Abstract
Hemodynamic conditions play a critical role in embryonic cardiovascular development, and altered blood flow leads to congenital heart defects. Chicken embryos are frequently used as models of cardiac development, with abnormal blood flow achieved through surgical interventions such as outflow tract (OFT) banding, in which a suture is tightened around the heart OFT to restrict blood flow. Banding in embryos increases blood pressure and alters blood flow dynamics, leading to cardiac malformations similar to those seen in human congenital heart disease. In studying these hemodynamic changes, synchronization of data to the cardiac cycle is challenging, and alterations in the timing of cardiovascular events after interventions are frequently lost. To overcome this difficulty, we used ECG signals from chicken embryos (Hamburger-Hamilton stage 18, ∼3 days of incubation) to synchronize blood pressure measurements and optical coherence tomography images. Our results revealed that, after 2 h of banding, blood pressure and pulse wave propagation strongly depend on band tightness. In particular, while pulse transit time in the heart OFT of control embryos is ∼10% of the cardiac cycle, after banding (35% to 50% band tightness) it becomes negligible, indicating a faster OFT pulse wave velocity. Pulse wave propagation in the circulation is likewise affected; however, pulse transit time between the ventricle and dorsal aorta (at the level of the heart) is unchanged, suggesting an overall preservation of cardiovascular function. Changes in cardiac pressure wave propagation are likely contributing to the extent of cardiac malformations observed in banded hearts.
Keywords: cardiovascular development, pulse wave propagation, micropressure measurement, electrocardiography, optical coherence tomography, Hamburger-Hamilton
the heart provides circulatory support to the embryo even during cardiovascular morphogenesis. Dynamic forces in the developing cardiac walls, generated by cardiac contraction and relaxation and the interaction between heart tissue and blood flow, have strong influence in the processes of growth, remodeling, and morphogenesis of the heart (7, 21, 32–34). At early stages of vertebrate embryonic development, the heart first forms as a straight tube that then loops and twists. The heart outflow tract (OFT) connects the ventricle with the aortic sac (AOS), which leads distally to the aortic arches and then the dorsal aorta (DA) (see Fig. 1A). Previous studies have provided strong evidence that hemodynamic conditions play a critical role in the regulation of embryonic cardiovascular development and that disturbed flows give rise to cardiac defects, including interventricular septum defects and semilunar valve defects that originate from the early OFT (9, 11, 12, 14, 15, 18, 22, 24).
Fig. 1.
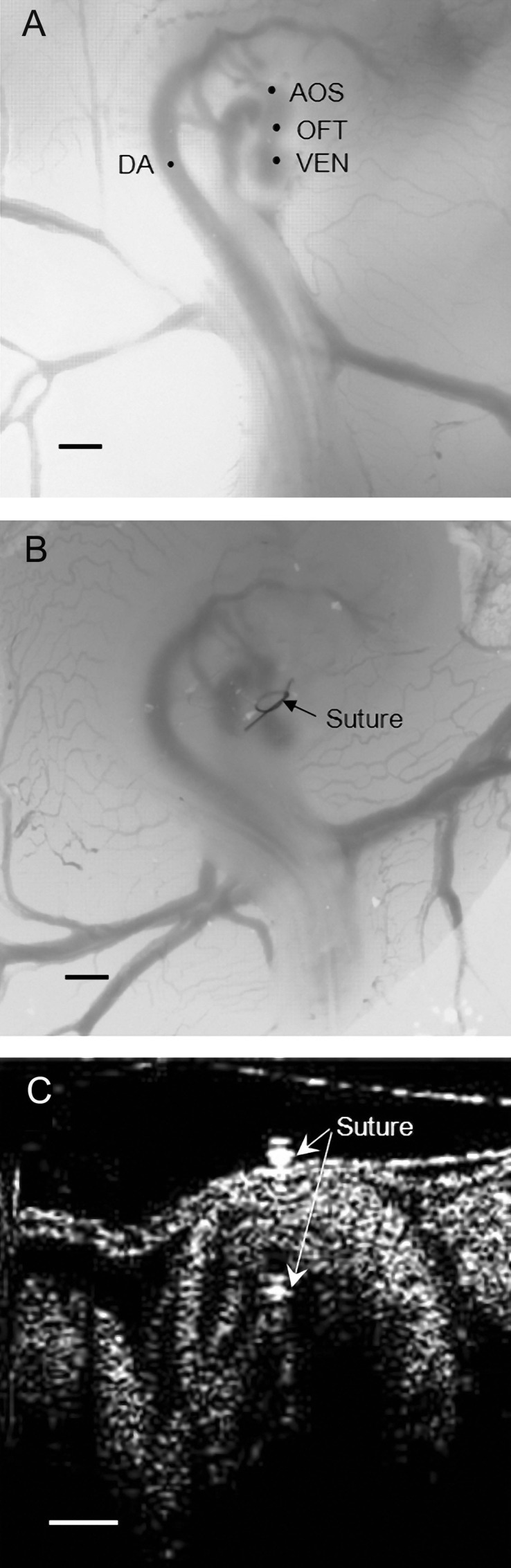
Chicken embryonic images showing heart morphology and the banding procedure in chick embryos at Hamburger-Hamilton stage 18 (HH18). A: microscopic image of a normal chicken embryo showing its heart and part of its circulation. VEN, ventricle; OFT, outflow tract; AOS, aortic sac; DA, dorsal aorta. Scale bar = 1 mm. B: OTF banding (OTB) in a HH18 heart showing the suture around the heart OFT. Scale bar = 1 mm. C: longitudinal tomographic section of the chick heart OFT after OTB at HH18 imaged with optical coherence tomography (OCT). Scale bar = 250 μm.
The chicken embryo–which is easy to access, manipulate, and image–has often been used as a model to study heart development (3, 4, 6, 14, 29, 35). Surgical interventions made to alter blood flow conditions in the embryonic chick cardiovascular circulation have been extensively shown to result in cardiac malformations, which resemble those of human patients with congenital heart disease (7, 12, 14, 29, 31, 35).
The periodic beating of the developing heart generates pulsatile blood flow. Pressure waves originating in the heart travel through the entire chick cardiovascular system. Wave amplitude, pulse transit time (PTT), and pulse wave velocity (PWV) are frequently used, even clinically, to quantify propagating waves and the impedance characteristics of the circulatory system. Since the embryonic heart and circulation (the vitelline circulation) form a closed system, pressure wave propagation in the vitelline circulation is coupled to pressure pulse generation in the heart. Therefore, an acute change of blood flow in the embryonic heart also affects vitelline pressure wave propagation, particularly in the arterial circulation. PTT and PWV can thus be used to determine the effects of hemodynamic interventions on the cardiovascular circulation.
