Abstract
We hypothesized that maternal separation (MS), an early life stress model, induces a sensitization of the sympathetic system. To test this hypothesis, we evaluated the renal and systemic sympathetic system in 12- to 14-wk-old male control or MS rats with the following parameters: 1) effect of renal denervation on conscious renal filtration capacity, 2) norepinephrine (NE) content in key organs involved in blood pressure control, and 3) acute systemic pressor responses to adrenergic stimulation or ganglion blockade. MS was performed by separating pups from their mothers for 3 h/day from day 2 to 14; controls were nonhandled littermates. Glomerular filtration rate (GFR) was examined in renal denervated (DnX; within 2 wk) or sham rats using I125-iothalamate plasma clearance. MS-DnX rats showed significantly increased GFR compared with MS-SHAM rats (3.8 ± 0.4 vs. 2.4 ± 0.2 ml/min, respectively, P < 0.05), whereas DnX had no effect in controls, indicating that renal nerves regulate GFR in MS rats. NE content was significantly increased in organ tissues from MS rats (P < 0.05, n = 6–8), suggesting a sensitization of the renal and systemic sympathetic system. Conscious MS rats displayed a significantly greater increase in mean arterial pressure (MAP) in response to NE (2 μg/kg ip) and a greater reduction in MAP in response to mecamylamine (2 mg/kg ip, P < 0.05, n = 4) monitored by telemetry, indicating that MS rats exhibit exaggerated responses to sympathetic stimulation. In conclusion, these data indicate that MS sensitizes the renal and systemic sympathetic system ultimately impairing blood pressure regulation.
Keywords: early life stress, denervation, glomerular filtration rate, maternal separation
developmental programming is now recognized as an important determinant of adult chronic diseases such as diabetes, obesity, and hypertension (1, 4, 44, 47). This phenomenon has been defined as the adaptation to a specific insult during a critical timeframe in life that induces permanent changes in the adult phenotype. Compelling studies showing a relationship between a diverse number of adverse factors in the early life environment and the increased susceptibility to develop cardiovascular disease later in life have provided consistent supporting evidence (7, 47).
Maternal separation (MS) is a rodent model that mimics the effects of early life stress (ELS) in humans (22, 24, 39). Systematic separation from the dams during this period induces higher anxiety and an exaggerated response to behavioral stress during adulthood as shown in several reports (11, 30, 36, 39). A number of investigations demonstrate that MS induces exaggerated responses to acute stress from the hypothalamic-pituitary-adrenal axis and sympathetic nervous system (SNS) hyperactivity (11, 24, 28), although only a few studies have assessed pressor responses (26, 45). Previously, we observed that MS does not manifest a change in baseline metabolic parameters, blood pressure, and heart rate but it sensitizes rats to a chronic prohypertensive stimulus (26).
It is well-established that the SNS is important in the acute and chronic regulation of arterial blood pressure. The role of the SNS in the development of hypertension has been described in several experimental models such as spontaneously hypertensive rats and Dahl salt-sensitive rats (6, 43). In terms of fetal programming, Samuelsson et al. (37, 38) showed that the hypertension observed in weanlings from obese dams is from sympathetic origin. Additionally, kidneys play a critical role in the regulation of fluid homeostasis and chronic blood pressure control (8, 15). Using a model of intrauterine growth restriction (IUGR), Alexander et al. (35) showed that the renal nerves play a causative role in the early onset of IUGR-induced hypertension, although established hypertension involves interaction of other regulatory systems in addition to the renal nerves. In humans, the use of renal denervation (DnX) as a therapy for resistant hypertension is becoming a possible lifelong treatment (9, 12, 20, 40), although the mechanism(s) leading to overactivation of the renal nerves in humans with resistant hypertension are unknown.
Many investigations have shown that alterations in normal renal development are associated with changes in renal structure/function and subsequently result in hypertension (14, 31, 34, 47). However, there is a gap in the literature regarding the impact of behavioral stress early in life and the mechanisms underlying the programming of the adult renal phenotype. Recently, we showed that creatinine clearance, a parameter of renal function, is significantly reduced in MS rats (27). Thus, reduced renal filtration capacity is a potential mechanism by which MS impairs chronic blood pressure control.
We hypothesized that MS sensitizes the renal and systemic sympathetic system. The aims of the current study were to test this hypothesis by evaluating three parameters. First, we examined whether renal nerves play a role in the MS-induced loss of renal filtration capacity in conscious rats. Second, we determined the norepinephrine (NE) content to test the status of a primary mediator of sympathetic postganglionic fibers in kidney, heart, spleen, adrenal, and large arteries. Finally, we elucidated the acute systemic pressor responses to adrenergic stimulation and ganglion blockade in telemetry-instrumented, conscious rats.
