Abstract
The process of renal regeneration after acute kidney injury is thought to recapitulate renal development, and proliferation of renal proximal tubular cells (RPTCs) is a critical step in the regenerative response. Recent studies indicate that class I histone deacetylases (HDACs) are required for embryonic kidney gene expression, growth, and differentiation. The role and underlying mechanisms of class I HDAC activation in RPTC proliferation, however, remain unclear. In this study, we used cultured RPTCs to examine this issue since four class I HDAC isoforms (1, 2, 3, and 8) are abundantly expressed in this cell type. Blocking class I HDAC activity with a highly selective inhibitor, MS-275, induced global histone H3 hyperacetylation, reduced RPTC proliferation, and diminished expression of cyclin D1 and proliferating cell nuclear antigen. Silencing HDAC1, 3, or 8 with small interfering RNA resulted in similar biological effects. Activation of epidermal growth factor receptor (EGFR) and signal transducers and activators of transcription 3 (STAT3) was required for RPTC proliferation, and STAT3 functioned downstream of EGFR. Treatment with MS-275 or knockdown of HDAC1, 3, or 8 suppressed EGFR expression and phosphorylation, and silencing HDAC1 and 3 also reduced STAT3 phosphorylation. However, HDAC2 downregulation did not affect RPTC proliferation and phosphorylation of EGFR and STAT3. Collectively, these data reveal a critical role of class I HDACs in mediating proliferation of renal epithelial cells through activation of the EGFR/STAT3 signaling pathway.
Keywords: class I histone deacetylases, renal epithelial cells, proliferation, epidermal growth factor receptor, signal transducers and activators of transcription 3
acute kidney injury (AKI) is a serious problem among hospitalized patients characterized by an abrupt decrease in glomerular filtration and associated with severe morbidity and mortality. It can be induced by multiple causes such as ischemia/reperfusion (I/R), trauma, sepsis, rhabdomyolysis, and nephrotoxin exposure (31, 47). After injury, surviving tubular cells are thought to undergo three steps in the regenerative processes, dedifferentiation, migration, and proliferation that restore renal tubular epithelium integrity (4). The kidney possesses a remarkable regenerative capacity under conditions of mild injury. Recent fate mapping studies indicated that renal repair and regeneration after AKI occur predominantly by proliferation of surviving intrinsic tubular epithelial cells (13). Thus elucidating signaling events that regulate renal tubular epithelial cell proliferation is critical for understanding the regulatory mechanism of renal regeneration after AKI.
Recent studies have indicated that the activity of histone deacetylases (HDACs) is required for growth and differentiation of the embryonic kidney and regulation of essential genes involved in these processes (6, 28). HDACs are a group of enzymes that deacetylate lysine residues in histones as well as nonhistones. HDACs are classified into four groups: class I HDACs contain HDAC1, 2, 3, and 8; class II HDACs include HDAC 4, 5, 6, 7, 9, and 10; class III HDACs consist of SIRT1–7; and class IV HDACs contain HDAC 11. Among these HDACs, class I HDACs, in particular, HDAC1 and 2, have been shown to be critically involved in the regulation of a number of key developmental pathways (6). Inhibition of class I HDAC activity by HDAC inhibitors, such as MS-275, Mgcd0103, and SK7041, also induces cell cycle arrest and suppresses cell proliferation in various cell types (19, 26, 42). This suggests the essential role of class I HDACs in the regulation of cell proliferation.
How class I HDACs act on cell proliferation is incompletely understood. Several studies indicated that inhibition of HDAC activity reduces the expression and activation of epidermal growth factor receptor (EGFR) in tumor cells (7, 27). EGFR is a transmembrane receptor with intrinsic tyrosine kinase activity that has been shown to be involved in renal development and regeneration. For example, inactivation of EGFR suppresses branching of cultured ureteric bud (50). Also, genetic disruption of EGFR in mice leads to epithelial development defects in several organs including kidney (45). Genetic reduction of EGFR activity or conditional knockout of EGFR in renal proximal tubular cells delayed renal functional recovery and lessened the regenerative response in animal models of AKI induced by insults that included mercury chloride, folic acid, or I/R (5, 10, 46). EGFR activation requires interaction with multiple ligands. Ligand binding to EGFR induces phosphorylation of specific tyrosine residues within its cytoplasmic domains, which serve as docking sites for signaling molecules. This in turn initiates activation of multiple intracellular signaling pathways, including the signal transducers and activators of transcription 3 (STAT3) pathway. Activated STAT3 translocates to the nucleus, where it drives expression of numerous genes needed for several biological cellular responses, including cell proliferation (14, 39, 49). One of the target genes of STAT3 is cyclin D, which is critically involved in cell cycle progression (41).
We have recently demonstrated that HDAC1 and HDAC2, two isoforms of class I HDACs, mediate proliferation of cultured renal interstitial fibroblasts, expression of cell cycle proteins, and activation of STAT3 (37). Blocking class I HDACs with MS-275 also inhibits renal fibroblast activation and STAT3 phosphorylation and attenuates development of renal interstitial fibrosis in a murine model of unilateral ureteral obstruction (25). These studies suggest that class I HDAC activity is required for activation and proliferation of renal interstitial fibroblasts. It remains unknown whether class I HDACs are needed for the regulation of renal epithelial cell proliferation and, if so, what mechanisms are involved. In this study, we investigated the effect of class I HDAC inhibition on proliferation of cultured mouse renal proximal tubular cells (RPTCs) and activation of the EGFR/STAT3 signaling pathway.
MATERIALS AND METHODS
Chemicals and antibodies.
Antibodies to p-EGFR, p-STAT3, STAT3, cyclin D1, acetyl-histone H3, cleaved caspase-3, and cleaved poly(ADP-ribose)polymerase (PARP) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to EGFR, HDAC1, HDAC2, HDAC3, HDAC8, proliferating cell nuclear protein (PCNA), and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). MS-275, anti-α-tubulin antibody, and all other chemicals were from Sigma (St. Louis, MO).
Cell culture and treatments.
Immortalized mouse RPTCs were kindly provided by Dr. E. Bello-Reuss (9) and cultured in DMEM (Sigma-Aldrich, St. Louis, MO) containing 5% FBS, 0.5% penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. To determine the effect of MS-275 on tubular cell proliferation, MS-275 was directly added to subconfluent RPTCs and then incubated for 48 h. For proliferation studies, cells were seeded in 12-well plates. Gefitinib and S3I-201 were added directly to subconfluent RPTCs for the indicated concentration as described (see Fig. 7) and incubated for 48 h. Control cells were treated with an equivalent volume of vehicle.
