Abstract
To investigate the responses of descending vasa recta (DVR) to deformation of the abluminal surface, we devised an automated method that controls duration and frequency of stimulation by utilizing a stream of buffer from a micropipette. During stimulation at one end of the vessel, fluorescent responses from fluo4 or bis[1,3-dibutylbarbituric acid-(5)] trimethineoxonol [DiBAC4(3)], indicating cytoplasmic calcium ([Ca2+]CYT) or membrane potential, respectively, were recorded from distant cells. Alternately, membrane potential was recorded from DVR pericytes by nystatin whole cell patch-clamp. Mechanical stimulation elicited reversible [Ca2+]CYT responses that increased with frequency. Individual pericyte responses along the vessel were initiated within a fraction of a second of one another. Those responses were inhibited by gap junction blockade with 18 β-glycyrrhetinic acid (100 μM) or phosphoinositide 3 kinase inhibition with 2-morpholin-4-yl-8-phenylchromen-4-one (50 μM). [Ca2+]CYT responses were blocked by removal of extracellular Ca2+ or L-type voltage-gated channel blockade with nifedipine (10 μM). At concentrations selective for the T-type channel blockade, mibefradil (100 nM) was ineffective. During mechanostimulation, pericytes rapidly depolarized, as documented with either DiBAC4(3) fluorescence or patch-clamp recording. Single stimuli yielded depolarizations of 22.5 ± 2.2 mV while repetitive stimuli at 0.1 Hz depolarized pericytes by 44.2 ± 4.0 mV. We conclude that DVR are mechanosensitive and that rapid transmission of signals along the vessel axis requires participation of gap junctions, L-type Ca2+ channels, and pericyte depolarization.
Keywords: rat, kidney, medulla, microcirculation, electrophysiology, calcium
descending vasa recta (DVR) are ∼15-μm vessels that supply the renal medulla with blood flow to accommodate countercurrent exchange, nutrient supply, and oxygenation. They are comprised of a continuous endothelium and a surrounding layer of contractile pericytes that respond to many autocrine and paracrine agents (4, 5, 7, 28, 30, 37). We demonstrated that DVR are mechanosensitive and release nitric oxide (NO) in response to luminal flow and wall stretch (44, 49). They conduct electrical signals along their axis and gap junction proteins, connexins 37, 40, 43, and 45 are present in the kidney vasculature and DVR wall (14, 15, 44). Voltage clamp depolarizations of the endothelial layer reveal large capacitance transients reflecting simultaneous access of the patch electrode to adjacent cells. Similarly, introduction of Lucifer yellow to the endothelial cytoplasm leads to rapid spread between cells along the vessel axis. Similar experiments in DVR pericytes revealed lesser cell-to-cell coupling between pericytes or between pericytes and endothelium (44).
To further investigate vascular responses to cell deformation, we devised a method to automate abluminal mechanostimulation of DVR under computer control. Brief streams of buffer are forced from the ∼1-μm orifice of a patch-clamp pipette onto the vessel surface for specified duration and frequency. Imaging of fluo4 fluorescence along the vessel axis during stimulation revealed that cytoplasmic calcium ([Ca2+]CYT) transients are largely confined to pericytes rather than endothelia. Individual pericytes along the vessel axis respond coincidently, within milliseconds of one another. The [Ca2+]CYT responses increase with stimulation frequency and can be reversibly prevented by gap junction blockade, removal of extracellular Ca2+, inhibition of L-type voltage-gated Ca2+ channels (CaV) with nifedipine, or kinase blockade with LY294002. Graded depolarization occurs in distant pericytes in proportion to the magnitude and frequency of mechanical stimulation.
METHODS
Isolation of DVR.
Investigations involving animal use were performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland. Kidneys were harvested from Sprague-Dawley rats (120–200 g; Harlan) that had been anesthetized by an intraperitoneal injection of ketamine/xylazine (80 mg/kg/10 mg/kg). Tissue slices were stored at 4°C in a physiological saline solution (PSS; in mM) 145 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES, 10 glucose, pH 7.4 at room temperature. For patch-clamp studies, wedges of renal medulla were enzymatically digested in Blendzyme 1 (0.27 mg/ml, Roche) in high-glucose DMEM media without inclusion of serum (Invitrogen) at 37°C for 30 min. In all cases, whether enzymatic digestion was used for patch-clamp, medullary tissue was transferred to PSS and held at 4°C. At intervals, DVR were isolated by hand dissection and transferred to an inverted microscope (Nikon TE300) for fluorescence microscopy or electrophysiological measurements. Immobilization on coverslip glass was achieved by scrubbing the glass with soap and water using a small piece of flexible silicone tubing cut at an angle and placed on the end of a yellow pipette tip. DVR adhere well to clean glass without the need for precoating.
Mechanostimulation.
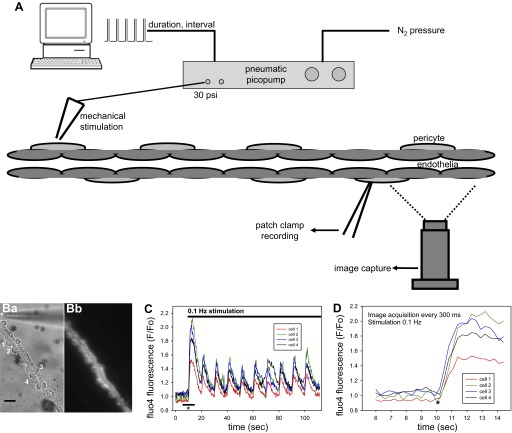
A diagram of the method used to automate mechanostimulation of isolated DVR is illustrated in Fig. 1A. A patch-clamp micropipette (orifice ∼1 μm) containing PSS was positioned close to an immobilized DVR near a pericyte cell body on the abluminal wall. The pipette was pressurized by World Precision Instruments PV830 pneumatic picopump. The PV830 regulates pressure to an adjustable level and applies it to the micropipette in response to either manual trigger or computer-controlled digital pulse trains. In these studies, the stimulation pipette was pressurized to a predefined level, generally 30 PSI, for 1 s. The stimulation protocol was programmed using the digital output of a Digidata interface with pClamp software (Molecular Devices). The tip of the stimulation pipette was positioned within a few microns of the DVR surface at one end of the vessel. Motion artifacts occurred only adjacent to the stimulation pipette. Elsewhere, visible cell motion or individual cell contractions were not observed. Away from the stimulation pipette tip, stable measurements are possible by either fluorescence imaging or patch-clamp recording. In vessels that are immobilized without luminal microperfusion, quantifiable wall motions are not observable even when contractile agonists are applied. This permits quantification of fluorescence in regions of interest. Moreover, long-term stable gigaseals needed for electrophysiological recording during mechanical stimulation herein, or in the presence of contractile agonists, are possible (24, 25, 45).
Fig. 1.
Automated mechanical stimulation of isolated descending vasa recta (DVR). A: DVR is stimulated by puffs of buffer released from the orifice of a patch-clamp pipette positioned within a few microns of the vessel wall. Pressurization of the pipette occurs when an internal valve of a pneumatic picopump is opened either by manual control or by a programed train of pulses from a computer-controlled analog-to-digital interface. In the latter case, duration of the pulse and pulse frequency (interpulse interval) are programmable. The pressure is supplied from a nitrogen tank and regulated to a set level (generally 30 PSI) by the picopump. Responses of the vessel are recorded at sites distant from the stimulation pipette either by loading fluorescent probes and performing digital image acquisitions or by electrophysiological recording from an abluminal pericyte. B: white light (Ba) and fluo4 fluorescent (Bb) images are shown along with adjacent numbering of 4 pericytes from which fluorescence was quantified by “region of interest” (ROI) image analysis. The tip of the stimulation pipette (*) is observed at the top portion of the image near one end of the vessel. The black bar is 10 μm. C: responses of the individual pericytes to stimulation are displayed as normalized fluorescence (F/Fo, ordinate) where Fo is the fluorescent signal from the first image. At 10 s (abscissa, *), stimulation begins at a rate of 1 every 10 s (0.1 Hz, pulse duration 1 s). The separate cytoplasmic calcium ([Ca2+]CYT) responses of the 4 pericytes are coincident. The black bar shows the region of the abscissa that is expanded to generate D. D: data show coincidence of onset of the separate [Ca2+]CYT responses of the 4 pericytes.
