Abstract
CTRP3 is a secreted plasma protein of the C1q family that helps regulate hepatic gluconeogenesis and is downregulated in a diet-induced obese state. However, the role of CTRP3 in regulating lipid metabolism has not been established. Here, we used a transgenic mouse model to address the potential function of CTRP3 in ameliorating high-fat diet-induced metabolic stress. Both transgenic and wild-type mice fed a high-fat diet showed similar body weight gain, food intake, and energy expenditure. Despite similar adiposity to wild-type mice upon diet-induced obesity (DIO), CTRP3 transgenic mice were strikingly resistant to the development of hepatic steatosis, had reduced serum TNF-α levels, and demonstrated a modest improvement in systemic insulin sensitivity. Additionally, reduced hepatic triglyceride levels were due to decreased expression of enzymes (GPAT, AGPAT, and DGAT) involved in triglyceride synthesis. Importantly, short-term daily administration of recombinant CTRP3 to DIO mice for 5 days was sufficient to improve the fatty liver phenotype, evident as reduced hepatic triglyceride content and expression of triglyceride synthesis genes. Consistent with a direct effect on liver cells, recombinant CTRP3 treatment reduced fatty acid synthesis and neutral lipid accumulation in cultured rat H4IIE hepatocytes. Together, these results establish a novel role for CTRP3 hormone in regulating hepatic lipid metabolism and highlight its protective function and therapeutic potential in attenuating hepatic steatosis.
Keywords: adipokine, CTRP, C1q/TNF, fatty liver, hepatic steatosis, NAFLD, triglyceride synthesis
hepatic steatosis, or fatty liver, results from an imbalance between production and removal of hepatic triglycerides (TAGs) (10). This imbalance can result from excessive alcohol consumption (alcoholic fatty liver disease) or through other means (nonalcoholic fatty liver disease, NAFLD). In NAFLD, elevated hepatic TAG is caused by a combination of excess dietary lipids and de novo fatty acid synthesis (6, 10, 45). Fat oxidation and TAG export (in the form of very low-density lipoprotein, VLDL) aid in removal of hepatic TAGs. NAFLD is one of the primary causes of abnormal liver function (10) and is frequently linked to hepatic insulin resistance and uncontrolled gluconeogenesis in the diabetic state (6, 21, 22, 25, 26, 49). Indeed, up to 70% of clinically obese patients have NAFLD (31). Furthermore, obese patients with NAFLD are at a significantly higher risk of developing obesity-associated comorbidities (e.g., heart disease and Type 2 diabetes) (52). For reasons still poorly understood, a subset of patients with NAFLD will go on to develop NASH (nonalcoholic steatohepatitis) and cirrhosis (10). Despite the prevalence of NAFLD in the general population (28, 50), therapeutic options are limited.
As part of an effort to discover novel secreted metabolic regulators, we recently identified and characterized a family of 15 secreted proteins of the C1q family, designated as C1q/TNF-related proteins (CTRP1–15) (48, 55–57, 60–62). Several of these proteins play important and distinct roles in regulating insulin sensitivity and energy balance (11, 42, 43, 54–56, 60, 61). We demonstrated that CTRP3 acts on liver to suppress hepatic glucose output by modulating the expression of gluconeogenic enzymes (43). A cardioprotective function of CTRP3 was recently demonstrated in an animal model of myocardiac infarction (67). In addition, several other functions attributable to CTRP3, derived from in vitro studies, have been reported (1–3, 16, 23, 24, 33, 34, 58). In the present study, we sought to address the role of CTRP3 in regulating lipid metabolism and its protective function in a pathophysiological context of high-fat feeding. Using a transgenic (Tg) mouse model, along with short-term recombinant protein supplementation, we established an important and novel role for CTRP3 in regulating hepatic TAG metabolism and highlighted its protective function in attenuating diet-induced hepatic steatosis.
EXPERIMENTAL PROCEDURES
Animals.
All animal protocols were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University School of Medicine. CTRP3 Tg mice (on a C57BL/6 genetic background) and control littermates were housed in polycarbonate cages on a 12-h light-dark photocycle with ad libitum access to water and food. Littermates were used throughout the study as wild-type (WT) controls. Mice were fed a high-fat diet (HFD; 60% kcal derived from fat, Research Diets; D12492) or the isocaloric-matched low-fat diet (LFD; 10% kcal derived from fat, Research Diets; D12450B). Diet was provided for a period of 14 wk, beginning at 4 wk of age. Metabolic parameters and food intake were measured by using the Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments), and body composition was determined by using a whole-body NMR instrument (EchoMRI) as previously described (42). At termination of the study, animals were fasted overnight and euthanized, when tissues were collected, snap frozen in liquid nitrogen, and kept at −80°C until analysis.
Antibodies and chemicals.
Mouse monoclonal anti-FLAG M2 antibody was obtained from Sigma. Antibodies that recognize phospho-Akt (Thr-308), phospho-AMPKα (Thr-172), Akt, and AMPKα were obtained from Cell Signaling Technology. Antibody that recognizes actin (sc1616) was obtained from Santa Cruz Biotechnology. Polyclonal rabbit antibody recognizing CTRP3 was obtained from Novus Biologicals (NBP1-02995).
Generation of CTRP3 transgenic mouse line.
Carboxy-terminal FLAG epitope (DYKDDDDK)-tagged CTRP3 was cloned into the EcoRI site of pCAGGS vector (40). Expression of Ctrp3 transgene was driven by the ubiquitous CAG promoter, containing a CMV enhancer element with a chicken β-actin promoter. Plasmid construct was digested with SalI and NotI restriction enzymes, and resulting DNA fragments (∼3.5 and 2.5 kb) were separated on 1% agarose gel. The ∼3.5-kb linear DNA fragment containing the CAG promoter and enhancer, Ctrp3 transgene, and rabbit β-globin polyA adenylation signal was excised from the agarose gel, purified, and verified by DNA sequencing. Pronuclear injections were performed, and several founder lines (on a C57BL/6 genetic background) expressing the Ctrp3 transgene were obtained. One of these mouse lines was maintained and expanded for phenotypic analysis. Tg mice are fertile with no gross abnormality observed.
Mouse serum analysis.
Mouse serum samples were collected at times indicated by using microvette CB 300 (Sarstedt). Glucose concentrations were determined at time of blood collection with a glucometer (BD Biosciences). Serum/tissue TAGs (Thermofisher), nonesterified fatty acids (NEFA; Wako), insulin, tumor necrosis factor-α (TNF-α), and adiponectin (Millipore) were determined with commercially available kits. For Western blot analysis, serum samples were diluted 1:20 in SDS loading buffer (50 mM Tris·HCl, pH 7.4, 2% SDS wt/vol, 6% glycerol wt/vol, 1% 2-mercaptoethanol vol/vol, and 0.01% bromophenol blue wt/vol).
Intraperitoneal glucose and insulin tolerance tests.
