Abstract
The Brunner's glands of the proximal duodenum exert barrier functions through secretion of glycoproteins and antimicrobial peptides. However, ion transporter localization, function, and regulation in the glands are less clear. Mapping the subcellular distribution of transporters is an important step toward elucidating trafficking mechanisms of fluid transport in the gland. The present study examined 1) changes in the distribution of intestinal anion transporters and the aquaporin 5 (AQP5) water channel in rat Brunner's glands following second messenger activation and 2) anion transporter distribution in Brunner's glands from healthy and disease-affected human tissues. Cystic fibrosis transmembrane conductance regulator (CFTR), AQP5, sodium-potassium-coupled chloride cotransporter 1 (NKCC1), sodium-bicarbonate cotransporter (NBCe1), and the proton pump vacuolar ATPase (V-ATPase) were localized to distinct membrane domains and in endosomes at steady state. Carbachol and cAMP redistributed CFTR to the apical membrane. cAMP-dependent recruitment of CFTR to the apical membrane was accompanied by recruitment of AQP5 that was reversed by a PKA inhibitor. cAMP also induced apical trafficking of V-ATPase and redistribution of NKCC1 and NBCe1 to the basolateral membranes. The steady-state distribution of AQP5, CFTR, NBCe1, NKCC1, and V-ATPase in human Brunner's glands from healthy controls, cystic fibrosis, and celiac disease resembled that of rat; however, the distribution profiles were markedly attenuated in the disease-affected duodenum. These data support functional transport of chloride, bicarbonate, water, and protons by second messenger-regulated traffic in mammalian Brunner's glands under physiological and pathophysiological conditions.
Keywords: ion transporters, Brunner's glands, regulated traffic
duodenal brunner's glands are distinct structures consisting of clusters of serous cells and branching tubules that are located in the submucosal layer of the proximal duodenum in mammals. Brunner's glands are innervated by cholinergic vagal and polypeptidergic nerves that release vasoactive intestinal peptide (VIP) (42, 54, 68). The glands were thought to be either an extension of the pyloric glands or an accessory pancreas because they first appear at the pyloro-duodenum junction and extend along the proximal-distal duodenal axis in decreasing abundance up to the entry site of the biliary/pancreatic duct. Brunner's glands arise from the base of the crypts in 19-day-old rat embryos (29, 51). The ducts of the glands drain into the base of crypts in rats and humans and into the duodenal lumen in cats (58, 105).
The factors that mediate secretion from duodenal crypt and villus epithelial cells are better understood than those regulating Brunner's gland acinar cells (23). Secretion of glycoproteins, trypsin inhibitors, and antimicrobial peptides including lysozyme was demonstrated in a number of studies indicating an important role for the gland in duodenal epithelial barrier functions (Tables 1 and 2) (9, 16, 46). Acetylcholine and VIP stimulate the secretion of epidermal growth factor from Brunner's glands (44) that can inhibit gastric acid secretion (10). Similarly, secretion of insulin-like growth factor 1 (88) promotes immunological defense, cellular proliferation, and differentiation and contributes factors that promote pancreatic secretions (50).
Table 1.
Factors present in the Brunner's glands
| Factors Detected in the Brunner's Glands | References |
|---|---|
| IgA and IgM | 16 |
| Trefoil peptides | 59, 61, 87 |
| Lipase | 65, 101 |
| Adenosine deaminase complex proteins | 91 |
| Pepsinogens | 60 |
| Enterokinase and duodenase | 50, 116 |
| Pancreatic secretory trypsin inhibitor | 9 |
| Proteinase inhibitor | 46 |
| Prostaglandin H synthase (Cox-1) | 37 |
| Thyroid hormone-binding protein | 111 |
| Intestinal cholecystokinin (CCK)-releasing factor | 102 |
Table 2.
Summary of domain specific localization of proteins and factors in Brunner's glands
| Localization | References | |
|---|---|---|
| Ion transporters | ||
| CFTR | Apical membrane, subapical endosomes | 6, 40, 63, 96 |
| NKCC1 | Basolateral membrane | 40 |
| NBCe1 | Basolateral membrane | 40 |
| AE2 | Apical and basolateral membrane, subapical structures | 2 |
| NHE3 | Absent | 53 |
| Water channel | ||
| Aquaporin 5 | Apical and lateral membranes, subapical vesicles | 67, 77, 78 |
| Trafficking proteins | ||
| Syntaxin 4 | Apical membrane | 15 |
| SNAP-23 | Apical and basal membrane, intracellular structures | 15 |
| Munc18c | Apical membrane, cytosol | 15 |
| Rab3D | Apical secretory granules, cis-Golgi | 107 |
| Mucins | ||
| Class III | Basolateral, supranuclear region of cytoplasm | 41, 75 |
| Mucin6 | Detected but localization not fully described | 8, 34, 61 |
| Mucin5AC | Absent | 61 |
| Mucin2 | Absent | 61 |
| Growth factors | ||
| Epidermal/Urogastrone | Granules, cytoplasm, supranuclear region of cytoplasm | 19, 32, 43, 82 |
| Insulin-like | Detected but localization not fully described | 88 |
| Additional factors | ||
| Glutamate transporter | Lateral membrane | 38 |
| Carbonic anhydrase | Cytoplasmic | 76, 97 |
| Adrenocorticotropin | Endocytic vesicles | 113 |
| Lectins | Apical and basolateral surface, secretory granules | 17, 26 |
| Cytoplasm | ||
| Breast cancer resistance protein | Granular staining in cytoplasm | 106 |
| Multidrug resistance-associated protein 1 | Rough endoplasmic reticulum | 106 |
| Lysozyme | 47, 89 |
In contrast to its demonstrated role in mucus secretion and barrier functions, little is understood regarding ion transport functions of Brunner's glands. Early studies of mammalian duodenum suggested that alkaline secretions originated from the gland (24). Bicarbonate transport proteins are present in Brunner's glands (2, 6, 40, 76), but direct evidence for bicarbonate secretion emanating from the gland has not been provided. The cellular architecture of mammalian proximal duodenum in higher species is complex and comprised of distinct ion-transporting epithelial cell types (Brunner's glands, crypt, and villus) that presents a challenge for functional isolation of Brunner's glands. Moreover, duodenal villus epithelial cells secrete bicarbonate (93, 94, 110) and possess similar transporters to the Brunner's glands (Table 3). cAMP and gastrointestinal hormones including VIP, secretin, gastric inhibitory peptide, and CCK have been shown to stimulate bicarbonate secretion in the proximal duodenum but not specifically from the Brunner's glands (22, 45, 114). Secretion was reported in isolated Brunner's glands following prostaglandin E2, and hormone stimulation, but without direct confirmation that bicarbonate emanated from the glands (68). Indirect approaches using species lacking Brunner's glands have provided some functional insights into transport functions of the gland. Amphibian proximal duodenums devoid of Brunner's glands display high rates of bicarbonate secretion from surface epithelial cells (21). Moreover, HCl and other luminal agonists, prostaglandin E2, and VIP stimulate bicarbonate secretion in duodenum lacking Brunner's glands (1). These data do not support a major role for bicarbonate secretion from the glands.
Table 3.
Comparison of distribution of transporters in the proximal duodenum
| Brunner's glands | Crypts | Villi | |
|---|---|---|---|
| CFTR | + | + | + |
| NKCC1 | + | + | + |
| NBCe1 | + | −/+ | + |
| AE2 | + | + | + |
| NHE3 | − | −/+ | + |
| AQP5 | + | − | − |
| V-ATPase | + | + | + |
+Upper crypt.
Recent studies have contributed important insights into factors that control secretion and fluid transport in mammalian Brunner's glands (Table 2). Whether cystic fibrosis transmembrane conductance regulator (CFTR) channel transports chloride or bicarbonate in Brunner's gland is unknown. High levels of apical CFTR coupled with abundant basolateral potassium-coupled chloride cotransporter 1 (NKCC1) and low expression of basolateral sodium bicarbonate cotransporter (NBCe1) were observed in rat Brunner's glands, suggesting a more predominant role for chloride rather than bicarbonate secretion (40). Chloride/bicarbonate exchangers (AE2) were localized to the basolateral membranes of mouse Brunner's glands and suggested to be in the subapical pole of Brunner's gland neck cells that empty into the crypt, although convincing evidence for the latter localization was less clear (2). The water channel aquaporin 5 (AQP5) was recently identified on the apical and lateral domains of acinar cells in rat Brunner's glands, supporting a role in water transport by the gland (67, 77). The apical sodium hydrogen exchanger 3 (NHE3), which regulates sodium absorption and proton secretion in small intestinal villus enterocytes, is absent from human and guinea pig Brunner's glands (53). Whether the gland regulates luminal pH by secreting protons through an alternate pathway such as the vacuolar ATPase proton pump is unknown.
In crypt and villus enterocytes of the intestine, second messenger-regulated traffic from endosomes into the plasma membrane is an important mechanism regulating anion transport (4–6, 18, 39). Whether second messenger-regulated traffic is also important for fluid transport functions in Brunner's gland is unknown. A recent in vitro study provided evidence for VIP-induced apical traffic of AQP5 in water transport (77), and cholinergic-dependent trafficking of the exocytic membrane fusion machinery protein Munc18 was demonstrated in Brunner's gland (15). Furthermore, the presence of CFTR in subapical endosomes (6) and exocytic cellular trafficking machinery including Rab3D, SNAP-23, Munc18c, and syntaxin 4 (15, 107) provide strong evidence in support of exocytic trafficking in regulating ion transport in Brunner's gland.
Detailed mapping of anion transporter distribution and the cellular machinery that regulates transporter functions in the Brunner's glands will be beneficial for elucidating the role of the glands in fluid transport in health and disease. Duodenal luminal pH is abnormally low in cystic fibrosis (CF) because of absent CFTR and defective bicarbonate secretion from the apical membranes of duodenal enterocytes (112, 115). Although high levels of CFTR were identified in the Brunner's glands, the role of the glands in the pathophysiology of CF disease is unknown. Similarly, the role of Brunner's gland in celiac disease, a disease that targets the duodenum and results in diarrhea and ion transport abnormalities, is unknown.
To gain further insight into the role that transporter trafficking may play in physiological and pathophysiological processes in the Brunner's glands, the present study examined localization patterns of CFTR, NKCC1, NBCe1, the water channel (AQP5) and the proton pump [vacuolar ATPase (V-ATPase)] in rat Brunner's glands. Following treatment with second messengers (cAMP or carbachol), anion transporters undergo trafficking to the apical or basolateral membranes in rat Brunner's glands, suggesting that regulated traffic is an important mechanism that controls anion and water transport in the gland. The distribution profiles of CFTR, NKCC1, NBCe1, V-ATPase, and AQP5 were also examined in human duodenal tissues from 1) healthy controls, 2) celiac disease, and 3) the most common disease-producing mutation in CF (ΔF508CFTR). The data indicate that CFTR, NKCC1, NBCe1, AQP5, and V-ATPase protein are present in rat and human Brunner's gland. However, their profiles appear to be downregulated in CF and celiac disease.
MATERIALS AND METHODS
Reagents and antibodies.
Three independent anti-CFTR antibodies were used in this study. AME4991 is an affinity-purified, rabbit polyclonal antibody raised against the carboxy-terminal peptide of rat CFTR (3). The anti-CFTR mouse monoclonal antibody 217 was obtained from Dr. John Riordan (University of North Carolina-Chapel Hill, and Cystic Fibrosis Foundation Therapeutics). The monoclonal mouse antibody M3A7 raised against the COOH-terminus of human CFTR was purchased from Chemicon International (Temecula, CA). The electrogenic Na+/HCO3− cotransporter 1 (NBCe1) antibody was a gift from Dr. Walter Boron (Case Western Reserve University). The human monoclonal antibody (T4) generated against the human K+/Cl− cotransporter 1 (NKCC1) was acquired from the Developmental Hybridoma Bank (University of Iowa). The V-ATPase subunit V1E2, chicken IgY polyclonal antibody (anti-ATP6V1E1) and β-actin were purchased from Sigma Aldrich (St. Louis, MO). AQP5 was purchased from Alomone Labs (Jerusalem, Israel), and the rabbit polyclonal early endosome antibody EEA1 was purchased from Affinity BioReagents (Golden, CO). Fluorescent-tagged secondary antibodies were purchased from Invitrogen (Carlsbad, CA) and Jackson ImmunoResearch Laboratories (West Grove, PA). N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (dibutyryl cAMP) and carbamoylcholine chloride (carbachol) were obtained from Sigma Aldrich. The PKA inhibitor H-89 dihydrochloride was purchased from Calbiochem/EMD Millipore (Billerica, MA).