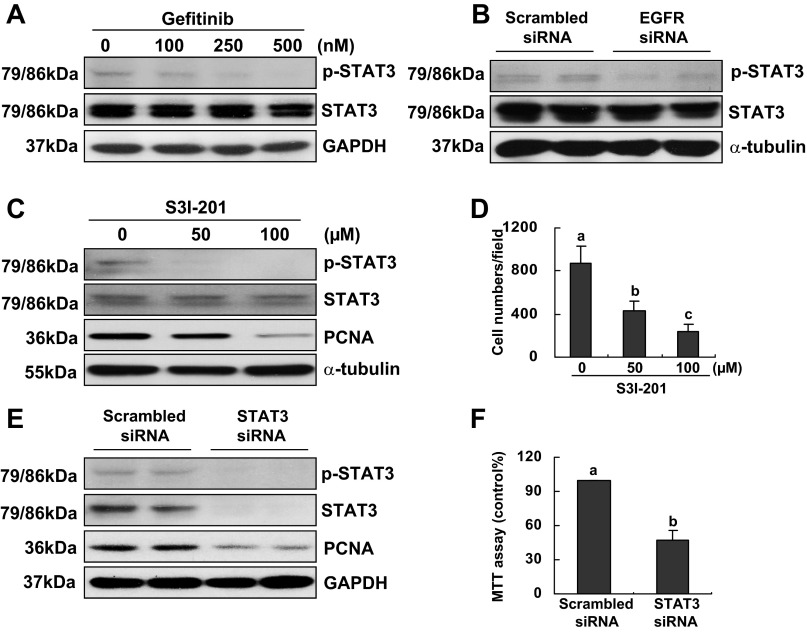
Fig. 7.
Effect of inhibition or knockdown of EGFR on signal transducers and activators of transcription 3 (STAT3) activation and the effect of S3I-201 on RPTC proliferation. RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of gefitinib (100–500 nM; A) or transfected with scrambled siRNA or specific siRNA to EGFR (B) or STAT3 (E and F) and incubated for 48 h in the same medium. Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to p-STAT3, STAT3, and GAPDH or α-tubulin. Representative immunoblots from 3 or more experiments are shown (A and B). RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of S3I-201 (100 and 200 μM). Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to p-STAT3, STAT3, PCNA, and α-tubulin. Representative immunoblots from three or more experiments are shown (C and E). Cell proliferation was determined by counting cell numbers (D and F). Means with different superscript letters are represented as significant difference from one another (P < 0.05).
Cell proliferation.
Cell proliferation was assessed by the 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and cell counting under a microscopy. MTT was added to individual cultures for 1 h at the end of experiments (final concentration, 0.5 mg/ml). Dimethyl sulfoxide released tetrazolium. The optical density was detected with a Spectramax M5 plate reader (Molecular Devices, Sunnyvale, CA) at 570 nm. For counting cell numbers, at least five pictures from each well of a 12-well plate were taken and cell numbers in five wells were counted for a sample under the microscope.
Transfection of small interfering RNA into cells.
The small interfering (si)RNA oligonucleotides targeted specifically to HDAC1, HDAC2, HDAC3, or HDAC8 were used in this study. RPTCs were seeded to 30–40% confluence in antibiotic-free medium, and 24 h later siRNA (750 pmol) was transfected into cells with Lipofectamine 2000 (Invitrogen). In parallel, scrambled siRNA (750 pmol) was used to control for off-target changes in RPTCs. After transfection, cells were cultured in antibiotic-free DMEM-F-12 with 5% FBS for 48 h before being used for the experiments.
Immunoblot analysis.
After treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Equal amounts of cellular protein lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% skim milk for 1 h in room temperature, membranes were incubated with primary antibodies overnight at 4°C and then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h in room temperature. Bound antibodies were visualized by chemiluminescence detection on autoradiographic films. The densitometry analysis of immunoblot results was conducted with NIH Image software (National Institutes of Health, Bethesda, MD).
Immunofluorescent staining.
Cells cultured on coverslips were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and then incubated 30 min in PBS containing 5% BSA. Cells were then treated with primary antibodies at 4°C overnight. After being washed with PBS, cells were exposed to Texas red-labeled anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA) for 1 h at room temperature. Morphological analysis was performed by using light and fluorescent microscopy.
Statistical analysis.
Data depicted in graphs represent the means ± SE for each group. Comparisons between intergroups were made using one-way ANOVA. Multiple means were compared using Tukey's test. The differences between two groups were conducted by Student t-test. Statistical significant difference were considered at P < 0.05.
RESULTS
Expression and location of class I HDACs in RPTCs.
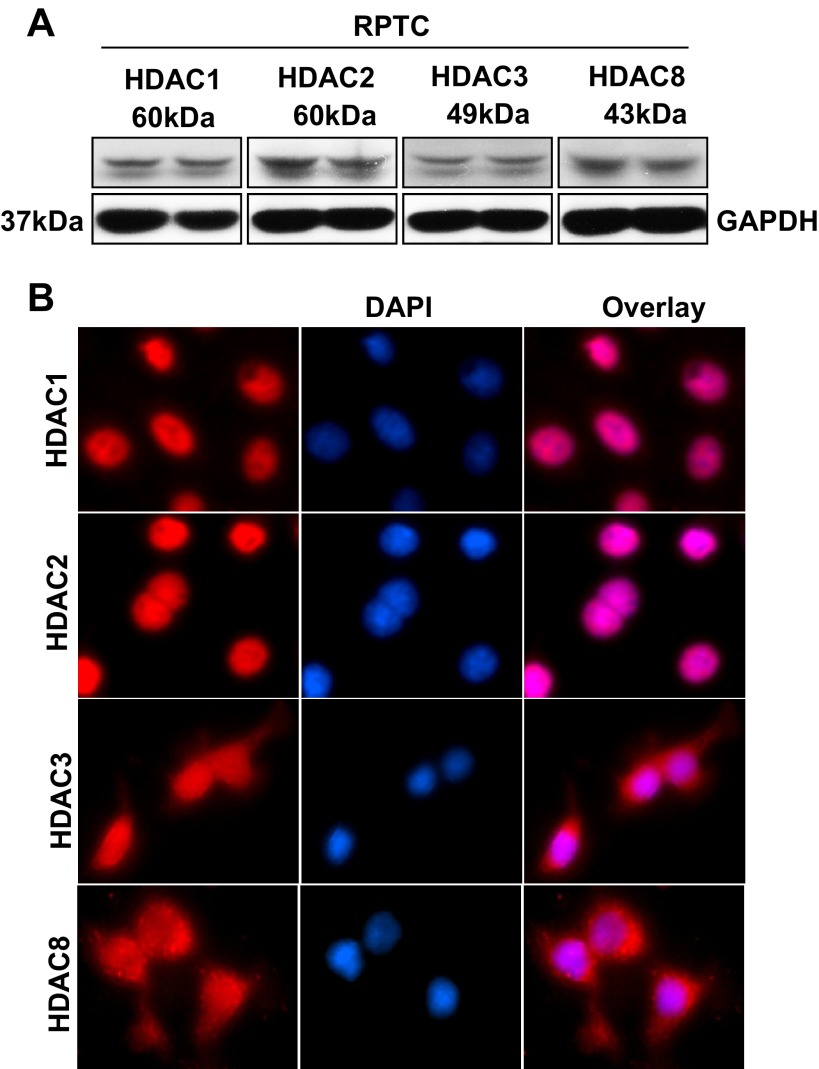
Recent studies demonstrated that class I HDACs are required for kidney development and proliferation of embryonic proximal tubular cells (6). As the first step towards understanding the role of class I HDACs in renal tubular cell proliferation, we examined their expression in RPTCs. Immunoblot analysis indicated that HDAC1, HDAC2, HDAC3, and HDAC8 were abundantly expressed in this cell type (Fig. 1A). Immunofluorescent staining demonstrated that HDAC1 and HDAC2 were only located in the nuclei, whereas HDAC3 and HDAC8 were distributed in both the cytoplasm and nucleus of RPTCs (Fig. 1B). These data illustrated that class I HDACs are expressed in renal epithelial cells, with HDAC1 and HDAC2 located in the nucleus whereas HDAC3 and HDAC8 were in both the nucleus and cytoplasm.
