Abstract
Skeletal muscle fibrosis can be a devastating clinical problem that arises from many causes, including primary skeletal muscle tissue diseases, as seen in the muscular dystrophies, or it can be secondary to events that include trauma to muscle or brain injury. The cellular source of activated fibroblasts (myofibroblasts) may include resident fibroblasts, adult muscle stem cells, or inflammatory or perivascular cells, depending on the model studied. Even though it is likely that there is no single source for all myofibroblasts, a common mechanism for the production of fibrosis is via the transforming growth factor-β/phosphorylated Smad3 pathway. This pathway and its downstream targets thus provide loci for antifibrotic therapies, as do methods for blocking the transdifferentiation of progenitors into activated fibroblasts. A structural model for the extracellular collagen network of skeletal muscle is needed so that measurements of collagen content, morphology, and gene expression can be related to mechanical properties. Approaches used to study fibrosis in tissues, such as lung, kidney, and liver, need to be applied to studies of skeletal muscle to identify ways to prevent or even cure the devastating maladies of skeletal muscle.
Keywords: myofibroblast, passive mechanics, muscle mechanics, stiffness
skeletal muscle extracellular matrix (ECM) not only plays the general supportive role that is typical of other tissues, such as lung, liver, and heart, but the proper functioning of the skeletal muscle matrix is critical to maintain the normal locomotor ability of an organism. Alterations in the ECM perturb functional properties of muscle in a way that can be directly measured, and, in fact, the health of skeletal muscle, which makes up ∼50% of the body mass (74), is tantamount to the health of the organism itself.
In this review, we focus on unique aspects of the structural and functional changes in muscles that undergo fibrosis. While skeletal muscle retains many of the fibrotic mechanisms of other tissues, the functional effects of fibrosis, such as in muscle contractures, in which a joint takes on a permanently fixed position that requires relief by surgery (75), or retracted rotator cuff muscles, which are separated from the bone and are very difficult to reattach (133), can be devastating. Because the organization of skeletal muscle ECM is closely linked to its mechanical function, this tissue provides a unique opportunity to study the mechanics of fibrosis in a way that is not possible with other tissues.
Skeletal Muscle ECM
Skeletal muscle tissue is dominated by large, multinuclear muscle fibers that extend along the muscle's length. Thus it is perhaps not surprising that the field of skeletal muscle physiology has focused on the properties of these fibers, including their size (44), function (19), gene regulation (108), and development (130), with a surprising paucity of studies that define the structural and functional properties of the ECM. The majority of microscopic studies of skeletal muscle are performed on tissue cross sections, which reveal tightly packed polygonal fibers surrounded by a very small amount of ECM, typically only ∼5% (27) (Fig. 1A). While muscle fiber type specialization among mammalian muscles (12, 122) and within muscles (69) is well characterized, the surrounding extracellular tissue has been treated more or less generically. Thus students are typically taught that skeletal muscle fibers are surrounded by endomysial connective tissue, bundles of muscle fibers are surrounded by perimysial connective tissue, and the entire muscle is surrounded by epimysial connective tissue (28). However, inspection of a muscle cross section reveals that these designations are relatively arbitrary (Fig. 1A). Thus, while understanding the cellular mechanics and biology of muscle cells represents one of the triumphs of modern science, there is a lack of understanding of the noncontractile skeletal muscle ECM.
Fig. 1.
A: micrograph of a cross section of healthy skeletal (rat tibialis anterior) muscle demonstrating normal morphology that consists of tightly packed polygonal fibers with a small amount (∼5%) of extracellular material. Traditional location of endomysial connective tissue is outlined with a solid line; perimysial tissue is outlined with a dashed line. However, as can be seen elsewhere in the micrograph, this distinction can be arbitrary. B: micrograph of a cross section of skeletal muscle demonstrating fibrotic morphology in which extracellular material is increased to ∼20% of the cross section, fibers are loosely packed, extracellular space is hypercellular, and fiber sizes are highly variable. This muscle was injected twice with botulinum toxin type A (Botox, Allergan; 6 U/kg in 100 μl) at a 3-mo interval and tested after 6 mo.
ECM Composition
Numerous noncontractile proteins have been isolated from skeletal muscle homogenates and are believed to make up the ECM. Many of the details of these protein populations have recently been reviewed (38, 77) and are only briefly outlined here. As with most tissues, the major ECM muscle protein is collagen, dominated by the type I and type III isoforms. Muscle cells themselves are surrounded by a basement membrane composed of type IV collagen, which is intimately associated with an integrin focal adhesion complex. This complex includes primarily α7β1- and other α-integrin subunits, the identity of which depends on the precise developmental time point investigated and whether the integrin is in the region of the sarcolemma (muscle plasma membrane), the muscle-tendon junction, or the neuromuscular junction (81, 107). A second focal adhesion between myofibrils and the ECM is formed by the dystroglycan complex (94), an array of proteins that may perform a role similar to that of integrins; abnormalities of the dystroglycan complex are associated with a large number of primary myopathies (31). The transmission of force between muscle cells and the ECM is not unique to muscle tissue, since it likely occurs in all cells but, in skeletal muscle, is essentially its primary function (97).
The intimate association between the extracellular and intracellular milieus creates a tissue that acts as an excellent mechanotransducer of the environment and is very sensitive to alterations in use patterns. Many of these “altered-use” patterns have profound clinical relevance and are active areas of study. These include muscle hypertrophy due to exercise, stretch, and hormonal treatment, as well as muscle atrophy due to tenotomy, spaceflight, aging, spinal cord injury, denervation, and regeneration. Each of these models is discussed in detail elsewhere (74, 98, 102, 109). Nearly all these altered-use models result in muscle fibrosis. Unlike the generally opposite direction of muscle fiber adaptation to increased use (where muscle fibers hypertrophy and decrease speed) compared with decreased use (where muscle fibers atrophy and increase speed), almost all altered-use patterns result in increased skeletal muscle ECM (Fig. 1B).
Extracellular Collagen Matrix Structure
The structure of the extracellular collagen network of skeletal muscle is poorly understood. Apart from the classic mathematical models and vivid images of the endomysial connective tissue network published decades ago by Trotter and colleagues (100, 123, 124), there are almost no other quantitative physiological or mechanical studies of skeletal muscle ECM that permit a mechanistic understanding of the way in which skeletal muscles bear passive tension. One recent development in this area relates to the rediscovery of the so-called perimysial cables in skeletal muscles that were originally described by Borg and Caulfield (10). The highly organized longitudinal nature of these cables (Fig. 2A) identifies them as likely candidates for major passive load-bearing structures in skeletal muscles. These cables are composed of collagen fibrils that are stereotypically arranged in bundles of 25–100 fibrils and occupy the region between muscle fibers and fiber bundles (Fig. 2, B and C). These cables can traverse extremely long distances (>150 μm) within the muscle ECM and provide a parallel elastic element to the muscle tissue itself (38). The extent to which loading is borne inside and outside the muscle cell is not known.
