Abstract
Matrix protein accumulation is a prominent feature of diabetic nephropathy that contributes to renal fibrosis and decline in renal function. The pathogenic mechanisms of matrix accumulation are incompletely characterized. We investigated if the matrix metalloprotease a disintegrin and metalloprotease1 7 (ADAM17), known to cleave growth factors and cytokines, is activated in the kidney cortex of OVE26 type 1 diabetic mice and the potential mechanisms by which ADAM17 mediates extracellular matrix accumulation. Protein expression and activity of ADAM17 were increased in OVE26 kidney cortex. Using a pharmacological inhibitor to ADAM17, TMI-005, we determined that ADAM17 activation results in increased type IV collagen, Nox4, and NADPH oxidase activity in the kidney cortex of diabetic mice. In cultured mouse proximal tubular epithelial cells (MCTs), high glucose increases ADAM17 activity, Nox4 and fibronectin expression, cellular collagen content, and NADPH oxidase activity. These effects of glucose were inhibited when cells were pretreated with TMI-005 and/or transfected with small interfering ADAM17. Collectively, these data indicate a novel mechanism whereby hyperglycemia in diabetes increases extracellular matrix protein expression in the kidney cortex through activation of ADAM17 and enhanced oxidative stress through Nox enzyme activation. Additionally, our study is the first to provide evidence that Nox4 is downstream of ADAM17.
Keywords: ADAM17, Nox4, diabetic nephropathy, OVE26 mice, extracellular matrix
diabetic nephropathy (DN) is characterized by diverse pathophysiological changes in the kidney including extracellular matrix (ECM) accumulation in glomeruli and tubulointerstitium (50). Matrix metalloproteases (MMPs) are a large and diverse family of proteins implicated in regulating ECM turnover and tissue remodeling. Several studies have suggested that metalloproteases contribute to ECM turnover in diabetes. However, relatively little information is available regarding the role of the a disintegrin and a metalloprotease, or ADAM, family of MMPs. Members of this family are modular membrane proteins involved in proteolytic processing or shedding of membrane-bound proteins and receptors (24, 25, 32, 43). ADAM metalloproteases have adhesive and proteolytic properties that modulate cell signaling and biological responses of cells (5, 12, 19). ADAM17, a member of this ADAM family of MMPs, was originally described as the sheddase of tumor necrosis factor-α, referred to as TNFα-converting enzyme (4, 34). In addition, ADAM17 is also responsible for cleaving membrane-bound growth factors and receptors such as transforming growth factor-α (TGFα), heparin-bound epidermal growth factor (HB-EGF), TNF receptor I and II, adhesion molecules, proinflammatory molecules, amyloid precursor protein, and ErbB4 (4, 12, 17, 28, 34, 39, 41).
There is evidence that ADAM17 plays a role in the pathogenesis of DN (1, 3, 10, 11, 20, 21, 26, 29, 33, 35, 40, 42, 47, 48). Studies in mesangial cells showed that glucose activates ADAM17 and EGF receptor (EGFR) and regulates profibrotic TGFβ and the accumulation of matrix proteins (48, 51–53). However, the mechanisms by which glucose-mediated ADAM17 activity contributes to increased matrix production and fibrosis are still not fully understood.
Reactive oxygen species (ROS) originating from nicotinamide adenine dinucleotide phosphate (NADPH) oxidases participate in the pathogenesis of DN including increased ECM accumulation (2, 6, 8, 22, 38, 44). The Nox family of NADPH oxidases consists of different isoforms, which are homologues of gp91phox (or Nox2), the catalytic subunit of the phagocyte isoform. Nox4 was originally cloned from the kidney (18) and increased levels of ROS derived from Nox4 enhance matrix accumulation in cultured mesangial, tubular, and interstitial cells (2, 6, 8).
In this study, we demonstrate that ADAM17 expression and activity are increased in the kidney cortex of OVE26 mice with type 1 diabetes. Pharmacological inhibition of ADAM17 indicated that the activation of ADAM17 is responsible at least partially for the increase in Nox4 protein expression, NADPH oxidase activity, and collagen IV expression in the diabetic kidney cortex. Exposure of cultured MCT mouse proximal tubular cells to 25 mM d-glucose increases ADAM17 activity, fibronectin, collagen, and Nox4 protein expression, and NADPH oxidase activity. Pharmacological inhibition or knockdown of ADAM17 with small interfering (si)RNA prevented the effects of high glucose. Collectively, the data suggest that ADAM17 contributes to matrix accumulation through oxidative stress.
MATERIALS AND METHODS
Materials.
TMI-005 was a gift from Pfizer-Wyeth Pharmaceuticals. Tween-80, PEG-400, dimethyl sulfoxide, and d-glucose were purchased from Sigma-Aldrich (St. Louis, MO). Pools of siRNAs targeting ADAM17 and nontargeting siRNAs were purchased from Dharmacon (Lafayette, CO). RiboJuice siRNA transfection reagent was purchased from EMD Millipore (Billerica, MA). Sircol collagen colorimetric dye-binding assay was purchased from Accurate Chemical & Scientific Corporation (Westbury, NY).
Animals and tissue culture.
Age- and weight-matched male type 1 diabetic OVE26 mice (14) and nondiabetic FVB control mice were purchased from Jackson Laboratories. Five-month-old mice were randomly assigned to three groups that received vehicle or drug by oral gavage: group 1: FVB mice given vehicle buffer (2% Tween 80 and 0.5% methylcellulose) twice daily; group 2: OVE26 mice that received vehicle buffer twice daily; and group 3: OVE26 mice that received the ADAM17 MMP inhibitor TMI-005 at a dose of 10 mg/kg twice daily. After 3 wk of drug administration, 24-h urine was collected in metabolic cages and urine albumin was measured by a mouse albumin ELISA kit (Bethyl Laboratories; Montgomery, TX). Following urine collection, animals were killed by exsanguination while the were under anesthesia. Both kidneys were removed and weighed, and portions of kidney cortex were flash-frozen in liquid nitrogen for microscopy and biochemical analyses or formalin fixed for morphometric imaging.
All animal studies followed a protocol that was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Immortalized mouse renal cells (glomerular endothelial cells, glomerular epithelial cells, mesangial cells, and proximal tubular epithelial cells) were cultured in DMEM supplemented with fetal bovine serum and antibiotic/antifungal solution or RPMI medium supplemented with fetal bovine serum, antibiotic/antifungal solution (glomerular epithelial cells). When cells reached 90% confluence, tissue culture plates were serum starved overnight before pretreatment with or without 1 μM TMI-005 for 1 h followed by exposure to 25 mM d-glucose for 4 or 24 h. Immortalized mouse proximal tubular epithelial cells (MCTs) were a gift from Eric Neilson (23). Transient transfection using RiboJuice siRNA transfection reagent and 20 nM siRNA was performed when tissue culture plates reached 30% confluence. Cells were incubated with siRNA and transfection reagent for 24 h before overnight serum starvation and subsequent d-glucose exposure (25 mM).
Western blot analysis.