METHODS
MS protocol.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the Georgia Regents University Institutional Animal Care and Use Committee. With the use of Wistar-Kyoto breeders, pups were separated from their mothers and littermates for 3 h a day in the morning time and identified as “MS” at postnatal days 2 to 14 of life (26). Nonhandled littermates served as control group. Each experimental group was comprised of adult male rats (12–14 wk old) from at least three different litters.
Chronic catheter implantation and DnX.
Chronic in-dwelling catheters were placed in the femoral vein and abdominal aorta artery (3, 5, 21). Sterile catheters were implanted in the femoral vein and abdominal aorta and passed through a stainless steel button that was implanted subcutaneously in the scapular region. Aseptic techniques were used throughout, and all incisions were infiltrated with penicillin G, procaine, and Marcaine. The rats were allowed to recover in a warm, clean cage without bedding. In addition, both kidneys were approached retroperitoneally via a flank incision and the renal vessels were stripped of all visible nerves and were painted with a 10% phenol solution (in 95% ethanol). Sham consisted of identical anesthetic and all surgical procedures, but the renal nerves were left intact. This denervation procedure reduces dramatically renal cortex NE content (2), consistent with complete DnX. We verified in a subset of control and MS rats that this protocol did indeed reduce renal NE content in the denervated group more than 10-fold (12.4 ± 1.5 and 20.9 ± 3.2 ng/g renal cortex, respectively). Following recovery, the rats were moved to metabolic cages and a stainless steel spring was used to connect the catheters to a sterile infusion swivel. All glomerular filtration rate (GFR) experiments were performed within 2 wk of the DnX protocol. Total sodium intake throughout the experiment was maintained constant at ∼3.5 mmol/day by continuous intravenous infusion of ∼22 ml/day sterile 0.9% saline combined with Teklad sodium-deficient rat chow (0.006 mmol sodium/g food). Food and drinking water were available ad libitum. A sodium-deficient diet was used to achieve a normal-sodium intake. Blood pressure and heart rate were monitored daily using PowerLab (ADinstruments). Continuous recordings over 6 h (8 AM to 2 PM) were collected and averaged.
Measurement of GFR.
Measurement of GFR was accomplished by 24-h infusion of (125I) iothalamate (Glofil). Glofil (∼7 μCi) was added to the infusion syringe the day before the scheduled blood sample (1 ml). In the morning time, aliquots of (125I) iothalamate in plasma and infusate were measured in a gamma counter (Packard, Perkin Elmer). GFR was calculated as [Clearance Glofil (ml/min) = Infusion Rate Glofil (μCi/min)/Plasma Glofil (μCi/ml)] and expressed in milliliters per minute. All measurements were corrected to the background counts (3, 5).
Metabolic parameters.
A separate group of SHAM and denervated operated rats was placed in metabolic cages after 1-wk recovery. Water intake, food intake, and urine excretion were monitored daily. Urinary volume was assessed during 24 h and expressed in milliliters per day. Urinary sodium and potassium were measured in 24-h urine collections (Easy Lyte, Medica).
NE content in plasma and tissues.
Thoracic aortas, abdominal aortas, left ventricles, spleens, and adrenals were harvested and snap-frozen. Kidney vessels were isolated as previously described (42). In a separate set of animals, kidneys were dissected into cortex, outer medulla, and inner medulla and snap-frozen. Tissues were homogenized in 0.01 mol/l of HCl in the presence of EDTA (1 mmol/l), sodium metabisulfite (4 mmol/l), and centrifuged (8,000 g, 30 min); supernatant was removed for further analysis (38). Plasma or supernatants were extracted following the manufacturer's instructions to determine NE (Bi-CAT ELISA; Rocky Mountain Diagnostic). NE was expressed as nanograms per gram of tissue weight.
Acute mean arterial pressure responsiveness.
Rats were implanted with telemetry transmitters at 10 wk of age (Data Sciences, St. Paul, MN) as previously described (26). Mean arterial pressure (MAP) and heart rate (HR) were continuously recorded throughout the study using the Dataquest ART Acquisition program (Data Sciences International, St. Paul, MN). After recovery (10 days) and a baseline period (7 days), separate groups of rats were subjected to intraperitoneal injections of mecamylamine (2 mg/kg) or NE (2 μg/kg). Telemetry data were collected every 10 min for 30 s and averaged for the mecamylamine experiment and baseline blood pressure, and every 30 s for 4 s for the NE experiment.
Statistical analysis.