Quantification of PTT and PWV, however, requires simultaneous measurements of pressure or flow at different locations in the cardiovascular system (e.g. 17, 25, 38) or synchronization of measurements with a cardiac signal, such as ECG signals. PTT and PWV can also be quantified from imaging data, since blood pressure waves affect cardiac and arterial wall motion. Optical coherence tomography (OCT), in particular, is a powerful imaging technique that offers high-resolution, and noninvasive imaging up to a depth of 2 mm in biological tissues (10, 37). OCT is ideal for studying early heart development in chicken embryos because its resolution allows the visualization of heart microstructures at image acquisition rates fast enough to capture the beating motion of the heart (26, 28).
In this study, we demonstrate the feasibility of using simultaneous ECG and blood pressure measurements, as well as simultaneous ECG and OCT imaging, to quantify pulse wave propagation in the chicken embryonic heart and vitelline circulation. The study also analyzes changes in wave propagation due to an acute change of blood flow in the heart introduced by cardiac outflow tract banding (OTB) at Hamburger-Hamilton (HH) stage 18 (13). In OTB, a suture is tightened around the heart OFT, restricting the motion of the OFT wall and producing increased afterload (see Fig. 1B). It is well established that OTB increases ventricular blood pressure and blood flow velocities through the constriction and that OTB leads to cardiac malformations (7, 8, 29, 36, 38). Our data indicate that the degree of banding determines hemodynamic conditions in the chick embryonic circulation.
METHODS
Embryonic Preparation and Hemodynamic Intervention
Fertilized White Leghorn chicken eggs were incubated at 38°C to HH18 (∼3 days) (13). For physiological measurements, each egg was placed in a custom-made, pad-heating system for manipulation. The egg shell was opened at the air sac end, and a small section of the shell membrane was carefully removed.
Chicken embryos were divided into three groups: 1) the normal (NL) group, in which no interventions were performed; 2) the OTF banding (OTB) group, in which the embryo heart was banded at HH18 by passing a 10-0 nylon suture under the midsection of the OFT and tying the suture in a knot to constrict the OFT cross-sectional area (see Fig. 1B); and 3) the control (CON) sham-operated group, in which the suture was passed around the OFT at HH18 but not tightened. After interventions (OTB and CON groups), eggs were placed back into the incubator and extracted ∼2 h later for measurements.
In OTB embryos, band tightness was measured by imaging the OFT longitudinally before and after banding using OCT (see Fig. 1C). Band tightness was defined as follows:
| (1) |
where Da and Db are the maximum external diameters of the OFT at the place where the band was located after and before banding, respectively.
Experimental System Setup
ECG system.
Cardiac electrical signals that induce heart contraction propagate through the chick embryo body and extraembryonic fluid. These signals, which are referred to as the ECG, can be measured in the egg at early stages of development (2). In this study, we collected ECG data by inserting three monopolar needle electrodes, which were connected to a differential bioamplifier (FE-136 Animal Bio Amp, AD Instruments, Colorado Springs, CO) and sampled at a rate of 1,000 Hz. The amplified signal was then filtered at cutoff frequencies of 10 Hz to eliminate baseline drift. The needle electrode position was optimized to achieve strong ECG signals, with the reference electrode placed in the extraembryonic fluid, the positive (+) electrode close to the heart, and the negative (−) electrode in the embryo tail. Typically, ECG signals have three peaks: P, R, and T. The R peak of the ECG corresponds to ventricular depolarization and is the strongest peak within the ECG signal. Here, we used the R peak as a reference to synchronize measured data (pressure, flow, and images) acquired simultaneously with ECG data.
Doppler flow system.
To determine the sensitivity of electrode positioning on ECG signals and optimize ECG electrode positioning, we conducted simultaneous ECG and blood flow measurements in the DA. Blood flow data were acquired using a noninvasive laser-Doppler flow monitor (Moor) with a 40-Hz maximum sampling rate. ECG and Doppler blood flow data acquisition were simultaneously triggered by a PowerLab Analog-to-Digital (A/D) Converter (ML750, AD Instruments). ECG-flow measurements were also used to assess the effect of temperature changes on phase measurements. Both ECG and Doppler flow signals were digitized with the PowerLab A/D Converter and recorded for further processing.
Servo-null pressure system.
Blood pressure was measured by a servo-null pressure system (model 5A-LN, Instrumentation for Physiology and Medicine, San Diego, CA) using standard procedures (3, 17, 19). Simultaneous ECG and blood pressure signals (see Fig. 2A) were digitized at a sampling rate of 1,000 Hz with the PowerLab A/D Converter (ML750, AD Instruments) and recorded for further processing.
Fig. 2.
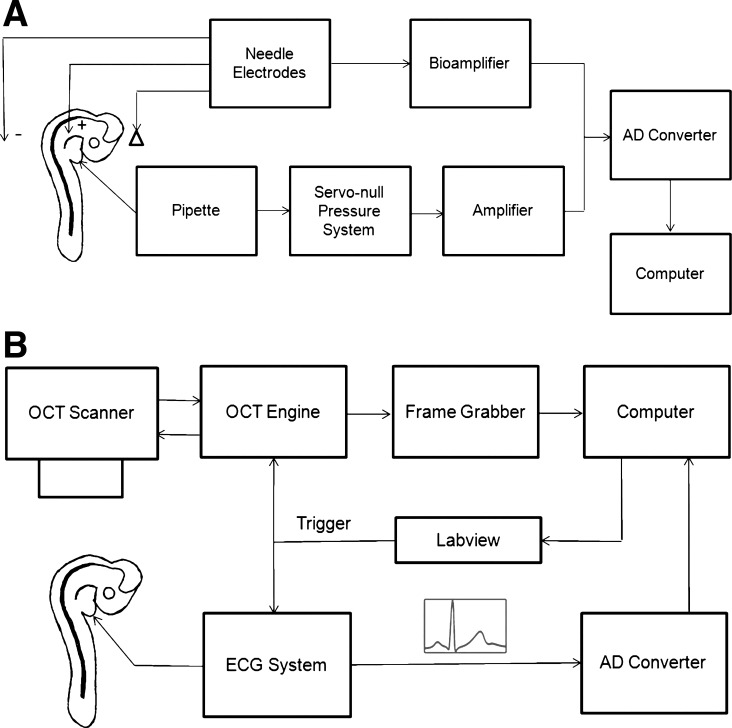
Schematic representation of the simultaneous acquisition of ECG signals and hemodynamic data. A: system setup for simultaneous ECG-pressure data acquisition. B: system setup for simultaneous ECG-OCT acquisition. AD, analog to digital.
OCT system.
Our custom-made OCT system consisted of a 1,325-nm source centered at 100 nm from Thorlabs (Newton, NJ) and a 1,024-pixel infrared InGaAs line-scan camera with a maximal line scan rate of 92 kHz from Goodrich (Charlotte, NC). This system can acquire image sequences (512 × 512 pixels, 512 A-scans) at ∼140 frames/s with a resolution of <10 μm. We have previously used a similar OCT system to image the chicken embryo heart (23, 26, 28). In this study, we integrated ECG data with OCT imaging. The configuration of the ECG-OCT system is shown in Fig. 2B.
