Abstract
The essential components of the immune system that control primary and chronic infection with herpes simplex virus type 1 (HSV-1) in mice were investigated. Infection within the first few days can be controlled by alpha/beta interferon (IFN-α/β) alone without significant contribution of B, T, or NK cells. IFN-α/β and IFN-γ cooperate in the elimination of virus in the absence of these lymphocytes. In contrast, B, T, or NK cells appear to be required to control persistent infection with HSV-1. These results suggest that distinct and essential immune elements are recruited in a time-dependent fashion to control acute and persistent HSV-1 infection.
The main immunological elements that control infection with herpes simplex virus type 1 (HSV-1) include interferon (IFN), NK cells, and specific T and B cells (36).
Both IFN-α/β and IFN-γ have been shown to be essential for virus control (33). In addition to their direct antiviral properties, IFNs are potent regulators of cell growth and thus indirectly influence virus replication. Global gene expression analysis has estimated that several hundred genes are regulated within a single cell following IFN stimulation in vitro. The complexity of the “IFN transcriptome” will likely increase when different cell populations are analyzed and global gene expression of organs or even whole organisms can be achieved (24). Besides effects on cell regulation, inflammation, and stress, IFNs dominantly influence cells that belong to the innate and adaptive immune system (2). However, it is not known at what time point after viral infection IFN-α/β, IFN-γ, and NK, T, and B cells are required; how these immune elements cooperate; or whether they are functionally redundant.
Natural and gene-targeted mice are useful for assigning biological roles for genes in vivo. We have concentrated on gene deletions that inactivate the receptor for IFN-α/β and -γ and thus abort the function of IFN-α/β and -γ; the common cytokine receptor gamma chain (γc) for interleukin-2 (IL-2), -4, -7, -9, -15, and -21 that is required for the development of NK cells; and the recombination activating gene (RAG) required for the development of mature T and B cells. Our main findings are that (i) IFN-α/β is able to control acute HSV-1 infection in the absence of NK cells or specific immunity, (ii) IFN-α/β and -γ systems participate to eliminate HSV-1 without the need for NK cells or specific immunity, and (iii) lymphocytes (either B, T, or NK cells) appear to be required to control persistent virus.
MATERIALS AND METHODS
Animals and virus.
Six- to 8-week-old 129Sv/Ev or C57BL/6 mice and congenic strains with gene-targeted disruptions of the IFN-α/β receptor, IFN-γ receptor, recombination activating gene (RAG), as well as combinations thereof obtained by breeding were used (Table 1) (18, 25, 47). Mice bred on the 129Sv/Ev genetic background contain “129” as part of the name (Table 1). All other mice are on a C57BL/6 genetic background. In p40−/− mice, p35−/− IL-12 and IL-23 are inactivated (4, 46). In mice deficient in RAG and the common cytokine receptor chain (γc; [RAG−/−γc−/−]), mature T and B cells as well as NK cells are absent, and IL-2, -4, -7, -9, -15, and -21 are nonfunctional (7, 22). All gene-altered C57BL/6 mice were backcrossed at least 10 generations. Mice of both sexes were used for the experiments. The animals were bred and maintained under specific-pathogen-free conditions in the Labortierkunde, Universität Zurich, Zurich, Switzerland.
TABLE 1.
Mouse strains used in this study
HSV-1 strain F was originally obtained from B. Roizman (University of Chicago) and propagated on Vero cells (11, 44). For all experiments, virus particles were used after purification by ultracentrifugation on a sucrose density gradient, and the virus titer (PFU) was determined as described previously (44).
Animals were infected by intraperitoneal inoculation of 100 μl of virus suspension. The 50% lethal dose (LD50) was calculated as described previously (23). We euthanized infected animals when they were terminally ill or at 21 days postinfection, unless stated otherwise. In some experiments, mice were treated with neutralizing monoclonal antibody (MAb) 10F6 (specific for IL-12 p40), which neutralizes IL-12-dependent bioactivity (29). Neutralizing MAb XMG1.2 specific for murine IFN-γ was used (29) until animals were terminally ill. All experiments were conducted at least twice, and different doses of virus were used where appropriate.