Fig. 1.
Expression of histone deacetylase (HDAC)1, 2, 3, and 8 in cultured renal proximal tubular cells (RPTCs). Cultured RPTCs were harvested and cell lysates were subjected to immunoblot analysis with antibodies against HDAC1, 2, 3, or, 8 and GAPDH. Representative immunoblot from 3 or more experiments are shown (A). RPTCs were seeded on coverslips and then cultured with 5% FBS for 24 h. Cells were stained with antibodies against HDAC1, 2, 3, or 8 (×200) and DAPI (B).
Class I HDAC activity is required for renal epithelial cell proliferation.
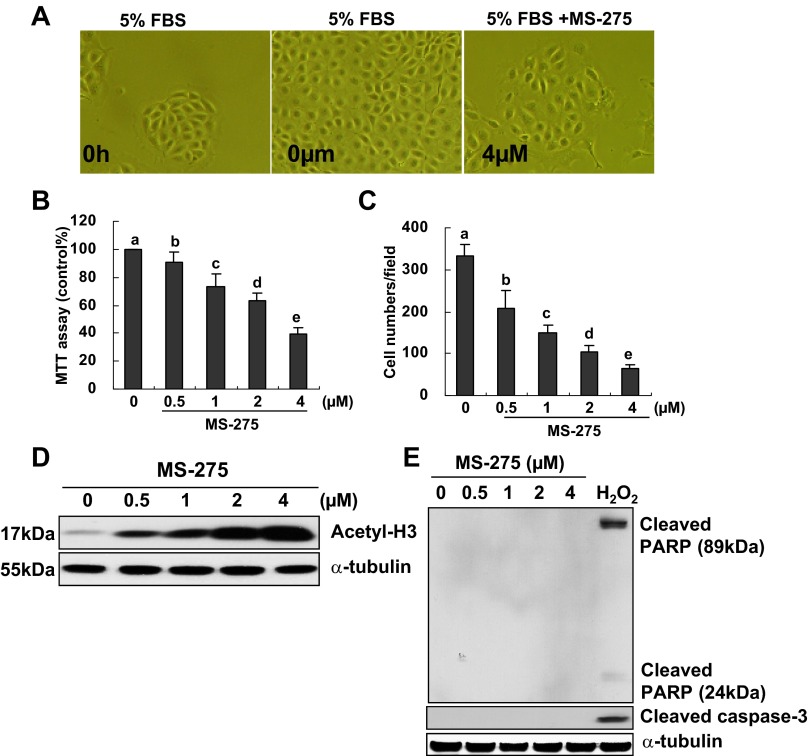
MS-275 is a highly selective inhibitor for class I HDACs, which preferentially inhibits HDAC1, 2, and 3 with an ED50 value from 0.18∼2.3 μM (11, 16). To demonstrate the role of class I HDACs in renal epithelial cell proliferation, MS-275 (0.5–4 μM) was added to normally cultured RPTCs and incubated for 48 h. Cell proliferation was then measured by the MTT assay and cell counting. As shown in Fig. 2, A–C, MS-275 treatment did not change the morphology of RPTCs but significantly reduced cell proliferation in a dose-dependent manner with the maximum inhibition at 4 μM. MS-275 dose dependently inhibited class I HDAC activity as indicated by increased expression of acetylated histone H3 (acetyl-H3; Fig. 2D). MS-275 at these doses (0.5–4 μM) did not cause apoptosis of RPTCs as shown by absence of cleaved PARP and cleaved caspase-3 (Fig. 2E). As a positive control, we also treated cells with a potent inducer of apoptosis, H2O2. In response to this oxidative stress, cleaved PARP and cleaved caspase-3 were clearly induced in RPTCs (Fig. 2E). These data indicate that activation of class I HDACs is necessary for proliferation of renal epithelial cells.
Fig. 2.
Effect of MS-275 on RPTC proliferation. RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of MS-275 (0.5–4 μM; A–E). Cells were treated with H2O2 (1 mM) for 4 h as positive control of apoptosis (E). Phase-contrast photographs (×200) showing the effect of MS-275 on cell proliferation (A). Cell proliferation was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (B) and cell counting (C). Data are expressed as means ± SE. Means with different superscript letters are represented as significant difference from one another (P < 0.05). Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to acetylated histone H3 (acetyl-H3) and α-tubulin (D) or cleaved poly(ADP-ribose)polymerase (PARP), cleaved caspase-3, and α-tubulin (E). Representative immunoblots from 3 or more experiments are shown.
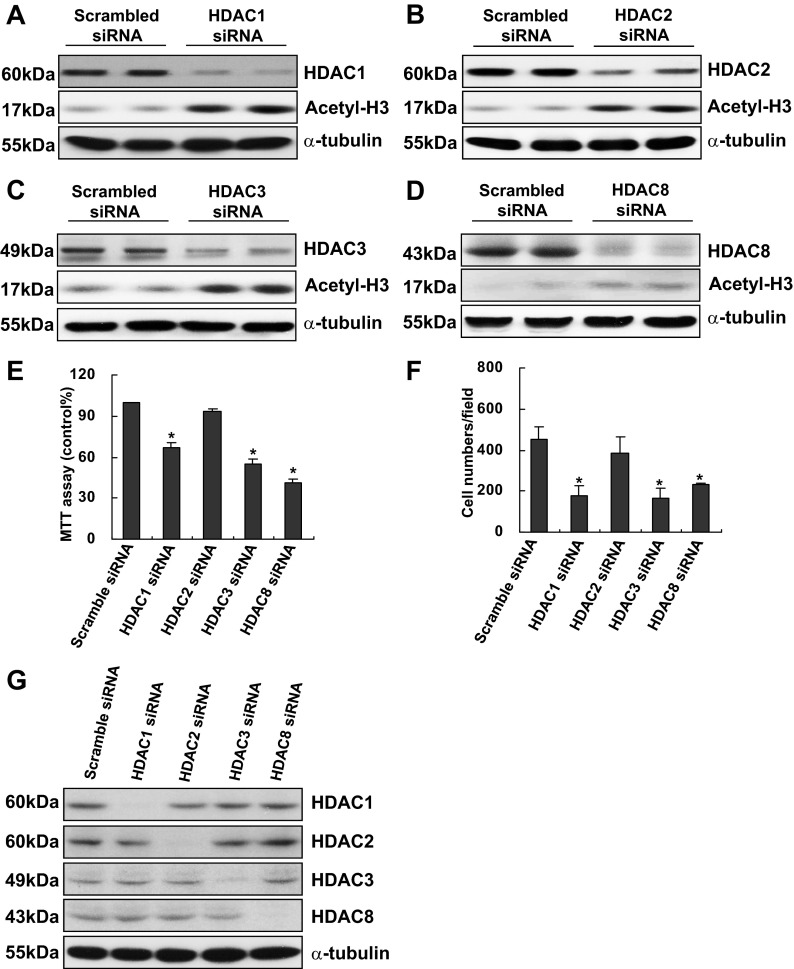
Knockdown of HDAC1, HDAC3, and HDAC8, but not HDAC2, reduces RPTC proliferation.