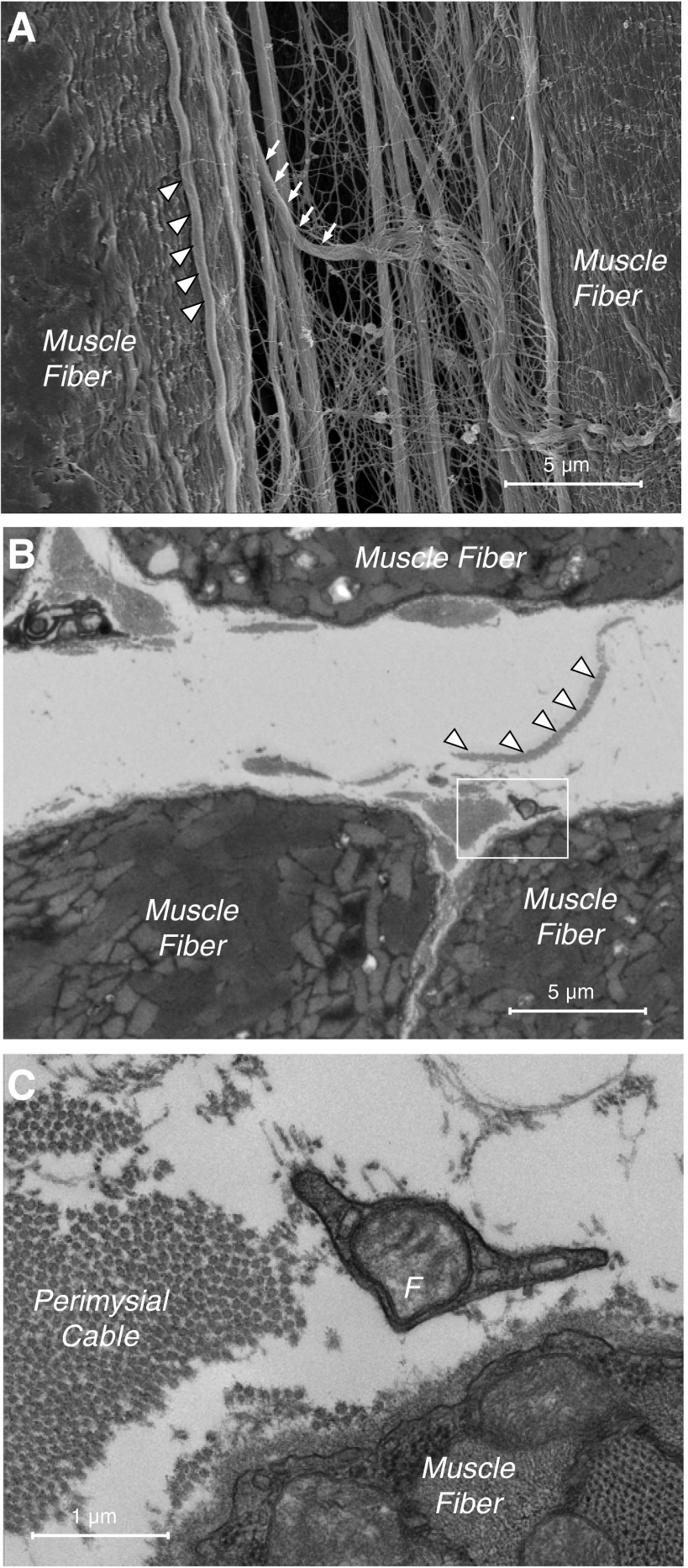
Fig. 2.
A: scanning electron micrograph of perimysial cables in a stretched mouse extensor digitorum longus (EDL) muscle. These cables run longitudinally along the length of the muscle fiber without noticeable connections to the fiber itself over ∼100-μm distances (arrowheads). Cables consist of bundles of collagen fibrils, as can be seen in the cable that has frayed (arrows). (Micrograph modified from Ref. 38.) B: transmission electron micrograph of wild-type mouse EDL muscle in the extracellular space region. A perimysial cable is demarcated with the box, and a wide ribbonlike perimysial cable is marked with arrowheads. C: higher-magnification view of the region in B indicates that the perimysial cable is composed of collagen fibrils. (This is most easily seen on longitudinal sections that demonstrate the characteristic striation pattern of type I collagen fibrils.) Also shown are superficial aspects of the muscle cell and a section of an extracellular-resident fibroblast (F). (Micrographs courtesy of Allison Gillies.)
Measures of Skeletal Muscle Fibrosis
An important and unresolved issue regarding skeletal muscle fibrosis is the definition of “muscle fibrosis.” As a general rule, fibrosis is characterized by abnormal accumulation of ECM, but time and severity of disease are important contributors to fibrosis. For example, accumulation of ECM can be seen in nearly all models of muscle injury or damage, but this is often transient and thought to stabilize the contractile apparatus while normal adaptive or regenerative processes proceed. In contrast, long-term accumulation of ECM interferes with function, does not resolve under normal physiological conditions, and is therefore considered an end-stage process. To be consistent with other tissues, skeletal muscle fibrosis could be defined as an abnormal and unresolvable, chronic increase in extracellular connective tissue that interferes with function.
The precise quantification of skeletal muscle fibrosis can be difficult. This is due in part to the fact that, as mentioned above, a deterministic quantitative model of collagen arrangement for muscle ECM is not available. Additionally, situations in which fibrosis occurs (e.g., exercise-induced injury, severe atrophy, chronic inflammation, and dystrophies) dramatically alter muscle fiber size, which complicates expressing fibrosis as a “number” or even a relative fraction. Typically, skeletal muscle assays quantify the cross-sectional area fraction of ECM by excluding muscle fibers using image-processing algorithms and then report the amount of ECM as “area fraction,” as suggested in stereology (128). The problem with this approach is that if muscle fibers atrophy and ECM structure remains the same, ECM will occupy a greater fraction of the muscle cross section. In addition, this approach gives no information regarding collagen isoforms and cross-linking, which can also affect function. For normal muscle, the ECM area fraction is typically ∼5%, but this value can increase dramatically in diseased or injured states. In addition to increased fractional area (area fraction) of ECM in fibrotic muscles, because the pathological response often includes fiber degeneration and regeneration, muscle fibrosis is also accompanied by a large increase in muscle fiber size variation. Thus pathologists often highlight the increase in the area fraction of ECM, as well as the increased fiber size variability, as the defining factor in describing a tissue sample as pathological (16, 23).
Skeletal muscle fibrosis can also be expressed in terms of the total amount of collagen present in the tissue, as measured by the content of hydroxyproline, a major component of collagen derived from hydroxylation of the amino acid proline by prolyl oxidase. While this assay has been used for decades (48), expression of collagen mass relative to a known muscle protein is only rarely reported. In the majority of studies of skeletal muscle, collagen contents (typically expressed as micrograms of collagen per wet or dry muscle mass) of experimental and control groups are compared. While this content provides some insight into a tissue's response to treatment, in the few cases where collagen content has been quantified along with skeletal muscle mechanical stiffness, the two values show only a weak correlation (Fig. 3). Thus the method used to quantify fibrosis in skeletal muscle will depend on whether one uses a morphological assay such as area fraction, a biochemical assay such as collagen content, or a functional assay such as stiffness. It is not possible to quantitatively interchange results of these assays, although they usually change in the same direction. Thus a muscle with increased collagen content also typically has an increased stiffness, but a quantitative relationship between the two has not been established.
Fig. 3.
Correlation between collagen content as measured by hydroxyproline assay and mechanical tangent stiffness measured biomechanically in human muscle fiber bundles, as described in Ref. 111. Note that collagen content alone is a very poor predictor of bundle stiffness.