Fluorescence microscopy.
As previously described, we recorded propagated [Ca2+]CYT transients along the DVR wall with the Ca2+-sensitive fluorescent probe, fluo4 (45). As a single wavelength probe with bright emissions, fluo4 enabled image acquisition to be performed at a rapid rate. Fluo4 (Molecular Probes) was loaded by incubation of the AM ester (2 μmol/l, 20 min, 37°C) and excited at 485 nm (DeltaRam, PTI). Fluorescent emissions were monitored at 530 nm (B-2E/C filter cube, Nikon) using a Nikon ×60 1.45 N.A. plan apo oil immersion objective. Using fluo4 it was possible to capture sequential images at 300-ms intervals with a low-light CCD camera with on-chip multiplication gain (Photometrics Cascade 512B, Roper Scientific) using ImageMaster software (PTI). The [Ca2+]CYT transients were quantified off-line from the images by specifying regions of interest (ROI) in NIH ImageJ. In some experiments, pericyte membrane potential was recorded using the voltage-sensitive fluorescent probe bis[1,3-dibutylbarbituric acid-(5)] trimethineoxonol [DiBAC4(3); 5 μM], using methods we previously described (47). DiBAC4(3) was excited at 485 nm, using a DeltaRAM illuminator (PTI). Fluorescent emission at 530 nm was isolated with a band pass filter (Omega Optical, Brattleboro, VT).
Whole cell patch-clamp recording.
Patch pipettes were made from borosilicate glass (PG52151-4, external diameter 1.5 mm, internal diameter 1.0 mm; World Precision Instruments, Sarasota, FL), using a two-stage vertical pipette puller (Narshige PP-830) and heat polished. Membrane potential was measured using the nystatin-perforated patch configuration by zero current clamp recording (19) as previously described (25, 50). For patch-clamp, the electrode solution was (in mmol/l) 120 Kaspartate, 20 KCl, 10 NaCl, 10 HEPES, pH 7.2, and nystatin (100 μg/ml with 0.1% DMSO) in ultrapure water. Recordings were obtained with a CV201AU headstage and Axopatch 200 amplifier (Molecular Devices, Foster City, CA). Patch-clamp pipettes used for mechanostimulation (Fig. 1) were filled with PSS.
Reagents.
Fluo4 and DiBAC4(3) were from Molecular Probes. 18 β-Glycyrrhetinic acid (18βGRA), 2-morpholin-4-yl-8-phenylchromen-4-one (LY294002) nystatin, collagenase 1A, protease XIV, and other chemicals were from Sigma (St. Louis, MO). Liberase Blenzyme 1 was from Roche Applied Science. 18βGRA was dissolved in DMSO. Reagents were thawed and diluted on the day of the experiment and excess was discarded daily. Blendzyme was stored in 40-μl aliquots of 4.5 mg/ml in water and diluted into high-glucose DMEM lacking serum on the day of the experiment.
Statistics.
Data in the text and figures are reported as means ± SE. The significance of differences was evaluated with SigmaStat 3.11 (Systat Software, Point Richmond, CA) using parametric or nonparametric tests as appropriate for the data. Comparisons between two groups were performed with Student's t-test (paired or unpaired, as appropriate) or the Rank Sum Test (nonparametric). Comparisons between multiple groups were performed with repeated-measures ANOVA, or repeated-measures ANOVA on ranks (nonparametric). Post hoc comparisons were performed using Tukey's test. P < 0.05 was used to reject the null hypothesis.
RESULTS
DVR [Ca2+]CYT responses occur in pericytes.
Abluminal mechanostimulation led to elevations of [Ca2+]CYT along the vessel axis. Figure 1 illustrates the method and typical responses. Figure 1Ba shows a white-light image of a DVR on a coverslip with abluminal pericyte cell bodies labeled 1–4. A corresponding fluorescent image is provided as Fig. 1Bb. The fluo-4 [Ca2+]CYT response of each cell is displayed in Fig. 1C as fluo4 fluorescence normalized to that quantified from the first image (Fo). During stimulation at 0.1 Hz (duration 1 s, every 10 s), simultaneous elevations of [Ca2+]CYT occur in the four pericytes. The region of the abscissa defined by the horizontal dark line at the onset of stimulation in Fig. 1C is expanded as Fig. 1D to show that individual pericyte [Ca2+]CYT responses begin within a fraction of a second of one another (Fig. 1D, *).
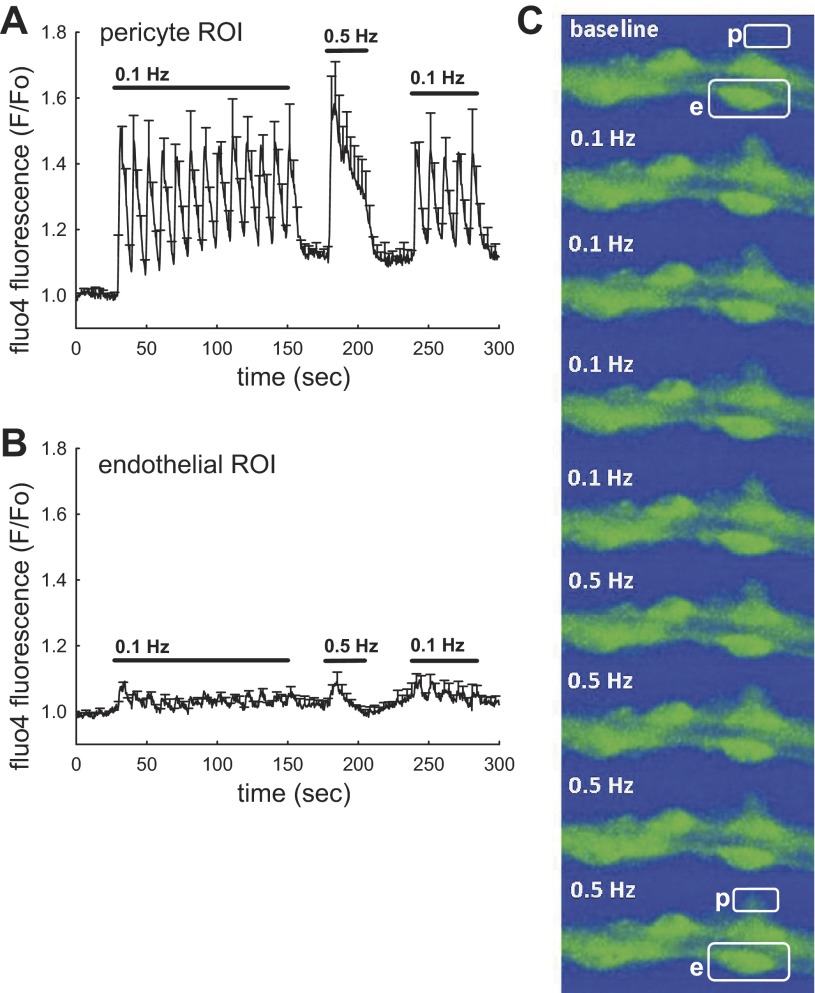
As shown in Fig. 2, when the frequency of stimulation was increased from 0.1 to 0.5 Hz, the [Ca2+]CYT response was sustained rather than oscillatory. Figure 2A shows normalized fluo4 fluorescence from pericytes, analyzed by placing ROI over abluminal protrusions of the pericyte cell bodies. The means ± SE of pericyte responses are shown at baseline and during subsquent applications of 0.1- and 0.5-Hz stimulations. At 0.1 Hz, the pericyte [Ca2+]CYT repsonses are rapidly reversible, nearly returning to baseline. In contrast, more rapid 0.5-Hz stimulation (1-s duration separated by 1-s interval) leads to larger and more persistent [Ca2+]CYT elevations. Endothelial effects are shown in Fig. 2B where fluorescence has been quantified by placing ROI over adjacent endothelial cell bodies. The image analysis is exemplified in Fig. 2C where “p” and “e” represent a pericyte and an endothelial cell, respectively. We cannot rule out that the small [Ca2+]CYT elevations in Fig. 2B arise from fluo4 fluorescence emanating from pericytes that wrap their extensions around the vessel wall. Nonetheless, it is clear from comparison of the data in Fig. 2, A and B, derived from the same series of vessels, that the predominant [Ca2+]CYT elevations occur in pericytes and not the endothelium. This is illustrated by the succession of images in Fig. 2C where a fluorescing pericyte (p) is nearly invisible at baseline but appears during 0.1- or 0.5-Hz stimulation. Fluorescence of an adjacent endothelial cell (e) is greater at onset, but remains stable. As we previously described, the greater baseline fluorescence of endothelia occurs because they load fluorescent dyes by deesterification more efficiently than the adjacent pericytes (26, 27).
