Abstract
As a typical matricellular protein, thrombospondin (TSP)-1, binds to the structural matrix and regulates cellular behavior by modulating growth factor and cytokine signaling. Obesity and diabetes are associated with marked upregulation of TSP-1 in adipose tissue. We hypothesized that endogenous TSP-1 may play an important role in the pathogenesis of diet-induced obesity and metabolic dysfunction. Accordingly, we examined the effects of TSP-1 gene disruption on weight gain, adiposity, and adipose tissue inflammation in mice receiving a high-fat diet (HFD: 60% fat, 20% carbohydrate) or a high-carbohydrate low-fat diet (HCLFD: 10% fat, 70% carbohydrate). HFD mice had significantly higher TSP-1 expression in perigonadal adipose tissue; TSP-1 was predominantly localized in the adipose interstitium. TSP-1 loss attenuated weight gain and fat accumulation in HFD and HCLFD groups. Compared with corresponding wild-type animals, TSP-1-null mice had decreased insulin levels but exhibited elevated free fatty acid and triglyceride levels, suggesting impaired fatty acid uptake. TSP-1 loss did not affect adipocyte size and had no effect on adipose vascular density. However, TSP-1-null mice exhibited attenuated tumor necrosis factor-α mRNA expression and reduced macrophage infiltration, suggesting a role for TSP-1 in mediating obesity-associated inflammation. In vitro, TSP-1 enhanced proliferation of 3T3-L1 preadipocytes but did not modulate inflammatory cytokine and chemokine synthesis. In conclusion, TSP-1 upregulation contributes to weight gain, adipose growth, and the pathogenesis of metabolic dysfunction. The effects of TSP-1 may involve stimulation of adipocyte proliferation, activation of inflammatory signaling, and facilitated fatty acid uptake by adipocytes.
Keywords: matricellular proteins, adipocyte, obesity, inflammation, macrophage
tissue remodeling requires the active participation of the extracellular matrix. In injured and remodeling tissues, activated cells produce and secrete matricellular proteins, macromolecules that do not play a structural role but bind to the matrix and serve as molecular bridges that transduce growth factor-mediated signals and mediate cellular responses (2, 30). Thrombospondin (TSP)-1, a prototypical matricellular protein (1), is not expressed in most normal adult tissues but is markedly upregulated in injury, neoplasia, and repair (11, 21, 25). When bound to the matrix, TSP-1 modulates cellular phenotype either through direct integrin-mediated actions or via activation of growth factor cascades. Extensive in vitro and in vivo evidence suggests that TSP-1 is a crucial activator of transforming growth factor (TGF)-β (7), exerts potent angiostatic effects (20), and modulates inflammatory cascades through effects on monocytes/macrophages (8) and on T lymphocytes (14).
Obesity is associated with extensive remodeling of the adipose tissue. The marked expansion of fat requires a highly plastic and dynamic extracellular matrix to support and accommodate the growth (40). The role of the matrix in the adipose tissue is not limited to providing structural support. Alterations in the composition of the adipose extracellular matrix modulate phenotype and function of both adipocytes and nonadipocytes, facilitating transduction of cytokine and growth factor signals. Several matricellular proteins are upregulated in activated adipose tissue and may modulate weight gain and adipocyte function. Expression of osteonectin/secreted protein acidic and rich in cysteine (SPARC) is upregulated in both genetic and diet-induced models of obesity (41) and suppresses adipocyte morphogenesis by enhancing β-catenin signaling (3, 32). Members of the TSP family are also highly expressed in adipose tissue in both experimental rodent models and in obese human patients (46, 18, 37, 43, 44); however, their role in weight gain and metabolic dysfunction remains controversial. Studies in human patients demonstrated that TSP-1 expression in visceral adipose tissue was strongly associated with obesity, insulin resistance, and adipose inflammatory activity (44). In an experimental model of high-fat diet-induced obesity, TSP-1 loss did not affect weight gain but improved glucose tolerance and attenuated insulin resistance (28). In contrast, another recent study demonstrated that TSP-1 worsened glucose tolerance despite an increase in pancreatic islet mass (34).
To investigate the role of TSP-1 in weight gain and metabolic dysfunction in diet-induced obese mice we systematically compared the effects of two different diets, a high-fat diet (HFD) and a high-carbohydrate low-fat diet (HCFLD), on adiposity, metabolic function, adipose tissue morphology, inflammation, and fibrosis in TSP-1-null and WT mice. We found that TSP-1 loss significantly attenuated weight gain and adiposity while reducing metabolic dysfunction in diet-induced obese animals. TSP-1-null animals had reduced adipose tissue inflammation and attenuated TGF-β expression but exhibited comparable adipose vascular density. In vitro, TSP-1 increased proliferative activity in 3T3-L1 preadipocytes without affecting their inflammatory gene expression profile. Our findings suggest that TSP-1 upregulation in adipose tissue increases weight gain and accentuates adipose inflammatory activity; its effects may be mediated, at least in part, through enhanced adipocyte proliferation.
MATERIALS AND METHODS
Animal models of diet-induced obesity.
We used wild-type (WT) C57BL/6J mice and mice with a targeted disruption of TSP-1 (12, 27) in a C57BL/6J background. TSP-1-null mice (originally donated by Dr. Jack Lawler, Harvard Medical School) and WT animals were not littermates but were all derived from our own colonies and were bred and maintained under identical conditions to test the effects of distinct dietary interventions. TSP-1-null mice in a C57BL/6J background survive past 2 yr of age and do not develop a systemic illness (11, 19). All animal studies were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine and the Animal Protocol Review Committee at Baylor College of Medicine. Experimental feeding was initiated at 1 mo of age. WT and TSP-1-null (−/−) mice were randomized to receive ad libitum a high-carbohydrate low-fat diet (HCLFD, D12450B, OpenSource Diets; 20% protein, 70% carbohydrate, 10% fat) or a high-fat diet (HFD, D12492, Open Source Diets; 20% protein, 20% carbohydrate, 60% fat[lard]) for 5 mo (female HCLFD: WT n = 21, −/− n = 9; male HCLFD: WT n = 19, −/− n = 8; female HFD: WT n = 24, −/− n = 8; male HFD: WT n = 15, −/− n = 5). At 6 mo of age, the animals were euthanized, and perigonadal adipose tissue was used for histological studies or RNA extraction. Subcutaneous adipose tissue was also harvested and was used for histological analysis.
Additional groups of WT C57BL/6J and TSP-1-null mice were fed a regular chow diet (Laboratory Rodent Diet 5001, LabDiets, St. Louis, MO; 28.5% protein, 58% carbohydrate, 13,5% fat) for 6 mo and underwent indirect calorimetry experiments to assess food intake and energy expenditure studies (n = 7 per group). Subcutaneous and perigonadal adipose tissues were harvested from 2-mo- and 6-mo-old WT and TSP-1-null mice fed a chow diet (n = 13) and were used for RNA extraction.
Assessment of adiposity.