Cohorts of 8–10 Tg and WT control littermates were injected with glucose (1 g/kg) or insulin (0.8 units/kg for LFD-fed mice, 1.2 units/kg for HFD-fed mice). Animals were fasted overnight (16 h) prior to the glucose tolerance test. For the insulin tolerance test, food was removed 2 h prior to insulin injection. Serum samples were collected at the indicated time points. Insulin and glucose tolerance tests were performed when mice were 16 and 17 wk of age, respectively.
Measurement of tissue triglyceride levels.
Lipids were extracted as described by Bligh and Dyer (5). Samples were weighed then homogenized in PBS (100 mg/ml) and 1 ml of the sample was added to 3.75 ml of 1:2 (vol/vol) chloroform-methanol. Next, an additional 1.25 ml chloroform were added; subsequently, 1.25 ml distilled water were added to the solution. Samples were vortexed for 30 s between each addition. Samples were then centrifuged at 1,100 g for 10 min at room temperature to give a two-phase solution (aqueous phase on top and organic phase below). The lower phase was collected with a glass Pasteur pipette with gentle positive pressure. This phase was then washed three times with dH2O, and each time the upper phase was collected. Samples were then dried under nitrogen gas at 60°C and dissolved in tert-butyl alcohol-Triton X-100 (3:2). Triglycerides were then quantified colorimetrically as glycerol by use of a commercial enzymatic assay (Infinity Triglycerides, Fisher Diagnostics).
Quantitative real-time PCR.
Total RNAs from mouse tissues were isolated with TRIzol (Invitrogen), and 2 μg of total RNA were reverse transcribed by use of Superscript III (Invitrogen). Quantitative PCR analyses were performed on an Applied Biosystems Prism 7500 Sequence Detection System. Samples were analyzed in 25-μl reactions according to the standard protocol provided in the SYBR Green PCR Master Mix (Applied Biosystems). All expression levels were normalized to the corresponding 18 S rRNA levels. Primer sequences can be found in Supplemental Table S1 (Supplemental Material for this article is available online at the Journal website.).
Quantifying the rate of VLDL-triglyceride secretion.
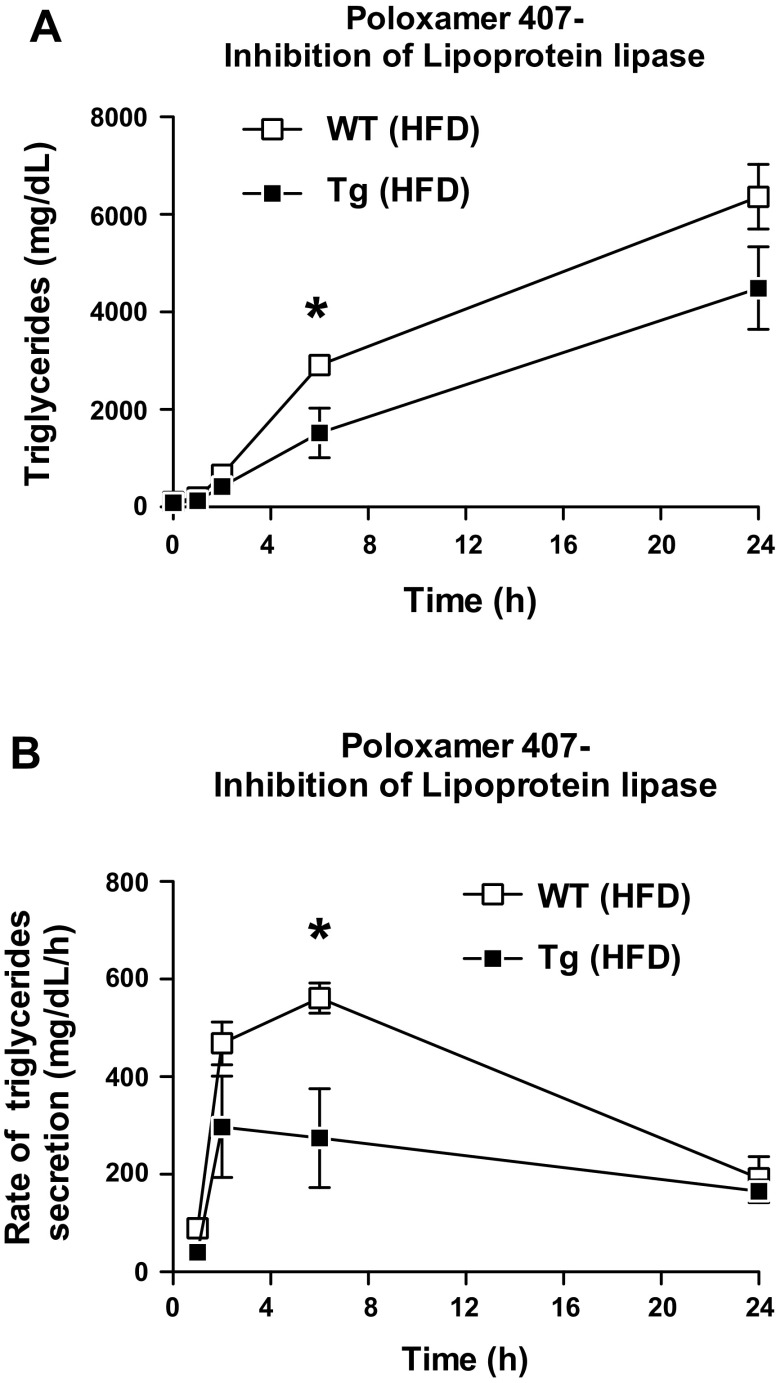
To measure hepatic TAG production rate, a separate cohort of HFD-fed mice (Tg and WT littermates) were given an intraperitoneal injection of 1,000 mg/kg poloxamer 407 (Sigma) in saline ∼4 h into the light cycle, as described by Millar et al. (35). Poloxamer 407 is an inhibitor of lipoprotein lipase and it blocks TAG hydrolysis, thus allowing VLDL-TAG molecules to accumulate over time. This process allows for the calculation of hepatic VLDL-triglyceride secretion rates (35). Serum samples were collected at time 0 and at 1, 2, 6, and 24 h and analyzed for triglyceride concentration. The TAG production rate was calculated from the differences in plasma TAG levels over a given interval following Poloxamer 407 injection.
Immunoblot analysis.
Tissue and cell culture homogenates were prepared by using Tissue Protein Extraction buffer (Pierce) supplemented with phosphatase and protease inhibitors (Calbiochem). Protein concentrations were determined by Bradford assay (Thermo Scientific). Ten micrograms of protein from tissue lysates or 1 μl serum were loaded and separated on a 10% Bis-Tris NuPAGE gel (Invitrogen) and transferred to Protran BA8 nitrocellulose membranes (Whatman). Membranes were blocked in 2% nonfat milk and probed with primary and horseradish peroxidase-conjugated secondary antibodies, and chemiluminescence signals were visualized via ECL (GE Healthcare) with MultiImage III FluorChem Q (Alpha Innotech). Quantification of signal intensity was performed with Alphaview Software (Alpha Innotech). SeeBlue Plus 2 molecular weight markers (Invitrogen) were used in all immunoblot analysis.
