Abstract
Human cytomegalovirus (CMV) is a viral pathogen that infects both genders, who remain asymptomatic unless they receive immunosuppressive drugs or acquire infections that cause reactivation of latent virus. CMV infection also causes serious birth defects following primary maternal infection during gestation. A safe and effective vaccine to limit disease in this population continues to be elusive. A well-studied antigen is glycoprotein B (gB), which is the principal target of neutralizing antibodies (NAb) towards CMV in humans and has been implicated as the viral partner in the receptor-mediated infection by CMV in a variety of cell types. Antibody-mediated virus neutralization has been proposed as a mechanism by which host immunity could modify primary infection. Towards this goal, an attenuated poxvirus, modified vaccinia virus Ankara (MVA), has been constructed to express soluble CMV gB (gB680-MVA) to induce CMV NAb. Very high levels of gB-specific CMV NAb were produced after two doses of the viral vaccine. NAb were durable within a twofold range for up to 6 months. Neutralization titers developed in immunized mice are equivalent to titers found clinically after natural infection. This viral vaccine, expressing gB derived from CMV strain AD169, induced antibodies that neutralized CMV strains of three different genotypes. Remarkably, preexisting MVA and vaccinia virus (poxvirus) immunity did not interfere with subsequent immunizations of gB680-MVA. The safety characteristics of MVA, combined with the robust immune response to CMV gB, suggest that this approach could be rapidly translated into the clinic.
Human cytomegalovirus (HCMV) is a member of the herpesvirus family. It is a major cause of congenital disease, resulting in an estimated 4,000 cases of symptomatic congenital cytomegalovirus (CMV) infection per year in the United States (58). An effective CMV vaccine that can prevent or reduce CMV-associated disease is highly desirable. Early studies have indicated that HCMV gB is the major target of NAb that are induced by naturally acquired CMV infection (16, 39). It is the most highly conserved envelope glycoprotein of human herpesviruses (38). Thus, CMV gB has been an attractive candidate for CMV vaccine development. CMV gB vaccines using recombinant gB protein expressed from plasmid DNA and gB expressed in several different viral vectors (ALVAC, adenovirus, and vaccinia virus [VV]) have been investigated with animal models (9, 13, 23, 26, 31, 40, 54). Safety and moderate immunogenicity have been demonstrated with these vaccines, but no licensed CMV vaccine is available. A live attenuated Towne strain of CMV, either alone or with a gB subunit vaccine as a prime-boost, have also been evaluated in human subjects (1, 2, 48).
Full-length CMV gB is synthesized as a 907-amino acid (aa) precursor in CMV-infected cells with a predicted molecular mass of 105 kDa, but it can be glycosylated to form a 170-kDa modified protein (17). To enable pharmaceutical development, truncated and secretable forms of gB were derived. These include the original design of the Chiron gB vaccine, a molecular fusion protein of 807 aa, that was mutagenized at the protease cleavage site and which contained an internal deletion of the putative membrane-spanning (TM) domain between aa 715 and 772 (48, 54, 55). This molecule and variant constructs of 680 (gB680) and 692 aa, from which the entire carboxyl terminus was deleted, were shown to be immunogenic in animals and humans and induced virus-neutralizing antibodies (NAb) (7, 48, 53, 54). In fact, a plasmid expressing gB680 induced higher levels of CMV NAb than full-length gB in mice, confirming reports that it is more immunogenic than full-length gB, making it a suitable candidate for further vaccine development (26, 27).
Modified VV Ankara (MVA) was derived from the Ankara strain of VV due to safety concerns associated with using VV as a primary immunization against smallpox (41). During more than 570 passages in chicken embryo fibroblasts, MVA became host restricted and highly attenuated. Although there is replication, little or no packaging of infectious virus takes place in primate and other mammalian cells (59). Towards the end of the smallpox eradication era, MVA was administered as a primary immunogen to lessen the potential morbidity of receiving the more virulent VV as a vaccine against smallpox in more than 120,000 individuals (56). Many of the MVA recipients were considered high risk, including children and the elderly (56). Furthermore, a recent preclinical study has shown that MVA is safe in macaques with immune suppression induced by anti-thymocyte globulin, total body irradiation, or measles virus (57). The clinical utility of MVA is being explored in two phase I safety and immunogenicity clinical trials of MVA-based human immunodeficiency virus and malaria vaccines, either alone or in combination with a DNA vaccine in both Oxford and Nairobi (33, 42).
In addition to having a good safety profile, MVA also has many other advantages as a live viral vaccine vector: (i) large foreign gene capacity; (ii) high levels of recombinant protein expression in most human and mammalian cells; (iii) potent inducer of humoral and cellular immune responses; (iv) long-term stability in frozen or lyophilized state. In fact, several MVA vector-based experimental vaccines have demonstrated efficacy in animal models in protecting against challenge from pathogens such as influenza, malaria, simian immunodeficiency virus, and human immunodeficiency virus (3, 24, 60). Thus, MVA's safety profile, along with its ability to provide protection against a challenge pathogen, makes MVA an ideal live viral vector choice for constructing a CMV gB vaccine. In this report, we describe the construction of a recombinant MVA that expresses soluble CMV gB (gB680), its immunogenicity, evaluation of mice with and without preexisting poxvirus immunity, and effectiveness of induced NAb against multiple strains of CMV.
MATERIALS AND METHODS
Cells, viruses, and animals.
BHK-21 (ATCC CCL-10), MRC-5 (ATCC CCL-171), and CV-1 (ATCC CCL-70) cells were purchased from American Type Culture Collection. Various lots of primary chicken embryo fibroblast (CEF) cells, prepared from 8- to 11-day-old specific-pathogen-free chicks, were purchased from Charles River SPAFAS (North Franklin, Conn.). MVA virus stock was kindly provided by Linda Wyatt and Bernard Moss at the National Institutes of Health (NIH) (National Institute of Allergy and Infectious Diseases [NIAID]). John Zaia at the Beckman Research Institute of the City of Hope, Duarte, Calif., kindly provided the AD169, Towne, Davis, and Toledo strains of HCMV. BALB/c and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice used in this study were 6 to 12 weeks old. Mice were bred and maintained in the Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility at City of Hope National Medical Center. The institutional animal care committee approved all experimental procedures.
Construction of homologous recombination plasmid, gB680-pLW51.
pLW51 was provided by Linda Wyatt and Bernard Moss (Laboratory of Viral Diseases, NIAID, NIH). pLW51 has the following features: (i) Flanking regions of deletion III that allow it to be inserted into the deletion III region of MVA via homologous recombination (60) (Fig. 1); (ii) marker gene for color screening, β-glucuronidase (gus), under control of the early-late VV promoter P11 (19, 20); (iii) two direct repeats flanking the gus gene, allowing it to be removed by deletion recombination (Fig. 1); and (iv) two VV promoters, PsynII and modified H5 (mH5) (64), to allow the expression of two separate foreign genes simultaneously. A manuscript in preparation more fully describes the structure and properties of this vector (L. Wyatt and B. Moss, unpublished data).
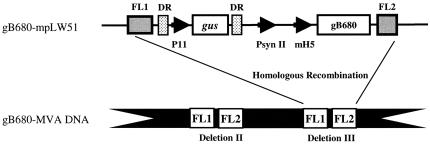
FIG. 1.
Schematic map of the gB680-mpLW51 plasmid. pLW51 has four features: (i) flanking regions (FL1 and FL2); (ii) color screen marker gene, gus under control of VV promoter P11; (iii) identical direct repeats (DR); and (iv) two VV promoters, PsynII and mH5, for recombinant gene expression. The gB680 gene was cloned behind the mH5 promoter to create gB680-mpLW51. gB680-mpLW51 recombined into deletion III of wt MVA to create gB680-MVA by homologous recombination. More details can be found in Materials and Methods.
pLW51 has been modified in our laboratory to include PmeI and AscI restriction enzyme sites behind the mH5 promoter to facilitate the cloning of the HCMV gB680 gene. Briefly, pLW51 was modified from its original form by using PCR primers to knock out the AscI site at nucleotide 941, and PmeI and AscI sites were added behind the mH5 promoter. The following primers were used for PCR (5′ primer, GATTAAGATTGCTCTTTCGGTGGCTGGGTACCAGGCGCGCATTTCATTTTG; and 3′ primer, AGCATTGGTTCTGCAGGGCGCGCCGTTTAAACGTCGACTCTAGAGGATCCCCGGG). The PCR fragment was gel purified and digested with PstI and BssHII. pLW51 was digested with PstI and BssHII. The digested DNA was separated on an agarose gel, and the larger band was excised and gel purified. These purified DNA fragments were ligated together to create modified pLW51 (mpLW51).
CMV cDNA was reverse transcribed using mRNA derived from CMV (AD169)-infected MRC-5 cells using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.). The gB680 gene was made by PCR amplification using the following primer pair (5′ primer, ATAAGAATGCGGCCGCCCCGGGGTTTAAACGCCACCACC; and 3′ primer, CGGGATCCGGCGCGCCTTTATTTCAGAGGTCAAAAACGTTCC). The PCR product was cut with PmeI and AscI, gel purified, and then cloned into mpLW51 to yield gB680-mpLW51. gB680 gene expression was driven by the mH5 viral promoter, which has strong early transcription activity (64). The sequences of mpLW51 and gB680-mpLW51 were verified by restriction enzyme digestion and DNA sequence analysis.