Given the importance of class I HDACs in regulating RPTC proliferation, we further examined which isoforms of HDACs are responsible for this biological process by using siRNA interference technique. Transfection of isoform-specific siRNA led to a similar decline in the expression of individual class I HDACs and an increase in the expression of acetyl-H3, indicating the effectiveness of these siRNA (Fig. 3, A–D). Figure 3, E and F, demonstrated that silencing HDAC1, 3, or 8, significantly reduced RPTC proliferation, whereas knockdown of HDAC2 had no such an effect, as measured by both the MTT assay and cell counting. Figure 3F shows that a specific siRNA for a class I HDAC does not affect the other three class I HDACs, indicative of the specificity of these siRNAs. These data suggest that HDAC1, 3, and 8, but not HDAC2, activation is required for RPTC proliferation.
Fig. 3.
Effect of small interfering (si)RNA specific to HDAC1, 2, 3, or 8 on RPTC proliferation. RPTCs were transfected with scrambled siRNA or specific siRNA to HDAC1, 2, 3, or 8, respectively, and incubated for 48 h in the DMEM with 5% FBS. Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to HDAC1, 2, 3, or 8 and acetyl-H3 or α-tubulin. Representative immunoblots from 3 or more experiments are shown (A–D and G). Cell proliferation was assessed by the MTT assay (E) and counting cells (F). Data are expressed as mean ± SE. *P < 0.05, significantly different from controls.
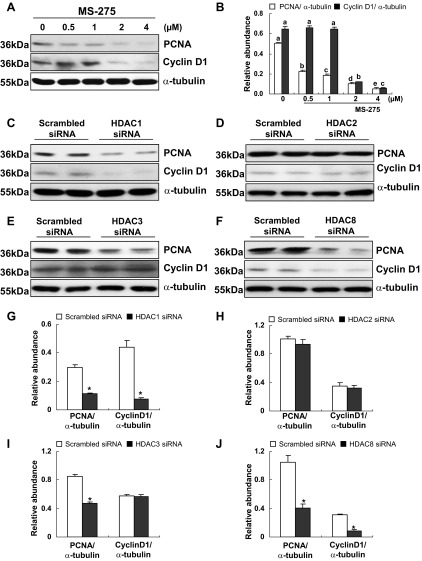
Class I HDACs regulate the expression of cell cycle proteins.
PCNA is a cell proliferation marker and is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle (17). Cyclin D1 is another nuclear protein critically involved in cell cycle G1/S transition (34). To confirm the functional significance of class I HDACs in RPTC proliferation, we further examined the effect of MS-275 and class I HDAC isoform-specific siRNA on the expression of PCNA and cyclin D1. As shown in Fig. 4, A and B, exposure of RPTCs to MS-275 resulted in a significant decrease in the expression of PCNA and cyclin D1 in a dose dependent manner. Silencing of HDAC1 and 8 also inhibited expression of PCNA and cyclin D1 (Fig. 4, C, F, G, and J). However, depletion of HDAC3 reduced expression of PCNA, but not cyclin D1 (Fig. 4, E and I). Knockdown of HDAC2 had no effect on expression of PCNA or cyclin D1 (Fig. 4, D and H). These data suggest that HDAC 1, 3, and 8 are required for regulation of PCNA while HDAC1 and 8 are associated with expression of cyclin D1.
Fig. 4.
Effects of MS-275 or knockdown of HDAC1, 2, 3, or 8 on the expression of proliferating cell nuclear antigen (PCNA) and cyclin D1 in RPTCs. RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of MS-275 (0.5 to 4 uM). Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies against PCNA, cyclin D1, or α-tubulin. Representative immunoblots from 3 or more experiments are shown (A). Expression levels of PCNA and cyclin D1 were calculated by densitometry and normalized with α-tubulin (B). Data are expressed as means ± SE. Means with different superscript letters are represented as significant difference from one another (P < 0.05). RPTCs were transfected with scrambled siRNA or specific siRNA to HDAC1, 2, 3, or 8 and incubated for 48 h in the DMEM with 5% FBS. Prepared cell lysates were subjected to immunoblot analysis with antibodies to PCNA, cyclin D1, or α-tubulin. Representative immunoblots from 3 or more experiments are shown (C–F). Expression levels of PCNA and cyclin D1 were calculated by densitometry and normalized with α-tubulin (G–J). Data are expressed as means ± SE. *P < 0.05, significantly different from controls.
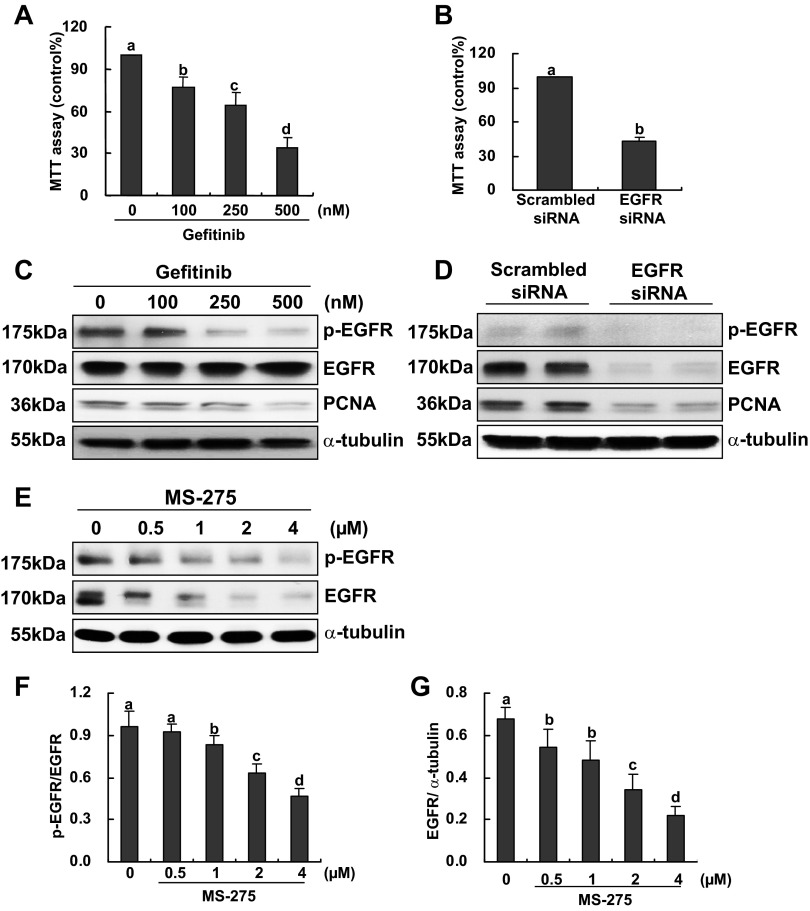
Role of EGFR in RPTC proliferation and effect of MS-275 on EGFR expression and phosphorylation.
