Abstract
Vascular endothelial cell (EC) inflammation is a key event in the pathogenesis of multiple vascular diseases. We tested the hypothesis that interleukin-19 (IL-19), an anti-inflammatory Th2 interleukin, could have a direct anti-inflammatory effect on ECs to decrease inflammation. IL-19 can significantly decrease tumor necrosis factor (TNF)-α-driven intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 mRNA and protein abundance in cultured human coronary artery ECs (P < 0.01). IL-19 treatment of ECs, but not monocytes, significantly reduced monocyte adhesion to EC monolayers (P < 0.01). In vivo, systemic administration of IL-19 could significantly reduce TNF-α-induced leukocyte rolling and adhesion in wild-type mice as assayed by intravital microscopy (P < 0.05). IL-19 does not reduce TNF-α-stimulated NF-κB activation in ECs but does decrease serine phosphorylation and cytoplasmic translocation of the mRNA stability factor HuR and significantly reduces stability of ICAM-1 and VCAM-1 mRNA (P < 0.01). These data are the first to report that IL-19 can reduce leukocyte-endothelial cell adhesion and the first to propose reduction in HuR-mediated mRNA stability of ICAM-1 and VCAM-1 as a mechanism. Expression of IL-19 by ECs may represent a protective mechanism to promote resolution of the vascular response to inflammation. Function of IL-19 outside of the immune system is a novel concept, suggesting that resident vascular cells can adopt a Th2 phenotype, and has important ramifications for numerous inflammatory diseases.
Keywords: interleukin-19, endothelial cell, ICAM-1, VCAM-1, leukocyte adhesion
cardiovascular disease accounts for over half of all mortality and continues to grow in the Western world. Vascular endothelial cells (ECs) actively participate in the pathogenesis of inflammation in general and many vascular diseases in particular. Extravasation of circulating leukocytes, which is important physiologically for immune surveillance, is upregulated during vascular inflammation through changes in EC activity (4). In response to injury or proinflammatory mediators, ECs express higher levels of cell adhesion molecules (CAMs) with the capability to tether and firmly adhere to circulating leukocytes. ECs also actively participate in recruitment of leukocytes by generating a chemotactic gradient of chemokines that attract leukocytes to the site of injury (4, 32). While the effects of proinflammatory cytokines on EC dysfunction and adhesion molecule upregulation are fairly well characterized, much less is understood about soluble factors that may attenuate this process. Most studies of inhibition of leukocyte-EC interaction tend to focus on leukocytes as the effector cell, with less attention paid to ECs in this process. The identification of an anti-inflammatory cytokine that reduces leukocyte-EC interaction by targeting ECs has obvious clinical potential to attenuate many vascular diseases.
Interleukin (IL)-19, an IL-10 family member, was initially identified in 2000 (11) and is classified in a subfamily that includes IL-20 and IL-24. IL-19 is distinct from these interleukins and IL-10 in terms of tissue-specific expression, signal transduction, receptor utilization, and mode of activity (13, 15, 22). IL-19 is considered to be anti-inflammatory because in T-lymphocytes it promotes the Th2 (regulatory) rather than the Th1 (proinflammatory) response (12, 13). We previously reported that IL-19 is expressed in ECs and vascular smooth muscle cells (VSMCs) in injured, but not in naïve, arteries, and in inflammatory cytokine-stimulated, but not in naïve, cultured ECs and VSMCs (14, 31). This was novel and unexpected because IL-19 expression was previously assumed to be restricted to immune cells (11, 12). Importantly, IL-19 can reduce the proliferation and migration of VSMCs (10, 31). While these studies suggested a potential protective role for IL-19 in vascular disease, nothing has been reported on the potential of IL-19 to dampen EC inflammation and leukocyte-EC interactions.
The purpose of the present study was to test the hypothesis that IL-19 has anti-inflammatory effects on ECs in vivo and ex vivo and to identify a potential mechanism for this activity.1 We have previously reported IL-19 expression in endothelium; however, an anti-inflammatory function for this cytokine in EC has not been reported. Pretreatment of cultured ECs with IL-19 can reduce the abundance of tumor necrosis factor (TNF)-α-induced intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 mRNA and protein in a nuclear factor (NF)-κB-independent process. IL-19 can decrease stability of ICAM-1 and VCAM-1 mRNA, can attenuate TNF-α-driven monocyte adhesion to cultured ECs, and can attenuate leukocyte rolling and adhesion in vivo. This study demonstrates a direct, anti-inflammatory effect of a Th2 interleukin on EC pathophysiology. This suggests that endogenous expression of IL-19 by ECs may represent an autoregulatory autocrine or paracrine homeostatic mechanism to promote resolution of the local vascular response to injury.
MATERIALS AND METHODS
Cells and culture.
Primary human coronary artery vascular endothelial cells (hCaECs) were obtained as cryopreserved secondary culture from Cascade (Portland, OR) and subcultured as we described (14). Cells were used from passages 3–5. ECs were incubated in Endothelial Basal Medium-2 (EBM-2; Lonza Group, Basel, Switzerland) supplemented with 1.0% FBS for 24 h and then exposed to recombinant human IL-19 (100 ng/ml, R&D, Minneapolis, MN) for various times, then stimulated with recombinant human TNF-α (10 ng/ml, Sigma, St. Louis, MO).
Western blotting.
Cells were cultured and extracts were made as we have described (14). Briefly, cells were rinsed with PBS and extract proteins were separated by SDS-PAGE. Membranes were incubated with a 1:4,000–5,000 dilution of primary antibody and a 1:5,000 dilution of secondary antibody. Primary antibodies used were ICAM-1 (Abcam, Cambridge, UK), VCAM-1, HuR, Hsc70 (Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH, IkB, phospho-serine, and phospho- and total p65 from (Cell Signaling, Danvers, MA). Reactive proteins were visualized using enhanced chemiluminescence (Amersham) according to the manufacturer's instructions. Relative intensity of bands was normalized to GAPDH and Hsc70 and quantitated by scanning image analysis and the ImageJ densitometry program (National Institutes of Health, Bethesda, MD).
Cellular fractionation and immunoprecipitation.
Cell extracts were fractionated as we have described (5). Briefly, ECs were washed in PBS, scraped off the plates, pelleted by low-speed centrifugation (4,500 rpm), lysed by addition of 100 μl of ice-cold lysis buffer (10 mM HEPES, pH 7.9, 1.0 mM EDTA, 60 mM KCl, 0.5% NP-40, and complete Mini EDTA-free protease inhibitor cocktail tablet, Roche). Nuclei were pelleted by centrifugation (8,000 rpm) for 5 min at 4°C, and the supernatant was isolated as cytoplasmic proteins. Nuclear proteins were isolated by brief sonication of nuclear pellets in SDS sample buffer. HuR immunoprecipitation was performed as described (5). Briefly, ECs were lysed by addition of 500 μl of ice-cold immunoprecipitation (IP) buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 6 mM β-octyl glucoside, 1 mM sodium orthovanadate, 10 mM sodium fluoride, and complete Mini EDTA-free protease inhibitor cocktail tablet), cell debris was pelleted by centrifugation at 8,000 rpm at 4°C for 15 min, supernatant was precleared with protein A/G conjugate (Santa Cruz) and HuR antibody (Santa Cruz), and protein A/G conjugate was added to supernatant and incubated with gentle rocking overnight at 4°C. Pellets were washed three times in IP buffer, and Western blotting with anti-phospho-serine antibody (Abcam) was carried out as described above.
Immunocytochemistry.
hCaECs were grown to confluence in chamber slides, serum starved for 16 h, and treated with either 16 h IL-19, 6 h TNF-α, or both. ECs were fixed in 10% NB formalin, permeabilized for 10 min in 0.2% Triton X-100, and blocked for 25 min in 5% goat serum. ECs were incubated with either anti-HuR primary antibody or negative control mouse IgG for 1 h, followed by incubation with goat anti-mouse Alexa Fluor (R) green fluorescent secondary antibody (AF-488; Invitrogen) for 30 min, and DAPI counterstain for 5 min.
Transfection and siRNA knockdown.