Kidney cortex homogenates were prepared in 500 μl of radioimmune precipitation assay buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 20 μg/ml leupeptin, and 1% NP-40) using a Dounce homogenizer. Samples containing equivalent amounts of protein were separated by 7.5% SDS-polyacrylamide gel electrophoresis and transferred to 0.45-μm nitrocellulose membranes before immunoblotting with antibodies. Blots were incubated with anti-collagen IV α5 (1:1,000; Santa Cruz Biotechnology), anti-Nox4 (1:500; Santa Cruz Biotechnology), anti-fibronectin (1:1,000; Sigma Aldrich), and polyclonal anti-ADAM17 (1:250; Abcam). Secondary antibodies conjugated with horseradish peroxidase were employed to detect signals (1:5,000; Bio-Rad). Results were visualized with enhanced chemiluminescence detection reagents (Amersham GE Life Sciences and Pfenix X-ray film). Quantitation of Western blot data was performed using ImageJ software analysis (Wayne Rasband, National Institutes of Health).
Immunoperoxidase and immunofluorescent staining.
Frozen kidney cortex samples were sectioned with a Leica cryostat at a thickness of 5 μm and processed for immunoperoxidase staining. Digital images were obtained with an Olympus AX70 research microscope and a DP70 digital camera. Immunoperoxidase staining was performed using Vector Laboratories DAB substrate kit SK-4100, avidin/biotin blocking kit (SP-2001), and ABC Vectastain standard kit (PK-4000). Anti-collagen IV antibodies (Millipore) were used at 1:500. Image Pro Plus software (Media Cybernetics) was utilized to quantify staining within the glomeruli and interstitium.
ADAM17 enzymatic activity assay.
Kidney cortex homogenates were prepared in 200 μl of assay buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, and 1% NP-40) using a Dounce homogenizer. Protein estimation was performed by the Bradford method, and 25 μg of homogenate were used in the assay. ADAM17 activity was measured using EMD Bioscience's InnoZyme ADAM17 activity kit. The kit utilizes an internally quenched fluorogenic substrate, MCA-KPLGL-Dpa-AR-NH2, which when cleaved specifically by ADAM17, causes fluorophore release. The resultant fluorescence is measured at an excitation wavelength of 320 nm and an emission wavelength of 405 nm. Activity was expressed as relative fluorescence units per milligrams of protein.
NADPH oxidase assay.
NADPH-dependent superoxide production was determined using the lucigenin-enhanced chemiluminescence method as described previously (7, 13). Kidney cortex or MCT cell homogenates were prepared in 1 ml or 250 μl, respectively, of lysis buffer (20 mM KH2PO4, pH 7.0, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 0.5 μg/ml leupeptin) by using a Dounce homogenizer. Protein content was estimated using the Bradford method, and 25 μg of homogenate were added to 50 mM phosphate buffer, pH 7.0, containing 1 mM EGTA, 150 mM sucrose, 5 μM lucigenin, and 100 μM NADPH at a final volume of 1 ml. Photon emission was measured every 30 s for 5 min in a luminometer. Superoxide production was expressed in relative light units per milligrams of protein (RLU/mg).
Statistical analysis.
Results are expressed as the means + SE. Statistical significance was calculated by either student's unpaired t-test (see Fig. 4D) or one-way ANOVA with post hoc Tukey analysis (see Figs. 2A; 3, B, C, and E; 4, A, C, G, and H; 5, B and C; 6, B, C, E, and F) and determined as P < 0.05.
Fig. 4.
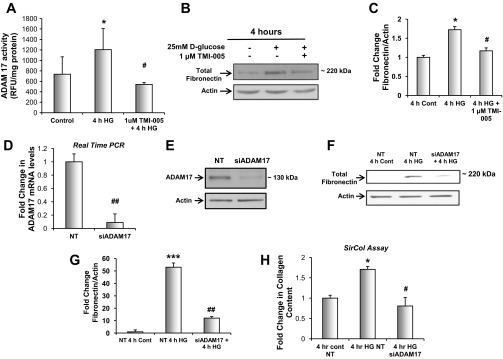
ADAM17 activation mediates the effect of glucose on fibronectin protein expression and collagen content in mouse proximal tubular epithelial cells (MCTs). A: proximal tubular epithelial cells were serum-starved for 24 h and pretreated with 1 μM TMI-005 before exposure to 25 mM d-glucose for 4 h. Dounce homogenized samples were used to determine ADAM17 activity levels by measuring cleavage of the internally quenched fluorogenic substrate MCA-KPLGL-Dpa-AR-NH2. Shown are means of 6 experiments ± SE. *P < 0.05, compared with the control cells; #P < 0.05, compared with HG-treated cells. B: proximal tubular epithelial cells were serum-starved for 24 h and pretreated with 1 μM TMI-005 before exposure to 25 mM d-glucose for 4 h; 20 μg of cellular lysates were resolved on a 7.5% SDS-PAGE and immunoblotted for anti-fibronectin. C: pixel densitometry was performed using ImageJ software to quantitate differences in protein expression levels. Values are representative of the means of 4 experiments ± SE. *P < 0.05, compared with 4-h control, #P < 0.05, compared with 4 h HG. D: Proximal tubular epithelial cells were transfected with a pool of specific small interfering (si)RNAs for ADAM17 or non-targeting (NT) siRNA. mRNA expression analysis was performed using real-time qPCR to determine the efficiency of ADAM17 knockdown. Shown are the means of 5 experiments + S.E. Statistics were determined by Student's unpaired t-test. ##P < 0.01, compared with NT transfected cells. E: proximal tubular epithelial cells were transfected with a pool of specific siRNAs for ADAM17 or NT siRNA; 20 μg of cellular lysates were resolved on a 7.5% SDS-PAGE and immunoblotted for anti-ADAM17 to determine ADAM17 knockdown efficiency. F: proximal tubular epithelial cells were transfected with a pool of specific siRNAs for ADAM17 or NT siRNA and serum-starved for 24 h before exposure to 25 mM d-glucose for 4 h; 20 μg of cellular lysates were resolved on a 7.5% SDS-PAGE and immunoblotted for anti-fibronectin. G: pixel densitometry was performed using ImageJ software to quantitate differences in protein expression levels. Values are representative of the means of 5 experiments from each group ± SE. ***P < 0.001, compared with NT 4-h control; ##P < 0.01, compared with NT 4 h HG. H: proximal tubular epithelial cells were transfected with a pool of specific siRNAs for ADAM17 or NT siRNA and serum-starved for 24 h before exposure to 25 mM d-glucose for 4 h. Collagen content was determined using picrosirius-red staining with a Sircol colorimetric assay. Shown are the means of 3 experiments ± SE. *P < 0.05, compared with the NT transfected control cells; #P < 0.05, compared with NT transfected HG-treated cells.