Our previous studies demonstrated that EGFR is expressed in primary cultured rabbit RPTCs and is required for their proliferation and migration (53). It was also reported that inhibition of HDAC activity with trichostatin A reduced phosphorylation and expression of EGFR in a number of tumor cells (7), suggesting the importance of HDAC activation in maintaining EGFR stability. We hypothesized that class I HDACs also play a role in regulating EGFR phosphorylation and expression in renal epithelial cells. To test this hypothesis, we first attempted to ensure the role of EGFR in mouse RPTC proliferation. Figure 5A showed that incubation of RPTCs with gefitinib, a specific inhibitor of EGFR, inhibited RPTC proliferation in a dose dependent fashion. Silencing EGFR with siRNA also reduced RPTC proliferation (Fig. 5B). Consistently, gefitinib treatment dose dependently suppressed phosphorylation and expression of EGFR as well as expression of PCNA. Similarly, EGFR knockdown resulted in a dramatic decrease in PCNA expression as well as EGFR phosphorylation. These results confirm the role of EGFR in regulating mouse RPTC proliferation.
Fig. 5.
Effect of epidermal growth factor receptor (EGFR) inhibition or knockdown on RPTC proliferation and the effect of MS-275 on EGFR expression and phosphorylation. RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of gefitinib (100 to 500 nM; A and C) or transfected with scrambled siRNA or specific siRNA to EGFR and incubated for 48 h in the same medium (B and D). Cell proliferation was determined by the MTT assay (A and B). Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to p-EGFR, EGFR, PCNA, or α-tubulin. Representative immunoblots from 3 or more experiments are shown (C and D). RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of MS-275 (0.5–4 uM). Prepared cell lysates were subjected to immunoblot analysis with antibodies against p-EGFR, EGFR, and α-tubulin. Representative immunoblots from 3 or more experiments are shown (E). Expression levels of p-EGFR, EGFR, and α-tubulin were calculated by densitometry and the ratio between p-EGFR and total EGFR was determined (F). Total EGFR levels were normalized with α-tubulin (G). Data are expressed as mean ± SE. Means with different superscript letters are represented as significant difference from one another (P < 0.05).
Next, we examined the effect of class I HDAC inhibition on EGFR expression and phosphorylation. As shown in Fig. 5, E–G, treatment with MS-275 not only suppressed EGFR expression but also inhibited its phosphorylation. Calculation of the ratio of p-EGFR to total EGFR indicated that EGFR dephosphorylation is not only due to reduced expression of total EGFR but that phosphorylation itself is also inhibited.
Taken together, these data suggest that class I HDAC-mediated RPTC proliferation is associated with activation of EGFR signaling.
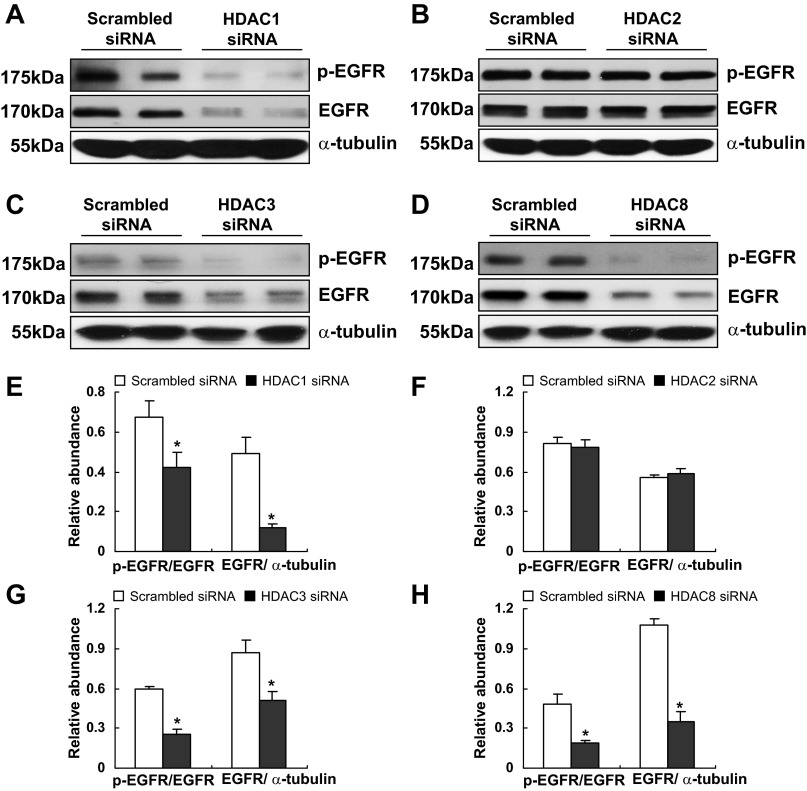
Knockdown of HDAC1, 3, and 8, but not 2, inhibits the phosphorylation and expression of EGFR in RPTCs.
To determine the isoform of class I HDACs responsible for EGFR phosphorylation and expression, we further examined the effect of isoform-specific siRNA on the expression and phosphorylation of EGFR. As shown in Fig. 6, phosphorylation and expression levels of EGFR were significantly reduced in RPTCs when HDAC1, 3, and 8 were specifically silenced with individual siRNA, whereas knockdown of HDAC2 had no such effects. Hence, we suggest that activation and expression of EGFR is controlled by HDAC 1, 3, and 8 but not HDAC 2.
Fig. 6.
Effect of knockdown of HDAC1, 2, 3, or 8 on the phosphorylation and expression of EGFR. RPTCs were transfected with scrambled siRNA or specific siRNA to HDAC1, 2, 3, or 8 and incubated for 48 h in the DMEM with 5% FBS. Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies to p-EGFR, EGFR, and α-tubulin. Representative immunoblots from 3 or more experiments are shown (A–D). Expression levels of p-EGFR, EGFR, and α-tubulin were calculated by densitometry and the ratio between p-EGFR and total EGFR was determined (E–H). Total EGFR levels were normalized with α-tubulin (E–H). *P < 0.05, significantly different from controls.
