Abstract
Activation of the transcription factor NFAT5 by high NaCl involves changes in phosphorylation. By siRNA screening, we previously found that protein targeting to glycogen (PTG), a regulatory subunit of protein phosphatase1 (PP1), contributes to regulation of high NaCl-induced NFAT5 transcriptional activity. The present study addresses the mechanism involved. We find that high NaCl-induced inhibition of PTG elevates NFAT5 activity by increasing NFAT5 transactivating activity, protein abundance, and nuclear localization. PTG acts via a catalytic subunit PP1γ. PTG associates physically with PP1γ, and NaCl reduces both this association and remaining PTG-associated PP1γ activity. High NaCl-induced phosphorylation of p38, ERK, and SHP-1 contributes to activation of NFAT5. Knockdown of PTG does not affect phosphorylation of p38 or ERK. However, PTG and PP1γ bind to SHP-1, and knockdown of either PTG or PP1γ increases high NaCl-induced phosphorylation of SHP-1-S591, which inhibits SHP-1. Mutation of SHP-1-S591 to alanine, which cannot be phosphorylated, increases inhibition of NFAT5 by SHP-1. Thus high NaCl reduces the stimulatory effect of PTG and PP1γ on SHP-1, which in turn reduces the inhibitory effect of SHP-1 on NFAT5. Our findings add to the known functions of PTG, which was previously recognized only for its glycogenic activity.
Keywords: protein targeting to glycogen, TonEBP, PP1γ, PPP1R3C, hypertonicity, protein phosphatase 1
hypertonicity, such as produced by elevation of extracellular NaCl, shrinks cells, which increases intracellular ionic strength, stresses the cells, and can even kill them. In response to hypertonic stress, cells accumulate organic osmolytes, which replace elevated intracellular inorganic ions and normalize cell volume (3). In mammalian cells, accumulation of organic osmolytes and of protective heat shock proteins results from increased gene transcription. The transcription factor NFAT5 (also called TonEBP or OREBP) activates expression of osmoprotective genes (3). Cells in many organs are normally exposed to hypertonic milieu, the extreme case being cells in the kidney medulla. NFAT5 plays an indispensable role in protecting these cells from hypertonic injury (13, 23, 26). NFAT5 also activates expression of numerous other genes, whose role in osmoprotection is uncertain (24). Phosphorylation is a prominent mechanism by which hypertonicity activates NFAT5 (3). Regulatory increase of phosphorylation of NFAT5 depends on both kinase and phosphatase activity (12, 41).
Protein phosphatase 1 encompasses a large family of serine/threonine phosphatases, each member of which contains a separate regulatory and catalytic subunit (30). Protein targeting to glycogen (PTG), encoded by the gene PPP1R3C, is one of the regulatory subunits (7, 29). It is expressed in a wide variety of tissues, including the kidney medulla (7). PTG acts as a molecular scaffold. It bridges a catalytic subunit to glycogen synthase and glycogen phosphorylase in intracellular glycogen particles. This leads to activation of glycogen synthase and inhibition of glycogen phosphorylase through dephosphorylation of these two enzymes, resulting in increased glycogen synthesis and decreased glycogenolysis (14, 29). Homozygous knockout of PTG is embryonically lethal (5). Heterozygous deletion of the PTG gene impairs glycogen synthesis in many tissues and causes developmental insulin resistance in mice (5). Mutation of PTG is associated with a mild phenotype of Lafora disease, an autosomal recessive disease due to presence of intracellular polyglucosan bodies (15). Glycogenic activity is the only function previously assigned to PTG.
SHP-1, encoded by the gene PTPN6, is involved in inhibition of T-cell activation (27). Many of the known substrates of SHP-1 are plasma membrane receptors for antigens, cytokines, and growth factors (27). Our screen of a genome-wide siRNA library against phosphatases identified probable negative roles of PTG and SHP-1 in regulation of NFAT5 activity (41). We previously confirmed the negative role of SHP-1 (41). SHP-1 inhibits high NaCl-induced increase of NFAT5 transcriptional activity by reducing NFAT5 nuclear localization and transactivating activity (41). Here, we report further studies that link regulation of NFAT5 to combined activity of PTG and SHP-1. We find that PTG and PP1γ, its catalytic subunit, activate SHP-1 by dephosphorylating SHP-1-S591. We also find that high NaCl inhibits PTG and PP1γ, resulting in attenuation of their stimulatory effect on SHP-1. The net effect of NaCl inhibition of PTG and PP1γ is to reduce the negative effect of SHP-1 on NFAT5.
MATERIALS AND METHODS
Cells and chemicals.
Human embryonic kidney 293 (HEK293) cells (ATCC) and HEK293FT cells (Invitrogen) were incubated in Eagle's minimal essential medium plus 10% fetal bovine serum in 5% CO2/95% air at 37°C. HEK293 cells were used between passages 38 and 48. HEK293FT cells were used between passages 3 and 13. HEK293 cells stably expressing osmotic response element (ORE) luciferase reporter or mutated ORE luciferase reporter (39) were used between passages 40 and 45. HeLa cells (ATCC) were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum in 5% CO2/95% air at 37°C and used between passages 7 and 10. HEK293 cells stably expressing PTG-V5 were established by transfection of pcDNA3.1-PTG-V5 plasmid and selection with 600 μg/ml G418. The population of stable cells (without subcloning) was used between passages 39 and 49. The initial osmolality of medium for HEK293 cells and HEK293FT cells was 290 mosM (control), and the initial osmolality of medium for HeLa cells was 300 mosM (control). All experiments were performed on sub-confluent cells.
Plasmids, siRNAs, transfections, and luciferase activity.
Plasmids coding for PTG-V5 [gift from Dr. Jack E. Dixon (36)], SHP-1-HA [gift of Dr. Stephen Shaw (25)], SHP-2-myc [gift of Dr. Gibbes R. Johnson (6)], human ORE-X, and NFAT5-V5 (19) were transfected into HEK293 cells with Effectene, according to the manufacturer's protocol (Qiagen). Plasmids coding for SHP-1-HA, SHP-1-S591A-HA (25), and ORE-X were transfected into HEK293 cells with Lipofectamine 2000 (Invitrogen). Combinations of two siRNAs against human PTG (SI02658978 and SI02658985, Qiagen) and of four siRNAs against human PP1γ (SI02225776, SI02759211, SI02225769, and SI04436411, Qiagen) were used. The control siRNA was described previously (17). Transfection of siRNAs was done with Lipofectamine 2000 in the recommended ratio of siRNA to Lipofectamine 2000 (Invitrogen). All transfections were done by adding cell suspensions to a plated complex of DNA or siRNA plus transfection reagent. Luciferase activity was measured as previously described (39).
Quantitative PCR.
HEK293 cells were treated with high NaCl or replaced by fresh control medium for 16 h in a 96-well plate, and then cells were lysed and cDNAs were synthesized using the Cells-to-Ct kit (Applied Biosystems) and amplified by Taqman 7900HT (Applied Biosystems). Primers and probes for human aldose reductase and betaine/GABA transporter were described previously (9). Fold difference in RNA abundance between conditions (F) was calculated, as described previously (9).
Western analysis and antibodies.
