene is linked to the Z promoter) in combination with either the SG5 control vector or the SG5-R expression vector in the presence (+) or absence (−) of cerulenin. Cells were harvested for CAT activity assay 72 h posttransfection.
B-cell receptor engagement activates FAS expression.
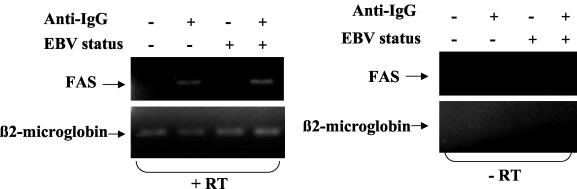
Currently, little is known regarding the regulation of FAS expression in B cells. Since activation of the B-cell receptor induces lytic EBV gene expression and we previously found that R induces many of the same signal transduction pathways that are activated by B-cell receptor engagement (3, 13), we wished to determine whether B-cell receptor engagement, like R, also results in enhanced FAS expression. The level of FAS RNA in Akata cells (both EBV positive and EBV negative) was quantitated by RT-PCR analysis with or without anti-IgG treatment. As shown in Fig. 8, B-cell receptor stimulation resulted in an increased level of FAS RNA expression in both EBV-negative and EBV-positive Akata cells. Thus, enhanced FAS expression may be a downstream component of the B-cell receptor signaling pathway which is required for induction of lytic EBV gene expression.
FIG. 8.
FAS is activated by B-cell receptor engagement in EBV-negative and EBV-positive Akata cells. EBV-negative and EBV-positive Akata cells were treated with anti-human IgG (0.1 mg/ml) for 24 h (+) or not treated with anti-IgG (−), and cells were then harvested for RNA isolation. RT-PCR was performed to detect FAS or β2-microglobulin RNA expression. Control experiments without reverse transcriptase (−RT) were also performed.
DISCUSSION
Expression of the EBV IE protein R is sufficient to initiate the switch from the latent to lytic form of viral infection in many EBV-positive cell lines. In this report, we demonstrate that R activates the expression of the cellular FAS gene in HeLa and TIK cells. In addition, we show that FAS expression is elevated within lytically infected epithelial cells of OHL lesions in patients. Most importantly, we show that FAS activity is essential for efficient induction of lytic EBV gene transcription by a variety of different stimuli. The ability of R to induce FAS expression in cells which would normally express minimal FAS (such as primary epithelial cells and B cells) may therefore be important for efficient lytic EBV infection. Our finding that B-cell receptor stimulation in both EBV-positive and EBV-negative Akata cells also results in enhanced FAS expression suggests that increased FAS activity may likewise play a role in anti-IgG mediated lytic EBV induction.
Although many transformed cell lines constitutively express high levels of FAS in vitro, high-level expression of FAS in vivo is restricted to liver, lung, brain, and adipose tissue (50). FAS expression is tightly regulated at the transcriptional level and is induced in normal cells primarily in response to low sterol levels. Sterol-responsive FAS transcription is mediated by SREBPs. SREBPs exist in an inactive form bound to the endoplasmic reticulum (26, 50). Intracellular lipid depletion triggers proteolytic cleavage of SREBPs, allowing the amino terminus to enter the nucleus and activate the expression of genes encoding enzymes involved in de novo fatty acid synthesis (26, 50). In contrast, FAS overexpression in cancer cell lines is mediated primarily through activation of the PI3 kinase and MAP kinase signal transduction pathways (57, 67). Our results here suggest that R activation of FAS requires the p38 stress MAP kinase pathway.
FAS activity is required for de novo synthesis of palmitate, a fatty acid. At present, it is not clear how cellular FAS activity contributes to lytic EBV gene induction. However, our results here suggest that this requirement for FAS activity occurs at the most proximal step in the activation of the lytic EBV gene transcription cascade, the induction of IE gene transcription. In addition, it appears that cellular FAS activity is required for the effectiveness of a variety of different lytic inducing stimuli and in a variety of different cell types. FAS inhibitors not only prevented the ability of R to induce lytic EBV gene expression in Jijoye cells but also prevented lytic EBV gene expression after ligation of the B-cell receptor in Akata cells, as well as the spontaneous lytic viral gene expression that normally occurs in EBV-infected gastric carcinoma cells.
Whatever the mechanism, our results suggest that FAS activity may be primarily required for activation of Z transcription, rather than R transcription, from the intact viral genome. A Z expression vector in which the Z gene product is driven by a strong heterologous promoter can activate R transcription (as well as early gene transcription) from the endogenous viral genome even in the presence of FAS inhibitors. In contrast, an expression vector containing the R gene product driven by the same heterologous promoter cannot activate Z transcription (or downstream early genes).