Muscle Function and Fibrosis
Because fibrosis occurs in the ECM, functional measurements of fibrosis can be made in the absence of muscle fiber activation and muscle contraction. It has been known for centuries that skeletal muscles bear load when passively lengthened (8), but a full understanding of the origin of passive skeletal muscle tension is lacking. This vagueness is in stark contrast to the highly detailed molecular description of active muscle sarcomere force generation elucidated almost 50 years ago (26, 46) and recent descriptions of the intracellular myofibrillar passive load bearing via the molecule titin (49, 92, 93). Passively elongated muscle cells typically bear stresses of ∼50 kPa (units of kPa are used to express stress, or force per unit area: 1 Pa = 1 N/m2; biological tissues typically bear stresses in the kilo- to megapascal range; Table 1). Passively elongated bundles of muscle cells (that contain ECM as well as fibers) bear stresses on the order of 250 kPa. Comparisons of fiber and bundle stresses can be difficult, as the method of cross-sectional area measurement (which is required for the stress calculation) can be subject to error, especially in muscle fibers, which can have highly variable shapes. However, on the basis of the 5- to 25-fold difference in fiber stress compared with bundle stress and the small amount of ECM relative to muscle fibers, modulus values (i.e., the intrinsic stress-bearing ability of ECM) for the ECM of skeletal muscle are relatively high, on the order of hundreds of megapascals, or even in the gigapascal range (37, 76, 100).
Table 1.
Tensile modulus values for various connective tissues
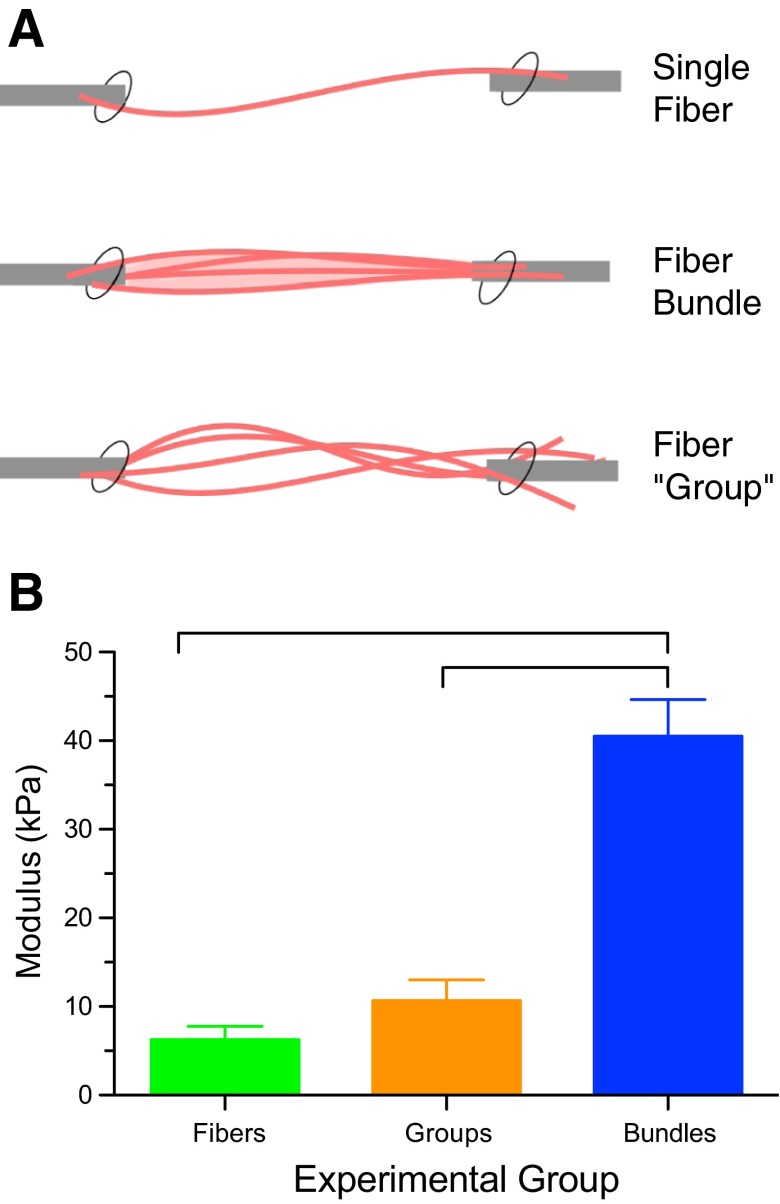
To definitively show that the ECM was the major source of passive load bearing, we compared what we called a “group” of muscle fibers (single muscle fibers that had each been dissected free of the ECM and then tied together) with a naturally occurring bundle (the same number of fibers, but in their native configuration, containing both fibers and ECM; Fig. 4A). Stretching each of these samples in small increments and measuring both force and cross-sectional area revealed that the natural muscle fiber bundle had much higher stress, even though it had the same cross-sectional area as the group of fibers (Fig. 4B). Furthermore, the fiber groups bore the same stress (force/area), even though they were 10–20 times larger than the single fibers (86). Given that the ECM in these tissues makes up only 5–10% of the cross-sectional area, an overall muscle modulus of 40 kPa (Fig. 4B) corresponds to an ECM modulus of ∼1 GPa. Therefore, while it is clear that the intracellular protein titin bears much of the force in muscle cells (99), from a tissue point of view, the major muscle passive load bearing occurs in the ECM. Current estimates of ECM modulus are 5–25 times the modulus of muscle cells.
Fig. 4.
A: schematic illustration of the arrangement of 3 specimen types. Single fibers (curved pink lines) were isolated from the muscle and tested individually or secured in groups. Bundles of a similar number of fibers embedded in ECM (light pink) were isolated and similarly secured. (Modified from Ref. 86 with permission from Elsevier). B: normalized stiffness of fibers, fiber groups, and fiber bundles. Fiber bundles have a significantly higher modulus than either individual fibers or fiber groups, demonstrating that the ECM provides a large fraction of the load bearing. Fiber and fiber group moduli were not significantly different from each other. Connecting bars represent significant differences (P < 0.05). (Data replotted from Ref. 86 with permission from Elsevier.)
Model Systems of Skeletal Muscle Fibrosis
There are numerous models of fibrosis in skeletal muscle (Table 2), probably the most dramatic of which are the muscular dystrophies, which have cyclic degeneration and regeneration. In different types of dystrophy, ECM area fraction increases as much 10-fold, and the associated muscle increases in stiffness. These models are highly complex, since cellular infiltration, muscle atrophy, fiber size variability, and regenerating fibers accompany fibrosis and fill the tissue area (Fig. 5). Since this type of fibrosis is extreme and clinically relevant, studies of muscle fibrosis and of potential therapeutic interventions for its prevention are often performed using dystrophic models (see below).
Table 2.