After treatment, cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl. For measuring phosphorylation, the samples were collected with phosphosafe buffer (EMD Chemicals) or M-PER buffer (Pierce) plus 2.0 μM NaF and 2.0 μM Na3VO4. For measuring NFAT5 protein abundance, the samples were collected with 1% Triton X-100, 150 mM NaCl, and 50 mM Tris·HCl pH7.4. All buffers were supplemented with a protease inhibitor tablet (Roche) immediately before use. Samples were analyzed by Western blotting and quantified by infrared imaging (Odyssey, Li-Cor). Antibodies against NFAT5 (catalog no. sc-13035, rabbit), PP1γ (catalog no. sc-6108, goat), Brg-1 (catalog no. sc-17796, mouse), and SHP-1 (catalog no. sc-287, rabbit) were from Santa Cruz Biotechnologies. Antibody against SHP-1-S591-P (catalog no. sp-1531, rabbit) was from ECM Biosciences. This antibody worked best in our hands. For measuring phosphorylation of SHP-1-S591-P, the mouse anti-SHP-1 (catalog no. 610126, BD Biosciences) was combined with the rabbit anti-SHP-1-S591-P (ECM Biosciences), and the antibodies were identified simultaneously by secondary anti-rabbit and anti-mouse antibodies conjugated with different wavelengths of Alexa Fluor. The mouse β-tubulin antibody (catalog no. T8660) was from Sigma. The mouse anti-V5 antibody (catalog no. MCA1360) was from AbD Serotec. The rabbit anti-V5 antibody (catalog no. RV5–45A) was from Immunology Consultants Laboratory. The rabbit anti-PP1γ antibody (catalog no. 07-1218) was from Millipore. Antibodies against p38 (catalog no. 9212), p38-P (catalog no. 9216), ERK (catalog no. 9102), ERK-P (catalog no. 9106), and SHP-2 (catalog no. 3397) were from Cell Signaling.
NFAT5 transactivating activity and nuclear localization.
HEK293 cells stably expressing a yeast binary GAL4 reporter assay system (9) were used to analyze the effect of PTG siRNAs on NFAT5 transactivating activity. For measuring NFAT5-V5 nuclear localization, cells were co-transfected with plasmids coding for NFAT5-V5 and PTG-V5 for 24 h, then divided into two dishes and incubated for an additional 22 h. In one dish, the medium was replaced with fresh control culture medium and the other with medium at 500 mosM (NaCl added) for 2 h. Cells were washed once with ice-cold PBS adjusted to the osmolality of the medium by adding NaCl. NE-PER kit (Pierce) was used to extract cytoplasmic and nuclear proteins. NFAT5-V5 in each fraction was measured by Western analysis, and nuclear to cytoplasmic ratio was calculated as previously described (8). To exclude the possibility that the ratio was affected by inadequate separation of nuclear and cytoplasmic proteins, cytoplasmic marker protein β-tubulin and nuclear marker protein Brg-1 were monitored in each extract.
Co-immunoprecipitation.
After treatment with the control or high NaCl medium for 2 h, cells were washed once with ice-cold PBS (adjusted to the experimental NaCl concentration), then collected in a lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, and 1% Triton X-100 plus protease inhibitor tablet (Roche). The supernatant was cleared with an agarose-conjugated IgG from the same species as the antibody at 4°C for 1 h and then incubated with the agarose-conjugated antibody at 4°C overnight. After incubation, the agarose beads were gently washed twice with ice-cold lysis buffer. Proteins were stripped from the beads by SDS-loading buffer at 95°C for 5 min and analyzed by immunoblotting. To minimize background, antibodies from a different species than the antibody used to perform co-immunoprecipitation were used to probe membranes.
Duolink in situ.
PTG-V5 transfected or non-transfected HeLa cells were seeded in Lab-Tek chamber slides (Nalge Nunc International) for 24 h. After treatment, cells were fixed with 4% formaldehyde for 15 min at room temperature and for additional 45 min on ice, permeabilized with 0.5% Triton X-100 in PBS at room temperature for 15 min, blocked with Odyssey blocking buffer (Li-Cor) at room temperature for 30 min, and incubated with two primary antibodies from two different species. The rest of the analysis was performed according to the manufacturer's protocol (Olink Bioscience).
PTG-V5-associated PP1γ activity.
PTG-V5-associated PP1γ activity was assayed with the Ser/Thr phosphatase assay kit (Millipore, catalog no. 17–127). Briefly, HEK293 cells stably expressing PTG-V5 were treated with control or high NaCl medium for 2 h. After being washed once with ice-cold PBS, adjusted to the experimental NaCl concentration, cells were collected in a lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% β-mercaptoethanol, and 1% Triton X-100 plus protease inhibitor tablet (Roche). Supernatant containing 1 mg of protein was incubated with agarose-conjugated mouse anti V5 antibody at 4°C for 2 h, and then the agarose beads were gently washed once with ice-cold lysis buffer and once with the assay buffer (50 mM HEPES, pH 7.5, 100 mM NaCl, 2 mM dithiothreitol, 0.1 mM EGTA, 0.025% Tween-20, and 1 mM MnCl2) (1). The beads were resuspended in 35 μl of the assay buffer. The reaction was started by adding the substrate KR-pT-IRR at a final concentration of 20 μM and incubated at room temperature for 15 min. The liberated phosphorus was reacted with mixture of malachite green solution A and B, and absorption was measure at 630 nM.
Statistics.
Data are expressed as means ± SE. Data were transformed to log10 scale before statistical analyses, which were performed by paired t-test or repeated-measures ANOVA (with Student-Newman-Keuls post hoc comparison) as appropriate. P < 0.05 is considered significant.
RESULTS
Effect of PTG on the transcriptional activity of NFAT5.
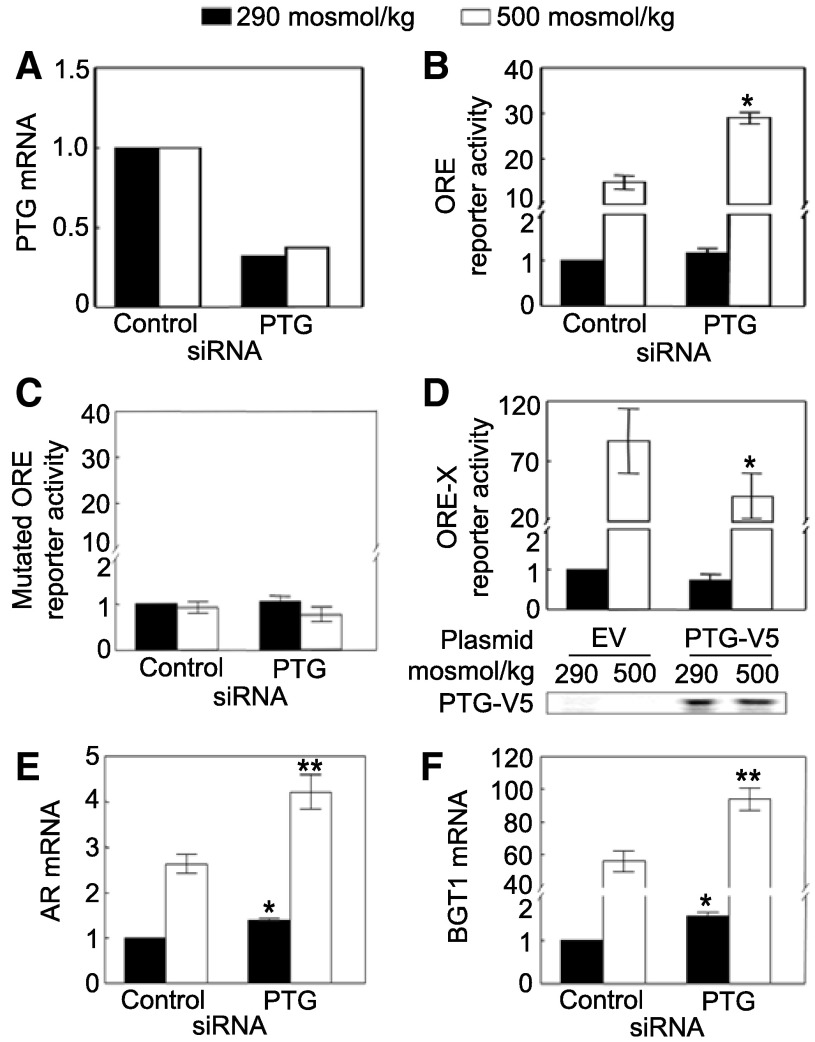
We previously screened a library of siRNAs against human phosphatases for their effects on NFAT5 transcriptional activity using human embryonic kidney (HEK) 293 cells that stably express a luciferase reporter containing the NFAT5 ORE (41). We found that siRNA knockdown of PTG increases NFAT5 transcriptional activity (41). In the present studies, our attempts to measure the extent to which the PTG siRNAs reduce PTG protein abundance were unsuccessful since none of four different commercially available anti-PTG antibodies worked satisfactorily in our hands. Nevertheless, effectiveness of the knockdown is confirmed by ∼80% reduction of PTG mRNA (Fig. 1A). PTG siRNAs significantly increase high NaCl-induced NFAT5 transcriptional activity (Fig. 1B). As a control, the siRNAs have no significant effect on the activity using an ORE reporter in which the ORE elements are mutated to prevent NFAT5 binding (Fig. 1C). As an additional test, we transiently overexpressed PTG-V5 together with ORE-X luciferase reporter of NFAT5 activity and find that overexpression of PTG reduces high NaCl-induced NFAT5 transcriptional activity (Fig. 1D). We conclude that PTG contributes to regulation of NFAT5 transcriptional activity.