Lean and fat tissue mass was measured in WT (HCLFD, n = 15; HFD, n = 15) and TSP-1−/− mice (HCLFD, n = 10; HFD, n = 19) at 2, 4, and 6 mo of age by dual-energy X-ray absorptiometry (DEXA) using the PIXImus Densitometer (Lunar, Madison, WI). Each mouse was anesthetized in a chamber for the entire procedure (5% isoflurane and 95% oxygen at 4 l/min). Once anesthetized, each mouse was placed in the densitometer in the prone position, with the limbs and tail extended. Data were analyzed with the PIXImus software (v. 2.0; GE/Lunar).
Assessment of metabolic parameters.
Animals used for assessment of metabolic parameters in serum were euthanized at 6 mo of age, and blood was collected by aortic puncture into EDTA anticoagulant-coated tubes. Plasma was extracted by centrifugation at 850 g for 15 min at 4°C and stored at −80°C. Mice were fasted for 12 h before collection of the blood. Serum leptin and insulin were measured using mouse enzyme-linked immunosorbent assay kits (from Minipore and Crystal Chem, respectively). Glucose levels were measured by the glucose oxidase method (GM7; Analox, London, UK). HOMA-IR was calculated as fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5. Total cholesterol was measured using a colorimetric enzymatic method (Wako Cholesterol E, Wako Pure Chemicals Industries) according to manufacturers' instructions. Triglycerides were quantified using Thermo DMA reagent (cat. no. TR22203; Thermo Electron, Middletown, VA). Nonesterified fatty acids were determined by enzymatic colorimetric assay [NEFA-HR (2) kit, Wako Diagnostics, Richmond, VA].
Indirect calorimetry.
Metabolic rate was assessed in 6-mo-old WT and TSP-1-null animals fed a chow diet (n = 7 per group) using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments, Columbus, OH). Data on O2 consumption (V̇o2) and CO2 production (V̇co2) were monitored and collected at the inlet and outlet of the sealed chambers collected every 5 min for 24 h. Feeding episodes were monitored with precision balances and were continuously recorded to calculate food intake. Energy expenditure was calculated as follows: energy expenditure = (3.815 +1.232 × respiratory quotient) × V̇o2.
Processing of adipose tissue, immunohistochemistry, and quantitative histology.
Subcutaneous and perigonadal adipose tissues from 6-mo-old animals were fixed in zinc formalin and embedded in paraffin, and 5-μm sections were cut. To quantitate adipocyte size, three sections from each sample stained with hematoxylin-eosin were used to measure the cross-sectional area of 50 adipocytes with Axiovision image analysis software. The mean adipocyte area was determined for each mouse. For quantitative analysis of macrophage density, Mac2 staining was used as previously described (5), and the ratio of macrophage to adipocyte numbers was measured. For quantitation of microvascular density, Griffonia simplicifolia-I lectin staining was used as previously described (36), and the number of microvascular profiles to adipocytes was counted. TSP-1 immunohistochemistry was performed using a mouse monoclonal antibody to TSP-1 (1:25, NeoMarkers) and the UltraVision LP Detection System (Thermo Scientific) as previously described (47). Ten mice from each group were used for quantitative histological analysis.
RNA extraction and qPCR.
Total RNA was extracted from perigonadal adipose tissue or from stimulated cells by use of a RNeasy Lipid Tissue Mini Kit (Qiagen). cDNA was amplified using the EvaGreen Supermix reagent and the C1000 thermal cycler apparatus from Bio-Rad following the manufacturer's recommendations. TSP-1, leptin, TNF-α, IL-1β, IL-6, monocyte chemoattractant protein (MCP)-1/CCL2, interferon-γ-inducible protein (IP)-10/CXCL10, TGF-β1, and collagen type Iα1 mRNA levels were assessed. Primer sequences are listed in Table 1. The housekeeping gene GAPDH was used as internal control. The qPCR procedure was repeated three times in independent runs; expression levels were calculated using the ΔΔCT method.
Table 1.
Primers used for qPCR analysis
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| TSP-1 | GCAGCACACACAGAAGCATT | CAATCAGCTCTCACCAGCAG |
| TNF-α | GATTATGGCTCAGGGTCCAA | CTCCCTTTGCAGAACTCAGG |
| IL-6 | TCCTTCCTACCCCAATTTCC | TGACCACAGTGAGGAATGTC |
| MCP-1 | CCCAATGAGTAGGCTGGAGA | TCTGGACCCATTCCTTCTTG |
| IL-1β | CAGGCAGGCAGTATCACTCA | AGCTCATATGGGTCCGACAG |
| CXCL10 | CAAGTGGCTGGGATGGCTG | GGTTCCTCTGAGTATCTTGA |
| TGF-β1 | AATCAAGTGTGGAGCAACATG | AGCCCTGTATTCCGTCTCCT |
| CD31 | AACAGAAACCCGTGGAGATG | GTCTCTGTGGCTCTCGTTCC |
| VE-cadherin | CCTGGTATAACCTGACTGTG | GGCACCACATCCTTGTCTGT |
| Leptin | GAGTGTACCAGGCACCCTTG | TGCAAACGTCTACCTGCTTAA |
| CD68 | ACTCATAACCCTGCCACCAC | GGGTATAGGATTCGGATTTG |
| GAPDH | AACGACCCCTTCATTGACCT | CACCAGTAGACTCCACGACA |
| 18S | TCAGATACCGTCGTAGTT | CTTTAAGTTTCAGCTTTGCA |
TSP-1, thrombospondin-1.
Stimulation of 3T3-L1 preadipocytes, proliferation studies and assessment of inflammatory gene expression.
3T3-L1 preadipocytes (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagles's Medium (DMEM) containing 10% fetal bovine serum (FBS) in a 37°C incubator with 5% CO2. Proliferation of 3T3-L1 preadipocytes was assessed using a colorimetric BrDU cell proliferation ELISA kit (Roche) following the manufacturer's instructions. Cells were seeded in 96-well plates, after 1-day serum starvation and then stimulated with 100 ng/ml leptin, 300 ng/ml leptin, 100 ng/ml TSP-1, 300 ng/ml TSP-1, 100 ng/ml leptin + 300 ng/ml TSP-1, or 300 ng/ml leptin + 300 ng/ml TSP-1 in DMEM, in the presence or absence of 1% FBS. Each condition was run in triplicate in three independent experiments. Cell proliferation measurements for each experiment were normalized to the mean proliferation of cells cultured without FBS.
For assessment of inflammatory gene expression, 3T3-L1 preadipocytes were grown 2 days after complete confluence, and then differentiation was induced by incubation in DMEM containing 10% FBS plus 0.5 mM isobutylmethylxanthine (IBMX), 1 mM dexamethasone, and 167 nM insulin. After 3 days of incubation, the cells were maintained in DMEM containing 10% FBS and 167 nM insulin for 3 days followed by culturing with DMEM plus 10% FBS for an additional 2 days, at which time more than 90% of cells were mature adipocytes with accumulated fat droplets. Mature adipocytes were serum-starved overnight and then stimulated with vehicle, TSP-1 (300, 600 ng/ml), leptin (100, 300 ng/ml), TNF-α (10 ng/ml), IL-1β (10 ng/ml), or TGF-β1 (10 ng/ml) for 4 h. Cells were then harvested and used to quantitate inflammatory gene expression.
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed with one-way ANOVA and Tukey's multiple comparison test using GraphPad Prism software. P < 0.05 was considered to be significant.
RESULTS
TSP-1 upregulation in mouse adipose tissue.