Protein purification.
Recombinant full-length mouse CTRP3, containing a COOH-terminal FLAG epitope tag, was produced in HEK 293 mammalian cells (GripTite 293; Invitrogen) and purified as described previously (43). The mammalian expression system ensures proper posttranslational modification and assembly of CTRP3 protein into its correct higher-order structure (61). Sufficient quantity of recombinant protein was purified from ∼6 liters of serum-free conditioned media to enable repeated administration into mice. Purified proteins were dialyzed against 20 mM HEPES buffer (pH 8.0) containing 135 mM NaCl in a 10-kDa cutoff Slide-A-Lyzer dialysis cassette (Pierce). Protein concentration was determined by use of a Coomassie Plus protein assay reagent (Thermo Scientific) before samples were aliquoted and stored at −80°C. The purity of recombinant protein was judged to be >95% by Coomassie blue-stained gel.
Cell culture.
Rat H4IIE hepatoma cells were maintained in Dulbecco's modified Eagle medium containing 10% newborn calf serum (Gemini Bio-product). All cell culture experiments were performed in triplicate. Free fatty acid/BSA (bovine serum albumin) conjugates were prepared as described previously (30). Briefly, a 20 mM solution of free fatty acids in 0.01 M NaOH were incubated at 70°C for 30 min, and the fatty acid soaps were then complexed with 5% BSA in PBS at an 8:1 ratio of fatty acid to BSA. Conjugates were administered to cultured cells at concentrations indicated.
In vitro Oil Red O staining.
Total neutral lipid content was determined by Oil Red O staining as described by Huang et al. (19). H4IIE hepatocytes were grown to confluence. Cells were then treated overnight with 200 μM palmitate, in the presence of vehicle buffer or CTRP3 (5 μg/ml). Cell were then washed with PBS and fixed for 2 min with 4% formaldehyde. A working solution was prepared from a stock solution of 0.5% Oil Red O isopropanol, diluted with water (3:2), and filtered through a 0.45-μm filter. The cells were incubated for 1 h at room temperature. After incubation the cells were washed with water and the Oil Red O staining was extracted in isopropanol. Absorbance was measured at 500 nm with a fluorescent plate reader (model SynergyMxl; Biotek).
Fatty acid uptake assay.
H4IIE cells were washed twice in PBS and placed in stimulation media (0.1% BSA low-glucose, fatty acid-free DMEM) at 37°C and 5% CO2 incubator for 2 h. Next, medium was replaced with the same DMEM containing vehicle control, CTRP3 (5 μg/ml), or insulin (50 nM) and incubated overnight. Cells were transferred to a 37°C water bath where 1 μCi/well (in a 24-well format) of 3H-labeled palmitate (dissolved previously for 1 h in the fatty-acid-free DMEM containing 0.1% BSA) was added for either 10, 30, or 60 s. Medium was then aspirated out and cells were washed twice in cold PBS. Cells were lysed in 10% SDS and transferred to a scintillation vial. Radioactive counts were measured and normalized to protein concentration of cell lysate.
Fatty acid synthesis.
Fatty acid synthesis was determined via measurement of [3H]acetate incorporation into cells as previously described (44). Briefly, H4IIE hepatocytes were grown to confluence in a 24-well plate. Cells were then treated with vehicle buffer or CTRP3 (5 μg/ml) for 2 h. Next, cold acetic acid (100 mM) and 0.2 μCi/well [3H]acetic acid (American Radiolabeled Chemicals) were added to the media. After 2 h incubation, cells were washed in PBS and lipids were extracted with varying amounts of chloroform-methanol and MgCl2. Sample was resuspended in a liquid scintillation vial and the amount of 3H radioactivity was determined by use of a Beckman Coulter counter (model LS6000SC).
Recombinant protein injection.
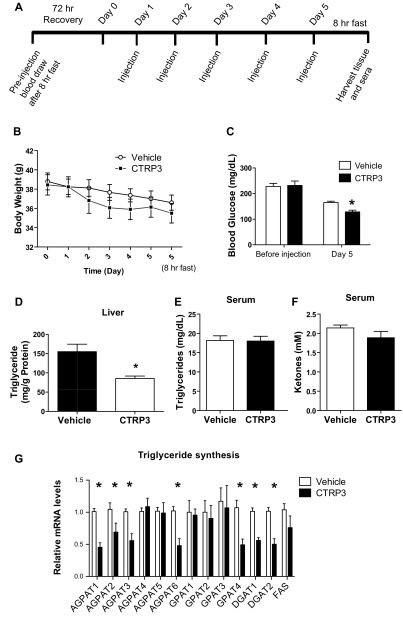
A separate cohort of 4-wk-old C57BL/6 male mice was obtained from the Jackson Laboratory. After 1 wk of acclimatization the mice were placed on an HFD for 12 wk. Mice were fasted for 8 h to obtain initial blood draw (2 h into dark cycle) and then allowed to recover for 72 h with ad libitum access to food. After recovery, initial body weight (considered day 0) was determined. Body weight was measured daily and CTRP3 (2 μg/g body wt) or vehicle buffer was administered daily via intraperitoneal route for the next 5 days. Injections were given at the same time each day (6 h into light cycle). After the fifth injection, food was immediately removed and mice were fasted for 8 h before final blood and tissue collections were performed.
Statistical analyses.
Body weights, glucose and insulin tolerance test, and pre/post data from CTRP3 injection experiments were analyzed by a repeated-measures analysis of variance followed by Tukey post hoc analysis. All remaining statistical analyses were performed by a one-way analysis of variance. Statistical analyses were performed with GraphPad Prism 5 statistical software. Statistical significance was accepted at P < 0.05. All data are reported as means ± SE.
RESULTS
Generation of CTRP3 Tg mouse line.
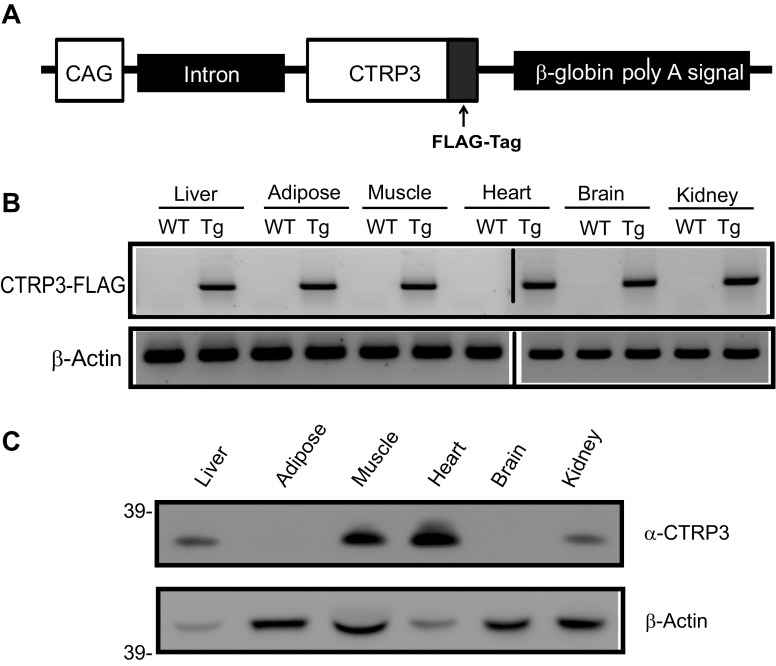
We generated a Tg mouse model overexpressing FLAG epitope-tagged CTRP3. Because CTRP3 is a secreted protein and is normally expressed in multiple tissues and cell types in both mouse and human (32, 47, 61), we chose to express the Ctrp3 transgene using a ubiquitous promoter (Fig. 1A). As expected, the Tg mouse line has greater than fivefold higher circulating levels of CTRP3 over baseline serum levels found in wild-type mice (data not shown). At the mRNA level, Ctrp3 transgene was expressed in all tissues examined (Fig. 1B). At the protein level, we detected FLAG-tagged CTRP3 in the liver, heart, muscle, and kidney, but not in brain or adipose tissue (Fig. 1C).