Animal preparation.
Rodent studies were approved by the Institutional Animal Care and Use Committee at Yale University School of Medicine. Adult male Sprague-Dawley rats (250–300 g body wt, Charles River Laboratories, Wilmington, MA) were fasted overnight with access to water. Each rat was anesthetized with Inactin (thiobutabarbital sodium salt) (120 mg/kg), administered by intraperitoneal injection. The abdomen of anesthetized animals was opened, the duodenum was identified, and loops were created with sutures. The loops were filled by injection with warm saline, 1 mM dibutyryl cAMP, or 10 μM carbachol for 30 min. Loops were also pretreated with the PKA inhibitor H-89 (10 μM) for 30 min prior to treatment with dibutyryl cAMP (1 mM) for 30 min. The abdomen was closed and the animal was kept warm (37°C) on a 37°C heating pad. Duodenal loops were removed 30 or 60 min later and flushed with cold phosphate buffer solution (PBS). Tissues were processed for immunofluorescence microscopy. Each animal was euthanized at the end of the experiment with an overdose of Inactin.
Tissue preparation.
The preparation of tissue blocks was performed as previously described (40). Rat duodenum segments were fixed with 2% (wt/vol) paraformaldehyde for 1 h at room temperature. Tissues were cryo-protected with 30% sucrose in PBS overnight at 4°C, embedded in Tissue-Tek OCT embedding medium (Miles Laboratory, Elkhart, IN) and frozen in isopentane precooled with liquid nitrogen. Tissue blocks were stored at −20°C until sectioned. Blocks were sectioned on a Leica 1900 microtome into 5-μm sections, placed on coated slides (Fisher Scientific), and stored for future use at −20°C.
Immunofluorescence labeling and confocal microscopy.
Fluorescence immunolabeling was performed as described previously (40). Cryostat sections were rehydrated in PBS. To minimize autofluorescence, sections were treated with 1% sodium borohydride for 20 min and washed with PBS. Sections were blocked in 10% goat serum, 0.5% bovine serum albumin, 0.15% glycine, and 0.1% Triton-X for 1 h, followed by washes with PBS. Sections were incubated with primary antibodies overnight at 4°C, with the exception of NKCC1 antibodies, which were incubated for 15 min at room temperature. Control sections were labeled with the respective nonspecific IgG antibodies. The following day, sections were washed with PBS, incubated with the appropriate secondary antibodies for 1 h at room temperature, stained with 1% Hoechst nuclear stain, and mounted in Slow Fade medium (Molecular Probes, Eugene, OR). When monoclonal CFTR and polyclonal AQP5 antibodies were employed on the same tissue section, sequential labeling was performed as follows: sections were incubated with the mouse monoclonal anti-CFTR antibody overnight at 4°C, labeled with the appropriate secondary antibody the following day, and incubated with Fab IgG fragments overnight. The next day, the same sections were incubated with the rabbit polyclonal AQP5 antibody for 2 h at room temperature and the appropriate secondary antibody for 1 h at room temperature. Immunolabeled sections were viewed on a Zeiss LSM 510-META laser scanning confocal microscope using ×20 and ×63 objectives.
Fluorescence intensity image analysis.
Confocal images were taken at ×63 magnification with uniform exposure times. CFTR, NKCC1, NBCe1, AQP5, and V-ATPase fluorescence intensities were determined in at least four different images, each containing at least four Brunner's glands. Analyses were performed as previously described by use of Adobe Photoshop CS4 (40). Fluorescence intensity levels were measured by selecting regions of interest in the apical membrane (AQP5, CFTR, and V-ATPase), the basolateral membrane (AQP5, NKCC1, and NBCe1), and the intracellular pole (AQP5, NKCC1, NBCe1, and V-ATPase). To select the region of interest, the circular brush tool was set at 100% hardness, and 2.0-μm-thick areas were traced on the apical or basolateral membrane. For intracellular membrane fluorescence, 3.0-μm-thick areas were traced closely to the basolateral membrane. Using the advanced “Analysis and Record Measurements” tools in Adobe Photoshop CS4 Extended, we recorded the pixel intensities (mean gray value) for the selected areas. Analysis of colocalization was performed on confocal images of double-labeled duodenum sections containing Brunner's glands by using Image J software as described previously (52). CFTR/F-actin or CFTR/AQP5 colocalization at the apical domain was measured by comparing the CFTR mean pixel levels to CFTR/F-actin or CFTR/AQP5 mean pixel levels as described previously (52).
Statistics.
Measured values are expressed as means + SE. Significant differences were determined by a Student t-test, and a P value of <0.05 was determined to be significant.
Human duodenum tissues.
Human tissues were collected with the approval of the Yale University Institutional Review Board. Healthy controls and tissues from diagnosed celiac disease were identified from the Yale University Department of Pathology databases. CF (Δ508 CFTR) duodenal biopsy specimens were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA). For this study, human duodenal biopsies (three from each condition) from healthy controls, celiac disease, and CF disease were analyzed.
Histology.
Proximal rat duodenum, healthy controls (normal) and disease-affected human duodenum were fixed in 10% buffered formalin, embedded in paraffin, sectioned (5-μm-thick sections), and mounted onto Superfrost-coated slides (Fisher Scientific, Pittsburgh, PA). Conventional hematoxylin and eosin stains were performed as described previously (3).
Immunohistochemistry.
Sections from healthy controls and disease-affected human proximal duodenum were deparaffinized in xylene and rehydrated through ethanol to distilled water. Antigen retrieval was performed with citrate buffer (DAKO, Carpinteria, CA) for 30 min. Endogenous peroxidase was blocked with 3% hydrogen peroxide for 5 min at room temperature. The slides were rinsed with Tween-20-Tris-buffered saline solution between each of the following steps. The sections were incubated with primary antibodies (CFTR, NKCC1, NBCe1, V-ATPase, and AQP5) for 30 min or overnight at 4°C. The antibodies were then detected by use of Envision+ (K4001, DAKO). Diaminobenzidine was used to detect the antibody complex (K3468, DAKO). Negative control sections were incubated with isotype-matched immunoglobulins. The slides were subsequently counterstained with hematoxylin, dehydrated, and coverslipped with resin mounting media. Immunolabeled sections were examined by light microscopy on an Olympus BX51. Digital images were acquired with an Olympus DP72 camera using Olympus DP2-BSW software.
RESULTS
Histology of rat and human proximal duodenum.
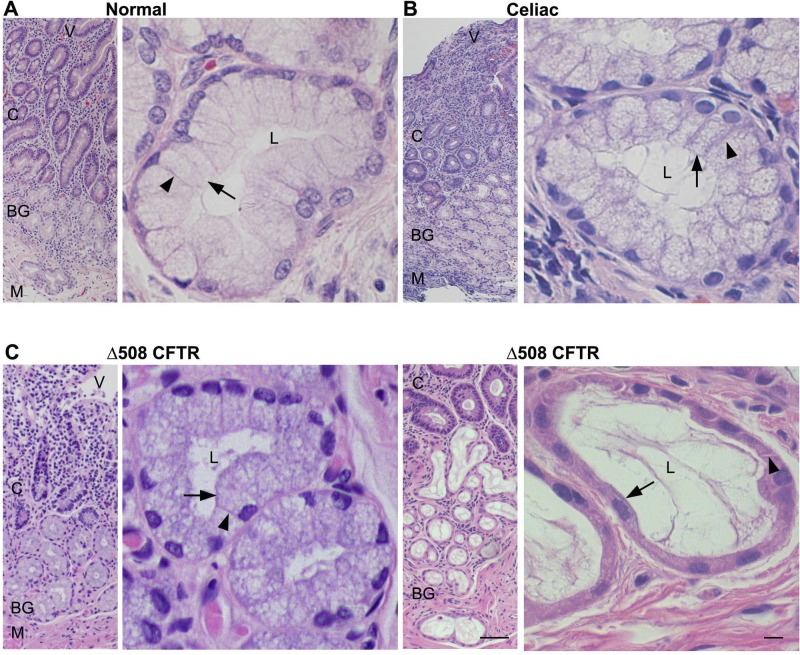
Rat and human Brunner's gland histology have been documented (50, 58). In this study, histological examination was performed to provide orientation of Brunner's gland morphology in normal rat, in healthy human duodenum, and in tissues from two human diseases that affect the duodenum: CF and celiac disease. Examination of hematoxylin and eosin-stained sections from rat and normal human proximal duodenum revealed no major morphological defects (Fig. 1 and 2A). In rat and human proximal duodenum, Brunner's glands were observed deep within the submucosal layer below the crypts, consisting of clusters of branched and coiled tubules with the lumen that opened into the base of the duodenal crypts. Acinar cell size and shape were heterogeneous with the nuclei positioned at the base of cells. One of the characteristic features of celiac disease is inflammation that results in blunted villi and crypt hyperplasia that was evident in stained sections (Fig. 2B). In human celiac and CF (Δ508 CFTR)-affected duodenum, the Brunner's glands and crypt appeared intact, but villus architecture was not preserved (Fig. 2, B and C, left). Similar to studies that reported dilations and distention of duodenal Brunner's glands in human CF (74) and CFTR−/− (95) mice, we also observed that human CF Brunner's glands were elongated with flattened nuclei (Fig. 2C, right).
Fig. 1.
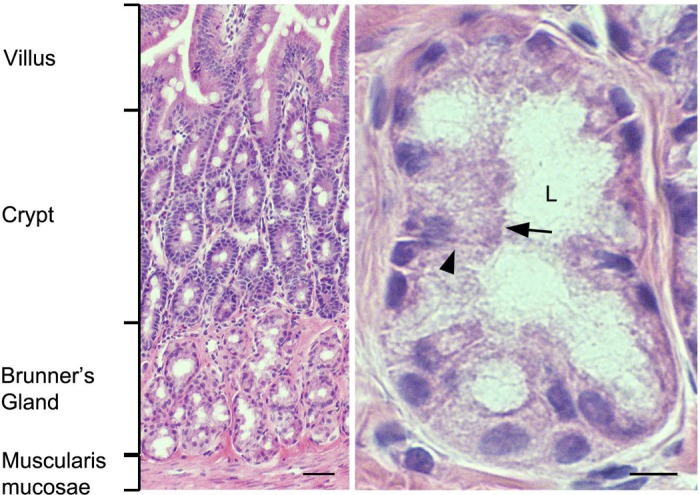
Histological appearance of rat duodenum Brunner's glands. Hematoxylin and eosin-stained sections from rat proximal duodenum. Low-magnification image (left) shows location of Brunner's glands relative to the crypt-villus axis. High-magnification image (right) of representative Brunner's gland. Black arrow indicates apical membrane; black arrowhead indicates basolateral membrane. L, lumen. Scale bar = 10 or 100 μm.
Fig. 2.
Histological appearance of Brunner's glands in human proximal duodenum tissues from healthy controls, celiac disease, and cystic fibrosis (CF; Δ508 CFTR). Hematoxylin and eosin-stained sections from healthy (A) and celiac (B) and CF (Δ508) (C, left and right) disease-affected human duodenum. Low-magnification images (left) show location of Brunner's glands relative to the crypt-villus axis. High-magnification images (right) of representative Brunner's gland. Black arrow indicates apical membrane. Black arrowhead indicates basolateral membrane. M, Muscularis mucosae; C, crypts; V, villi; BG, Brunner's gland. Scale bar = 10 or 100 μm.
cAMP and carbachol-induced apical trafficking of CFTR in rat Brunner's glands.
Previous studies from this laboratory demonstrated high levels of CFTR in the apical domain of rat Brunner's glands and confirmed CFTR localization on the membranes of subapical vesicles within the gland at steady state (6, 40). This subcellular distribution for CFTR in Brunner's gland is consistent with its regulation by second messenger traffic, similar to what was demonstrated in crypt and villus enterocytes (4, 6, 39). Since 1) the glands possess cellular machinery to regulate transporter traffic (15, 107); 2) cAMP and carbachol regulate anion secretion and trafficking of ion transporters, including CFTR in the intestine (40); and 3) carbachol has been shown to stimulate protein traffic in the glands (15), the present study used confocal microscopy with immunofluorescence techniques to examine whether CFTR undergoes cAMP and carbachol-regulated trafficking in the Brunner's glands.