Nevertheless, we found that FAS inhibitors reduce expression of both IE proteins (Z and R) in spontaneously lytic AGS cells and after ligation of the B-cell receptor in Akata cells. The ability of FAS inhibitors to reduce R, as well as Z, expression under these conditions could be due to either of the following explanations. If we assume that induction of lytic EBV transcription is initially mediated through cellular activation of Zp, then the ability of cellular transcription factors to induce Z expression from the latent viral genome (in the absence of any R expression) may also require FAS activity. In this model, the loss of Z expression induced by FAS inhibitors then results in the loss of R expression, since the Z gene product is not available to activate R transcription. If lytic induction is primarily initiated through cellular activation of the R promoter and the R gene product then activates Z expression, we can still explain the loss of both R and Z expression in the presence of the FAS inhibitors, since an essential aspect of lytic EBV gene induction is the positive autoregulatory cascade resulting from the ability of Z to activate R transcription and vice versa. By preventing even one part of this autoregulatory cascade (the ability of R to induce Z transcription), lytic EBV induction by a variety of different stimuli may be effectively attenuated.
Although we do not currently understand why FAS activity is required for activation of Z transcription, there are a variety of potential possibilities. One possibility is that the level of palmitate in the cell is limiting in the absence of de novo fatty acid synthesis. A variety of proteins are known to be posttranslationally modified by palmitoylation over cysteine residues, and in some cases this modification is important for the localization of proteins into lipid rafts. Palmitoylation has been shown to be required for the correct cellular localization and/or function of a variety of proteins, including p21 Ras (6, 14), and we have previously shown that Ras is required for efficient lytic EBV infection (13). Fyn and Lck (the Src family kinases), which are important for the signal transduction pathways resulting from activation of the B-cell and T-cell receptors, are also palmitoylated (58, 59, 66). In addition, enhanced FAS activity could potentially lead to activation of signal transduction pathways dependent upon fatty acid precursors. For example, de novo synthesis of the lipid mediator ceramide requires palmitate, and exogenous palmitate treatment of cells has been shown to induce de novo ceramide synthesis in some cells (5, 41, 49). Ceramide-induced signal transduction pathways include some of the same pathways (PI3 kinase, JNK, and p38 stress MAP kinase) (5, 45, 65) activated by R (3, 13). However, we have not found that an inhibitor of de novo ceramide synthesis, fumonisin, inhibits the ability of R to induce lytic infection (unpublished data).
Perhaps most importantly, our results here suggest a completely novel approach for inhibiting the lytic form of EBV replication. FAS inhibitors, such as C75 and cerulenin, inhibit EBV replication at a much earlier step (prevention of IE gene transcription) than the currently used antiviral drugs, such as acyclovir, which block viral DNA replication but do not prevent IE and early gene transcription. Furthermore, C75 is apparently nontoxic in mice (other than causing obese mice to lose weight) and is being investigated as an agent to treat obesity (31, 34). Interestingly, the active agent in green tea (epigallocatechin gallate) was recently shown to inhibit expression of lytic EBV genes in response to 12-O-tetradecanoylphorbol-13-acetate induction (7) and was also found to directly bind to FAS and inhibit its activity (61). Thus, inhibition of FAS activity may prove to be an effective and potentially nontoxic method for inhibiting lytic EBV replication in patients.
Acknowledgments
This work was supported in part by grants 2-R01-CA58853, and P01-CA19014 from the National Institutes of Health.
We thank Erik Flemington and S. Diane Hayward for providing plasmids, A. J. Klingelhutz and H.-J. Delecluse for providing cell lines, the UNC Gene Therapy Core for preparing the adenovirus vectors, and Rosalind Coleman at the UNC School of Public Health for helpful discussions.