Statistical comparisons of MAP and GFR in SHAM and DnX rats were made by two-way ANOVA followed by a Tukey post hoc test. For statistical comparisons of MAP, HR, and NE levels between control and MS, unpaired Student's t-test was utilized. All statistical analyses were conducted using Prism 5.01 (GraphPad Software, 2007). A value of P < 0.05 was considered statistically significant.
RESULTS
Effect of DnX on MAP and GFR.
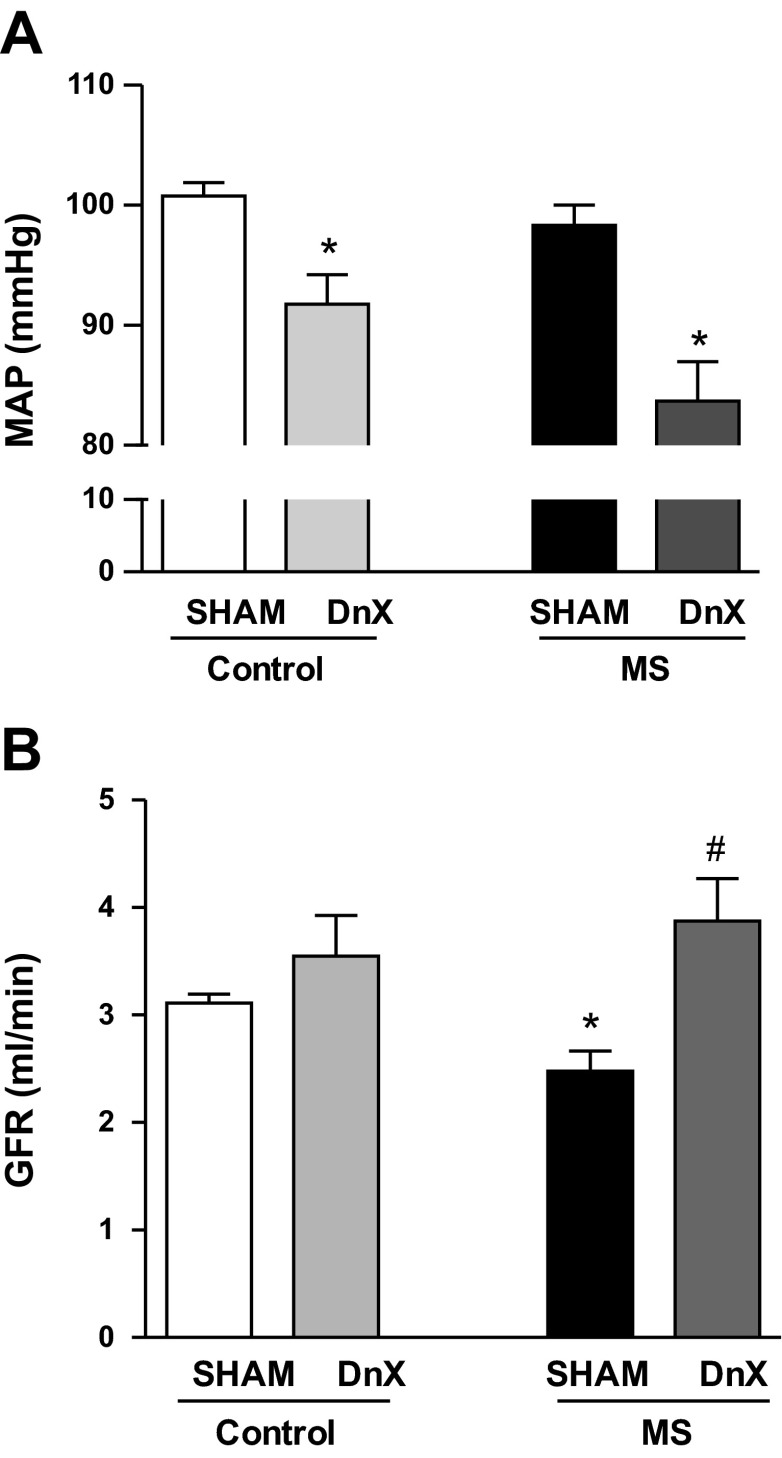
MAP directly measured from the abdominal aorta was not different in control-SHAM or MS-SHAM rats similar to our previous reports using telemetry-implanted rats (26) (Fig. 1A). Renal DnX significantly reduced MAP in both control and MS groups (Fig. 1A). GFR was significantly reduced in MS-SHAM rats compared with control-SHAM rats as previously reported using creatinine clearance (27) (Fig. 1B). DnX did not affect GFR notably in control rats but significantly increased GFR in MS rats (Fig. 1B).
Fig. 1.