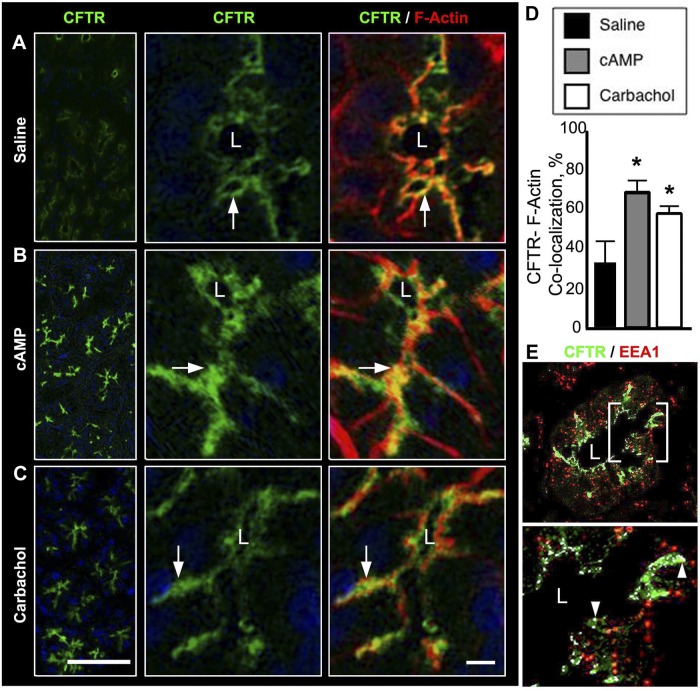
The distribution of anion transporters was examined in cryosections from proximal rat duodenum doubled labeled with CFTR antibodies and phalloidin (to delineate apical F-actin and the brush border) following treatment with saline, 1 mM dibutyryl cAMP, or 10 μm carbachol in vivo. Consistent with our previous observations (40), CFTR was observed in the apical domain of the Brunner's glands (Fig. 3A). cAMP and carbachol treatment increased apical CFTR fluorescence intensity (Fig. 3, B and C), similar to our observations in the stimulated small intestine (39). Stimulation with agonists significantly increased CFTR/F-actin colocalization (Fig. 3D). To confirm that CFTR was present in endosomes, rat duodenum sections were double labeled with the early endosome marker EEA1. CFTR was confined to early endosomes near the lateral membranes of the glands (Fig. 3E).
Fig. 3.
cAMP and carbachol-induced trafficking of CFTR to the apical domain in rat Brunner's glands. Cryostat sections from rat proximal duodenum treated with normal saline, 1 mM dibutyryl cAMP, or 10 μM carbachol were immunolabeled for CFTR and F-actin or CFTR and early endosome (EEA1) and examined by confocal microscopy as described in materials and methods. A–C: low (left)- and high (middle and right)-magnification images are shown. Images of Brunner's glands in saline- (A), dibutyryl cAMP- (B), or carbachol (C)-treated tissues. CFTR (green, white arrow), F-actin (red), and merged images show CFTR/F-actin colocalization (yellow, white arrow). D: graphs of quantification analysis of CFTR/F-actin colocalization in the apical domain of saline-, dibutyryl cAMP-, or carbachol-treated Brunner's glands. Data are shown as percentage of apical CFTR colocalized with F-actin. For each treatment group, the fluorescence intensity was measured from minimum of 4 glands from 3 different animals. Data represent +SE (n > 10). *P < 0.05. E: image of a single Brunner's gland shows the distribution of CFTR and EEA1 (top) with enlarged region (bottom) indicating colocalization (white arrowhead). Scale bar = 10 or 100 μm.
AQP5 colocalizes with CFTR and undergoes cAMP-regulated traffic to the apical membrane of rat Brunner's glands.
The aquaporin family of membrane-bound water channels regulates fluid transport across the apical surface of polarized epithelium (62, 108, 109). Several aquaporin (AQP) isoforms (AQP 1, 3, 5, 7, 8, and 9) have been localized to select membrane domains of intestinal enterocytes (13, 20, 49, 55, 56, 66, 73, 78, 86, 99). AQP5 was localized to the apical and lateral membranes of rat Brunner's glands (77, 78). Consistent with previous observations (77, 78), AQP5 was localized to the Brunner's glands but was distinctly absent from the crypts in the duodenum (Fig. 4A). Detailed examination revealed that AQP5 predominately localized to the apical domain of the gland (Fig. 4B), with staining at the lateral membrane (Fig. 4, A and B). AQP5 was also identified in vesicular compartments within the gland in separate studies (77). To determine whether AQP5 is in endosomes, similar to CFTR (6), rat sections containing Brunner's glands were double labeled with antibodies against the early endosome marker EEA1 and against AQP5. AQP5 was detected in early endosomes in the subapical domain and near the lateral membranes (Fig. 4C). Endosomes are abundant in the gland; however, the ability to detect colocalization of membrane transporters by immunolabel depends on many factors including the kinetics and abundance of endocytosis of the transporter in question, antigenicity, fixation, and orientation of section.
Fig. 4.
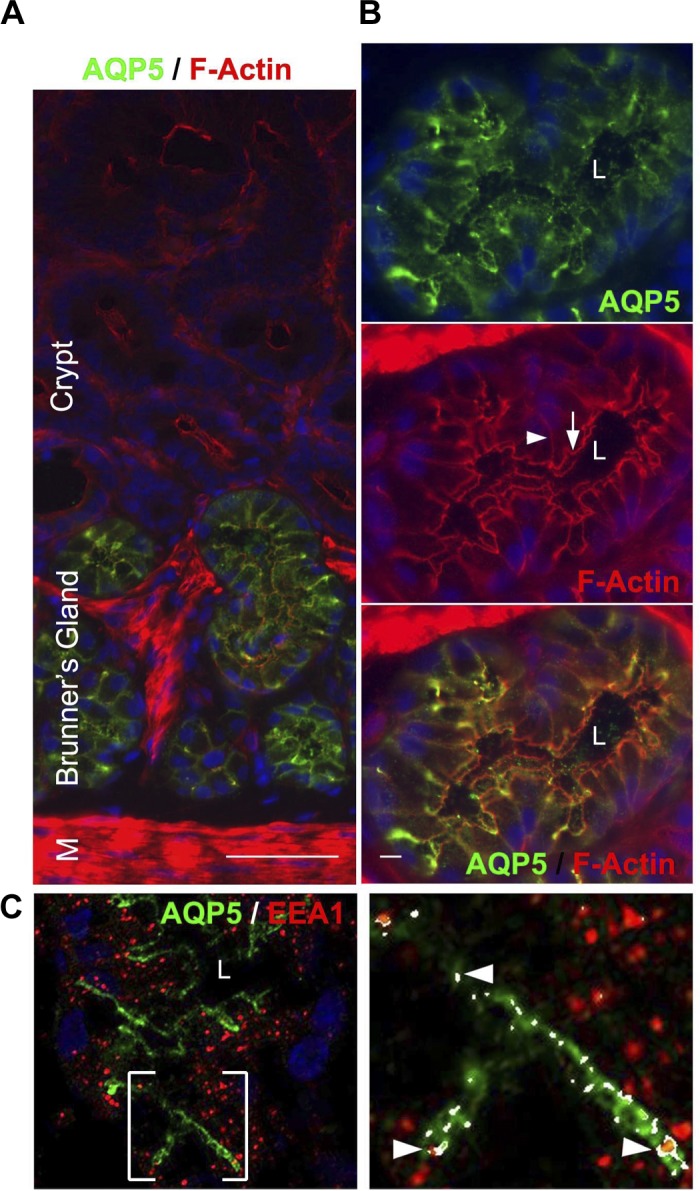
Distribution of aquaporin 5 (AQP5) in rat Brunner's glands. Cryostat sections of rat proximal duodenum were immunolabeled with antibodies against AQP5, F-actin, and/or EEA1 and viewed by confocal microscopy as described in materials and methods. A: low-magnification image of immunolabeled section shows the distribution of AQP5 (green) and F-actin (red) along the Brunner's gland-crypt axis. B: high-magnification image of a single Brunner's gland shows the distribution of AQP5 (top, green) in the apical domain and F-actin (middle). Merged image shows areas of AQP5/F-actin colocalization (bottom). White arrow indicates apical membrane. White arrowhead indicates basolateral membrane. C: image of a single Brunner's gland showing the distribution of AQP5 and EEA1 (left); enlarged area shows colocalization (right, arrowheads). Hoechst stain labels the nuclei blue. Scale bar = 10 or 100 μm.
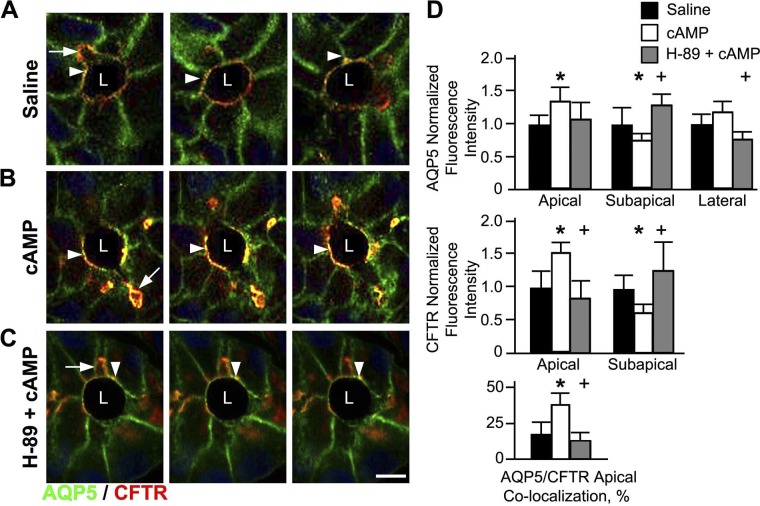
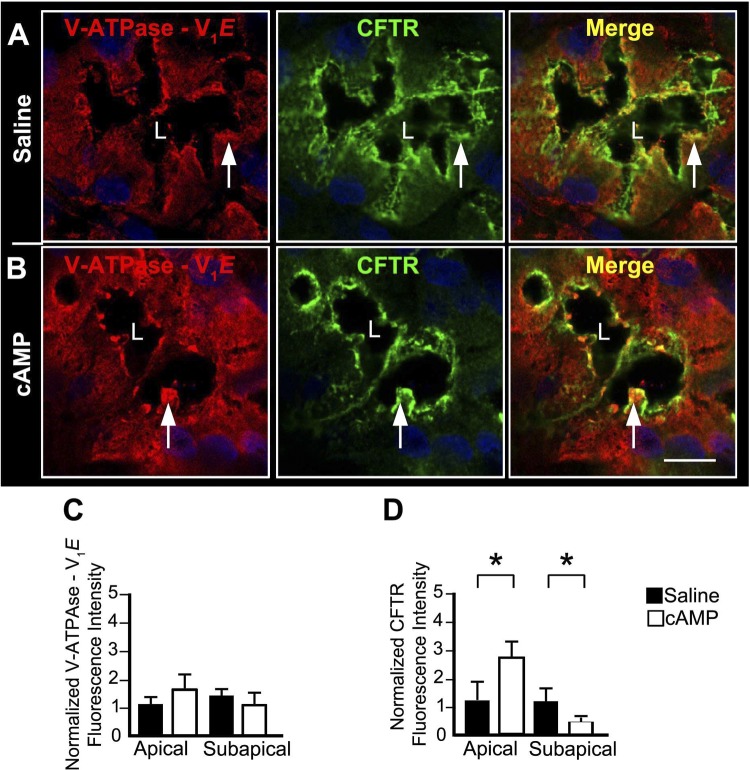
Both CFTR and AQP5 possess target sequences for cAMP PKA, suggesting that PKA may regulate cAMP-stimulated traffic of CFTR and AQP5 to the cell surface (27, 77). The shared apical distribution of AQP5 and CFTR suggests that both transporters could be in the same compartments within the gland and function synergistically to regulate fluid secretion. To test whether AQP5 and CFTR are in the same compartments, functionally linked and undergo regulated traffic to the apical membrane, colocalization studies examined the distribution of AQP5 and CFTR in Brunner's gland following treatment of rat proximal duodenum with saline or 1 mM dibutyryl cAMP. To examine the role of PKA in regulated trafficking of CFTR and AQP5 in the Brunner's glands, rat duodenum was pretreated with the protein kinase A inhibitor H-89, followed by dibutyryl cAMP, and the distribution patterns of CFTR and AQP5 were examined. At steady state (saline), CFTR colocalized with AQP5 at the apical domain but not at the lateral membranes of the gland (Fig. 5A). cAMP treatment resulted in robust recruitment of CFTR and AQP5 to the apical domain of the gland that was reflected by increased colocalization of AQP5 and CFTR at the membranes of Brunner's gland cells (Fig. 5B). Increased apical membrane label for CFTR was accompanied by concomitant decrease in subapical CFTR fluorescence labeling (Fig. 5B). AQP5 trafficking was primarily observed in the apical domain, but some lateral membrane labeling was also detected. The PKA inhibitor was effective in inhibiting cAMP-dependent trafficking of CFTR and AQP5 to the apical surface (Fig. 5C). Figure 5D reflects the percentage of fluorescence intensity of CFTR or AQP5 at the apical and lateral membranes as well as in the subapical compartments at steady state and under stimulated conditions. As predicted, there was a significant increase in CFTR/AQP5 colocalization at the apical membrane in cAMP-stimulated glands that was abrogated in the presence of the PKA inhibitor.