We used qPCR analysis to assess TSP-1 mRNA expression in adipose tissue of diet-induced obese mice. Mice fed the regular chow diet had significantly increased TSP-1 mRNA expression in perigonadal adipose tissue at 6 mo of age compared with 2-mo-old animals (Fig. 1A). A trend toward an age-related increase in TSP-1 expression was also noted in subcutaneous fat (Fig. 1A). In age-matched animals, TSP-1 expression was comparable between perigonadal and subcutaneous fat. At 6 mo of age, mice fed the HFD had higher TSP-1 mRNA levels in perigonadal fat than animals fed the HCLFD (Fig. 1B); in contrast, in subcutaneous fat TSP-1 expression was comparable between the groups. Immunohistochemical staining of adipose tissue from HFD and HCLFD mice showed TSP-1 localization in adipose interstitium and in perivascular areas but not in adipocytes (Fig. 1C). In vitro, undifferentiated 3T3-L1 preadipocytes, but not differentiated adipocytes, expressed high TSP-1 mRNA levels (Fig. 1D). In differentiated adipocytes, TGF-β1 stimulation (10 ng/ml) significantly upregulated TSP-1 mRNA synthesis. In contrast, stimulation of 3T3-L1 adipocytes with leptin (100–300 ng/ml), TNF-α (10 ng/ml), or IL-1β (10 ng/ml) did not significantly affect TSP-1 synthesis (Fig. 1E).
Fig. 1.
Thrombospondin-1 (TSP-1) expression in mouse adipose tissue. A: TSP-1 mRNA expression was assessed using qPCR in perigonadal (PG) and subcutaneous (SC) adipose tissue harvested from 2- and 6-mo-old wild-type (WT) C57BL/6J mice fed a regular chow diet (**P < 0.01 vs. 2 mo old). A significant increase in TSP-1 mRNA expression was noted at 6 mo of age. B: mice fed a high-fat diet (HFD) had higher TSP-1 mRNA expression in perigonadal adipose tissue than animals fed a high-carbohydrate low-fat diet (HCLFD) (*P < 0.05). In contrast, in subcutaneous fat, TSP-1 mRNA expression was comparable between groups (HFD, n = 8; HCLFD, n = 8; chow diet, n = 13). C: immunohistochemical staining localized TSP-1 immunoreactivity in adipose interstitium in HFD and HCLFD groups; however, staining was more intense in HFD animals (B). Adipose tissue harvested from TSP-1-null mice showed minimal staining for TSP-1. D: undifferentiated 3T3-L1 preadipocytes showed high levels of TSP-1 mRNA expression. Adipocyte differentiation markedly reduced TSP-1 mRNA expression in 3T3-L1 cells (**P < 0.01, n = 3). E: in differentiated 3T3-L1 adipocyte stimulation, TSP-1 expression was not induced by leptin (100–300 ng/ml) or proinflammatory cytokines IL-1β and TNF-α. In contrast, TGF-β significantly increased TSP-1 synthesis by 3T3-L1 cells (*P < 0.05 vs. control, n = 3). F–I: TSP-1-null mice exhibit reduced weight gain. Both female (F) and male TSP-1-null mice (H) fed HCLFD gained significantly less weight than WT controls. Male TSP-1-null mice fed HFD had reduced weight gain after 5–6 mo of feeding (I); in contrast, weight gain in female mice fed HFD was not affected by loss of TSP-1 (G) (female HCLFD: WT n = 21, −/− n = 9; male HCLFD: WT n = 19, −/− n = 8; female HFD: WT n = 24, −/− n = 8; male HFD: WT n = 15, −/− n = 5).
TSP-1 loss attenuates weight gain in mice fed HCLFD or HFD.
Both male and female TSP-1-null mice had significantly attenuated weight gain when fed the HCLFD diet, exhibiting reduced weight after 1–2 mo of feeding (Fig. 1, F and H). The effects of TSP-1 loss were less impressive in animals fed the HFD: only male mice had a late attenuation of weight gain (after 4 mo of feeding), whereas female TSP-1-null animals had comparable weight curves with corresponding TSP-1-null mice (Fig. 1, G and I).
TSP-1-null mice exhibit reduced adiposity when fed HCLFD or HFD.
In both diet groups, the age-related increase in length was reduced in the absence of TSP-1, possibly reflecting the spinal abnormalities observed in TSP-1-null animals (27). After 5 mo of feeding (at 6 mo of age), TSP-1 deficiency significantly attenuated the increase in adiposity in both HCLFD and HFD groups (Fig. 2, A–D, and Tables 2, 3, and 4). Between 2 and 6 mo of age, both HCLFD and HFD mice exhibited significant increases in fat weight; TSP-1 loss was associated with a marked reduction in adipose tissue gain (see Table 4). Over this 4-mo period, male TSP-1−/− mice fed the HCLFD had a 26.2+6.7% increase in fat weight (vs. a 172.7+38.1% increase in WT animals, P < 0.01). Female mice on the HCLFD also exhibited reduced adipose tissue growth (%change in fat content: TSP-1-null 60.6+14.2 vs. WT 135.5%+17.1, **P < 0.01; Table 4 and Fig. 2, A–D). Feeding with the HFD significantly increased adiposity. At 6 mo of age, WT mice fed a HFD had significantly increased fat content compared with HCLFD animals (Tables 2 and 3). Much as in HCLFD animals, TSP-1 loss markedly attenuated adipose tissue growth in both male and female mice fed the HFD (Table 4). The rate of fat weight increase in HFD animals was 3.8–3.9 times lower in male and female TSP-1-null mice compared with corresponding sex-matched WT groups. Abdominal fat weight and percent abdominal fat content were also significantly reduced in TSP-1-null animals.
Fig. 2.
TSP-1 loss reduces adiposity without affecting food intake and energy expenditure. DEXA studies demonstrated that total body fat was significantly reduced in TSP-1-null mice fed HCLFD (A) or HFD (B). TSP-1 loss also attenuated abdominal adiposity in both HCLFD (C) and HFD (D) groups (*P < 0.05, **P < 0.01 vs. corresponding WT) (HFD: WT n = 15, −/− n = 19; HCLFD: WT n = 15, −/− n = 10). In a cohort of mice fed regular chow diet for 6 mo (n = 7), TSP-1 loss did not affect food intake (E) or energy expenditure (F).
Table 4.
Percent changes in weight and adiposity in WT and TSP-1 KO mice
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| HCLFD |
HFD |
HCLFD |
HFD |
|||||
| %Change | WT | KO | WT | KO | WT | KO | WT | KO |
| ΔLength | 6.37 ± 2.00 | −0.40 ± 1.56* | 10.07 ± 3.11 | 0.84 ± 1.52** | 5.14 ± 0.84 | 3.00 ± 0.84 | 10.18 ± 2.35 | 4.39 ± 0.96* |
| ΔWeight | 45.70 ± 9.48 | 19.95 ± 1.44 | 70.13 ± 4.97 | 22.16 ± 1.41* | 44.80 ± 3.63 | 21.54 ± 3.55** | 59.72 ± 11.24 | 28.23 ± 4.02* |
| ΔLean weight | 23.13 ± 1.85 | 18.78 ± 1.96 | 30.59 ± 4.06 | 11.12 ± 3.46* | 23.34 ± 1.41 | 11.42 ± 1.96** | 15.17 ± 4.78 | 16.73 ± 7.97 |
| ΔFat weight | 172.67 ± 38.12 | 26.18 ± 6.65* | 206.45 ± 7.00 | 53.32 ± 11.30** | 135.45 ± 17.06 | 60.58 ± 14.24** | 227.88 ± 43.33 | 60.03 ± 14.40** |
| ΔAbdominal fat | 350.39 ± 73.73 | 86.59 ± 16.46* | 311.77 ± 20.83 | 82.37 ± 19.32** | 291.40 ± 36.89 | 154.87 ± 35.85* | 459.46 ± 83.88 | 165.38 ± 41.39** |
Values are means ± SE.