Gene silencing was performed using ON-TARGET plus SMARTpool HuR siRNA, which contains a mixture of four siRNAs that target HuR (30 nM) purchased from Dharmacon, as we have described (5). EC transfection was performed using the Human EC Nucleofector Kit (Amaxa) following the manufacturer's instructions. Transfection efficiency was 70–90% as assayed by dual transfection of a GFP reporter plasmid (14). Lysates were immunoblotted for HuR 72 h posttransfection, and RNA was extracted 72 h posttransfection. HuR cDNA was purchased in an expression vector (Origene Technologies) and was overexpressed by transfection of 2 μg/106 ECs using the Nucleofector kit as described for siRNA.
Monocyte adhesion assay.
Adhesion was assayed as described (16). Briefly, hCaECs were cultured on glass coverslips at a density of 6 × 105 cells/chamber. Confluent ECs were treated with IL-19 (100 ng/ml) for 16 h, then in the presence or absence of TNF-α (10 ng/ml) for an additional 6 or 24 h, followed by extensive washing with PBS. THP-1 human monocytes were purchased from the American Type Culture Collection (catalog no. TIB-202) and cultured according to vendors instructions. THP-1 monocytes (5 × 105 cells/well) were labeled with 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM, 10 μM, Sigma) and incubated with hCaECs for 30 min at 37°C. Unbound THP-1 cells were removed by gently washing them twice with PBS, and adherent cells were fixed with 4% formalin, photographed by an inverted fluorescent microscope (Eclipse TS-100, Nikon), and counted per high-power field (HPF). For some experiments, anti-IL-20Rα (catalog no. MAB11762, R&D) or negative control antibody was added to ECs 2 h before addition of IL-19 to neutralize the IL-19 receptor. Results are expressed as percentages of the controls and represent means ± SE from triplicate experiments.
Intravital microscopy, animal care.
Leukocyte rolling and adhesion were assayed in mesenteric postcapillary venules by intravital microscopy as described previously (27). Mice (20 g body wt) were then injected intraperitoneally with 10.0 ng/g IL-19 followed 16 h later by intraperitoneal injection with 20.0 ng/g body wt TNF-α. Rolling and adhesion were quantitated 4 h following TNF-α injection. Wild-type C57BL/6 mice were injected with 120 mg/kg pentobarbital sodium by intraperitoneal injection. Depth of anesthesia was monitored by toe-pinch and blood pressure. Three to four straight, unbranched segments of postcapillary venules with lengths of >100 μm and diameters between 25 and 40 μm were studied in each mouse using an Eclipse FN1 Microscope (Nikon), and the images were recorded and analyzed on A WIN XP Imaging Workstation. Leukocyte rolling was defined as the number of leukocytes rolling past a fixed point per minute; leukocyte adherence was defined as the number of leukocytes firmly adhered to 100-μm length of endothelium for at least 30 s. Venular blood velocity (V) was measured using the Microvessel Velocity OD-RT optical Doppler velocimeter (Circusoft Instrumentation) with corresponding software. Venular wall shear rate (γ) was calculated using the formula: γ = 4.9 × 8(Vmean/D), where D is the venule diameter (23). Euthanasia was by injection of 160 mg/kg pentobarbital, then exsanguination under anesthesia. Systemic leukocytes counts were determined using the Leuko-TIC Kit (Bioanalytic) according to manufacturer instructions with an enhanced Neubauer hemocytometer. All animal procedures were approved by the Institutional Animal Care and Use Committee of Temple University and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH, 8th ed., 2011).
RNA extraction and quantitative RT-PCR.
RNA was isolated using the RNeasy Mini Kit (Qiagen) and reverse transcribed into cDNA using the Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) as we have described, and target genes were amplified using Maxima SYBR Green/ROX qPCR Master Mix (Fermentas) in an Eppendorf Realplex4 Mastercycler (5). Multiple mRNAs (Ct values) were quantitated simultaneously by the Eppendorf software. Primer pairs were purchased from Integrated DNA Technologies (Coralville, IA). The following primer pairs were used: GAPDH forward (F): CGAGAGTCAGCCGCATCTT, reverse (R): CCCCATGGTGTCTGAGCG; ICAM-1 F: CTCCAATGTGCCAGGCTTG, R: CAGTGGGAAAGTGCCATCCT; VCAM-1 F: TTCCCTAGAGATCCAGAAATCGAG, R: CTTGCAGCTTACAGTGACAGAGC; VLA-4 β1-integrin F: GCGGAAAAGATGAATTTACAACCA, R: TTCTCCACATGATTTGGCATTTGGCATTTG; E-selectin F: TCTGGGAATTGGGACAACG, R: CCTCACAGGTGAAGTTGCAG; P-selectin F: TTCCATTGTCTAGAGGGCC, R: AATGGTCCTTGGCAGGTTG; CD18 (Mac-1 and LFA-1 shared β2-integrin) F: GTGTCAGGACTTTACGACCC, R: CTTTGCTACCAGTCTGCCC; CD11B (Mac-1 αM-integrin) F: GTGATGCTGTTCTCTACGGG, R: TCAGCTTGTCCCCATTTACG; CD11A (LFA-1 αL-integrin) F: CCCCACAGATGGAAGCATTT, R: GAAGAGGTAACACAGGCCAC; TNFR1 F: TTCACCGCTTCAGAAAACCA, R: AAGAGATCTCCACCTGACCC.
Statistical analysis.
Results are expressed as means ± SE. Differences between groups were evaluated using one-way ANOVA. Individual mean differences were evaluated using the Newman-Keuls post test and confirmed by paired t-tests where appropriate. Differences were considered significant at a level of P < 0.05.
RESULTS
IL-19 reduces TNF-α-induced cell adhesion molecule expression in human ECs.
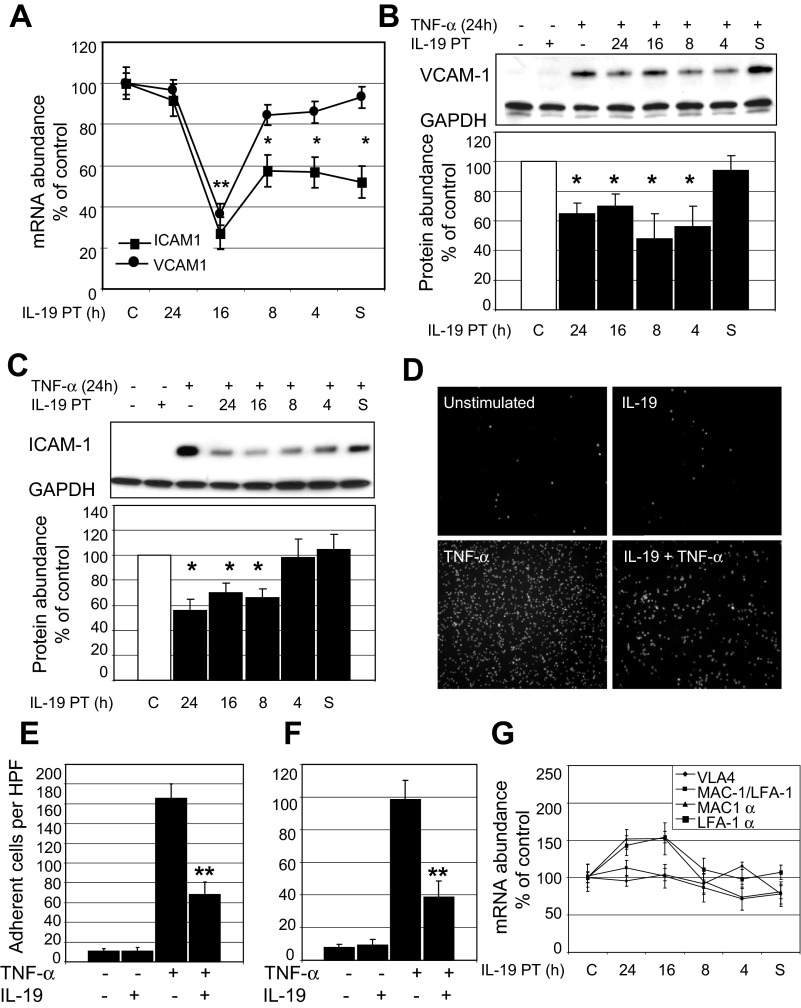
We previously demonstrated that IL-19 is expressed in cultured human endothelial cells in response to inflammatory stimuli (14). Considering that IL-19 has putative anti-inflammatory effects, we tested whether IL-19 treatment could decrease expression of adhesion molecules on human endothelial cells. Human ECs were pretreated with IL-19 at different times before stimulation with TNF-α to induce expression of ICAM-1 and VCAM-1. Figure 1 demonstrates that IL-19 pretreatment can significantly decrease both ICAM-1 and VCAM-1 mRNA abundance as measured by qRT-PCR. Sixteen-hour IL-19 pretreatment is the most effective time for reduction of mRNA abundance (26.0 ± 7.7% and 36.4 ± 5.2% of control cells for ICAM-1 and VCAM-1, respectively; P < 0.01). The inhibitory effect of a single addition of IL-19 to culture media is transient, as the mRNA abundance of both molecules returned to basal levels with 24 h IL-19 pretreatment. VCAM-1 mRNA suppression is less sensitive to IL-19 treatment than ICAM-1, as 4 h pretreatment and addition of IL-19 simultaneously with TNF-α have no suppressive effect on VCAM-1 mRNA levels. Figure 1, B and C, shows that IL-19 pretreatment between 4 and 24 h significantly decreases VCAM-1 protein abundance and IL-19 pretreatment between 8 and 24 h significantly decreases ICAM-1 abundance on ECs.