Fig. 5.
cAMP-regulated apical trafficking of AQP5 and CFTR in rat Brunner's glands. Cryostat sections from rat proximal duodenum treated with normal saline or with 1 mM dibutyryl cAMP, or pretreated with 10 μM PKA inhibitor (H-89, 10 μM) prior to 1 mM dibutyryl cAMP, were immunolabeled with antibodies against AQP5 and CFTR and examined by confocal microscopy as described in materials and methods. Consecutive images were isolated from a stack and representative images taken at 0.25-μm intervals. High-magnification images of immunolabeled Brunner's glands from saline- (A), cAMP- (B), or H-89 pretreated prior to cAMP (C)-treated tissues show apical (white arrowhead) and lateral (white arrow) distribution of AQP5 (green) and apical localization of CFTR (red, arrow). Merged images show areas of AQP5/CFTR colocalization in yellow. D: graphs of normalized AQP5 and CFTR fluorescence intensity at the apical membrane, subapical region, or lateral membrane and percent of apical CFTR colocalization with AQP5 in Brunner's gland. For each treatment group, the fluorescence intensity was measured from minimum of 4 glands from 3 different animals. Data represent +SE (n > 10) significance between saline and cAMP groups *P < 0.05. Data represent +SE (n > 10) significance between cAMP- and PKA/cAMP-treated groups +P < 0.05. Scale bar = 10 or 100 μm.
cAMP regulates trafficking of NKCC1 and NBCe1 to the basolateral domain in rat Brunner's glands.
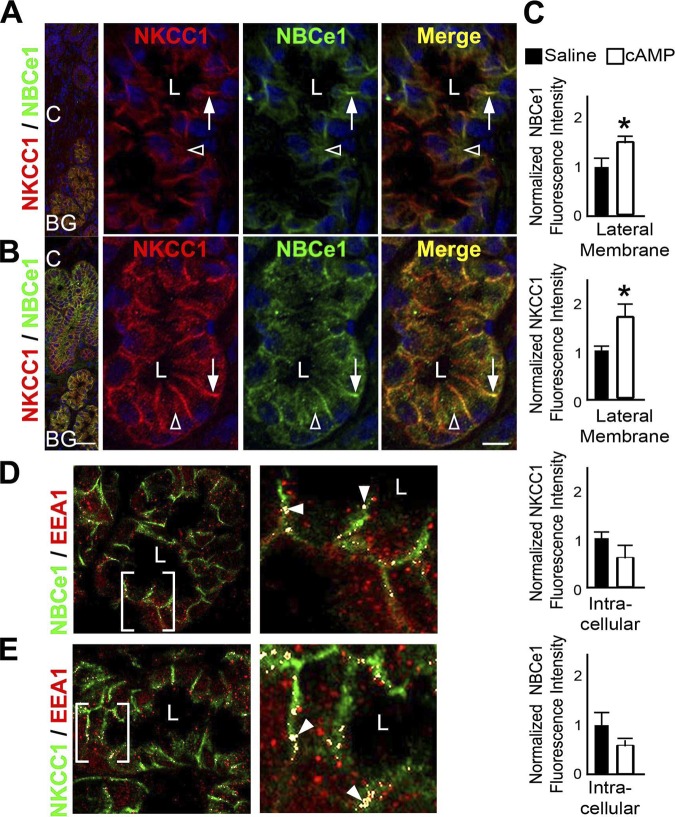
Rat Brunner's glands express the basolateral sodium- and potassium-coupled chloride cotransporter NKCC1 and low levels of the sodium bicarbonate cotransporter NBCe1 (40). But the role of NKCC1 and NBCe1 in regulating fluid secretion from the gland is unknown. Because cAMP activates fluid transport by NKCC1 and NBCe1 traffic in enterocytes, the distribution of both transporters was examined in the glands following treatment of rat proximal duodenal tissues with saline or 1 mM dibutyryl cAMP. Cryosections of rat duodenum were immunolabeled to detect NKCC1 and NBCe1 and were examined by confocal microscopy (Fig. 6). Similar to our previous observations (40), NKCC1 and NBCe1 staining were observed on the lateral membrane and in intracellular compartments in the Brunner's glands in saline-treated tissues. cAMP treatment resulted in significantly increased NKCC1 and NBCe1 fluorescence intensity at the basolateral domain that was accompanied by decreased fluorescence intensity in intracellular compartments (Fig. 6, A–C), as confirmed by quantification analysis (Fig. 6C). NKCC1 and NBCe1 were detected in EEA1-positive endosomes in close proximity to basolateral membranes (Fig. 6, D and E).
Fig. 6.
cAMP-regulated basolateral trafficking of sodium-potassium-coupled chloride cotransporter 1 (NKCC1) and sodium-bicarbonate cotransporter (NBCe1) and localization in endosomes in rat Brunner's glands. Cryostat sections of rat proximal duodenum treated with normal saline and 1 mM dibutyryl cAMP were immunolabeled with antibodies against NKCC1, NBCe1, and the endosome marker EEA1. Immunolabeled sections were viewed by confocal microscopy as described in materials and methods. A and B: low- and high-magnification images of immunolabeled duodenum with Brunner's glands treated with saline (A) or dibutyryl cAMP (B). The distribution of NKCC1 (red) and NBCe1 (green) in the basolateral domains (white arrow) and in intracellular label (white open arrowhead) in saline- (A) or dibutyryl cAMP (B)-treated rat Brunner's gland. Merged images show areas of colocalization (yellow). Nuclei (blue) are labeled with Hoechst stain. C: quantification of normalized fluorescence intensity of NKCC1 and NBCe1 at the basolateral membrane and in basolateral intracellular compartments from saline- and dibutyryl cAMP-treated Brunner's glands. For each treatment group, fluorescence intensity was measured from a minimum of 4 glands from 3 different animals. Data represent +SE (n > 10). *P < 0.05. D: Distribution of NBCe1 (green) and EEA1 (red) in rat Brunner's gland. Bracketed area at left is magnified at right. Colocalization, white arrowheads. E: distribution of NKCC1 (green) and EEA1 (red) in rat Brunner's gland. Bracketed area at left is magnified at right. Colocalization, white arrowheads. Scale bar = 10 or 100 μm.
cAMP-induced trafficking of CFTR and V-ATPase in rat Brunner's gland.
CFTR undergoes cAMP-mediated trafficking from subapical endosomes into the apical membrane of crypt and villus enterocytes in the intestine (4–6). In the epididymis, cAMP stimulates traffic of the vacuolar proton pump V-ATPase into the apical membrane to regulate luminal pH (79). We observed that CFTR and V-ATPase colocalize and undergo cAMP-regulated trafficking to the apical membrane in the intestine (A. Collaco and N. Ameen, unpublished observations). Since the Brunner's glands lack the apical sodium-proton exchanger NHE3 (53), and no other proton pump has been identified in the gland, antibodies were used to examine the distribution of the V-ATPase proton pump. Following treatment of proximal rat duodenum with saline or 1 mM dibutyryl cAMP, tissue sections were immunolabeled with CFTR and V-ATPase (V1E) antibodies and examined by confocal microscopy (Fig. 7). In saline-treated tissues, V-ATPase staining was detected throughout the cytoplasm with increased label in the apical region of Brunner's gland (Fig. 7A, left). CFTR was observed in the apical domain and in subapical compartments in the gland (Fig. 7A, top middle). Following cAMP treatment, V-ATPase fluorescence intensity increased and colocalized with CFTR at the apical domain of the gland (Fig. 7B). As expected, apical label for CFTR also increased following cAMP treatment (Fig. 7B, middle). Merged images illustrate regions of CFTR and V-ATPase colocalization at the apical domain. Quantification of changes in CFTR and V-ATPase fluorescence intensity in the apical domain and subapical compartment are shown in Fig. 7, C and D. Although we observed an increase in apical CFTR/V-ATPase colocalization by labeling in the Brunner's glands following cAMP, quantitative analysis revealed that the difference in V-ATPase fluorescence intensity in the apical domain or subapical compartments of the Brunner's gland was not significant compared with saline-treated tissues (Fig. 7C). The changes in CFTR fluorescence intensity in saline- vs. cAMP-treated duodenum (Fig. 7D) were similar to our findings for the experiments examining the distribution of CFTR/AQP5 in saline- and cAMP-treated duodenum (Fig. 5).
Fig. 7.
cAMP-induced trafficking of vacuolar ATPase (V-ATPase) and CFTR to the apical domain of rat Brunner's glands. Cryostat sections of rat proximal duodenum treated with normal saline and 1 mM dibutyryl cAMP were immunolabeled with antibodies against V-ATPase V1E and CFTR and examined by confocal microscopy as described in materials and methods. A and B: images of immunolabeled Brunner's gland from saline- (A) or dibutyryl cAMP (B)-treated tissues show apical distribution of V-ATPase V1E (red) (left, white arrow) and CFTR (green) (middle, white arrow) and merged images (right). Merged images show areas of colocalization (right, yellow). Quantification of average fluorescence intensities of V-ATPase (C) and CFTR (D) in the apical and subapical domains of saline- and dibutyryl cAMP-treated rat Brunner's glands. For each treatment group, fluorescence intensity was measured from a minimum of 4 glands in 3 different animals. Data represent +SE (n > 10). *P < 0.01. Scale bar = 10 or 100 μm.
AQP5 is on the apical domain of human Brunner's glands.
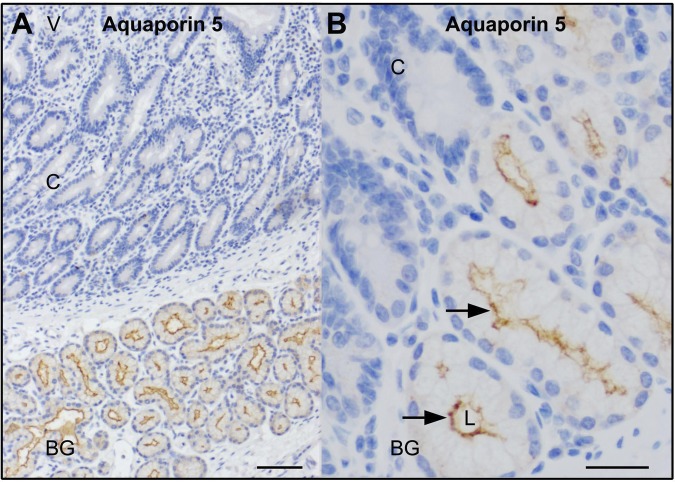
The data from this study and other published studies reveal that AQP5 is localized to the apical and lateral membranes of rat Brunner's glands (77, 78). AQP5 is also on the apical membranes of rat salivary and lacrimal glands, apical and basolateral membranes of mouse, and human excretory portions of sweat ducts (70). The distribution of AQP5 in human duodenum and Brunner's glands is unknown. Immunohistochemical staining was performed on normal human duodenum with the same anti-AQP5 antibody employed in rat localization studies. Consistent with the observations in rat tissues, AQP5 staining was detected at the apical domain but not on the lateral membranes of the glands in normal human duodenum and, similar to the observations in rat duodenum, AQP5 staining was absent from human duodenal crypts (Fig. 8).
Fig. 8.
Immunohistochemical distribution of AQP5 staining in healthy human proximal duodenum. Immunohistochemical staining of AQP5 in normal proximal human duodenum was performed as described in materials and methods. A: low-magnification image shows location of Brunner's glands relative to the crypt-villus axis. B: high-magnification image at the Brunner's gland-crypt junction shows apical distribution of AQP5 (brown stain, black arrow). Scale bar = 10 or 100 μm.