P < 0.05,
P < 0.01.
Table 2.
Comparison of weight gain and adiposity in male WT and TSP-1-KO mice
| HCLFD |
HFD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo |
4 mo |
6 mo |
2 mo |
4 mo |
6 mo |
|||||||
| WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | |
| Length, cm | 8.6 ± 0.14 | 8.8 ± 0.12 | 9.6 ± 0.15 | 9.1 ± 0.07* | 9.12 ± 0.17 | 8.7 ± 0.10 | 8.5 ± 0.13 | 8.7 ± 0.18 | 9.1 ± 0.17 | 9.2 ± 0.08 | 9.1 ± 0.17 | 8.8 ± 0.11 |
| Weight, g | 29.4 ± 0.77 | 26.7 ± 0.52* | 34.5 ± 1.79 | 31.5 ± 0.63 | 43.0 ± 3.26 | 32 ± 0.57* | 26.5 ± 0.93 | 29.9 ± 0.36* | 33.4 ± 1.74 | 36.0 ± 0.27 | 43.5 ± 2.16 | 36.5 ± 0.60 |
| Lean weight, g | 19.6 ± 0.85 | 18.4 ± 0.35 | 22.1 ± 1.01 | 21.6 ± 0.46 | 24.1 ± 1.11 | 21.9 ± 0.46 | 16.8 ± 0.45 | 19.0 ± 0.2** | 19.4 ± 0.45 | 21.0 ± 0.64 | 21.4 ± 0.72 | 21.2 ± 0.86 |
| Fat weight, g | 4.4 ± 0.28 | 3.8 ± 0.25 | 7.1 ± 0.88 | 4.4 ± 0.29* | 12.2 ± 2.03 | 4.8 ± 0.36* | 5.4 ± 0.47 | 6.2 ± 0.42 | 9.2 ± 1.21 | 9.5 ± 0.81 | 14.9 ± 1.54 | 9.6 ± 1.19* |
| Fat content, % | 18.2 ± 0.3 | 17.3 ± 0.93 | 24.1 ± 2.11 | 16.9 ± 0.96* | 32.5 ± 4.02 | 18.1 ± 1.23* | 24.2 ± 1.45 | 24.5 ± 1.39 | 31.8 ± 2.53 | 31.0 ± 2.44 | 40.5 ± 2.38 | 31.1 ± 3.46 |
| Abdominal fat weight, g | 1.3 ± 0.12 | 1.2 ± 0.14 | 2.9 ± 0.52 | 1.5 ± 0.15 | 5.96 ± 1.12 | 2.2 ± 0.25* | 2.2 ± 0.26 | 2.8 ± 0.29 | 3.9 ± 0.56 | 4.8 ± 0.55 | 7.7 ± 0.79 | 5.1 ± 0.83 |
| Abdominal fat content, % | 15.7 ± 0.8 | 16.0 ± 1.34 | 26.3 ± 3.3 | 16.9 ± 1.5* | 34.0 ± 4.81 | 17.4 ± 1.73* | 27.7 ± 1.88 | 28.9 ± 2.34 | 34.4 ± 2.81 | 31.9 ± 2.87 | 43.0 ± 1.97 | 36.4 ± 4.33 |
Values are means ± SE. HCLFD, high-carb, low-fat diet; HFD, high-fast diet; KO, TSP-1-null. WT HCLFD: n = 5, KO HCLFD: n = 5, WT HFD: n = 5, KO HFD: n = 9.
P < 0.05,
P < 0.01.
Table 3.
Comparison of weight gain and adiposity in female WT and TSP-1 KO mice
| HCLFD |
HFD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo |
4 mo |
6 mo |
2 mo |
4 mo |
6 mo |
|||||||
| WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | WT | KO | |
| Length, cm | 8.3 ± 0.06 | 8.5 ± 0.08 | 8.8 ± 0.10 | 9.2 ± 0.03** | 8.7 ± 0.08 | 8.7 ± 0.04 | 7.8 ± 0.12 | 8.4 ± 0.1** | 8.6 ± 0.08 | 9.0 ± 0.12* | 8.6 ± 0.10 | 8.4 ± 0.14 |
| Weight, g | 22.0 ± 0.3 | 21.7 ± 0.2 | 26.7 ± 0.7 | 24.9 ± 0.59 | 31.9 ± 1.0 | 26.4 ± 0.8** | 22.9 ± 0.7 | 26.2 ± 1.1* | 27.7 ± 0.9 | 32.3 ± 1.8 | 36.3 ± 2.1 | 34.4 ± 1.8 |
| Lean weight, g | 14.5 ± 0.2 | 14.5 ± 0.2 | 16.3 ± 0.3 | 15.9 ± 0.4 | 17.8 ± 0.3 | 16.2 ± 0.4** | 14.6 ± 0.4 | 17.2 ± 0.7** | 16.0 ± 0.3 | 19.2 ± 0.1* | 16.7 ± 0.4 | 20.7 ± 1.9 |
| Fat weight, g | 3.7 ± 0.1 | 3.4 ± 0.1 | 6.1 ± 0.4 | 4.4 ± 0.2** | 8.7 ± 0.73 | 5.4 ± 0.41** | 4.3 ± 0.25 | 5.8 ± 0.6* | 7.6 ± 0.6 | 7.9 ± 0.9 | 13.9 ± 1.6 | 9.7 ± 1.2 |
| Fat content, % | 20.2 ± 0.5 | 19.0 ± 0.8 | 27.0 ± 1.8 | 21.5 ± 0.6** | 32.2 ± 2.1 | 24.5 ± 1.0** | 22.6 ± 0.8 | 24,8 ± 1.4 | 31.8 ± 1.5 | 28.9 ± 2.0 | 43.7 ± 3.0 | 32.9 ± 2.8* |
| Abdominal fat weight, g | 1.0 ± 0.1 | 1.0 ± 0.04 | 2.2 ± 0.19 | 1.6 ± 0.16* | 4.1 ± 0.4 | 2.6 ± 0.27* | 1.4 ± 0.13 | 2.0 ± 0.36 | 3.0 ± 0.28 | 3.5 ± 0.54 | 7.3 ± 0.94 | 5.2 ± 0.81 |
| Abdominal fat content, % | 16.8 ± 0.8 | 16.3 ± 0.68 | 25.8 ± 1.6 | 19.6 ± 1.2** | 32.1 ± 2.56 | 24.5 ± 1.5* | 23.6 ± 1.8 | 23.9 ± 2.3 | 33.2 ± 1.7 | 31.8 ± 2.8 | 46.3 ± 3.9 | 36.4 ± 3.0 |
Values are means ± SE. WT HCLFD: n = 10, KO HCLFD: n = 5, WT HFD: n = 10, KO HFD: n = 10.