Fig. 1.
Protein targeting to glycogen (PTG) inhibits NFAT5 transcriptional activity. A–C: HEK293 cells stably expressing a luciferase reporter containing the osmotic response element (ORE) DNA element that binds NFAT5 or containing an ORE mutated to prevent NFAT5 binding were transfected with PTG siRNAs for 32 h, then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 16 h, before PTG mRNA or luciferase activity was measured. A: PTG siRNAs reduce PTG mRNA. B: PTG siRNAs significantly increase high NaCl-induced NFAT5 transcriptional activity. C: PTG siRNAs have no significant effect on the mutated ORE reporter activity. D: overexpression of PTG-V5 reduces high NaCl-induced NFAT5 transcriptional activity. PTG-V5 plasmid was transfected together with the ORE-X reporter of NFAT5 transcriptional activity into HEK293 cells for 32 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 16 h. *Significant difference compared with reporter activity with empty vector (P < 0.05; n = 3). E and F: PTG siRNAs increase mRNA expression of aldose reductase (AR) and betaine/GABA transporter1 (BGT1), which are transcriptional targets of NFAT5. HEK293 cells were transfected with PTG siRNAs for 32 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 16 h. *Signficant difference vs. control at 290 mosM (P < 0.05). **Signficant difference vs. control at 500 mosM (P < 0.05; n = 3).
Effect of PTG on expression of AR and BGT1 mRNAs.
Aldose reductase (AR) and betaine/GABA transporter 1 (BGT1) are transcriptional targets of NFAT5 responsible for accumulation of sorbitol and betaine (3). We find that PTG siRNAs significantly increase AR and BGT1 mRNA expression at both 290 and 500 mosM (Fig. 1, E and F), confirming that PTG regulates NFAT5 activity.
Effects of PTG on high NaCl-induced NFAT5 nuclear localization, transactivating activity, and protein abundance.
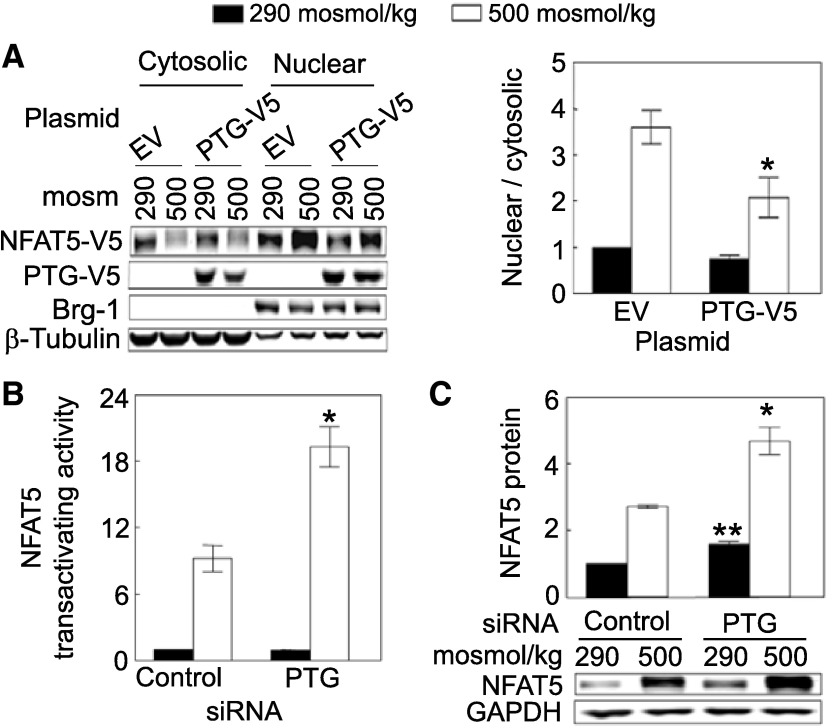
To identify the mechanism by which PTG inhibits NFAT5 transcriptional activity, we examined the effect of PTG on NFAT5 nuclear localization, transactivating activity, and protein abundance. Overexpression of PTG reduces high NaCl-induced increase of the nuclear to cytoplasmic ratio of NFAT5 (Fig. 2A). On the other hand, high NaCl does not significantly affect nuclear localization of PTG-V5 itself (nuclear/cytoplasmic ratio of PTG-V5, normalized to 1.0 at 290 mosM, is 1.14 ± 0.09 at 500 mosM; n = 4). High NaCl increases the transactivating activity of NFAT5 in HEK293 cells stably expressing a reporter that contains the NFAT5 transactivation domain (Fig. 2B). PTG siRNAs significantly elevate the high NaCl-induced increase of NFAT5 transactivating activity (Fig. 2B). Note that reporter activity in this assay is dependent on expression of the NFAT5 transactivation domain in the reporter and is independent of native NFAT5 (9). Also, PTG siRNAs significantly increase NFAT5 protein expression both at 290 and 500 mosM (Fig. 2C). We conclude that PTG negatively regulates high NaCl-dependent NFAT5 transcriptional activity by inhibiting its nuclear localization, transactivating activity, and protein expression.
Fig. 2.
A: overexpression of PTG reduces high NaCl-induced NFAT5 nuclear localization. HEK293FT cells were transiently co-transfected with NFAT5-V5 and PTG-V5 or empty vector for 46 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 2 h. Proteins were extracted separately from cytoplasm and nucleus, and then analyzed by Western blotting. The cytoplasmic and nuclear proteins were consistently separated, as supported by the subcellular distributions of β-tubulin (cytoplasmic marker) and Brg-1 (nuclear marker), which are unaffected by NaCl concentration. *Signficant difference vs. control (P < 0.05; n = 4). B: siRNAs against PTG increase high NaCl-induced NFAT5 transactivating activity. As in Fig. 1B, except that we measured NFAT5 transactivating activity in HEK293 cells stably expressing a yeast binary GAL4 reporter assay system. High NaCl increases NFAT5 transactivating activity, and knockdown of PTG by its siRNAs further increases high NaCl-induced NFAT5 transactivating activity. *Signficant difference vs. control at 500 mosM (P < 0.05; n = 3). C: siRNAs against PTG increase NFAT5 protein abundance. HEK293 cells were transfected with siRNA against PTG for 32 h, and then the medium was changed for 16 h, either maintaining it at 290 mosM or increasing it to 500 mosM (NaCl added). In B and C, the PTG siRNAs decrease PTG mRNA abundance by ∼68%. *Significant difference compared with control at 500 mosM (P < 0.05; n = 3). **Significant difference compared with control at 290 mosM (P < 0.05; n = 3).