Generation of gB680-MVA.
Recombinant MVA expressing the HCMV gB680 protein (gB680-MVA) was generated by transfecting 10 μg of gB680-mpLW51 plasmid into 5 × 105 BHK-21 cells previously infected with wild-type (wt) MVA (multiplicity of infection of 0.01) in six-well culture plates. gB680-MVA was selected by color screening in the presence of X-glcA (5-bromo-4-chloro-3-indolyl-β-d-glucuronide) (Sigma-Aldrich, St Louis, Mo.). After each round of color selection, a portion of lysate from each plaque was immunostained using a gB-specific monoclonal antibody (MAb) (7-17) to distinguish gB680-positive and gus+ MVA from gB680-negative gus+ recombinant MVA (rMVA). After 8 to 10 rounds of plaque purification, gB680-MVA virus was grown and amplified on BHK-21 or CEF cells. The virus-cell pellet was resuspended in minimum essential medium (Mediatech, Herndon, Va.) containing 2% fetal calf serum and subjected to three freeze-thaw and sonication cycles. The virus stocks, typically between 109 and 1010 PFU/ml, were divided into aliquots and stored at −80°C. For gB680-MVA virus stock used for immunization, the virus stock was purified by sucrose density ultracentrifugation and resuspended in phosphate-buffered saline (PBS) containing 5% lactose and stored at −80°C (44).
Purification of soluble gB680 by affinity chromatography.
Soluble gB680 protein (S-gB680) was purified by affinity chromatography according to previously described methods (18). Briefly, 20 mg of affinity purified anti-gB MAb (clone 7-17) (14) was coupled with 2 mg of protein G-Sepharose beads by a chemical cross-linking method (34). Prior to use, the anti-gB protein G affinity column (2 ml) was prewashed with eluting buffer (100 mM glycine [pH 2.7]) to remove non-cross-linked or weakly cross-linked antibodies and then washed with PBS. To purify S-gB680, culture medium was passed through the column and the beads were washed with 1× PBS at 4°C. The S-gB680 was eluted with 0.1 M glycine (pH 2.7). The eluate was neutralized with 1.0 M Tris-HCl buffer (pH 8.0) and stored at −20°C.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Lysates were prepared from VV- or MVA-infected cells using standard methods (44). Between 20 to 40 μl of lysate or affinity-purified S-gB680 protein was separated on a mini-acrylamide separating gel (10%) with a 4% stacking gel (Mini-PROTEAN 3 cell system; Bio-Rad, Hercules, Calif.). The protein expression levels of gB680 in MVA-infected BHK-21 cells were evaluated by Western blotting. After the run, the acrylamide gel was placed in a transfer apparatus with a polyvinylidene difluoride membrane (Bio-Rad), and conditions for transfer were according to the manufacturer's protocol. Development of the polyvinylidene difluoride membrane and detection of the gB proteins were done using the primary antibody (7-17) together with an ECL bioluminescence kit, using a goat anti-rabbit alkaline phosphatase-conjugated secondary antibody according to the manufacturer's conditions (Pharmacia Amersham, Piscataway, N.J.). The dried membrane was exposed to Kodak X-omat autoradiography film and developed in a standard film developer (model M35A; Kodak, Rochester, N.Y.).
Immunization procedure.
gB680-MVA, wt MVA, wt VV, and gus-MVAs used for mouse inoculation studies were all purified and resuspended in PBS. Six- to eight-week-old BALB/c mice were injected with 5 × 107 PFU of purified gB680-MVA or PBS (as a control) in 100 μl by either intramuscular (i.m.) or subcutaneous (s.c.) routes. Immunized animals were boosted after 3 weeks and 6 months with the same dose of virus and by the same route(s). To generate immunity to wt MVA or VV, 10 BALB/c mice were immunized with 5 × 107 PFU of MVA virus via intraperitoneal (i.p.) injection followed by a booster immunization with wt MVA of the same dosage and route. Serum samples were collected 6 weeks after initial wt MVA immunization. Systemic VV immunity was generated by inoculating 10 BALB/c mice with 5 × 105 PFU of wt VV via by i.p. injection. Serum samples were collected 4 weeks after VV inoculation. Groups of mice either with preexisting MVA immunity or with VV immunity were further immunized either i.m. or s.c. with 5 × 107 PFU of gB680-MVA, followed by a booster immunization with the same dose and route after 3 weeks. Serum samples were collected 3 and 6 weeks after initial gB680-MVA immunization. Serum samples from mice were collected from the orbital plexus using microhematocrit tubes for gB antibody level assessment.
Immunologic assays for evaluation of immune response after immunization. (i) gB-specific enzyme-linked immunosorbent assays (ELISA).
Purified S-gB680 or irrelevant control protein diluted in PBS was used to coat 96-well Costar (Corning, N.Y.) microplates (100 ng per well) at 37°C for 1 h. The plate was washed three times with PBS containing 0.05% Tween 20 and blocked with PBS containing 2% bovine serum albumin and 0.05% Tween 20 for 1 h at 37°C. The plate was incubated with preimmune mouse sera and postimmune mouse sera diluted 1:50 or 1:100 in PBS at 37°C for 1 h. Following three washing cycles, the plate was incubated for 30′ with 1:20,000 goat anti-mouse immunoglobulin G (IgG) conjugated with peroxidase (Sigma-Aldrich). One milligram of O-phenylendiamine (Sigma-Aldrich) per milliliter in 0.1 M citrate-phosphate buffer (pH 5) and 0.015% H2O2 was used as a substrate for color development. The reaction was stopped after 15 min with 4.0 M sulfuric acid and read at 490 nm by a microplate reader (DYNEX Technologies, Inc., Chantilly, Va.). An optical density (OD) reading greater than the geometric mean OD of preimmune mouse sera plus three standard deviations was considered positive. Titer of gB680-specific antibody is defined as the highest serum dilution which gives a positive OD in ELISA.
(ii) ELISA to measure gB-specific IgG subclasses.
gB-specific IgGs from sera from immunized mice were isotyped by using a kit from Zymed Laboratories Inc. (San Francisco, Calif.). Ninety-six-well microtiter plates were coated with 100 ng of purified S-gB680 protein in each well and incubated overnight at 4°C. The plate was then washed once and blocked with 1% bovine serum albumin in PBS. Pre- and postimmune sera diluted 1:5,000 were added to the plates and incubated at 37°C for 30 min. The plates were then washed four times. Biotinylated antibodies specific for mouse IgG1, IgG2a, IgG2b, IgG3, IgM, IgA, Igκ, and Igλ subclasses, or an irrelevant biotinylated antibody as negative control, and PBS were incubated for 15 min at room temperature. Horseradish peroxidase-streptavidin was then incubated in each well for 15 min at room temperature. ABTS substrate (2,2-azino-di-[3-ethylbenzthiazoline sulfonic acid]) was incubated in each well for 45 min at room temperature. The plates were read at 405 nm. The negative control ODs were subtracted from the sample ODs, and the percentage of each subclass was taken as the OD of each individual subclass divided by the OD of IgG1 plus IgG2a plus IgG2b plus IgG3.
(iii) CMV microneutralization assay.
CMV neutralizing titer (NT) of mouse sera was measured by a rapid microneutralization assay described previously (4). In brief, MRC-5 fibroblast monolayers were seeded onto flat-bottom wells of a 96-well microtiter plate. Sera from immunized mice, diluted in PBS, were mixed and incubated with CMV AD169 (100 foci) for 1 h at 37°C. The mixture of sera and virus was then transferred to a 96-well plate and incubated for 4 h. After being washed, the plates were further incubated for 16 h in a 37°C incubator. Cells were fixed in 100% ethanol and reacted with anti-IE1 MAb (p63-27) and fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma-Aldrich). Finally, wells were counterstained with 0.02% Evans blue containing 30% glycerol. Nuclei were counted using fluorescence microscopy. The 50%-inhibition endpoint calculated using the Reed-Münch method was considered the NT NT = reciprocal sera dilution [>50% inhibition] × [(% inhibition greater than 50% − 50%)/(% inhibition greater than 50% − % inhibition less than 50%)].
(iv) VV neutralization assay.
MVA and VV immunity was determined by performing a VV neutralization assay. VV (100 PFU) was mixed and incubated with serially diluted mouse sera for 2 h at 37°C. The mixture was transferred onto confluent monolayers of CV-1 cells on a six-well plate and incubated for 1 h at 37°C. After aspirating the mixture from the well, fresh culture medium was added and the plate was incubated overnight. On day 2, VV plaques were visualized by crystal violet staining and counted. The VV NT was defined as the highest dilution of mouse serum that generated at least 85% VV plaque reduction.
(v) T-cell proliferation assay.
Fresh spleen cells collected from immunized mice were resuspended at 2 × 106 cells/ml in RPMI medium containing 5% fetal calf serum. Cells (2 × 105) in 100 μl were added to wells of a 96-well U-bottom plate. One hundred microliters of affinity-purified S-gB680 or control antigen (MRC-5 cell lysate) was added to the cells in each well of a 96-well microtiter plate at a final concentration of 1, 10, and 100 μg/ml. The plates were incubated at 37°C for 4 days. After the incubation, 0.25 μCi of [3H]thymidine was added to each well, and the plates were incubated for an additional 7 h at 37°C. They were then harvested by a Micro96 Harvester (SKATRON, Sterling, Va.). [3H]thymidine incorporated into the cells was measured with a Wallac TRILUX scintillation counter (Perkin-Elmer, Torrance, Calif.).