Experimental Procedure and Data Analysis
Simultaneous ECG-flow measurements.
Blood flow data were obtained by placing the laser-Doppler flow probe near the proximal DA (see Fig. 1A) while simultaneously acquiring ECG data. To analyze variations in the phase of the R peak (relative to the cardiac cycle) due to electrode position, ECG needle electrodes were moved while the laser-Doppler flow probe remained in one location. We selected five combinations of electrode positions to test. The reference ECG electrode was always placed in the extraembryonic fluid. The positive electrode was placed either close to the heart in the embryo body (at two different depths) or a bit farther from the heart (closer to the eye). The negative electrode was inserted either in the embryo tail or in the extraembryonic fluid (away from the heart). For each embryo (n = 4), data were acquired for each of the five electrode positions, and care was taken not to move the embryo or flow system during measurements to facilitate data comparisons. From simultaneous ECG-flow measurements, we calculated the time lag (Φ) for flow to peak after cardiac depolarization marked by the R peak in the ECG data. We then compared Φ values obtained when electrode positions were changed.
We further investigated whether the phase of the ECG is affected by small temperature variations, which also affect the period of the cardiac cycle. To this end, for each embryo (n = 4), we measured Φ (as described above) as the embryo temperature was varied.
We simultaneously collected at least five cardiac cycles of flow and ECG data from each embryo and each measurement (at each ECG electrode configuration). For each data set, we calculated the average Φ from five cardiac cycles. Only NL embryos were considered for simultaneous ECG-flow data acquisition.
Simultaneous ECG-pressure measurements.
Embryos from each group (NL, CON, and OTB) were placed on an XYZ translational stage. A micromanipulator was used to insert the servo-null pressure pipette into the heart or vasculature (ventricle, AOS, and DA) with the aid of the OCT system. After the pressure pipette had been placed, needle electrodes were inserted to acquire ECG signals.
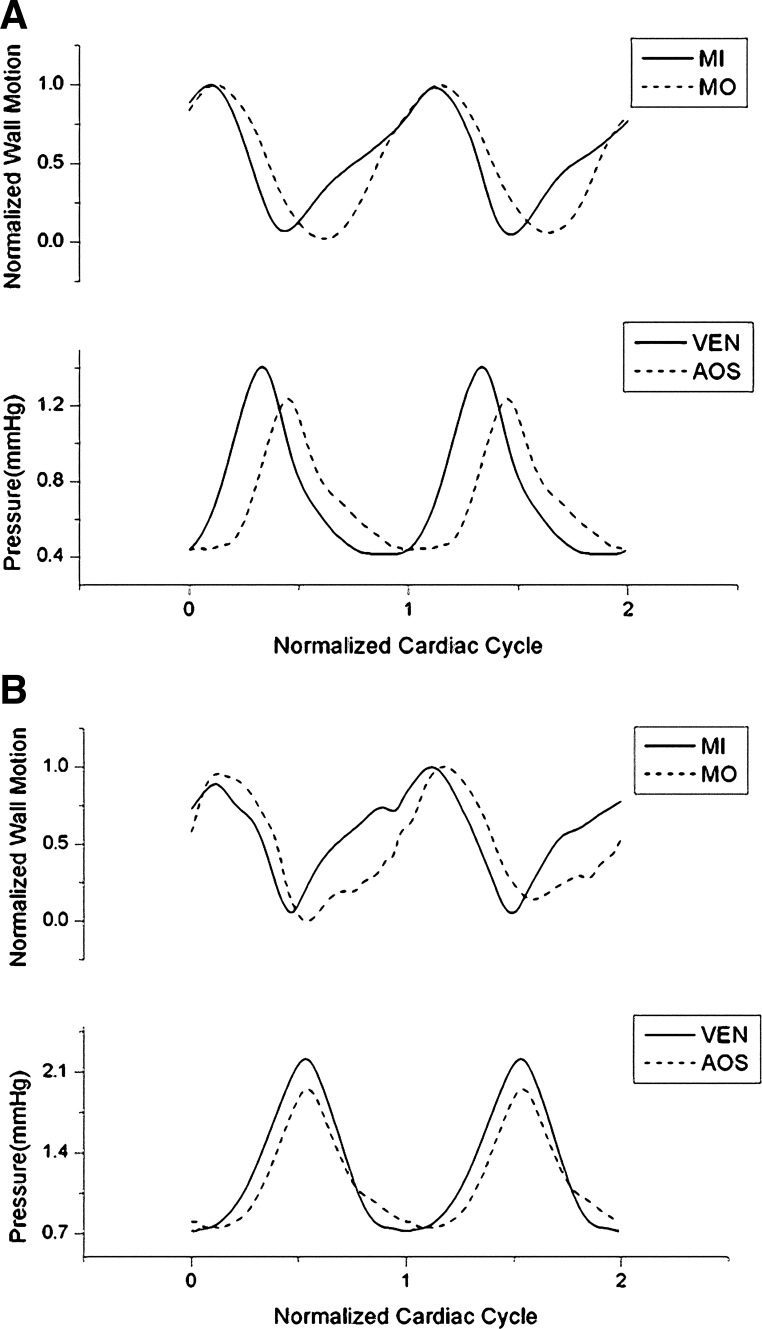
Simultaneously acquired ECG and pressure data were recorded for at least five consecutive cardiac cycles for each embryo in each group (n ≥ 8). Pressure and ECG data were processed using a custom Matlab program to quantify, from each data set, average pressure, pressure pulse amplitude (ΔP; the difference between systolic and diastolic pressure), average period of the cardiac cycle (T), and average Φ for blood pressure to peak after cardiac depolarization marked by the R peak in the ECG data (see Fig. 3). Because the pressure pulse originates in the ventricle, travels through the OFT to the AOS, and then moves through the aortic arches to the DA, we expected peak pressures in the ventricle, AOS, and DA to occur in sequence. Thus, in our data, we added a delay of one cardiac cycle to the Φ between the ECG R peak and DA pressure peak (see Fig. 3) to avoid a negative, unrealistic PTT between the AOS and DA or between the ventricle and DA.
Fig. 3.