Models of in vivo skeletal muscle fibrosis and common features
| Model | Animal | Muscle | Stiffness | Connective Tissue | Inflammation | MHCs | Myofibroblasts (α−SMA) | Atrophy | Regeneration | Fat | Sample References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical | |||||||||||
| Laceration | Mouse | Gastrocnemius | I | I | I/F | I | I | 17, 34, 71 | |||
| Puncture | Mouse | TA | I | I | I/F | I | I | 21 | |||
| Chronic stretch | Rabbit | EDII | I | NC | 115 | ||||||
| Immobilization | Mouse | Soleus | I | I | I | 114 | |||||
| Rat | TA | I | 61 | ||||||||
| Soleus | I | ||||||||||
| Gastrocnemius | I | ||||||||||
| Exercise | Human | Vastus lateralis | I | 53 | |||||||
| Hindlimb suspension | Rat | Soleus | NC | I | I | 52, 54, 118 | |||||
| Tenotomy | Mouse | Supraspinatus | I | I | 105 | ||||||
| Rat | Supraspinatus | I | I | I | I | I | 5, 39, 127 | ||||
| Dog | Infraspinatus | I | I | I/F | I | 104 | |||||
| Sheep | Infraspinatus | I | I/F | I | 85 | ||||||
| Human | Supraspinatus | I | I | I | 36, 55 | ||||||
| Chemical or toxin | |||||||||||
| Cardiotoxin | Mouse | TA | I | I | I/F | I | I | 71 | |||
| Glycerol | |||||||||||
| Botulinum toxin | Rat | I | 119 | ||||||||
| hrTGF-β1 | Mouse | TA | I | I | I/F | I | I | D | 71 | ||
| BaCl2 | Mouse | Gastrocnemius | I | I | I | 66 | |||||
| TA | I | I | I | 66 | |||||||
| Genetic | |||||||||||
| Desmin KO | Mouse | I | I | I | S | I | I | 71 | |||
| Mdx | Mouse | Diaphragm | I | I | I | I/F | I | I | 58, 60 | ||
| Biceps femoris | I | I | I | I/F | I | I | |||||
| DMD | Human | I | I | I | I | I/F | I | I | I | 29, 33, 79, 82, 103 | |
| Acquired disease | |||||||||||
| Cerebral palsy | Human | Semitendinosus | I | I | S | 111 | |||||
| Gracilis | I | I | S | 111 | |||||||
| Stroke | Human | Plantarflexors | I | F | I | 40, 50 | |||||
| Spinal cord injury | Human | Vastus lateralis | I | I | F | 7, 13 | |||||
| Osteoarthritis | Human | Vastus medialis | I | I | I | 32 | |||||
| Diabetes (type 2) | Human | I | I | I | |||||||
| Aging | |||||||||||
| Rat | EDL | I | I | 3 | |||||||
| Soleus | I | I | |||||||||
| Human | Vastus lateralis | NC | S | I | I | 45, 51, 67 | |||||
MHCs, myosin heavy chains; α−SMA, α−smooth muscle actin; TA, tibialis anterior; EDII, 2nd toe digital extensor; hrTGF-β1, human recombinant transforming growth factor-β1; KO, knockout; DMD, Duchenne muscular dystrophy; EDL, extensor digitorum longus; I, increased; D, decreased; S, slower; F, faster; NC, no change. Blank entry indicates no data available.
Fig. 5.
Micrograph of a cross section of a regenerated skeletal muscle from a control mouse (A) and a mouse model of Duchenne muscular dystrophy, the mdx mouse (B). In contrast with the control muscle and the muscle shown in Fig. 1, the mdx muscle has more loosely packed fibers of highly varying size with a large amount (>25%) of hypercellular extracellular material. Scale bars, 60 μm. (From Ref. 121.)
However, skeletal muscle fibrosis can also occur in settings where the treatment of the muscle is extremely simple and does not involve a large degree of degeneration or regeneration. One approach is to simply stretch a muscle-tendon unit and secure it at a fixed length. This maneuver results in acute muscle lengthening, addition of serial sarcomeres (116), muscle fiber atrophy, increased collagen content and collagen area fraction (65), and increased muscle stiffness (115). This fibrotic response occurs with negligible muscle fiber regeneration. Thus, while cellular regeneration often accompanies fibrosis, the two are not necessarily causally related. In a recent study, we chronically stretched skeletal muscles (by securing the distal tibialis anterior tendon to the ankle extensor retinaculum) and measured muscle stiffness and sarcomeric properties (115). This protocol dramatically increased muscle stiffness far more than would be expected on the basis of muscle fiber length change alone, and, importantly, the relationship between active and passive muscle force generation was largely disrupted (Fig. 6). Specifically, active force produced by control and stretched muscle was approximately the same (Fig. 6, dotted line), whereas the fibrotic, stretched muscle was much stiffer than the control muscle.
Fig. 6.
Active (dotted lines) and passive (red and blue symbols) length-tension curves of a control rabbit muscle and a rabbit muscle subjected to chronic stretch at a fixed length (Fibrotic). Chronic surgical stretch results in a leftward shift in passive mechanical properties, demonstrating the functional mechanical effects of fibrosis. (Data replotted from Ref. 115.)
Muscle unloading that results from tenotomy [i.e., cutting the muscle insertion tendon and allowing the muscle to retract (1)] is accompanied by significant muscle fiber atrophy, which is mediated by catabolic processes involving the muscle ubiquitin-proteasome pathway (68, 90) and its associated E3 ligase partners (106). This raises the intriguing possibility that muscle fiber atrophy may be associated with fibrosis. Indeed, it is provocative that most fibrotic skeletal muscles are also atrophied or vice versa. It thus appears that resetting a muscle to an altered length initiates the fibrotic process. Whether this is an attempt to restore length or fix length or is simply a secondary response to muscle atrophy is not known.
Another interesting model of skeletal muscle fibrosis has been created with the deletion (by homologous recombination) of the intermediate filament protein desmin (89). Loss of this protein decreases muscle fiber stiffness but also increases ECM stiffness and fibrosis (87). Since the process of fibrosis in this model is relatively slow and involves very little fiber regeneration, we investigated the timing of the change in muscle fiber properties relative to the change in ECM properties in the absence of regeneration. Measurement of fiber and bundle properties of neonatal and adult mice revealed that muscle fibrosis developed after the fibers changed their properties, which suggests that the biomechanical properties of the muscle cell and its ECM may be mutually sensed. In this way, a change in the ECM may compensate for changes in cellular mechanical properties. This is potentially a system in which the tissue mechanical properties are regulated by communication among the various tissue domains, which has been suggested by others (130).
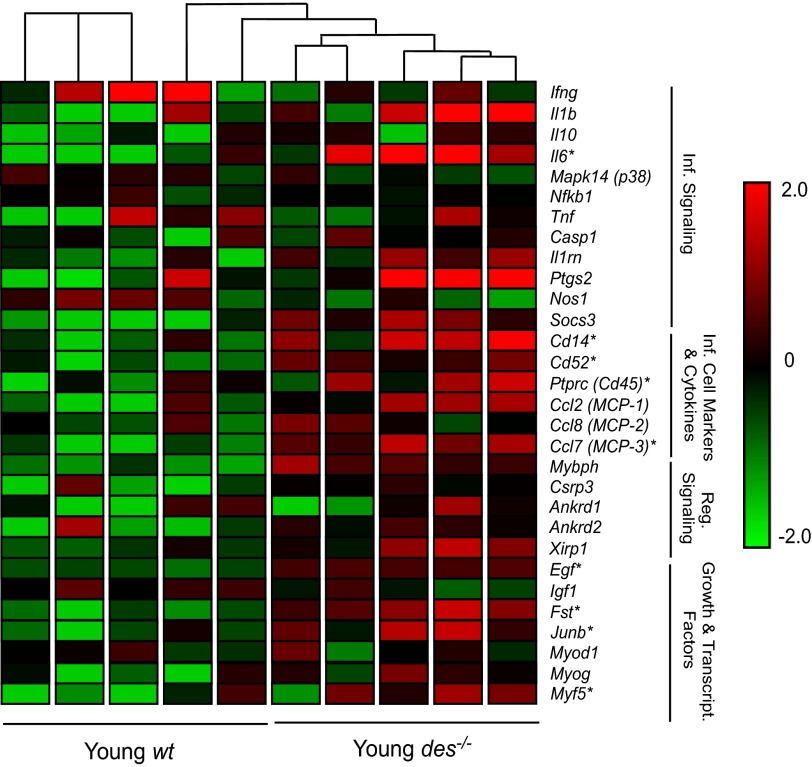
In an attempt to understand the mechanism of fibrosis in the intermediate filament knockout model, we performed transcriptional profiling of this fibrotic tissue (88) and found that intramuscular inflammation was one of the most dramatically altered systems. Specifically, a group of 18 genes were differentially expressed between desmin-deficient (Des−/−) and wild-type (WT) samples (Fig. 7), suggesting that inflammation contributes to Des−/− muscle ECM remodeling. Consistent with these results, gene ontology (GO) pathways involved in inflammation and response to stress, including response to wounding (GO:0009611), inflammatory response (GO:0006954), and regulation of tumor necrosis factor (GO:0032680), were overrepresented in the Des−/− compared with the WT muscle. Since tissue inflammation in general (131) and skeletal muscle inflammation in particular (129) are associated with fibrosis, this provides another potential tissue-level explanation for the fibrotic response. Moreover, inflammatory cells can be a source of cytokines that lead to fibrotic tissue (see below).