P < 0.05,
P < 0.01.
TSP-1 loss does not affect food intake and energy expenditure.
To examine whether reduced weight gain in TSP-1-null mice was due to alterations in food intake or energy expenditure, we performed indirect calorimetry in 6-mo-old WT and TSP-1-null mice fed the regular chow diet. We found no significant differences in food intake (Fig. 2E) or energy expenditure (Fig. 2F) between groups.
TSP-1 loss is associated with reduced insulin levels and HOMA-IR but increased FFA and triglyceride levels in mice fed HFD.
Metabolic function was compared between WT and TSP-1-null animals at 6 mo of age. HFD mice had significantly higher serum insulin levels and HOMA-IR than HCLFD animals, reflecting worse metabolic function. TSP-1 loss was associated with reduced glucose levels in mice fed the HCLFD but not in animals fed the HFD (Fig. 3A). Compared with WT animals, TSP-1-null mice had lower insulin levels (Fig. 3B) and decreased HOMA-IR (Fig. 3C) in both HCLFD and HFD groups, suggesting that TSP-1 loss improved insulin resistance. Plasma cholesterol levels were reduced in TSP-1-null mice fed the HCLFD but not in animals fed the HFD (Fig. 3D). In contrast, serum FFA and triglyceride levels were significantly increased in TSP-1-null mice fed the HFD compared with corresponding WT animals (Fig. 3, E and F). Plasma leptin levels (Fig. 3G) were also reduced in TSP-1-null mice fed the HCLFD but not in animals fed the HFD compared with corresponding diet-matched WT controls. Reduced serum leptin levels in TSP-1-null mice fed the HCLFD were associated with attenuated leptin mRNA transcription in the perigonadal adipose tissue (Fig. 3H).
Fig. 3.
TSP-1 loss attenuates insulin resistance and metabolic dysfunction in diet-induced obese mice. Serum glucose (A) and insulin (B) levels and HOMA-IR (C) were compared between WT and TSP-1-null animals (n = 10 per group). A: compared with WT animals, TSP-1−/− mice had reduced plasma glucose levels when fed HCLFD (but not when fed HFD). In HCLFD (B) and HFD (C) groups, TSP-1 loss was associated with reduced plasma insulin levels and HOMA IR, reflecting attenuated insulin resistance. D: serum cholesterol levels were also lower in TSP-1-null mice fed HCLFD compared with corresponding WT animals. E and F: serum FFA levels were significantly increased in TSP-1-null mice fed HCLFD or HFD (E); TSP-1 absence was associated with increased serum triglyceride levels only in animals fed HFD. G and H: serum leptin levels were markedly lower in TSP-1−/− mice fed HCLFD compared with WT animals (E); reduced serum leptin in TSP-1-null mice was associated with attenuated expression of adipose tissue leptin mRNA (F).
TSP-1 loss does not significantly affect adipocyte size.
To examine whether reduced adiposity in TSP-1-null mice was due to changes in adipocyte size, we compared the mean adipocyte area in subcutaneous and perigonadal adipose tissue harvested from WT and TSP-1-null mice (Fig. 4A). In perigonadal fat, WT and TSP-1-null mice fed the HFD had significantly larger adipocytes than corresponding animals fed the HCLFD (Fig. 4B). TSP-1 absence did not significantly affect adipocyte size in HCFLD or HFD mice. In the subcutaneous fat, mean adipocyte size was not significantly different between animals fed the HFD or the HCLFD and was not affected by TSP-1 loss (Fig. 4C).
Fig. 4.
Effects of TSP-1 loss on adipocyte size. A: hematoxylin-eosin-stained sections were used to quantitate adipocyte size in perigonadal and subcutaneous fat in 6-mo-old animals (n = 10 per group). B: mice fed HFD had higher adipocyte size in perigonadal fat than those fed HCLFD in both WT and TSP-1-null groups (^P < 0.05, ^^P < 0.01 vs. corresponding HCLFD). However, TSP-1 loss did not affect adipocyte size in both HFD and HCLFD groups. C: in subcutaneous fat no significant differences in adipocyte size were noted.
WT and TSP-1-null mice have comparable vascular density in adipose tissue.
Because TSP-1 is a potent angiostatic mediator (26), we examined whether the effects of TSP-1 loss on weight gain, adiposity, and metabolic dysfunction were due to alterations in the vascular supply of the adipose tissue. To quantitate the density of the adipose vasculature, we used two independent strategies: 1) quantitative histochemical analysis of GS-I+ microvascular profiles and 2) assessment of mRNA expression of the endothelial-specific genes CD31 and VE-cadherin. WT and TSP-1-null mice had comparable microvessel-to-adipocyte ratios when fed the HCLFD or the HFD, suggesting that TSP-1 loss did not significantly affect adipose vascular density (Fig. 5, A and B). Feeding with the HFD significantly increased CD31 (Fig. 5C) and VE-cadherin (Fig. 5D) mRNA levels; however, TSP-1 loss did not affect endothelial-specific gene expression in adipose tissue.
Fig. 5.
TSP-1 loss does not affect adipose tissue vascular density. A: GS-I lectin histochemistry was used to identify vascular profiles in adipose tissue. B: in perigonadal fat, the microvessel:adipocyte ratio was not significantly affected by TSP-1 loss. C and D: to compare angiogenic responses between WT and TSP-1−/− animals, expression of endothelial-specific genes CD31 and VE-cadherin was assessed in perigonadal adipose tissue (HCLFD: WT n = 17, −/− n = 18; HFD: WT n = 20, −/− n = 14). Adipose tissue CD31 and VE-cadherin mRNA expression was significantly higher in mice fed HFD; however, TSP-1 loss did not affect endothelial gene transcription.
TSP-1 loss is associated with attenuated adipose tissue macrophage infiltration in mice fed HCLFD.
Because TSP-1 is a modulator of the inflammatory response, we examined whether reduced metabolic dysfunction in TSP-1-null mice was due to decreased macrophage accumulation in the adipose tissue. Animals fed the HFD had a significantly higher macrophage:adipocyte than mice fed the HCLFD (Fig. 6, A and B). TSP-1 absence reduced macrophage infiltration in mice fed the HCLFD but not in animals on the HFD (Fig. 6B). Assessment of CD68 mRNA expression in the adipose tissue confirmed the immunohistochemical findings showing that mice on the HFD had increased CD68 mRNA levels and that TSP-1 loss attenuated adipose CD68 mRNA expression in HCLFD mice but not in HFD animals (Fig. 6C).
Fig. 6.
TSP-1 loss attenuates infiltration of adipose tissue with macrophages. A: immunofluorescent staining for macrophage marker Mac2 was used to quantitatively assess macrophage density in adipose tissue. B: macrophage-to-adipocyte ratio was significantly increased in animals fed HFD compared with HCLFD animals (*P < 0.05 vs. HCLFD). TSP-1 loss reduced macrophage infiltration in mice fed HCLFD but not in animals fed HFD (n = 7 for HFD mice; n = 10 for HCLFD groups). C: mRNA expression of macrophage-specific gene CD68 was assessed in perigonadal adipose tissue (HCLFD: WT n = 17, −/− n = 18; HFD: WT n = 20, −/− n = 14). Findings were consistent with immunohistochemical observations. TSP-1-null mice fed HCLFD exhibited significantly lower CD68 mRNA expression than corresponding WT mice, suggesting attenuated macrophage infiltration.