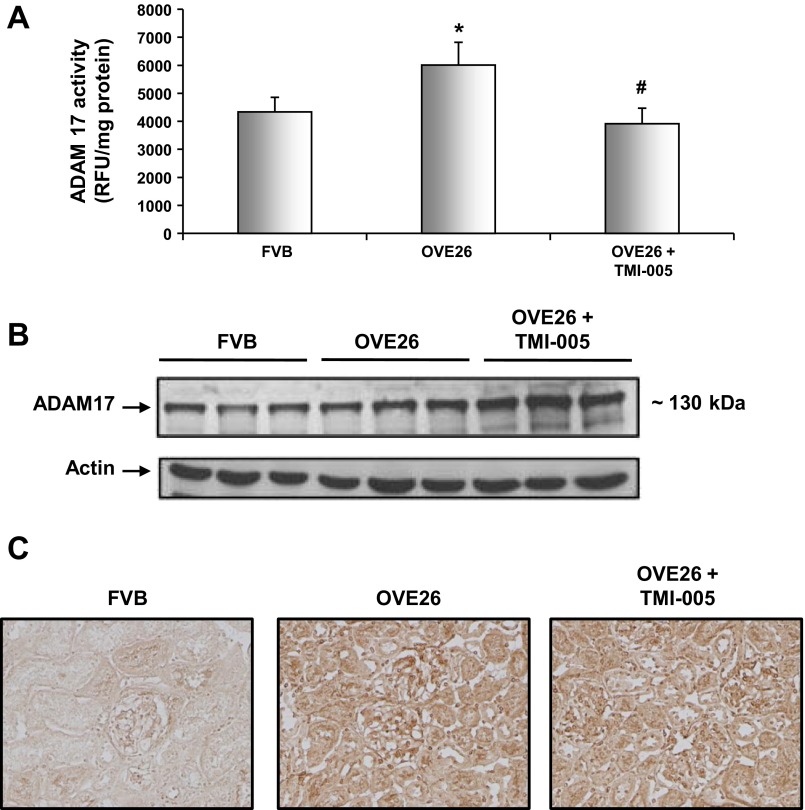
Fig. 2.
ADAM17 protein expression and enzymatic activity are increased in OVE26 kidney cortex. Five-month-old diabetic OVE26 mice were administered TMI-005, a pharmacological inhibitor to ADAM 17 (Pfizer-Wyeth), or vehicle control (2% TWEEN 80 and 0.5% methylcellulose) by oral gavage for an interval of 4 wk. FVB nondiabetic mice were only given vehicle control during the same duration. A: dounce homogenized whole kidney cortex was used to determine ADAM 17 activity levels by measuring cleavage of the internally quenched fluorogenic substrate MCA-KPLGL-Dpa-AR-NH2. Shown are means of 4 animals/group ± SE. *P < 0.05 vs. the FVB control group; #P < 0.05 vs. the OVE26 diabetic group. B: kidney cortex lysates were subjected to Western blot analysis and immunoblotted with anti-ADAM 17 antibody and anti-actin for loading control. Western blot is representative of 4 animals from each group. C: 5-μm thick frozen kidney cortex sections were stained with anti-ADAM 17 antibody using an immunoperoxidase method. Digital images are representative of 4 animals from each group.
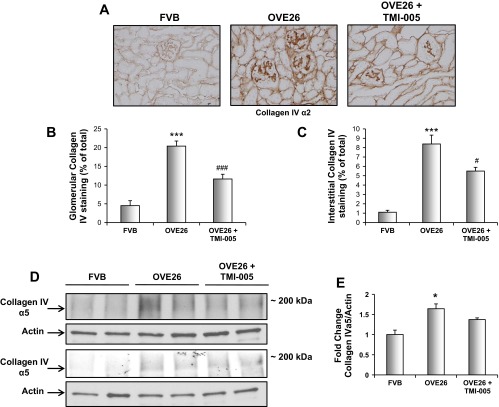
Fig. 3.
Inhibition of ADAM17 with TMI-005 reduces collagen IV protein expression in OVE26 kidney cortex. Five-month-old diabetic OVE26 mice were administered TMI-005, a pharmacological inhibitor to ADAM 17 (Pfizer-Wyeth) or vehicle control (2% TWEEN 80 and 0.5% methylcellulose) by oral gavage for an interval of 4 wk. FVB nondiabetic mice were only given vehicle control during the same duration. A: frozen kidney cortex sections were stained with anti-type IV collagen α2 by immunoperoxidase method. Digital images are representative of 4 animals from each group. B: glomerular collagen IV α2-protein expression was calculated using Image-Pro Plus software. Shown are means ± SE of 20 imaged glomeruli per treatment group. ***P < 0.001, compared with FVB control group; ###P < 0.001, compared with OVE26 diabetic group. C: interstitial collagen IV α2-protein expression was calculated using Image-Pro Plus software. Shown are means ± SE of 20 imaged fields per treatment group. ***P < 0.001, compared with FVB control group; #P < 0.05, compared with OVE26 diabetic group. D: kidney cortex lysates were subjected to Western blot analysis and immunoblotted with anti-collagen IV α5-antibody and anti-actin for loading control. Western blots represent 4 animals from each group. E: pixel densitometry was performed using ImageJ software to quantitate differences in protein expression levels. Values are representative of the means of 4 animals from each group ± SE. *P < 0.05, compared with FVB control group.
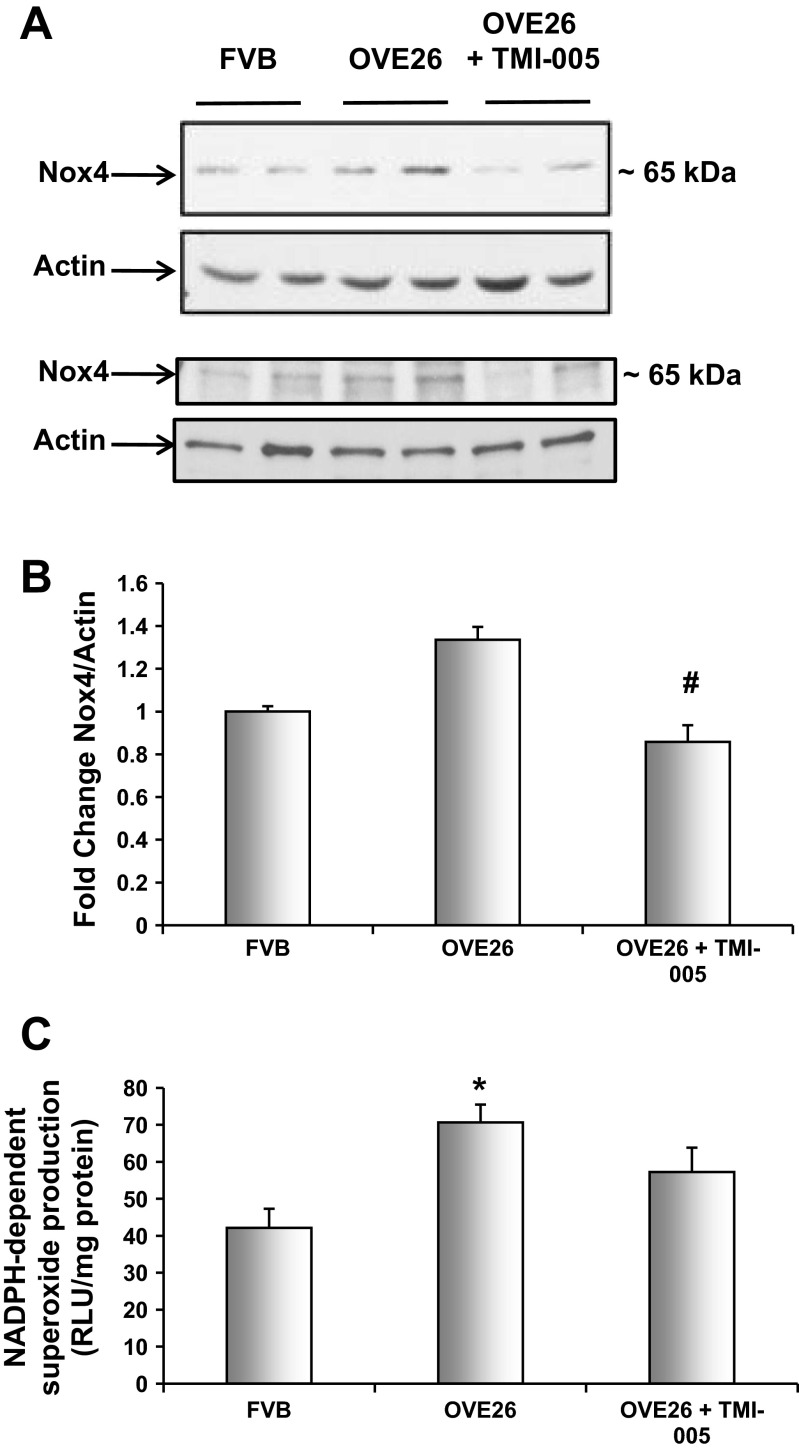
Fig. 5.