Dissection of trigeminal and spinal ganglia and PCR.
Trigeminal and spinal ganglia and brain tissues were isolated for detection of HSV-1 DNA by PCR. Trigeminal ganglia were separated from brain tissue after opening and removing the upper skull. Spinal ganglia were surgically removed by opening the spinal cord under microscopic observation. To detect HSV-1 genomic sequences in spleen, liver, lung, kidney, or brain, DNA was isolated by standard procedures, and HSV-specific genes were amplified by PCR. Primers were directed to the HSV-1 glycoprotein B (gB) gene. The forward primer was 5′-TCCCGGTACGAAGACCAG, and the reverse primer was 5′-AGCAGGCCGCTGTCCTTG. The conditions for PCR and sensitivity testing were described previously (39, 48).
RESULTS
IFN-α/β controls high doses of HSV-1 for a short time in the absence of NK cells and mature T and B cells.
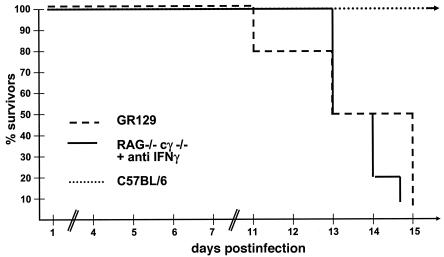
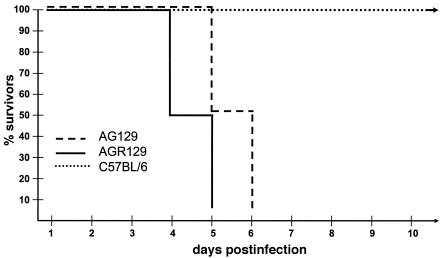
IFN-α/β is considered crucial for the control of acute viral infections (33). However, IFN-α/β can cooperate with IFN-γ and regulate lymphocytes of innate or specific immunity (24). In order to analyze the effect of the IFN-α/β system in the absence of T and B cells, we infected GR129 mice (Table 1), which lack IFN-γ receptors as well as mature T and B cells, with 103 PFU of HSV-1. The infected mice survived the early phase of infection (up to 15 days) but succumbed after this time (Fig. 1). Infection of GR129 mice with higher HSV-1 doses (105 PFU) was lethal for all mice within 9 days (data not shown). Mice lacking both IFN-α/β and -γ systems had no appreciable resistance against infection with HSV-1 (Fig. 2). These data highlight the contribution of the IFN-α/β system in the defense against HSV-1 but left unanswered the question of the importance of NK cells in the control of viral replication.
FIG. 1.
IFN-α/β controls acute infection with HSV-1 independent of NK cells. C57BL/6, GR129, or RAG−/−γc−/− mice were infected with 103 PFU of HSV-1. RAG−/−γc−/− mice were additionally treated with neutralizing antibodies to IFN-γ throughout the experiment. The survival of mice after viral infection is shown. A typical experiment with 10 mice in each group is shown.
FIG. 2.
IFN is essential for protection against HSV-1. C57BL/6, AGR129, and AG129 mice were infected with 103 PFU of HSV-1. The survival of mice after viral infection is shown.
To evaluate the role of NK cells during acute HSV-1 infection, we utilized RAG−/−γc−/− mice, which are devoid of all lymphocyte subsets (B, T, and NK cells, Table 1) (7). In this experiment, RAG−/−γc−/− mice were treated with neutralizing antibodies against IFN-γ (29). Under these conditions, mice have an intact IFN-α/β system, lack all lymphocytes, and have no capacity to respond to IFN-γ. RAG−/−γc−/− mice infected with 103 PFU of HSV-1 in the presence of neutralizing antibodies specific to IFN-γ also died within 15 days (Fig. 1). These data indicated that NK cells do not contribute to survival under the experimental conditions used. To further substantiate these data, RAG−/− mice were also treated with neutralizing antibodies specific to IFN-γ and infected with 103 PFU of HSV-1. Again, none of the infected mice survived longer than 16 days (data not shown). Naïve untreated RAG−/− mice survived infection with 103 PFU of HSV-1 (Table 2) (data not shown). Therefore, data from three different mouse strains indicated that the IFN-α/β system alone can control infections with HSV-1 for several days without the need for NK cells and mature B and T cells.