Chronic measurements in conscious rats. A: mean arterial pressure (MAP) was similar in control-SHAM and maternal separation (MS)-SHAM rats. Renal denervation (DnX) reduced MAP in control and MS rats. B: glomerular filtration rate (GFR) was significantly reduced in MS-SHAM rats. Denervation restored GFR in MS rats. *P < 0.05 vs. control. #P < 0.05 vs. SHAM, n = 4–5.
Metabolic variables in renal denervated rats.
Renal DnX did not significantly affect food and water intake, urine volume, or sodium excretion in control or MS rats (Table 1).
Table 1.
Metabolic variables in SHAM and DnX rats
| Control SHAM (n = 4) | Control DnX (n = 6) | MS-SHAM (n = 4) | MS-DnX (n = 6) | |
|---|---|---|---|---|
| Body wt, g | 376 ± 8 | 369 ± 6 | 370 ± 7 | 371 ± 6 |
| Food intake, g/day | 21.0 ± 1.1 | 18.7 ± 1.8 | 21.2 ± 0.5 | 21.4 ± 0.3 |
| Water intake, ml/day | 40.1 ± 3.0 | 43.7 ± 4.3 | 42.2 ± 4.3 | 44.5 ± 1.7 |
| Urine flow rate, ml/day | 27.3 ± 3.4 | 24.9 ± 5.8 | 26.1 ± 2.2 | 26.7 ± 2.4 |
| Na excretion, meq/day | 1.67 ± 0.06 | 1.34 ± 0.17 | 1.73 ± 0.11 | 1.45 ± 0.09 |
Values are means ± SE. MS, maternal separation; DnX, denervated.
Plasma and tissue NE content.
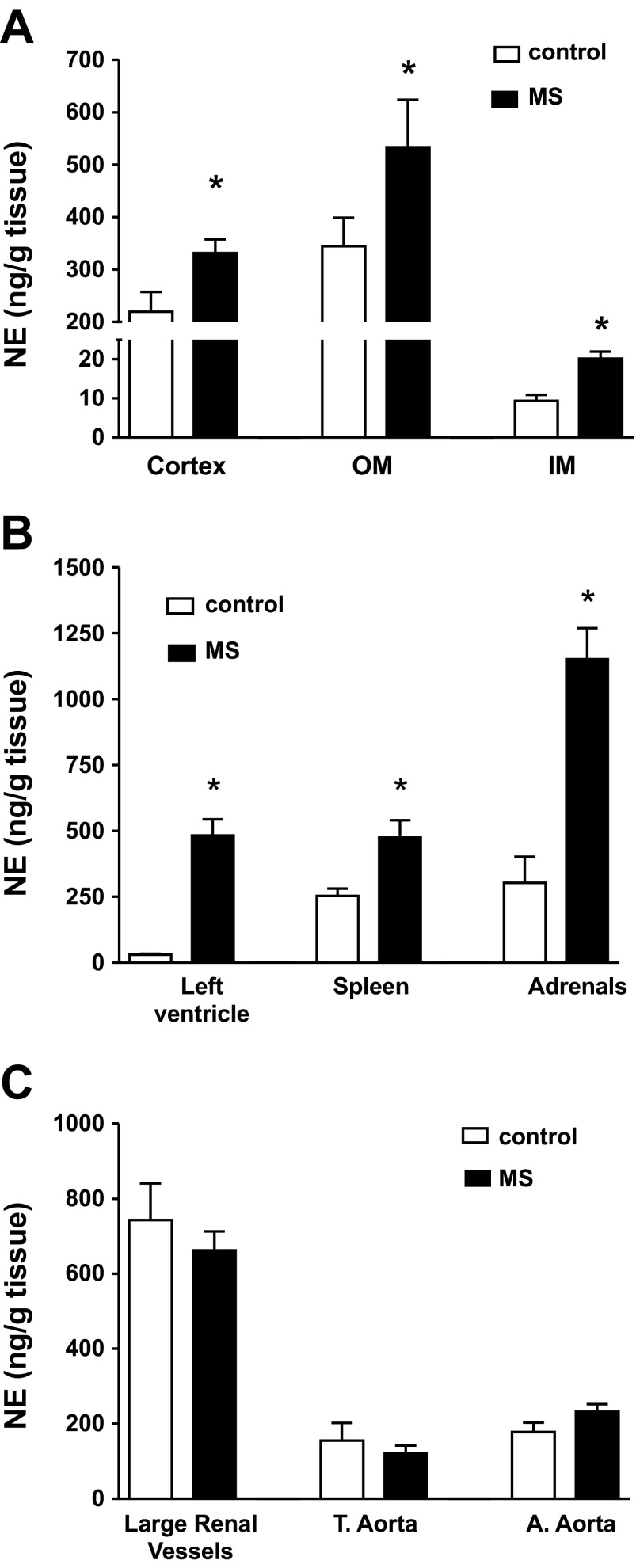
In MS rats, the renal content of NE was significantly increased in cortical, outer medullary, and inner medullary regions compared with control rats (Fig. 2A). Other organs regulated in part by the SNS such as left ventricle, spleen, and adrenals also showed significantly increased NE content in the MS group compared with control (Fig. 2B). NE content in isolated large vessels from the kidney, thoracic aorta, and abdominal aorta was not different in control and MS rats (Fig. 2C). Plasma levels of NE in control and MS rats were not significantly different (211.0 ± 24.4 and 222.9 ± 25.2 pg/ml, respectively).