ADAM17 mediates Nox4 protein expression and NADPH oxidase activity in OVE26 kidney cortex. Five-month-old diabetic OVE26 mice were administered TMI-005, a pharmacological inhibitor to ADAM 17 (Pfizer-Wyeth) or vehicle control (2% TWEEN 80 and 0.5% methylcellulose) by oral gavage for an interval of 4 wk. FVB nondiabetic mice were only given vehicle control during the same duration. A: kidney cortex lysates were subjected to Western blot analysis and immunoblotted with anti-Nox4 antibody and anti-actin for loading control. Western blots represent 4 animals from each group. B: pixel densitometry was performed using ImageJ software to quantify protein expression levels. Values are representative of the means of 4 animals from each group ± SE. #P < 0.05, compared with OVE26 diabetic group. C: dounce homogenized kidney cortex samples were used to determine NADPH oxidase levels using a lucigenin-enhanced chemiluminescent assay. Shown are means of 4 animals/group ± SE. *P < 0.05 vs. the FVB control group by one-way ANOVA with post hoc Tukey analysis.
Fig. 6.
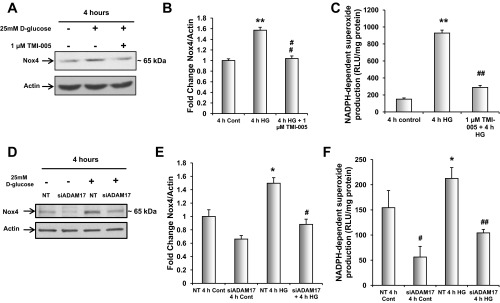
Glucose-induced ADAM17 activation mediates Nox4 protein expression and NADPH oxidase activity in MCTs. A: proximal tubular epithelial cells were serum starved for 24 h and pretreated with 1 μM TMI-005 before exposure to 25 mM d-glucose for 4 h; 40 μg of cellular lysates were resolved on a 7.5% SDS-PAGE and immunoblotted for anti-Nox4. B: pixel densitometry was performed using ImageJ software to quantitate differences in protein expression levels. Values are representative of the means of 3 experiments from each group ± SE. *P < 0.05, compared with 4-h control, #P < 0.05, compared with 4-h HG. C: proximal tubular epithelial cells were serum-starved for 24 h and pretreated with 1 μM TMI-005 before exposure to 25 mM d-glucose for 4 h. Dounce homogenized samples were used to determine NADPH oxidase levels using a lucigenin-enhanced chemiluminescence assay. Shown are means of 4 experiments ± SE. **P < 0.01 vs. control cells; ##P < 0.01 vs. HG-treated cells. D: proximal tubular epithelial cells were transfected with a pool of specific siRNAs for ADAM17 or NT siRNA and serum-starved for 24 h before exposure to 25 mM d-glucose for 4 h; 20 μg of cellular lysates were resolved on a 7.5% SDS-PAGE and immunoblotted for anti-Nox4. E: pixel densitometry was performed using ImageJ software to quantitate differences in protein expression levels. Values are representative of the means of 5 experiments from each group ± SE. *P < 0.05, compared with NT 4-h control, #P < 0.05 compared with NT 4 h HG. F: proximal tubular epithelial cells were transfected with a pool of specific siRNAs for ADAM17 or NT siRNA and serum-starved for 24 h before exposure to 25 mM d-glucose for 4 h. Dounce homogenized samples were used to determine NADPH oxidase levels using a lucigenin-enhanced chemiluminescent assay. Shown are means of 5 experiments + SE. #P < 0.05 vs. NT control cells; *P < 0.05 vs. NT control cells; ##P < 0.01 vs. siADAM17 transfected 4-h HG-treated cells.
RESULTS
ADAM17 expression is upregulated in response to high glucose exposure in renal cells.