TABLE 2.
HSV-1-specific LD50s for different mouse strains
| Mouse straina | IL-12 | IFN γ | RAG | LD50 (PFU) |
|---|---|---|---|---|
| Wild type | + | + | + | 5 × 106 |
| IL-12−/− | − | + | + | 5 × 106 |
| G129 (F) | + | − | + | 5 × 106 |
| G129 (M) | + | − | + | 5 × 105b |
| RAG−/− | + | + | − | 5 × 105b |
At least 20 mice of each sex were used. F, female; M, male.
Statistical difference from wild-type animals at P ≤ 0.05 (Mann-Whitney U test).
In the absence of IFN-α/β, IL-12 is required for the defense against HSV-1.
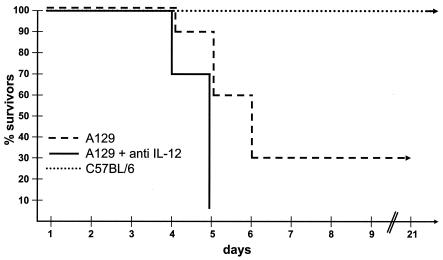
We next analyzed whether mice without an IFN-α/β system but with intact IFN-γ and mature B and T cells (A129, Table 1) were also able to control infection with 103 PFU of HSV-1. Surprisingly, about 30% of them survived the infection, and the mice stayed healthy for more than 6 months (Fig. 3 and Table 3). The presence of neutralizing antibody specific to IL-12 during infection abolished the protective effect completely (Fig. 3), as seen previously with AR129 mice (48). As expected, neutralizing antibodies specific to IFN-γ present during virus challenge led to a lethal outcome for the infected A129 mice (data not shown). Furthermore, mice without functional IFN (AG129 mice, Table 1) did not resist infective doses as low as 50 to 100 PFU of virus. Interestingly immunization (16) or transfer of neutralizing antibodies against HSV-1 (48) prolonged survival but did not lead to protection against viral infection. Therefore, the lack of IFN-α/β responses during the early period after HSV-1 infection can be compensated for, to some extent, by elements of specific immunity (48), provided that IL-12 is functional (Fig. 3). In the presence of functional IFN-α/β and -γ, the absence of IL-12 had no deleterious effect (Table 2).
FIG. 3.
IFN-γ and T and B cells provide IL-12-dependent protection against HSV-1. C57BL/6 and A129 mice were infected with 103 PFU of HSV-1 or additionally injected with neutralizing antibodies to IL-12 p40. The survival of mice after viral infection is shown.
TABLE 3.
Persistence of HSV-1 DNA in neuronal tissue
NK cells or mature T and B cells are not required to resist infection with HSV-1.
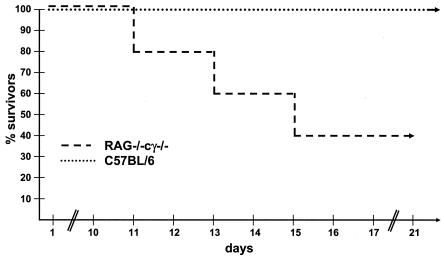
Components of the adaptive immune system, including antibodies and in particular CD8+ T cells, have been postulated to be essential for long-term protection against HSV-1 infection (35). RAG-deficient mice, which are unable to generate mature T and B cells, were 10 times more susceptible to infection with HSV-1 than C57BL/6 or 129Sv/Ev mice (Tables 1 and 2). However, HSV-1-infected mice that controlled virus during the first 10 to 14 days survived for several months until the experiment was terminated. To investigate the role of NK cells in this process, RAG−/−γc−/− mice that have no significant numbers of these cells were infected with virus. Interestingly, 20 to 40% of RAG−/−γc−/− mice infected with 103 or 104 PFU of HSV-1 survived the infection for more than 6 months, the longest period analyzed (Fig. 4 and Table 3).