Fig. 2.
Norepinephrine (NE) content in large vessels and kidney tissue. A: levels of NE in renal cortex, inner medulla, and outer medulla were increased in MS rats, n = 6. B: levels of NE in several organs, n = 6–8. C: levels of NE in vascular tissue; T=thoracic, A=abdominal, n = 4–6. *P < 0.05.
Acute hemodynamic response to NE and mecamylamine.
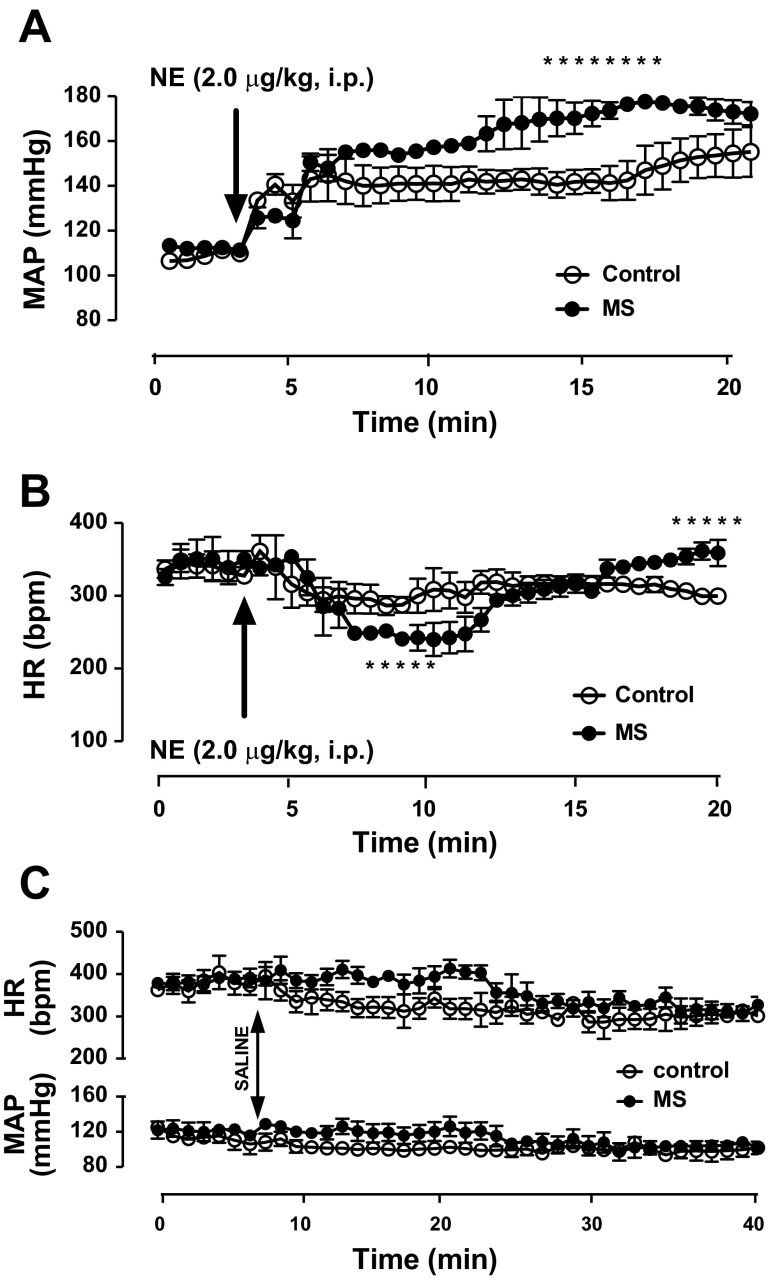
Baseline MAP and HR monitored in conscious telemetry-instrumented rats were not different between control and MS groups as previously reported (26). Acute administration of NE (2 μg/kg ip), a nonselective adrenergic agonist, significantly increased MAP and decreased HR in control and MS rats, while acute saline injection did not change either hemodynamic parameter (Fig. 3). The acute increase in MAP to NE was significantly exaggerated (Fig. 3A) and the HR response displayed an enhanced biphasic response (Fig. 3B) in MS rats compared with control rats.