REFERENCES
- 1.Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. [DOI] [PubMed] [Google Scholar]
- 2.Adamson, A. L., and S. Kenney. 1999. The Epstein-Barr virus BZLF1 protein interacts physically and functionally with the histone acetylase CREB-binding protein. J. Virol. 73:6551-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson, A. L., D. Darr, E. Holley-Guthrie, R. A. Johnson, A. Mauser, J. Swenson, and S. Kenney. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 74:1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthiaume, L. G. 2002. Insider information: how palmitoylation of Ras makes it a signaling double agent. Sci. STKE 2002:PE41. [DOI] [PubMed] [Google Scholar]
- 5.Blazquez, C., I. Galve-Roperh, and M. Guzman. 2000. De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J. 14:2315-2322. [DOI] [PubMed] [Google Scholar]
- 6.Cadwallader, K. A., H. Paterson, S. G. Macdonald, and J. F. Hancock. 1994. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol. Cell. Biol. 14:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, L. K., T. T. Wei, Y. F. Chiu, C. P. Tung, J. Y. Chuang, S. K. Hung, C. Li, and S. T. Liu. 2003. Inhibition of Epstein-Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem. Biophys. Res. Commun. 301:1062-1068. [DOI] [PubMed] [Google Scholar]
- 8.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conti, G., P. Portincasa, and C. Chezzi. 1995. Cerulenin inhibits production of mature virion particles in chick embryo fibroblasts infected by influenza A viruses. Res. Virol. 146:141-149. [DOI] [PubMed] [Google Scholar]
- 10.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Agnolo, G., I. S. Rosenfeld, J. Awaya, S. Omura, and P. R. Vagelos. 1973. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim. Biophys. Acta 326:155-156. [DOI] [PubMed] [Google Scholar]
- 13.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol 3-kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudler, T., and M. H. Gelb. 1996. Palmitoylation of Ha-Ras facilitates membrane binding, activation of downstream effectors, and meiotic maturation in Xenopus oocytes. J. Biol. Chem. 271:11541-11547. [DOI] [PubMed] [Google Scholar]
- 15.Dunphy, J. T., and M. E. Linder. 1998. Signalling functions of protein palmitoylation. Biochim. Biophys. Acta 1436:245-261. [DOI] [PubMed] [Google Scholar]
- 16.Farrell, P. J., D. T. Rowe, C. M. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farwell, D. G., K. A. Shera, J. I. Koop, G. A. Bonnet, C. P. Matthews, G. W. Reuther, M. D. Coltrera, J. K. McDougall, and A. J. Klingelhutz. 2000. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 156:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemington, E., and S. H. Speck. 1990. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenspan, J. S., D. Greenspan, E. T. Lennette, D. I. Abrams, M. A. Conant, V. Petersen, and U. K. Freese. 1985. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 313:1564-1571. [DOI] [PubMed] [Google Scholar]
- 22.Gruffat, H., E. Manet, A. Rigolet, and A. Sergeant. 1990. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 18:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guinea, R., and L. Carrasco. 1990. Phospholipid biosynthesis and poliovirus genome replication, two coupled phenomena. EMBO J. 9:2011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardwick, J. M., L. Tse, N. Applegren, J. Nicholas, and M. A. Veliuona. 1992. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J. Virol. 66:5500-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton, J. D. 2002. Sterol regulatory element-binding proteins: transcriptional activators of lipid synthesis. Biochem. Soc. Trans. 30:1091-1095. [DOI] [PubMed] [Google Scholar]
- 27.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 63:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 29.Kuhajda, F. P. 2000. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition 16:202-208. [DOI] [PubMed] [Google Scholar]
- 30.Kuhajda, F. P., E. S. Pizer, J. N. Li, N. S. Mani, G. L. Frehywot, and C. A. Townsend. 2000. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl. Acad. Sci. USA 97:3450-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar, M. V., T. Shimokawa, T. R. Nagy, and M. D. Lane. 2002. Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice. Proc. Natl. Acad. Sci. USA 99:1921-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman, P. M., J. M. Hardwick, and S. D. Hayward. 1989. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J. Virol. 63:3040-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, P., and S. H. Speck. 2003. Synergistic autoactivation of the Epstein-Barr virus immediate-early BRLF1 promoter by Rta and Zta. Virology 310:199-206. [DOI] [PubMed] [Google Scholar]
- 34.Loftus, T. M., D. E. Jaworsky, G. L. Frehywot, C. A. Townsend, G. V. Ronnett, M. D. Lane, and F. P. Kuhajda. 2000. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288:2379-2381. [DOI] [PubMed] [Google Scholar]
- 35.Magana, M. M., and T. F. Osborne. 1996. Two tandem binding sites for sterol regulatory element binding proteins are required for sterol regulation of fatty-acid synthase promoter. J. Biol. Chem. 271:32689-32694. [DOI] [PubMed] [Google Scholar]