FIG. 4.
NK, T, and B cells are not required to resist infection with HSV-1. C57BL/6 and RAG−/−γc−/− mice were infected with 103 PFU of HSV-1. The survival of mice after viral infection is shown.
Immunological requirements to detect persistent viral DNA in neuronal tissue.
We have previously shown that persistent virus can be controlled in the absence of both specific immunity and IFN-α/β (48). Therefore, it was interesting to see whether the different gene-deleted animals that had survived infection harbored viral DNA, indicating the presence of persistent virus (Tables 1 and 3). Trigeminal and spinal ganglia were removed at least 3 weeks after infection with a viral dose that led to 30 to 50% survival (Table 3). HSV-1 DNA was detected in ganglia of mice with the IFN-α/β and -γ genes deleted as well as in C57BL/6 wild-type mice. Interestingly, HSV-1 gB-specific DNA was only detected in four of eight RAG gene-deficient mice but none of the nine RAG−/−γc−/− mice (Table 3). Therefore, the presence of mature T and B cells or NK cells is correlated with the persistence of viral DNA. It would therefore appear that RAG−/−γc−/− mice, which do not have these cells, either eliminate the virus during the acute phase or succumb to infection.
DISCUSSION
IFN in acute infection of HSV-1.
It is well established that treatment with IFN-α, IFN=β, and IFN-γ is very effective against HSV-1 infections in mice (34). To further understand the plethora of interactions between IFN and various immune elements, we monitored animals with defects in IFN receptors, NK cells, and/or mature T and B cells during the course of infection with HSV-1.
We have shown that the IFN-α/β system is the most effective innate immune element able to control acute HSV-1 infection and can operate in the absence of NK cells and mature T and B cells (Fig. 1). In the presence of IFN-α/β, treatment of mice with neutralizing antibody to IL-12 p40 did not influence the course of the infection (data not shown). Therefore, after infection with HSV-1, IFN-α/β, IL-12, and possibly direct cell-cell contact (14) may not activate NK cells. The role of NK cells in acute infections with HSV-1 in mice is still controversial (1, 3, 5, 15, 19, 38), and the role of NK cells in chronic infections should be evaluated separately (see below). Evaluation of NK cell function in vivo by eliminating cells with antibodies to NK1.1 or asialo-GM1 is difficult if not impossible to assess. NK1.1 is not expressed on all NK cells (37). In contrast, NK1.1 and asialo-GM1 are not only expressed on NK cells, but also on macrophages (30) and some dendritic cells (DC) (41). This is important to note, because IFN produced during infection may prime these cells for enhanced resistance and control of HSV-1 replication (20, 24).
This early IFN-α/β production may control virus replication long enough to enable initiation of specific immunity. Indeed, priming of HSV-1-specific CD8+ T cells in C57BL/6 mice requires approximately 30 h (32). Significant virus-specific cytotoxic T-cell activity in draining lymph nodes is detectable within 2 to 3 days (32). IFN-α/β controls infection with 105 PFU of HSV-1 for the first 6 to 9 days. This time appears sufficient for T-cell priming and expansion to induce a protective HSV-1-specific immune response in the absence of IFN-γ (Table 2).
In the absence of IFN-α/β, mice are very susceptible to infection with HSV-1. The remaining protection was strictly IL-12 dependent. In the absence of IFN-α/β, IL-12 may activate NK cells, macrophages, and possibly T cells (14, 16, 48).
Synergies with IFN for long-term survival after HSV-1 infection.