Fig. 2.
Frequency dependence of [Ca2+]CYT responses. A and B: stimulation was performed in a series of DVR (n = 7), at 0.1 Hz, 0.5 Hz, and followed by return to 0.1 Hz. Means ± SE of normalized fluo4 fluorescence is shown for ROI corresponding to pericytes (A, 14 cells) or endothelia (B, 15 cells). Pericytes show reversible, oscillatory [Ca2+]CYT elevations at 0.1 Hz that merge to become a sustained response 0.5 Hz (1-s pulses every 2 s). Endothelia show little response. C: example shows an image sequence with outline of a pericyte (p) and adjacent endothelial cell (e) in the first and final images. Stimulation at 0.1 or 0.5 Hz leads to an increase in pericyte fluorescence with little change in the adjacent endothelium.
Pericyte [Ca2+]CYT responses require gap junctions and kinase activation.
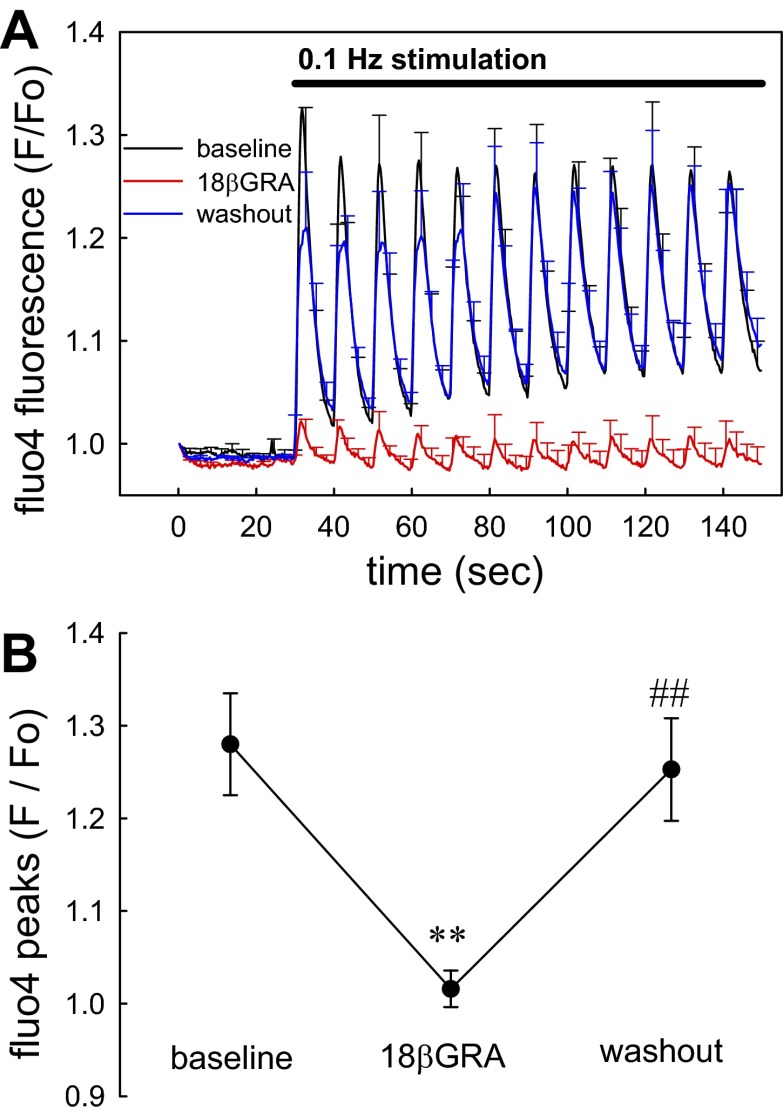
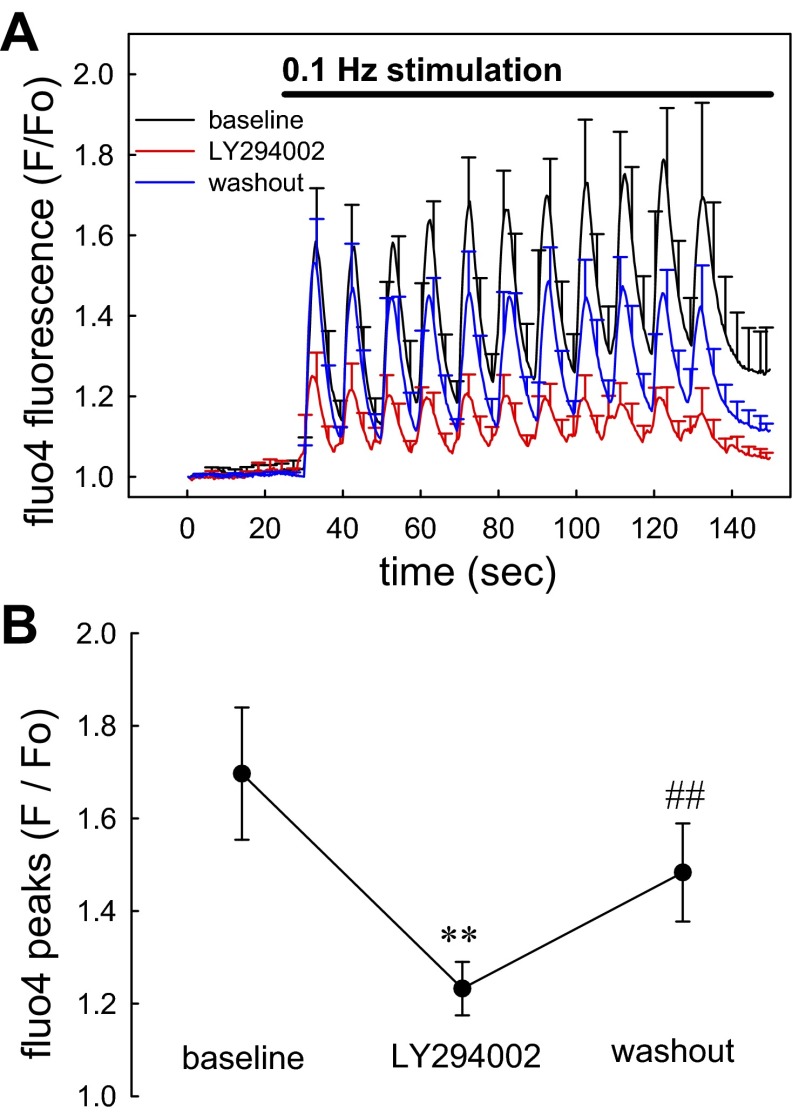
Figure 3A illustrates the ability of gap junction blockade with 18βGRA (100 μM) to prevent transmission of mechanosensitive signaling. Sequential images (300-ms intervals) were captured at 30-s baseline upon which 0.1-Hz stimulation was initiated for 120 s. Subsequently, 18βGRA was exchanged into the bath and the stimulation protocol was reinitiated and repeated again after washout. Statistical comparison of the peak [Ca2+]CYT elevations before, during, and after 18βGRA is summarized in Fig. 3B. 18βGRA effectively and reversibly blocked the pericyte [Ca2+]CYT responses. Mechanosensitivity of blood vessels has been traced to integrin signaling through phophoinositide-3-kinase (PI3K) (1, 2). Accordingly, we examined the effects of PI3K blockade with LY294002. As shown in Fig. 4, A and B, using a stimulation protocol identical to that in Fig. 3, LY294002 reversibly blocked pericyte responses.