(vi) In vitro expansion of cytotoxic T lymphocytes (CTLs).
Naive spleen cells for preparation of blasts as stimulator cells were obtained according to a method described previously (36). Lipopolysaccharide (LPS) blasts (2.5 μg of LPS/ml and 7 μg of dextran sulfate/ml) were pulsed for 8 h with 5 μg of purified S-gB680 protein/ml in serum-free in vitro stimulation (IVS) medium (RPMI 1640; Gibco-BRL Life Technologies, Rockville, Md.), supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 10 mM HEPES buffer solution, 5 mM glutamine, 50-U/ml penicillin-50-μg/ml streptomycin, and 50 μM 2-mercaptoethanol, and subsequently irradiated. Splenocytes from immunized animals and from PBS-injected control mice were cocultured with S-gB680-pulsed blasts at a ratio of 3:1 in IVS medium for 7 days, with the addition of 10% rat T-stim (Collaborative Biomedical Products, Bedford, Mass.) on day 3 of stimulation.
(vii) Cytotoxicity assay.
Cytolytic activity was determined using a 4-h chromium release assay (CRA) following one IVS. Syngeneic splenocytes pulsed for 8 h with 5 μg of gB680/ml were used as targets. Target cells were labeled with 200 μCi of Na51CrO4− (ICN, Costa Mesa, Calif.) for 1 h in a 37°C water bath, washed three times with IVS medium, and plated in 96-well round-bottom plates at a concentration of 2,000 target cells/well. IVS immune splenocytes were added at different effector-to-target ratios (E/T) in a final volume of 200 μl per well. Following incubation in a 5% CO2 incubator for 4 h at 37°C, supernatants were harvested using the microplate harvesting system (Skatron, Lier, Norway) and analyzed for gamma emission using a Cobra II auto γ-counter (Packard, Downers Grove, Ill.). Experimental evaluations were performed in triplicate, and percent specific lysis was determined as described previously (36).
(viii) Intracellular IFN-γ expression.
Intracellular cytokine staining (ICC) for IFN-γ was detected using a Cytofix/Cytoperm Plus kit with GolgiStop (BD Pharmingen, San Jose, Calif.). LPS blasts were incubated with 5 μg of S-gB680 protein/ml for 8 h and then irradiated (36). Splenocytes (2 × 106) from 7-day IVS cultures were incubated in 96-well round-bottom plates together with pulsed blasts for 6 h. GolgiStop was added after 1 h of stimulation. Cells were washed and incubated with Fc Block (BD Pharmingen) for 15 min at 4°C and washed. This was followed by staining with CD4 or CD8 fluorescein isothiocyanate-conjugated MAb (BD Pharmingen) for 15 min at 4°C. Cells were washed and then fixed with Cytofix/Cytoperm for 20 min at 4°C, after which cells were washed twice with 1× Perm/Wash and stained with allophycocyanin or phycoerythrin-labeled-anti-gamma interferon (IFN-γ) or matched control antibodies.
Statistical analysis methods.
Measurements of gB-specific antibodies were summarized as geometric mean titer (GMT). Titers of antibody were compared using nonparametric tests. Specifically, Wilcoxon signed rank tests were used for two-group comparisons, Kruskal-Wallis tests for comparing three groups, and Wilcoxon rank sum tests for testing change in a single set of mice.
RESULTS
Generation and characterization of gB680-MVA.
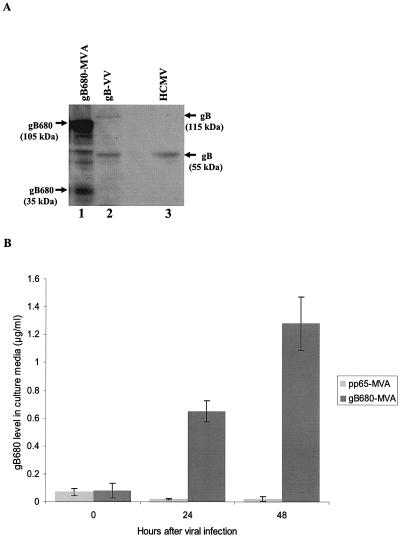
Preceding studies with ALVAC, VV, and adenovirus utilized full-length CMV gB as the recombinant expression molecule. We proceeded initially by inserting full-length CMV gB into several different MVA recombination plasmids (pSC11, pLW22, and pLW51) (44) and attempted to make recombinant viruses unsuccessfully (data not shown). No virus was obtained by using the highly efficient pSyn II promoter in pLW22 (data not shown). We observed that rMVA incorporating full-length CMV gB was unstable utilizing pLW51 employing moderately active (mH5) or pSC11 employing weaker (p7.5) pox promoters, exhibiting a phenotype of progressively diminished frequency at each successive round of screening (data not shown). We hypothesized that a secretable form of gB might be less toxic to cells infected with recombinant MVA expressing it, as discussed earlier by Moss and Earl with reference to difficulties in purifying MVA expressing membrane glycoproteins (44). A recombinant MVA expressing gB680 (gB680-MVA) was generated using the plasmid vector (gB680-mpLW51) that inserted the gB680 gene into deletion III of MVA (Fig. 1). The expression of gB680 after gB680-MVA infection was characterized by Western blot (WB) analysis (Fig. 2A), immunofluorescence (data not shown), and ELISA (Fig. 2B). WB analysis detected high-level expression of precursor intracellular gB680 protein (105 kDa), which is not found in gB-VV-infected (16) and HCMV-infected cell lysate samples (Fig. 2A). Since gB680 and full-length gB both contain a cleavage site, RTK/RR, representing the recognition motif of the subtilisin-like serine endoprotease furin, both proteins can be proteolytically cleaved at aa 460 to generate two fragments (55). As expected, carboxyl-terminal fragments of 30 kDa in gB680-MVA-infected cell lysates and 55 kDa in gB-VV- and HCMV-infected cell lysates were detected by MAb 7-17, which recognized the epitope located at the carboxyl terminus of the gB protein (14).
FIG. 2.
Characterization of gB680-MVA. (A) Western blot detection of intracellular gB680 protein from gB680-MVA-infected BHK-21 cells. Lane 1, cell lysate from gB680-MVA-infected BHK-21 cells; lane 2, cell lysate from gB-VV-infected BHK-21 cells; lane 3, cell lysate from HCMV (AD169)-infected MRC-5 cells. All lanes were loaded with the same amount of protein, as determined by the Bradford protein measurement method. (B) ELISA measurement of S-gB680 protein in culture medium collected from gB680-rMVA-infected BHK-21 cells. pp65-MVA served as a negative control (28). Concentration values were determined by using an internal reference standard of commercially bought soluble gB protein.
S-gB680 is predicted to be secreted into culture medium, because it contains a signal peptide at the amino terminus of the protein and the TM region has been removed (55). A sandwich ELISA was set up to measure the S-gB680 protein level in culture medium collected from gB680-MVA-infected cells (55). S-gB680 in culture media of gB680-MVA-infected BHK-21 cells was detected at 24 and 48 h after virus infection, with levels reaching as high as 1.4 μg/ml in culture medium at 48 h postinfection (Fig. 2B). In contrast, no S-gB680 signal was detectable in culture medium from pp65-MVA virus infection (Fig. 2B). The impact of deletion of the TM on cellular localization of the gB680 protein was evaluated after infection of BHK-21 cells and staining with the gB-specific MAb 7-17. Expressed S-gB680 protein was found in abundance in the cytoplasm of infected cells and secreted in medium (see above), whereas in accord with results in previous work, processed full-length gB (55 kDa) localized predominately in the cytoplasm (data not shown).
Purification of the S-gB680 protein from culture medium of gB680-MVA-infected cells.
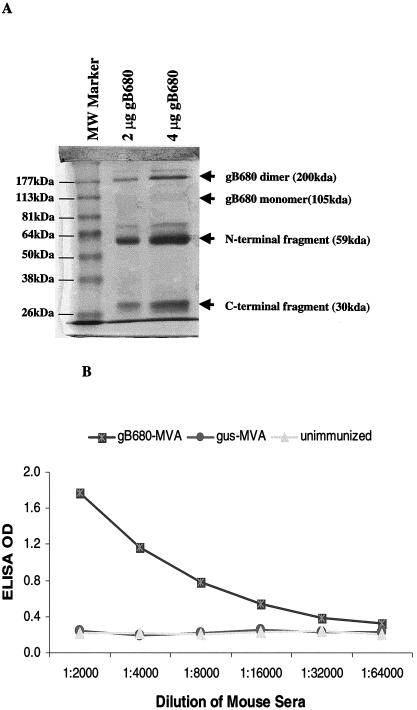
Because of abundant S-gB680 produced in culture medium of gB680-MVA-infected BHK-21 cells, it was possible to purify the S-gB680 protein from culture medium using gB antibody affinity chromatography. Eluted S-gB680 was analyzed by SDS-PAGE. The Coomassie blue-stained protein bands on the SDS-PAGE gel showed that purified S-gB680 was a mixture of a possible disulfide-bonded dimer form (200 kDa), a minimal level of the monomer form (105 kDa), and abundant proteolytically cleaved (presumably at aa 460) amino-terminal (59 kDa) and carboxyl-terminal (30 kDa) fragments, in agreement with previous studies (18) (Fig. 3A). The amino-terminal fragment copurified with the carboxyl-terminal fragment on the gB-MAb affinity column, as a likely result of the interchain disulfide bonding of the gB dimer (37). The specificity of gB-associated bands on the gel was confirmed by WB (data not shown).