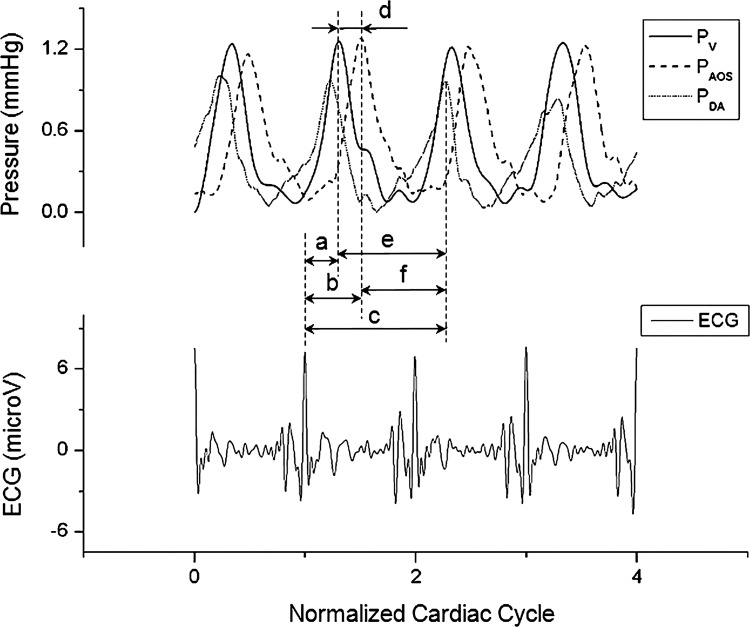
Example of synchronized pressure data from the ventricle, AOS, and DA of HH18 chick embryos acquired simultaneously with ECG signals. PV, ventricular pressure; PAOS, AOS pressure; PDA, DA pressure. The plot also shows the normalized time lag (φ = Φ/T, where Φ is the time lag and T is the period) for pressure to peak after cardiac depolarization: a, b, and c correspond to φV, φAOS, and φDA, respectively. Furthermore, normalized pulse transit times (NP = PTT/T, where PTT is the pulse transit time) are also shown: d, e, and f correspond to NPV-AOS, NPV-DA, and NPAOS-DA, respectively.
Based on ECG-pressure data, we calculated PTT as the time it takes for the peak pressure to travel between two sites in the cardiovascular system. To this end, we used the computed Φs from ventricular, AOS, and DA data (ΦV, ΦAOS and ΦDA, respectively) as follows (see also Fig. 3):
| (2) |
| (3) |
| (4) |
where PTTV-AOS, PTTV-DA, and PTTAOS-DA are PTTs between the ventricle and AOS (V-AOS), ventricle and DA (V-DA), and AOS and DA (AOS-DA), respectively. For additional comparisons, we normalized Φ and PTT to T.
To synchronize pressure data from different locations in the cardiovascular system (ventricle, AOS, and DA) and different embryos, we first normalized time to T and then aligned the pressure data to the R peaks of the ECG data.
Simultaneous ECG-OCT measurements.
Simultaneous ECG-OCT data were also collected from the three groups (NL, CON, and OTB, n ≥ 8). To investigate pulse wave propagation from OFT wall motion, we obtained OCT images of a longitudinal section of the OFT while collecting synchronized ECG data from the embryos. We acquired a total of 300 frames, at 280 frames/s (256 A-scans/frame), which corresponded to ∼2.5 cardiac cycles. Simultaneous ECG-OCT measurements enabled the synchronization of pressure data and OFT cardiac wall motion.
To analyze the OCT imaging data, we selected a line close to the OFT inlet and another line close to the OFT outlet (the distance between these two lines was ∼600 μm; see Fig. 4A). We then generated M-mode images, which show grayscale intensity data along the line in the vertical directions versus time in the horizontal direction (see Fig. 4, B and C). From the M-mode images, we could visualize and trace the motion of the OFT upper wall. Note that, due to insertion of ECG electrodes into the embryo, the embryo descended slightly into the extraembryonic fluid, and, because of OCT imaging depth limitations, we could no longer visualize the lower myocardium wall. By analyzing the M-mode images, however, we found the time difference between maximal wall contraction in the OFT inlet and outlet. This time difference is the OFT PTT measured using wall motion (PTTMI-MO). We then compared PTTMI-MO obtained from OCT with the corresponding PTTV-AOS obtained from pressure data.
Fig. 4.
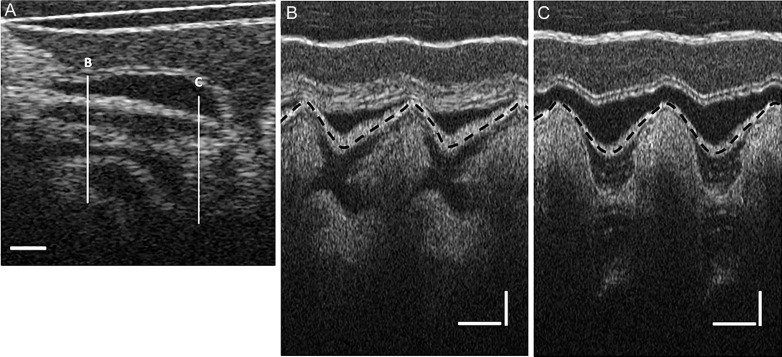
OCT images of the HH18 chick heart OFT. A: longitudinal section of the OFT. Scale bar = 200 μm. B and C: M-mode images obtained from a series of longitudinal OFT images along lines B and C in A, respectively. Horizontal scale bar = 200 ms; vertical scale bar = 200 μm.
Statistics.
Statistical analysis was performed on all data. Results are expressed as means ± SD. Statistical significance was determined by Student's t-test with statistically significant differences defined as P values of <0.05.
RESULTS
ECG-Flow Measurements
To study the effect of ECG needle electrode placement on ECG signals, ECG and DA blood flow data were simultaneously collected from normal HH18 chicken embryos (n = 4). Changing the position of the ECG electrodes minimally affected Φ: variations were <25 ms (<7% of the cardiac cycle), which is within the flow data uncertainty range. This result demonstrates the robustness of the embryonic ECG data to synchronize signals. Stronger ECG signals were obtained when the negative electrode was placed in the embryo tail and the positive electrode was close to the heart. Thus, for the remaining measurements, we used this electrode configuration, making sure that electrode position did not interfere with pressure and OCT data acquisition.
The robustness of using ECG signals to synchronize cardiac data when cardiac cycle changed due to temperature variations was also determined. Even large changes in cardiac cycle (from 300 to 600 ms, n = 4) did not result in variations of Φ (differences were <25 ms, within the flow data uncertainty range).
ECG-Pressure Measurements
At least 8 embryos/group (NL, CON, and OTB) were used to acquire simultaneous ECG-pressure data. During measurements, T was kept close to 400 ms, with variations of <40 ms (∼10% of the cardiac cycle; Table 1). Results from the acquired data are shown in Table 1.
Table 1.