Effect of PP1γ on the transcriptional activity of NFAT5.
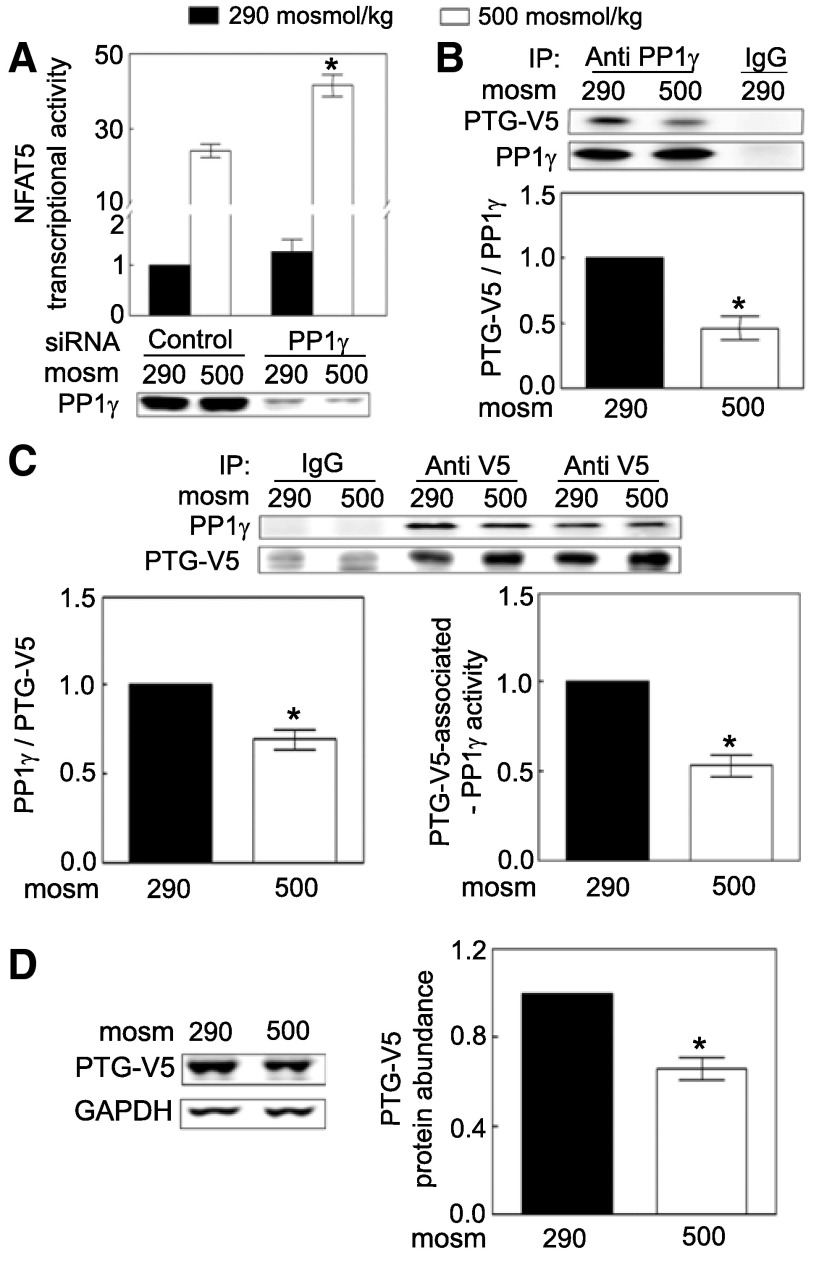
The catalytic subunit of PP1 has α, β, and γ isoforms, encoded by genes PPPCA, PPP1CB, and PPP1CC, respectively (30). In our previous screen of a phosphatase siRNA library, siRNAs against PPP1CC increased NFAT5 transcriptional activity, whereas siRNAs against PPP1CA and PPP1CB did not (41). Here, we confirm the effect of knocking down PPP1CC (Fig. 3A). We conclude that PP1γ contributes to regulation of NFAT5 transcriptional activity.
Fig. 3.
A: siRNA against PP1γ increases NFAT5 transcriptional activity, as in Fig. 1B except that PP1γ siRNAs were transfected. PP1γ siRNAs reduce PP1γ protein abundance by ∼69%. *Signficant difference vs. control at 500 mosM (P < 0.05; n = 3). B: PTG-V5 coimmunoprecipitates with PP1γ, and the coimmunoprecipitation is reduced by high NaCl. Medium bathing HEK293 cells stably expressing PTG-V5 was exchanged for medium at 290 or 500 mosM (NaCl added) for 30 min. The cell lysates were immunoprecipitated with the goat anti-PP1γ antibody or goat IgG overnight at 4°C. Immunoprecipitates were probed with mouse anti-V5 and rabbit anti PP1γ. *Signficant difference compared with 290 mosM (P < 0.05; n = 3). C: high NaCl decreases association of PP1γ with PTG-V5 (left) and inhibits activity of remaining PTG-V5-associated PP1γ (right) as in B except that the cell lysates were immunoprecipitated with mouse anti-V5 or IgG. The PP1γ activity was normalized by the amount of PP1γ protein that was co-immunoprecipitated. *Signficant difference compared with 290 mosM (P < 0.05; n = 4). D: high NaCl decreases PTG-V5 abundance. Medium bathing HEK293 cells stably expressing PTG-V5 at 290 mosM was changed to the identical medium or to an otherwise identical one at 500 mosM (NaCl added) for 30 min. Expression of PTG-V5 was determined with mouse anti-V5 antibody. *Signficant difference compared with 290 mosM (P < 0.05; n = 4).
High NaCl reduces PP1 activity by separating PTG from its catalytic subunit PP1γ by inhibiting remaining PTG-associated PP1γ activity and by reducing protein abundance of PTG.
PP1 forms a heterodimer with one regulatory subunit and one catalytic subunit (30). To determine whether PP1γ is the catalytic subunit PTG regulates, we performed co-immunoprecipitation and reciprocal co-immunoprecipitation. We find that PTG-V5 physically associates with PP1γ, since the anti-V5 antibody immunoprecipitated PTG-V5 together with the native PP1γ (Fig. 3B) and vice versa (Fig. 3C). High NaCl reduces association of PTG-V5 with PP1γ and decreases remaining PTG-V5-associated PP1γ activity (Fig. 3C, right). High NaCl also rapidly (≤30 min) reduces protein abundance of PTG-V5 (Fig. 3D). (We were unable to test directly whether high NaCl also reduces endogenous PTG because of an inability to obtain a satisfactory antibody against PTG.) We conclude that high NaCl-induced inhibition of PP1 activity contributes to activation of NFAT5 activity by reducing abundance of PTG by disassociating PTG from its catalytic subunit PP1γ and by inhibiting remaining PTG-associated PP1γ activity.
Effect of PTG and PP1γ on high NaCl-induced phosphorylation of SHP-1-S591.