Fig. 3.
Acute bolus of a nonselective adrenergic agonist (NE, 2 mg/kg ip) in telemetry-implanted rats. A: MAP showed a greater MAP in MS rats. B: heart rate (HR) was similar in MS and control rats. C: no significant changes in MAP were observed in response to a saline injection. *P < 0.05, n = 3.
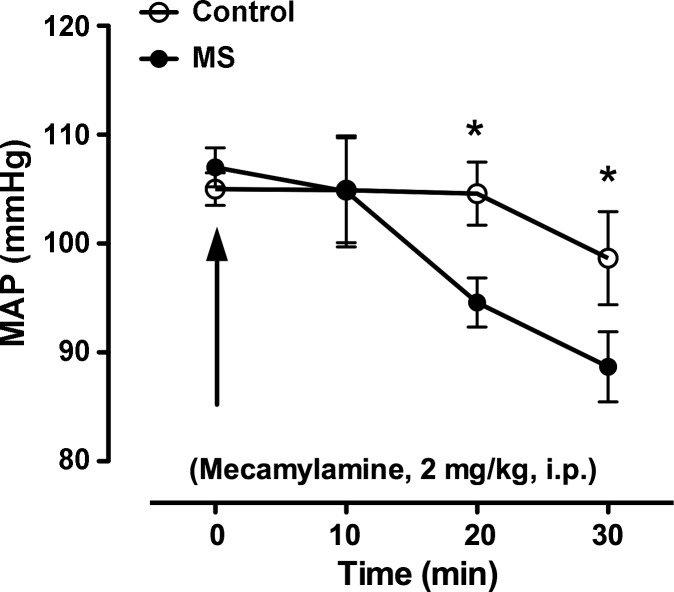
Acute administration of the ganglion blocker, mecamylamine (2 mg/kg ip), lowered blood pressure in both control and MS rats. However, the drop in MAP was exaggerated in MS rats (Fig. 4). Mecamylamine increased HR similarly in control and MS rats after 30 min (382 ± 14 and 353 ± 12 beats/min, respectively).
Fig. 4.
Acute bolus of a ganglion blockade (2 mg/kg ip) induced a greater drop in MAP in MS telemetry-implanted rats compared with control rats. *P < 0.05, n = 4.
DISCUSSION
This study supports the hypothesis that ELS, utilizing the rat model of MS, sensitizes the renal and systemic sympathetic system. At the kidney level, we found that MS induces decreased GFR that is mediated by renal nerve activation. MS rats also display an exaggerated pressor response to adrenergic stimulation and a greater reduction in blood pressure to ganglion blockade providing evidence for increased basal sympathetic tone modulating baseline blood pressure. Furthermore, NE content was significantly greater in renal tissue, as well as left ventricle, spleen, and adrenal, from MS rats compared with control rats. However, NE levels were not significantly different in the large conduit vessels, or plasma from control and MS rats. We propose that sensitization of the renal and systemic sympathetic system may provide one of the mechanism(s) for exaggerated responses to secondary hypertensive stimuli observed in this model of ELS.
Classic sympathetic activation, seen in many hypertensive models including fetal programming models, is linked to heightened basal blood pressure, increases in HR, sodium retention, as well as increased plasma and tissue catecholamine levels. In this model of postnatal chronic behavioral stress, adult MS rats display many cardiovascular parameters within the normal range at basal conditions—for example, blood pressure, HR, plasma levels of glucose, insulin, and vasoactive peptides such as catecholamines, angiotensin II, angiotensin 1–7, and endothelin-1 (25, 26). We found that the NE content in large vessels was similar in both control and MS rats, which is consistent with our previous findings showing that phenylephrine-induced constriction of thoracic aorta (using ex vivo wire myography) was not different in control and MS rats (25). Nevertheless, we demonstrated increased NE content in all regions of the kidney, left ventricle, spleen, and adrenal tissues. This observation may signify that the sympathetic system is “primed” in the MS model of ELS. Even though MS rats basally do not demonstrate sympathetic activation in the classical sense, in vivo studies showed that acute systemic activation or blockade of the sympathetic system produced exaggerated responses that led us to conclude that the sympathetic system may be a critical mediator of the detrimental cardiovascular responses to secondary environmental challenges in adult life.