Fig. 7.
Data from a transcriptional profile of control muscles [Young wild-type (wt), n = 4] and muscles with a desmin intermediate filament deletion [desmin-deficient (Des−/−), n = 4]. Des−/− muscle demonstrates increased ECM-specific gene expression, as shown in the normalized gene expression pattern for 42 genes involved in ECM structure and maintenance. Expression levels are shown on a color scale, with green and red representing low and high expression, respectively. Hierarchical clustering is represented by connecting lines at the top of the grid, with lines closest to the grid denoting the most similar samples. Des−/− muscle samples have a distinct expression pattern of ECM genes compared with wild-type muscles, showing a higher expression of the majority of the listed genes indicated by the red color scheme. ECM-related genes are subdivided into 4 categories: basement membrane, ECM constituents, proteases, and protease inhibitors. Inf, inflammatory; Reg, regulatory. *Significantly higher expression values, as determined by 2-way ANOVA. (Data from Ref. 87.)
Cellular and Molecular Basis of Skeletal Muscle Fibrosis
Not only are the structural and functional properties of the skeletal muscle ECM poorly understood, but also there is a lack of insight regarding mechanisms that lead to fibrosis, at least relative to those previously reported in this series (15, 41, 131). While functional effects of fibrosis and many of its morphological signs have been well studied in skeletal muscle, the cellular and molecular determinants of fibrosis are less well defined for skeletal muscle than for other tissues (note that skeletal muscle is not among the tissues listed in Table 1 of Ref. 129). The majority of the studies in this area involve muscular dystrophies, a hallmark of which is skeletal muscle fibrosis, as noted above.
Transforming Growth Factor-β in Skeletal Muscle Fibrosis
The cellular signaling processes that dominate skeletal muscle fibrosis follow the general pattern for other tissues, as previously discussed in this series of articles (15, 41, 131). Of particular importance is the cytokine transforming growth factor-β (TGFβ), which, in skeletal muscle, takes the form of TGFβ1 (80). TGFβ1 is released from injured muscle fibers or, in an autocrine fashion, from fibers exposed to TGFβ1 (71) and accompanies muscle inflammation (78), whereby inflammatory cells can deposit this cytokine. TGFβ receptor activation leads to phosphorylation of receptor-regulated Smad (R-Smad), and, in turn, transcriptional pathways are activated or repressed (22). The quantity and type of Smad partners determine which genes will be targeted and if they will be activated or repressed. Variations in the expression of TGFβ receptors, the presence of receptor antagonists, and the variety of mechanisms downstream of Smad explain why TGFβ can be involved in muscle fibrosis, as well as other processes, such as cell proliferation, differentiation, cancer, and immunity (80). Because of the central role of TGFβ1 in skeletal muscle fibrosis, anti-TGFβ1 therapy is a strategy used to minimize or ameliorate the effects of fibrosis (34, 132).
Myostatin in Skeletal Muscle Fibrosis
Another member of the TGFβ superfamily and a muscle-specific factor is myostatin, also known as growth differentiation factor 8 (83). Myostatin has a well-known role in regulating muscle mass: animals lacking the myostatin gene (84) or humans with mutated myostatin proteins (110) have extremely large muscles. Skeletal muscle regenerates more effectively and develops less fibrosis in the absence of myostatin or in the presence of myostatin inhibitors (132). The dystrophic mdx mouse model has less fibrosis and increased muscle size in the absence of myostatin, suggesting synergistic actions between myostatin and other fibrotic pathways (126), apparently via apoptotic mechanisms (9). Myostatin can directly stimulate muscle fibroblasts to proliferate and expresses ECM proteins (73). In the latter experiments, myostatin stimulated proliferation of fibroblasts from dystrophic muscle and increased the resistance of fibroblasts to apoptosis.
ANG II in Skeletal Muscle Fibrosis
While anti-TGFβ-based therapies have proven effective in animal models, clinical application is hampered by severe side effects, lack of oral dosing profiles, and regulatory hurdles. In this regard, fibrosis occurs in the presence of the peptide hormone ANG II, which also causes vasoconstriction and increases blood pressure. Because blood pressure regulation is a well-studied area of cardiovascular physiology and of major clinical significance, there is interest in using agents that block actions of ANG as an antifibrotic therapy. ANG II, which is derived from the action of ANG II-converting enzyme (ACE) on ANG I, acts on smooth muscle (and other types of) cells via ANG receptors. Importantly, the use of ACE inhibitors and ANG receptor blockers decreases fibrosis in heart, liver, kidney, lung, and muscle (6). Patients treated with ANG-modulating drugs can display decreased muscle atrophy and muscle tissue adipogenesis (96), implying that antifibrotic therapy has positive effects on muscle health. However, no change in muscle properties were observed in experiments in which rats were treated with ACE inhibitors for 12 wk (4), so it is not clear if modulation of ANG directly affects muscle cells.
Collagen Triple Helix Repeat-Containing 1 Protein in Skeletal Muscle Fibrosis
A fascinating recent development in the muscular dystrophy field is the increased expression of collagen triple helix repeat-containing 1 (Cthrc1) protein in response to TGFβ1/Smad3 activation. Cthrc1 was first identified as a unique transcript in balloon-injured arteries, along with deposition of collagen and the presence of myofibroblasts (101). Increased Cthrc1 expression has also been associated with increased cell migration, motility, and invasion in cancer models (117) and, importantly, with inhibition of TGFβ1-stimulated collagen I synthesis (70). These findings suggest that tissue matrix remodeling and repair could be affected by Cthrc1 by limiting collagen I synthesis and promoting cell migration, with Cthrc1 playing a part in the physiological machinery that reverses the fibrotic reaction after termination of the TGFβ stimulus. Moreover, where collagen I and Cthrc1 colocalized, the collagen fibers appeared smaller, suggesting that Cthrc1 is also involved in collagen turnover. In the dystrophic mouse models, infiltrating myofibroblasts were the source of the Cthrc1. Cthrc1 is thus another attractive therapeutic target to prevent fibrosis.
Wnt Signaling in Skeletal Muscle Fibrosis
Similar to the relationship between muscle atrophy and fibrosis is the relationship between muscle aging and fibrosis (42). Using a surgical model in which the circulatory systems of older and younger mice are connected, Brack et al. (11) demonstrated that systemic factors are involved in the aging process, which results in increased collagen deposition in muscles. Since it had been observed that the Wnt pathway participates in fibrogenic conversion of pulmonary cells (18) and liver cells (62), this pathway was tested in the skeletal muscle aging model. Intriguingly, it was determined that not only was the Wnt pathway involved (since use of the specific Wnt inhibitors Dickkopf homolog 1 and secreted frizzled-related protein 3 reversed the effect), but the cells that became fibrogenic (as indicated by the ER-TR7 marker) were those that had been myogenic, as demonstrated using lineage tracing. In the older animals, myogenic cells (identified on the basis of their expression of Pax7) were more likely to move toward fibrogenesis than myogenesis. The extent to which myogenic precursors are involved in other models of fibrogenesis is not known. Other components of the Wnt signaling cascade, including glycogen synthase kinase 3β and its substrate β-catenin, which translocates to the muscle cell nucleus upon activation, were also involved in this process. Because the Wnt signaling pathway is so highly conserved across species, these experiments suggest that the fibrosis pathway is also highly conserved. In addition, since almost all the signaling pathways described here are also involved in embryonic development, as well as carcinogenesis, these results demonstrate the delicate balance and context-dependent nature of the fibrotic processes (80). Of course, given the highly mechanically active nature of skeletal muscle, it is also likely that many (if not all) of these signaling pathways and morphogenic transitions will themselves be mechanosensitive.