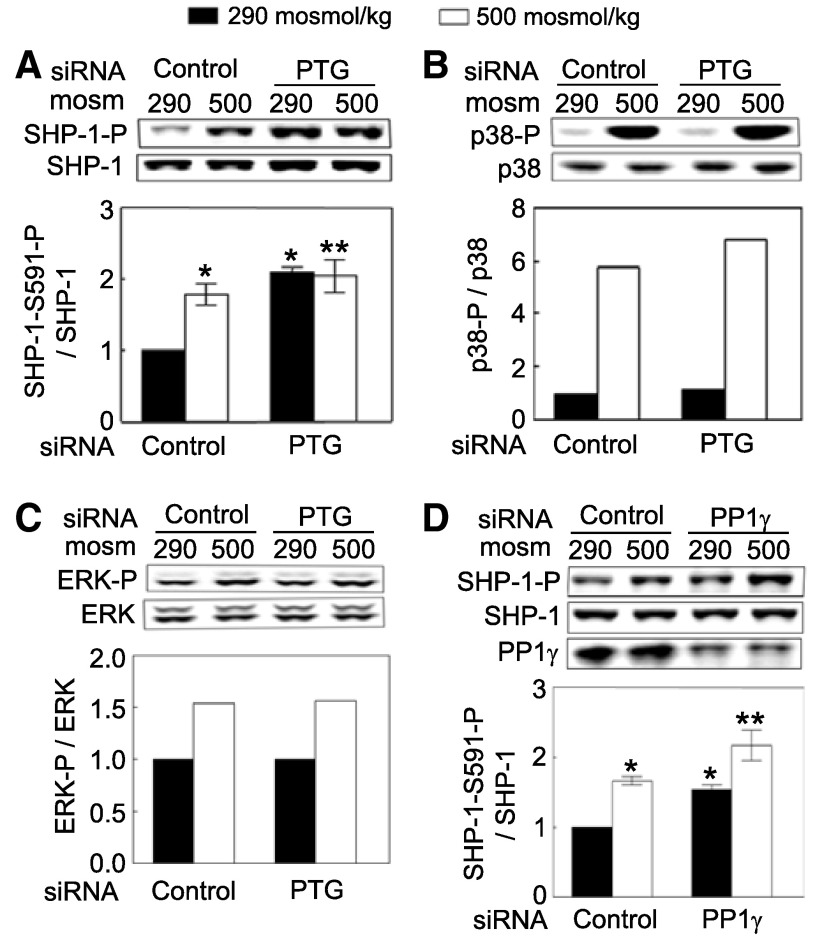
To identify phosphorylated proteins that PTG-regulated PP1γ might affect, we examined the effect of PTG siRNAs on high NaCl-induced phosphorylation of p38, ERK, and SHP-1, which are kinases and a phosphatase known to contribute to regulation of NFAT5 activity (22, 34, 40, 41). siRNAs against PTG significantly increase phosphorylation of SHP-1-S591 both at 290 and 500 mosM (Fig. 4A) but do not affect high NaCl-dependent phosphorylation of p38 or ERK (Fig. 4, B and C). siRNA-mediated knockdown of PP1γ also significantly increases phosphorylation of SHP-1 on serine 591 (Fig. 4D). We conclude that PTG, in conjunction with PP1γ, ordinarily dephosphorylates SHP-1-S591 and that high NaCl prevents the dephosphorylation.
Fig. 4.
PTG and PP1γ reduce phosphorylation of SHP-1-S591. A–C: HEK293 cells at 290 mosM were transfected with either PTG or PP1γ siRNA for 48 h. Then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 30 min before Western analysis of phosphorylation of SHP-1-S591, p38, and ERK and of their protein abundance. A: siRNA knock down of PTG increases phosphorylation of SHP-1-S591. B and C: siRNA knock down of PTG does not affect phosphorylation of p38 (B) or ERK (C). In A–C, PTG siRNAs reduce PTG mRNA abundance by ∼71%. D: siRNA knockdown of PP1γ increases phosphorylation of SHP-1-S591. PP1γ siRNAs reduce PP1γ protein abundance by ∼57%. *Signficant difference compared with 290 mosM (P < 0.05; n = 4). **Signficant difference compared with 500 mosM (P < 0.05; n = 4).
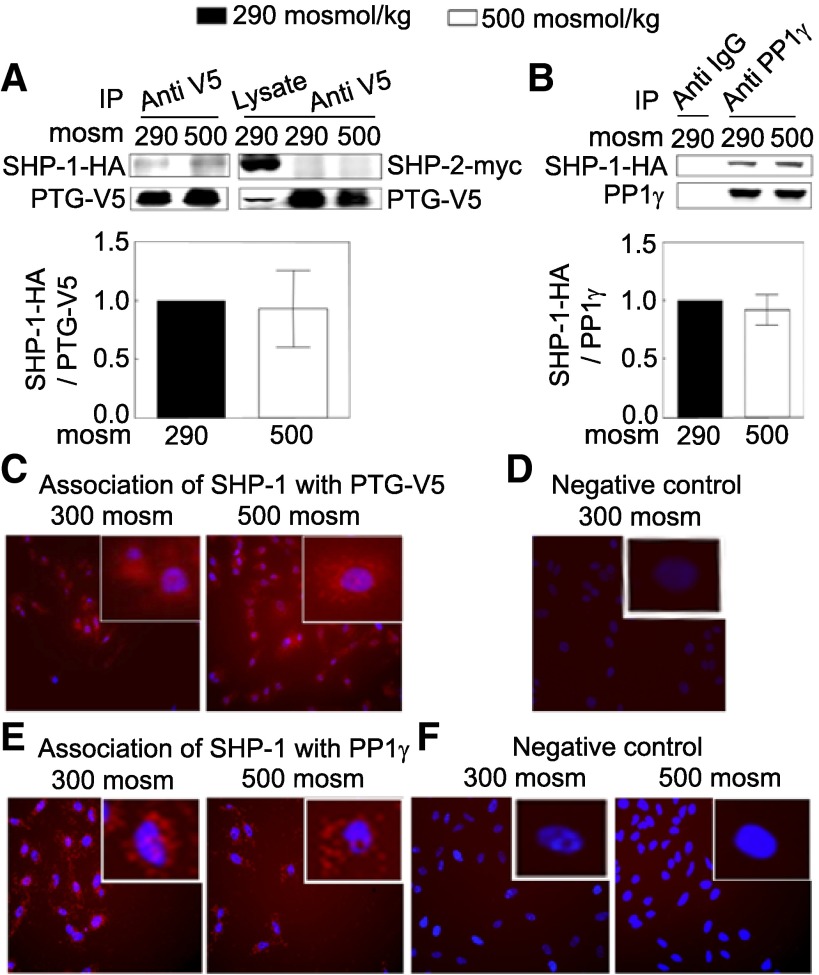
Association of PTG-V5 and PP1γ with SHP-1.
To determine whether PTG-V5 and PP1γ physically interact with SHP-1, we performed co-immunoprecipitation and Duolink in situ assays. SHP-1-HA, but not SHP-2-myc, co-immunoprecipitates with PTG-V5 (Fig. 5A). SHP-1-HA also co-immunoprecipitates with endogenous PP1γ (Fig. 5B). Duolink in situ assay is an alternative way to test protein association (32). In contrast to co-immunoprecipitation, this assay does not require lysis of cells. Instead, the two proteins are bound in fixed, permeabilized cells to specific primary antibodies raised in two different species. Then, the cells are incubated with two species-specific secondary antibodies; each of them has a unique DNA strand attached to it. When the secondary antibodies are in close proximity, the two DNA oligonucleotides ligate and are amplified via rolling-circle amplification, during which a fluorescent-labeled probe is incorporated, resulting in red fluorescence (Fig. 5, C and E) (32). Use of this assay confirms that PTG-V5 and endogenous PP1γ both physically associate with endogenous SHP-1 in HeLa cells (Fig. 5, C–F). Since high NaCl does not affect the association of PTG with SHP-1, activation of SHP-1 by PTG/PP1γ may or may not be affected by changes in their association but by changes in PTG/ PP1γ activity. We conclude that PTG and PP1γ both physically associate with SHP-1.
Fig. 5.