Fig. 3.
Blockade of pericyte [Ca2+]CYT responses by 18 β-glycyrrhetinic acid (18βGRA). A: means ± SE of normalized fluo4 fluorescence are shown for pericytes stimulated before (black), during (red), and after washout (blue) of the gap junction blocker 18βGRA (100 μM). In each sequence, images were acquired for 30 s before onset of stimulation at 0.1 Hz. The bath was exchanged between sequence acquisitions and the data were superimposed for display. Each vessel was analyzed as the mean of its pericyte responses and the means ± SE of individual vessels were displayed from that data (total, n = 47 pericytes from 7 DVR). B: statistical comparison of data from A performed by comparing the mean of peaks from each vessel before, during, and after washout of 18βGRA (**P < 0.01 18βGRA vs. control; ##P < 0.01 washout vs. 18βGRA).
Fig. 4.
Blockade of pericyte [Ca2+]CYT responses by LY294002. A: means ± SE of normalized fluo4 fluorescence are shown for pericytes stimulated before (black), during (red), and after washout (blue) of LY294002 (50 μM). In each sequence, images were acquired for 30 s before onset of stimulation at 0.1 Hz. The bath was exchanged between sequence acquisitions and the data were superimposed for display. Each vessel was analyzed as the mean of its pericyte responses and the means ± SE of individual vessels were displayed from that data (total, n = 33 pericytes from 5 DVR). B: statistical comparison of data from A performed by comparing the mean of peaks from each vessel before, during, and after washout of LY294002 (**P < 0.01 LY294002 vs. control; ##P < 0.01 washout vs. LY294002).
Pericyte [Ca2+]CYT responses require external calcium entry.
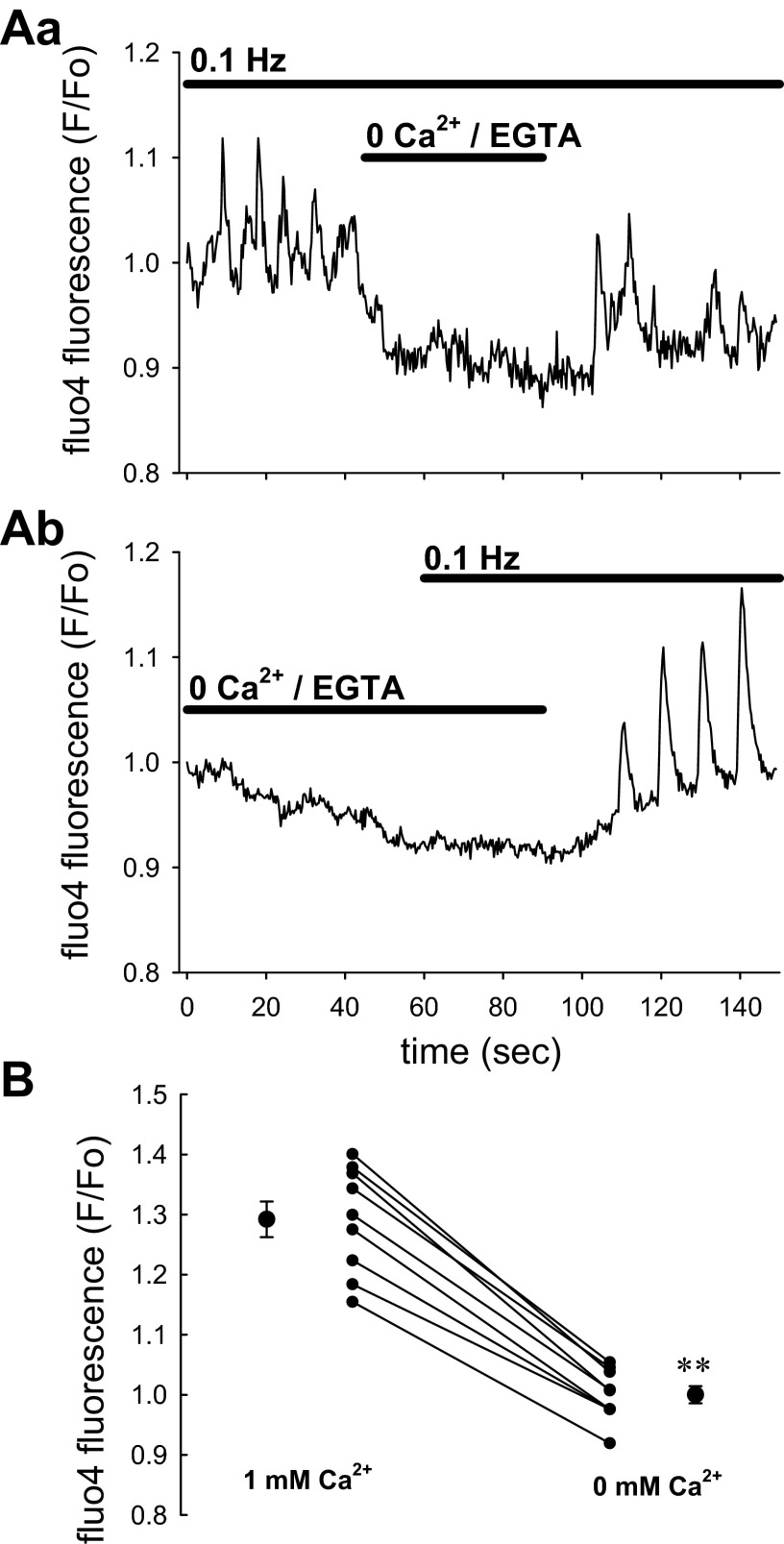
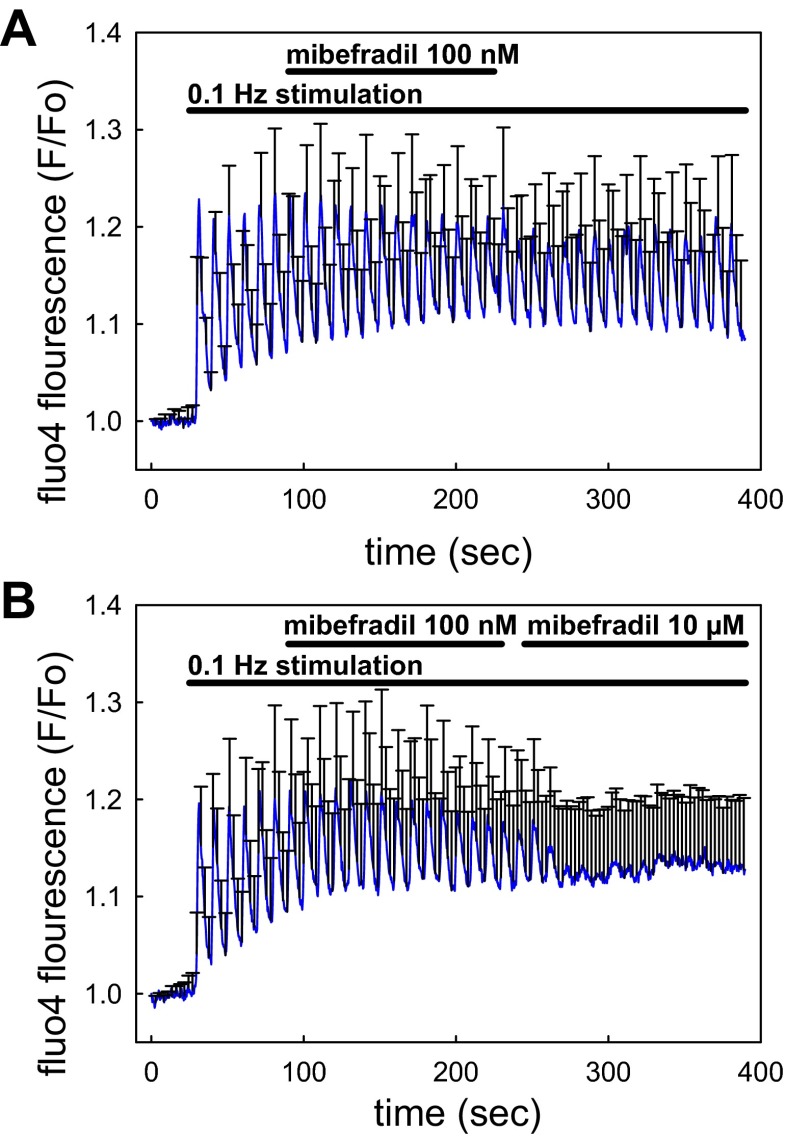
As illustrated in Fig. 5, removal of Ca2+ ions from the bath (0 CaCl2/100 μM EGTA) lowered baseline fluo4 fluorescence and prevented any pericyte [Ca2+]CYT responses to mechanostimulation from occurring. This supports dependence of responses on Ca2+ entry. We previously showed that DVR pericyte Ca2+ entry is partially mediated by nifedipine- and diltiazem-sensitive L-type CaV (45, 48, 50). In this study, we tested whether that pathway also mediates Ca2+ entry during mechanical stimulation. As shown in Fig. 6A, nifedipine effectively reduced the responses but was slow to reverse during washout. The stimulation protocol used in Figs. 3 and 4 was repeated before, during, and after nifedipine application (Fig. 6B) with results summarized in Fig. 6C. Although T-type CaV have been observed in DVR and participate in efferent arteriolar contractile responses (18, 33), we and others have been unable to detect T-type currents during patch-clamp studies (39, 48). Herein, we tested whether T-type channels participate in Ca2+ entry during mechanostimulation by examining the effectiveness of a low concentration of mibefradil (Fig. 7A). At a concentration of 100 nM, selective for T-channel blockade, it was ineffective. As illustrated in Fig. 7B, raising mibefradil concentration to 10 μM partially blocked pericyte [Ca2+]CYT responses. Those results mirror blockade of angiotensin II-induced contraction of isolated-perfused DVR by high concentrations where L-type channel inhibition is also likely to occur (48).