Fig. 1.
IL-19 reduces ICAM-1 and VCAM-1 expression and leukocyte-endothelial cell interaction. A: IL-19 significantly reduces ICAM-1 and VCAM-1 mRNA expression. Endothelial cells (ECs) were pretreated (PT) with IL-19, then stimulated with TNF-α for 6 h. mRNA was quantitated by qRT-PCR and normalized to GAPDH. C, control. **P < 0.001 for 16 h pretreatment; *P < 0.05 for other times. B and C: IL-19 significantly reduces ICAM-1 and VCAM-1 protein abundance. ECs were pretreated with IL-19 as for mRNA, then stimulated with TNF-α for 24 h. Western blot analysis was quantitated by densitometry from at least 3 experiments. *P < 0.05 for times indicated. Data are representative of at least 3 experiments. D: ECs grown on glass coverslips were pretreated with IL-19 for 16 h, then TNF-α for 24 h. Adherent THP-1 cells were quantitated by count per high-power field (HPF); photo shown is representative (magnification ×200). E: results represent means ± SE. **P < 0.01 for five high-power fields from triplicate experiments. F: ECs grown on glass coverslips were pretreated with IL-19 for 16 h, then TNF-α for 6 h. Results represent means ± SE. **P < 0.01 for five high-power fields from triplicate experiments. G: IL-19 does not reduce integrin mRNA expression in monocytes. THP-1 cells were pretreated with IL-19, then stimulated with TNF-α for 6 h. mRNA was quantitated by qRT-PCR and normalized to GAPDH. There was no significant difference in any of the IL-19-treated groups compared with controls. All samples were pretreated with IL-19 for different times. S, ECs treated with IL-19 at the same time as TNF-α.
IL-19 can reduce leukocyte-endothelial cell adhesion.
Adhesion molecules are responsible for leukocyte-endothelial cell interactions, and we tested the hypothesis that IL-19 could reduce leukocyte-endothelial cell adhesion using an endothelial cell monolayer adhesion assay (16). ECs were cultured on glass cover slides, and were pretreated with IL-19 for 16 h, as this was the optimal time for decrease in ICAM-1 and VCAM-1 protein abundance (Fig. 1, B and C), then with TNF-α for an additional 24 h. THP-1 human monocytes were fluorescently labeled with BCECF, added to EC monolayers, and adherent cells quantitated by microscopy (images shown in Fig. 1D). Figure 1E shows that IL-19 significantly decreases THP-1 adhesion to human ECs (165.3 ± 15.1 vs. 68.3 ± 12.3 adherent cells per HPF for TNF-α and IL-19 pretreated, respectively, P < 0.01). IL-19 also significantly reduces THP-1 adhesion when ECs are stimulated with TNF-α for 6 h (98.3 ± 11.7 vs. 38.7 ± 9.5 adherent cells per HPF for TNF-α and IL-19 pretreated, respectively, P < 0.01)(Fig. 1F). We also tested the possibility that IL-19 treatment of THP-1 monocytes could reduce their surface expression of the VCAM-1 coreceptor VLA-4. THP-1 cells were pretreated with IL-19, then stimulated with TNF-α for 6 h to induce expression, and VLA-4 β1-integrin subunit mRNA abundance was quantitated by qRT-PCR. Figure 1G shows that IL-19 does not decrease VLA-4 mRNA abundance. IL-19 also does not decrease mRNA abundance of other leukocyte adhesion molecules, including LFA-1 and MAC-1 integrins (4). These data suggest that IL-19 can reduce leukocyte-EC interaction by a reduction of adhesion molecule abundance on ECs.
IL-19 reduces leukocyte-endothelial cell adhesion in vivo.
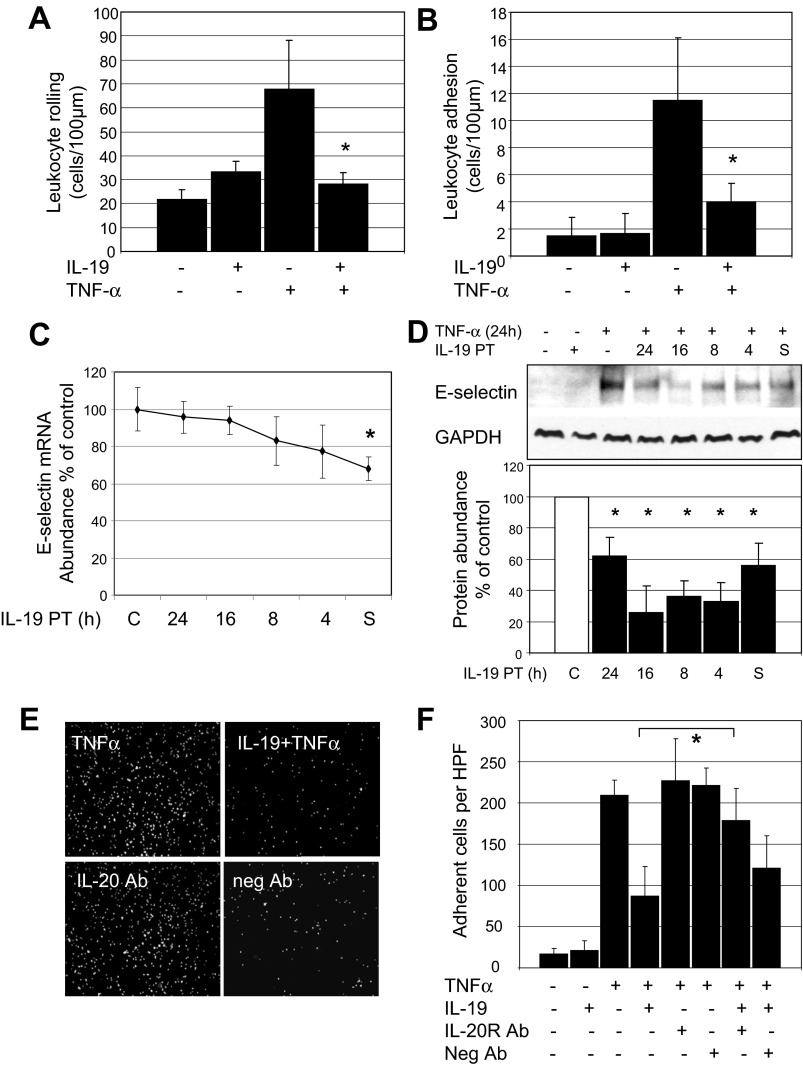
Intravital microscopy was used to determine whether IL-19 treatment could reduce leukocyte-EC adhesion in vivo. Wild-type C57BL/6 mice were injected with a single dose of 10.0 ng/g ip IL-19 for 16 h before injection of TNF-α. Mice were then injected with a single dose of 20.0 ng/g body wt (400 ng) TNF-α to elicit leukocyte adhesion to endothelium (20, 33). Four hours later, rolling and adhesion in postcapillary venules were quantitated by intravital microscopy (27). Figure 2, A and B, shows that similar to our observations in cultured endothelial cells, IL-19 pretreatment can significantly reduce TNF-α-stimulated leukocyte rolling (67.6 ± 20.4 vs. 28.2 ± 4.5 cells/min for TNF-α and TNF-α pretreated with IL-19, P < 0.01), and adhesion (11.5 ± 5.6 vs. 4.0 ± 1.4 cells/100 μm for TNF-α and TNF-α pretreated with IL-19, P < 0.05). There was no statistical difference in adhesion between IL-19-pretreated and control mice. There were no significant differences in microvascular and hemodynamic parameters between the groups of mice (not shown). Leukocyte rolling is mediated by selectin expression on ECs (4). The observed reduction in in vivo rolling prompted us to investigate whether IL-19 could modulate selectin expression in cultured ECs. IL-19 could not significantly decrease abundance of P-selectin in ECs (not shown). However, IL-19 can moderately but significantly decrease TNF-α-driven expression of E-selectin mRNA (68.0 ± 6.3% of control) and protein (Fig. 2, C and D).