A: PTG-V5 is physically associated with SHP-1 but not SHP-2, and the association is not affected by high NaCl. HEK293 cells were co-transfected at 290 mosM with plasmids coding for PTG-V5 and with SHP-1-HA or SHP-2-myc for 48 h. Then, osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 30 min before cell lysates were prepared. The lysates were immunoprecipitated with mouse anti-V5 antibody, and Western blots were probed with rabbit anti SHP-1 or SHP-2 antibody (n = 3). B: PP1γ is physically associated with SHP-1, and the association is not affected by high NaCl as in A, except that cells were transfected with the plasmid coding for SHP-1-HA but not for SHP-2-myc. The lysates were immunoprecipitated with the goat anti-PP1γ antibody, and Western blots were probed with rabbit anti-SHP-1 antibody (n = 3). C–F: Duolink in situ assays. Red stain indicates protein-protein association. Blue stain indicates nuclei, detected by DAPI. C: SHP-1 associates with PTG-V5 at 300 and 500 mosM. HeLa cells were transfected with the plasmid coding for PTG-V5 for 24 h. Cells were plated on a chamber slide (NUNC) for an additional 24 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 300 mosM for 30 min before Duolink in situ assay. Mouse anti-V5 antibody (1:250) and rabbit anti-SHP-1 (1:50) were used. D: negative control. Same as in C, except the HeLa cells were not transfected with the PTG-V5 plasmid. E: SHP-1 associates with native PP1γ at 300 and 500 mosM. Nontransfected HeLa cells were incubated with the goat anti-PP1γ (1:50) and rabbit anti-SHP-1 (1:50) for the Duolink in situ assay. F: negative control. Same as in E, except no anti-SHP-1 antibody was included in the Duolink in situ assay. (All Duolink assay results are representative of two independent experiments).
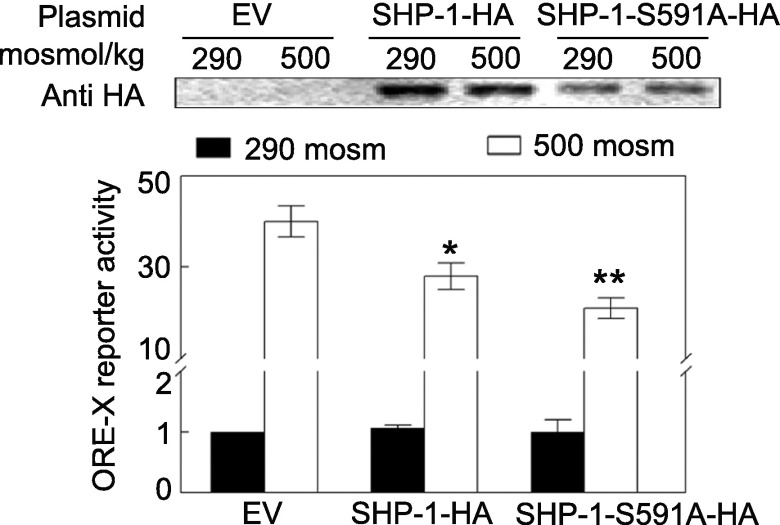
Effect of mutation of SHP-1-S591 on NFAT5 transcriptional activity.
We previously found that SHP-1 negatively regulates NFAT5 and that high NaCl-induced phosphorylation of SHP-1-S591 reduces its inhibition of NFAT5 (41). Phosphorylation of SHP-1 at S591 results in reduced phosphatase activity (20, 25). Here, we further test the importance of the phosphorylation by comparing the effect of overexpressing wild-type SHP-1-HA to that of SHP-1-HA in which serine 591 is mutated to alanine to prevent phosphorylation. Consistent with our previous report (41), overexpression of wild-type SHP-1 reduces NFAT5 transcriptional activity (Fig. 6). Most likely, high NaCl-induced phosphorylation of SHP-1-S591 is not complete, so sufficient activity remains to at least partially inhibit NFAT5 (41). Overexpression of SHP-1-S591A-HA, in which phosphorylation at amino acid 591 cannot occur, inhibits transcriptional activity of NFAT5 more than overexpression of wild-type SHP-1-HA (Fig. 6).
Fig. 6.
Mutation of SHP-1 serine 591 to alanine enhances the inhibitory effect of SHP-1 on NFAT5 transcriptional activity. HEK293 cells were co-transfected with ORE-X luciferase reporter plasmid and either empty vector (EV), SHP-1-HA, or SHP-1-S591A-HA plasmid for 32 h, and then osmolality was increased to 500 mosM (NaCl added) or left at 290 mosM for 16 h. *Signficant difference compared with EV at 500 mosM (P < 0.05; n = 3). **Signficant difference compared with SHP-1-HA at 500 mosM (P < 0.05; n = 3).
DISCUSSION
Activation of NFAT5 by high NaCl involves changes in phosphorylation of several amino acids within NFAT5, including, importantly, increased phosphorylation of NFAT5 tyrosine 143 (18). High NaCl increases phosphorylation of NFAT5-Y143 by concurrent activation of c-Abl kinase (12) and inhibition of SHP-1 phosphatase (41). High NaCl reduces the inhibitory effect of SHP-1 on NFAT5 by increasing phosphorylation of SHP-1-S591 (41). PP1 phosphatase constitutively reduces phosphorylation of SHP-1-S591 (Fig. 4), so, when high NaCl inhibits PP1 by dissociating its regulatory PTG subunit from its catalytic PP1γ subunit and reducing the remaining PTG-associated PP1γ activity (Fig. 3), phosphorylation of SHP-1-S591 increases and its negative effect is reduced, contributing to the increase of NFAT5 activity.
High NaCl increases transcriptional activity of NFAT5 by increasing its nuclear localization, transactivating activity, and protein abundance (3). High NaCl-induced nuclear localization of NFAT5 is rapid (≤30 min) (3). Phosphorylation within the amino terminus of NFAT5 plays a critical role in regulating its nuclear localization. High NaCl-induced phosphorylation of tyrosine 143 (12, 18, 41) and threonine 135 (11) increases NFAT5 nuclear localization, whereas low NaCl-induced phosphorylation of serines 155 and 158 (38) has the opposite effect. Since SHP-1 dephosphorylates NFAT5-Y143, inhibition of SHP-1 by high NaCl increases the phosphorylation, which contributes to nuclear localization of NFAT5 (41).
High NaCl-induced increase of NFAT5 transactivating activity is associated with increased phosphorylation in the region of its carboxy terminus that contains the NFAT5 transactivating domain (9). Increased activity of several kinases, including p38α (22), ERK (34), c-Abl (12), PI3 kinase (17), and ATM (19) contribute to the high NaCl-induced increase of NFAT5 transactivating activity, as does decreased activity of SHP-1 (41); however, we do not know what amino acid sites are involved. In the present paper, we find that siRNA-mediated knockdown of PTG increases NFAT5 transactivating activity indirectly by increasing phosphorylation of SHP-1-S591 and thus inhibiting SHP-1. c-Abl (12) and SHP-1 (41), which regulate NFAT5 transactivating activity, both regulate phosphorylation of NFAT5-Y143, but the reporter of NFAT5 transactivating activity does not contain NFAT5-Y143, so some additional activity of these factors apparently is involved. In this respect, it is cogent that PLC-γ1 has two such independent actions (18). Phospholipase activity of PLC-γ1 affects NFAT5 nuclear localization via NFAT5-Y143, whereas a lipase-independent activity of PLC-γ1 affects transactivating activity of NFAT5 (18).