Fig. 5.
Elimination of pericyte [Ca2+]CYT responses by removal of extracellular Ca2+ ions. A: examples show the effect of removing Ca2+ ions from extracellular buffer during (Aa) or before (Ab) 0.1-Hz stimulation. In the experiments, Ca2+ was nominally reduced to 0 concentration in the external buffer and chelated by addition of 100 μM EGTA (total, n = 12 pericytes from 9 DVR). B: statistical comparison of data by comparing the mean of peaks from each vessel in 1 and 0 mM CaCl2/EGTA. Residual peaks were never detectable in 0 Ca2+ so that the fluorescence of the baseline was used for comparison to peaks in 1 mM CaCl2 (**P < 0.01, 1 vs. 0 mM CaCl2/EGTA).
Fig. 6.
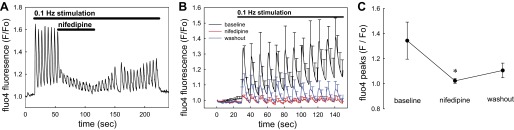
Blockade of pericyte [Ca2+]CYT responses by nifedipine. A: example shows the mean response of 5 pericytes to introduction and washout of nifedipine (10 μM) during 0.1-Hz stimulation. Nifedipine reversibly suppressed [Ca2+]CYT responses but its effects were slow to reverse. B: means ± SE of normalized fluo4 fluorescence are shown for pericytes stimulated before (black), during (red), and after washout (blue) of nifedipine (10 μM). In each sequence, images were acquired for 30 s before onset of 0.1-Hz stimulation. The bath was exchanged between sequence acquisitions and the data were superimposed for display. Each vessel was analyzed as the mean of its pericyte responses and the means ± SE of individual vessels were displayed from that data (total, n = 34 pericytes from 5 DVR). C: statistical comparison of data from B performed by comparing the mean of peaks from each vessel before, during, and after washout of nifedipine (*P < 0.05 nifedipine vs. control).
Fig. 7.
Blockade of pericyte [Ca2+]CYT responses by mibefradil. A: means ± SE of normalized fluo4 fluorescence are shown for pericytes stimulated at 0.1 Hz before, during, and after washout of mibefradil (100 nM, n = 29 pericytes, 8 vessels). At 100 nM aimed at selectively blocking T-type voltage-gated Ca2+ channels, mibefradil was without effect. B: means ± SE of normalized fluo4 fluorescence are shown for pericytes stimulated at 0.1 Hz before and during sequential introduction of mibefradil at 100 nM and 10 μM (n = 25 pericytes, 5 vessels). Mibefradil was only effective at the higher nonselective concentration (P < 0.05, mean of peaks in 10 μM mibefradil vs. either 0 or 100 nM mibefradil).
Mechanostimulation depolarizes the pericyte cell membrane.
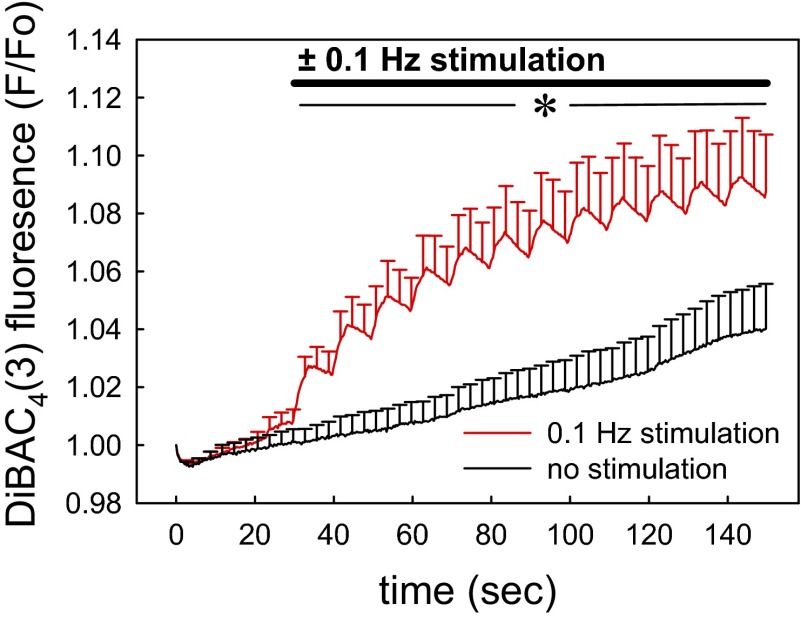
Since Ca2+ entry via nifedipine-sensitive L-type channels is gated by membrane depolarization, we tested for pericyte depolarization during mechanostimulation. Using DiBAC4(3) as a voltage-sensitive probe, baseline fluorescence in the absence of stimulation tended to slowly increase as we previously observed (47). DiBAC4(3) responds too slowly to resolve the detailed kinetics of voltage fluctuations, but did confirm pericyte depolarization (Fig. 8).