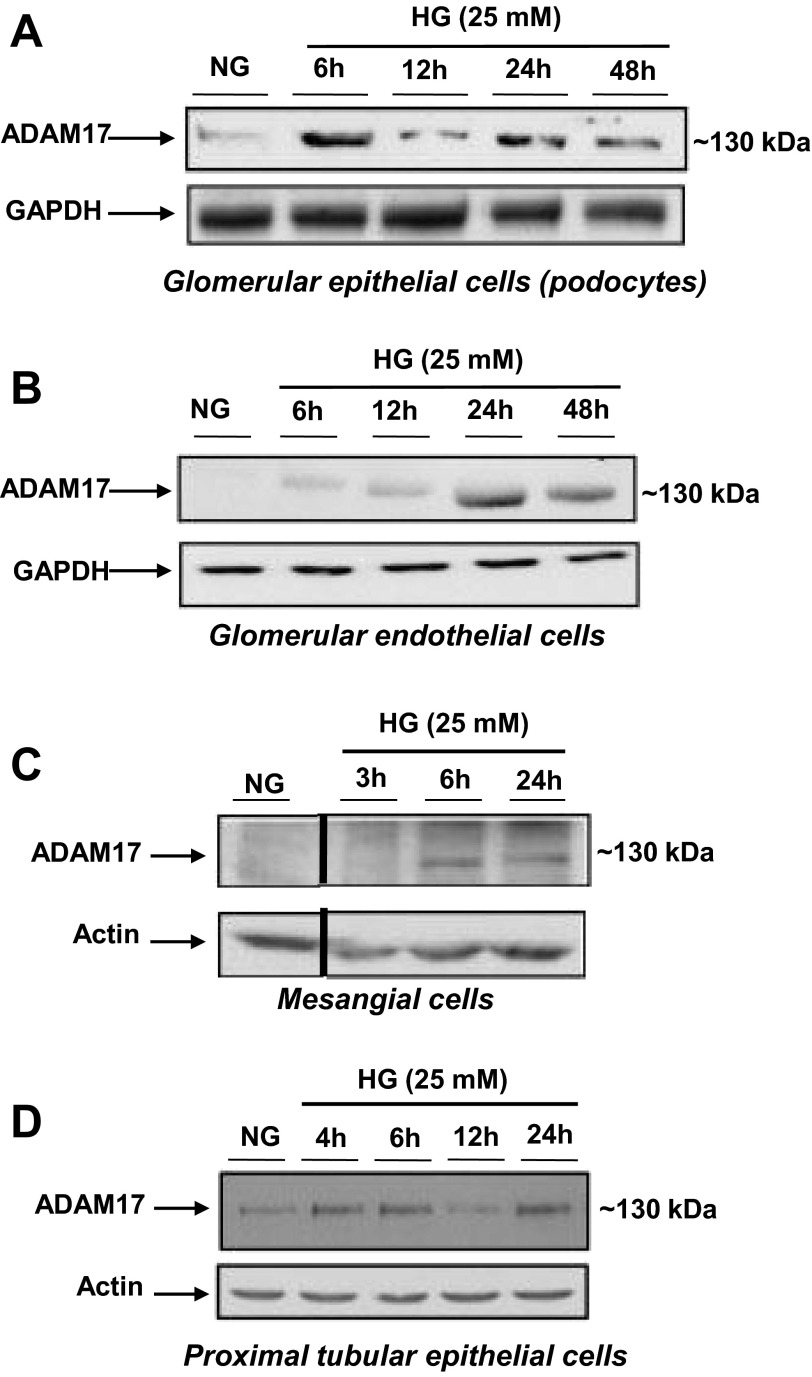
To first determine if glucose regulates ADAM17, mouse renal cells were exposed to a high concentration of d-glucose (25 mM) to mimic the diabetic milieu. Glomerular epithelial cells or podocytes showed a robust increase in ADAM17 protein expression after 6-h exposure to high glucose, and its expression decreased at 12-h exposure but increased once more at 24 h and maintained expression at 48-h exposure to high glucose (Fig. 1A). Western blot analysis of glomerular endothelial cells incubated with a high concentration of glucose revealed a slight increase in ADAM17 protein expression at 6- and 12-h exposure to high glucose and a strong increase 24 and 48 h (Fig. 1B). Mouse mesangial cells also showed increased ADAM17 expression at 3-, 6-, and 24-h exposure to 25 mM d-glucose with the highest expression levels at 6 and 24 h (Fig. 1C). Proximal tubular epithelial cells exhibited an increase in ADAM17 protein expression in response to high glucose incubation at 4, 6, and 24 h (Fig. 1D). Collectively, these experiments show that ADAM17 is expressed ubiquitously in the kidney cortex and that it is induced in all four cell types with high glucose.
Fig. 1.
A disintegrin and metalloprotease1 7 (ADAM17) expression is upregulated in response to high glucose (HG) exposure in renal cells. A: glomerular epithelial cells were exposed to 25 mM d-glucose for varying time points before lysis and collection of cells for Western blot analysis. Normal glucose (NG) was used as a control (5 mM); 50 μg protein were resolved on a 7.5% SDS-PAGE and immunoblots were probed for anti-ADAM17. B: glomerular endothelial cells were exposed to 25 mM d-glucose for varying time points before lysis and collection of cells for Western blot analysis. NG was used as a control (5 mM); 50 μg protein were resolved on a 7.5% SDS-PAGE and immunoblots were probed for anti-ADAM17. C: glomerular mesangial cells were exposed to 25 mM d-glucose for varying time points before lysis and collection of cells for Western blot analysis. NG was used as a control (5 mM); 50 μg protein were resolved on a 7.5% SDS-PAGE and immunoblots were probed for anti-ADAM17. Images were cropped from the same membrane to show only normal glucose and HG treatments as another irrelevant agonist was used simultaneously in this experiment. D: proximal tubular epithelial cells were exposed to 25 mM d-glucose for varying time points before lysis and collection of cells for Western blot analysis. NG was used as a control (5 mM); 50 μg protein were resolved on a 7.5% SDS-PAGE and immunoblots were probed for anti-ADAM17.
ADAM17 protein expression and enzymatic activity are increased in OVE26 kidney cortex.
Initial reports indicate ADAM17 and cleavage of its substrates may be involved in kidney disease and can also contribute to the progression of diabetes (1, 3, 9, 10, 15, 20, 21, 26, 29, 33, 35, 40, 42, 45, 47). We hypothesized that ADAM17 plays a key role in ECM accumulation in DN. To examine this hypothesis, 5-mo-old OVE26 transgenic mice and FVB nondiabetic control mice were segregated into the following groups with four mice per group: FVB control mice, OVE26 diabetic mice, and OVE26 diabetic mice receiving 10 mg/kg TMI-005 by oral gavage twice daily. TMI-005 is a potent and selective thiomorpholine sulfonamide hydroxymate inhibitor of ADAM17, generously provided by Pfizer (formerly Wyeth-Ayerst Pharmaceuticals). TMI-005 has an IC50 of 20 nM and exhibits 89% inhibition of lipopolysaccharide-induced TNFα production in THP-1 monocytic cells (30).
Table 1 displays the blood glucose levels, body and kidney weights, and urine albumin (albuminuria) levels in the three different groups of mice (Table 1). After 3 wk of TMI-005 administration, there was no significant difference in blood glucose levels and body weight between the OVE26 group and OVE26 mice treated with TMI-005. Interestingly, a significant reduction in the urine albumin levels was observed in OVE26 mice treated with TMI-005. ADAM17 activity and protein expression were assayed in kidney cortex samples of OVE26 type 1 diabetic mice (Fig. 2A). OVE26 kidney cortex exhibited a significant increase in ADAM17 activity compared with the control FVB group. Mice treated with TMI-005 had a significant reduction in ADAM17 activity (Fig. 2A). Moreover, ADAM17 protein expression increased in OVE26 kidney cortex compared with FVB nondiabetic kidney cortex (Fig. 2B) and treatment with TMI-005, while inhibiting enzyme activity, increased ADAM17 protein expression (Fig. 2B). The increase in ADAM17 protein levels was confirmed by immunoperoxidase staining (Fig. 2C).
Table 1.