Brunner's gland anion transporter and AQP5 distribution profiles are downregulated in human CF (Δ508 CFTR) and celiac disease.
Little is understood regarding fluid and water transport in human Brunner's glands and alterations that may contribute to pathophysiological disease states. To gain insight into the role of anion transporters in human Brunner's gland in health and disease states, anion transporters (CFTR, NKCC1 and NBCe1, V-ATPase) and AQP5 distribution profiles were examined in tissues from healthy human duodenum, CF (Δ508 CFTR), and celiac disease tissues (Figs. 9–11). Both CF and celiac disease target the proximal duodenum. In the genetic disease CF, failure of mutant CFTR to traffic to into the apical membrane of epithelial cells leads to lack of bicarbonate secretion and hyperacidity in the proximal duodenum (31, 115). The most common disease-producing mutation in human CF is Δ508 CFTR. In celiac disease, sensitivity to gliadin- and gluten-containing foods leads to duodenal inflammation, villus blunting, crypt hyperplasia, malabsorption, and diarrhea (28, 81).
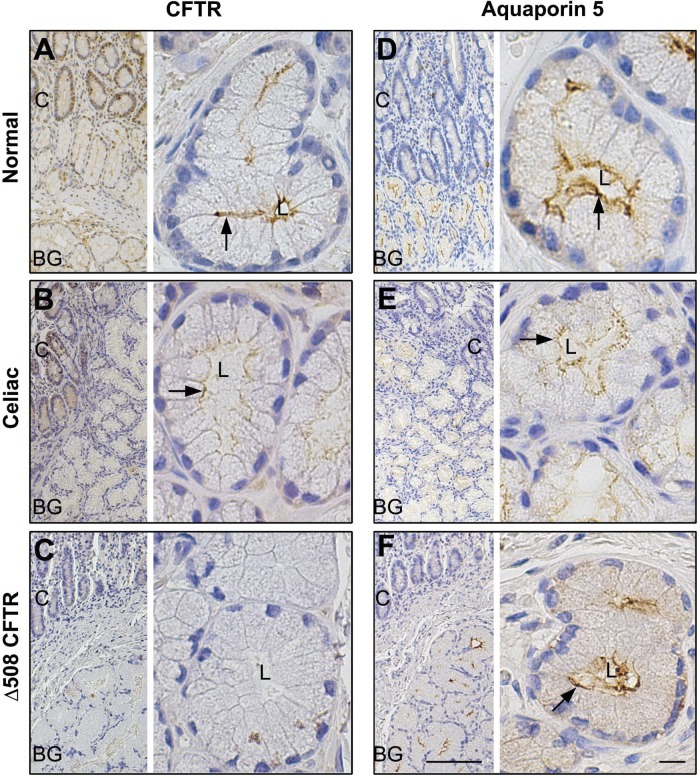
Fig. 9.
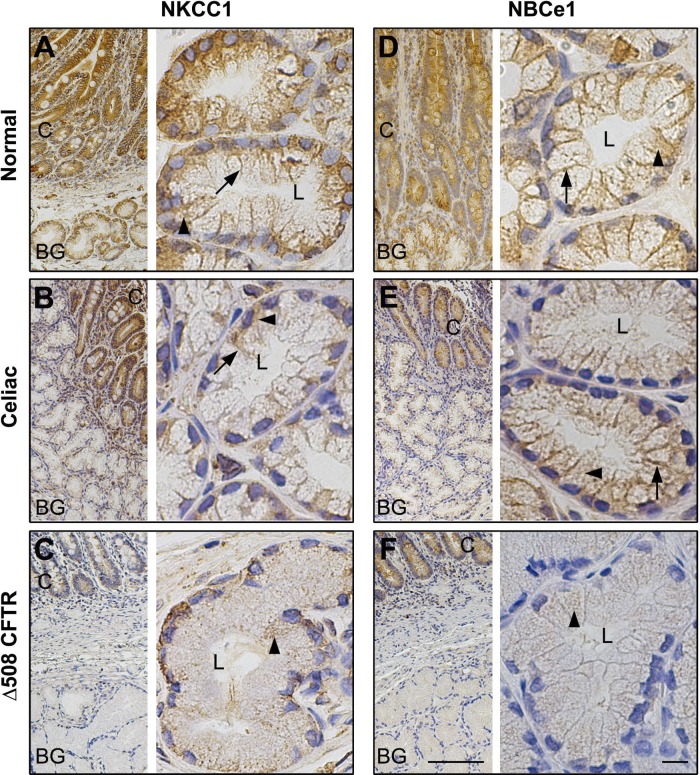
Distribution of CFTR and AQP5 in healthy control and celiac disease- and CF (ΔF508 CFTR)-affected human proximal duodenum. Immunohistochemical staining for CFTR and aquaporin 5 (AQP5) in healthy controls, celiac disease, and CF (ΔF508 CFTR) proximal human duodenum tissue sections was performed as described in materials and methods. Low-magnification (A–F, left) and high-magnification (A–F, right) images show the distribution of CFTR (A–C) and AQP 5 (D–F) in healthy (A and D), celiac disease (B and E), and CF (ΔF508 CFTR) (C and F) proximal human duodenum and Brunner's gland. Apical membrane staining (brown) in Brunner's gland, is indicated by black arrows. Scale bar =10 or 100 μm.
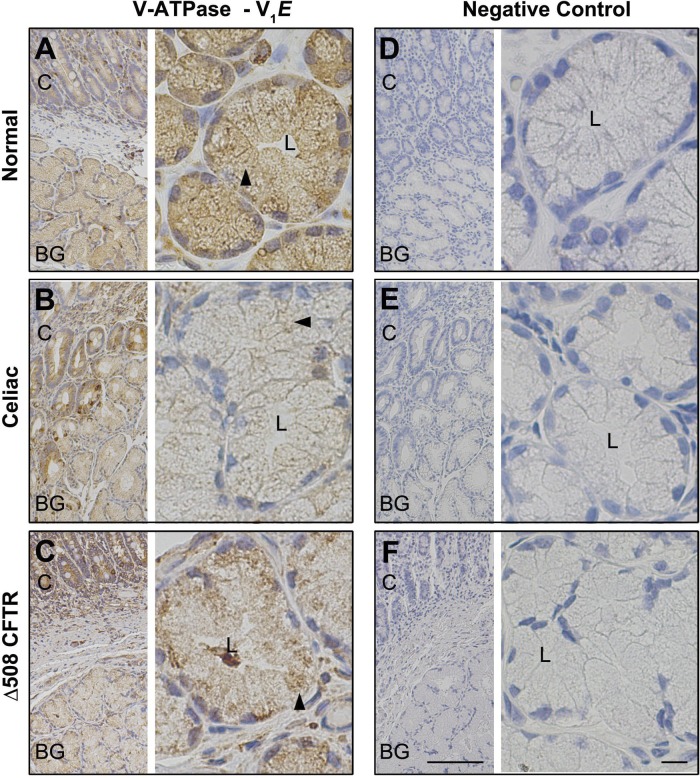
Fig. 11.
Distribution of V-ATPase in healthy control, celiac disease, or CF (Δ508 CFTR) human proximal duodenum. Immunohistochemical staining of V-ATPase V1E in healthy control, celiac disease, and CF (Δ508 CFTR) proximal human duodenum was performed as described in materials and methods. Low- (A–F, left) and high-magnification (A–F, right) images show the distribution of V-ATPase V1E (A–C) in healthy (A), celiac disease (B), and CF (ΔF508 CFTR) (C) proximal human duodenum and Brunner's gland. Intracellular staining (brown) in the Brunner's gland is indicated by black arrowhead. Negative control sections stained with isotype matched immunoglobulins in healthy (D), celiac disease (E), and CF (ΔF508 CFTR) (F) proximal human duodenum and Brunner's gland. Scale bar =10 or 100 μm.
To compare localization and staining patterns of transporters, immunohistochemical staining was performed on all duodenal sections under uniform conditions and antibody dilutions. In tissues from normal healthy controls and celiac disease, CFTR labeling was detected in the apical domain of the crypts and the Brunner's glands, but apical label was reduced in celiac Brunner's gland (Fig. 9, A and B). As expected, no CFTR staining was detected in epithelial cells, including Brunner's gland in CF (Δ508 CFTR), affected human duodenum (Fig. 9C). In normal, celiac disease, and proximal human CF duodenum, AQP5 label was detected at the apical membranes but not at the basolateral membranes of Brunner's glands or in the crypts (Fig. 9, D–F). AQP5 labeling was reduced in CF and celiac Brunner's gland. Labeling for the chloride and bicarbonate cotransporters NKCC1 and NBCe1 was detected at the basolateral membranes and intracellular compartments of both crypts and Brunner's glands (Fig. 10, A, B, D, and E) in normal and celiac disease duodenum. In CF duodenum tissues, weak staining for NKCC1 and NBCe1 was observed in the basal but no label was detected on the lateral membranes of Brunner's gland, although some intracellular staining was observed within acinar cells (Fig. 10, C and F). More intense staining of NKCC1 and NBCe1 was observed in intracellular compartments in the crypts. Consistent with our immunofluorescence localization in rat Brunner's gland, strong diffuse intracellular staining of acinar cells was observed in normal human Brunner's gland with V-ATPase proton pump antibody, with reduced staining in the crypt. Levels of V-ATPase staining were reduced in Brunner's glands from tissues affected with celiac disease, although crypt staining was not reduced compared with normal (Fig. 11, A and B). The pattern of V-ATPase staining in CF tissues was different from normal and celiac disease. Staining was cytoplasmic and higher in crypt compared with that of Brunner's gland. V-ATPase staining was reduced compared with normal but detected diffusely within the acinar cells but more prominent at the cell bases in CF Brunner's gland (Fig. 11, A–C). Images of negative control sections confirmed specificity of antibody staining (Fig. 11, D–F). Overall, the distribution of anion transporters in celiac disease and CF proximal human duodenum, including the Brunner's glands, resembled that of normal tissues except that staining was weaker under the same conditions. However, the Brunner's gland staining for AQP5 was reduced in celiac disease and CF compared with healthy controls.
Fig. 10.
Distribution of NKCC1 and NBCe1 in healthy controls, celiac disease, and CF (Δ508 CFTR) human proximal duodenum. Immunohistochemical staining of basolateral transporters NKCC1 and NBCe1 in healthy controls and celiac- and CF (Δ508 CFTR)-affected proximal human duodenum tissue sections was performed as described in materials and methods. Low- (A–F, left) and high (A–F, right)-magnification images show the distribution of NKCC1 (A–C) and NBCe1 (D–F) in healthy (A and D), celiac disease (B and E), and CF (Δ508 CFTR) (C and F) proximal human duodenum and Brunner's gland. Basolateral membrane staining (brown) in the Brunner's glands is indicated by black arrow and intracellular staining is indicated by black arrowhead. Scale bar = 10 or 100 μm.
DISCUSSION
Brunner's glands in the proximal duodenum secrete glycoproteins and antimicrobial peptides that are important for epithelial barrier functions. Although advancements have been made in understanding the role of the gland in fluid transport and bicarbonate secretion (48, 69), information is limited. The anatomic location of the glands deep beneath the crypts within the proximal duodenum presents technical challenges for directly accessing the glands for functional ion transport investigations. Furthermore, confirmation that Brunner's glands is the direct source of bicarbonate secretion is difficult since duodenal villus enterocytes within the same epithelium are capable of robust bicarbonate secretion in response to agonists including cyclic nucleotides, VIP, and luminal acid (1, 36, 43). Despite the recognized limitations, localization studies have recently provided important insights supporting a role for the glands in regulated fluid and water transport.