Fig. 1.
Generation of CTRP3 Tg mice. A: schematic of Ctrp3 transgenic construct. FLAG-tagged Ctrp3 transgene is driven by a ubiquitous CAG promoter. B: semiquantitative RT-PCR analysis of Ctrp3 transgene expression in mouse tissues; β-actin was included as control. C: immunoblot analysis for the presence of CTRP3-FLAG protein in mouse tissues. β-Actin levels serve as loading control. WT, wild-type; Tg, Transgenic.
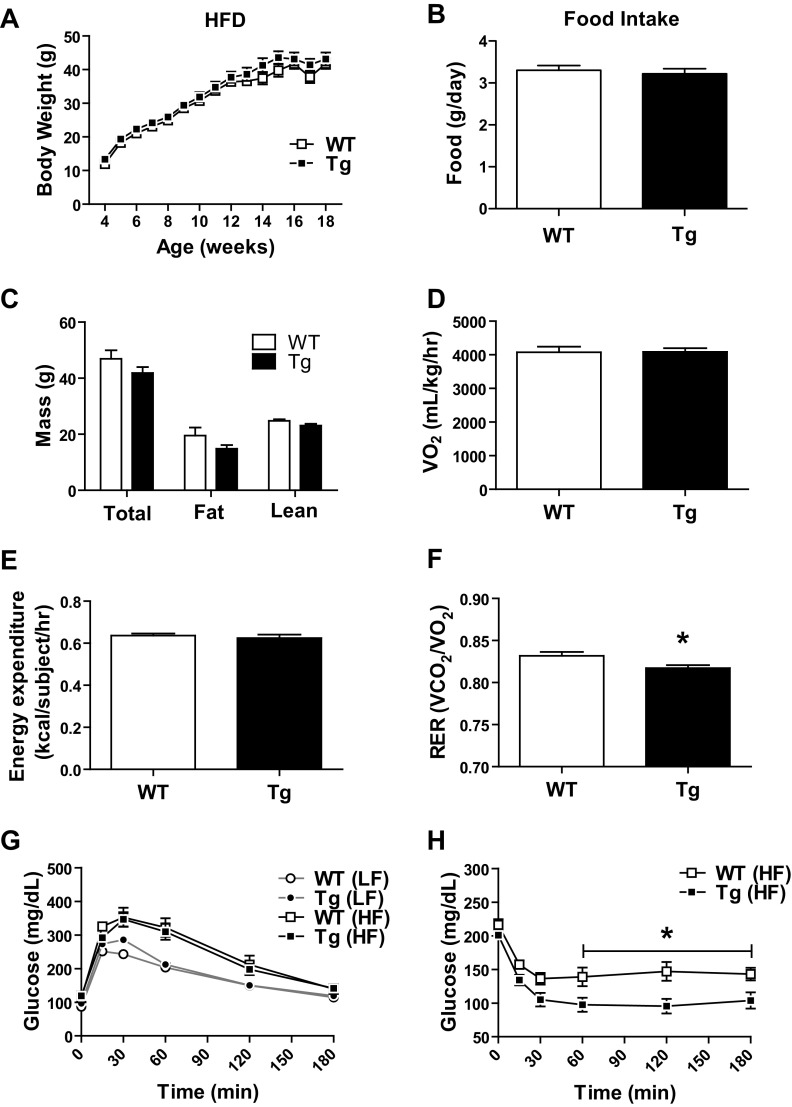
Body weight gain and energy expenditure in response to HFD.
The CTRP3 Tg mice developed normally with no obvious phenotype. Body weight gains on LFD (data not shown) and HFD (Fig. 2B) over a period of 14 wk were indistinguishable between Tg and WT mice. No differences were observed in food intake, total fat, or lean body mass between Tg and WT mice fed an LFD (data not shown) or an HFD (Fig. 2, B and C). Oxygen consumption (indicative of basal metabolic rate) and energy expenditure were also similar between HFD-fed Tg and WT mice (Fig. 2, D and E). However, we did observe a modest, but significant, reduction in respiratory exchange ratio (RER) in Tg mice relative to littermate controls (Fig. 2F), indicating a greater utilization of fatty acids as fuel source. No differences in glucose tolerance were observed between Tg and WT mice fed an LFD (data not shown) or an HFD (Fig. 2G), nor were there any differences in the magnitude of insulin secretion between the two groups in response to glucose injection (data not shown). When subjected to insulin tolerance test, however, Tg mice on an HFD clearly demonstrated greater insulin sensitivity relative to WT controls, as indicated by a sustained and significantly greater reduction in blood glucose levels after insulin administration (Fig. 2H).
Fig. 2.
Improved insulin tolerance in Tg mice without changes in other metabolic parameters. A: no differences in body weight gain over time between WT and Tg male mice fed a high-fat diet (HFD). B: food intake in Tg and WT mice. C: total body mass, fat mass, and lean mass of HFD-fed WT and Tg mice. D–F: indirect calorimetry analysis of oxygen consumption (D), energy expenditure (E), respiratory exchange ratio (RER = V̇co2/V̇o2; F) in HFD-fed Tg and WT mice. G: glucose tolerance test on HFD-fed Tg and WT mice. H: insulin tolerance test on HFD-fed Tg and WT mice. Body weight measurements and glucose and insulin tolerance tests were repeated with multiple cohorts of HFD-fed WT and Tg mice (n = 8–10 per group). Data reported are the results from 1 cohort, with results similar across cohorts. Data are reported as means ± SE of 8–10 mice per group. *P < 0.05 vs. WT. LF, low-fat diet; HF, High-fat diet. V̇o2, volume of oxygen consumption; V̇co2, volume of carbon dioxide produced; RER, respiratory exchange ratio.
Fasting serum analysis of HFD-fed Tg and WT mice.