Glucose level, body weight, kidney weight, kidney weight/body weight ratio, and urine albumin (albuminuria) levels in vehicle-dosed FVB and OVE26 mice and TMI-005-dosed OVE26 mice
| Blood Glucose, mg/dl | Body Weight, g | Kidney Weight, mg | Kidney Weight/Body Weight, mg/g | Albuminuria, μg/24 h | |
|---|---|---|---|---|---|
| FVB vehicle | 151 ± 8.14 | 28.66 ± 1.39 | 227.86 ± 7.05 | 7.95 ± 3.17 | 0.08 ± 0.01 |
| OVE26 vehicle | 565 ± 6.00* | 17.29 ± 1.07 | 241.25 ± 12.74 | 13.95 ± 1.02* | 10.73 ± 4.05† |
| OVE26 + TMI-005, 10 mg/kg | 571.5 ± 23.63 | 16.55 ± 0.60 | 241.67 ± 15.75 | 14.60 ± 1.43 | 5.34 ± 2.56‡ |
Values are the means ± SE from 4 animals from each group.
P < 0.05 vs. FVB group;
P < 0.01 vs. FVB group;
P < 0.05 vs. OVE26 vehicle-treated group.
Inhibition of ADAM17 with TMI-005 reduces collagen IV protein expression in OVE26 kidney cortex.
To determine the role of ADAM17 activation in ECM accumulation, the basement membrane protein collagen IV was investigated. Immunoperoxidase staining demonstrated a significant increase in collagen IV within the glomeruli and tubule/interstitium of OVE26 kidney cortex (Fig. 3, A–C). OVE26 mice treated with TMI-005 exhibited a significant reduction in collagen IV α2-protein expression in both compartments (Fig. 3, A–C). Additionally, these findings were supported by Western blot analysis where collagen IV α5-expression was significantly increased in kidney cortex of OVE26 mice compared with control mice. Collagen IV α5 had a trend to decrease in TMI-005-treated mice (Fig. 3D). These data suggest that ADAM17 plays a role in ECM accumulation in DN.
ADAM17 activation mediates the effect of glucose on fibronectin protein expression and collagen content in mouse proximal tubular epithelial cells (MCTs).
To determine the role of ADAM 17 in matrix accumulation in mouse proximal tubular epithelial cells (MCTs; Ref. 23) were exposed to a high concentration of glucose (25 mM) and ADAM17 activity was assayed. After 4 h of exposure to 25 mM d-glucose, ADAM17 activity was significantly increased and pretreatment with TMI-005 inhibited enzyme activity (Fig. 4A). Fibronectin protein expression was also examined using Western blot analysis. Fibronectin expression increased after 4-h glucose exposure, and inhibition of ADAM17 with TMI-005 prevented the glucose-induced increase in fibronectin (Fig. 4, B and C). To confirm the role of ADAM17 in mediating ECM accumulation in cells treated with glucose, a pool of siRNAs targeting ADAM17 was transfected into MCTs. ADAM17 mRNA and protein expression were reduced with siRNA-targeted knockdown as assessed by quantitative real-time PCR and Western blot, respectively (Fig. 4, D and E). Knockdown of ADAM17 blocked the increase in fibronectin protein expression in response to glucose (Fig. 4, F and G). RNA interference of ADAM17 also blocked the accumulation of all collagen isoforms as evaluated by a Sircol colorimetric assay (Fig. 4H). These data strongly implicate ADAM17 in the accumulation of ECM in mouse proximal tubular epithelial cells exposed to glucose.
ADAM17 mediates Nox4 protein expression and NADPH oxidase activity in OVE26 kidney cortex.
To identify a potential downstream target of ADAM17 that results in increased ECM expression in DN, we examined Nox4, a member of the NADPH oxidase family of enzymes. Numerous studies, including several from our group, have identified Nox4 in mediating renal injury including renal hypertrophy and ECM accumulation in mesangial cells and proximal tubular epithelial cells as well as glomerular epithelial cell apoptosis and glomerular endothelial cell dysfunction (2, 7, 8, 13, 31, 44). We hypothesized that ADAM17 increases ECM accumulation in the diabetic kidney, at least partially, through the actions of Nox4. To investigate this possibility, Western blot analysis of Nox4 was performed on cortical lysates of FVB, OVE26, and OVE26 mice treated with TMI-005. Nox4 protein expression was increased in OVE26 kidney cortex and significantly reduced when mice were administered the ADAM17 inhibitor (Fig. 5, A and B). It is important to note that previous work has shown Nox4 exists in a conformation that allows for spontaneous transfer of electrons from NADPH to FAD conferring constitutive activity; hence, the enzyme is regulated partially at the level of protein expression with an increase in Nox4 protein expression translating to an increase in enzymatic activity (46). NADPH oxidase activity was also measured using a lucigenin-enhanced chemiluminescence assay. There was a significant decrease in NADPH-dependent superoxide production in kidney cortex homogenate from TMI-005-treated OVE26 mice compared with kidney cortex from the OVE26-vehicle-treated mice (Fig. 5C). These data suggest that ADAM17 is an upstream mediator of the Nox oxidases in the diabetic kidney.
Glucose-induced ADAM17 activation mediates Nox4 protein expression and NADPH oxidase activity in MCTs.
After determining that glucose increased ADAM17 activity in mouse proximal tubular epithelial cells and that ADAM17 mediated amplified Nox4 protein expression and NADPH oxidase activity in diabetic mouse kidney cortex, we sought to determine if glucose is the factor involved in ADAM17-dependent NADPH oxidase activity and Nox4 protein expression. MCTs were pretreated with 1 μM TMI-005 before exposure to 25 mM d-glucose for 4 h. ADAM17 inhibition blocked glucose-induced Nox4 protein expression (Fig. 6, A and B). Concomitant with the increase in Nox4 protein expression, NADPH oxidase activity was significantly increased after 4-h exposure to high glucose (Fig. 6C). When MCTs were pretreated with TMI-005, there was an attenuation of the glucose-induced NADPH oxidase activity (Fig. 6C). Consistent with our pharmacological inhibitor results, siRNA-mediated knockdown of ADAM17 resulted in a reduction of glucose-induced Nox4 protein expression by Western blot analysis (Fig. 6, D and E). It is also noteworthy that ADAM17 knockdown also resulted in a reduction in basal Nox4 levels (Fig. 6, D and E). Finally, NADPH oxidase activity was measured in MCT cells transfected with nontargeting or ADAM17 siRNA with or without 4-h high glucose treatment (Fig. 6F). Basal NADPH-dependent superoxide production was diminished when ADAM17 was knocked down and siRNA-mediated knockdown of ADAM17 significantly decreased high glucose-induced NADPH oxidase activity (Fig. 6F). Taken together these data indicate that glucose is responsible for ADAM17-dependent increase in Nox4 expression and NADPH oxidase activity.