FIG. 3.
Purified S-gB680 protein does not cross-react with sera from MVA-immunized mice. (A) Coomassie blue-stained SDS-PAGE profile of affinity purified S-gB680 (see Materials and Methods). Lane 1, molecular mass markers; lane 2 is 2 μg of affinity-purified S-gB680, and lane 3 is 4 μg of affinity-purified S-gB680. The gel pattern shows possible dimers (200 kDa), monomer form (105 kDa), and proteolytically cleaved amino-terminal (59 kDa) and carboxyl-terminal (30 kDa) fragments, indicated by arrows (see the text for further description). (B) Sera from mice immunized with gB680-MVA and gus-MVA were used in ELISA as described in Materials and Methods. gB680-MVA-immunized sera were used as positive controls (▪). Sera from gus-MVA-immunized mice (• symbol) and unimmunized mice (▴ symbol) were still negative in ELISA even when serum dilution was less than 1:100 (data not shown in graph).
Affinity-purified S-gB680 was collected to be used as a coating antigen in ELISA to measure gB-specific antibodies in immunized mouse sera. It was necessary to ensure that purified S-gB680 did not cross-react with serum antibodies from either naive or poxvirus-infected mice. Sera generated from gB680-MVA- or gus-MVA-immunized mice or preimmune sera were assayed for the presence of gB680-specific antibodies (Fig. 3B). ELISA results unequivocally demonstrate that IgG antibodies measured using plates coated with affinity-purified S-gB680 were gB specific. There was no evidence of cross-reaction with sera from gus-MVA-immunized mice or preimmune sera. These results validate the use of affinity-purified S-gB680 protein for detection of gB-specific antibodies in sera from gB680-MVA-immunized mice.
High level of gB-specific antibodies produced by gB680-MVA immunization.
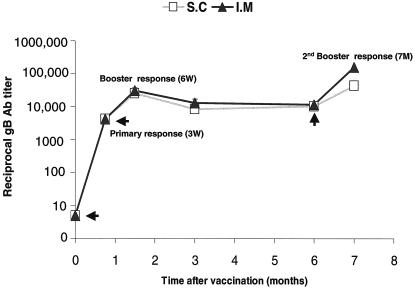
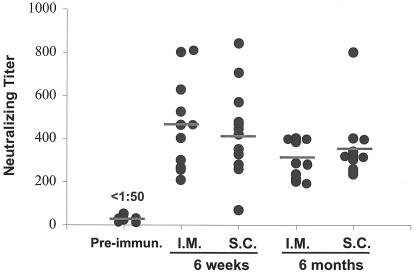
To evaluate immunogenicity of gB680-MVA, groups of 10 BALB/c mice were immunized with 5 × 107 PFU of gB680-MVA via by i.m. or s.c. injection. Three weeks later, all mice were boosted with the same dosage of vaccine and by the same route (i.m. or s.c.). A second boost immunization was administered 6 months after the initial immunization. Sera were collected at different time points for gB antibody level measurement, as shown on the time-scale (x axis) of Fig. 4. gB-specific IgG antibodies were detected by ELISA in all mice immunized with gB680-MVA. High levels of gB-specific antibody were produced after a single administration of gB680-MVA. The first boost immunization after 3 weeks generated a significantly higher gB antibody level than primary immunization for both the i.m. and s.c. routes (P = 0.0015 and P = 0.0037, respectively, by two-sided signed rank tests, with no significant difference between the routes [P = 0.59]). In fact, the GMT of gB-specific antibody in the boost response is at least fivefold higher than that for the primary response. The gB-specific antibody level declined about twofold at the 3-month time point after primary immunization and held steady for up to 6 months. A second boost immunization was administered 6 months after the initial immunization, and remarkably, gB antibody levels reached yet another higher peak level, compared to the primary immunization and first booster response for both i.m. (P = 0.0086) and s.c. (P = 0.053) routes. These results suggest that gB680-MVA is very immunogenic in mice, even after repeated administration (three times) over a 7-month period (Fig. 4).
FIG. 4.
Time course of gB-specific antibody levels after immunization. Mice were immunized and boosted with gB680-MVA as indicated by arrows in the graph. Serum samples were collected at 3 weeks (primary response), 6 weeks (booster response), and 3, 6, and 7 months (second booster response) after initial immunization and analyzed by ELISA as described in Materials and Methods. The gB-specific antibody ELISA titer is expressed on a log scale as the reciprocal of the highest dilution of mouse serum that gives positive OD. The y axis represents the gB ELISA antibody titer on a log10 scale. The x axis indicates the time point (months) after initial immunization.
Similar titers of gB-specific antibody were produced by gB680-MVA immunization in BALB/c and C57BL/6J mice.
To test if haplotype plays a role in the humoral response to the gB680 MVA vaccine, groups of BALB/c (H-2d) and C57BL/6J (H-2b) mice were twice immunized with 5 × 107 PFU of gB680-MVA via i.m. and s.c. routes. Sera were collected at 3 and 6 weeks after initial immunization, and gB-specific serum IgG antibodies were measured (Table 1). Similar gB-specific antibody levels were produced in both BALB/c and C57Bl/6J mice after both immunizations. There was no significant difference between strains at 3 weeks (P = 0.35), nor at 6 weeks (P = 0.67). Pooling strains, there was weak evidence for a slight difference between routes at 3 weeks (P = 0.053), but this minor difference did not persist at 6 weeks (P = 0.67). These results demonstrate that the robust antibody response is not specific to a single haplotype after immunization by either i.m. or s.c. routes.
TABLE 1.
gB-specific antibody titer of BALB/c and C57BL/6J mice after gB680-MVA immunizationa
| Route | Wk after initial immunization | GMT of gB antibody, BALB/c group (n = 4) | GMT of gB antibody, C57BL/6J group (n = 4) |
|---|---|---|---|
| s.c. | 3 | 2,828 (2,000-4,000) | 2,378 (2,000-4,000) |
| 6 | 11,314 (8,000-16,000) | 11,314 (8,000-16,000) | |
| i.m. | 3 | 4,000 (4,000-4,000) | 3,364 (2,000-4,000) |
| 6 | 11,314 (8,000-16,000) | 9,514 (8,000-16,000) |
gB-specific antibody titers, determined as the GMT for four immunized mice. gB-specific antibody titer ranges are given in parentheses.
Durable HCMV NAb produced by gB680-MVA immunization.
CMV neutralization induced by antibody responses to gB680-MVA were analyzed by performing a microneutralization assay to measure NAb titers (Fig. 5). It has been demonstrated that the CMV microneutralization assay is comparable with a conventional 14-day CMV plaque reduction assay (4). Serum samples from 6-week and 6-month time points were used in this assay to measure titers of CMV NAb. Average titers of CMV NAb at the 6-week time point are above 1:400 and remain above 1:300 at the 6-month time point. The i.m. and s.c. routes were not significantly different at 6 weeks (P = 0.98), nor at 6 months (P = 0.38). These levels exceed the titer of CMV NAb found after immunization with gB protein or the native response to CMV in seropositive healthy adults (29, 48, 51). Pooling data from both routes, there was a small but discernible drop in titers from 6 weeks to 6 months (P = 0.017). The titer of CMV NAb is reduced only within a twofold range between 6 weeks and 6 months, demonstrating that gB680-MVA immunization induces CMV NAb that are durable for at least 6 months.
FIG. 5.
Titer of HCMV NAb 6 weeks and 6 months after initial immunization. Ten serum samples from the 6-week (short-term) and 6-month (long-term) immunizations were used in the CMV microneutralization assay. Sera from preimmunized mice were used as negative controls. The CMV NT is expressed as the reciprocal of the highest dilution of mouse sera that inhibits 50% of virus input compared to the control. Filled circles represent NT for each individual mouse. Horizontal bars in the graph represent geometric means of the NT. The i.m. and s.c. routes were examined.
gB680-MVA-immunized-mouse sera neutralizes CMV strains with different gB genotypes.
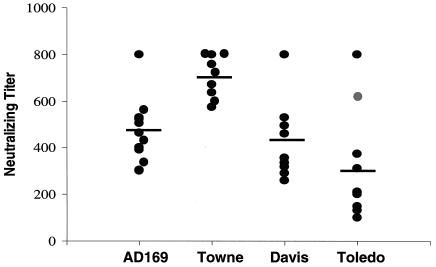
We evaluated the ability of gB680-MVA to induce antibodies that neutralize heterologous CMV strains, a property that is likely to be crucial to an efficacious CMV vaccine (5, 25, 61). To test whether sera from gB680-MVA-immunized mice were able to neutralize heterologous CMV strains, four different CMV strains were used in microneutralization assays as described in Materials and Methods. CMV AD169 and Towne strains represent different gB genotypes and also have sequence variations in other virion structural genes, including envelope glycoprotein genes (21). Davis and Toledo are low-passage strains that are similar to clinically circulating CMV isolates. Serum samples from all 10 gB680-MVA-immunized mice were found to effectively neutralize all four CMV strains (Fig. 6). There was no significant difference between strains at 3 weeks (P = 0.35), nor at 6 weeks (P = 0.67). These data suggest that gB680-MVA is able to induce broadly cross-reactive gB-specific antibodies to neutralize a wide variety of CMV strains.