Effects of TSP-1 loss on adipose tissue inflammatory gene expression.
To dissect the effects of TSP-1 loss on adipose tissue inflammation, we assessed cytokine and chemokine expression in perigonadal adipose tissue. In both HFD and HCLFD groups, TSP-1-null mice had significantly lower adipose TNF-α mRNA expression levels than corresponding WT animals (Fig. 7A). In contrast, TSP-1 loss did not significantly affect IL-1β, IL-6, MCP-1, and IP-10 mRNA expression (Fig. 7, B–E). Moreover, mRNA expression of type I collagen and of the profibrotic growth factor TGF-β were significantly reduced in TSP-1-null animals fed the HCLFD compared with corresponding controls (Fig. 7, F and G).
Fig. 7.
Effects of TSP-1 on adipose tissue inflammatory gene expression. A: TSP-1 loss significantly attenuated adipose TNF-α mRNA expression in HFD and HCLFD groups. In contrast, IL-6 (B), IL-1β (C), MCP-1 (D), and IP-10 (E) mRNA levels were comparable between WT and corresponding TSP-1-null animals. F and G: TSP-1 disruption significantly reduced expression of TGF-β1 (F) and collagen Iα1 (G) mRNA in HCLFD but not HFD mice (HCLFD: WT n = 17, −/− n = 18; HFD: WT n = 20, −/− n = 14).
In vitro, TSP-1 stimulates 3T3-L1 preadipocyte proliferation.
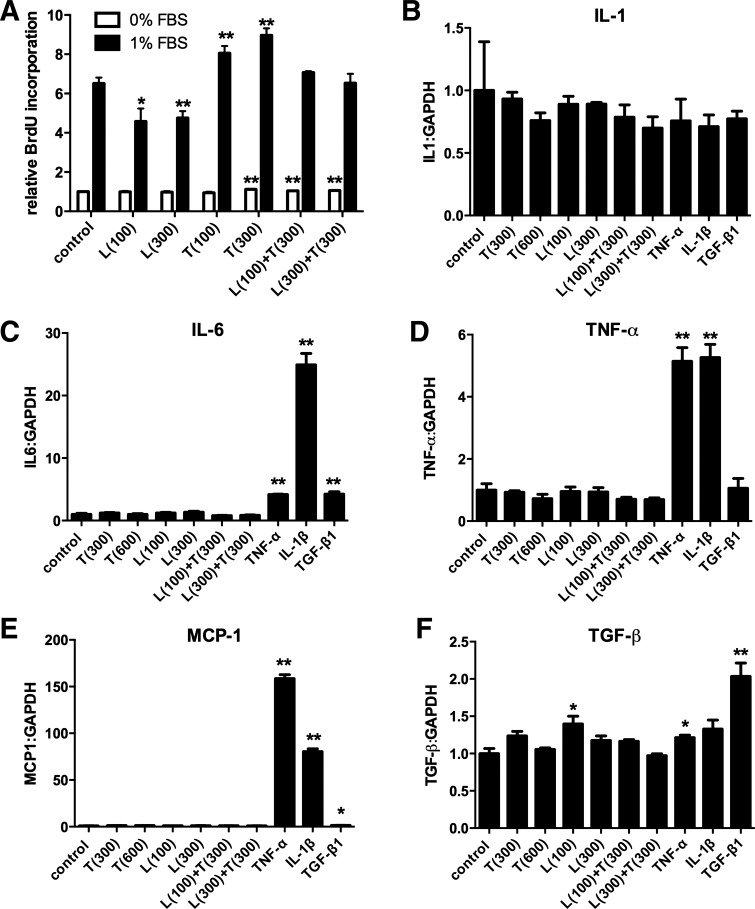
Loss of TSP-1 attenuates weight gain and adiposity without significant effects on adipocyte size. This finding suggests that TSP-1 loss may affect adipocyte number by modulating their proliferation. To test this hypothesis, we studied the in vitro effects of TSP-1 on proliferation of 3T3-L1 preadipocytes, using a BrdU proliferation assay. In serum-starved cells, high-concentration TSP-1 (300 ng/ml) had a modest (11% increase) but statistically significant stimulatory effect on preadipocyte proliferation (Fig. 8A). Serum-stimulated cells (1% FBS) showed markedly increased proliferative activity compared with serum-starved cells. TSP-1 stimulation significantly increased 3T3-L1 cell proliferation by 20–30% (**P < 0.01 vs. unstimulated cells; Fig. 8A). In contrast, leptin stimulation reduced the proliferative activity of 3T3-L1 cells and abrogated the proliferative effects of TSP-1 (Fig. 8A).
Fig. 8.
TSP-1 enhances proliferative activity but does not modulate inflammatory gene expression in 3T3-L1 preadipocytes. A: stimulation with TSP-1 (T, 300 ng/ml) modestly but significantly increased proliferative activity of 3T3-L1 cells in the absence of FBS. In the presence of 1% FBS, TSP-1 increased 3T3-L1 proliferation by 20–30%; in contrast, leptin (L) stimulation exerted antiproliferative actions (n = 6). B: expression of IL-1β by 3T3-L1 cells was not modulated by TSP-1 or inflammatory cytokine stimulation. C: in contrast, IL-6 mRNA synthesis was markedly upregulated by IL-1β and significantly increased by TNF-α and TGF-β. TSP-1 and leptin stimulation had no significant effects on IL-6 transcription. D and E: TNF-α and IL-1β, but not TSP-1, increased TNF-α (D) and MCP-1 (E) mRNA expression by 3T3-L1 adipocytes. F: leptin (100 ng/ml) and TNF-α modestly but significantly increased TGF-β1 mRNA synthesis. TGF-β1 stimulation was the most potent stimulator, increasing its own expression by 100%. TSP-1 had no effects on TGF-β1 mRNA expression by 3T3-L1 cells (n = 3).
TSP-1 has no effects on inflammatory gene synthesis by 3T3-L1 cells.
Because TSP-1 absence is associated with attenuated adipose tissue inflammation, we tested whether TSP-1 directly stimulates inflammatory gene expression in 3T3-L1 cells. Stimulation with TNF-α and IL-1β had no effects on IL-1β mRNA synthesis by 3T3-L1 cells but markedly upregulated TNF-α, IL-6, and MCP-1 mRNA expression (Fig. 8, B–E). In contrast, TSP-1 did not significantly modulate inflammatory cytokine and chemokine gene expression in either the presence or the absence of leptin (Fig. 8, B–E). TNF-α, leptin, and TGF-β, but not TSP-1, induced significant upregulation of TGF-β1 mRNA synthesis in 3T3-L1 cells (Fig. 8F).