DISCUSSION
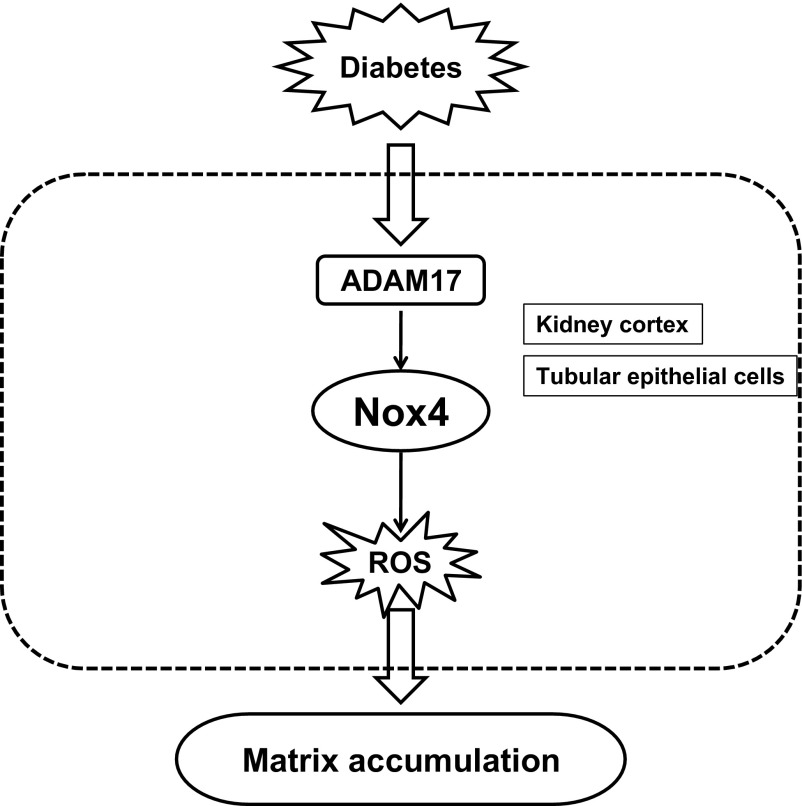
This study provides strong evidence that hyperglycemia in diabetes results in the activation of the metalloprotease ADAM17 to increase matrix protein expression in kidney cortex. Furthermore, ADAM 17 appears to exert its effect on matrix accumulation through upregulation of Nox4 and increased generation of reactive oxygen species. Our conclusions are based on the following findings: ADAM17 is upregulated and activated in the kidney cortex of mice with type 1 diabetes; and inhibition of ADAM17 downregulated Nox4 expression, decreased NADPH oxidase activity, and reduced fibronectin expression. In cultured proximal tubular epithelial cells, high glucose concentration activated ADAM17, increased Nox4 protein expression and NADPH oxidase activity, and increased the expression of matrix proteins. The increase in fibronectin expression or cellular collagen content observed after glucose exposure was reversed when ADAM17 or Nox4 was blocked with pharmacological inhibitors and/or siRNA-mediated knockdown. This report is the first to show that the MMP ADAM17 is activated in the kidney in diabetes and that it acts upstream of Nox4 to enhance matrix accumulation (Fig. 7).
Fig. 7.
Proposed mechanism of HG/diabetes-induced extracellular matrix accumulation in proximal tubular epithelial cells. See discussion for details.
Nox4 involvement in ECM accumulation has been reported by several groups (2, 6, 8, 22, 38, 44). Additionally, there is evidence that ROS activates ADAM17 (16, 37, 49). However, only one publication has shown upregulation of NADPH oxidases and subsequent ROS production, which results in shedding of known ADAM17 substrates (36). In this particular report, rat liver hepatocytes exposed to the growth factor TGFβ exhibited an increase in HB-EGF and TGFα transcript levels. Pretreatment with the flavoprotein inhibitor diphenyleneiodonium or the NADPH oxidase inhibitor apocynin reversed the increase in TGFα and HB-EGF mRNA (36). To the best of our knowledge, the present study provides the first evidence that ADAM17 also functions as an upstream regulator of Nox enzymes. ADAM17 activity is required for high glucose-induced Nox4 protein expression and subsequent ROS production. Note that ADAM17 appears to regulate Nox4 protein expression. This is important since Nox4 is constitutively active (2) and thereby ROS production by Nox4 is governed by changes in its expression levels. Utilizing pharmacologic and genetic approaches, we show that inhibition of ADAM17 in cultured tubular cells decreases Nox4 expression/activity and reduces matrix protein expression. Of interest is that while administration of ADAM17 inhibitor to the diabetic mice blocked enzyme activity, ADAM17 protein levels in the kidney cortex increased. It is possible that the inhibitor stabilizes enzyme protein levels by inhibiting its degradation by the proteasome; other mechanisms cannot be excluded at this time.
The mechanism by which ADAM17 regulates Nox oxidases in the diabetic kidney cortex or in response to glucose in cultured tubular cells remains to be determined. It is likely that ADAM17 activation by glucose results in cleavage of growth factors that in turn activate and upregulate Nox4. A potential direct effect of ADAM17 on the oxidase is unlikely but cannot be excluded.
Glucose increases ADAM17 activity and/or EGFR transactivation causing increased ECM or fibrotic factors within the kidney (48, 51–53). Exposure of primary cultures of rat mesangial cells to a high concentration of d-glucose activates ADAM17 and results in an increase in TGFβ promoter activity and an increase in TGFβ transcripts (48). Additional data in mesangial cells suggest that the increase in collagen I and fibronectin expression in response to glucose depends on the activation of ADAM17, EGFR transactivation, and activation of downstream signals such as the phosphoinositide 3-kinase-Akt pathway (48, 51–53). Other than these limited publications, glucose-induced ADAM17 expression or activity influencing the progression of DN has not been thoroughly investigated.
In addition to high glucose, several groups demonstrated a role for ADAM17 in mediating the profibrotic effects of angiotensin II (ANG II). In 2005, Lautrette et al. (29) published a seminal article linking ANG II-induced EGFR transactivation to ADAM17. Chronic infusion of ANG II in mice resulted in glomerulosclerosis and interstitial fibrosis, while mice deficient in EGFR (dominant negative isoform of EGFR) or TGFα−/− mice were protected from these lesions. Importantly, ANG II-induced renal lesions were reduced in wild-type mice administered a pharmacological inhibitor to ADAM17, WTACE2 (29). Interestingly, it appears that there may be cross-talk between glucose and AngII signaling in EGFR transactivation. In vascular smooth muscle cells cultured with high glucose, EGFR glycosylation increased. HG sensitized EGFR transactivation through AngII-mediated angiotensin type 1 receptor activation (27).
In conclusion, this study establishes a role for ADAM17 activation in ECM accumulation in DN in vivo and in mouse proximal tubular epithelial cells exposed to HG. Additionally, it provides a novel mechanism whereby the activation of ADAM17 in diabetes or in a high glucose medium induces oxidative stress through the upregulation of Nox4 protein expression and increased NADPH oxidase activity contributing to ECM accumulation.