High NaCl-induced increase of NFAT5 protein expression contributes to sustaining activity of NFAT5. p38 kinase contributes to the high NaCl-induced increase of NFAT5 protein expression but not its increased nuclear localization (42). PTG is the first serine/threonine phosphatase recognized to inhibit NFAT5 protein expression and is exceptional in that it affects NFAT5 protein abundance, as well as its nuclear localization and transactivating activity. High NaCl increases NFAT5 mRNA, as well as protein (4). Further studies are necessary to determine whether PTG/PP1γ affect NFAT5 mRNA abundance. In this regard, it is significant that SHP-1 itself is not involved in the high NaCl-induced increase of NFAT5 protein expression (41), so the effect of PTG on NFAT5 abundance must be by some other mechanism besides its effect on SHP-1. Furthermore, PTG-regulated phosphorylation of SHP-1 clearly affects high NaCl-induced NFAT5 transcriptional activity, but we cannot rule out the possibility that PTG also has this effect independent of SHP-1.
High NaCl reduces protein abundance of recombinant PTG-V5 within 30 min (Fig. 3D). We could not confirm that high NaCl similarly affects endogenous PTG protein since the available commercial antibodies do not work satisfactorily in our hands. Considering that transcription of recombinant PTG-V5 is driven by a strong promoter, unlikely to be affected by high NaCl, increased degradation of PTG is a likely possibility, particularly considering that hypertonicity activates AMP-activated protein kinase (10, 16), and AMP-activated protein kinase increases degradation of PTG by phosphorylating PTG-S8 (35).
Despite the fact that PP1 has only three catalytic subunits, PP1α, PP1β, and PP1γ, it regulates a wide variety of cellular activities (30). PP1 achieves diverse effects by using at least 50 regulatory subunits (30). In response to a particular stimulus, a specific regulatory subunit brings a catalytic subunit to or away from a specific site of action to achieve a specific effect (30). High NaCl reduces the association of PTG with PP1γ (Fig. 3, B and C), which would remove PP1γ from its site of action. The concentration of PTG also affects the activity of its regulated catalytic subunits. For example, overexpression of PTG increases PP1α activity by decreasing the Km of PP1α (2). Thus decreased protein abundance of PTG (Fig. 3D) provides an additional mechanism by which high NaCl could contribute to inhibition of PP1γ activity. PTG regulates PP1α (2). However, PP1α apparently is not involved in high NaCl-induced activation of NFAT5 because our siRNA library screen of phosphatases did not indicate an effect of PP1α on NFAT5 activity (41). Before accepting this negative conclusion, however, it should be confirmed that the PP1α siRNAs that we used for screening actually knock down PP1α, which was not done at the time.
SHP-1 activity is regulated by two amino-terminal SH2 domains as well as by sites in the carboxyl terminus (28). Phosphorylation of tyrosine 536 or 564 in the carboxyl terminus increases the phosphatase activity, whereas phosphorylation of serine 591 does the opposite (28). For example, abrogation of phosphorylation of SHP-1-Y536-P and -Y564-P reduces SHP-1 activity, leading to constitutive activation of STAT5 (37). On the other hand, increase of phosphorylation of serine 591 reduces SHP-1 activity during T-cell activation (25) and activation of thrombin receptors (20), and possibly during mycophenolic acid treatment (33). Also, cdk2-induced phosphorylation of serine 591 increases proteasome-dependent truncation of SHP-1 (31). We ascribe the effect of high NaCl on SHP-1 activity toward NFAT5 to increased phosphorylation of SHP-1-S591 (41). In support of this conclusion, overexpression of SHP-1 in which serine 591 is mutated to alanine to prevent phosphorylation inhibits NFAT5 transcriptional activity (Fig. 6). Previous studies showed that PKC kinases, including PKCα, phosphorylate SHP-1-S591 (28). We now find additional regulation of SHP-1-S591 phosphorylation by protein phosphatase activity (Fig. 4, A and D) and physical interaction of both PTG and PP1γ with SHP-1 (Fig. 5).
In summary, PTG negatively regulates high NaCl-induced activation of NFAT5, as measured by luciferase reporter assay of transcriptional activity and by expression of NFAT5-targeted gene expression (Fig. 1). The effect of PTG is mediated by reducing NFAT5 transactivating activity, nuclear localization, and protein abundance (Fig. 2). PP1γ is the catalytic subunit regulated by PTG (Fig. 3, A–C). PTG and PP1γ affect NFAT5 by dephosphorylating SHP-1-S591 (Fig. 4, A and D), which increases the inhibitory effect of SHP-1 on NFAT5 (Fig. 6). High NaCl reduces both association of PTG with PP1γ and the remaining PTG-associated PP1γ activity (Fig. 3, B and C), contributing to activation of NFAT5.
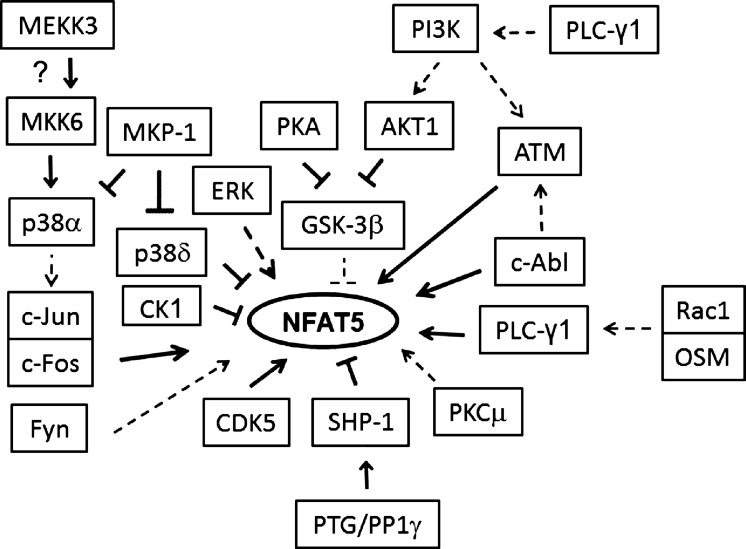
NFAT5 is regulated by a network of signaling pathways. Such redundancy provides robustness against other internal and external perturbations (21). None of these pathways is alone sufficient to activate NFAT5 (19). As depicted in Fig. 7, more than a dozen kinases regulate NFAT5 activity (43). In contrast to kinases, the role of phosphatases in regulation of NFAT5 has been studied less. We previously demonstrated that a dual-specificity MAP kinase phosphatase, MKP-1, has little effect on high NaCl-induced activation of NFAT5, because it inhibits both p38α, which stimulates NFAT5 activity, and p38δ, which inhibits NFAT5 activity (40). The PTG-SHP-1 pathway depicted in Fig. 7 is the first phosphatase pathway identified to contribute to regulation of NFAT5 activity.
Fig. 7.
Summary of kinases and phosphatases that are known to regulate NFAT5 and of proteins that interact with them when NaCl is elevated. Direct interaction with another component of a pathway or with NFAT5 itself is depicted by bold lines; broken lines indicate that the effect is not known to be direct.
GRANTS
This study was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, Department of Health and Human Services, and by a grant-in-aid from the National Kidney Foundation/National Capital Area.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.Z., M.B.B., and J.D.F. conception and design of research; X.Z. and H.W. performed experiments; X.Z., M.B.B., and J.D.F. analyzed data; X.Z., M.B.B., and J.D.F. interpreted results of experiments; X.Z. prepared figures; X.Z. drafted manuscript; X.Z., M.B.B., and J.D.F. edited and revised manuscript; X.Z., M.B.B., and J.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Authors thank Dr. Jack E. Dixon (University of California at San Diego) for pcDNA3.1-PTG-V5 plasmid, Dr. Stephen Shaw (National Institutes of Health, Bethesda, MD) for pCMV5-SHP-1-HA and pCMV5-SHP-1-S591A-HA plasmids, Dr. Gibbes R. Johnson (FDA, Bethesda, MD) for SHP-2-myc plasmid, and Dr. Sergei Nekhai (Howard University, Washington, DC) for help in measuring PP1γ activity.