In the absence of mature T and B cells and either IFN system (GR129 or AR129, Table 1) mice did not survive infection with HSV-1 (Fig. 1). Synergistic actions between innate and specific immune elements were required for long-term survival. Neutralizing antibodies specific to HSV-1 transferred to AR129 mice (48) or GR129 mice (data not shown) and the presence of adaptive immunity represented such elements (Table 2 and Fig. 3).
Mice without mature B and T cells (RAG−/− mice, Table 1) but intact IFN systems were remarkably resistant against infection with HSV-1 (Table 2). Direct cooperative effects between the two IFN system as well as NK cells may explain this observation (1, 40). To directly analyze the effect of NK cells, we infected mice that have no significant numbers of NK cells and lack RAG (RAG−/−γc−/− mice, Table 1). In addition, the IL-2, IL-15, and possibly IL-21 systems in these mice required for efficient activation of NK cells are nonfunctional (7, 8, 10, 17). Some of the RAG−/−γc−/− mice survived infection with HSV-1 (Fig. 4 and Table 3). Because treatment of RAG−/−γc−/− or RAG−/− mice with neutralizing antibodies to IFN-γ aborted long-term survival after HSV-1 infection (Fig. 1), we suggest that in these mice, the cooperative effect between the two IFN systems is mandatory, whereas the contribution of NK cells is synergistic (Table 2, Table 3, and Fig. 4).
Requirements for persistent viral DNA in CNS.
“True” latent HSV-1 infections in humans and in animal models, including mice, have been extensively studied, and the need for immune cells and cytokines—in particular IFN-γ—was postulated (6, 12, 13, 27, 31, 45, 49). Latent HSV-1 infections have three separable phases: establishment, maintenance, and reactivation. In this report, we have determined the general potential of the various mice to maintain the HSV-1 genome in tissues of the central nervous system (CNS). The presence of HSV-1 gB DNA was analyzed by PCR following more than 3 weeks of infection (Table 3). After infection with high doses of HSV-1, both wild-type mice and mice with the genes coding for the IFN-α/β or -γ receptor deleted can harbor viral DNA. In only four of eight RAG−/− animals was HSV-1 gB-specific DNA amplified. In various organs from two groups of RAG−/−γc−/− mice infected with different doses of HSV-1, no virus-specific DNA was detected. The role of T cells, NK cells, and macrophages in persistent viral infection has been discussed previously (26, 28, 42, 43, 48). In RAG−/−γc−/− mice, which have no mature T and B cells and no significant numbers of NK cells, the absence of viral DNA may indicate that persistent virus is incompatible with survival. HSV-1-infected RAG−/−γc−/− mice either eliminate the virus or die. The elimination of virus is most likely the result of a cooperative effect between IFN-α/β and -γ, as shown previously in vitro (40). The cells able to produce IFN-γ in RAG−/−γc−/− mice have yet to be determined. One possible source may be DC (21).
The mice described in this article may be the basis for a detailed in situ analysis of the immune elements required to control viral replication, the route of viral spread, and the sites and conditions of virus maintenance as well as reactivation.
Acknowledgments
We thank the team of the Labortierkunde, University of Zurich, headed by Frank Bootz and Martin Mörter, for breeding the animals and maintaining the correct genetic background of each strain. Bogdana Salathé carefully monitored the infection experiments. We appreciate the comments and suggestions by Hans Peter Hefti from our institute.
This work was supported by the Kanton of Zürich and a grant from the European Union (ERBFMRXCT960053) financed by the Swiss Federal Office for Education and Research.