Serum levels of hormones and metabolites are tightly linked to metabolic state. We therefore performed blood chemistry analysis on WT and Tg mice. We did not observe any improvements in fasting glucose, insulin, glucagon, NEFA, TAGs, or adiponectin levels following HFD in Tg mice relative to control littermates (Table 1). However, we observed a substantial reduction in serum cholesterol (22%), LDL (31%), and HDL (13%) levels in Tg mice compared with littermate controls (Table 1). Low-grade chronic inflammation, reflected in elevated plasma levels of TNF-α, is frequently associated with obesity (17). Strikingly, we also observed a marked reduction (66%) in the circulating levels of TNF-α in Tg mice relative to controls (Table 1).
Table 1.
Blood chemistry analysis of WT and Tg mice
| Serum Marker | WT | Tg | P Value |
|---|---|---|---|
| Insulin, ng/ml | 1.3 ± 0.18 | 1.7 ± 0.18 | ns |
| Glucose, mg/dl | 109.6 ± 6.5 | 101.9 ± 10.1 | ns |
| Glucagon, pM | 13.3 ± 0.32 | 13.8 ± 0.26 | ns |
| Adiponectin, μg/ml | 12.5 ± 1.3 | 13.0 ± 1.3 | ns |
| TNF-α, pg/ml | 6.4 ± 2.0 | 2.2 ± 0.3 | P < 0.01 |
| Cholesterol (total), mg/dl | 138.0 ± 8.6 | 108.5 ± 6.3 | P < 0.01 |
| LDL, mg/dl | 67.2 ± 6.2 | 46.4 ± 3.9 | P < 0.01 |
| HDL, mg/dl | 59.4 ± 2.3 | 51.5 ± 2.8 | P < 0.01 |
| NEFA, mEq/l | 0.89 ± 0.06 | 0.97 ± 0.07 | ns |
| Triglycerides, mg/dl | 41.9 ± 3.6 | 43.9 ± 3.2 | ns |
WT, wild-type; Tg, transgenic; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NEFA, nonesterified fatty acids.
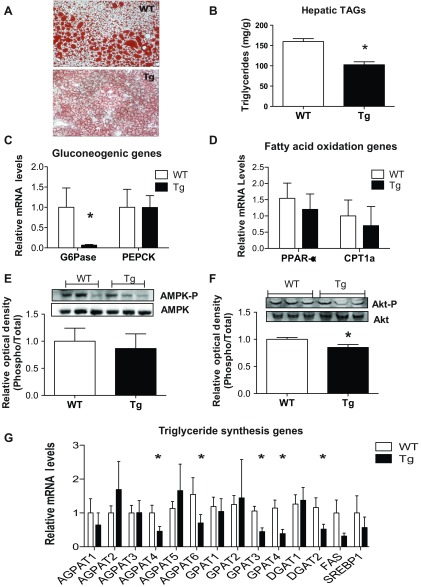
Reduced expression of lipid synthesis genes and hepatic TAG levels in Tg mice.
When liver sections were stained with Oil Red O to detect the presence of neutral lipids, dramatic differences were observed between Tg and WT mice (Fig. 3A), clearly indicating a striking resistance of Tg mice to developing hepatic steatosis in response to HFD. Quantification of hepatic TAG levels confirmed a 38% reduction in TAG levels in Tg mice relative to control littermates (Fig. 3B). Expression of hepatic glucose-6-phosphatase (G6pase), a key gluconeogenic enzyme, was reduced by 90% in Tg mice (Fig. 3C), confirming our previous study based on recombinant CTRP3 protein administration (43). Expression of hepatic Ppar-α, a major transcriptional regulator of fat oxidation genes, was not changed between Tg and WT mice (Fig. 3D), nor were there any differences in the expression of genes directly involved in fat oxidation (e.g., Cpt1a, Acoxs, Acads) (Fig. 3D and data not shown). As observed following acute CTRP3 protein administration (43), no significant differences in the phosphorylation levels of AMPKα were detected in the liver of Tg and WT mice (Fig. 3E). In contrast to acute recombinant protein administration (43), when plasma CTRP3 protein was chronically elevated as in Tg mice, we observed a modest reduction in hepatic Akt phosphorylation (Fig. 3F). Importantly, the expression levels of a number of genes involved in TAG synthesis were substantially reduced in the liver of Tg mice relative to control littermates (Fig. 3G).
Fig. 3.
Reduced hepatic triglyceride content and synthesis in CTRP3 Tg mice. A: representative Tg and WT mouse liver sections stained with Oil Red O. B: quantification of hepatic triglyceride content. C: quantification of mRNA expression of gluconeogenic genes in liver, normalized against 18 S rRNA. D: quantification of mRNA expression of representative fatty acid oxidation genes in liver, normalized against 18 S rRNA. E and F: quantitative immunoblot analysis of liver AMPKα (Thr-172) (E) and Akt (Ser-473) (F) phosphorylation in WT and Tg mice. G: quantification of mRNA expression of enzymes involves in triglyceride synthesis. All data are reported as comparisons between WT and Tg mice on an HFD (n = 8–10 per group). Phosphorylated protein levels were normalized to total protein levels. All data are reported as means ± SE. *P < 0.05 vs. WT.
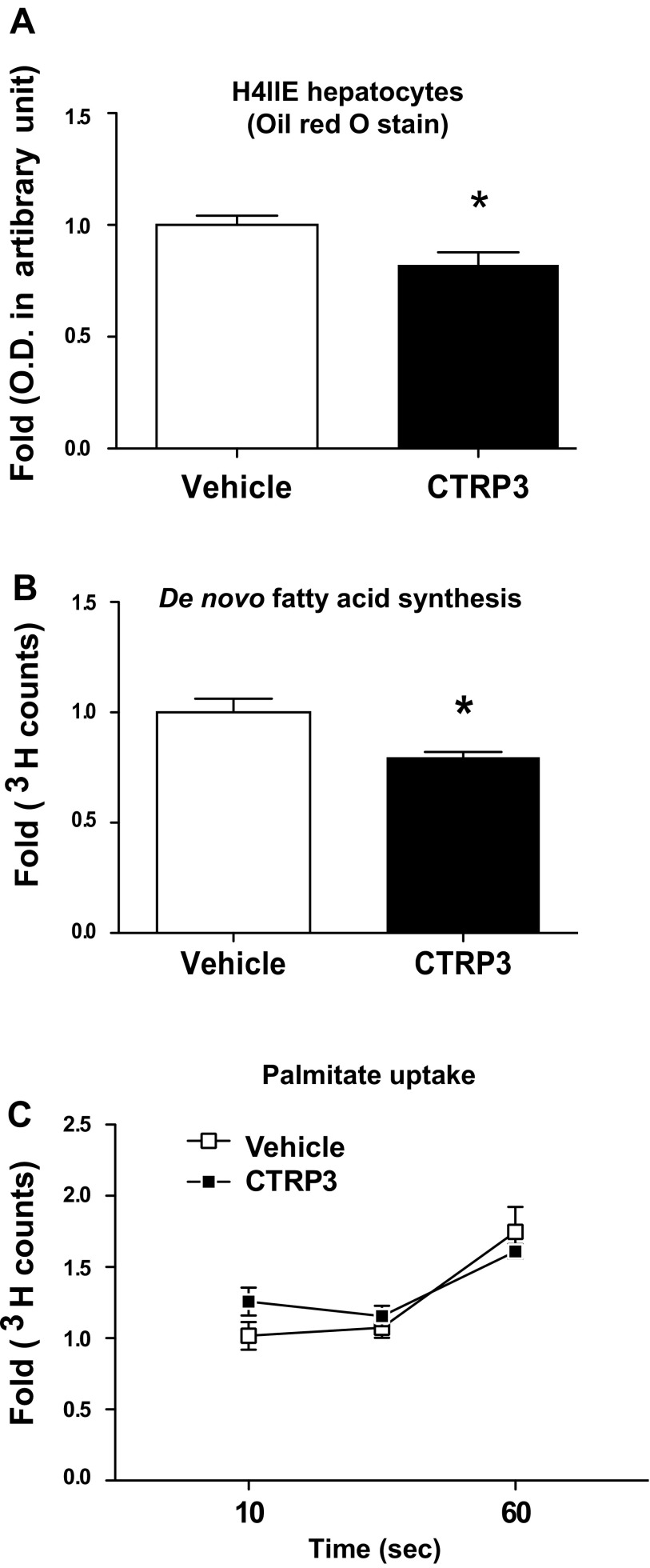
CTRP3 reduces fatty acid synthesis and neutral lipid accumulation in cultured hepatoma cells.