DISCUSSION
Adipose tissue growth in obese subjects is associated with extensive alterations in the extracellular matrix. In rodent models of obesity, collagen genes are markedly upregulated in perigonadal fat, leading to expansion of the adipose interstitial space (23). In obese human patients, pericellular fibrosis is noted in both omental and subcutaneous adipose tissue (9). Matrix proteins in the remodeling fat not only serve to provide mechanical support, but also to regulate signaling cascades, thus modulating phenotype and function of both adipocytes and nonadipocytes. Our study provides new insights into the role of the extracellular matrix in weight gain, adiposity, and metabolic dysfunction in diet-induced obesity. Using genetically targeted mice, we found that the prototypical matricellular protein TSP-1 mediates adipose tissue growth and metabolic dysfunction in mice fed a HCLFD or a HFD. Moreover, we report for the first time that the effects of TSP-1 are not related to its potent angiostatic properties but may reflect inflammatory actions and direct effects on adipocyte proliferation.
Matricellular proteins in adipose tissue.
Because adipose tissue in obese subjects is actively remodeling, it is not surprising that obesity is associated with upregulation of several members of the matricellular family. SPARC expression is increased both in animal models of obesity (41) and in obese subjects (24); SPARC upregulation may reduce adipogenesis by attenuating mitotic expansion of preadipocytes (3) and by inhibiting adipocyte differentiation (32). Serum and adipose tissue osteopontin levels are markedly increased in obese individuals (13); osteopontin upregulation may mediate infiltration of fat tissue with proinflammatory macrophages, contributing to the development of metabolic dysfunction (33). Tenascin-C expression is also increased in visceral adipose tissue harvested from obese individuals; however, its potential role in the pathogenesis of adipogenesis remains unknown (4).
TSP-1 upregulation in adipose tissue.
Induction of TSP-1 in adipose tissue has been consistently reported in mouse (46) and rat (18) models of obesity. In tissue harvested from obese human subjects, TSP-1 expression was markedly upregulated (35) in both adipocytes and infiltrating macrophages (44); TSP-1 levels were associated with adipose tissue inflammation and metabolic dysfunction (44). In our study, mice fed a HFD exhibited significant upregulation of TSP-1 mRNA in perigonadal fat but not in subcutaneous adipose tissue (Fig. 1B). In perigonadal fat, TSP-1 protein was predominantly localized in the adipose interstitium and in perivascular areas. The mechanism responsible for TSP-1 induction in activated adipose tissue remains unknown. Our immunohistochemical and in vitro findings suggest that differentiated adipocytes are not a major source of TSP-1. Undifferentiated 3T3-L1 preadipocytes, but not differentiated adipocytes, exhibit high levels of TSP-1 expresssion (Fig. 1). Shitaye et al. (37) have previously reported that adipocyte differentiation decreases TSP-2 expression in adipocytes. Taken together, these observations may indicate that adipocyte maturation is associated with decreased synthesis of matricellular genes. Moreover, cytokine stimulation does not significantly upregulate TSP-1 expression in adipocytes (Fig. 1D). In contrast, adipose vascular cells and macrophages are capable of secreting large amounts of TSP-1. In vitro, high glucose levels potently stimulate TSP-1 synthesis, inducing a 30-fold increase in TSP-1 expression in isolated endothelial cells, smooth muscle cells, and fibroblasts (39). Thus, increased adipose TSP-1 levels in mice fed a HFD may reflect accentuated synthesis of the matricellular protein by nonadipocytes in response to elevated glucose levels or an increase in the number of undifferentiated adipocyte progenitors.
Role of TSP-1 in weight gain, adiposity, and metabolic dysfunction.
Three recently published studies have suggested somewhat contradictory effects of TSP-1 loss on metabolic function. Olerud et al. (34) systematically compared pancreatic islet function in TSP-1-null and WT mice. Despite an increase in β-cell mass, TSP-1-null animals were glucose intolerant, exhibiting decreased glucose-stimulated insulin release and proinsulin biosynthesis. Defective islet function in TSP-1-null mice was due to impaired TGF-β activation. The exclusive localization of TSP-1 in the islet endothelium suggested that endothelial-derived TSP-1 activates islet TGF-β, thus providing crucial functional support for pancreatic β-cells through a paracrine mechanism (34). On the other hand, Voros and Lijnen (45) compared adipose tissue development between WT and TSP-1-null animals (both male and female) fed a high-fat diet (42% fat content) and found no significant effects of TSP-1 loss on body and adipose tissue weight and on metabolic function. A third study, that by Li et al. (28), found that male TSP-1-null mice fed a HFD (60% fat) had improved glucose tolerance compared with WT controls. The conflicting findings on the role of TSP-1 in obesity and metabolic dysfunction likely reflect the complex and context-dependent actions of the TSP-1 molecule, which is capable of modulating a wide range of cellular effects and molecular interactions in many tissues. As a multidomain matricellular protein, TSP-1 transduces integrin-mediated signals, activates TGF-β, regulates growth factor signaling, and modulates inflammatory pathways; the relative significance of each one of these interactions is dependent on the experimental context. Thus, differences in the composition and duration of the diets and in genetic background, sex, and age of the animals may account for the divergent observations. Our work shows that in animals fed a HFD (60% fat content) or a HCLFD (10% fat content), TSP-1 loss was associated with a marked reduction in weight gain and significantly decreased accumulation of adipose tissue (Fig. 2 and Table 4). TSP-1-null mice also had reduced insulin resistance and attenuated metabolic dysfunction: glucose, insulin levels, and HOMA-IR were significantly lower in TSP-1-null animals (Fig. 3). Taken together, the findings suggest that in both high-carbohydrate and high-fat diet groups, TSP-1 mediates weight gain and adipose tissue growth.
Cellular basis of TSP-1-mediated weight gain and metabolic dysfunction.
The effects of TSP-1 loss on weight gain were not associated with alterations in food intake and energy expenditure (Fig. 2 and Ref. 28) but may involve actions on adipose tissue growth and metabolic function. First, TSP-1 may exert direct effects on adipocytes, promoting their proliferation and activation. Our in vivo experiments showed no statistically significant differences in adipocyte size between WT and TSP-1-null groups. Considering the marked reduction in adiposity, this finding suggests that TSP-1 loss may be associated with reduced adipocyte numbers. Our in vitro experiments provide, for the first time, evidence suggesting proliferative effects of TSP-1 on adipocytes. Second, TSP-1 may induce metabolic dysfunction by enhancing adipose inflammatory activity. In our diet-induced obese mice, we found that TSP-1 loss attenuated macrophage infiltration (Fig. 6) and reduced adipose TNF-α mRNA expression, suggesting proinflammatory actions of TSP-1 (Fig. 7). Reported associations between adipose TSP-1 expression and inflammatory activity in obese human subjects (44) provide further support for this potential mechanism. TSP-1 may promote inflammation by enhancing macrophage recruitment and activity (29) and by promoting activation and clonal expansion of inflammatory T cells (42). Any proinflammatory actions of TSP-1 likely involve nonadipocytes; our in vitro experiments did not reveal direct effects of TSP-1 on cytokine and chemokine expression by 3T3-L1 cells. Third, TSP-1 may act through its potent angiostatic functions (26), thus potentiating adipocyte hypoxia and enhancing adipose inflammation. TSP-1 may be responsible for pathological expansion of the adipose tissue after exposure to HFD (40), limiting angiogenesis and accentuating inflammatory signaling. However, quantitative assessment of the adipose vasculature using both histochemical staining and measurement of mRNA levels of the endothelial-specific genes CD31 and VE-cadherin (Fig. 5) suggest that TSP-1 loss does not significantly modulate angiogenic activity in the adipose tissue. Fourth, the significant hyperglycemia observed in TSP-1-null animals fed a HFD, despite reduced adiposity and lower insulin levels, may indicate a defect in insulin release. Olerud and coworkers have demonstrated that TSP-1 loss is associated with a defect β-cell function that persists in aged mice and is due, at least in part, to impaired TGF-β activation (10, 34). Finally, TSP-1 may play an important role in uptake of fatty acids; increased serum FFA and triglyceride levels in TSP-1-null mice (Fig. 3) may reflect impairment in fatty acid uptake by adipocytes. Lack of CD36, a key ligand of TSP-1, is also associated with defective uptake of FFAs by adipocytes (16, 17), suggesting that TSP-1 deposition in the adipose interstitium may facilitate CD36-mediated uptake of fatty acids. Loss of the inhibitory effects of fatty acids on basal and insulin-stimulated leptin release by adipocytes may also explain the relatively high leptin levels observed in TSP-1-null mice fed a HFD despite a significantly reduced adiposity (Fig. 3). Much like TSP-1-null mice, CD36 knockouts also exhibit increased circulating leptin levels (16).