Fig. 2.
IL-19 reduces leukocyte-endothelial interactions in vivo. Wild-type C57BL/6 mice were injected a single time with 10.0 ng/g ip IL-19 for 16 h before a single injection with 20.0 ng/g ip TNF-α. A and B: after 4 h, leukocyte rolling (A) and adhesion (B) were quantitated by intravital microscopy. IL-19 treatment significantly reduces leukocyte rolling and adhesion. *P < 0.05; n = 5 mice/group. C and D: IL-19 reduces expression of E-selectin mRNA (C) and protein (D) in cultured ECs. Blot shown is representative of at least 3 experiments. *P < 0.05. E: antibody neutralization of the IL-20 receptor negates the inhibitory effect of IL-19 on THP-1 monocyte adhesion. Anti-IL-20Rα or negative control antibody was added to ECs 2 h before addition of IL-19. F: results represent means ± SE. *P < 0.05 for five high-power fields from triplicate experiments. All samples were pretreated with IL-19 for different times. S, ECs treated with IL-19 at the same time as TNF-α.
IL-19 signals through the IL-20 receptor complex (IL20Rα/IL20Rβ) (12). To determine specificity of IL-19 effects, for some experiments, anti-IL-20Rα or negative control antibody was added to ECs 2 h before addition of IL-19. Neutralization of the IL-20 receptor with specific antibody negates IL-19-induced inhibition of THP-1 adhesion (87.4 ± 36.1 vs. 179 ± 30.4 adherent cells per HPF for TNF-α + IL-19 and TNF-α + IL-19 and IL-20R antibody; Fig. 2, E and F).
IL-19 reduces cell adhesion molecule abundance in an NF-κB-independent mechanism.
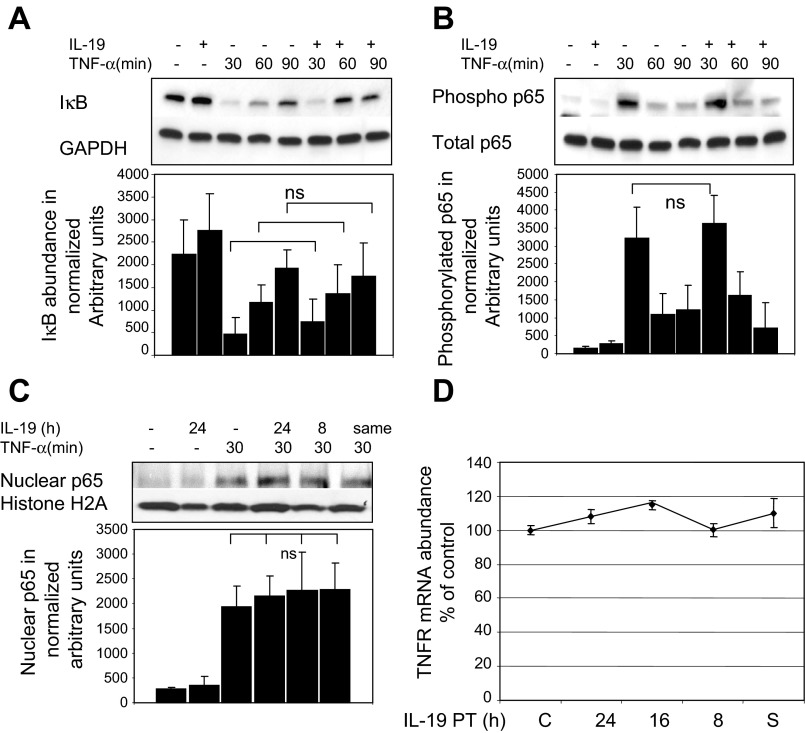
To determine a molecular mechanism for IL-19 reduction of EC adhesion molecules, three experiments were performed to determine whether IL-19 reduced CAM expression by inhibition of NF-κB. TNF-α was used to activate NF-κB. First, TNF-α-induced degradation of inhibitor of κB (IκB), which allows activation and nuclear translocation of NF-κB, is not altered by IL-19 treatment (Fig. 3A). Second, IL-19 has no inhibitory effect on phosphorylation of the NF-κB p65 subunit (Fig. 3B). Third, TNF-α-driven nuclear translocation of p65 was not inhibited by IL-19 pretreatment (Fig. 3C). All three of these readouts are canonical events in TNF-α signal transduction. Furthermore, IL-19 does not reduce abundance of the TNF-α receptor (Fig. 3D). These data suggest that IL-19 does not decrease TNF-α signal transduction while still decreasing cell adhesion molecule abundance in an NF-κB-independent mechanism, and prompted us to investigate other potential mechanisms for IL-19 inhibitory effects.
Fig. 3.
IL-19 does not inhibit NF-κB activation. ECs were cultured in serum-reduced medium, preincubated with IL-19 for 16 h, then stimulated with TNF-α. A: IL-19 does not decrease degradation of IκB in response to TNF-α stimulation. Lysates were immunoblotted with anti-IκB and GAPDH antibody. There is no statistical (ns) difference between IL-19-treated and untreated ECs at any time point. B: IL-19 does not inhibit phosphorylation of NF-κB p65 subunit. Lysates were blotted with anti-phospho NF-κB p65 subunit. There is no statistical difference between IL-19-treated and untreated ECs. C: IL-19 does not inhibit nuclear translocation of p65. ECs were pretreated with IL-19, then stimulated with TNF-α for 30 min to induce NF-κB translocation. Lysates were fractionated, and nuclear fraction was immunoblotted with p65 antibody. Proteins were detected by Western blotting. Images are representative of at least 4 experiments. There was no significant difference in intensity of bands between TNF-α controls and IL-19-pretreated ECs for any experiment. D: IL-19 does not decrease abundance of TNF-α receptor (TNFR). ECs were pretreated with IL-19, then stimulated with TNF-α for 6 h. mRNA was quantitated by qRT-PCR and normalized to GAPDH. There was no significant difference between any of the times compared with TNF-α-stimulated control. S, ECs treated with IL-19 at the same time as TNF-α.
IL-19 reduces TNF-α-driven HuR translocation and serine phosphorylation.