Fig. 8.
Effect of 0.1-Hz stimulation on pericyte membrane potential recorded with bis[1,3-dibutylbarbituric acid-(5)] trimethineoxonol [DiBAC4(3)]. DVR, isolated and adherent to coverslips as in Fig. 1, were loaded with the voltage-sensitive probe DiBAC4(3). Images were captured with and without 0.1-Hz stimulation (n = 9 DVR, 40 pericytes). Fluorescence was normalized to that in the first image (F/Fo). Each vessel was analyzed as the mean of its pericyte responses and the means ± SE of individual vessels were displayed from that data (*P < 0.05, no stimulation vs. 0.1-Hz stimulation for all time points beyond 30 s).
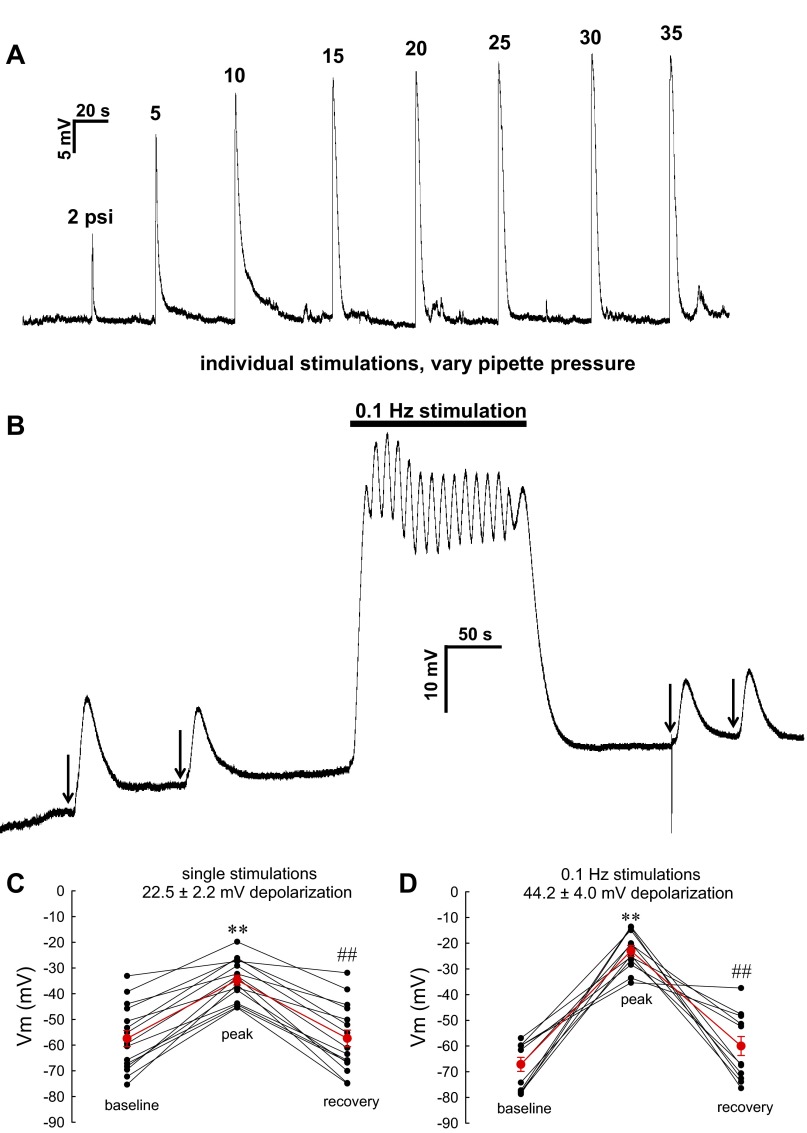
In contrast to DiBAC4(3), electrophysiological measurement can track rapid changes of membrane potential but dialyzes the cell cytoplasm with electrode buffer (19). Despite this, the nystatin-perforated patch-clamp with I=0 current recording also confirmed that mechanostimulation depolarizes pericytes. As shown in Fig. 9A, solitary stimulations yielded rapid and reversible increases of membrane potential the magnitude of which correlated with the pressure applied to the stimulation pipette. Contrast of the effect of solitary stimulation vs. 0.1-Hz repetitive stimulation is provided in Fig. 9B and summarized in Fig. 9, C and D. Single stimulations yielded a 22.5 ± 2.2-mV rise in membrane potential while repetitive stimulation nearly doubled the depolarization to 44.2 ± 4.0 mV.
Fig. 9.
Effect of stimulation on pericyte membrane potential recorded by nystatin-perforated patch-clamp. A: example in which membrane potential was recorded by I = 0 current clamp in a DVR pericyte. Transient peak elevations correspond to mechanical stimulation as illustrated in Fig. 1. The pipette pressure was adjusted to a series of levels between 2 and 35 PSI. B: example in which membrane potential was recorded in response to manually triggered single stimulations (arrows) or during computer-controlled 0.1-Hz stimulation (30 PSI pipette pressure, 1-s duration in all cases). The extent of depolarization was reversible and increased with frequency. C: summary of pericyte membrane potential before, at peak, and after single, manually triggered stimulations in individual pericytes of DVR (n = 16). The means ± SE depolarization was 22.5 ± 2.2 mV. D: summary of pericyte membrane potential before, at peak, and after 0.1-Hz stimulations in pericytes of DVR (n = 12). The means ± SE depolarization was 44.2 ± 4.0 mV (**P < 0.01 peak response vs. baseline; ##P < 0.01 recovery vs. peak response).
DISCUSSION
DVR are small microvessels (∼13-μm internal diameter on average) that derive from juxtamedullary efferent arterioles to supply the medulla of the kidney with blood flow. Luminal pressures are typically 10 to 12 mmHg by servo nulling measurements in the accessible, papillary inner medulla. When DVR are blocked with wax and perfused retrograde from the papilla toward the outer medulla, pressures rise to 20 to 22 mmHg providing a probable upper limit on luminal pressures of DVR that lie in outer medullary vascular bundles (29). Such pressures are well below the range that elicits myogenic contraction of renal intralobar or afferent arterioles. Despite this, DVR have been found to be very sensitive to mechanical stimuli. Luminal pressurization or step changes in rate of microperfusion elicit [Ca2+]CYT responses and modify NO generation. Due to the small size of these vessels, it has not been possible to reliably separate luminal shear from wall stretch as the mechanical stimulus (49). As an alternative, we demonstrated that manually puffing a buffer stream from a micropipette onto the abluminal DVR wall elicits [Ca2+]CYT responses that propagate along the vessel axis (44). In this study, we automated that method and reduced the stimulation pipette orifice to that typical of a patch-clamp pipette (∼1 μm). These alterations improve reproducibility of experimental conditions and remove variations that result from manual control of pipette pressure by the investigator (Fig. 1). The automation enables reliable superposition and averaging of results from individual vessels to enhance power of statistical comparisons (Figs. 2–8). Immobilization of the vessel away from the stimulation pipette prevents motion artifacts and cell contractions are not observed to an observable degree. This is consistent with our prior observation that luminal pressurization with microperfusion is needed to quantify wall motion and vasoactivity in these small vessels. Finally, it is clear that motion artifacts away from the site of stimulation are sufficiently small for gigaohm seals to be maintained for electrophysiological measurements. Similarly, in past studies, we have been readily able to sustain such seals when vasoactive agonists are applied (24, 25, 45).