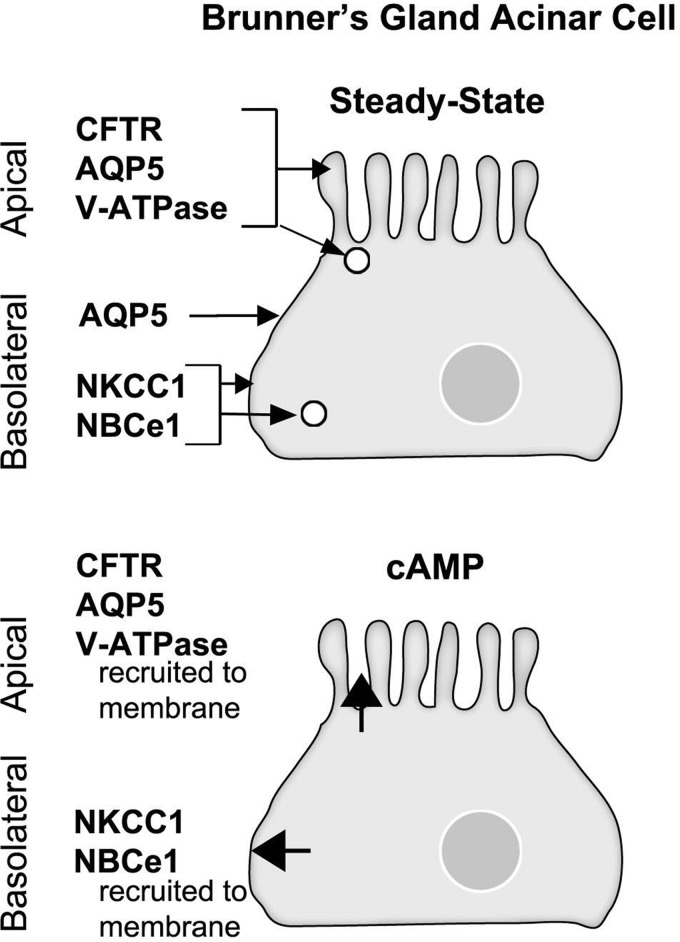
Second messenger-regulated membrane traffic is an important mechanism regulating ion transporter localization and functions in the native intestine. To gain further insight into potential fluid transport functions of Brunner's gland in health and disease, and to understand mechanisms that may regulate anion transporter functions, the present study examined the distribution patterns of clinically relevant anion transporters that are present in the duodenum: CFTR, NKCC1, NBCe1, the water channel AQP5 and the vacuolar ATPase proton pump. To determine whether trafficking regulated transporters in the gland the distribution of transporters was examined in rat Brunner's glands under steady state conditions and following cAMP or cholinergic stimulation (carbachol). The study also examined and compared the distribution of anion transporters, AQP5 and V-ATPase in tissues from healthy human proximal duodenum, and human duodenum in celiac disease and CF (Δ508 CFTR). Overall, the localization patterns of transporters and channels and their redistribution patterns following second messenger stimulation (Fig. 12) support functional transport of chloride, bicarbonate, water, and protons by second messenger-regulated traffic in mammalian Brunner's glands under physiological and pathophysiological conditions.
Fig. 12.
Schematic diagram summarizing the localization and redistribution patterns of the ion transporters, AQP5, and V-ATPase proton pump in the rat Brunner's gland. Top: distribution of CFTR, NKCC1, NBCe1, AQP5, and V-ATPase at steady state conditions (saline). At steady state, CFTR, V-ATPase, and AQP5 are localized to the apical domain and subapical endosomes of the gland. AQP5 was also detected on the lateral membrane of the gland. NKCC1 and NBCe1 localized to the basolateral membrane and endosomes in Brunner's glands at steady state. Bottom: trafficking patterns in the Brunner's gland acinar cell following cAMP treatment. CFTR, V-ATPase, and AQP5 are all recruited to the apical membrane, whereas NKCC1 and NBCe1 traffic to the basolateral membrane of the gland.
Distribution of anion transporters in Brunner's glands.
To better understand how Brunner's gland transport functions may differ from crypt and villus enterocytes in the proximal duodenum, the distribution of transporters was examined and compared (Table 3). CFTR, NKCC1, NBCe1, AE2, and V-ATPase were present in the Brunner's glands, crypt, and villus enterocytes, albeit at varying levels of expression. NHE3 was distinctly absent in the Brunner's glands and lower crypt, whereas AQP5 was present in the Brunner's glands but absent from crypt and villus enterocytes. Our previous studies identified high levels of CFTR in the apical domain and used ultrastructural localization to confirm that CFTR is in subapical endosomes within acinar cells of rat Brunner's gland (6, 40). Similar to what was demonstrated in enterocytes, the subcellular distribution of CFTR in rat Brunner's gland is consistent with a role for regulated traffic in CFTR-mediated anion secretion from the apical membrane. High levels of apical CFTR chloride channels and NKCC1, coupled with low levels of NBCe1, were identified in rat Brunner's gland (40). CFTR can transport chloride and/or bicarbonate through the apical membrane of intestinal epithelial cells. Preference for chloride or bicarbonate secretion appears to be dependent on apical chloride-bicarbonate exchangers and basolateral counterpart chloride/bicarbonate transporters in specific cell types. In duodenal villus enterocytes that express high levels of NBCe1, apical CFTR regulates bicarbonate secretion in response to cyclic AMP and luminal acid (35, 92). Identification of chloride/bicarbonate exchangers on the basolateral membranes provides important pathways for regulating bicarbonate transport in the glands. The presence of both apical and basolateral membrane chloride and bicarbonate transporters in the gland suggest that Brunner's gland possesses functional plasticity to transport both anions upon appropriate agonist stimulation similar to cells along the crypt-villus axis (40).
The transepithelial movement of water in epithelial cells is dependent on water channels or aquaporins (108). In the intestine, distinct aquaporin family members (AQP1, AQP3, AQP4, AQP7, AQP8 and AQP9) (but not AQP5) are present in goblet cells, endothelial cells, villus, and crypt enterocytes (49, 55, 62, 66, 100). However, AQP5 appears to be specific to the Brunner's gland and has not been localized to enterocytes. Consistent with previous observations (67, 77, 78), we found that AQP5 was detected primarily at the apical membrane and in intracellular endosomes in the rat Brunner's glands and on the apical and lateral membranes of human Brunner's glands. AQP5 is likely to be in endosomes in human glands, similar to the observations in rat, but was not detected, most likely because of limits in antigen detection by immunohistochemical approaches.
The V-ATPase pump is a multisubunit enzyme complex that mediates ATP-driven proton transport across membranes (11, 64, 72). Functional expression of subunits and isoforms of V-ATPase is cell and tissue specific (98). V-ATPase performs a number of housekeeping functions in the cell and is an important regulator of intraorganelle pH (71, 72). The identification of high expression levels of V-ATPase proton pumps in both human and rat Brunner's glands in this study is a new finding. Diffuse staining for V-ATPase pumps was observed throughout the cytoplasm of Brunner's gland acinar cells, with enrichment of apical membrane staining. Brunner's glands secrete glycoproteins, possess abundant protein synthesis machinery and organelles, thus V-ATPase pumps may contribute important functions for maintaining organelle homeostasis and pH regulation in the gland. Proton movement across the membrane and the maintenance of luminal pH in the small intestine is dependent on Na+/H+ exchangers. Inhibition of NHE3 in the small intestine by amiloride failed to eliminate luminal acidification, suggesting the presence of an alternative H+ pump such as V-ATPase (83). This scenario may also apply in the Brunner's gland, where V-ATPase may function to balance intracellular and luminal pH in the absence of apical NHE3, but this requires further investigation.
Trafficking of anion transporters, proton pumps, and water channels by cAMP or carbachol into the plasma membrane of rat Brunner's gland.
The relationships or mechanisms linking specific anion transporters to Brunner's gland functions have not been demonstrated. Although the nature of this study precluded direct measurements of anion transport on basolateral or apical membranes, this study demonstrated anion transporters in intracellular endosomes and regulated traffic into the plasma membranes of rat Brunner's glands following cAMP or cholinergic stimulation. Data provided here indicated that CFTR undergoes traffic from subapical endosomes into the apical membranes of rat Brunner's gland following both cAMP and carbachol. This response is similar to what was observed along the crypt-villus axis in the rat proximal small intestine, indicating that activation of CFTR occurs following both second messengers (40). Whether carbachol-stimulated CFTR traffic results from cross activation of cAMP pathways is unknown. However, Brunner's glands are under the control of the sympathetic nervous system and innervated by vagal nerves. Both cholinergic and acetylcholinesterase axons are found close to the gland and could regulate secretory activity (54). Carbachol or carbamylcholine activates acetylcholine receptors to release acetylcholine and activate secretion, since cholinergic stimulation leads to exocytosis of mucins from the glands (15).
CFTR anion secretion has been linked to aquaporin family members in epithelial tissues including the bile duct epithelium (103) and epididymis (14, 80). AQP5 associates with CFTR in the intercalated ducts of the pancreas, an epithelial tissue that actively secretes bicarbonate (12). Whether intestinal aquaporins (AQP 7, 8, and 11) are functionally linked to CFTR in enterocytes remains unknown. CFTR and AQP5 were previously identified in subapical vesicles within rat Brunner's gland (40, 77) in separate studies, although the specific vesicular compartments housed by each transporter was unknown. Regulated traffic of AQP5 into the apical plasma membrane with concomitant apical exocytosis of granules was demonstrated following administration of the cAMP-mediated hormone VIP in rat Brunner's gland, indicating an important role for cAMP-regulated traffic in water secretion from the apical membrane of the glands (77). Interestingly, intravenous VIP stimulated robust traffic of CFTR from subapical endosomes into the apical membrane of duodenal villus enterocytes (5). The data provided in this study, that CFTR and AQP5 colocalize and traffic together into the apical membrane of rat Brunner's gland following cAMP stimulation and that movement into the apical membrane is reversed by PKA inhibition, indicate that CFTR-mediated anion secretion is linked to AQP5 in Brunner's gland and regulated by cAMP-dependent traffic. This observation is consistent with published studies of CFTR translocation in the jejunum (27) and the suggestion that PKA may regulate AQP5 redistribution in the glands (77). These data suggest that PKA-dependent traffic of CFTR and AQP5 to the apical membranes of the Brunner's glands provides a parallel pathway for electrolyte secretion and osmotic water movement.
The novel observations in the present study that NKCC1 and NBCe1 undergo trafficking into the basolateral membranes of rat Brunner gland cells is consistent with chloride and bicarbonate secretory responses of both transporters in enterocytes (7, 18, 30, 39, 40, 84). Interestingly, cAMP-activated recruitment of NKCC1 and NBCe1 to the basolateral membranes in rat Brunner's glands was robust but a significant fraction of NKCC1 and NBCe1 label remained in the cytoplasm of acinar cells following stimulation. This suggests that some vesicles may not respond to cAMP and perhaps respond to calcium or cholinergic agonists. Alternatively, these vesicles may recycle to and from the plasma membrane. Further characterization of the vesicle population is required, since the early endosome marker EEA1 labeled only a small fraction of the vesicles associated with either transporter.
In other epithelial tissues, V-ATPase regulates luminal pH by cAMP-mediated trafficking of the pumps into the plasma membrane (79). Assembly of V-ATPase subunits appears to be important for its activity on the plasma membrane of epithelia. Antibodies raised against the E subunit were associated with cAMP-regulated apical membrane traffic and luminal acidification, although other membrane-associated subunits (Voa1, Voa2, Voa3) are classically associated with membrane traffic of the pump in epithelia (33, 90, 104). There was no apparent association of V-ATPase pumps with CFTR in the cytoplasm, but some colocalization was observed with CFTR at the apical domain at steady state. cAMP treatment resulted in some visible increase in CFTR/V-ATPase colocalization compared with saline-treated tissues by immunostaining although the difference was not statistically significant. The lack of difference could be due to the relatively imprecise methods used for measuring fluorescence intensity that may exclude apical brush border regions. The behavior of V-ATPase and CFTR in response to cAMP activation in rat Brunner's gland is similar to our observations in rat enterocytes, where we observed a dependence of V-ATPase pump function on CFTR channels (Collaco AM, Ameen NA, unpublished observations). The observations in this study suggest that there is a common trafficking pathway for CFTR and V-ATPase in the Brunner's gland.
Relevance of transporter localization in disease affected human Brunner's glands.
Diseases that specifically target the Brunner's glands are rare. Clinical studies have shown that adenomas and hyperplasia of the glands can lead to tumors. Furthermore, histological examination of tissues from patients with gastric ulcers identifies hyperplasia of the glands near these ulcers that is associated with impaired duodenal bicarbonate secretion (25). Studies examining the localization of anion transporters and channels in human Brunner's glands are lacking but necessary to elucidate the role of the gland in the pathophysiology of human intestinal diseases. Although AE2 has been identified in mouse Brunner's gland, the data in this study demonstrate that the glands possess specific transporters (CFTR and NBCe1) for bicarbonate entry and exit paths in human Brunner's glands. AQP5 was almost absent in celiac disease-affected human Brunner glands, whereas AQP5 staining in CF was reduced compared with healthy controls. NKCC1, NBCe1, and V-ATPase were markedly downregulated in both celiac disease- and CF-affected human Brunner's glands, but V-ATPase accumulated on the basal pole of CF acinar cells.