Summary of results from simultaneous blood pressure and ECG measurements in HH18 chicken embryos
| Variables | NL | OTB | CON |
|---|---|---|---|
| TV, ms | 407 ± 21 | 414 ± 21 | 405 ± 20 |
| TAOS, ms | 390 ± 20 | 383 ± 19 | 406 ± 18 |
| TDA, ms | 400 ± 31 | 393 ± 13 | 401 ± 30 |
| ΔPV, mmHg | 1.05 ± 0.19 | 1.66 ± 0.19 | 1.09 ± 0.14 |
| ΔPAOS, mmHg | 0.94 ± 0.12 | 1.26 ± 0.16 | 0.94 ± 0.11 |
| ΔPDA, mmHg | 0.79 ± 0.15 | 0.98 ± 0.08 | 0.83 ± 0.13 |
| PV, mmHg | 0.33 ± 0.10 | 0.65 ± 0.11 | 0.34 ± 0.15 |
| PAOS, mmHg | 0.33 ± 0.13 | 0.68 ± 0.14 | 0.35 ± 0.11 |
| PDA, mmHg | 0.46 ± 0.09 | 0.75 ± 0.11 | 0.49 ± 0.12 |
| Maximum dPV/dt | 5.04 ± 0.93 | 5.41 ± 0.36 | 5.02 ± 1.08 |
| Maximum dPAOS/dt | 5.31 ± 0.56 | 5.79 ± 1.04 | 5.21 ± 0.46 |
| Maximum dPDA/dt | 2.45 ± 0.32 | 3.33 ± 0.89 | 2.53 ± 0.42 |
| ΦV, ms | 132 ± 13 | 218 ± 9 | 146 ± 13 |
| ΦAOS, ms | 174 ± 7 | 212 ± 14 | 182 ± 16 |
| ΦDA, ms | 503 ± 14 | 577 ± 13 | 505 ± 9 |
| φV | 0.33 ± 0.03 | 0.55 ± 0.03 | 0.36 ± 0.03 |
| φAOS | 0.45 ± 0.03 | 0.53 ± 0.03 | 0.45 ± 0.03 |
| φDA | 1.26 ± 0.02 | 1.44 ± 0.03 | 1.26 ± 0.02 |
Values are means ± SD. Data are shown for the following three groups of embryos considered: 1) normal (NL) embryos, 2) outflow tract (OFT)-banded (OTB) embryos, and 3) control (CON) embryos. Subscripts V, AOS, and DA correspond to measurements performed in the ventricle, aortic sac, and dorsal aorta, respectively. HH18, Hamburger-Hamilton stage 18; T, period of the cardiac cyle; ΔP, amplitude of the pulse pressure wave; P, diastolic pressure (minimum pressure); maximum dP/dt, maximum rate of pressure change; Φ, time lag for blood pressure to peak after cardiac depolarization marked by the R peak of the ECG signal; φ, normalized Φ (φ = Φ/T). Note that ΦDA and φDA reported here include a delay of one cardiac cycle.
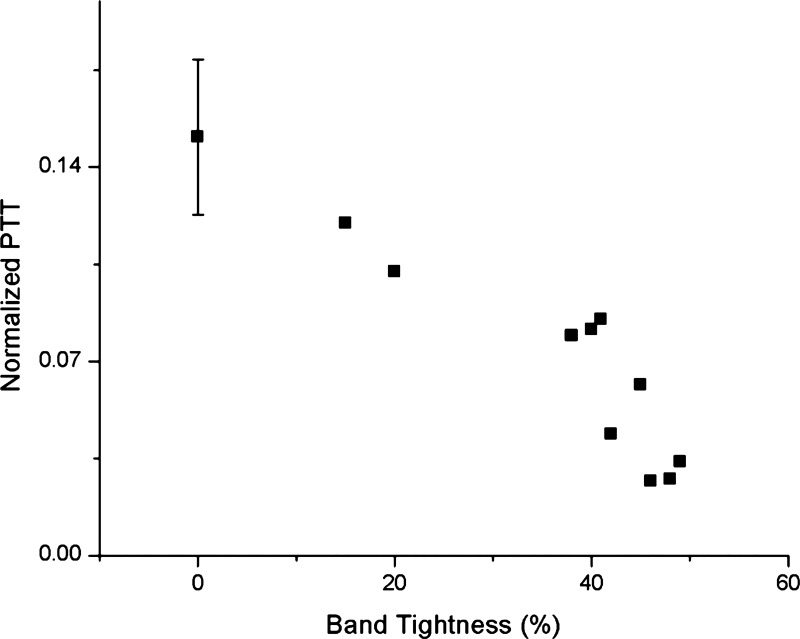
Note that for analysis, we considered OTB embryos with a band tightness ranging from 35% to 50% (n = 8; see Table 1), mainly because below 35% tightness there was no significant change in blood pressure or Φ and above 50% tightness embryo survival was severely compromised. However, we added results from additional embryos with band tightness of 10–20% (n = 2) in some plots to show trends (see Figs. 5 and 6).
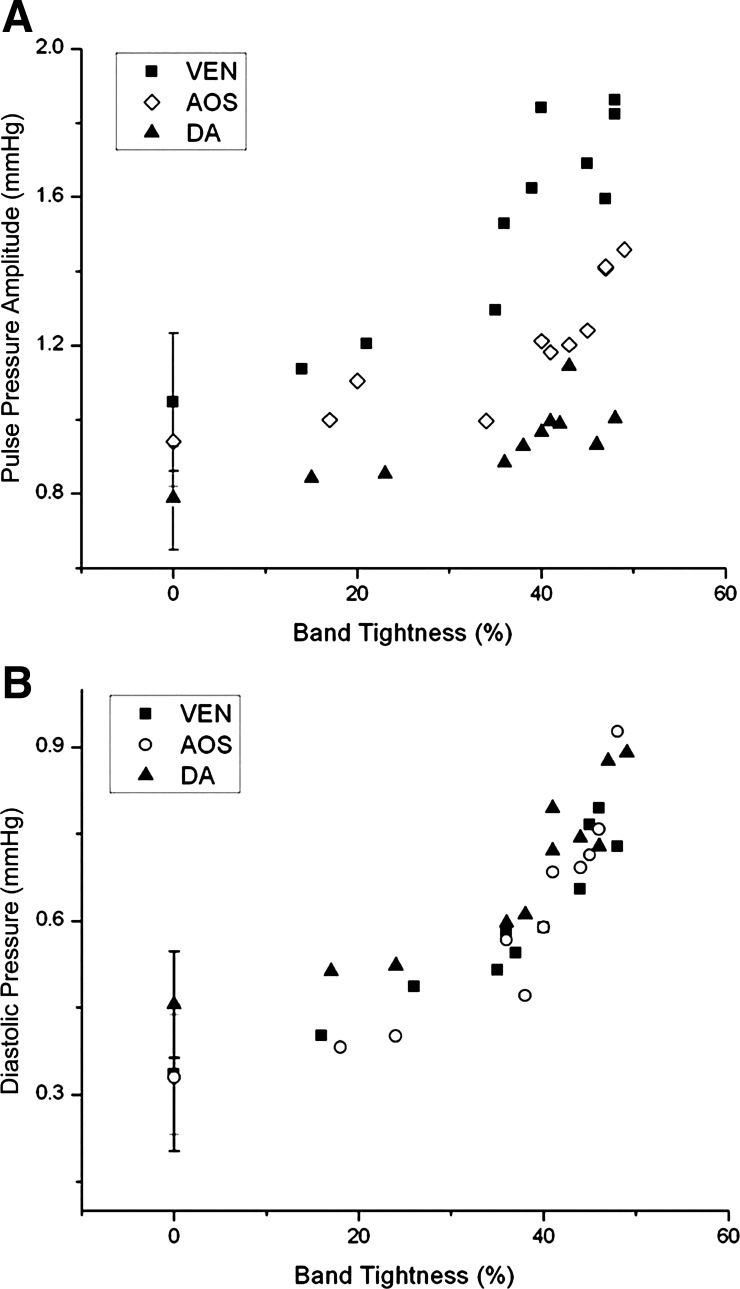
Fig. 5.