Cells Involved in Skeletal Muscle Fibrosis
While collagen deposition by myofibroblasts is involved in skeletal muscle fibrosis models, the cellular source of myofibroblasts in various conditions is less clear (Fig. 8). As noted previously in this series, certain cell types transdifferentiate into myofibroblasts (see Fig. 2 of Ref. 131). The detailed fate-mapping and lineage-tracing experiments in studies of other tissues have not been done in studies of skeletal muscle. Numerous cell sources have been suggested for myofibroblasts in muscle, including muscle-derived stem cells (72), resident fibroblasts (120), and, as observed in injured arteries and skin wounds (25), fibrocytes (56), perivascular cells (20), and nerve-associated cells (57).
Fig. 8.
A: schematic illustration of the location of cells involved in skeletal muscle fibrosis. Skeletal muscle contains numerous sources of mononuclear cells that can ultimately become activated fibroblasts (myofibroblasts; multicolored). These sources include inflammatory cells (black), resident fibroblasts (blue), fibroadipogenic progenitor (FAP) cells (orange), and pericytes (black). There is no evidence that satellite cells (green) become myofibroblasts. B: after an insult that results in fibrosis (cf. Table 2), muscle tissue is characterized by muscle fiber atrophy, increased collagen content, presence of myofibroblasts and inflammatory cells, increased muscle mechanical stiffness, and myofibrillar disorganization that presents primarily as disrupted muscle cell z disks.
Recently, skeletal muscle fibrosis and adipogenesis have been linked by the discovery of an adult muscle progenitor cell population: fibroadipogenic progenitors (FAPs) (63, 125). These cells, which are uniquely identified by their expression of the PDGF receptor-α (PDGFRα), do not differentiate into muscle fibers in vivo or in vitro. Transplantation experiments revealed that the differentiation of FAP cells into fat or connective tissue was strongly affected by the local environment (Fig. 9). The precise molecular cues for this differentiation are not known. The central role in fibrosis of cells that express PDGFRα was vividly demonstrated by experiments in which chronic PDGFRα activation resulted in widespread organ fibrosis (95), but the extent to which FAPs play a role in skeletal muscle fibrosis is not clear. In fact, the role of all these cell types in muscle fibrosis is not clear. Perhaps conditions with different causes recruit different populations of cells.
Fig. 9.

Two different progenitor cells differentiate into either muscle or fat. A: myogenic precursor cells (identified as CD34+/Sca1−; see Ref. 63) that express green fluorescent protein (GFP) were injected into muscle and engrafted to existing muscle fibers that were damaged along the needle track. Red outline of the fiber indicates laminin immunostaining. B: adipogenic and fibrogenic precursor cells (identified as PDGCα+/CD34+/Sca1+; see Ref. 63) that express GFP were injected subcutaneously and differentiated into adipocytes, as evidenced by the positive perlipin stain. Scale bars, 50 μm. (Images from Ref. 63.)
With regard to skeletal muscle as a mechanotransducer of the environment, it is interesting to suggest that the differentiation process relates in part to the biomechanical microenvironment in muscle tissue. Perhaps altered ECM mechanical properties help direct the fate of resident progenitor cells. Of note are the experiments of Engler et al. (30), who found that mesenchymal stem cells differentiate into neural, muscular, or bony cells, depending on the biomechanical properties of the substrate, thus demonstrating the strong effect of local mechanics on progenitor cells. Mesenchymal stem cells grown on substrates that approximate the elasticity of muscle (∼10 kPa) express a wide array of myogenic genes, such as MyoD, Myf5, myogenin, and Pax7. This expression was largely inhibited by the contractile inhibitor blebbistatin. A substrate mechanical property that is more bonelike (∼100 kPa) favors expression of osteogenic genes, including Bmp4, Sox9, and Col1A. It would be interesting to titrate elasticity to more closely approximate ECM properties and test if the expression pattern is more fibrogenic (Table 1). We recently found that the ECM of muscle from children with cerebral palsy is compromised in its mechanical properties relative to the ECM of children with normal development (76). It is intriguing to conjecture that part of the defect in these muscles that leads to contracture directly results from changes in ECM properties that are secondary to the neural insult. Clearly, more experiments are needed to sort out the cellular source, cellular mechanisms, and functional significance of fibrosis in normal skeletal muscle as well as the numerous diseases and model systems of fibrosis.
A recent, novel finding is that activated fibroblasts can arise from the endothelial cells that line the skeletal muscle microvasculature (24). Dulauroy et al. (24) focused on the metalloprotease ADAM12 (a disintegrin and metalloprotease 12), since it is expressed in the tissues of human diseases that involve fibrosis of liver, muscle, and skin. In view of this expression of ADAM12, they used genetic ablation of ADAM12, along with inducible fate mapping, to show that the majority of myofibroblasts arose from the muscle's perivascular cells that transiently expressed ADAM12 during development. This finding complements evidence that subsets of pericytes are the major sources of myofibroblasts and scar tissue in kidney (59) and spinal cord (47). If ADAM12 is selectively overexpressed in muscle, one finds increased muscle fibrosis and suppressed skeletal muscle regeneration (64). Since ADAM12 is rapidly induced by TGFβ (24, 112), this would represent a potential positive-feedback loop for the fibrotic response.
Conclusions
The physiological basis of skeletal muscle fibrosis is more poorly understood than processes that occur in other organs, such as kidney (15), liver (131), and heart (41). The most obviously measurable effects of skeletal muscle fibrosis are the increase in ECM collagen and increased mechanical stiffness of muscle fiber bundles. An explicit quantitative relationship between collagen content and passive mechanical properties of skeletal muscle is not available due to limited understanding of the structural organization of ECM collagen.
Skeletal muscle fibrosis can represent a debilitating clinical problem that may even require surgical intervention for correction. Nonsurgical therapeutic approaches involve targeting the classic TGFβ pathway, as well as other systems involved in muscle ECM homeostasis, such as Cthrc1 expression (113), Wnt signaling (11), and perivascular cell turnover (24). As in other fibrotic tissues, myofibroblasts deposit collagen, and these cells may derive from transdifferentiation of resident fibroblasts, FAPs, pericytes, and mesoangioblasts. Given this large number of cell sources and myriad underlying biological processes, future studies will require prodigious use of fate-mapping and molecular and cellular techniques to create powerful rescue and prevention strategies that inhibit or even reverse skeletal muscle fibrosis.
GRANTS
This work was supported by Department of Veterans Affairs Grant 101 RX000670 and National Institutes of Health Grants R24 HD-050837 and P30 AR-061303.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L.L. and S.R.W. prepared the figures, R.L.L. and S.R.W. drafted the manuscript, R.L.L. and S.R.W. edited and revised the manuscript, R.L.L. and S.R.W. approved the final version of the manuscript.