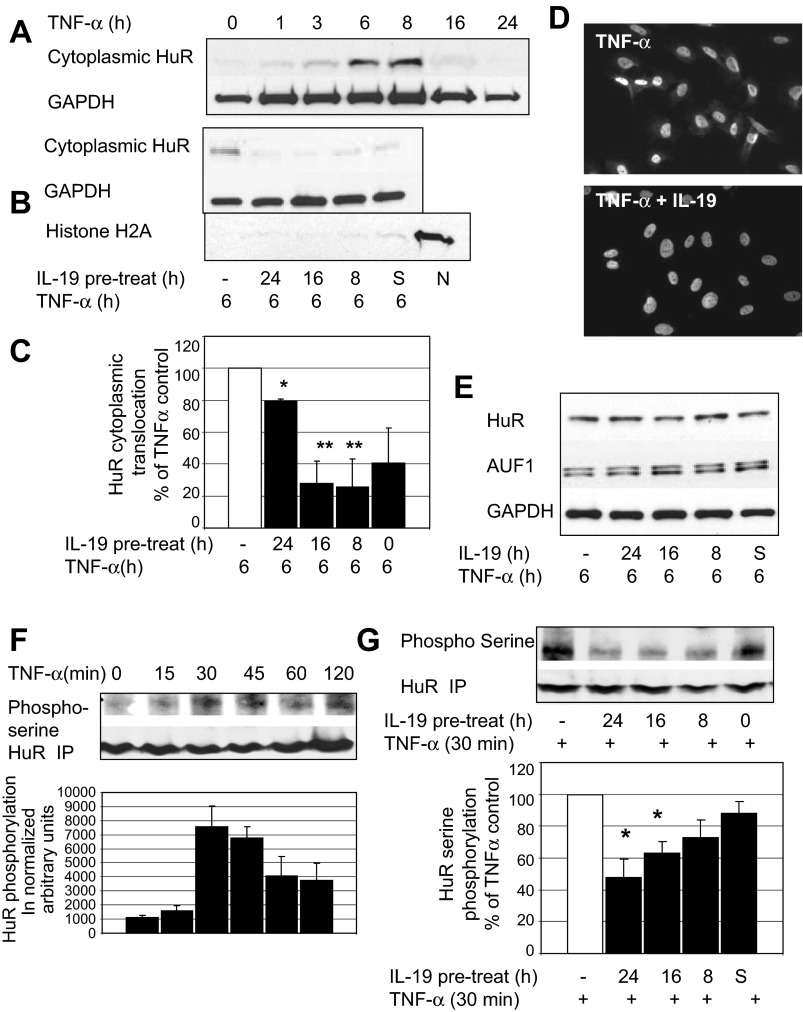
HuR (human R antigen) is an mRNA stability protein that regulates the half-life of transcripts that contain AU-rich elements (AREs) in their 3′-untranslated regions (UTRs) (8). HuR has recently been implicated as a mediator of ICAM-1 and VCAM-1 expression in umbilical vein ECs (26). Normally sequestered in the nucleus, nuclear-to-cytoplasmic translocation is required for HuR's mRNA stabilizing effects. In the absence of stimulus, HuR is relocated to the nucleus, causing its mRNA-stabilizing effects to be transient (6). We tested to see whether IL-19 can decrease HuR nuclear-to-cytoplasmic translocation. First, we determined that 6–8 h were optimal for TNF-α-driven HuR translocation (Fig. 4A). Next, ECs were pretreated with IL-19 for the indicated times, then stimulated with TNF-α for 6 h, and the cytoplasmic fraction was immunoblotted with anti-HuR antibody. Figure 4B shows that IL-19 can significantly reduce TNF-α-driven HuR cytoplasmic translocation, with 16 and 8 h of pretreatment being the most effective (28.0 ± 13.9% and 25.6 ± 17.7% of control for 16 and 8 h pretreatment, respectively, P < 0.05). Immunocytochemistry confirms that IL-19 inhibits HuR nuclear-to-cytoplasmic translocation (Fig. 4D). IL-19 treatment does not reduce whole cell HuR protein abundance, nor does it increase AUF-1 (Fig. 4E), an mRNA destabilizing protein that also regulates ARE-bearing transcripts (3).
Fig. 4.
IL-19 significantly reduces TNF-α-driven HuR cytoplasmic translocation in ECs. A: time course of TNF-α-driven HuR cytoplasmic translocation. B: ECs were pretreated with IL-19, then stimulated with TNF-α for 6 h to induce HuR translocation. The cytoplasmic fraction of lysates was immunoblotted with anti-HuR antibody, and Histone H2A as a nuclear protein control to show potential for unintended mixing of nuclear and cytoplasmic fractions. N, Histone H2A-positive control lane containing only nuclear fraction. C: densitometry from at least 3 experiments determined significance. *P < 0.05; **P < 0.01. D: immunocytochemistry showing that 16 h of IL-19 pretreatment reduces HuR cytoplasmic translocation. ECs grown on glass slides were treated as in B, then immunostained using HuR-specific antibody. E: IL-19 does not decrease total HuR or increase total AUF-1 abundance. Whole cell extract was blotted with shown antibody. Blot shown is representative of at least 4 experiments. F: TNF-α induces serine phosphorylation of HuR. ECs were serum reduced, then stimulated with TNF-α for the indicated times. HuR was immunoprecipitated with HuR antibody, then blotted with anti-phospho-serine or HuR antibody as a loading control. G: IL-19 decreases HuR serine phosphorylation. Serum-reduced ECs were pretreated with IL-19, then stimulated with TNF-α for 30 min. HuR was immunoprecipitated with HuR antibody, then blotted with anti-phospho-serine or HuR antibody. Blots shown are representative of at least 3 experiments. *P < 0.05. All samples were pretreated with IL-19 for different times. S, ECs treated with IL-19 at the same time as TNF-α.
It was important to determine a mechanism for IL-19 inhibition of HuR cytoplasmic translocation. In mesangial cells, HuR cytoplasmic translocation is associated with phosphorylation on serine residues (24), but this has not been reported in ECs. Time course studies in ECs established that 30 min of TNF-α stimulation resulted in maximal HuR phosphorylation (Fig. 4F). To determine whether IL-19 treatment could reduce HuR serine phosphorylation, ECs were cultured in serum-reduced media, pretreated with IL-19, then stimulated with TNF-α for 30 min. HuR was immunoprecipitated with HuR antibody, then blotted with phospho-serine antibody. Figure 4G demonstrates that 24 and 16 h of IL-19 pretreatment significantly reduced TNF-α-driven serine phosphorylation of HuR (48.3 ± 11.1 and 63.0+ ± 7.7% of TNF-α-phosphorylated control for 24 and 16 h pretreatment, respectively).
IL-19 decreases ICAM-1 and VCAM-1 mRNA stability.
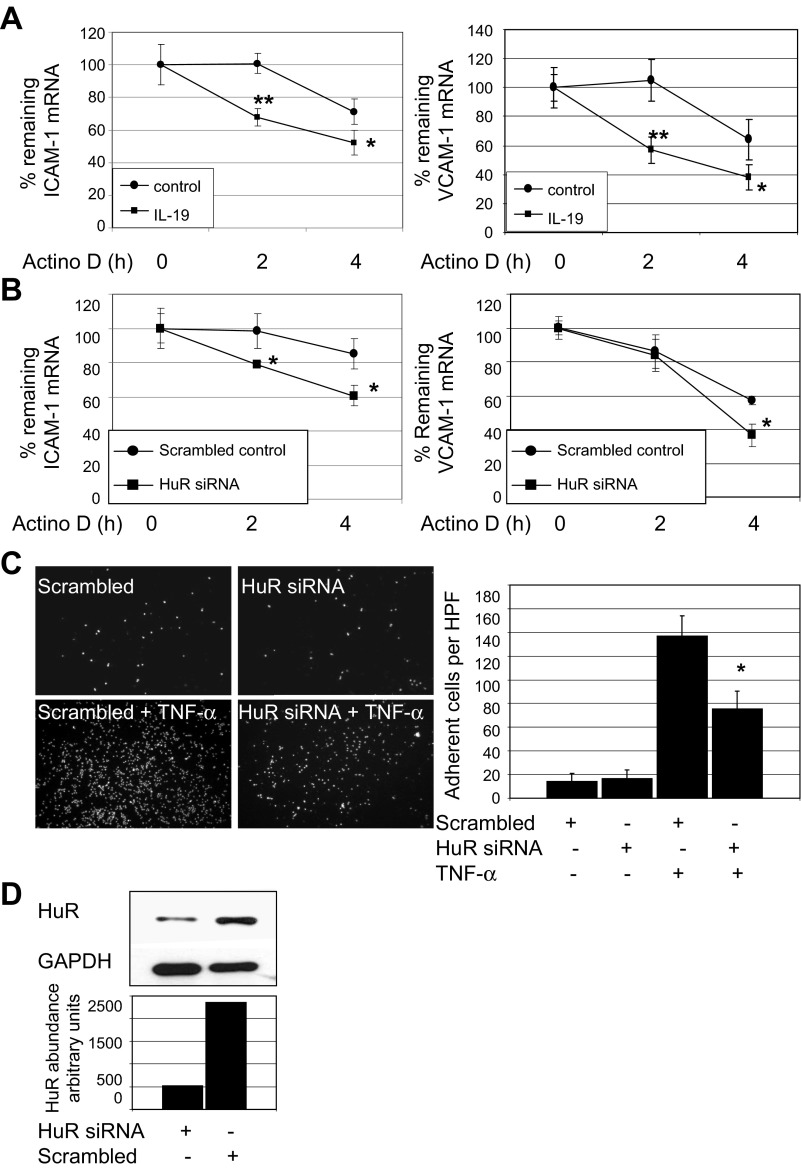
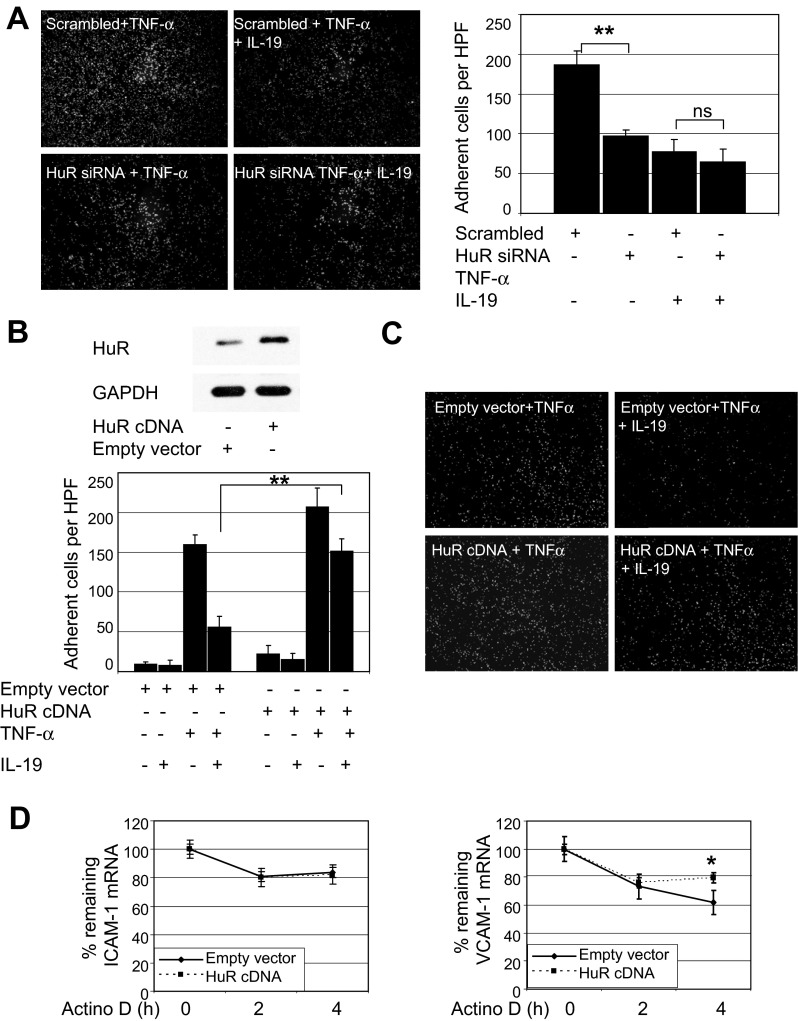
IL-19 inhibition of HuR translocation suggested that one mechanism whereby IL-19 could reduce CAM abundance was by promoting a decrease in mRNA stability. Three approaches were used to test this hypothesis. First, ECs were pretreated with IL-19, then stimulated with TNF-α for 6 h to induce expression of CAM mRNA, then treated with the transcription inhibitor actinomycin D. Target mRNA was quantitated by qRT-PCR. Figure 5A shows that IL-19 can significantly reduce stability of adhesion molecule mRNA at all times tested (ICAM-1, 100.4 ± 6.6% vs. 67.8 ± 5.2% for control and IL-19 treated; VCAM-1, 105.7 ± 14.0% vs. 57.3 ± 6.8%; at 2 h, P < 0.05 or P < 0.01 for all). To establish a relationship between CAM mRNA stability and HuR function, ECs were transfected with HuR siRNA or scrambled control siRNA, then treated with actinomycin D as described in the preceding