A cell culture system was used to confirm our in vivo findings and to demonstrate that CTRP3 protein directly regulates lipid metabolism in liver cells. When rat H4IIE hepatocytes were coincubated overnight with recombinant CTRP3 protein and 200 μM oleic acid conjugated to BSA to promote lipid loading, the amount of neutral lipids (mainly TAGs) accumulated in cells was significantly reduced (∼20%) compared with vehicle-treated controls (Fig. 4A). Whereas the uptake of exogenous fatty acids was not affected by CTRP3 protein treatment (Fig. 4C), de novo fatty acid synthesis, as measured by radiolabeled acetate incorporation, was suppressed (∼22%) in H4IIE cells treated with CTRP3 protein (Fig. 4B).
Fig. 4.
Recombinant CTRP3 treatment reduces lipid accumulation in vitro. A: CTRP3 treatment reduces the accumulation of neutral lipids in rat H4IIE hepatocytes treated overnight with 200 μM palmitate and CTRP3 (5 μg/ml), as quantified by Oil Red O staining. B: CTRP3 decreases de novo lipid synthesis in H4IIE hepatocytes, as quantified by [3H]acetate incorporation. C: no change in lipid uptake as measured by [3H]palmitate uptake by H4IIE hepatocytes pretreated with vehicle or CTRP3. Values are mean fold ± SE. *P < 0.05 vs. vehicle.
Measurement of VLDL-TAG export in Tg and WT mice.
To assess the rate and magnitude of VLDL-TAG secretion from the liver, a separate cohort of HFD-fed mice was injected with poloxamer 407, an inhibitor of lipoprotein lipase that blocks VLDL-TAG hydrolysis and clearance (35). Tg mice given poloxamer 407 had a significantly reduced TAG accumulation in the blood (Fig. 5A) and a reduced rate of TAG secretion from the liver (Fig. 5B). Since TAGs are mainly secreted from the liver as VLDL particles, these results suggest that the reduction in hepatic TAG accumulation in Tg mice is indeed due to the suppression of TAG synthesis (Fig. 3) and not caused by increased hepatic VLDL-TAG export.
Fig. 5.
Reduced export of VLDL-triglycerides from the liver of Tg mice. A: triglyceride content was measured in plasma samples taken at 0, 1, 2, 6, and 24 h after poloxamer 407 (lipoprotein lipase inhibitor) administration. B: rate of triglyceride accumulation was calculated for each time frame indicated. *P < 0.05 vs. vehicle (n = 8 mice per group).
Short-term administration of recombinant CTRP3.
Next, we conducted a short-term recombinant protein supplementation study to further ensure that the remarkable phenotype we observed in the liver of Tg mice is directly attributable to elevated plasma CTRP3 levels and not due to potential secondary effects of transgene overexpression. To address this issue, a separate cohort of WT mice was placed on an HFD for 12 wk to induce obesity and the development of fatty liver. DIO mice have similar starting body weights to one another and were given a daily injection of vehicle or recombinant CTRP3 protein (2 μg/g body wt) for 5 consecutive days as outlined (Fig. 6A). Both vehicle- and CTRP3-treated DIO mice lost ∼2 g of body weight during the course of the experiment (Fig. 6B). Consistent with our previous findings, in which a single dose of CTRP3 injection acutely reduces blood glucose levels (43), DIO mice that received a 5-day injection also had a 22% reduction in blood glucose levels (Fig. 6C). Strikingly, recombinant protein administration over 5 days resulted in a 43% reduction in hepatic TAGs (vehicle, 155.2 ± 19.4 mg/g vs. CTRP3, 88.6 ± 6.3 mg/g). Serum levels of TAGs and ketones were not different between the two groups of DIO mice (Fig. 6, E and F). Serum ketone levels reflect the extent of hepatic fat oxidation; thus unchanged ketone levels provide further support that hepatic fat oxidation may not be responsible for the reduction of TAG content in the liver of mice injected with recombinant protein. As with the Tg mice, reduced hepatic TAG in CTRP3-injected DIO mice was due to major reduction in the expression of most hepatic enzyme genes involved in TAG synthesis (Fig. 6G).
Fig. 6.
Short-term administration of recombinant CTRP3 reduces hepatic triglyceride levels in diet-induced obese (DIO) mice. A: time line depicting the daily injection study. After 12 wk on a high-fat diet, wild-type DIO mice were fasted for 8 h before initial blood draw. After 72-h recovery from the initial fast (considered day 0), body weight of DIO mice was determined. CTRP3 (2 μg/g body wt) or vehicle injection was given every 24 h for the next 5 days. After the 5th injection, food was immediately removed, and after an 8-h fast animals were euthanized and liver tissues and sera were harvested. B: daily body weight of vehicle- and CTRP3-injected DIO mice. C: pre- and posttreatment fasting (8 h) blood glucose levels. D: hepatic triglyceride contents in vehicle- and CTRP3-injected DIO mice. E and F: serum triglyceride (E) and ketone (F) levels in vehicle- and CTRP3-injected DIO mice.
DISCUSSION
In the present study, we provided multiple lines of evidence to establish the role of CTRP3 in regulating hepatic lipid metabolism. Tg mice with elevated plasma levels of CTRP3 are strikingly resistant to the development of HFD-induced hepatic steatosis, independent of other metabolic parameters such as food intake, body weight, adiposity, and energy expenditure. Three possible mechanisms involving production and/or removal of TAG could account for the marked reduction in liver TAG content in Tg mice on an HFD: 1) increased hepatic fat oxidation; 2) increased TAG export from liver in the form of VLDL-TAG particles; 3) decreased synthesis of TAG in liver. Our in vivo and in vitro data suggest that CTRP3-mediated suppression of TAG synthesis is primarily responsible for reduced hepatic TAG content seen in Tg mice.