GRANTS
Support for these studies was provided by the following sources: Veteran Administration Merit Review grant; National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-078971 (to H. E. Abboud) and DK-079996 (to Y. C. Gorin); and Juvenile Diabetes Research Foundation multi-project grant (to H. E. Abboud, Y. C. Gorin, and J. L. Barnes).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.M.F., A.A.E., M.G., J.L.B., Y.C.G., and H.E.A. conception and design of research; B.M.F. and A.A.E. performed experiments; B.M.F., J.L.B., and Y.C.G. analyzed data; B.M.F., A.A.E., M.G., J.L.B., and Y.C.G. interpreted results of experiments; B.M.F. prepared figures; B.M.F. drafted manuscript; M.G., Y.C.G., and H.E.A. edited and revised manuscript; H.E.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to acknowledge Pfizer-Wyeth Pharmaceuticals for providing TMI-005 inhibitor and Katalin Suztak for mouse podocytes used in our studies. We would also like to thank Chakradhar Velagapudi, Aaron Carranza, Andrea Barrentine-Fourcaudot, Sergio Garcia, and Fredyne Springer for technical assistance.
REFERENCES
- 1.Akool e Gauer S, Osman B, Doller A, Schulz S, Geiger H, Pfeilschifter J, Eberhardt W. Cyclosporin A and tacrolimus induce renal Erk1/2 pathway via ROS-induced and metalloproteinase-dependent EGF-receptor signaling. Biochem Pharmacol 83: 286–295, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Barnes JL, Gorin Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79: 944–956, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell HL, Gooz M. ADAM-17 is activated by the mitogenic protein kinase ERK in a model of kidney fibrosis. Am J Med Sci 339: 105–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385: 729–733, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283: 24061–24076, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal 8: 1497–1508, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol 21: 93–102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58: 2396–2401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell KM, Nemo R, Sweeney WE, Jr, Levin JI, Frost P, Avner ED. A novel inhibitor of tumor necrosis factor-alpha converting enzyme ameliorates polycystic kidney disease. Kidney Int 60: 1240–1248, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dey M, Baldys A, Sumter DB, Gooz P, Luttrell LM, Raymond JR, Gooz M. Bradykinin decreases podocyte permeability through ADAM17-dependent epidermal growth factor receptor activation and zonula occludens-1 rearrangement. J Pharmacol Exp Ther 334: 775–783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med 29: 258–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285: 37503–37512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein PN, Overbeek PA, Means AR. Calmodulin-induced early-onset diabetes in transgenic mice. Cell 58: 1067–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest 115: 3494–3505, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer OM, Hart S, Gschwind A, Prenzel N, Ullrich A. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol Cell Biol 24: 5172–5183, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J Biol Chem 278: 37459–37464, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol 45: 146–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooz M, Gooz P, Luttrell LM, Raymond JR. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem 281: 21004–21012, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gooz P, Dang Y, Higashiyama S, Twal WO, Haycraft CJ, Gooz M. A disintegrin and metalloenzyme (ADAM) 17 activation is regulated by alpha5beta1 integrin in kidney mesangial cells. PLoS One 7: e33350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280: 39616–39626, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, Kefalides NA, Neilson EG. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 107: 1359–1368, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J 321: 265–279, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper NM, Turner AJ. Protein processing mechanisms: from angiotensin-converting enzyme to Alzheimer's disease. Biochem Soc Trans 28: 441–446, 2000 [PubMed] [Google Scholar]
- 26.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol 20: 1223–1235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi A, Berk BC. Epidermal growth factor receptor transactivation is regulated by glucose in vascular smooth muscle cells. J Biol Chem 278: 35049–35056, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA 96: 3922–3927, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Levin JI, Chen JM, Laakso LM, Du M, Schmid J, Xu W, Cummons T, Xu J, Jin G, Barone D, Skotnicki JS. Acetylenic TACE inhibitors. Part 3: Thiomorpholine sulfonamide hydroxamates. Bioorg Med Chem Lett 16: 1605–1609, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mariappan MM, Feliers D, Mummidi S, Choudhury GG, Kasinath BS. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes 56: 476–485, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem 62: 515–541, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van Goor H. ADAM17 upregulation in human renal disease: a role in modulating TGF-alpha availability? Am J Physiol Renal Physiol 297: F781–F790, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385: 733–736, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Mulder GM, Melenhorst WB, Celie JW, Kloosterhuis NJ, Hillebrands JL, Ploeg RJ, Seelen MA, Visser L, van Dijk MC, van Goor H. ADAM17 upregulation in renal transplant dysfunction and non-transplant-related renal fibrosis. Nephrol Dial Transplant 27: 2114–2122, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Murillo MM, Carmona-Cuenca I, Del Castillo G, Ortiz C, Roncero C, Sanchez A, Fernandez M, Fabregat I. Activation of NADPH oxidase by transforming growth factor-beta in hepatocytes mediates upregulation of epidermal growth factor receptor ligands through a nuclear factor-kappaB-dependent mechanism. Biochem J 405: 251–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers TJ, Brennaman LH, Stevenson M, Higashiyama S, Russell WE, Lee DC, Sunnarborg SW. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol Biol Cell 20: 5236–5249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.New DD, Block K, Bhandhari B, Gorin Y, Abboud HE. IGF-I increases the expression of fibronectin by Nox4-dependent Akt phosphorylation in renal tubular epithelial cells. Am J Physiol Cell Physiol 302: C122–C130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Reddy AB, Ramana KV, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology 150: 63–74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem 275: 14608–14614, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Richards WG, Sweeney WE, Yoder BK, Wilkinson JE, Woychik RP, Avner ED. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J Clin Invest 101: 935–939, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci 112: 3603–3617, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299: F1348–F1358, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Serino M, Menghini R, Fiorentino L, Amoruso R, Mauriello A, Lauro D, Sbraccia P, Hribal ML, Lauro R, Federici M. Mice heterozygous for tumor necrosis factor-alpha converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 56: 2541–2546, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchiyama-Tanaka Y, Matsubara H, Nozawa Y, Murasawa S, Mori Y, Kosaki A, Maruyama K, Masaki H, Shibasaki Y, Fujiyama S, Nose A, Iba O, Hasagawa T, Tateishi E, Higashiyama S, Iwasaka T. Angiotensin II signaling and HB-EGF shedding via metalloproteinase in glomerular mesangial cells. Kidney Int 60: 2153–2163, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Uttarwar L, Peng F, Wu D, Kumar S, Gao B, Ingram AJ, Krepinsky JC. HB-EGF release mediates glucose-induced activation of the epidermal growth factor receptor in mesangial cells. Am J Physiol Renal Physiol 300: F921–F931, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Robertson JD, Walcheck B. Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J Biol Chem 286: 38980–38988, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf G, Ziyadeh FN. Molecular mechanisms of diabetic renal hypertrophy. Kidney Int 56: 393–405, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Wu D, Peng F, Zhang B, Ingram AJ, Gao B, Krepinsky JC. Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia 50: 2008–2018, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Krepinsky JC. PKC-beta1 mediates glucose-induced Akt activation and TGF-beta1 upregulation in mesangial cells. J Am Soc Nephrol 20: 554–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE, Gao B, Kumar S, Krepinsky JC. EGFR-PLCgamma1 signaling mediates high glucose-induced PKCbeta1-Akt activation and collagen I upregulation in mesangial cells. Am J Physiol Renal Physiol 297: F822–F834, 2009 [DOI] [PubMed] [Google Scholar]