Celiac disease is a common chronic inflammatory disorder of the small bowel caused by ingestion of proline-rich proteins from wheat, rye, and barley. In the duodenum, celiac disease is characterized by markedly increased inflammatory cells in the lamina propria, flattened villi, and crypt hyperplasia that is associated with malabsorption and diarrhea in susceptible individuals (28, 81). Very little is known regarding the role of intestinal transporters in the pathophysiology of human celiac disease. The reduced anion transporter profiles observed in human Brunner's glands from celiac disease-affected duodenum in this study are consistent with the findings of a recent study of human duodenal tissues from patients with active celiac disease, although Brunner's gland was not examined in that study (57). The reduction in expression of absorptive transporters including NHE3 and SGLT1 in celiac disease-affected duodenum (excluding the Brunner's glands) is consistent with a decrease in absorptive functions and loss of villi that could account for diarrhea in celiac disease (57). The celiac disease-affected duodenal tissues examined in this study lacked intact villi and contained numerous inflammatory cells, which is consistent with the morphology observed in celiac disease. The functional implications of our findings of reduced transporter expression (CFTR, V-ATPase, AQP5) in celiac disease-affected human Brunner's glands are unclear at this time, since the ion transport functions of the healthy Brunner's glands are not known.
CF is an autosomal recessive genetic disorder that affects a variety of organs, including the small intestine. In CF patients, lack of functional CFTR on the apical membrane of epithelial cells leads to duodenal luminal hyperacidity and contributes to malabsorption and bacterial overgrowth in the small intestine (112, 115). In addition, duodenal bicarbonate secretions are reduced in tissues from CF patients (85). But nothing is known regarding the role of the Brunner's glands in CF pathophysiology. Mutations of CFTR impair chloride and bicarbonate transport that could be critical for normal Brunner's gland physiology and function. The observed downregulation of NKCC1, NBCe1, V-ATPase, and AQP5 in CF Brunner's gland in this study could account for some of the fluid transport defects observed in duodenal CF disease.
The present study provides information about localization of anion transporters in normal and diseased human tissue as a potential basis for future studies to define functional relevance of altered localization or expression. These data provide important insights that advance our understanding of the importance of Brunner's gland in health and disease and suggest an important role for anion transporters and AQP5 in the gland.
GRANTS
The study was supported by National Institute of Health Grant R01-DK-077065 to N. Ameen, by National Institute of Health Grant 5F32-DK-091014 to A. Collaco, and by National Institute of Health Grant P30-DK-34989 to the Yale Liver Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M.C., K.M., and N.A.A. conception and design of research; A.M.C., R.L.J., N.H., and A.B. performed experiments; A.M.C. and N.A.A. analyzed data; A.M.C. and N.A.A. interpreted results of experiments; A.M.C. prepared figures; A.M.C. drafted manuscript; A.M.C., R.L.J., N.H., K.M., A.B., and N.A.A. edited and revised manuscript; A.M.C., R.L.J., and N.A.A. approved final version of manuscript.
REFERENCES
- 1. Ainsworth MA, Koss MA, Hogan DL, Isenberg JI. Higher proximal duodenal mucosal bicarbonate secretion is independent of Brunner's glands in rats and rabbits. Gastroenterology 109: 1160–1166, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Alper SL, Rossmann H, Wilhelm S, Stuart-Tilley AK, Shmukler BE, Seidler U. Expression of AE2 anion exchanger in mouse intestine. Am J Physiol Gastrointest Liver Physiol 277: G321–G332, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Ameen NA, Marino C, Salas PJ. cAMP-dependent exocytosis and vesicle traffic regulate CFTR and fluid transport in rat jejunum in vivo. Am J Physiol Cell Physiol 284: C429–C438, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Ameen NA, Martensson B, Bourguinon L, Marino C, Isenberg J, McLaughlin GE. CFTR channel insertion to the apical surface in rat duodenal villus epithelial cells is upregulated by VIP in vivo. J Cell Sci 112: 887–894, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3− cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol 284: G37–G45, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bartman AE, Buisine MP, Aubert JP, Niehans GA, Toribara NW, Kim YS, Kelly EJ, Crabtree JE, Ho SB. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol 186: 398–405, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Bohe H, Bohe M, Lindstrom C, Ohlsson K. Pancreatic secretory trypsin inhibitor in human Brunner's glands. J Gastroenterol 30: 90–95, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Bower JM, Camble R, Gregory H, Gerring EL, Willshire IR. The inhibition of gastric acid secretion by epidermal growth factor. Experientia 31: 825–826, 1975 [DOI] [PubMed] [Google Scholar]
- 11. Breton S, Brown D. New insights into the regulation of V-ATPase-dependent proton secretion. Am J Physiol Renal Physiol 292: F1–F10, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut 52: 1008–1016, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calamita G, Mazzone A, Bizzoca A, Cavalier A, Cassano G, Thomas D, Svelto M. Expression and immunolocalization of the aquaporin-8 water channel in rat gastrointestinal tract. Eur J Cell Biol 80: 711–719, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Cheung KH, Leung CT, Leung GP, Wong PY. Synergistic effects of cystic fibrosis transmembrane conductance regulator and aquaporin-9 in the rat epididymis. Biol Reprod 68: 1505–1510, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Cosen-Binker LI, Morris GP, Vanner S, Gaisano HY. Munc18/SNARE proteins' regulation of exocytosis in guinea pig duodenal Brunner's gland acini. World J Gastroenterol 14: 2314–2322, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coutinho HB, Robalinho TI, Coutinho VB, Amorin AM, Almeida JR, Filho JT, Walker E, King G, Sewell HF, Wakelin D. Immunocytochemical demonstration that human duodenal Brunner's glands may participate in intestinal defence. J Anat 189: 193–197, 1996 [PMC free article] [PubMed] [Google Scholar]
- 17. Crescenzi A, Barsotti P, Anemona L, Marinozzi V. Carbohydrate histochemistry of human Brunner's glands. Histochemistry 90: 47–49, 1988 [DOI] [PubMed] [Google Scholar]
- 18. D'Andrea L, Lytle C, Matthews JB, Hofman P, Forbush B, 3rd, Madara JL. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem 271: 28969–28976, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Elder JB, Williams G, Lacey E, Gregory H. Cellular localisation of human urogastrone/epidermal growth factor. Nature 271: 466–467, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Elkjaer ML, Nejsum LN, Gresz V, Kwon TH, Jensen UB, Frokiaer J, Nielsen S. Immunolocalization of aquaporin-8 in rat kidney, gastrointestinal tract, testis, and airways. Am J Physiol Renal Physiol 281: F1047–F1057, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Flemstrom G. Stimulation of HCO3− transport in isolated proximal bullfrog duodenum by prostaglandins. Am J Physiol Gastrointest Liver Physiol 239: G198–G203, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Flemstrom G, Garner A. Gastroduodenal HCO3− transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol Gastrointest Liver Physiol 242: G183–G193, 1982 [DOI] [PubMed] [Google Scholar]
- 23. Flemstrom G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 16: 23–28, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Florey HW, Harding HE. Further observations on the secretion of Brunner's glands. J Pathol Bacteriol 39: 255–276, 1934 [Google Scholar]
- 25. Fuse Y, Tsuchihashi Y, Takamasu M, Kodama T, Fujita S, Kashima K. Thickness of Brunner's glands and its clinical significance in duodenal ulcer disease. Scand J Gastroenterol 25: 165–172, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Geleff S, Bock P. Pancreatic duct glands. II. Lectin binding affinities of ductular epithelium, ductular glands, and Brunner glands. Histochemistry 80: 31–38, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Golin-Bisello F, Bradbury N, Ameen N. STa and cGMP stimulate CFTR translocation to the surface of villus enterocytes in rat jejunum and is regulated by protein kinase G. Am J Physiol Cell Physiol 289: C708–C716, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Green PH, Cellier C. Celiac disease. N Engl J Med 357: 1731–1743, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Grossman ML. The glands of Brunner. Physiol Rev 38: 675–690, 1958 [DOI] [PubMed] [Google Scholar]
- 30. Grubb BR, Lee E, Pace AJ, Koller BH, Boucher RC. Intestinal ion transport in NKCC1-deficient mice. Am J Physiol Gastrointest Liver Physiol 279: G707–G718, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heitz PU, Kasper M, van Noorden S, Polak JM, Gregory H, Pearse AG. Immunohistochemical localisation of urogastrone to human duodenal and submandibular glands. Gut 19: 408–413, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, Cao Y, Zhou Y, Kunz J, Bellen HJ. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121: 607–620, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 109: 735–747, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology 113: 533–541, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. CFTR mediates cAMP and calcium activated duodenal epithelial bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 272: G872–G878, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Iseki S. Immunocytochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the rat stomach. Histochem J 27: 323–328, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Iwanaga T, Goto M, Watanabe M. Cellular distribution of glutamate transporters in the gastrointestinal tract of mice: an immunohistochemical and in situ hybridization approach. Biomed Res (Tokyo) 26: 271–278, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Jakab RL, Collaco AM, Ameen NA. Cell-specific effects of luminal acid, bicarbonate, cAMP, and carbachol on transporter trafficking in the intestine. Am J Physiol Gastrointest Liver Physiol 303: G937–G950, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Katsuyama T, Spicer SS. Histochemical differentiation of complex carbohydrates with variants of the concanavalin A-horseradish peroxidase method. J Histochem Cytochem 26: 233–250, 1978 [DOI] [PubMed] [Google Scholar]
- 42. Kirkegaard P, Lundberg JM, Poulsen SS, Olsen PS, Fahrenkrug J, Hokfelt T, Christiansen J. Vasoactive intestinal polypeptidergic nerves and Brunner's gland secretion in the rat. Gastroenterology 81: 872–878, 1981 [PubMed] [Google Scholar]
- 43. Kirkegaard P, Olsen PS, Nexo E, Holst JJ, Poulsen SS. Effect of vasoactive intestinal polypeptide and somatostatin on secretion of epidermal growth factor and bicarbonate from Brunner's glands. Gut 25: 1225–1229, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirkegaard P, Olsen PS, Poulsen SS, Nexo E. Exocrine secretion of epidermal growth factor from Brunner's glands. Stimulation by VIP and acetylcholine. Regul Pept 7: 367–372, 1983 [DOI] [PubMed] [Google Scholar]
- 45. Kirkegaard P, Skov Olsen P, Seier Poulsen S, Holst JJ, Schaffalitzky de Muckadell OB, Christiansen J. Effect of secretin and glucagon on Brunner's gland secretion in the rat. Gut 25: 264–268, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kittas C, Aroni K, Matani A, Papadimitriou CS. Immunocytochemical demonstration of a1-Antitrypsin and a1-Antichymotrypsin in human gastrointestinal tract. Hepatogastroenterology 29: 275–277, 1982 [PubMed] [Google Scholar]
- 47. Klockars M, Reitamo S. Tissue distribution of lysozyme in man. J Histochem Cytochem 23: 932–940, 1975 [DOI] [PubMed] [Google Scholar]
- 48. Kovac J, Moore B, Vanner S. Potassium currents regulating secretion from Brunner's glands in guinea pig duodenum. Am J Physiol Gastrointest Liver Physiol 286: G377–G384, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Koyama Y, Yamamoto T, Tani T, Nihei K, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K, Kihara I. Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol Cell Physiol 276: C621–C627, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Krause WJ. Brunner's glands: a structural, histochemical and pathological profile. Prog Histochem Cytochem 35: 259–367, 2000 [PubMed] [Google Scholar]