FIG. 6.
Sera from gB680-MVA-immunized mice neutralized different CMV strains. The AD169, Towne, Davis, and Toledo CMV strains with different gB genotypes were used in CMV microneutralization assays. Filled circles represent NT for each mouse. Horizontal bars in the graph represent geometric means of the NT from 10 individual mice at the 6-week time point.
gB-specific IgG2as are predominant antibodies induced by gB680-MVA immunization.
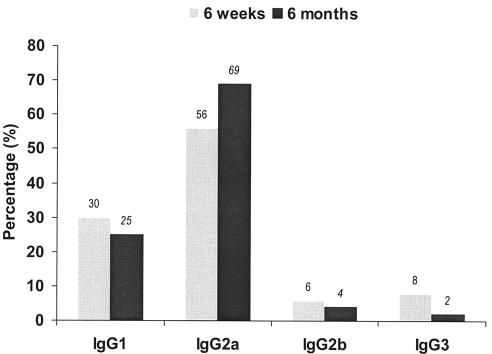
gB-specific IgG1 is the major subtype induced in response to natural HCMV infection (62). Human IgG1 is the functional analogue of murine IgG2a. In mice immunized with purified gB protein, levels of gB-specific IgG2a antibody are significantly correlated with CMV NAb (13). Earlier studies showed that gB-specific IgG2a is less abundant than IgG1 in mice immunized with DNA plasmid expressing the soluble form of gB (27). To measure gB-specific IgG subclass distribution produced by gB680-MVA immunization, an ELISA was set up to determine the presence of HCMV gB-specific IgG1, IgG2a, IgG2b, and IgG3 from sera samples collected at 6 weeks and 6 months (Fig. 7). In contrast to the earlier results with DNA vaccines, gB-specific IgG2a is abundant and accounted for more than 50% of gB-specific IgG at both 6 weeks and 6 months after initial immunization (27). The combination of IgG1 and IgG2a accounted for about 90% of total IgG produced at 6 weeks and 6 months after initial gB680-MVA immunization. No significant change of the IgG subclass percentage was found between 6 weeks and 6 months after gB680-MVA immunization. The high levels of these antibodies stimulated by gB680-MVA may be indicative of the robust level of CMV neutralization that we found.
FIG. 7.
IgG subclass distribution of gB-specific antibodies after immunization. Serum samples from 6 weeks and 6 months after gB680-MVA immunization were used for an isotype-specific ELISA assay. Purified S-gB680 was used as the coating antigen. Biotinylated anti-mouse IgG1, IgG2a, IgG2b, and IgG3 were used as secondary antibodies. The percentage of gB-specific IgG subclasses was calculated as the ratio of the individual subclass OD divided by the total OD of all subclasses.
Proliferative responses to purified S-gB680 protein induced by gB680-MVA immunization.
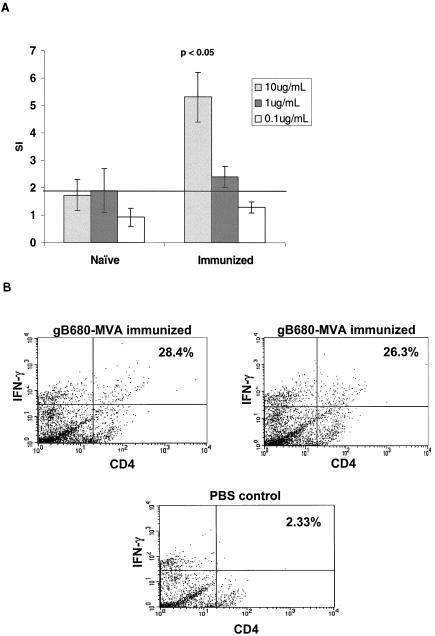
We examined whether gB680-MVA immunization stimulates T-cell proliferation in response to gB antigen. Freshly prepared spleen cells were collected 6 weeks after gB680-MVA immunization as described in Materials and Methods and exposed to purified S-gB680 antigen for 4 days. The magnitude of the T-cell proliferation was expressed as a stimulation index (SI) as described in Materials and Methods. Each of 10 mice after gB680-MVA immunization developed an SI value greater than 4 towards purified S-gB680 antigen (10 μg/ml). The average SI value for the gB680-MVA immunized group in the T-cell proliferation assay was significantly higher than that for the unimmunized group (P < 0.05) (Fig. 8A). Proliferative responses to purified gB680 protein were also measured in a group of mice immunized for 6 months (data not shown). The SI values exceeded those for mice evaluated after 6 weeks by 2.5-fold (data not shown), which agree with the continued robust IgG and NAb titers measured at 6 months (Fig. 4 and 5). ICC was used to measure IFN-γ as being indicative of a CD4+ TH response to S-gB680 recall antigens after immunization with gB680-MVA. Examination of splenocytes stimulated with S-gB680-pulsed blasts from immunized mice showed ∼27.2% average CD4+ IFN-γ cells in two mice compared to 2.35% for one representative PBS-injected control mouse (Fig. 8B). The CD4+-T-cell recall response to purified gB antigen remained detectable for up to 6 months after the initial gB680-MVA immunization (data not shown).
FIG. 8.
T-cell proliferation after gB680-MVA immunization. (A) Ten BALB/c mice were immunized with 5 × 107 PFU of gB680-MVA via the s.c. route, followed with a booster immunization with the same dosage by the same route. Fresh spleen cells were harvested 6 weeks after the initial immunization and incubated with purified S-gB680 at the concentrations indicated. The SI is defined as a ratio of [3H]thymidine incorporated into cells in the presence of purified S-gB680 antigen over mock antigen (MRC-5 cell lysate). Each bar represents the average SI of 10 immunized mice. (B) ICC of IFN-γ expressed in CD4+ T-cells after IVS in the presence of S-gB680 protein in immunized mice. Representative plots are shown for each group. Numbers reflect the percentages of CD4+ T cells.
gB-specific CTL responses in gB680-MVA-immunized mice.
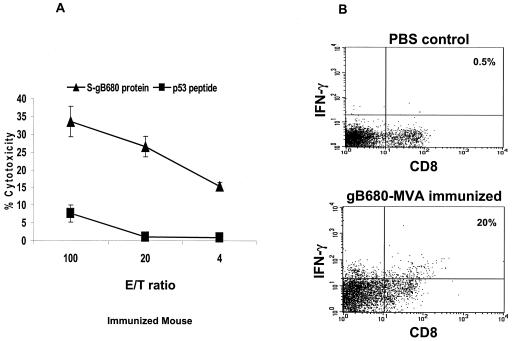
Earlier studies showed that gB-specific CTL can be detected in the peripheral blood of CMV-positive individuals, although they were found in one study to be major histocompatibility complex (MHC) class II restricted (35), or were found only very late after infection at low levels (10, 52). gB-ALVAC in vitro restimulation detects MHC class I-restricted CTL responses in a minority (33% compared to 92% for pp65) of normal CMV-seropositive donors (32). Previous animal studies showed that adenovirus expressing CMV gB elicited weak gB-specific CTL responses in BALB/c mice (9), while ALVAC-gB caused substantial cytotoxic responses in CBA mice only if repeatedly administered or given at high concentrations (1 × 108 to 2 × 108 PFU) (31). However, MVA is a potent inducer of CTL responses and may enhance the ability of gB to induce cytotoxic responses (49). We examined cytotoxic T-cell responses against S-gB680 in BALB/c mice immunized with gB680-MVA as described in Materials and Methods. LPS-stimulated syngeneic splenocytes were cocultured with 5 μg of S-gB680/ml for 8 h, presumably sufficient time for class I MHC recognition. They were then irradiated and cocultured with splenocytes from immunized or naive mice for 1 week. Upon one round of IVS, all three immunized mice showed a significant CTL response to S-gB680 protein-sensitized targets in a standard CRA assay (Fig. 9A). We also carried out ICC assays of IFN-γ expressed in CD8 T cells after IVS in the presence of the gB680 protein. The high frequency of CD8 T cells that are IFN-γ positive substantially agrees with cytotoxicity data shown in Fig. 9A. After one IVS, splenocytes from a gB680-MVA-immunized mouse contained 20% (x (mean) = 16.5%; n = 3) CD8+ IFN-γ+ T cells compared with 0.5% for a representative control mouse injected with PBS (Fig. 9B).
FIG. 9.
Induction of gB-specific CTL immune responses after immunization. Three BALB/c mice were immunized with gB680-MVA, and followed by booster immunization 3 weeks later. Spleens were removed 3 weeks after the booster. (a) An IVS was performed and was followed by a CRA as described in Materials and Methods. Purified S-gB680 protein (▴ symbol) and p53149-157 peptide (▪ symbol) sensitized splenocytes were used as targets. Means and SE were calculated at each effector-target (E/T) ratio for all evaluated mice. (b) ICC of IFN-γ expressed in CD8+ T-cells after IVS in the presence of S-gB680 protein in immunized or control PBS injected mice. One representative plot is shown for each group. Numbers reflect the percentages of CD8+ T cells.
gB680-MVA immunization of mice with preexisting MVA immunity or VV immunity.