Which molecular signals may mediate TSP-1 actions in adipose tissue?.
The TSP-1 molecule has multiple functional domains and, in a typical matricellular fashion, interacts with cytokines and growth factors, or modulates cell phenotype, through activation of specific receptors. Many biological effects of TSP-1 are mediated through binding to CD36, a widely expressed surface glycoprotein that functions as a scavenger receptor but also acts as a fatty acid translocase with an important role in lipid uptake in fat and other tissues (6, 38). CD36 absence protects mice from diet-induced insulin resistance and hyperlipidemia attenuating adipose inflammation (22); the beneficial actions may be due to decreased recruitment of proinflammatory macrophages in the adipose tissue (31). The similarities between our findings in TSP-1-null animals and the reported phenotype of CD36-null mice in diet-induced obesity models (16, 22, 31) may reflect the involvement of CD36 in TSP-1 signaling. Binding and activation of β1- and β3-integrin-mediated pathways is another common mechanism of TSP-1-mediated responses. Although the involvement of integrins in modulation of adipocyte behavior by TSP-1 has not been documented, adipocytes are known to engage tyrosine kinase signaling in response to β1-integrin activation (15). Modulation of growth factor responses may represent an additional molecular mechanism of action for TSP-1 in adipose tissue. As a crucial TGF-β activator (7), TSP-1 may modulate the phenotype of both adipocytes and nonadipocytes in fat tissue by facilitating transduction of TGF-β signaling cascades.
Conclusions.
Diet-induced obesity triggers dynamic changes in the extracellular matrix of adipose tissue. The remodeling adipose matrix not only provides support for activated adipocytes but also regulates the function of both adipocytes and nonadipocytes by modulating signaling cascades. The matricellular protein TSP-1 is upregulated in adipose tissue and contributes to the dynamic events associated with adipose remodeling mediating weight gain, adipose tissue growth, and metabolic dysfunction. Inflammatory activation of macrophages, accentuated adipocyte fatty acid uptake, and increased adipocyte proliferation may be in part responsible for the effects of TSP-1 in weight gain and in obesity-associated metabolic dysfunction. Dissection of the functional domains responsible for the effects of TSP-1 in adipose tissue biology may allow development of peptide-based strategies to inhibit detrimental TSP-1-mediated responses in obese individuals.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-76246 and R01 HL-85440, the Wilf Family Cardiovascular Research Institute, and a Baylor College of Medicine clinical and translational grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.K., C.G.-Q., and N.G.F. conception and design of research; P.K., C.G.-Q., N.L., M.C., and D.-W.L. performed experiments; P.K., C.G.-Q., M.C., and D.-W.L. analyzed data; P.K., C.G.-Q., and N.G.F. interpreted results of experiments; P.K. and N.G.F. prepared figures; P.K. and N.G.F. drafted manuscript; P.K., C.G.-Q., N.L., M.C., D.-W.L., and N.G.F. approved final version of manuscript; N.G.F. edited and revised manuscript.
REFERENCES
- 1.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol 130: 503–506, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal 3: 163–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA 100: 6045–6050, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab 97: E1880–E1889, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol 32: 2598–2608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275: 32523–32529, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93: 1159–1170, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, Dana R, Masli S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med 208: 1083–1092, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, Bedossa P, Clement K. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59: 2817–2825, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drott CJ, Olerud J, Emanuelsson H, Christoffersson G, Carlsson PO. Sustained beta-cell dysfunction but normalized islet mass in aged thrombospondin-1 deficient mice. PLoS One 7: e47451, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 92: 635–688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation 111: 2935–2942, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Ambrosi J, Catalan V, Ramirez B, Rodriguez A, Colina I, Silva C, Rotellar F, Mugueta C, Gil MJ, Cienfuegos JA, Salvador J, Fruhbeck G. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab 92: 3719–3727, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol 177: 3534–3541, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Guilherme A, Czech MP. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J Biol Chem 273: 33119–33122, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes 56: 1872–1880, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest 109: 1381–1389, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hida K, Wada J, Zhang H, Hiragushi K, Tsuchiyama Y, Shikata K, Makino H. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res 41: 1615–1622, 2000 [PubMed] [Google Scholar]
- 19.Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol 27: 2582–2588, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6: 41–48, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci 65: 700–712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res 89: 604–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, Pinkney JH, Wilding JP. Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes 58: 1780–1788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal 3: 215–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6: 1–12, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101: 982–992, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One 6: e26656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 68: 7090–7099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 107: 785–790, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A, Febbraio M, Lancaster GI, Febbraio MA. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes 60: 1100–1110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem 284: 1279–1290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117: 2877–2888, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olerud J, Mokhtari D, Johansson M, Christoffersson G, Lawler J, Welsh N, Carlsson PO. Thrombospondin-1: an islet endothelial cell signal of importance for beta-cell function. Diabetes 60: 1946–1954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramis JM, Franssen-van Hal NL, Kramer E, Llado I, Bouillaud F, Palou A, Keijer J. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci 59: 1960–1971, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem 50: 71–79, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Shitaye HS, Terkhorn SP, Combs JA, Hankenson KD. Thrombospondin-2 is an endogenous adipocyte inhibitor. Matrix Biol 29: 549–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2: re3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation 107: 3209–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, Van Obberghen E. The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem 276: 22231–22237, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Vallejo AN, Mugge LO, Klimiuk PA, Weyand CM, Goronzy JJ. Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J Immunol 164: 2947–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Van Hul M, Frederix L, Lijnen HR. Role of thrombospondin-2 in murine adipose tissue angiogenesis and development. Obesity (Silver Spring) 20: 1757–1762, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, Kern EM, Nagarajan R, Spencer HJ, 3rd, Lee MJ, Fried SK, McGehee RE, Jr, Peterson CA, Kern PA. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes 57: 432–439, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voros G, Lijnen HR. Deficiency of thrombospondin-1 in mice does not affect adipose tissue development. J Thromb Haemost 4: 277–278, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 58: 902–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]