REFERENCES
- 1. Abrams RA, Tsai AM, Watson B, Jamali A, Lieber RL. Skeletal muscle recovery after tenotomy and 7-day delayed muscle length restoration. Muscle Nerve 23: 707–714, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Ahsan T, Sah RL. Biomechanics of integrative cartilage repair. Osteoarthritis Cartilage 7: 29–40, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139: 677–689, 1984 [PMC free article] [PubMed] [Google Scholar]
- 4. Bahi L, Koulmann N, Sanchez H, Momken I, Veksler V, Bigard AX, Ventura-Clapier R. Does ACE inhibition enhance endurance performance and muscle energy metabolism in rats? J Appl Physiol 96: 59–64, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Barton ER, Gimbel JA, Williams GR, Soslowsky LJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res 23: 259–265, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bedair HS, Karthikeyan T, Quintero A, Li Y, Huard J. Angiotensin II receptor blockade administered after injury improves muscle regeneration and decreases fibrosis in normal skeletal muscle. Am J Sports Med 36: 1548–1554, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve 40: 499–519, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Blix M. Die lange und die spannung des muskels. Skand Arch Physiol 5: 150–206, 1895 [Google Scholar]
- 9. Bo Li Z, Zhang J, Wagner KR. Inhibition of myostatin reverses muscle fibrosis through apoptosis. J Cell Sci 125: 3957–3965, 2012 [DOI] [PubMed] [Google Scholar]
- 10. Borg TK, Caulfield JB. Morphology of connective tissue in skeletal muscle. Tissue Cell 12: 197–207, 1980 [DOI] [PubMed] [Google Scholar]
- 11. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 220: 1–14, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord 35: 86–91, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. In: Exercise and Sport Sciences Review. Philadelphia, PA: Franklin Institute Press, 1978, p. 125–182 [PubMed] [Google Scholar]
- 15. Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular Mechanisms of Tissue Fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol 304: C591–C603, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carpenter S, Karpati G. Pathology of Skeletal Muscle. New York: Churchill Livingstone, 1984 [Google Scholar]
- 17. Chan YS, Li Y, Foster W, Horaguchi T, Somogyi G, Fu FH, Huard J. Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol 95: 771–780, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197, 1972 [DOI] [PubMed] [Google Scholar]
- 20. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Desguerre I, Arnold L, Vignaud A, Cuvellier S, Yacoub-Youssef H, Gherardi RK, Chelly J, Chretien F, Mounier R, Ferry A, Chazaud B. A new model of experimental fibrosis in hindlimb skeletal muscle of adult mdx mouse mimicking muscular dystrophy. Muscle Nerve 45: 803–814, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Droguett R, Cabello-Verrugio C, Santander C, Brandan E. TGF-β receptors, in a Smad-independent manner, are required for terminal skeletal muscle differentiation. Exp Cell Res 316: 2487–2503, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Dubowitz V, Brooke MH. Muscle Biopsy: A Modern Approach. Philadelphia, PA: Saunders, 1973 [Google Scholar]
- 24. Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. In press [DOI] [PubMed] [Google Scholar]
- 25. Durmus T, LeClair RJ, Park KS, Terzic A, Yoon JK, Lindner V. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1). Gene Expr Patterns 6: 935–940, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Edman K. The relation between sarcomere length and active tension in isolated semitendinosus fibres of the frog. J Physiol 183: 407–417, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisenberg BR. Quantitative ultrastructure of mammalian skeletal muscle. In: Handbook of Physiology. Skeletal Muscle. Bethesda, MD: Am. Physiol. Soc, 1983, sect. 10, p. 73–112 [Google Scholar]
- 28. Engel AG, Banker AQ. Myology. New York: McGraw-Hill, 1986, p. 1179 [Google Scholar]
- 29. Engel WK. Investigative approach to the muscular dystrophies. Adv Neurol 17: 197–226, 1977 [PubMed] [Google Scholar]
- 30. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Fink B, Egl M, Singer J, Fuerst M, Bubenheim M, Neuen-Jacob E. Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis Rheum 56: 3626–3633, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Foidart M, Foidart JM, Engel WK. Collagen localization in normal and fibrotic human skeletal muscle. Arch Neurol 38: 152–157, 1981 [DOI] [PubMed] [Google Scholar]
- 34. Foster W, Li Y, Usas A, Somogyi G, Huard J. γ-Interferon as an antifibrosis agent in skeletal muscle. J Orthop Res 21: 798–804, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Frank CB, Hart DA, Shrive NG. Molecular biology and biomechanics of normal and healing ligaments—a review. Osteoarthritis Cartilage 7: 130–140, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 8: 599–605, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Gao Y, Waas AM, Faulkner JA, Kostrominova TY, Wineman AS. Micromechanical modeling of the epimysium of the skeletal muscles. J Biomech 41: 1–10, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Gillies AM, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res Sep: 258–265, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Given JD, Dewald JP, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Neurol Neurosurg Psychiatry 59: 271–279, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldsmith EC, Bradshaw AD, Spinale FG. Cellular Mechanisms of Tissue Fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 304: C393–C402, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldspink G. Cellular and molecular aspects of muscle growth, adaptation and ageing. Gerodontology 15: 35–43, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Goldstein SA. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech 20: 1055–1061, 1987 [DOI] [PubMed] [Google Scholar]
- 44. Goodman CA, Mayhew DL, Hornberger TA. Recent progress toward understanding the molecular mechanisms that regulate skeletal muscle mass. Cell Signal 23: 1896–1906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science 333: 238–242, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Grant RA. Estimation of hydroxyproline by the autoanalyser. J Clin Pathol 17: 685–686, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Granzier H, Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve 36: 740–755, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Grimby G, Broberg C, Krotkiewska I, Krotkiewski M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med 8: 37–42, 1976 [PubMed] [Google Scholar]
- 51. Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Hauschka EO, Roy RR, Edgerton VR. Size and metabolic properties of single muscle fibers in rat soleus after hindlimb suspension. J Appl Physiol 62: 2338–2347, 1987 [DOI] [PubMed] [Google Scholar]
- 53. Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports 23: e150–e161, 2013 [DOI] [PubMed] [Google Scholar]
- 54. Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol 106: 178–186, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg 7: 393–396, 1998 [DOI] [PubMed] [Google Scholar]
- 56. Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol 38: 548–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180: 1340–1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hori YS, Kuno A, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J Pharmacol Exp Ther 338: 784–794, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y, Uchino K, Yamashita S, Maeda Y, Uchino M. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord 18: 342–348, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Jarvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J Muscle Res Cell Motil 23: 245–254, 2002 [DOI] [PubMed] [Google Scholar]
- 62. Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol 45: 401–409, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jorgensen LH, Jensen CH, Wewer UM, Schroder HD. Transgenic overexpression of ADAM12 suppresses muscle regeneration and aggravates dystrophy in aged mdx mice. Am J Pathol 171: 1599–1607, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Józsa L, Kannus P, Thöring J, Reffy A, Järvinen M, Kvist M. The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg 72: 293–297, 1990 [DOI] [PubMed] [Google Scholar]
- 66. Kafadar KA, Yi L, Ahmad Y, So L, Rossi F, Pavlath GK. Sca-1 expression is required for efficient remodeling of the extracellular matrix during skeletal muscle regeneration. Dev Biol 326: 47–59, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140: 41–54, 1990 [DOI] [PubMed] [Google Scholar]
- 68. Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Lexell J, Downham D, Sjöström M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci 72: 211–222, 1986 [DOI] [PubMed] [Google Scholar]