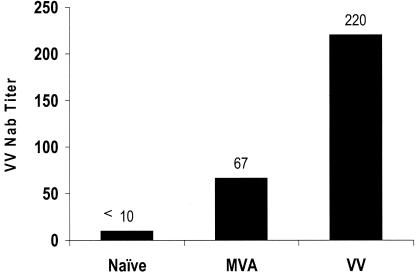
We evaluated the extent of interference due to host immune response from prior MVA or VV administration to future doses of gB680-MVA. Three groups of BALB/c mice were used in this study. One group consisted of naive mice with no prior MVA or VV immunity. A second group was immunized with wt MVA via i.p. injection to generate systemic MVA immunity. The third group was immunized with wt VV by i.p. injection to generate systemic VV immunity. Vector immunity generated by MVA and VV immunizations was determined by performing a VV neutralization assay (see Materials and Methods). As expected, the group of mice without MVA immunization had less than a 1:10 VV NT (Fig. 10). The groups of mice with MVA or VV immunization had an average VV NT of 1:67 and 1:220, respectively. MVA or VV immunity was successfully generated in every mouse by wt MVA or VV immunization (Fig. 10).
FIG. 10.
Preexisting MVA and VV immunity. Mice were immunized twice with 5 × 107 PFU of MVA or 1 × 106 PFU of VV once by i.p. injection. Serum samples were collected 6 weeks after initial inoculation for measurement of MVA or VV immunity. MVA or VV immunity was measured by performing VV plaque reduction assays as described in Materials and Methods. VV NT is the highest dilution of serum producing at least 85% inhibition of the VV input. Bars represent the geometric mean of VV NT for eight individual mice.
Three groups of mice as described above, with and without prior MVA or VV immunity, were further immunized with gB680-MVA, followed by a booster immunization. Sera were collected 3 and 6 weeks after initial gB680-MVA immunization. Table 2 shows that gB-specific antibody levels after one or two doses of gB680-MVA were very similar in mice with and without preexisting MVA immunity. Comparing mice with prior MVA immunity to controls, there was no significant difference at 3 weeks (P = 0.66) or at 6 weeks (P = 0.85). Similarly, for the comparison of mice with prior VV immunity to controls, there was no significant difference at 3 weeks (P = 0.29) or at 6 weeks (P = 0.38). The fact that similar titers of gB-specific antibody were produced with gB680-MVA immunization in both the presence and absence of preexisting MVA or VV immunity suggests that preexisting MVA immunity does not interfere with immune responses in subsequent immunizations with gB680-MVA. In comparing gB antibody levels of the primary and booster responses within the same groups of mice (see Table 2), it can be seen that the gB-specific antibody level was enhanced three- to fourfold by booster immunization in both groups. These results suggest that levels of gB-specific antibody can be enhanced with similar efficacy by booster immunization in the presence or absence of preexisting MVA immunity.
TABLE 2.
Effects of preexisting MVA and VV immunity on gB-specific antibody titer induced by gB680-rMVA immunization (P > 0.05)a
| Route | Wk after initial immunization | Control | With preexisting MVA immunity | With preexisting VV immunity |
|---|---|---|---|---|
| s.c. | 3 | 3,360 (2,000-4,000) | 2,540 (1,600-6,400) | 3,031 (1,000-8,000) |
| 6 | 11,314 (8,000-16,000) | 8,000 (4,000-16,000) | 9,190 (8,000-16,000) | |
| i.m. | 3 | 4,000 (4,000-4,000) | 1,903 (1,600-3,200) | 1,516 (1,000-2,000) |
| 6 | 11,314 (8,000-16,000) | 9,514 (4,000-16,000) | 12,126 (8,000-16,000) |
The GMT of gB-specific antibody titers are averages for four immunized mice in both the MVA and the VV immunity groups. gB-specific antibody titer ranges are given in parentheses.
DISCUSSION
The use of MVA as a live viral vector for vaccine development has been investigated in both experimental models and human clinical trials. The choice of MVA as a live viral vector to deliver CMV gB antigen was not solely based on its demonstrated safety in clinical trials but also on its capacity to induce strong humoral and cellular responses. It was anticipated that these properties would lead to the development of durable CMV-specific immune responses, including a boostable memory response against CMV. Our findings in this study have been consistent with these expectations for MVA as a vector to deliver CMV gB. In this study, we have successfully generated a recombinant MVA expressing S-gB680 protein regulated by the mH5 promoter with both early and late promoter function (gB680-MVA). In contrast, stable recombinant MVA viruses could not be recovered when full-length CMV gB was expressed under the control of promoters of various strengths. We found that gB680-MVA produced high levels of both intracellular and extracellular S-gB680 protein in mammalian cells, and gB680-MVA expressing gB680 under control of the mH5 promoter was stable and replicated to high titers in CEF cells. We found that gB680-MVA produced a broad range of immune responses, including gB-specific antibody, T-cell proliferation, and CTL responses, in immunized mice.
The average titer of CMV NAb induced by gB680-MVA immunization is similar to the titer of NAb detected following community-acquired CMV virus infection in humans (51). The NAb titer remained within a twofold range over the 6-month observation period. Consistent with production of CMV NAb, T-cell proliferation responses to purified S-gB680 antigen were also induced by gB680-MVA immunization and were sustained for 6 months. Both gB-specific CD8+ and CD4+ T cells were found in gB680-MVA-immunized mice. These results demonstrate that gB680-MVA vaccine can not only induce strong NAb responses but also produce long-term antigen-specific immunity.
Immunization strategies for evaluating recombinant MVA vaccines have been well established (49, 60, 65). Therefore, we followed published methods to evaluate immunogenicity of gB680-MVA by immunizing mice with one dose (5 × 107 PFU) of gB680-MVA by two routes (i.m. or s.c.). The results obtained in this study showed that i.m. inoculation of gB680-MVA is as effective as s.c. inoculation, and there is no significant difference in titer of gB-specific antibody, titer of neutralizing antibody, and IgG subclass distribution using either immunization route. The nature of the anti-CMV antibody response produced in immunized mice suggested that delivery of recombinant CMV gB by MVA was optimal for the induction of protective antibodies. First, significant titers of virus-specific NAb were produced even after primary immunization, and importantly, these antibodies were broadly reactive with unrelated strains of CMV. The latter consideration could be of considerable importance for vaccine protection against genetically unrelated strains of CMV because of the recent findings with several animal model systems and with humans that have demonstrated reinfection of seroimmune hosts (11, 12). Second, the induction of virus-specific IgG2a subclass antibodies likely represents an optimal humoral response. This subclass accounts for most of the documented biological effector functions of murine IgG antibodies, particularly those functions that are responsible for virus neutralization and recognition of virus-infected cells (22, 67).
Most importantly, the IgG anti-CMV antibody response induced by gB680-MVA immunization was boostable. This finding suggests that immunization of human hosts with this or similar vaccines would induce a durable immune response that could be boosted by natural exposure to CMV. Boosting gB680-MVA immunity following natural exposure with CMV would ensure maintenance of significant titers of virus-specific NAb in vaccine recipients. Although we are aware of the limitations of studies of potential human vaccines that are carried out with mice, our findings argue that gB680-MVA induces a robust immune response that exhibits all the characteristics associated with the generation of protective antibody responses in this species.
To determine the effects of booster immunization with gB680-MVA, gB-specific antibodies were measured in mice after each dose of gB680-MVA. High levels of gB-specific antibodies were produced after administration of a single dose of virus. The second administration of gB680-MVA after 3 weeks generated significantly higher gB-specific antibodies than the primary immunization. Interestingly, gB680-MVA immunization could stimulate high levels of gB-specific antibodies within 3 to 6 weeks with only two doses of the virus. Induction of such responses shortly after immunization raises the possibility that a simplified vaccine schedule could induce protective antibody responses without the requirement for adjuvants or repeated immunizations. The third dose of gB680-MVA produced an impressive booster effect that was measured at 7 months (Fig. 4), which has been sustained for an additional 5 months with minimal decay to 6-month levels (data not shown). In addition, data in this study showed that as a vaccine, gB680-MVA could be boosted multiple times. Recent measurements of titers of NAb at the 7-month time point (data not shown) showed an impressive response to the third booster, almost twice the levels (GMT of 800) found at 6 weeks (Fig. 5). This is due not only to a high expression level of the gB680 protein and strong immunogenicity of the gB680 protein itself but also to low levels of anti-MVA circulating antibodies in MVA-immunized mice shown here and in previous reports (50).