The catecholamines, epinephrine and NE, are the primary SNS mediators (13, 41). Epinephrine is produced exclusively by the adrenal medulla, whereas a large amount of NE is produced by sympathetic postganglionic fibers. Thus, the effects of NE are largely mediated by the SNS primarily through activation of α- and β1-adrenergic receptors located near postganglionic sympathetic fiber terminals (10). Increased tissue NE content may be secondary to a greater sympathetic nerve activity (SNA) in many organs, although measurement of tissue NE content may not directly reflect the autonomic sympathetic fiber outflow to their effector organ. This may add a limitation to the physiological relevance of this finding in MS rats. Nevertheless, we would propose that normalization of GFR after denervation provides substantial support for the concept that MS induces a sensitization of the renal sympathetic system. Direct measurements of SNA in each organ will be the focus of future experiments to decipher the mechanistic role of the increased tissue NE.
The improvement in the renal filtration capacity with DnX in MS rats may be due to an MS-induced attenuation of the renal SNA. Moreover, if sympathetic-dependent changes in GFR are presumed to depend predominantly on afferent arteriolar resistance, renal vascular resistance (RVR) may be exaggerated in the MS rats. In an attempt to assess the RVR parameter in this study, we determined the ratio between MAP and GFR. MAP/GFR ratio was ∼30% greater in MS than in control rats at baseline. However, we found that denervation reduced this ratio ∼50% in the MS rats compared with ∼15% in control rats, suggesting that attenuation in RVR due to denervation might be greater in MS rats. Although this ratio is not a substitute for direct measurements of RVR, the calculation gives us confidence to pursue these measurements in conscious rats to probe the hypothesis further.
Resistance vessels provide a powerful mechanism to regulate regional blood flow (23, 46). The arterioles, which constitute the majority of resistance vessels, especially within the kidney, are the major contributors to total peripheral resistance (17) and are regulated mainly by autonomic mechanisms (32). The vasculature of MS rats may have specific alterations either in the nerve innervations density, adrenergic receptor number, subtype, or affinity that could result in greater sensitivity to second stressors that trigger the sympathetic response. However, whether resistance vessels of MS rats are sensitized to neural or pharmacologic adrenergic stimulation and play a role in enhancing the blood pressure response needs further investigation. Although future studies will require measurement of renal blood flow in conscious rats to determine accurately renal vasculature resistance, these renal and systemic hemodynamic data further support the concept that the impaired GFR displayed by MS rats may be secondary to an exacerbated sympathetic outflow to the kidney. Further study of the adrenergic receptor subtypes in the renal vasculature may provide mechanistic pathways to understand the difference between the chronic and acute responses observed in MS rats.
Sympathetic nerve fibers innervate all organs that are involved in peripheral control of cardiovascular function such as the heart, the peripheral vasculature, and the kidneys. Renal nerves contribute to basal control of blood pressure in both control and MS rats as demonstrated by the DnX intervention. Other reports in normotensive rats and humans found similar results (16, 29), thus the results obtained in this study were not surprising. In particular, DnX has been used in a large number of hypertensive experimental animal models (18, 19, 33). In humans, the SNS plays a crucial role in the development and progression of hypertension (9). The absence of an effective treatment using antihypertensive drugs in patients who are resistant to conventional pharmacological treatment led to the use of renal nerve ablation by selective catheter-based renal sympathetic denervation to attenuate renal sympathetic nerve activity. Outcomes from at least two clinical trials demonstrated efficacy and safety of this intervention (12, 20). In this regard, the results provided in a near future by the randomized controlled study Simplicity HTN-3 (NCT01418261 at http://clinicaltrials.gov) will be crucial for the approval of the therapy at the national level (9). This study demonstrates that the renal nerves contribute to the altered programming of renal function in this model of ELS. Therefore, the renal nerves may play a significant role in the mechanism by which ELS increases the risk to chronic disease in the adult life.
In humans, enhanced stress-induced responses predict future cardiovascular events (7). Although, it is unknown whether exaggerated responses to stimulation of the SNS are a predictor of cardiovascular disease and renal dysfunction in humans with childhood stressors. Autonomic and renal dysfunctions are common chronic conditions that frequently coexist in the same individual, and both are associated with significant morbidity and mortality. The understanding of the impact of ELS in the cardiovascular and renal sympathetic outcomes will contribute to design-personalized therapies to achieve blood pressure control in adulthood.
GRANTS
This study was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (J. S. Pollock and D. M. Pollock: Grant P01 HL69999 and A.S. Loria: K99HL111354) and from the American Heart Association (A. S. Loria Postdoctoral Fellowship: AHASE00027).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.L. and J.S.P. conception and design of research; A.S.L. and M.W.B. performed experiments; A.S.L., M.W.B., and J.S.P. analyzed data; A.S.L., M.W.B., D.M.P., and J.S.P. interpreted results of experiments; A.S.L. prepared figures; A.S.L. drafted manuscript; A.S.L., M.W.B., D.M.P., and J.S.P. edited and revised manuscript; A.S.L., M.W.B., D.M.P., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the outstanding technical support from Hiram Ocasio and Amy Dukes.