In addition to automation of stimulus, rapid acquisition, low-light imaging with a 16-bit camera is important to spatially analyze responses. As previously observed with fura2, deesterification of fluo4 during loading yields endothelial fluorescence that exceeds the baseline pericyte signal (Fig. 2C) (26, 45). Pericyte responses can nonetheless be quantified by analyzing cell bodies that jut out from the abluminal surface away from of the vessel wall (Fig. 2A). The 16-bit capabilities of modern CCD cameras (>65,000 gray levels) provide sufficient sensitivity to accurately quantify fluorescence changes in these small cells. Accurate quantification of endothelial signals without pericyte contamination is more problematic because pericyte extensions of submicron thickness wrap around the vessel wall. As a consequence, the small elevations of fluo4 fluorescence obtained by placing ROI over endothelia in Fig. 2B might arise from pericytes. Despite these limitations, comparison of the dramatic differences between Fig. 2A and Fig. 2B favors the interpretation that [Ca2+]CYT responses are largely confined to the pericytes.
The present study confirms that physiological responses can be rapidly transmitted along the DVR wall (Fig. 1) by mechanisms that are sensitive to blockade of gap junctions (Fig. 3). With regard to cell coupling within the DVR wall, prior studies have pointed to syncytial continuity of the endothelium. Dialysis of Lucifer yellow (457 Da) into an endothelial cell from a patch-clamp pipette leads to rapid diffusion along the endothelial layer. When that probe is dialyzed into a pericyte, there is less consistent diffusion to adjacent pericytes or the underlying endothelium. Measurements of capacitance transients that arise from abrupt voltage clamp depolarizations support the notion that endothelia but not pericytes are consistently coupled (44). Poor myoendothelial coupling has been frequently observed in other small vessels (11, 38) and gap junction proteins connexins 37, 40, 43, and 45 have been found in the wall of DVR and other renal vessels (14, 15, 44).
Observations cited above with Lucifer yellow imply possible diffusion of vasoactive mediators between cells through gap junctions. We previously reported that adjacent endothelia and pericytes depolarize or hyperpolarize in parallel when vasoconstrictors and dilators are applied (35). Thus, pericyte-endothelial (myoendothelial) electrical coupling in the DVR wall involving small ions seems possible and likely. In contrast, Ca2+ ions do not freely exchange across putative myoendothelial junctions because angiotensin II induces asynchronous oscillatory pericyte [Ca2+]CYT responses while lowering [Ca2+]CYT in the DVR endothelial layer (27, 34, 45). The present observations also support independence of Ca2+ signals in the two cell types because mechanosensitive [Ca2+]CYT responses are largely confined to pericytes (Fig. 2, A and B). Given the coincidence of [Ca2+]CYT responses in individual pericytes along the vessel wall (Fig. 1) and the sensitivity to gap junction blockade (Fig. 3), participation of the syncytial endothelial layer in voltage spreading that yields simultaneous depolarizations of pericytes seems very likely.
While the existence of mechanically stimulated DVR pericyte depolarization is clear (Figs. 8 and 9), the channel architecture and ion movements that underlie the response are uncertain. Those events might originate in pericytes or they might arise from endothelia through syncytial spread of monovalent charge to pericytes. The coincidence of pericyte responses in Fig. 1C shows that cells separated by distances of 100 μm depolarize within milliseconds of one another, likely too fast to invoke simple diffusion of a vasoactive mediator; it occurs with a speed reminiscent of neural transmission. Depolarization of isolated vascular smooth muscle generally involves cation entry, reduction of potassium conductance, an increase in chloride channel activity, or a combination thereof and we showed that such channel modulation occurs in response to angiotensin II stimulation of DVR pericytes (24, 25, 50). With regard to enhancement of cation conductance, activation of transient receptor potential channels by mechanical stretch has been implicated (12, 43). Increasing attention has been paid to plausible roles of epithelial Na+ channel/degenerin proteins (9, 10), although detection of epithelial Na channels currents in vascular smooth muscle has been lacking (8, 22, 40). Other possibilities exist. Direct control of L-type channels by integrin interactions (13, 42) or through PI3K (1, 21, 23, 31) has been described. With regard to the latter possibility, our finding of sensitivity to LY294002 (Fig. 4) suggests PI3K involvement; however, given the limitations of specificity of kinase inhibitors, this should be viewed with caution (3, 6, 41). It seems plausible that mechanical interactions are transduced to the cytoskeleton at the point of stimulation by mechanisms involving kinase activity, stretch activation of channels, or both and that subsequent coordinated pericyte responses rely on syncytial spread of depolarization via the endothelium with consequent L-type channel gating. The precise identity, control, and selectivity of pathways that couple cells to concomitantly depolarize DVR pericytes remain to be discerned. The ability to perform patch-clamp studies during mechanostimulation may provide means to eventually analyze the responsible ion movements and channel activity.
Prior studies of Ca2+ entry routes into DVR pericytes identified an important role for CaV in agonist-invoked contractions. Predominance of CaV as a route for Ca2+ entry varies along the renal vasculature and may not be invoked in a given segment by all contractile agonists (16, 17, 32). In our hands, electrophysiological studies of pericytes showed that voltage clamp depolarization gates sequential inward currents carried by tetrodotoxin-sensitive Na+ channels and nifedipine-sensitive L-type Ca2+ channels (20, 46, 48). We found that L-type blockers, nifedipine and diltiazem, interfere with agonist-induced vasoconstriction by angiotensin II and that oscillatory inward currents involving Cl− ions can be involved (48, 50). A role for L-type CaV in the mechanosensitive responses of this study is supported by the ability of nifedipine to block [Ca2+]CYT transients (Figs. 6 and 7). Concomitant depolarization of the cell membrane (Figs. 8 and 9) points to processes intended to gate CaV channels, but the means used to achieve pericyte depolarization may be quite different from that involved in agonist contractions.
The vasculature of the kidney and renal medulla is subject to various mechanical stimuli. Classically, endothelial shear favoring vasodilator formation and wall stretch favoring myogenic constriction have received greatest attention. Whether the method of stimulation we describe in Fig. 1 evokes responses related to myogenic mechanisms is highly uncertain. As described above, DVR exist in the low-pressure efferent juxtamedullary region of the renal circulation. We have been unable to detect overt myogenic contraction of DVR in response to raising luminal pressure (49); however, recent studies in human DVR by Sendeski and colleagues (37) identified such behavior. In addition to pressure-associated variations of luminal flow and pressure, ureteral peristalsis regularly compresses the papillary inner medulla to optimize urinary concentration (36). The mechanism by which such peristalsis modifies medullary gradients has been the subject of past research and much controversy. If DVR respond to mechanical compression from ureteral peristalsis, feedback mechanisms may exist to optimize medullary perfusion for urinary concentration as part of the process. Finally, the renal medulla is believed to have sparse innervation. The speed with which responses spread along the vessel axis (Fig. 1) raises the possibility that neural control in the deep cortex is relayed to DVR of the outer medulla through the endothelial syncytium rather than renal nerves, per se.
GRANTS
These studies were supported by National Institutes of Health Grants DK42495, DK67621, and an American Heart Association Scientist Development Award to C. Cao (09SDG2130035).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.Z., C.C., and T.L.P. conception and design of research; Z.Z. and K.P. performed experiments; Z.Z., K.P., and T.L.P. analyzed data; Z.Z. and T.L.P. prepared figures; Z.Z., K.P., C.C., and T.L.P. approved final version of manuscript; C.C. and T.L.P. interpreted results of experiments; T.L.P. drafted manuscript; T.L.P. edited and revised manuscript.