Since CMV strains are severely host restricted, there is no animal protection model available to test the protective value of a human CMV vaccine (15, 43). Therefore, it is difficult to translate the impressive levels of immunity induced by gB680-MVA immunization in mice into levels of protection against HCMV. A surrogate challenge animal model using an HCMV-gB-murine CMV chimera might be a valuable tool not only in the design of a gB680-MVA vaccine regime but also in optimizing gB680-MVA vaccination strategies, such as doses, routes, and timing of prime-boost administrations. In fact, the surrogate challenge animal model using recombinant VV has been used in hepatitis C vaccine development (6, 45, 47). However, the life cycle and tissue tropism of poxviruses are far different from those of CMV and other herpesviruses, so the utility of developing such an approach is minimal. Since gB is crucial to CMV infectivity, creating a chimeric strain may require manipulation of murine CMV gB to accommodate the human analogue (46). An alternative approach is to facilitate HCMV infection of a modified mouse, perhaps with the recently described erbB receptor genes (63).
gB680-MVA is derived from a poxvirus, raising the concern that MVA-based vaccines may be of limited value in human populations with preexisting poxvirus immunity that extends to MVA (30). We have investigated the impact of previous administration of MVA or VV on effects of subsequent doses of gB680-MVA. We have found that gB680-MVA immunization produced very similar levels of gB-specific antibody response and had similar booster efficacy regardless of preexisting MVA or VV immunity. These results suggest that preexisting MVA or VV immunity has little or no effect on subsequent immunization with gB680-MVA. Recent measurements of T-cell proliferative responses at 6 months showed a continuous and vigorous recognition of the insert antigen, gB680, that exceeded the levels measured at 6 weeks (data not shown). The impact of preexisting MVA immunity may have little or no effect on gB680-MVA immunization, because MVA is viral replication deficient and cannot spread in mammalian cells, and it is less immunogenic than replication-competent viral vectors, such as VV (Fig. 10). However, preexisting VV immunity is generally very strong; both strong titers of VV NAb and anti-VV CD8+ T cells could reduce and block secondary specific immune responses to recombinant protein antigens by limiting the extent of virus vaccine infection of target immune cells, such as antigen-presenting cells. In fact, previous reports indicated that preexisting VV vector immunity indeed has such an impact on MVA-based vaccine immunization (8, 50, 66). Remarkably, and in contrast to previous reports, preexisting VV immunity had little impact on the gB680-MVA immunization that followed. The most likely explanation is that gB680 is highly expressed in infected cells, and gB680 itself is highly immunogenic. A strong immune response to gB antigen delivered by MVA overcomes the impact of preexisting VV immunity.
In summary, we have demonstrated durability and effectiveness of CMV NAb induced by gB680-MVA and its ability to overcome the impact of preexisting MVA or VV immunity on subsequent gB680-MVA immunization in mice. A vigorous T-help response throughout the period of immunity evaluation further suggests that the MVA vaccine approach induces a comprehensive stimulation to immunity that could benefit a vaccine recipient by a combined NAb and T-cell response to viral infection. The safety characteristics of MVA, combined with the robust immune response to CMV gB, suggest that this approach is effective and could be rapidly translated into the clinic.
Acknowledgments
We thank Bernard Moss and Linda S. Wyatt (LVD, NIAID, NIH) for providing wt MVA and the pLW51 insertion plasmid. John Zaia and Ghislaine Hawkins (City of Hope) are acknowledged for the HCMV Davis and Toledo strains. Tom Lebon and Donna Packer (City of Hope) are gratefully acknowledged for editorial assistance and preparation of the manuscript, respectively. The assistance of Julia Santos in the administration of the laboratory has greatly aided the completion of this work.
These studies have been partially supported by grants from the NCI (RO1-CA77544 and PO1-CA30206, project III) and UARP (ID02-BRI-054) to D.J.D. The City of Hope Comprehensive Cancer Center is supported by the NCI (CA33572). D.J.D is partially supported by a Translational Research Award from the LLS and the LVR is partially supported by The Edwin and Bea Wolfe Charitable Foundation. W.J.B. is partially supported by R01 AI49537.
REFERENCES
- 1.Adler, S. P., S. H. Hempfling, S. E. Starr, S. A. Plotkin, and S. Riddell. 1998. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr. Infect. Dis. J. 17:200-206. [DOI] [PubMed] [Google Scholar]
- 2.Adler, S. P., S. A. Plotkin, E. Gonczol, M. Cadoz, C. Meric, J. B. Wang, P. Dellamonica, A. M. Best, J. Zahradnik, S. Pincus, K. Berencsi, W. I. Cox, and Z. Gyulai. 1999. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J. Infect. Dis. 180:843-846. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, et al. 2001. Control of a mucosal challenge and preventions of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 5.Aquino, V. H., and L. T. Figueiredo. 2000. High prevalence of renal transplant recipients infected with more than one cytomegalovirus glycoprotein B genotype. J. Med. Virol. 61:138-142. [PubMed] [Google Scholar]
- 6.Arribillaga, L., A. L. De Cerio, P. Sarobe, N. Casares, M. Gorraiz, A. Vales, O. Bruna-Romero, F. Borras-Cuesta, G. Paranhos-Baccala, J. Prieto, J. Ruiz, and J. J. Lasarte. 2002. Vaccination with an adenoviral vector encoding hepatitis C virus (HCV) NS3 protein protects against infection with HCV-recombinant vaccinia virus. Vaccine 21:202-210. [DOI] [PubMed] [Google Scholar]
- 7.Banks, T., B. Huo, K. Kousoulas, R. Spaete, C. Pachl, and L. Pereira. 1989. A major neutralizing domain maps within the carboxyl-terminal half of the cleaved cytomegalovirus B glycoprotein. J. Gen. Virol. 70:979-985. [DOI] [PubMed] [Google Scholar]
- 8.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by preexisting poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berencsi, K., R. F. Rando, C. deTaisne, E. Paoletti, S. A. Plotkin, and E. Gonczol. 1993. Murine cytotoxic T cell response specific for human cytomegalovirus glycoprotein B (gB) induced by adenovirus and vaccinia virus recombinants expressing gB. J. Gen. Virol. 74:2507-2512. [DOI] [PubMed] [Google Scholar]
- 10.Boppana, S. B., and W. J. Britt. 1996. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology 222:293-296. [DOI] [PubMed] [Google Scholar]
- 11.Boppana, S. B., K. B. Fowler, W. J. Britt, S. Stagno, and R. F. Pass. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55-60. [DOI] [PubMed] [Google Scholar]
- 12.Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. [DOI] [PubMed] [Google Scholar]
- 13.Britt, W., J. Fay, J. Seals, and C. Kensil. 1995. Formulation of an immunogenic human cytomegalovirus vaccine: responses in mice. J. Infect. Dis. 171:18-25. [DOI] [PubMed] [Google Scholar]
- 14.Britt, W. J. 1984. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology 135:369-378. [DOI] [PubMed] [Google Scholar]
- 15.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 16.Britt, W. J., L. Vugler, E. J. Butfiloski, and E. B. Stephens. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 64:1079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britt, W. J., and L. G. Vugler. 1989. Processing of the gp55-116 envelope glycoprotein complex (gB) of human cytomegalovirus. J. Virol. 63:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson, C., W. J. Britt, and T. Compton. 1997. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology 239:198-205. [DOI] [PubMed] [Google Scholar]
- 19.Carroll, M. W., and B. Moss. 1995. E. coli beta-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19:352-354, 356. [PubMed] [Google Scholar]
- 20.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou, S. W., and K. M. Dennison. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J. Infect. Dis. 163:1229-1234. [DOI] [PubMed] [Google Scholar]
- 22.Coutelier, J. P., J. T. van der Logt, F. W. Heessen, G. Warnier, and J. Van Snick. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degano, P., J. Schneider, C. M. Hannan, S. C. Gilbert, and A. V. Hill. 1999. Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: enhanced CD8+ T cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine 18:623-632. [DOI] [PubMed] [Google Scholar]
- 25.Drew, W. L., S. Chou, R. C. Miner, B. A. Mohr, M. P. Busch, C. M. van der Horst, D. M. Asmuth, and L. A. Kalish. 2002. Cytomegalovirus glycoprotein B groups in human immunodeficiency virus-infected patients with incident retinitis. J. Infect. Dis. 186:114-117. [DOI] [PubMed] [Google Scholar]
- 26.Endresz, V., K. Burian, K. Berencsi, Z. Gyulai, L. Kari, H. Horton, D. Virok, C. Meric, S. A. Plotkin, and E. Gonczol. 2001. Optimization of DNA immunization against human cytomegalovirus. Vaccine 19:3972-3980. [DOI] [PubMed] [Google Scholar]