Blood pressure increases in response to band tightness. A: pulse pressure amplitude (ΔP) as a function of band tightness. B: minimum (diastolic) pressure versus band tightness. Blood pressure is shown for the ventricle (VEN), AOS, and DA of HH18 chick embryos 2 h after banding. The data shown include 8 embryos with band tightness from 35% to 50% (used to generate the data shown in Table 1), 2 embryos with band tightness from 10% to 30%, and averages and SDs from 8 control embryos (0% band tightness).
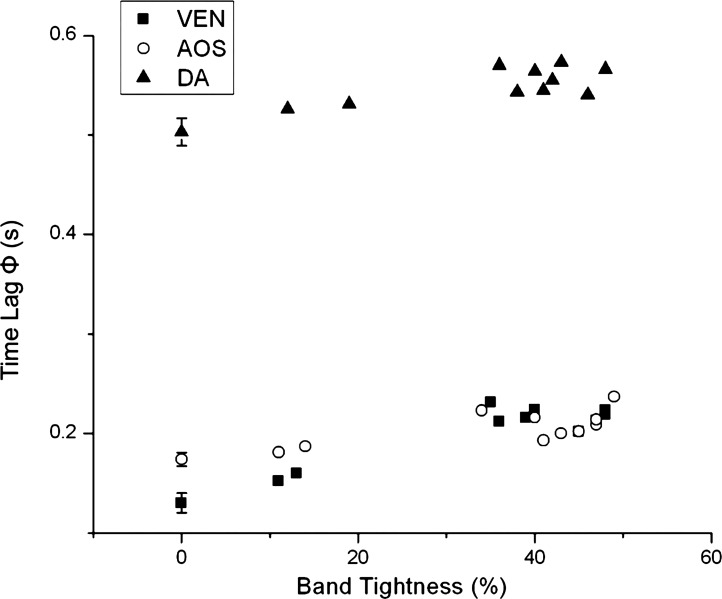
Fig. 6.
Values of Φ for blood pressure to peak after cardiac depolarization as a function of band tightness. Results are from the same embryos shown in Fig. 5; thus, the data correspond to blood pressure in the ventricle, AOS, and DA of HH18 chick embryos after 2 h of banding.
Blood pressure and Φ significantly increased in the OTB group when band tightness was 35–50% (see Table 1), in agreement with a previous study (7). Pressures in CON and NL groups were not significantly different. Compared with NL and CON embryos, OTB diastolic blood pressure (minimum pressure) almost doubled both in the ventricle and AOS and increased ∼60% in the DA. Together with an increase in diastolic pressure, pressure amplitude (ΔP) increased ∼60% in the ventricle and ∼30% in both the AOS and DA. Moreover, diastolic pressure and ΔP were dependent on OTB band tightness (see Fig. 5). ECG-pressure Φ values were similar between NL and CON groups (P = 0.62), whereas Φ values in the OTB group were larger and depended on band tightness (see Fig. 6).
Analysis of pressure data revealed that the shape of the blood pressure curves was generally consistent within each group (see Fig. 7). As expected due to variations in band tightness, however, the SD of the curves in the OTB group (band tightness: 35–50%) was higher than deviations in the NL and CON groups.
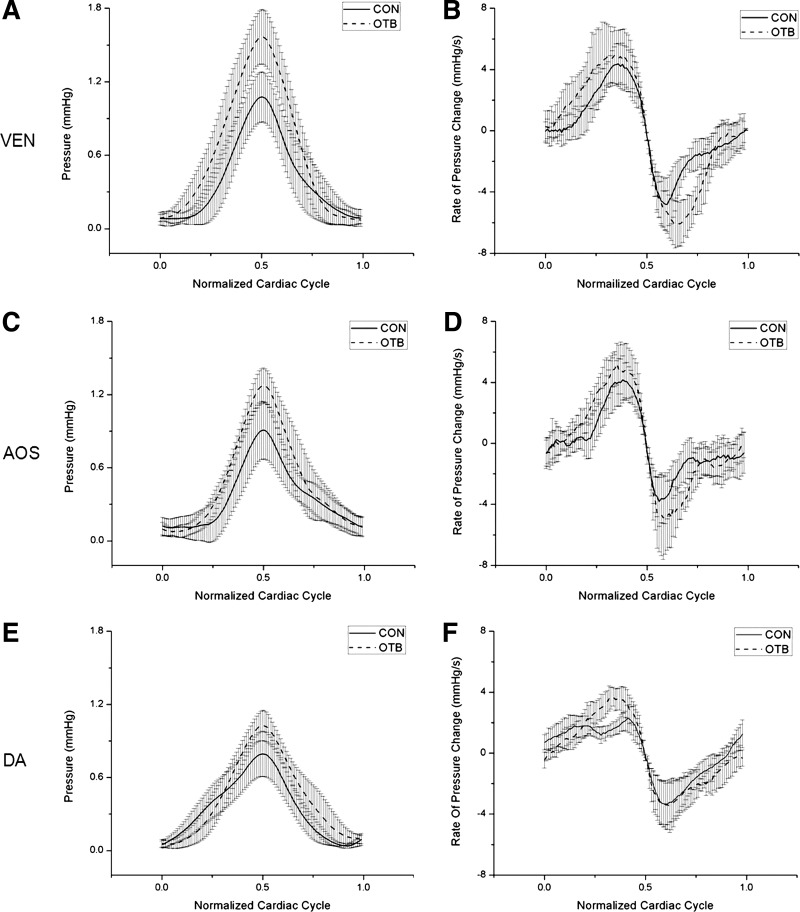
Fig. 7.
Comparison of pressure data acquired from HH18 control (CON) and OTB embryos. Results are shown as averages ± SD over the normalized cardiac cycle for CON (n = 8) and OTB (n = 8, 35–50% band tightness) embryos. A, C, and E: blood pressure (with respect to diastolic pressure). B, D, and F: rate of pressure change (dP/dt). Data are shown for the ventricle (A and B), AOS (C and D), and DA (E and F).
Rates of pressure change were also consistent within each group (see Fig. 7), with no significant differences found between NL and CON groups. Interestingly, there was no difference when we compared the rates of pressure change between NL and OTB groups in the ventricle and AOS. However, in the DA, there was a plateau-like phase in the rate of pressure change before systole in NL and CON embryos (see Fig. 7F), but this plateau disappeared after OTB.
Whereas ΔP significantly increased after OTB (see Fig. 5 and Table 1), the maximum rate of change in blood pressure was not significantly different in the ventricle and AOS (difference: <10%; see Fig. 7, B and D). However, in the DA (Fig. 7, E and F), the maximum rate of pressure change increased ∼40% after OTB (P < 0.001).