REFERENCES
- 1.Adler, H., J. L. Beland, N. C. DelPan, L. Kobzik, R. A. Sobel, and I. J. Rimm. 1999. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208-213. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A. 2001. Interferons alpha and beta as immune regulators—a new look. Immunity 14:661-664. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 4.Brombacher, F., A. Dorfmuller, J. Magram, W. J. Dai, G. Kohler, A. Wunderlin, K. Palmer-Lehmann, M. K. Gately, and G. Alber. 1999. IL-12 is dispensable for innate and adaptive immunity against low doses of Listeria monocytogenes. Int. Immunol. 11:325-332. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski, J. F., and R. M. Welsh. 1986. The role of natural killer cells and interferon in resistance to acute infection of mice with herpes simplex virus type 1. J. Immunol. 136:3481-3485. [PubMed] [Google Scholar]
- 6.Cantin, E., B. Tanamachi, and H. Openshaw. 1999. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J. Virol. 73:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colucci, F., C. Soudais, E. Rosmaraki, L. Vanes, V. L. J. Tybulewicz, and J. P. Di Santo. 1999. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J. Immunol. 162:2761-2765. [PubMed] [Google Scholar]
- 8.Cooper, R. N., A. Irintchev, J. P. Di Santo, M. Zweyer, J. E. Morgan, T. A. Partridge, G. S. Butler-Browne, V. Mouly, and A. Wernig. 2001. A new immunodeficient mouse model for human myoblast transplantation. Hum. Gene Ther. 12:823-831. [DOI] [PubMed] [Google Scholar]
- 9.DiSanto, J. P., W. Muller, D. Guy-Grand, A. Fischer, and K. Rajewsky. 1995. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc. Natl. Acad. Sci. USA 92:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty, T. M., R. A. Seder, and A. Sher. 1996. Induction and regulation of IL-15 expression in murine macrophages. J. Immunol. 156:735-741. [PubMed] [Google Scholar]
- 11.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 12.Ellison, A. R., L. Yang, C. Voytek, and T. P. Margolis. 2000. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 268:17-28. [DOI] [PubMed] [Google Scholar]
- 13.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 5:405-411. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald-Bocarsly, P., D. M. Howell, L. Pettera, S. Tehrani, and C. Lopez. 1991. Immediate-early gene expression is sufficient for induction of natural killer cell-mediated lysis of herpes simplex virus type 1-infected fibroblasts. J. Virol. 65:3151-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchini, M., C. Abril, C. Schwerdel, C. Ruedl, M. Ackermann, and M. Suter. 2001. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J. Virol. 75:83-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granucci, F., C. Vizzardelli, N. Pavelka, S. Feau, M. Persico, E. Virzi, M. Rescigno, G. Moro, and P. Ricciardi-Castagnoli. 2001. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2:882-888. [DOI] [PubMed] [Google Scholar]
- 18.Grob, P., V. E. C. J. Schijns, M. F. van den Broek, S. P. J. Cox, M. Ackermann, and M. Suter. 1999. Role of the individual interferon systems and specific immunity in mice in controlling systemic dissemination of attenuated pseudorabies virus infection. J. Virol. 73:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habu, S., K. Akamatsu, N. Tamaoki, and K. Okumura. 1984. In vivo significance of NK cell on resistance against virus (HSV-1) infections in mice. J. Immunol. 133:2743-2747. [PubMed] [Google Scholar]
- 20.Hendrzak, J. A., and P. S. Morahan. 1994. The role of macrophages and macrophage cytokines in host resistance to herpes simplex virus. Immunol. Ser. 60:601-617. [PubMed] [Google Scholar]
- 21.Hochrein, H., K. Shortman, D. Vremec, B. Scott, P. Hertzog, and M. O'Keeffe. 2001. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 166:5448-5455. [DOI] [PubMed] [Google Scholar]
- 22.Hölscher, C., R. Atkinson, B. Ardense, N. Brown, E. Myburgh, G. Alber, and F. Brombacher. 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J. Immunol. 167:6957-6966. [DOI] [PubMed] [Google Scholar]
- 23.Kaerber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480. [Google Scholar]
- 24.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 25.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethmann, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-690. [DOI] [PubMed] [Google Scholar]