- 27.Endresz, V., L. Kari, K. Berencsi, C. Kari, Z. Gyulai, C. Jeney, S. Pincus, U. Rodeck, C. Meric, S. A. Plotkin, and E. Gonczol. 1999. Induction of human cytomegalovirus (HCMV)-glycoprotein B (gB)-specific neutralizing antibody and phosphoprotein 65 (pp65)-specific cytotoxic T lymphocyte responses by naked DNA immunization. Vaccine 17:50-58. [DOI] [PubMed] [Google Scholar]
- 28.Espenschied, J., J. Lamont, J. Longmate, S. Pendas, Z. Wang, D. J. Diamond, and J. D. Ellenhorn. 2003. CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J. Immunol. 170:3401-3407. [DOI] [PubMed] [Google Scholar]
- 29.Frey, S. E., C. Harrison, R. F. Pass, E. Yang, D. Boken, R. E. Sekulovich, S. Percell, A. E. Izu, S. Hirabayashi, R. L. Burke, and A. M. Duliege. 1999. Effects of antigen dose and immunization regimens on antibody responses to a cytomegalovirus glycoprotein B subunit vaccine. J. Infect. Dis. 180:1700-1703. [DOI] [PubMed] [Google Scholar]
- 30.Frey, S. E., F. K. Newman, L. Yan, K. R. Lottenbach, and R. B. Belshe. 2003. Response to smallpox vaccine in persons immunized in the distant past. JAMA 289:3295-3299. [DOI] [PubMed] [Google Scholar]
- 31.Gonczol, E., K. Berensci, S. Pincus, V. Endresz, C. Meric, E. Paoletti, and S. A. Plotkin. 1995. Preclinical evaluation of an ALVAC (canarypox)-human cytomegalovirus glycoprotein B vaccine candidate. Vaccine 13:1080-1085. [DOI] [PubMed] [Google Scholar]
- 32.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meri, S. Plotkin, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-Exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 33.Hanke, T., A. J. McMichael, M. Mwau, E. G. Wee, I. Ceberej, S. Patel, J. Sutton, M. Tomlinson, and R. V. Samuel. 2002. Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine 20:1995-1998. [DOI] [PubMed] [Google Scholar]
- 34.Harlow, E., and D. Lane. 1988. Immunoaffinity purification, p. 519-551. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Hopkins, J. I., A. N. Fiander, A. S. Evans, M. Delchambre, D. Gheysen, and L. K. Borysiewicz. 1996. Cytotoxic T cell immunity to human cytomegalovirus glycoprotein B. J. Med. Virol. 49:124-131. [DOI] [PubMed] [Google Scholar]
- 36.La Rosa, C., Z. Wang, J. C. Brewer, S. F. Lacey, M. C. Villacres, R. Sharan, R. Krishnan, M. Crooks, S. Markel, R. Maas, and D. J. Diamond. 2002. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood 100:3681-3689. [DOI] [PubMed] [Google Scholar]
- 37.Lopper, M., and T. Compton. 2002. Disulfide bond configuration of human cytomegalovirus glycoprotein B. J. Virol. 76:6073-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall, G. S., M. Li, G. G. Stout, M. V. Louthan, A. M. Duliege, R. L. Burke, and L. A. Hunt. 2000. Antibodies to the major linear neutralizing domains of cytomegalovirus glycoprotein B among natural seropositives and CMV subunit vaccine recipients. Viral Immunol. 13:329-341. [DOI] [PubMed] [Google Scholar]
- 39.Marshall, G. S., G. P. Rabalais, G. G. Stout, and S. L. Waldeyer. 1992. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis. 165:381-384. [DOI] [PubMed] [Google Scholar]
- 40.Marshall, G. S., R. P. Ricciardi, R. F. Rando, J. Puck, R. W. Ge, S. A. Plotkin, and E. Gonczol. 1990. An adenovirus recombinant that expresses the human cytomegalovirus major envelope glycoprotein and induces neutralizing antibodies. J. Infect. Dis. 162:1177-1181. [DOI] [PubMed] [Google Scholar]
- 41.Mayr, A., H. Stickl, H. K. Muller, K. Danner, and H. Singer. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's translation). Zentbl. Bakteriol. B 167:375-390. (In German.) [PubMed] [Google Scholar]
- 42.McConkey, S. J., W. H. Reece, V. S. Moorthy, D. Webster, S. Dunachie, G. Butcher, J. M. Vuola, T. J. Blanchard, P. Gothard, K. Watkins, C. M. Hannan, S. Everaere, K. Brown, K. E. Kester, J. Cummings, J. Williams, D. G. Heppner, A. Pathan, K. Flanagan, N. Arulanantham, M. T. Roberts, M. Roy, G. L. Smith, J. Schneider, T. Peto, R. E. Sinden, S. C. Gilbert, and A. V. Hill. 2003. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9:729-735. [DOI] [PubMed] [Google Scholar]
- 43.Mocarski, E. S. 1996. Cytomegalovirus and their replication, p. 2447-2492. In B. N. Fields, D. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 44.Moss, B., and P. L. Earl. 1998. Expression of proteins in mammalian cells using vaccinia virus vectors, p. 16.15.1-16.21.9. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.
- 45.Murata, K., M. Lechmann, M. Qiao, T. Gunji, H. J. Alter, and T. J. Liang. 2003. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc. Natl. Acad. Sci. USA 100:6753-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarro, D., P. Paz, S. Tugizov, K. Topp, J. La Vail, and L. Pereira. 1993. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology 197:143-158. [DOI] [PubMed] [Google Scholar]
- 47.Pancholi, P., M. Perkus, N. Tricoche, Q. Liu, and A. M. Prince. 2003. DNA immunization with hepatitis C virus (HCV) polycistronic genes or immunization by HCV DNA priming-recombinant canarypox virus boosting induces immune responses and protection from recombinant HCV-vaccinia virus infection in HLA-A2.1-transgenic mice. J. Virol. 77:382-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pass, R. F., A. M. Duliege, S. Boppana, R. Sekulovich, S. Percell, W. Britt, and R. L. Burke. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970-975. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez, J. C., M. M. Gherardi, and M. Esteban. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramirez, J. C., M. M. Gherardi, D. Rodriguez, and M. Esteban. 2000. Attenuated modified vaccinia virus Ankara can be used as an immunizing agent under conditions of preexisting immunity to the vector. J. Virol. 74:7651-7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen, L., C. Matkin, R. Spaete, C. Pachl, and T. C. Merigan. 1991. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infection in humans. J. Infect. Dis. 164:835-842. [DOI] [PubMed] [Google Scholar]
- 52.Riddell, S. R., M. Rabin, A. P. Geballe, W. J. Britt, and P. D. Greenberg. 1991. Class I MHC-restricted cytotoxic T lymphocyte recognition of cells infected with human cytomegalovirus does not require endogenous viral gene expression. J. Immunol. 146:2795-2804. [PubMed] [Google Scholar]
- 53.Rothe, M., S. Pepperl-Klindworth, D. Lang, R. Vornhagen, W. Hinderer, K. Weise, H. H. Sonneborn, and B. Plachter. 2001. An antigen fragment encompassing the AD2 domains of glycoprotein B from two different strains is sufficient for differentiation of primary vs. recurrent human cytomegalovirus infection by ELISA. J. Med. Virol. 65:719-729. [DOI] [PubMed] [Google Scholar]
- 54.Spaete, R. R. 1991. A recombinant subunit vaccine approach to HCMV vaccine development. Transpl. Proc. 23:90-96. [PubMed] [Google Scholar]
- 55.Spaete, R. R., R. M. Thayer, W. S. Probert, F. R. Masiarz, S. H. Chamberlain, L. Rasmussen, T. C. Merigan, and C. Pachl. 1988. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 167:207-225. [DOI] [PubMed] [Google Scholar]
- 56.Stickl, H., V. Hochstein-Mintzel, A. Mayr, H. C. Huber, H. Schafer, and A. Holzner. 1974. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's translation). Dtsch. Med. Wochenschr. 99:2386-2392. (In German.) [DOI] [PubMed] [Google Scholar]
- 57.Stittelaar, K. J., T. Kuiken, R. L. de Swart, G. van Amerongen, H. W. Vos, H. G. Niesters, P. van Schalkwijk, T. van der Kwast, L. S. Wyatt, B. Moss, and A. D. Osterhaus. 2001. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine 19:3700-3709. [DOI] [PubMed] [Google Scholar]
- 58.Stratton, K. R., J. S. Durch, and R. S. Lawrence. 2001. Vaccines for the 21st century: a tool for decisionmaking. National Academy Press, Bethesda, Md. [PubMed]
- 59.Sutter, G., and B. Moss. 1992. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 89:10847-10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutter, G., L. S. Wyatt, P. L. Foley, J. R. Bennink, and B. Moss. 1994. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine 12:1032-1040. [DOI] [PubMed] [Google Scholar]
- 61.Torok-Storb, B., M. Boeckh, C. Hoy, W. Leisenring, D. Myerson, and T. Gooley. 1997. Association of specific cytomegalovirus genotypes with death from myelosuppression after marrow transplantation. Blood 90:2097-2102. [PubMed] [Google Scholar]
- 62.Urban, M., T. Winkler, M. P. Landini, W. Britt, and M. Mach. 1994. Epitope-specific distribution of IgG subclasses against antigenic domains on glycoproteins of human cytomegalovirus. J. Infect. Dis. 169:83-90. [DOI] [PubMed] [Google Scholar]
- 63.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 64.Wyatt, L. S., S. T. Shors, B. R. Murphy, and B. Moss. 1996. Development of a replication-deficient recombinant vaccinia virus vaccine effective against parainfluenza virus 3 infection in an animal model. Vaccine 14:1451-1458. [DOI] [PubMed] [Google Scholar]
- 65.Wyatt, L. S., S. S. Whitehead, K. A. Venanzi, B. R. Murphy, and B. Moss. 1999. Priming and boosting immunity to respiratory syncytial virus by recombinant replication-defective vaccinia virus MVA. Vaccine 18:392-397. [DOI] [PubMed] [Google Scholar]
- 66.Yang, Z. Y., L. S. Wyatt, W. P. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zinkernagel, R. M. 2002. On differences between immunity and immunological memory. Curr. Opin. Immunol. 14:523-536. [DOI] [PubMed] [Google Scholar]