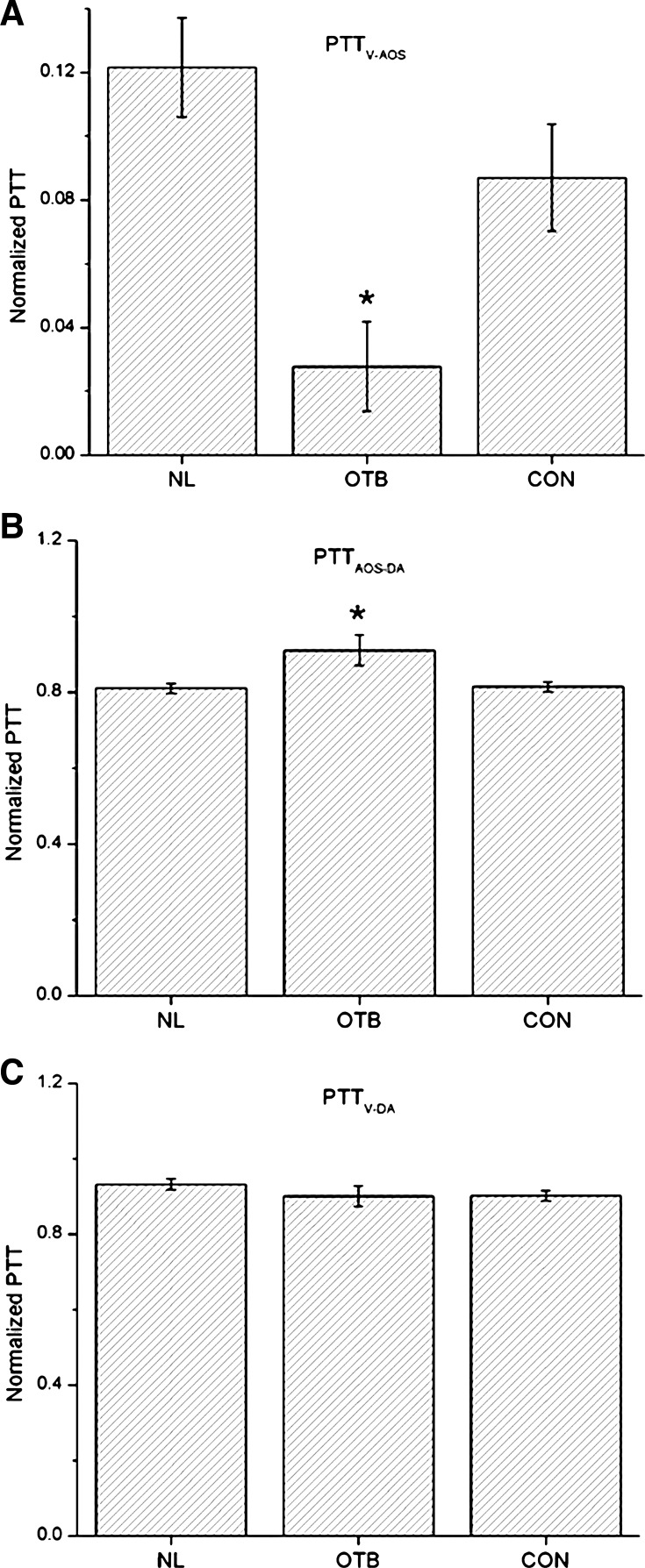
Using ECG-pressure data, PTTV-AOS, PTTV-DA, and PTTAOS-DA were calculated based on Eqs. 2–4 (see Fig. 8 and Table 1). Differences between the NL and CON groups were not significant (P = 0.06 for PTTV-AOS and PTTV-DA and P = 0.71 for PTTAOS-DA). However, PTTV-AOS significantly decreased, whereas PTTAOS-DA increased, in the OTB group (P < 0.01, 35–50% band tightness). PTTV-DA showed no significant differences among OTB, NL, or CON embryos.
Fig. 8.
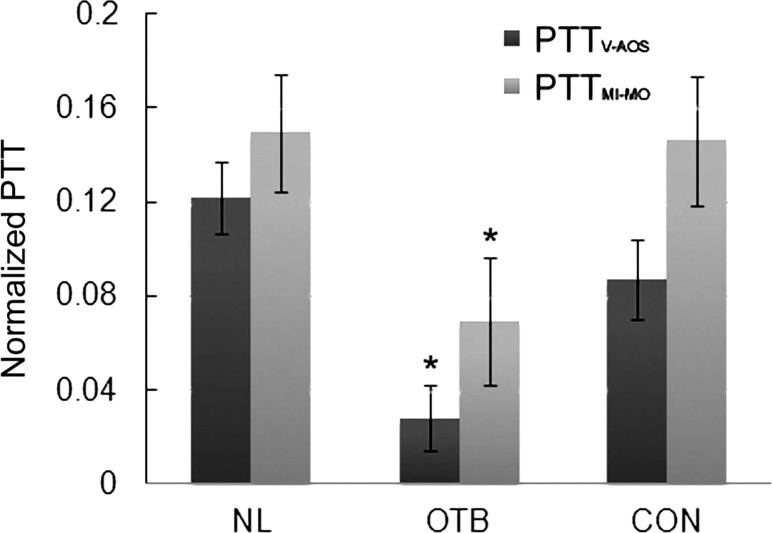
Comparison of normalized PTT (NP = PTT/T) obtained from simultaneous ECG-pressure measurements in HH18 chick embryos. A: NP between the ventricle and AOS (PTTV-AOS/T). B: NP between the AOS and DA (PTTAOS-DA/T). C: NP between the ventricle and DA (PTTV-DA/T). Results are shown as averages ± SD. Data are shown for the three groups of embryos considered: normal (NL), OTB (35–50% band tightness), and CON. *P < 0.05, statistically significant difference with respect to NL and CON embryos.
ECG-OCT Measurements
PTT was estimated based on Φ values between inlet and outlet OFT wall motion imaged with OCT (PTTMI-MO). The results are shown in Table 2. Normalized PTTMI-MO (see Fig. 9) changed significantly after OTB (P < 0.01), which is consistent with PTTV-AOS data obtained from ECG-pressure acquisitions, even though PTTMI-MO was slightly larger than PTTV-AOS.
Table 2.
Summary of results obtained from data analyses of optical coherence tomography images of the OFT of HH18 embryos acquired simultaneously with ECG data
| Variables | NL | OTB | CON |
|---|---|---|---|
| T, ms | 401 ± 34 | 404 ± 14 | 397 ± 21 |
| ΦMI, ms | 166 ± 15 | 188 ± 17 | 162 ± 12 |
| ΦMO, ms | 226 ± 18 | 212 ± 19 | 220 ± 14 |
| PTTMI-MO, ms | 60 ± 10 | 28 ± 11 | 58 ± 11 |
Values are means ± SD. Data are shown for the following three groups of embryos considered: 1) NL, 2) OTB, and 3) CON. Φ, time lag for maximal OFT wall contraction after cardiac depolarization marked by the R peak of the ECG signal; MI, inlet OFT myocardial wall; MO, outlet OFT myocardial wall; PTTMI-MO, pulse transit time between the OFT inlet and outlet myocardial wall motion.