- 26.Kodukula, P., T. Liu, N. V. Rooijen, M. J. Jager, and R. L. Hendricks. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J. Immunol. 162:2895-2905. [PubMed] [Google Scholar]
- 27.Lekstrom-Himes, J. A., R. A. LeBlanc, L. Pesnicak, M. Godleski, and S. E. Straus. 2000. Gamma interferon impedes the establishment of herpes simplex virus type 1 latent infection but has no impact on its maintenance or reactivation in mice. J. Virol. 74:6680-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattner, F., L. Ozmen, F. J. Podlaski, V. L. Wilkinson, D. H. Presky, M. K. Gately, and G. Alber. 1997. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect. Immun. 65:4734-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercurio, A. M., G. A. Schwarting, and P. W. Robbins. 1984. Glycolipids of the mouse peritoneal macrophage. Alterations in amount and surface exposure of specific glycolipid species occur in response to inflammation and tumoricidal activation. J. Exp. Med. 160:1114-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minagawa, H., and Y. Yanagi. 2000. Latent herpes simplex virus-1 infection in SCID mice transferred with immune CD4+ cells: a new model for latency. Arch. Virol. 145:2259-2272. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, S. N., C. M. Jones, C. M. Smith, W. R. Heath, and F. R. Carbone. 2002. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J. Exp. Med. 195:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 34.Pinto, A. J., P. S. Morahan, M. Brinton, D. Stewart, and E. Gavin. 1990. Comparative therapeutic efficacy of recombinant interferons-alpha, -beta, and -gamma against alphatogavirus, bunyavirus, flavivirus, and herpesvirus infections. J. Interferon Res. 10:293-298. [DOI] [PubMed] [Google Scholar]
- 35.Posavad, C. M., D. M. Koelle, and L. Corey. 1996. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J. Virol. 70:8165-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roizman, B., and W. Batterson. 1986. Herpesviruses and their replication, p. 607-636. In B. N. Fields and D. M. Knipe (ed.), Fundamental virology. Raven Press, New York, N.Y.
- 37.Rosmaraki, E., I. Douagi, C. Roth, F. Colucci, A. Cumano, and J. Di Santo. 2001. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 31:1900-1909. [DOI] [PubMed] [Google Scholar]
- 38.Rossol-Voth, R., S. Rossol, K. H. Schutt, S. Corridori, W. de Cian, and D. Falke. 1991. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J. Gen. Virol. 72:143-147. [DOI] [PubMed] [Google Scholar]
- 39.Ryncarz, A. J., J. Goddard, A. Wald, M.-L. Huang, B. Roizman, and L. Corey. 1999. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J. Clin. Microbiol. 37:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sainz, B., Jr., and W. P. Halford. 2002. Alpha/beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J. Virol. 76:11541-11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuler, G. 1984. The dendritic, Thy-1-positive cell of murine epidermis: a new epidermal cell type of bone marrow origin. J. Investig. Dermatol. 83:81-82. [DOI] [PubMed] [Google Scholar]
- 42.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, P. M., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma). Virology 202:76-88. [DOI] [PubMed] [Google Scholar]
- 44.Suter, M., A. M. Lew, P. Grob, G. J. Adema, M. Ackermann, K. Shortman, and C. Fraefel. 1999. BAC-VAC, a novel generation of (DNA) vaccines: a bacterial artificial chromosome (BAC) containing a replication-competent, packaging-defective virus genome induces protective immunity against herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 96:12697-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas, J., and B. T. Rouse. 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 16:375-386. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines. New players in the regulation of T cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 47.van den Broek, M. F., U. Müller, S. Huang, M. Aguet, and R. M. Zinkernagel. 1995. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 69:4792-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollstedt, S., M. Franchini, G. Alber, M. Ackermann, and M. Suter. 2001. Interleukin-12- and gamma interferon-dependent innate immunity are essential and sufficient for long-term survival of passively immunized mice infected with herpes simplex virus type 1. J. Virol. 75:9596-9600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner, E. D., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]