REFERENCES
- 1.Ammosova T, Obukhov Y, Kotelkin A, Breuer D, Beullens M, Gordeuk VR, Bollen M, Nekhai S. Protein phosphatase-1 activates CDK9 by dephosphorylating Ser175. PLos One 6: e18985, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady MJ, Printen JA, Mastick CC, Saltiel AR. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. J Biol Chem 272: 20198–20204, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Cai Q, Ferraris JD, Burg MB. High NaCl increases TonEBP/OREBP mRNA and protein by stabilizing its mRNA. Am J Physiol Renal Physiol 289: F803–F807, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Crosson SM, Khan A, Printen J, Pessin JE, Saltiel AR. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J Clin Invest 111: 1423–1432, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deb TB, Wong L, Salomon DS, Zhou G, Dixon JE, Gutkind JS, Thompson SA, Johnson GR. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J Biol Chem 273: 16643–16646, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Doherty MJ, Young PR, Cohen PT. Amino acid sequence of a novel protein phosphatase 1 binding protein (R5) which is related to the liver- and muscle-specific glycogen binding subunits of protein phosphatase 1. FEBS Lett 399: 339–343, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Ferraris JD, Burg MB. Tonicity-regulated gene expression. Methods Enzymol 428: 279–296, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ferraris JD, Williams CK, Persaud P, Zhang Z, Chen Y, Burg MB. Activity of the TonEBP/OREBP transactivation domain varies directly with extracellular NaCl concentration. Proc Natl Acad Sci USA 99: 739–744, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser S, Mount P, Hill R, Levidiotis V, Katsis F, Stapleton D, Kemp BE, Power DA. Regulation of the energy sensor AMP-activated protein kinase in the kidney by dietary salt intake and osmolality. Am J Physiol Renal Physiol 288: F578–F586, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gallazzini M, Heussler GE, Kunin M, Izumi Y, Burg MB, Ferraris JD. High NaCl-induced activation of CDK5 increases phosphorylation of the osmoprotective transcription factor TonEBP/OREBP at threonine 135, which contributes to its rapid nuclear localization. Mol Biol Cell 22: 703–714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallazzini M, Yu MJ, Gunaratne R, Burg MB, Ferraris JD. c-Abl mediates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP. FASEB J 24: 4325–4335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101: 10673–10678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg CC, Danos AM, Brady MJ. Central role for protein targeting to glycogen in the maintenance of cellular glycogen stores in 3T3-L1 adipocytes. Mol Cell Biol 26: 334–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero R, Vernia S, Sanz R, Abreu-Rodriguez I, Almaraz C, Garcia-Hoyos M, Michelucci R, Tassinari CA, Riguzzi P, Nobile C, Sanz P, Serratosa JM, Gomez-Garre P. A PTG variant contributes to a milder phenotype in Lafora disease. PLos One 6: e21294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Q, Zhang X, Xue R, Yang H, Zhou Y, Kong X, Zhao P, Li J, Yang J, Zhu Y, Guan Y. AMPK potentiates hypertonicity-induced apoptosis by suppressing NFkappaB/COX-2 in medullary interstitial cells. J Am Soc Nephrol 22: 1897–1911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irarrazabal CE, Burg MB, Ward SG, Ferraris JD. Phosphatidylinositol 3-kinase mediates activation of ATM by high NaCl and by ionizing radiation: role in osmoprotective transcriptional regulation. Proc Natl Acad Sci USA 103: 8882–8887, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irarrazabal CE, Gallazzini M, Schnetz MP, Kunin M, Simons BL, Williams CK, Burg MB, Ferraris JD. Phospholipase C-gamma1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 906–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 101: 8809–8814, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones ML, Craik JD, Gibbins JM, Poole AW. Regulation of SHP-1 tyrosine phosphatase in human platelets by serine phosphorylation at its C terminus. J Biol Chem 279: 40475–40483, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kitano H. Biological robustness. Nat Rev Genet 5: 826–837, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the OREBP/TonEBP. J Biol Chem 277: 46085–46092, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Lam AK, Ko BC, Tam S, Morris R, Yang JY, Chung SK, Chung SS. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem 279: 48048–48054, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lee SD, Choi SY, Lim SW, Lamitina ST, Ho SN, Go WY, Kwon HM. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: organic osmolyte-dependent and -independent pathways. Am J Physiol Renal Physiol 300: F707–F715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Kruhlak MJ, Hao JJ, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. J Leukoc Biol 82: 742–751, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA 101: 2392–2397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev 228: 342–359, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal 17: 1323–1332, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Printen JA, Brady MJ, Saltiel AR. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science 275: 1475–1478, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell 139: 468–484, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Simoneau M, Boulanger J, Coulombe G, Renaud MA, Duchesne C, Rivard N. Activation of Cdk2 stimulates proteasome-dependent truncation of tyrosine phosphatase SHP-1 in human proliferating intestinal epithelial cells. J Biol Chem 283: 25544–25556, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Thymiakou E, Episkopou V. Detection of signaling effector-complexes downstream of bmp4 using PLA, a proximity ligation assay. J Vis Exp 3: 2631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toubiana J, Rossi AL, Grimaldi D, Belaidouni N, Chafey P, Clary G, Courtine E, Pene F, Mira JP, Claessens YE, Chiche JD. IMPDHII protein inhibits Toll-like receptor 2-mediated activation of NF-kappaB. J Biol Chem 286: 23319–23333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res 22: 965–974, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Vernia S, Solaz-Fuster MC, Gimeno-Alcaniz JV, Rubio T, Garcia-Haro L, Foretz M, de Cordoba SR, Sanz P. AMP-activated protein kinase phosphorylates R5/PTG, the glycogen targeting subunit of the R5/PTG-protein phosphatase 1 holoenzyme, and accelerates its down-regulation by the laforin-malin complex. J Biol Chem 284: 8247–8255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worby CA, Gentry MS, Dixon JE. Malin decreases glycogen accumulation by promoting the degradation of protein targeting to glycogen (PTG). J Biol Chem 283: 4069–4076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao W, Ando T, Wang HY, Kawakami Y, Kawakami T. Lyn- and PLC-beta3-dependent regulation of SHP-1 phosphorylation controls Stat5 activity and myelomonocytic leukemia-like disease. Blood 116: 6003–6013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Wong CC, Tong EH, Chung SS, Yates JRIII, Yin Y, Ko BC. Phosphorylation by casein kinase 1 regulates tonicity-induced osmotic response element-binding protein/tonicity enhancer-binding protein nucleocytoplasmic trafficking. J Biol Chem 283: 17624–17634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Ferraris JD, Cai Q, Agarwal A, Burg MB. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 289: F377–F385, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Ferraris JD, Dmitrieva NI, Liu Y, Burg MB. MKP-1 inhibits high NaCl-induced activation of p38 but does not inhibit the activation of TonEBP/OREBP: opposite roles of p38alpha and p38delta. Proc Natl Acad Sci USA 105: 5620–5625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Gallazzini M, Burg MB, Ferraris JD. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 7072–7077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Izumi Y, Burg MB, Ferraris JD. Rac1/osmosensing scaffold for MEKK3 contributes via phospholipase C-gamma1 to activation of the osmoprotective transcription factor NFAT5. Proc Natl Acad Sci USA 108: 12155–12160, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou X, Wang H, Burg MB, Ferraris JD. Inhibitory phosphorylation of GSK-3beta by AKT, PKA, and PI3K contributes to high NaCl-induced activation of the transcription factor NFAT5 (TonEBP/OREBP). Am J Physiol Renal Physiol 304: F908–F917, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]