REFERENCES
- 1. Carnevale D, Vecchione C, Mascio G, Esposito G, Cifelli G, Martinello K, Landolfi A, Selvetella G, Grieco P, Damato A, Franco E, Haase H, Maffei A, Ciraolo E, Fucile S, Frati G, Mazzoni O, Hirsch E, Lembo G. PI3Kgamma inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc Res 93: 200–209, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Cary LA, Han DC, Guan JL. Integrin-mediated signal transduction pathways. Histol Histopathol 14: 1001–1009, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Cohen P. Guidelines for the effective use of chemical inhibitors of protein function to understand their roles in cell regulation. Biochem J 425: 53–54, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowley AW., Jr Role of the renal medulla in volume and arterial pressure regulation. Am J Physiol Regul Integr Comp Physiol 273: R1–R15, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351: 95–105, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dickhout JG, Mori T, Cowley AW., Jr Tubulovascular nitric oxide crosstalk: buffering of angiotensin II-induced medullary vasoconstriction. Circ Res 91: 487–493, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Drummond HA. Yes, no, maybe so: ENaC proteins as mediators of renal myogenic constriction. Hypertension 54: 962–963, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 19: 277–284, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Folgering JH, Sharif-Naeini R, Dedman A, Patel A, Delmas P, Honore E. Molecular basis of the mammalian pressure-sensitive ion channels: focus on vascular mechanotransduction. Prog Biophys Mol Biol 97: 180–195, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Gui P, Chao JT, Wu X, Yang Y, Davis GE, Davis MJ. Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling. Adv Exp Med Biol 674: 69–79, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol 298: R1143–R1155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanner F, von Maltzahn J, Maxeiner S, Toma I, Sipos A, Kruger O, Willecke K, Peti-Peterdi J. Connexin45 is expressed in the juxtaglomerular apparatus and is involved in the regulation of renin secretion and blood pressure. Am J Physiol Regul Integr Comp Physiol 295: R371–R380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen PB. The complex field of interplay between vasoactive agents. Kidney Int 76: 929–931, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Hansen PB. Functional and pharmacological consequences of the distribution of voltage-gated calcium channels in the renal blood vessels. Acta Physiol (Oxf) 207: 690–699, 2013 [DOI] [PubMed] [Google Scholar]
- 18. Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92: 145–159, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee-Kwon W, Goo JH, Zhang Z, Silldorff EP, Pallone TL. Vasa recta voltage-gated Na+ channel Nav1.3 is regulated by calmodulin. Am J Physiol Renal Physiol 292: F404–F414, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Liang W, Oudit GY, Patel MM, Shah AM, Woodgett JR, Tsushima RG, Ward ME, Backx PH. Role of phosphoinositide 3-kinaseα, protein kinase C, and L-type Ca2+ channels in mediating the complex actions of angiotensin II on mouse cardiac contractility. Hypertension 56: 422–429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loutzenhiser R, Aaronson PI. Role of epithelial sodium channels in the renal myogenic response? Hypertension 55: e6–9, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Lu Z, Jiang YP, Xu XH, Ballou LM, Cohen IS, Lin RZ. Decreased L-type Ca2+ current in cardiac myocytes of type 1 diabetic Akita mice due to reduced phosphatidylinositol 3-kinase signaling. Diabetes 56: 2780–2789, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Pallone TL, Cao C, Zhang Z. Inhibition of K+ conductance in descending vasa recta pericytes by ANG II. Am J Physiol Renal Physiol 287: F1213–F1222, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Pallone TL, Huang JM. Control of descending vasa recta pericyte membrane potential by angiotensin II. Am J Physiol Renal Physiol 282: F1064–F1074, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Pallone TL, Silldorff EP, Cheung JY. Response of isolated rat descending vasa recta to bradykinin. Am J Physiol Heart Circ Physiol 274: H752–H759, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Pallone TL, Silldorff EP, Zhang Z. Inhibition of calcium signaling in descending vasa recta endothelia by ANG II. Am J Physiol Heart Circ Physiol 278: H1248–H1255, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Pallone TL, Turner MR, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Pallone TL, Work J, Jamison RL. Resistance of descending vasa recta to the transport of water. Am J Physiol Renal Fluid Electrolyte Physiol 259: F688–F697, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol 284: F253–F266, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Pinho JF, Medeiros MA, Capettini LS, Rezende BA, Campos PP, Andrade SP, Cortes SF, Cruz JS, Lemos VS. Phosphatidylinositol 3-kinase-delta upregulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br J Pharmacol 161: 1458–1471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pollock DM, Jenkins JM, Cook AK, Imig JD, Inscho EW. L-type calcium channels in the renal microcirculatory response to endothelin. Am J Physiol Renal Physiol 288: F771–F777, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Poulsen CB, Al-Mashhadi RH, Cribbs LL, Skott O, Hansen PB. T-type voltage-gated calcium channels regulate the tone of mouse efferent arterioles. Kidney Int 79: 443–451, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Rhinehart K, Handelsman CA, Silldorff EP, Pallone TL. ANG II AT2 receptor modulates AT1 receptor-mediated descending vasa recta endothelial Ca2+ signaling. Am J Physiol Heart Circ Physiol 284: H779–H789, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Rhinehart K, Zhang Z, Pallone TL. Ca2+ signaling and membrane potential in descending vasa recta pericytes and endothelia. Am J Physiol Renal Physiol 283: F852–F860, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Schmidt-Nielsen B, Churchill M, Reinking LN. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney Int 18: 419–431, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Sendeski MM, Liu ZZ, Perlewitz A, Busch JF, Ikromov O, Weikert S, Persson PB, Patzak A. Functional characterization of isolated, perfused outermedullary descending human vasa recta. Acta Physiol (Oxf) 208: 50–56, 2013 [DOI] [PubMed] [Google Scholar]
- 38. Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res 97: 781–788, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Smirnov SV, Loutzenhiser K, Loutzenhiser R. Voltage-activated Ca2+ channels in rat renal afferent and efferent myocytes: no evidence for the T-type Ca2+ current. Cardiovasc Res 97: 293–301, 2013 [DOI] [PubMed] [Google Scholar]
- 40. Wang X, Takeya K, Aaronson PI, Loutzenhiser K, Loutzenhiser R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am J Physiol Renal Physiol 295: F272–F282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Welling A, Hofmann F, Wegener JW. Inhibition of L-type Cav1.2 Ca2+ channels by 2,(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) and 2-[1-(3-dimethyl-aminopropyl)-5-methoxyindol-3-yl]-3-(1H-indol-3-yl) maleimide (Go6983). Mol Pharmacol 67: 541–544, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol 143: 241–252, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys 56: 1–18, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Zhang Q, Cao C, Mangano M, Zhang Z, Silldorff EP, Lee-Kwon W, Payne K, Pallone TL. Descending vasa recta endothelium is an electrical syncytium. Am J Physiol Regul Integr Comp Physiol 291: R1688–R1699, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Zhang Q, Cao C, Zhang Z, Wier WG, Edwards A, Pallone TL. Membrane current oscillations in descending vasa recta pericytes. Am J Physiol Renal Physiol 294: F656–F666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Z, Cao C, Lee-Kwon W, Pallone TL. Descending vasa recta pericytes express voltage operated Na+ conductance in the rat. J Physiol 567: 445–457, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Z, Huang JM, Turner MR, Rhinehart KL, Pallone TL. Role of chloride in constriction of descending vasa recta by angiotensin II. Am J Physiol Regul Integr Comp Physiol 280: R1878–R1886, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Zhang Z, Lin H, Cao C, Khurana S, Pallone TL. Voltage-gated divalent currents in descending vasa recta pericytes. Am J Physiol Renal Physiol 299: F862–F871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Z, Pallone TL. Response of descending vasa recta to luminal pressure. Am J Physiol Renal Physiol 287: F535–F542, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am J Physiol Regul Integr Comp Physiol 283: R949–R957, 2002 [DOI] [PubMed] [Google Scholar]