REFERENCES
- 1. Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 290: R1– R10, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754– 758, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Bell TD, DiBona GF, Wang Y, Brands MW. Mechanisms for renal blood flow control early in diabetes as revealed by chronic flow measurement and transfer function analysis. J Am Soc Nephrol 17: 2184– 2192, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull 60: 103– 121, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 271: R276– R281, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Cabassi A, Vinci S, Quartieri F, Moschini L, Borghetti A. Norepinephrine reuptake is impaired in skeletal muscle of hypertensive rats in vivo. Hypertension 37: 698– 702, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status. A meta-analysis of prospective evidence. Hypertension 55: 1026– 1032, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA 103: 17985– 17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DiBona GF. Sympathetic nervous system and hypertension. Hypertension 61: 556– 560, 2013 [DOI] [PubMed] [Google Scholar]
- 10. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595– 638, 2000 [PubMed] [Google Scholar]
- 11. Enthoven L, Oitzl MS, Koning N, van der Mark M, de Kloet ER. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology 149: 6366– 6377, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903– 1909, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Furchgott RF. The receptors for epinephrine and norepinephrine (adrenergic receptors). Pharmacol Rev 11: 429– 441, 1959 [PubMed] [Google Scholar]
- 14. Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens 18: 123– 137, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl 55: S35–S41, 1996 [PubMed] [Google Scholar]
- 16. Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol 284: H2302– H2310, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Johnson PC. Autoregulation of blood flow. Circ Res 59: 483– 495, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893– 897, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Katholi RE, Winternitz SR, Oparil S. Decrease in peripheral sympathetic nervous system activity following renal denervation or unclipping in the one-kidney one-clip Goldblatt hypertensive rat. J Clin Invest 69: 55– 62, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275– 1281, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Kuo JJ, Jones OB, Hall JE. Chronic cardiovascular and renal actions of leptin during hyperinsulinemia. Am J Physiol Regul Integr Comp Physiol 284: R1037– R1042, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Lehmann J, Stohr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res 107: 133– 144, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Lewis DJ, Duncan CP. Expectation and resistance to extinction under partial reinforcement and risk-taking. Am J Psychol 75: 77– 84, 1962 [PubMed] [Google Scholar]
- 24. Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091– 3098, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619– 626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494– 499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121– R129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring's HPA and fear responses in rats. Eur J Neurosci 20: 1017– 1024, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Bohm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60: 419– 424, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev 31: 3– 17, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology 143: 4455– 4463, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Nyborg NC, Bevan JA. Increased alpha-adrenergic receptor affinity in resistance vessels from hypertensive rats. Hypertension 11: 635– 638, 1988 [DOI] [PubMed] [Google Scholar]
- 33. O'Hagan KP, Thomas GD, Zambraski EJ. Renal denervation decreases blood pressure in DOCA-treated miniature swine with established hypertension. Am J Hypertens 3: 62– 64, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Ojeda NB, Grigore D, Alexander BT. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv Chronic Kidney Dis 15: 101– 106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol 34: 1212– 1216, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18: 195– 200, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51: 383– 392, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 55: 76– 82, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res 183: 25– 30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schlaich MP, Krum H, Sobotka PA, Esler MD. Renal denervation and hypertension. Am J Hypertens 24: 635– 642, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Schmitz JM, Graham RM, Sagalowsky A, Pettinger WA. Renal alpha-1 and alpha-2 adrenergic receptors: biochemical and pharmacological correlations. J Pharmacol Exp Ther 219: 400– 406, 1981 [PubMed] [Google Scholar]
- 42. Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899– R906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shokoji T, Nishiyama A, Fujisawa Y, Hitomi H, Kiyomoto H, Takahashi N, Kimura S, Kohno M, Abe Y. Renal sympathetic nerve responses to tempol in spontaneously hypertensive rats. Hypertension 41: 266– 273, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc 64: 143– 151, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Tucker DC, Johnson AK. Influence of neonatal handling on blood pressure, locomotor activity, and preweanling heart rate in spontaneously hypertensive and Wistar Kyoto rats. Dev Psychobiol 17: 587– 600, 1984 [DOI] [PubMed] [Google Scholar]
- 46. Wright CE, Angus JA, Korner PI. Structural factors increase blood pressure through the interaction of resistance vessel geometry with neurohumoral and local factors: estimates in rabbits with renal cellophane-wrap hypertension with intact effectors and during neurohumoral blockade. J Hypertens 20: 471– 483, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47: 502– 508, 2006 [DOI] [PubMed] [Google Scholar]