EGFR mediates phosphorylation of STAT3 in RPTCs.
It has been reported that STAT3 is a transcriptional factor and its activation leads to expression of cyclin D1 and cell cycle progression in a variety of cell type (20, 23, 30, 43). To understand whether STAT3 acts downstream of EGFR to regulate cell proliferation, cultured RPTCs were treated with different doses (100–500 nM) of gefitinib for 48 h and then harvested to examine expression levels of p-STAT3 and total STAT3. As shown in Fig. 7A, gefitinib dose dependently inhibited expression of p-STAT3 whereas expression of total STAT3 was not affected. Similar results were observed in RPTCs transfected with EGFR siRNA (Fig. 7B). To confirm the role of STAT3 in RPTC proliferation, we also examined the effect of S3I-201 and STAT3 silencing on proliferation of RPTCs. Figure 7, C and D, showed that treatment of RPTCs with S3I-201 at 50 and 100 μM remarkably suppressed STAT3 phosphorylation and largely reduced expression of PCNA and proliferation of RPTCs. Knockdown of STAT3 with siRNA also significantly decreased PCNA expression and RPTC proliferation (Fig. 7, E and F), which was consistent with our previous observations (51). Collectively, we suggest that STAT3 is critically involved in transducing EGFR activation to RPTC proliferation.
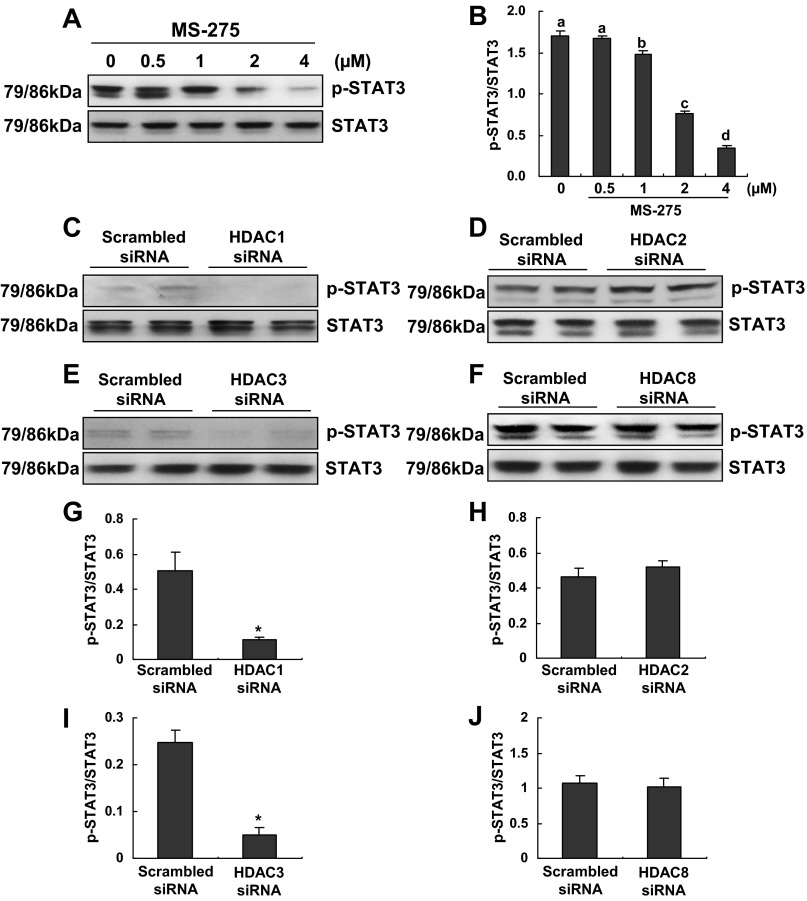
HDAC1 and 3 but not HDAC2 and 8 mediate STAT3 phosphorylation.
We further examined the role of class I HDACs in regulating STAT3 phosphorylation in RPTCs. Treatment of cells with MS-275 resulted in decreased phosphorylation of STAT3 in a dose-dependent manner (Fig. 8, A and B). Knockdown of HDAC1 and 3 also reduced STAT3 phosphorylation (Fig. 8, C, E, G, and I). However, HDAC2 and 8 downregulation did not affect STAT3 phosphorylation (Fig. 8, D, F, H, and J). In addition, neither MS-275 nor all isoform-specific siRNA affected expression of total STAT3. These data indicate that HDAC 1 and 3, but not HDAC2 and 8, are involved in the regulation of STAT3 activation.
Fig. 8.
Effect of MS-275 or knockdown of HDAC1, 2, 3, or 8 on the activation of STAT3. RPTCs were cultured in the DMEM with 5% FBS for 48 h in the absence or presence of MS-275 (0.5–4 uM; A and B) or transfected with scrambled siRNA or specific siRNA to HDAC1, 2, 3 or 8 and then incubated for 48 h in the DMEM with 5% FBS (C–F). Cells were harvested and cell lysates were subjected to immunoblot analysis with antibodies against p-STAT3 and STAT3. Representative immunoblots from 3 or more experiments are shown (A and C–F). The expression levels of p-STAT3 and STAT3 were calculated by densitometry, and the ratio between p-STAT3 and total STAT3 was determined (B and G–J). Data are expressed as means ± SE. Means with different superscript letters are represented as significant difference from one another (P < 0.05). *P < 0.05, significantly different from controls.
DISCUSSION
Proliferation is one of major regenerative responses of renal tubular cells after AKI. However, the regulatory mechanism of this process remains incompletely understood. In this study, we demonstrated that inhibition of class I HDAC activity with the selective inhibitor MS-275 and specific silencing of class I HDAC1, 3, and 8 isoenzymes with siRNA reduced proliferation of cultured RPTCs and expression of cell cycle proteins. Inhibition of class I HDACs also resulted in decreased phosphorylation and expression of EGFR and reduced phosphorylation of STAT3. Furthermore, blockade of EGFR suppressed phosphorylation of STAT3. Since both EGFR and STAT3 are required for renal tubular cell proliferation, these data suggest that class I HDAC activity is required for renal epithelial cell proliferation and activation of the EGFR/STAT3 signaling pathway.
Although it is well documented that blockade of class I HDACs can suppress cell cycle progression and cell proliferation in tumor cells (22, 38, 40, 42), their role in regulating proliferation of normal renal tubular cells is unclear. Recently, Chen et al. (6) revealed the importance of HDAC activity in the regulation of kidney gene expression, growth, and differentiation using mouse embryonic kidney. They demonstrated that class HDAC1–3 are highly expressed in nephron precursors and their inhibition or downregulation reduced proliferative activity of embryonic kidney cells during nephron differentiation. They further found that inhibition of HDACs with either Scriptaid, a general inhibitor of HDACs, or MS-275 reduced metanephric growth and blocked some essential genes involved in renal growth and differentiation. Since renal regeneration after AKI is a process similar to renal development, it is possible that class I HDACs are also involved in the regulation of renal regeneration. In support of this hypothesis, we revealed that blocking class I HDAC activity with MS-275 inhibited proliferation of renal epithelial cells, a primary regenerative response after AKI.