section. Figure 5D shows that HuR siRNA was effective in reducing HuR protein, and Fig. 5B demonstrates that ICAM-1 and VCAM-1 mRNA stability is reduced when HuR abundance is decreased (ICAM-1, 96.8 ± 11.8% vs. 79.1 ± 1.7% for control and siRNA transfected; VCAM-1, 57.2 ± 6.8% vs. 36.5 ± 2.7%; at 4 h, P < 0.05 or P < 0.01 for all). To strengthen the relationship between HuR and leukocyte-EC adhesion, we reduced HuR protein abundance by siRNA, then assayed THP-1 adhesion. Figure 5C shows that siRNA knockdown of HuR results in significantly less THP-1 adhesion compared with scrambled controls (137.1.3 ± 17.1 vs. 75.1 ± 15.3 adherent cells per HPF for scrambled versus HuR siRNA transfected, respectively; P < 0.05). To further demonstrate a fundamental role for HuR in IL-19-mediated abolition of leukocyte-EC interaction, three additional experiments were performed. First, we reduced HuR content in ECs by siRNA. Some were pretreated with IL-19 for 16 h, then stimulated with TNF-α. We then assayed THP-1 adhesion as described for Figs. 1D and 5C. Figure 6A shows that IL-19 reduced leukocyte-EC interaction, as did HuR siRNA knockdown, as expected. However, importantly, no additional decrease in THP-1 endothelial cell adherence is observed when IL-19 is added to ECs in which HuR is knocked down (77.6 ± 14.7 vs. 64.0 ± 17.3 adherent cells per HPF for HuR siRNA + TNF-α and HuR siRNA + IL-19 + TNF-α). Next, we transfected HuR cDNA into ECs and repeated the adhesion assay. HuR overexpression was verified by Western blotting (Fig. 6B). HuR overexpression increased THP-1 adhesion in the presence of TNF-α (159.9 ± 12.2 vs. 207.1 ± 23.3 for empty vector and HuR, P < 0.01), or 62.0% inhibition. Importantly, IL-19 was able to significantly inhibit THP-1 adhesion even in the presence of ECs in which HuR was overexpressed (207 ± 23.3 vs. 151.5 ± 15.5 for HuR + TNF-α and HuR + TNF-α + IL-19, P < 0.05), but to a lesser degree than in empty vector-transduced control ECs (29.0%). Inhibition of adhesion was significant but much less effective in the presence of HuR overexpression (56.0 ± 13.3 vs. 151.5 ± 15.1 for empty vector and HuR cDNA, P < 0.01). To further confirm our hypothesis that HuR overexpression may stabilize CAM mRNA, we, in a third experiment, repeated mRNA stability experiments in HuR-overexpressing ECs using the transcription inhibitor actinomycin D. Interestingly, HuR overexpression stabilizes VCAM-1 but not ICAM-1 mRNA (Fig. 6D), which may explain the partial antagonism observed in the adhesion assay. Together, these results implicate HuR activity with stability of ICAM-1 and VCAM-1 mRNA, and leukocyte-EC interactions, and suggest that IL-19-mediated decreases in leukocyte-EC interactions are likely mediated by HuR.
Fig. 5.
IL-19 significantly decreases ICAM-1 and VCAM-1 mRNA stability. A: ECs were pretreated with IL-19 for 16 h, stimulated with TNF-α for 6 h to induce expression of cell adhesion molecule (CAM) mRNA, then treated with the transcription inhibitor actinomycin D (Actino D). mRNA was quantitated by qRT-PCR, normalized to GAPDH at the times indicated. IL-19 reduces stability of CAM mRNA transcripts at all times tested. *P < 0.05 or **P < 0.01 for all. B: ECs were transfected with HuR siRNA or scrambled siRNA control and treated with actinomycin D. mRNA was quantitated by qRT-PCR. ICAM-1 and VCAM-1 mRNA stability is significantly reduced when HuR abundance is decreased by siRNA. *P < 0.05. C: HuR knockdown reduces leukocyte-endothelial cell interaction. Experiment was performed as described for Fig. 2, and the image shown is representative of 3 experiments. *P < 0.05. D: representative Western blot and densitometry of HuR knockdown by specific siRNA.
Fig. 6.
IL-19 regulation of adhesion is mediated by HuR. A: IL-19 does not further inhibit leukocyte interactions in HuR-depleted endothelial cells. ECs were transfected with scrambled or HuR siRNA. Some ECs were pretreated with IL-19 before stimulation with TNF-α. Adhesion was performed as in Fig. 1D. **P < 0.01. Adhesion in ECs pretreated with IL-19 was not significantly different from HuR siRNA ECs not treated with IL-19. Image shown is representative of at least 3 experiments. B: overexpression of HuR increases TNF-α-induced THP-1 adhesion. Representative Western blot shows that transfection of ECs with HuR cDNA increases expression of HuR protein. **P < 0.01. C: overexpression of HuR increases THP-1 adhesion in the presence of TNF-α, and IL-19 can significantly inhibit THP-1 adhesion even in ECs in which HuR is overexpressed, but to a lesser degree than in empty vector-transduced control ECs. D: HuR overexpression stabilizes VCAM-1, but not ICAM-1, mRNA. ECs transfected with empty vector or HuR cDNA were stimulated with TNF-α for 6 h to induce expression of CAM mRNA, then treated with the transcription inhibitor actinomycin D. mRNA was quantitated by qRT-PCR and normalized to GAPDH at the times indicated. *P < 0.05.