- 36.Mauser, A., E. Holley-Guthrie, A. Zanation, W. Yarborough, W. Kaufmann, A. Klingelhutz, W. T. Seaman, and S. Kenney. 2002. The Epstein-Barr virus immediate-early protein BZLF1 induces expression of E2F-1 and other proteins involved in cell cycle progression in primary keratinocytes and gastric carcinoma cells. J. Virol. 76:12543-12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moche, M., G. Schneider, P. Edwards, K. Dehesh, and Y. Lindqvist. 1999. Structure of the complex between the antibiotic cerulenin and its target, β-ketoacyl-acyl carrier protein synthase. J. Biol. Chem. 274:6031-6034. [DOI] [PubMed] [Google Scholar]
- 38.Namazue, J., T. Kato, T. Okuno, K. Shiraki, and K. Yamanishi. 1989. Evidence for attachment of fatty acid to varicella-zoster virus glycoproteins and effect of cerulenin on the maturation of varicella-zoster virus glycoproteins. Intervirology 30:268-277. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto, T., S. Terashima, M. Kogure, Y. Hoshino, T. Kusakabe, T. Suzuki, and M. Gotoh. 2001. Overexpression of fatty acid synthase in oesophageal squamous cell dysplasia and carcinoma. Pathobiology 69:297-303. [DOI] [PubMed] [Google Scholar]
- 40.Omura, S. 1976. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol. Rev. 40:681-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry, D. K. 2002. Serine palmitoyltransferase: role in apoptotic de novo ceramide synthesis and other stress responses. Biochim. Biophys. Acta 1585:146-152. [DOI] [PubMed] [Google Scholar]
- 42.Pizer, E. S., F. J. Chrest, J. A. DiGiuseppe, and W. F. Han. 1998. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 58:4611-4615. [PubMed] [Google Scholar]
- 43.Quinlivan, E. B., E. Holley-Guthrie, M. Norris, D. Gutsch, S. L. Bachenheimer, and S. C. Kenney. 1993. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 21:1999-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riboni, L., P. Viani, R. Bassi, A. Prinetti, and G. Tettamanti. 1997. The role of sphingolipids in the process of signal transduction. Prog. Lipid Res. 36:153-195. [DOI] [PubMed] [Google Scholar]
- 46.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In B. Fields, D. Knipe, and P. Howley (ed.), Fields virology, 4rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 47.Rooney, C., N. Taylor, J. Countryman, H. Jenson, J. Kolman, and G. Miller. 1988. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc. Natl. Acad. Sci. USA 85:9801-9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarisky, R. T., Z. Gao, P. M. Lieberman, E. D. Fixman, G. S. Hayward, and S. D. Hayward. 1996. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J. Virol. 70:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz-Peiffer, C., D. L. Craig, and T. J. Biden. 1999. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 274:24202-24210. [DOI] [PubMed] [Google Scholar]
- 50.Semenkovich, C. F. 1997. Regulation of fatty acid synthase (FAS). Prog. Lipid Res. 36:43-53. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sixbey, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 53.Slabas, A. R., A. Brown, B. S. Sinden, R. Swinhoe, J. W. Simon, A. R. Ashton, P. R. Whitfeld, and K. M. Elborough. 1994. Pivotal reactions in fatty acid synthesis. Prog. Lipid Res. 33:39-46. [DOI] [PubMed] [Google Scholar]
- 54.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urier, G., M. Buisson, P. Chambard, and A. Sergeant. 1989. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 8:1447-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van de Sande, T., E. De Schrijver, W. Heyns, G. Verhoeven, and J. V. Swinnen. 2002. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res. 62:642-646. [PubMed] [Google Scholar]
- 58.van't Hof, W., and M. D. Resh. 1997. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J. Cell Biol. 136:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van't Hof, W., and M. D. Resh. 1999. Dual fatty acylation of p59Fyn is required for association with the T cell receptor ζ chain through phosphotyrosine-Src homology domain-2 interactions. J. Cell Biol. 145:377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wakil, S. J. 1989. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523-4530. [DOI] [PubMed] [Google Scholar]
- 61.Wang, X., and W. Tian. 2001. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 288:1200-1206. [DOI] [PubMed] [Google Scholar]
- 62.Webster-Cyriaque, J., and N. Raab-Traub. 1998. Transcription of Epstein-Barr virus latent cycle genes in oral hairy leukoplakia. Virology 248:53-65. [DOI] [PubMed] [Google Scholar]
- 63.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 65.Willaime, S., P. Vanhoutte, J. Caboche, Y. Lemaigre-Dubreuil, J. Mariani, and B. Brugg. 2001. Ceramide-induced apoptosis in cortical neurons is mediated by an increase in p38 phosphorylation and not by the decrease in ERK phosphorylation. Eur. J. Neurosci. 13:2037-2046. [DOI] [PubMed] [Google Scholar]
- 66.Wolven, A., H. Okamura, Y. Rosenblatt, and M. D. Resh. 1997. Palmitoylation of p59fyn is reversible and sufficient for plasma membrane association. Mol. Biol. Cell 8:1159-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, Y. A., W. F. Han, P. J. Morin, F. J. Chrest, and E. S. Pizer. 2002. Activation of fatty acid synthesis during neoplastic transformation: role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res. 279:80-90. [DOI] [PubMed] [Google Scholar]
- 68.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, and A. B. Rickinson. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the IE BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]