Our studies indicated that all class I HDAC isoforms are abundantly expressed in RPTCs but play distinct roles in regulating RPTC proliferation. Silencing HDAC1, 3, and 8 isoforms reduced RPTC proliferation, whereas HDAC2 knockdown did not affect this process. The involvement of HDAC1 in RPTC proliferation is consistent with its critical role in regulating cell proliferation in other cell types in vitro and in vivo. For example, HDAC1-deficient embryos and embryonic stem cells are characterized by an overall reduced cellular proliferation rate (18); deletion of HDAC1, but not of HDAC2, caused a partial G1 arrest in mouse embryonic fibroblasts (48, 54); further, targeted deletion of HDAC1 in mice results in an early embryonic lethal phenotype due to severe developmental defects (18). HDAC3 has been reported to be acquired for normal mitotic process and cell cycle progression in mouse embryonic fibroblasts (2). HDAC8 has also been shown to play a role in the regulation of proliferation of sympathetic neuroblastoma cells (33). In contrast to the developmental significance of these three class I HDACs, the effect of HDAC2 gene deletion was much less. In primary mouse embryonic fibroblasts, deletion of HDAC2 has little or no effect on proliferation (48). In embryonic stem cell, loss of HDAC2 did not affect embryonic stem cell differentiation (8). However, selective inhibition of HDAC2, but not HDAC1, can increase the sensitivity of breast cancer cells to tamoxifen treatment (3) or topoisomerase inhibitor induced apoptosis (29). Thus HDAC2 seems to have a rather specific antiapoptotic function in cancer cells. Whether or not HDAC2 activation is also implicated to renal tubular cell survival needs further investigation.
Currently, the mechanism by which class I HDACs mediate proliferation is incompletely understood. It was reported that inhibition of HDAC activity downregulates the expression of EGFR in tumor cells (7). EGFR is a receptor tyrosine kinase that is critically implicated in kidney development and renal regeneration after AKI. Chen et al. (5) have recently demonstrated that recovery of renal function was markedly slowed following I/R injury in mice with a specific EGFR deletion in the renal proximal tubule or when given erlotinib, a specific EGFR inhibitor. We further showed that genetic and pharmacologic blockage of EGFR resulted in inhibition of renal tubular cell dedifferentiation and proliferation in a murine model of AKI induced by folic acid (10). In the current study, we examined the possible implication of class I HDACs in mediating expression and phosphorylation of EGFR in normally cultured RPTCs. Our results showed that inhibition of class I HDACs by MS-275 reduced both phosphorylation and expression of EGFR. Individual knockdown of class I HDAC isoforms indicated that silencing of HDAC1, 3, and 8, but not HDAC2, reduced the expression levels of p-EGFR and total EGFR in RPTCs. These data are consistent with the role of individual class I HDACs in the regulation of RPTC proliferation and suggest the importance of EGFR signaling in mediating growth-promoting actions of class I HDACs in renal epithelial cells.
Phosphorylation of EGFR results in activation of several intracellular signaling pathways including STAT3. STAT3 activation is associated with cell cycle progression and cell proliferation in a number of cell types, including epithelial cells (1, 32, 51, 52). Our previous studies indicated that knockdown of STAT3 by siRNA largely blocked RPTC proliferation (51), suggesting involvement of STAT3 in this process. In this study, we further demonstrated that inhibition of EGFR with a specific inhibitor, gefitinib, or specific siRNA suppressed STAT3 phosphorylation. In addition, blockade of STAT3 activation with S3I-201 or siRNA diminished RPTC proliferation, confirming that STAT3 acts downstream of EGFR to mediate RPTC proliferation. Supporting the idea that class I HDACs regulate RPTC proliferation through the EGFR/STAT3 signaling pathway, we also found that treatment with the class I HDAC inhibitor MS-275 dose dependently suppressed STAT3 phosphorylation in RPTCs. Furthermore, siRNA-mediated knockdown of HDAC1 and 3 leads to suppression of p-STAT3 levels. Although HDAC8 is also implicated in the regulation of EGFR expression, knockdown of this HDAC isoenzyme did not significantly affect STAT3 phosphorylation, suggesting that in addition to STAT3, EGFR also signals to other intracellular signaling pathways associated with cell proliferation. Additional studies are needed to address this issue.
It is well known that after AKI the kidney can either be completely or incompletely repaired, depending on the severity of injury (44). In the case of mild injury, the renal repair usually results in a return to a normal structural and functional state; however, severe injury is frequently accompanied by the development of renal fibrosis (44). Previously, our in vitro and in vivo data showed that class I HDACs and STAT3 are critically involved in the differentation of renal interstitial fibroblasts to myofibroblasts and the development of renal fiobrosis in a murine model of unilateral ureteral obstruction (25, 35, 36). Here, we showed that class I HDAC-mediated STAT3 activation is required for proliferation of renal epithelial cells. These data suggest that activation of class I HDACs and STAT3 contributes to both renal fibrogenesis and regeneration. In line with involvement of HDACs in renal regenerative response, other studies also showed that conditional knockownof STAT3 or pharmacological inhibition of HDACs with suberoylanilide hydroxyamic acid suppressed hepatocyte cell proliferation during liver regeneration after partial hepatectomy (12, 15, 21). Since our data indicate that EGFR activity is required for STAT3 phosphorylation and that class I HDACs mediates EGFR expression and phosphorylation, it is likely that EGFR serves as an intermediate for class I HDAC regulation of STAT3 activation. Further studies are needed to investigate the role of HDACs and STAT3 in renal regeneration and their relation to EGFR activity in animal models of AKI.
In conclusion, our studies demonstrated for the first time that class I HDAC activity is required for proliferation of renal epithelial cells as evidenced by the fact that inhibition of class I HDACs by the selective inhibitor MS-275 or knockdown of some specific class I HDAC isoforms decreases RPTC proliferation. Furthermore, the mechanism involved in this process is associated with suppression of the EGFR/STAT3 signaling pathway. This study provides new insights on the mechanism of renal tubular cell proliferation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-085065 (to S. Zhuang), National Nature Science Foundation of China (81270778 to S. Zhuang and 81170638 to H. Yan), and Pudong New District Foundation of China (PWZxk2010-02).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.T., T.C.Z., H.Y., and S.Z. conception and design of research; J.T. and Y.Y. performed experiments; J.T. and S.Z. prepared figures; J.T. and S.Z. drafted manuscript; G.B. and S.Z. edited and revised manuscript; S.Z. analyzed data; S.Z. interpreted results of experiments; S.Z. approved final version of manuscript.