DISCUSSION
Little has been reported on the direct effect of Th2 interleukins on endothelial cell pathophysiology, and this is the first study to report that IL-19 can decrease expression of cell adhesion molecules, decrease leukocyte-endothelial cell interaction in vivo, and diminish HuR activity in ECs. We propose that IL-19 reduces leukocyte-EC adhesion by an HuR-mediated decrease in ICAM-1 and VCAM-1 mRNA stability, leading to a reduction in protein levels of these adhesion molecules. IL-19 expression may represent a compensatory counterregulatory mechanism in inflamed endothelium.
We have previously reported that IL-19 could be induced in injured carotid arteries and that IL-19 is detected only at very low levels in unstimulated cultured ECs, but can be induced by inflammatory stimuli (14). However, no association of IL-19 expression with inflammation in endothelium has been reported. ICAM-1 and VCAM-1 are induced in ECs by proinflammatory conditions and subsequently promote the adhesion of leukocytes to the endothelium. Little has been reported on direct effects of Th2 interleukins on EC expression of adhesion molecules, and most work focuses on regulation of leukocytes. The prototypical Th2 cytokine, IL-10, did reduce IFN-γ-driven ICAM-1 mRNA expression in human monocytes at the transcriptional level (30). In the present study, pretreatment of human coronary ECs with IL-19 significantly decreases TNF-α-induced expression of ICAM-1 and VCAM-1 mRNA and protein. By contrast, a single IL-19 posttreatment after a single TNF-α stimulation had no inhibitory effect on mRNA abundance, similar to our observations in VSMCs (5, 10, 31). While in cultured cells a single dose of IL-19 appears to be transient, in vivo, IL-19 would be continuously expressed by inflamed ECs as well as inflammatory cells, suggesting therapeutic effects of IL-19. IL-19-mediated decreases in endothelial CAM expression were reflected functionally, as IL-19 can significantly reduce THP-1-EC interactions. IL-19 effects were EC specific, as IL-19 treatment of THP-1 cells did not reduce expression of monocyte VLA-4, nor was THP-1 adhesion to ECs affected in the reverse assay in which only THP-1 cells were treated with IL-19. Leukocyte integrin receptors are not typically regulated at the mRNA level but rather, through alterations in receptor conformation and avidity and we therefore were not surprised that IL-19 does not reduce leukocyte integrin mRNA abundance. Direct IL-19 treatment of THP-1 monocytes does not reduce their adhesion to endothelial cells, which strongly suggests that IL-19 does not directly affect leukocyte integrin receptor avidity. This study is the first to show a function for IL-19 in regulation of ICAM-1 and VCAM-1.
No data concerning IL-19 involvement in reduction of leukocyte-EC interaction have previously been reported. IL-10 can reduce IL-1β-driven monocyte-endothelial cell adhesion (21), although in this assay it was the monocytes that were treated, rather than the endothelial cells. Treatment of ECs with IL-10 had no inhibitory effect on leukocyte-human umbilical vein EC (HUVEC) adhesion (2). This is in contrast with our results indicating that IL-19 has no effect on adhesion when THP-1 cells are treated, further distinguishing IL-19 from IL-10 activity. In one interesting report, IL-10 did decrease ICAM-1 expression in ECs stimulated with LPS, but not when they were stimulated with TNF-α or IL-1β (18). Inhibition of human EC with IL-10 can inhibit minimally oxidized LDL (MM-LDL)-induced monocyte-endothelium interaction and, similar to IL-19, 18 h of pretreatment were necessary for significant reduction (25). However, in this manuscript, neither CAM expression levels nor a mechanism for these effects was elucidated. Taken in total, our present data are particularly intriguing in their demonstration that a Th2 interleukin can have direct anti-inflammatory effects on cells outside of the T cell lineage.
Extending these data into in vivo studies, we determined that IL-19 could reduce TNF-α-induced leukocyte rolling and adhesion as quantitated by intravital microscopy. This is likely due to effects on ECs, as IL-19 does not decrease counterreceptor abundance on THP-1 cells, nor does it reduce adhesion when THP-1 cells are incubated with IL-19. It is not yet known whether IL-19 can specifically affect adhesion molecule expression in neutrophils, which are known to comprise a portion of rolling and adhering cells in our model (1, 9). The reduction in in vivo rolling prompted us to investigate whether IL-19 could modulate selectin expression on cultured ECs. Surprisingly, IL-19 could not significantly decrease abundance of P-selectin in ECs. IL-19 did slightly, but significantly, reduce E-selectin mRNA and protein in ECs, providing a possible mechanism for reduced rolling in vivo. The possibility exists that IL-19 could effect a conformational change in either of these molecules, or inhibit an additional molecule on ECs or leukocytes. While the in vitro reverse-adhesion assay suggested that IL-19 did not affect monocytes, this assay cannot measure loose adhesion. Future studies need to determine the precise mechanism of IL-19 reduction in leukocyte rolling.
One major mechanism for transcription of ICAM-1 and VCAM-1 is activation of NF-κB (30). Though none was performed in ECs, other studies have shown that the anti-inflammatory effects ascribed to IL-10 are mediated by inhibition of NF-κB activity (9, 17), and IL-10 can decrease expression of NF-κB-dependent gene transcripts in both monocytes and VSMCs (7, 19, 29). IL-10 can inhibit IFN-γ-induced NF-κB activation in monocytes but, in contrast to our study, did not enhance the rate of ICAM-1 mRNA degradation, suggesting that IL-10 does not alter ICAM-1 mRNA stability (30). It was therefore somewhat unexpected that IL-19 did not inhibit or reduce TNF-α-driven NF-κB activation. HuR is an mRNA stability protein that is a member of the ELAV family of mRNA stability proteins that regulate mRNA half-life (8). The ability of HuR to stabilize mRNA corresponds with its translocation from a predominately nuclear location to the cytoplasm. IL-19 inhibition of TNF-α-driven nuclear-to-cytoplasmic translocation peaked at 16 h of pretreatment, which correlates very well with the observed 16 h of pretreatment necessary for efficient inhibition of ICAM-1 and VCAM-1 mRNA abundance. Interestingly, IL-19 treatment does not decrease the overall abundance of HuR, which is in contrast to our previous report using VSMCs and possibly reflects cell-specific differences (5). Nuclear-to-cytoplasmic translocation is essential for HuR activity and is regulated by serine phosphorylation (3, 24). A least one mechanism whereby IL-19 can decrease HuR translocation is by reduction of its serine phosphorylation, as IL-19 pretreatment can transiently decrease serine phosphorylation of HuR. This decrease requires at least 16 h of pretreatment and is transient, implying that synthesis of a labile factor or factors is necessary for the detected decrease in phosphorylation. Future studies are necessary to characterize IL-19-sensitive signaling pathways that may affect HuR cytoplasmic shuttling.
While many inflammatory cytokines have been shown to stabilize inflammatory gene transcripts, few have been shown to destabilize mRNA. This is the first report showing inhibition of HuR by an anti-inflammatory cytokine in ECs. In a broader sense, this also implicates HuR as a potential target of anti-inflammatory therapy. The 3′-UTRs of many inflammatory transcripts associated with inflammation contain AU-rich elements (AREs) that are target sites for HuR (2). It is not entirely unexpected that ICAM-1 and VCAM-1 transcripts are sensitive to HuR modulation. The ICAM-1 3′-UTR (GenBank NM_000201) has two Class 1 AREs, consisting of a conserved AUUUA pentamer, and the VCAM-1 3′-UTR (GenBank NM_001078) has three Class 1 AREs. This difference in the number of AREs in the 3′-UTRs of these transcripts may account for differential sensitivity to HuR overexpression noted in the mRNA stability experiments, as well as the partial antagonism of THP-1 adhesion observed when HuR is overexpressed. IL-19 treatment of ECs appears to reduce THP-1 adhesion between 59–62% in all experiments in which this was assayed (Figs. 1E, 1F, 2F, and 6A), but, although still significant, adhesion is inhibited by only 29% in ECs in which HuR is overexpressed. A limitation of the HuR overexpression study is that IL-19 regulates HuR in ECs not by reducing its abundance, but by reducing its nuclear-to-cytoplasmic translocation. Consequently, apparent partial antagonism of IL-19 effects by HuR overexpression may not fully represent the mechanistic relationship between IL-19 inhibition of HuR activity.