In liver, TAG is synthesizes via the glycerol phosphate pathway (4) through sequential acylation of glycerol-3 phosphate, lysophosphatidic acid, and diacylglycerol by multiple isoforms of GPAT, AGPAT, and DGAT enzymes (51, 66). We show that the expression of these enzymes in liver is significantly suppressed in HFD-fed CTRP3 Tg and wild-type DIO mice administered recombinant CTRP3, thus contributing to reduced hepatic lipid content seen in these animals relative to controls. Remarkably, daily supplementation of recombinant protein for 5 days is sufficient to markedly reduce hepatic TAG levels in wild-type DIO mice, confirming that the improved liver phenotype in Tg mice is due to elevated plasma CTRP3 levels and not a consequence of secondary effects of transgene overexpression. We also noted that serum adiponectin levels were not different between Tg and WT mice, indicating that decreased hepatic TAG content is unlikely due to adiponectin, an adipokine known to alleviate diet-induced hepatic steatosis in mice (64, 65). Adiponectin alleviates hepatic steatosis largely through increasing liver fat oxidation (64); expression of TAG synthesis genes (Gpat, Agpat, and Dgat) were not examined. In contrast, CTRP3 ameliorates fatty liver by reducing hepatic triglyceride synthesis. We cannot completely rule out the possibility that enhanced hepatic fat oxidation may also play a role, as indicated by the lower RER in Tg mice. Even a modest increase in fat oxidation, over time, could potentially result in reduced hepatic TAG content.
We observed a very modest improvement in insulin sensitivity, as judged by insulin but not glucose tolerance test, in HFD-fed CTRP3 Tg mice. This is likely due to improved insulin action in the liver but not skeletal muscle (43). Consistent with this, we did not observe any changes in Akt phosphorylation in the skeletal muscle of Tg mice or WT mice injected with recombinant CTRP3 protein (data not shown). Although the potential function of CTRP3 in skeletal muscle remains unclear, a cardioprotective function of CTRP3 was recently demonstrated in an animal model of myocardiac infarction (67), indicating that CTRP3 plays an important role in the heart.
Excessive fat deposition in hepatocytes, a hallmark of steatosis, is frequently associated with hepatic insulin resistance (25, 26, 46, 49). Whether hepatic steatosis causes or is a consequence of insulin resistance is a hotly debated issue (10, 13, 39, 46). Two recent studies using transgenic overexpression of diacylglycerol O-acyltransferase 2 (DGAT2) in mouse liver to alter hepatic lipid content have yielded contradictory results on hepatic insulin sensitivity (21, 37). Also, several other mouse models, with reduced fatty acid synthesis (8), mobilization (5a, 18, 36, 63), or oxidation (38), developed hepatic steatosis without accompanying insulin resistance. Given the very modest improvements in insulin sensitivity seen in the HFD-fed CTRP3 Tg mice compared with littermate controls, it is unclear whether this modest phenotype is due to reduced hepatic lipid content. The mechanistic link between hepatic steatosis and insulin resistance remains to be fully established (39) and is not the focus of present study. Rather, we aim here to establish the role of CTRP3 in regulating lipid metabolism.
We have previously shown that a single injection of recombinant CTRP3 acutely lowered blood glucose levels in WT and genetically obese (ob/ob) mice (43). The CTRP3-mediated suppression of hepatic gluconeogenesis is correlated with the activation of protein kinase B/Akt. In contrast, chronic overexpression of CTRP3 in Tg mice resulted in decreased Akt activation with no change in Pepck expression despite a marked suppression of G6Pase expression (Fig. 3, C and F). These results suggest that CTRP3 can inhibit hepatic G6Pase expression independent of Akt signaling whereas the suppression of Pepck expression is likely Akt dependent (29). Although chronic overexpression of CTRP3 in Tg mice did not lower fasting blood glucose levels (Table 1), short-term administration of recombinant CTRP3 (one injection per day for 5 days) significantly reduced fasting blood glucose levels in DIO mice (Fig. 6C). The glucose-lowering effect seen in DIO mice is similar to WT and ob/ob mice acutely injected with recombinant CTRP3 (43). Because blood glucose levels are tightly regulated, chronic overexpression of CTRP3 in Tg mice may result in homeostatic compensation to prevent hypoglycemia induced by CTRP3. This may account for the lack of differences in fasting blood glucose levels between WT and Tg mice.
Tg mice fed an HFD have reduced hepatic TAG content compared with WT mice. However, no differences were observed in serum TAG levels between the two groups. The blood chemistry analysis (Table 1) was conducted on sera harvested from overnight-fasted mice. In contrast to the fed state, in which TAG are secreted from liver in the form of VLDL, in the fasted state free fatty acids derived from adipose triglycerides were shunted to the liver to fuel gluconeogenesis and ketones production. Under the fasted state, we did not observe any difference in the steady-state levels of serum TAG between WT and Tg mice.
Interestingly, we observed a decrease in the circulating levels of TNF-α in Tg mice, likely reflecting a dampening of chronic low-grade systemic inflammation associated with high-fat feeding (15, 17). Our in vivo observation is consistent with a previous study demonstrating the ability of recombinant CTRP3 protein to inhibit TNF-α release from primary human macrophages isolated from healthy donors (24). Mice lacking TNF-α or its receptors are protected from obesity-induced insulin resistance (53). Therefore, lower serum levels of TNF-α seen in CTRP3 Tg mice may contribute to the modest improvement in systemic insulin sensitivity.
A reversal or improvement in hepatic steatosis is possible through lifestyle modifications such as reduced energy intake and/or weight loss (41), as well as gastric bypass surgery (31). However, lifestyle changes are often difficult to sustain, necessitating alternative treatment options. One way to reduce liver TAG content is by decreasing TAG synthesis. Previous proof-of-principle studies using siRNA targeting DGAT2 or small molecule inhibitor of GPAT or DGAT1 have demonstrated the feasibility of attenuating hepatic steatosis in rodent (7, 9, 27). In the present study, we show that increasing plasma CTRP3 levels can significantly suppress TAG synthesis through downregulation of TAG synthesis genes (i.e., Agpat, Gpat, and Dgat), thereby improving the fatty liver phenotype in mice without affecting food intake and body weight. This highlights the potential therapeutic value of recombinant CTRP3 protein supplementation in mitigating NAFLD in humans. Given that siRNA or small molecule inhibitor of enzyme often has unintended off-target effects (12, 14, 20), the use of recombinant protein therapy to treat obesity-linked fatty liver may prove to be advantageous.
In sum, we provide novel insights into the metabolic function of CTRP3 and reveal, for the first time, its protective function in liver in response to excess caloric intake. Our data suggest the utility of recombinant CTRP3 as a potential protein therapeutic for treating obesity-associated fatty liver disease.