REFERENCES
- 1. Arakawa T, Masaki T, Hirai T, Doi S, Kuratsune M, Arihiro K, Kohno N, Yorioka N. Activation of signal transducer and activator of transcription 3 correlates with cell proliferation and renal injury in human glomerulonephritis. Nephrol Dial Transplant 23: 3418–3426, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, Hiebert SW. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell 30: 61–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bicaku E, Marchion DC, Schmitt ML, Munster PN. Selective inhibition of histone deacetylase 2 silences progesterone receptor-mediated signaling. Cancer Res 68: 1513–1519, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14, Suppl 1: S55–61, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Chen JK, Harris RC. Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S, Bellew C, Yao X, Stefkova J, Dipp S, Saifudeen Z, Bachvarov D, El-Dahr SS. Histone deacetylase (HDAC) activity is critical for embryonic kidney gene expression, growth, and differentiation. J Biochem 286: 32775–32789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chou CW, Wu MS, Huang WC, Chen CC. HDAC inhibition decreases the expression of EGFR in colorectal cancer cells. PLoS One 6: e18087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dovey OM, Foster CT, Cowley SM. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. Proc Natl Acad Sci USA 107: 8242–8247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 10. He S, Liu N, Bayliss G, Zhuang S. EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid-induced acute kidney injury. Am J Physiol Renal Physiol 304: F356–F366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu E, Dul E, Sung CM, Chen Z, Kirkpatrick R, Zhang GF, Johanson K, Liu R, Lago A, Hofmann G, Macarron R, de los Frailes M, Perez P, Krawiec J, Winkler J, Jaye M. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther 307: 720–728, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Barr E, Rudnick DA. Characterization of the regulation and function of zinc-dependent histone deacetylases during rodent liver regeneration. Hepatology 57: 1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Ke Q, Yang RN, Ye F, Wang YJ, Wu Q, Li L, Bu H. Impairment of liver regeneration by the histone deacetylase inhibitor valproic acid in mice. J Zhejing Univ Sci B 13: 695–706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 409: 581–589, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kisielewska J, Lu P, Whitaker M. GFP-PCNA as an S-phase marker in embryos during the first and subsequent cell cycles. Biol Cell 97: 221–229, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee KW, Kim JH, Park JH, Kim HP, Song SH, Kim SG, Kim TY, Jong HS, Jung KH, Im SA, Kim NK, Bang YJ. Antitumor activity of SK-7041, a novel histone deacetylase inhibitor, in human lung and breast cancer cells. Anticancer Res 26: 3429–3438, 2006 [PubMed] [Google Scholar]
- 20. Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, Sakamaki T, Pestell R, Bromberg J. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res 66: 2544–2552, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biochem 277: 28411–28417, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Lin WH, Martin JL, Marsh DJ, Jack MM, Baxter RC. Involvement of insulin-like growth factor-binding protein-3 in the effects of histone deacetylase inhibitor MS-275 in hepatoma cells. J Biochem 286: 29540–29547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu B, Ren Z, Shi Y, Guan C, Pan Z, Zong Z. Activation of signal transducers and activators of transcription 3 and overexpression of its target gene cyclin D1 in laryngeal carcinomas. Laryngoscope 118: 1976–1980, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Liu N, He S, Ma L, Ponnusamy M, Tang J, Tolbert E, Bayliss G, Zhao TC, Yan H, Zhuang S. Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling. PLoS One 8: e54001, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S, Cheng H, Kwan W, Lubieniecka JM, Nielsen TO. Histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in clear cell sarcoma models. Mol Cancer Ther 7: 1751–1761, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Liu W, Fan LX, Zhou X, Sweeney WE, Jr, Avner ED, Li X. HDAC6 regulates epidermal growth factor receptor (EGFR) endocytic trafficking and degradation in renal epithelial cells. PloS One 7: e49418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Majumdar G, Adris P, Bhargava N, Chen H, Raghow R. Pan-histone deacetylase inhibitors regulate signaling pathways involved in proliferative and pro-inflammatory mechanisms in H9c2 cells. BMC Genomics 13: 709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchion DC, Bicaku E, Turner JG, Schmitt ML, Morelli DR, Munster PN. HDAC2 regulates chromatin plasticity and enhances DNA vulnerability. Mol Cancer Ther 8: 794–801, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res 62: 3351–3355, 2002 [PubMed] [Google Scholar]
- 31. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol 520: 4294–4311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oehme I, Deubzer HE, Wegener D, Pickert D, Linke JP, Hero B, Kopp-Schneider A, Westermann F, Ulrich SM, von Deimling A, Fischer M, Witt O. Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res 15: 91–99, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Ouyang W, Ma Q, Li J, Zhang D, Liu ZG, Rustgi AK, Huang C. Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res 65: 9287–9293, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268 [DOI] [PubMed] [Google Scholar]
- 37. Pang M, Ma L, Liu N, Ponnusamy M, Zhao TC, Yan H, Zhuang S. Histone deacetylase 1/2 mediates proliferation of renal interstitial fibroblasts and expression of cell cycle proteins. J Cell Biochem 112: 2138–2148, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JH, Jung Y, Kim TY, Kim SG, Jong HS, Lee JW, Kim DK, Lee JS, Kim NK, Bang YJ. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Can Res 10: 5271–5281, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Senese S, Zaragoza K, Minardi S, Muradore I, Ronzoni S, Passafaro A, Bernard L, Draetta GF, Alcalay M, Seiser C, Chiocca S. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol 27: 4784–4795, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shields BJ, Tiganis T. Replication checkpoint control by a PTK/STAT3/cyclin D1 axis. Cell Cycle 8: 223–230, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Sikandar S, Dizon D, Shen X, Li Z, Besterman J, Lipkin SM. The class I HDAC inhibitor MGCD0103 induces cell cycle arrest and apoptosis in colon cancer initiating cells by upregulating Dickkopf-1 and non-canonical Wnt signaling. Oncotarget 1: 596–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19: 5419–5427, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Tang J, Liu N, Zhuang S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Bernard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol 14: 3147–3154, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol 165: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi T, Cubizolles F, Zhang Y, Reichert N, Kohler H, Seiser C, Matthias P. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev 24: 455–469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Zhang Z, Pascuet E, Hueber PA, Chu L, Bichet DG, Lee TC, Threadgill DW, Goodyer P. Targeted inactivation of EGF receptor inhibits renal collecting duct development and function. J Am Soc Nephrol 21: 573–578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Z, Xing J, Ma L, Gong R, Chin YE, Zhuang S. Transglutaminase-1 regulates renal epithelial cell proliferation through activation of Stat-3. J Biol Chem 284: 3345–3353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao SH, Zhao F, Zheng JY, Gao LF, Zhao XJ, Cui MH. Knockdown of stat3 expression by RNAi inhibits in vitro growth of human ovarian cancer. Radiol Oncol 45: 196–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhuang S, Dang Y, Schnellmann RG. Requirement of the epidermal growth factor receptor in renal epithelial cell proliferation and migration. Am J Physiol Renal Physiol 287: F365–F372, 2004 [DOI] [PubMed] [Google Scholar]
- 54. Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, Rembold M, Meunier D, Egger G, Lagger S, Chiocca S, Propst F, Weitzer G, Seiser C. The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Mol Cell Biol 30: 1171–1181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]