There is one important study that demonstrated that siRNA depletion of HuR in HUVEC resulted in a significant decrease in LPS-stimulated HUVEC-monocyte binding and LPS-induction of ICAM-1 and VCAM-1 mRNA transcripts (26). Our present study in coronary artery ECs extends that work in two ways. First, it demonstrates that inhibition of HuR by IL-19 or HuR siRNA also decreases the stability of ICAM-1 and VCAM-1 mRNA transcripts. Second, our study also places HuR activity in a physiological context, with use of coronary artery ECs rather than human umbilical vein ECs, with ECs stimulated with the cytokine TNF-α rather than LPS, and inhibition of HuR translocation by an anti-inflammatory cytokine, rather than by knockdown with transfected siRNA. It is interesting to note that this same report suggested that HuR also decreased NF-κB activation, specifically through inhibition of NF-κB p65 phosphorylation, in contrast to our study in which IL-19 did not inhibit NF-κB p65 phosphorylation. One explanation may be that, in that study, total levels of HuR were decreased by specific siRNA, whereas IL-19 inhibits HuR nuclear-to-cytoplasmic translocation without reducing overall cellular HuR levels, which may allow remaining nuclear HuR to modulate NF-κB. One limitation of our present study is that other transcription factors that may play a role in adhesion molecule expression, such as Sp1 or GATA-binding proteins, have not been completely eliminated as potentially sensitive to IL-19 effects. However, if these other transcription factors are targets of IL-19, their contribution is likely very small, because when HuR is knocked down by siRNA, IL-19 pretreatment does not potentiate loss of adhesion, suggesting that IL-19 actions are overwhelmingly mediated by HuR. NF-κB activation is necessary for mediating the response to inflammatory stimuli and suggests that IL-19 actions on mRNA are distal to NF-κB and posttranscriptional. Nevertheless, this study does demonstrate the interesting and unanticipated findings that NF-κB is not sensitive to IL-19 inhibition and that HuR and mRNA stability are.
In summary, there are several novel findings from this study. First, IL-19 directly can decrease TNF-α-induced ICAM-1 and VCAM-1 mRNA and protein abundance in ECs. Second, IL-19 reduces adhesion molecule abundance by a posttranscriptional mechanism through inhibition of HuR and mRNA stability. Third, IL-19 can reduce HuR phosphorylation and subsequent cytoplasmic shuttling. And fourth, IL-19 reduces leukocyte-endothelial cell interaction in vitro and in vivo. Together, these data imply that IL-19 can impart a Th2 phenotype on ECs and also support the hypothesis that IL-19, or IL-19 signaling targets, may be a valuable anti-inflammatory therapeutic modality.
GRANTS
This work was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant HL-090885 and HL-115575 (to M. V. Autieri) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-064344 (to R. Scalia). R. N. England was supported by NIH Training Grant 5T32HL091804-04.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.N.E. and M.V.A. conception and design of the research; R.N.E., K.J.P., and M.V.A. performed the experiments; R.N.E., K.J.P., R.S., and M.V.A. analyzed the data; R.N.E., R.S., and M.V.A. interpreted the results of the experiments; M.V.A. prepared the figures; M.V.A. drafted the manuscript; M.V.A. edited and revised the manuscript; M.V.A. approved the final version of the manuscript.
Footnotes
This article is the topic of an Editorial Focus by David W. Scott and Rakesh P. Patel (28).
REFERENCES
- 1. Atherton A, Born GV. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol 222: 447–474, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res 33: 7138–7150, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci 58: 266–277, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol 77: 487–495, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cuneo AA, Herrick D, Autieri MV. IL-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol 49: 647–654, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20: 2165–2173, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 281: L1037–L1050, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17: 3448–3460, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiebig E, Ley K, Arfors KE. Rapid leukocyte accumulation by “spontaneous” rolling and adhesion in the exteriorized rabbit mesentery. Int J Microcirc Clin Exp 10: 127–144, 1991 [PubMed] [Google Scholar]
- 10. Gabunia K, Jain S, England RN, Autieri MV. Anti-inflammatory cytokine interleukin-19 inhibits smooth muscle cell migration and activation of cytoskeletal regulators of VSMC motility. Am J Physiol Cell Physiol 300: C896–C906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun 1: 442–450, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, Dickensheets H, Donnelly RP. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol 4: 615–626, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev 21: 345–352, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol 31: 167–175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 15: 991–1004, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Lee YW, Eum SY, Nath A, Toborek M. Estrogen-mediated protection against HIV Tat protein-induced inflammatory pathways in human vascular endothelial cells. Cardiovasc Res 63: 139–148, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lentsch AB, Shanley TP, Sarma V, Ward PA. In vivo suppression of NF-kappa B and preservation of I kappa B alpha by interleukin-10 and interleukin-13. J Clin Invest 100: 2443–2448, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lisinski TJ, Furie MB. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1beta or tumor necrosis factor alpha. J Leukoc Biol 72: 503–511, 2002 [PubMed] [Google Scholar]
- 19. Mazighi M, Pellé A, Gonzalez W, Mtairag EM, Philippe M, Hénin D, Michel JB, Feldman LJ. IL-10 inhibits vascular smooth muscle cell activation in vitro and in vivo. Am J Physiol Heart Circ Physiol 287: H866–H871, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Missiou A, Rudolf P, Stachon P, Wolf D, Varo N, Aichele P, Colberg C, Hoppe N, Ernst S, Münkel C, Walter C, Sommer B, Hilgendorf I, Nakano H, Bode C, Zirlik A. TRAF5 deficiency accelerates atherogenesis in mice by increasing inflammatory cell recruitment and foam cell formation. Circ Res 107: 757–766, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Mostafa Mtairag E, Chollet-Martin S, Oudghiri M, Laquay N, Jacob MP, Michel JB, Feldman LJ. Effects of interleukin-10 on monocyte/endothelial cell adhesion and MMP-9/TIMP-1 secretion. Cardiovasc Res 49: 882–890, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Oral HB, Kotenko SV, Yılmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol 36: 380–388, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117: 1718–1726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, Govoni S, Alkon DL, Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha-dependent pathway. Proc Natl Acad Sci USA 102: 12065–12070, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol 19: 2847–2853, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Rhee WJ, Ni CW, Zheng Z, Chang K, Jo H, Bao G. HuR regulates the expression of stress-sensitive genes and mediates inflammatory response in human umbilical vein endothelial cells. Proc Natl Acad Sci USA 107: 6858–6863, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scalia R. Evaluation of endothelial function by in vivo microscopy. Methods Mol Med 139: 225–235, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Scott DW, Patel RP. Targeting endothelial adhesion molecule mRNA to control inflammation: novel insights into potential anti-inflammatory effects of IL-19. Focus on “Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability.” Am J Physiol Cell Physiol (May 8, 2013). 10.1152/ajpcell.00120.2013 [DOI] [PubMed] [Google Scholar]
- 29. Selzman CH, Meldrum DR, Cain BS, Meng X, Shames BD, Ao L, Harken AH. Interleukin-10 inhibits postinjury tumor necrosis factor-mediated human vascular smooth muscle proliferation. J Surg Res 80: 352–356, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Song S, Ling-Hu H, Roebuck KA, Rabbi MF, Donnelly RP, Finnegan A. Interleukin-10 inhibits interferon-gamma-induced intercellular adhesion molecule-1 gene transcription in human monocytes. Blood 89: 4461–4469, 1997 [PubMed] [Google Scholar]
- 31. Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol 173: 901–909, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vestweber D. Lymphocyte trafficking through blood and lymphatic vessels: more than just selectins, chemokines and integrins. Eur J Immunol 33: 1361–1364, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Woollard KJ, Suhartoyo A, Harris EE, Eisenhardt SU, Jackson SP, Peter K, Dart AM, Hickey MJ, Chin-Dusting JPF. Pathophysiological levels of soluble P-selectin mediate adhesion of leukocytes to the endothelium through Mac-1 activation. Circ Res 103: 1128–1138, 2008 [DOI] [PubMed] [Google Scholar]