GRANTS
This work was supported by grants from the National Institutes of Health (DK084171 to G. W. Wong and F32DK084607 to J. M. Peterson) and the American Heart Association (SDG2260721 to G. W. Wong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.P. and G.W.W. conception and design of research; J.M.P., M.M.S., Z.W., and G.W.W. performed experiments; J.M.P., S.A., and G.W.W. analyzed data; J.M.P., S.A., and G.W.W. interpreted results of experiments; J.M.P. prepared figures; J.M.P. and G.W.W. drafted manuscript; J.M.P., M.M.S., Z.W., S.A., and G.W.W. approved final version of manuscript; M.M.S. edited and revised manuscript.
Supplementary Material
REFERENCES
- 1. Akiyama H, Furukawa S, Wakisaka S, Maeda T. Cartducin stimulates mesenchymal chondroprogenitor cell proliferation through both extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways. FEBS J 273: 2257–2263, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Akiyama H, Furukawa S, Wakisaka S, Maeda T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem 304: 243–248, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Akiyama H, Furukawa S, Wakisaka S, Maeda T. Elevated expression of CTRP3/cartducin contributes to promotion of osteosarcoma cell proliferation. Oncol Rep 21: 1477–1481, 2009. [DOI] [PubMed] [Google Scholar]
- 4. Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem 49: 459–487, 1980. [DOI] [PubMed] [Google Scholar]
- 5. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 5a. Brown JM, Betters JL, Lord C, Ma Y, Han X, Yang K, Alger HM, Melchior J, Sawyer J, Shah R, Wilson MD, Liu X, Graham MJ, Lee R, Crooke R, Shulman GI, Xue B, Shi H, Yu L. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J Lipid Res 51: 3306–3315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147–152, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao J, Zhou Y, Peng H, Huang X, Stahler S, Suri V, Qadri A, Gareski T, Jones J, Hahm S, Perreault M, McKew J, Shi M, Xu X, Tobin JF, Gimeno RE. Targeting acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J Biol Chem 286: 41838–41851, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309–322, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282: 22678–22688, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 332: 1519–1523, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 functions as an adipokine that improves glucose metabolism. J Biol Chem 286: 34552–34558, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fabian MA, Biggs WH, 3rd, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lelias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23: 329–336, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab 15: 570–573, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by siRNA can induce toxic phenotype. RNA 12: 1188–1196, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, Falk W, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 17: 2462–2471, 2011. [DOI] [PubMed] [Google Scholar]
- 17. Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Hoy AJ, Bruce CR, Turpin SM, Morris AJ, Febbraio MA, Watt MJ. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology 152: 48–58, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Huang H, Lane MD, Tang QQ. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3–L1 preadipocytes. Biochem Biophys Res Commun 338: 1185–1188, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread siRNA “off-target” transcript silencing mediated by seed region sequence complementarity. RNA 12: 1179–1187, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci USA 108: 5748–5752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ, Breslow JL, Shulman GI. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA 98: 7522–7527, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M, Scholmerich J, Schaffler A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 151: 5267–5278, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Kopp A, Bala M, Weigert J, Buchler C, Neumeier M, Aslanidis C, Scholmerich J, Schaffler A. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 49: 51–57, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia 51: 130–138, 2008. [DOI] [PubMed] [Google Scholar]
- 26. Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 293: E1709–E1715, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Kuhajda FP, Aja S, Tu Y, Han WF, Medghalchi SM, El Meskini R, Landree LE, Peterson JM, Daniels K, Wong K, Wydysh EA, Townsend CA, Ronnett GV. Pharmacological glycerol-3-phosphate acyltransferase inhibition decreases food intake and adiposity and increases insulin sensitivity in diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 301: R116–R130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 28: 339–350, 2008. [DOI] [PubMed] [Google Scholar]
- 29. Liao J, Barthel A, Nakatani K, Roth RA. Activation of protein kinase B/Akt is sufficient to repress the glucocorticoid and cAMP induction of phosphoenolpyruvate carboxykinase gene. J Biol Chem 273: 27320–27324, 1998. [DOI] [PubMed] [Google Scholar]
- 30. Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem 276: 14890–14895, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Luyckx FH, Desaive C, Thiry A, Dewe W, Scheen AJ, Gielen JE, Lefebvre PJ. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord 22: 222–226, 1998. [DOI] [PubMed] [Google Scholar]
- 32. Maeda T, Abe M, Kurisu K, Jikko A, Furukawa S. Molecular cloning and characterization of a novel gene, CORS26, encoding a putative secretory protein and its possible involvement in skeletal development. J Biol Chem 276: 3628–3634, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Maeda T, Jikko A, Abe M, Yokohama-Tamaki T, Akiyama H, Furukawa S, Takigawa M, Wakisaka S. Cartducin, a paralog of Acrp30/adiponectin, is induced during chondrogenic differentiation and promotes proliferation of chondrogenic precursors and chondrocytes. J Cell Physiol 206: 537–544, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Maeda T, Wakisaka S. CTRP3/cartducin is induced by transforming growth factor-beta1 and promotes vascular smooth muscle cell proliferation. Cell Biol Int 34: 261–266, 2010. [DOI] [PubMed] [Google Scholar]
- 35. Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res 46: 2023–2028, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Minehira K, Young SG, Villanueva CJ, Yetukuri L, Oresic M, Hellerstein MK, Farese RV, Jr, Horton JD, Preitner F, Thorens B, Tappy L. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J Lipid Res 49: 2038–2044, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007. [DOI] [PubMed] [Google Scholar]
- 38. Monsenego J, Mansouri A, Akkaoui M, Lenoir V, Esnous C, Fauveau V, Tavernier V, Girard J, Prip-Buus C. Enhancing liver mitochondrial fatty acid oxidation capacity in obese mice improves insulin sensitivity independently of hepatic steatosis. J Hepatol 56: 632–639, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res 50 Suppl: S74–S79, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108: 193–199, 1991. [DOI] [PubMed] [Google Scholar]
- 41. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54: 603–608, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem 287: 1576–1587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res 56: 2745–2747, 1996. [PubMed] [Google Scholar]
- 45. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest 118: 829–838, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375: 2267–2277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schaffler A, Ehling A, Neumann E, Herfarth H, Paul G, Tarner I, Gay S, Scholmerich J, Muller-Ladner U. Genomic organization, promoter, amino acid sequence, chromosomal localization, and expression of the human gene for CORS-26 (collagenous repeat-containing sequence of 26-kDa protein). Biochim Biophys Acta 1630: 123–129, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14: 804–810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int 32: 945–950, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389: 610–614, 1997. [DOI] [PubMed] [Google Scholar]
- 54. Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 287: 35804–35814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 286: 15652–15665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/TNF-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wolfing B, Buechler C, Weigert J, Neumeier M, Aslanidis C, Schoelmerich J, Schaffler A. Effects of the new C1q/TNF-related protein (CTRP-3) “cartonectin” on the adipocytic secretion of adipokines. Obesity (Silver Spring) 16: 1481–1486, 2008. [DOI] [PubMed] [Google Scholar]
- 60. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 23: 241–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54: 122–132, 2011. [DOI] [PubMed] [Google Scholar]
- 64. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. [DOI] [PubMed] [Google Scholar]
- 66. Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49: 2283–2301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 125: 3159–3169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.