- 70. Li J, Cao J, Li M, Yu Y, Yang Y, Xiao X, Wu Z, Wang L, Tu Y, Chen H. Collagen triple helix repeat containing-1 inhibits transforming growth factor-β1-induced collagen type I expression in keloid. Br J Dermatol 164: 1030–1036, 2011 [DOI] [PubMed] [Google Scholar]
- 71. Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol 161: 895–907, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem 283: 19371–19378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lieber RL. Skeletal Muscle Structure and Function and Plasticity. Baltimore, MD: Lippincott, Williams & Wilkins, 2010 [Google Scholar]
- 75. Lieber RL, Friden J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 25: 265–270, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28: 464–471, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Lund DK, Cornelison DD. Enter the matrix: shape, signal and superhighway. FEBS J. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P. Aberrant repair and fibrosis development in skeletal muscle. Skeletal Muscle 1: 21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol 34: 140–148, 2005 [DOI] [PubMed] [Google Scholar]
- 80. Massague J. TGFβ signalling in context. Nat Rev 13: 616–630, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003 [DOI] [PubMed] [Google Scholar]
- 82. McDouall RM, Dunn MJ, Dubowitz V. Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J Neurol Sci 99: 199–217, 1990 [DOI] [PubMed] [Google Scholar]
- 83. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 84. McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meyer DC, Gerber C, Von Rechenberg B, Wirth SH, Farshad M. Amplitude and strength of muscle contraction are reduced in experimental tears of the rotator cuff. Am J Sports Med 39: 1456–1461, 2011 [DOI] [PubMed] [Google Scholar]
- 86. Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J Biomech 44: 771–773, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Meyer GA, Lieber RL. Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol 302: C1609–C1620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Meyer GA, Schenk S, Lieber RL. Role of the cytoskeleton in muscle transcriptional responses to altered use. Physiol Genomics 45: 321–331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol 134: 1255–1270, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996 [DOI] [PubMed] [Google Scholar]
- 91. Myers BS, Woolley CT, Slotter TL, Garrett WE, Best TM. The influence of strain rate on the passive and stimulated engineering stress—large strain behavior of the rabbit tibialis anterior muscle. J Biomech Eng 120: 126–132, 1998 [DOI] [PubMed] [Google Scholar]
- 92. Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 24: 175–189, 2003 [DOI] [PubMed] [Google Scholar]
- 93. Oberhauser AF, Hansma PK, Carrion-Vazquez M, Fernandez JM. Stepwise unfolding of titin under force-clamp atomic force microscopy. Proc Natl Acad Sci USA 98: 468–472, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ohlendieck K, Ervasti JM, Snook JB, Campbell KP. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol 112: 135–148, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell 16: 303–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Onder G, Vedova CD, Pahor M. Effects of ACE inhibitors on skeletal muscle. Curr Pharm Des 12: 2057–2064, 2006 [DOI] [PubMed] [Google Scholar]
- 97. Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev 25: 321–363, 1997 [PubMed] [Google Scholar]
- 98. Pette D. The Dynamic State of Muscle Fibers. Berlin: de Gruyter, 1990 [Google Scholar]
- 99. Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol 126: 461–480, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Purslow PP, Trotter JA. The morphology and mechanical properties of endomysium in series-fibred muscles: variations with muscle length. J Muscle Res Cell Motil 15: 299–308, 1994 [DOI] [PubMed] [Google Scholar]
- 101. Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res 96: 261–268, 2005 [DOI] [PubMed] [Google Scholar]
- 102. Roy RR, Baldwin KM, Edgerton VR. The plasticity of skeletal muscle: effects of neuromuscular activity. In: Exercise and Sport Sciences Reviews. Baltimore, MD: Williams and Wilkins, 1991, p. 269–312 [PubMed] [Google Scholar]
- 103. Sabatelli P, Gualandi F, Gara SK, Grumati P, Zamparelli A, Martoni E, Pellegrini C, Merlini L, Ferlini A, Bonaldo P, Maraldi NM, Paulsson M, Squarzoni S, Wagener R. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol 31: 187–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am 87: 2662–2670, 2005 [DOI] [PubMed] [Google Scholar]
- 105. Samagh SP, Kramer EJ, Melkus G, Laron D, Bodendorfer BM, Natsuhara K, Kim HT, Liu X, Feeley BT. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res 31: 421–426, 2013 [DOI] [PubMed] [Google Scholar]
- 106. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278: 12601–12604, 2003 [DOI] [PubMed] [Google Scholar]
- 108. Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423, 1996 [DOI] [PubMed] [Google Scholar]
- 109. Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda, MD) 22: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 110. Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004 [DOI] [PubMed] [Google Scholar]
- 111. Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Solomon E, Li H, Duhachek Muggy S, Syta E, Zolkiewska A. The role of SnoN in transforming growth factor β1-induced expression of metalloprotease-disintegrin ADAM12. J Biol Chem 285: 21969–21977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spector I, Zilberstein Y, Lavy A, Genin O, Barzilai-Tutsch H, Bodanovsky A, Halevy O, Pines M. The involvement of collagen triple helix repeat containing 1 in muscular dystrophies. Am J Pathol 182: 905–916, 2013 [DOI] [PubMed] [Google Scholar]
- 114. Sutherland DH. Gait Disorders in Childhood and Adolescence. Baltimore, MD: Williams & Wilkins, 1984 [Google Scholar]
- 115. Takahashi M, Ward SR, Friden J, Lieber RL. Muscle excursion does not correlate with increased serial sarcomere number after muscle adaptation to stretched tendon transfer. J Orthop Res 30: 1774–1780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Takahashi M, Ward SR, Marchuk LL, Frank CB, Lieber RL. Asynchronous muscle and tendon adaptation after surgical tensioning procedures. J Bone Joint Surg Am 92: 664–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, Ijiri K, Akimitsu N. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett 584: 4575–4580, 2010 [DOI] [PubMed] [Google Scholar]
- 118. Templeton GH, Sweeney HL, Timson BF, Padalino M, Dudenhoeffer GA. Changes in fiber composition of soleus muscle during rat hindlimb suspension. J Appl Physiol 65: 1191–1195, 1988 [DOI] [PubMed] [Google Scholar]
- 119. Thacker BE, Tomiya A, Hulst JB, Suzuki KP, Bremner SN, Gastwirt RF, Greaser ML, Lieber RL, Ward SR. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J Orthop Res 30: 497–502, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 139: 871–890, 2009 [DOI] [PubMed] [Google Scholar]
- 121. Tidball JG, Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res 56: 831–841, 2004 [DOI] [PubMed] [Google Scholar]
- 122. Tirrell TF, Cook MS, Carr JA, Lin E, Ward SR, Lieber RL. Human skeletal muscle biochemical diversity. J Exp Biol 215: 2551–2559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Trotter JA, Hsi K, Samora A, Wofsy C. A morphometric analysis of the muscle-tendon junction. Anat Rec 213: 26–32, 1985 [DOI] [PubMed] [Google Scholar]
- 124. Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol 212: 109–122, 1992 [DOI] [PubMed] [Google Scholar]
- 125. Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124: 3654–3664, 2011 [DOI] [PubMed] [Google Scholar]
- 126. Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52: 832–836, 2002 [DOI] [PubMed] [Google Scholar]
- 127. Ward SR, Sarver JJ, Eng CM, Kwan A, Wurgler-Hauri CC, Perry SM, Williams GR, Soslowsky LJ, Lieber RL. Plasticity of muscle architecture after supraspinatus tears. J Orthop Sports Phys Ther 40: 729–735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weibel ER. Practical methods for biological morphometry. In: Stereological Methods. New York: Academic, 1980 [Google Scholar]
- 129. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 93: 23–67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zeisberg M, Kalluri R. Cellular Mechanisms of Tissue Fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol 304: C216–C225, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J. Relationships between transforming growth factor-β1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem 282: 25852–25863, 2007 [DOI] [PubMed] [Google Scholar]
- 133. Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am 90: 2423–2431, 2008 [DOI] [PubMed] [Google Scholar]