- 51. Krause WJ, Leeson CR. The origin, development and differentiation of Brunner's glands in the rat. J Anat 101: 309–320, 1967 [PMC free article] [PubMed] [Google Scholar]
- 52. Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 13: 1072–1082, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kulaksiz H, Bektas H, Cetin Y. Expression and cell-specific and membrane-specific localization of NHE-3 in the human and guinea pig upper gastrointestinal tract. Cell Tissue Res 303: 337–343, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Kyosola K. Innervation of the duodenal submucosal Brunner's glands. Acta Physiol Scand 101: 498–500, 1977 [DOI] [PubMed] [Google Scholar]
- 55. Laforenza U. Water channel proteins in the gastrointestinal tract. Mol Aspects Med 33: 642–650, 2012 [DOI] [PubMed] [Google Scholar]
- 56. Laforenza U, Gastaldi G, Grazioli M, Cova E, Tritto S, Faelli A, Calamita G, Ventura U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol Cell 97: 605–613, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Laforenza U, Miceli E, Gastaldi G, Scaffino MF, Ventura U, Fontana JM, Orsenigo MN, Corazza GR. Solute transporters and aquaporins are impaired in celiac disease. Biol Cell 102: 457–467, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Leeson TS, Leeson CR. The fine structure of Brunner's glands in man. J Anat 103: 263–276, 1968 [PMC free article] [PubMed] [Google Scholar]
- 59. Lefebvre O, Wolf C, Kedinger M, Chenard MP, Tomasetto C, Chambon P, Rio MC. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol 122: 191–198, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liebman WM, Samloff IM. Immunochemical characterization and cellular localization of pepsinogens in cat and dog. J Histochem Cytochem 26: 1115–1120, 1978 [DOI] [PubMed] [Google Scholar]
- 61. Longman RJ, Douthwaite J, Sylvester PA, Poulsom R, Corfield AP, Thomas MG, Wright NA. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut 47: 792–800, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol 517: 317–326, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manson AL, Trezise AE, MacVinish LJ, Kasschau KD, Birchall N, Episkopou V, Vassaux G, Evans MJ, Colledge WH, Cuthbert AW, Huxley C. Complementation of null CF mice with a human CFTR YAC transgene. EMBO J 16: 4238–4249, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20: 415–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martin BF. Serous cells in Brunner's glands of the rabbit. Nature 174: 1195–1196, 1954 [DOI] [PubMed] [Google Scholar]
- 66. Matsuzaki T, Tajika Y, Ablimit A, Aoki T, Hagiwara H, Takata K. Aquaporins in the digestive system. Med Electron Microsc 37: 71–80, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Matsuzaki T, Tajika Y, Suzuki T, Aoki T, Hagiwara H, Takata K. Immunolocalization of the water channel, aquaporin-5 (AQP5), in the rat digestive system. Arch Histol Cytol 66: 307–315, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Moore BA, Kim D, Vanner S. Neural pathways regulating Brunner's gland secretion in guinea pig duodenum in vitro. Am J Physiol Gastrointest Liver Physiol 279: G910–G917, 2000 [DOI] [PubMed] [Google Scholar]
- 69. Moore BA, Morris GP, Vanner S. A novel in vitro model of Brunner's gland secretion in the guinea pig duodenum. Am J Physiol Gastrointest Liver Physiol 278: G477–G485, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Nejsum LN, Kwon TH, Jensen UB, Fumagalli O, Frokiaer J, Krane CM, Menon AG, King LS, Agre PC, Nielsen S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc Natl Acad Sci USA 99: 511–516, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nelson N, Harvey WR. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol Rev 79: 361–385, 1999 [DOI] [PubMed] [Google Scholar]
- 72. Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat Rev Mol Cell Biol 3: 94–103, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Okada S, Misaka T, Matsumoto I, Watanabe H, Abe K. Aquaporin-9 is expressed in a mucus-secreting goblet cell subset in the small intestine. FEBS Lett 540: 157–162, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Oppenheimer EH, Esterly JR. Cystic fibrosis of the pancreas. Morphologic findings in infants with and without diagnostic pancreatic lesions. Arch Pathol 96: 149–154, 1973 [PubMed] [Google Scholar]
- 75. Ota H, Nakayama J, Momose M, Kurihara M, Ishihara K, Hotta K, Katsuyama T. New monoclonal antibodies against gastric gland mucous cell-type mucins: a comparative immunohistochemical study. Histochem Cell Biol 110: 113–119, 1998 [DOI] [PubMed] [Google Scholar]
- 76. Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut 35: 646–650, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Parvin MN, Kurabuchi S, Murdiastuti K, Yao C, Kosugi-Tanaka C, Akamatsu T, Kanamori N, Hosoi K. Subcellular redistribution of AQP5 by vasoactive intestinal polypeptide in the Brunner's gland of the rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1283–G1291, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Parvin MN, Tsumura K, Akamatsu T, Kanamori N, Hosoi K. Expression and localization of AQP5 in the stomach and duodenum of the rat. Biochim Biophys Acta 1542: 116–124, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pietrement C, Da Silva N, Silberstein C, James M, Marsolais M, Van Hoek A, Brown D, Pastor-Soler N, Ameen N, Laprade R, Ramesh V, Breton S. Role of NHERF1, cystic fibrosis transmembrane conductance regulator, and cAMP in the regulation of aquaporin 9. J Biol Chem 283: 2986–2996, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Polanco I. Celiac disease. J Pediatr Gastroenterol Nutr 47, Suppl 1: S3–S6, 2008 [DOI] [PubMed] [Google Scholar]
- 82. Poulsen SS, Nexo E, Olsen PS, Hess J, Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry 85: 389–394, 1986 [DOI] [PubMed] [Google Scholar]
- 83. Praetorius J, Andreasen D, Jensen BL, Ainsworth MA, Friis UG, Johansen T. NHE1, NHE2, and NHE3 contribute to regulation of intracellular pH in murine duodenal epithelial cells. Am J Physiol Gastrointest Liver Physiol 278: G197–G206, 2000 [DOI] [PubMed] [Google Scholar]
- 84. Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, Ainsworth MA, Johansen T. Molecular and functional evidence for electrogenic and electroneutral Na+-HCO3− cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280: G332–G343, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Pratha VS, Hogan DL, Martensson BA, Bernard J, Zhou R, Isenberg JI. Identification of transport abnormalities in duodenal mucosa and duodenal enterocytes from patients with cystic fibrosis. Gastroenterology 118: 1051–1060, 2000 [DOI] [PubMed] [Google Scholar]
- 86. Ramirez-Lorca R, Vizuete ML, Venero JL, Revuelta M, Cano J, Ilundain AA, Echevarria M. Localization of aquaporin-3 mRNA and protein along the gastrointestinal tract of Wistar rats. Pflügers Arch 438: 94–100, 1999 [DOI] [PubMed] [Google Scholar]
- 87. Rasmussen TN, Raaberg L, Poulsen SS, Thim L, Holst JJ. Immunohistochemical localization of pancreatic spasmolytic polypeptide (PSP) in the pig. Histochemistry 98: 113–119, 1992 [DOI] [PubMed] [Google Scholar]
- 88. Ryan J, Costigan DC. Determination of the histological distribution of insulin like growth factor 1 receptors in the rat gut. Gut 34: 1693–1697, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Saito H, Kasajima T, Masuda A, Imai Y, Ishikawa M. Lysozyme localization in human gastric and duodenal epithelium. An immunocytochemical study. Cell Tissue Res 251: 307–313, 1988 [DOI] [PubMed] [Google Scholar]
- 90. Saw NM, Kang SY, Parsaud L, Han GA, Jiang T, Grzegorczyk K, Surkont M, Sun-Wada GH, Wada Y, Li L, Sugita S. Vacuolar H+-ATPase subunit Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake and storage. Mol Biol Cell 22: 3394–3409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schrader WP, West CA. Adenosine deaminase complexing proteins are localized in exocrine glands of the rabbit. J Histochem Cytochem 33: 508–514, 1985 [DOI] [PubMed] [Google Scholar]
- 92. Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3− secretion. J Physiol 505: 411–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Simpson JE, Gawenis LR, Walker NM, Boyle KT, Clarke LL. Chloride conductance of CFTR facilitates basal Cl−/HCO3− exchange in the villous epithelium of intact murine duodenum. Am J Physiol Gastrointest Liver Physiol 288: G1241–G1251, 2005 [DOI] [PubMed] [Google Scholar]
- 94. Simpson JE, Schweinfest CW, Shull GE, Gawenis LR, Walker NM, Boyle KT, Soleimani M, Clarke LL. PAT-1 (Slc26a6) is the predominant apical membrane Cl−/HCO3− exchanger in the upper villous epithelium of the murine duodenum. Am J Physiol Gastrointest Liver Physiol 292: G1079–G1088, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992 [DOI] [PubMed] [Google Scholar]
- 96. Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sugai N, Oosaki T. Cytochemistry for carbonic anhydrase activity in rat Brunner's gland. Fukushima J Med Sci 29: 63–75, 1983 [PubMed] [Google Scholar]
- 98. Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16-kDa subunit c of vacuolar proton pump with the ileal Na+-dependent bile acid transporter: protein-protein interaction and intracellular trafficking. J Biol Chem 279: 16295–16300, 2004 [DOI] [PubMed] [Google Scholar]
- 99. Suzuki M. Expression and localization of aquaporin-1 on the apical membrane of enterocytes in the small intestine of bottlenose dolphins. J Comp Physiol B 180: 229–238, 2010 [DOI] [PubMed] [Google Scholar]
- 100. Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 39: 1–83, 2004 [DOI] [PubMed] [Google Scholar]
- 101. Takehana K, Masty J, Abe M, Yamaguchi M. Duodenal glands of the pony (Equus caballus). Anat Histol Embryol 20: 1–9, 1991 [DOI] [PubMed] [Google Scholar]
- 102. Tarasova N, Spannagel AW, Green GM, Gomez G, Reed JT, Thompson JC, Hellmich MR, Reeve JR, Jr, Liddle RA, Greeley GH., Jr Distribution and localization of a novel cholecystokinin-releasing factor in the rat gastrointestinal tract. Endocrinology 138: 5550–5554, 1997 [DOI] [PubMed] [Google Scholar]
- 103. Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper S, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem 278: 20413–20419, 2003 [DOI] [PubMed] [Google Scholar]
- 104. Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278: 22023–22030, 2003 [DOI] [PubMed] [Google Scholar]
- 105. Treasure T. The ducts of Brunner's glands. J Anat 127: 299–304, 1978 [PMC free article] [PubMed] [Google Scholar]
- 106. Tyden E, Bjornstrom H, Tjalve H, Larsson P. Expression and localization of BCRP, MRP1 and MRP2 in intestines, liver and kidney in horse. J Vet Pharmacol Ther 33: 332–340, 2010 [DOI] [PubMed] [Google Scholar]
- 107. Valentijn JA, van Weeren L, Ultee A, Koster AJ. Novel localization of Rab3D in rat intestinal goblet cells and Brunner's gland acinar cells suggests a role in early Golgi trafficking. Am J Physiol Gastrointest Liver Physiol 293: G165–G177, 2007 [DOI] [PubMed] [Google Scholar]
- 108. Verkman AS. Water channels in cell membranes. Annu Rev Physiol 54: 97–108, 1992 [DOI] [PubMed] [Google Scholar]
- 109. Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol 278: L867–L879, 2000 [DOI] [PubMed] [Google Scholar]
- 110. Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL. Role of down-regulated in adenoma anion exchanger in HCO3− secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Willingham MC, Rutherford AV, Cheng SY. Immunohistochemical localization of a thyroid hormone-binding protein (p55) in human tissues. J Histochem Cytochem 35: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 112. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut 56: 1153–1163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wolter HJ. Adrenocorticotropin immunoreactivity is contained within presumptive endocytotic vesicles of rat Brunner's gland cells. Brain Res 336: 143–147, 1985 [DOI] [PubMed] [Google Scholar]
- 114. Yao B, Hogan DL, Bukhave K, Koss MA, Isenberg JI. Bicarbonate transport by rabbit duodenum in vitro: effect of vasoactive intestinal polypeptide, prostaglandin E2, and cyclic adenosine monophosphate. Gastroenterology 104: 732–740, 1993 [DOI] [PubMed] [Google Scholar]
- 115. Youngberg CA, Berardi RR, Howatt WF, Hyneck ML, Amidon GL, Meyer JH, Dressman JB. Comparison of gastrointestinal pH in cystic fibrosis and healthy subjects. Dig Dis Sci 32: 472–480, 1987 [DOI] [PubMed] [Google Scholar]
- 116. Zamolodchikova TS, Sokolova EA, Alexandrov SL, Mikhaleva II, Prudchenko IA, Morozov IA, Kononenko NV, Mirgorodskaya OA, Da U, Larionova NI, Pozdnev VF, Ghosh D, Duax WL, Vorotyntseva TI. Subcellular localization, substrate specificity and crystallization of duodenase, a potential activator of enteropeptidase. Eur J Biochem 249: 612–621, 1997 [DOI] [PubMed] [Google Scholar]