Fig. 9.
Comparison of NPs to travel the heart OFT obtained from simultaneous ECG-pressure measurements (PTTV-AOS) and OCT imaging [time difference between maximal wall contraction in the OFT inlet and outlet (PTTMI-MO)] of HH18 embryos. Results are shown as averages ± SD. Data are shown for the three groups of embryos considered: NL, OTB (35–50% band tightness), and CON. *P < 0.05, statistically significant difference with respect to NL and CON embryos.
Similar to results obtained from the ECG-pressure data, Φ values between ECG and wall motion (maximal contraction) increased after OTB. To further analyze the data, wall motion and blood pressure curves were normalized to the cardiac cycle, and wall motion and blood pressure data were synchronized using ECG signals. Average data over time are shown for the group (n = 8/group) in Fig. 10. Both the ventricular pressure peak and AOS pressure peak occurred during local myocardium wall contraction for all groups (NL, OTB, and CON).
Fig. 10.
Synchronized wall motion and blood pressure across the OFT of HH18 chick embryos. A: NL embryos (n = 8). B: OTB embryos (n = 8, 35–50% band tightness). SDs are not shown. MI, OFT inlet myocardial wall displacement; MO, OFT outlet myocardial wall displacement.
DISCUSSION
Despite the importance of understanding the impact of blood flow conditions on the cardiovascular system during embryonic development, the relationship between flow in the heart and flow in the vitelline circulation is not well understood. This is in part due to limited methods to accurately quantify and synchronize hemodynamic data (pressure and flow) from different locations in the embryonic cardiovascular system. To overcome these limitations, in this study, we used ECG signals as a means of synchronizing data acquired at different sites (heart ventricle, AOS, and DA) and from different measurement systems (OCT and the servo-null pressure system).
OFT wall motion constriction in banded embryos alters pressure wave propagation. Banding is a procedure frequently used to alter hemodynamic conditions in chicken embryos, and it is known to lead to cardiac malformations (29). The exact mechanisms by which banding alters cardiac development is yet to be determined, but it is widely accepted that hemodynamic changes due to banding (increased blood pressure and increased wall shear stresses) trigger mechanotransduction mechanisms by which the heart adjusts to the new “banded” conditions. While previous studies have addressed changes in hemodynamic conditions over developmental stages (e.g., 5, 6, 16, 17, 19) and due to banding (e.g. 7, 8, 29), we present here, for the first time, the impact of OTB on the heart and circulation (Φ, PTT, and blood pressure data) and how changes depend on the degree of band tightness. This study provides data on the immediate changes (within 2 h) that occur after banding the embryonic chick heart at HH18. Presumably, these changes represent only a mechanical and perhaps fast biochemical adjustment to banding, as the heart and vitelline circulation tissues have not had enough time to remodel. We found that banding increases blood pressure in the chick circulation (ventricle, AOS, and DA) and delays peak ventricular pressure after cardiac depolarization (and thus increases ΦV) and that these changes strongly depend on the degree of band tightness. Moreover, after OTB, PTTV-AOS decreases, but, surprisingly, PTTV-DA is preserved.
Validation of ECG Data Acquisition
ECG signals can be reliably used to synchronize blood pressure and cardiac wall motion to the cardiac cycle in chicken embryos. To determine the robustness of ECG data from early chick embryos, we used a Doppler flow laser system, which provided a reliable, easy-to-analyze signal that could be acquired simultaneously with ECG. Because measurements were noninvasive, flow data were more reliable than pressure data, and continuous measurements of flow from the DA while changing the position of ECG electrodes were feasible. The results indicated that the chick embryonic ECG could be reliably used as a signal to synchronize cardiovascular events.
The effect of changes in temperature, and thus cardiac cycle T, on ECG signals was also investigated. Surprisingly, Φ between the ECG R peak and the peak of the flow signal was not significantly altered even with large (∼50%) changes in T. These data suggest that, upon cardiac depolarization, the speed of pulsatile waves do not vary with temperature. Rather, temperature affects the timing between contraction events. Thus, normalization of temporal data to T may be problematic. In the analysis presented here, T was tightly controlled (fluctuations were <10%; see Table 1) to avoid ambiguities in phase shift comparisons.
Pulse Wave Propagation in the HH18 Chick Circulation
Periodic wall contraction of the embryonic ventricle generates a pulsatile pressure wave that travels through the chick circulatory system. Previous studies (19, 31) have demonstrated that, even though the HH18 chick tubular heart has no valves or chambers, ventricular pressure-volume loops are remarkably similar to those of the mature system (but with much smaller pressure and volume values). These data show that the primitive heart is an efficient pump. The relationship between pumping mechanics and the mechanics of the resulting traveling wave in the vitelline circulation, however, had not been extensively studied. After ventricular depolarization (R peak of the ECG signal), maximum systolic pressure in the ventricle was achieved with a delay of ∼30% of the cardiac cycle. Pressure waves originating in the ventricle quickly propagated through the OFT (propagation took ∼12% of the cardiac cycle) and then through the aortic arches and DA (propagation was ∼80% of the cardiac cycle). Since pressure in the DA was measured at the level of the heart (see Fig. 1A), the pressure wave traveled a relatively long distance from the AOS to the site where DA pressure was measured (at least 5 mm from the AOS to DA vs. ∼600 μm from the ventricle to AOS). When accounting for changes in traveled lengths, it is interesting to note that the propagation velocity from the AOS to the DA is almost the same as that from the ventricle to the AOS (∼15 mm/s). Note however, that PWV can only be estimated, as in our measurements we did not accurately control distances but rather used morphological landmarks for the measurements. Measured PWV in the normal HH18 chicken embryonic DA has been previously found to be ∼500 mm/s (38), which is ∼30 times faster than the velocity we estimated for the wave to travel from the AOS to DA. The slower estimated velocity of pressure wave propagation is likely due to a slower propagation velocity in the aortic arches. Because of the complex geometry of the aortic arches, no studies have directly addressed pressure wave propagation inside them. A more detailed investigation of the effects of aortic arches on pulse wave propagation, however, is outside the scope of the present report.
Blood flows through the OFT by a combination of wave propagation and active wall contraction. Active pushing of blood through the OFT is suggested by synchronized analysis of OFT wall motion (from OCT images) and blood pressures (ventricle and AOS). Our results revealed that peak ventricular systolic pressure occurs immediately before maximum OFT-inlet wall contraction (see Fig. 10A). Similarly, peak AOS pressure occurs just before maximum OFT-outlet wall contraction (Fig. 10A). While surprising at first, these data are in agreement with our previous results (22, 23). We previously modeled blood flow through the OFT using wall motions and blood pressure data as boundary conditions and compared the predicted blood flow velocities with velocities measured using ultrasound and OCT. In those studies, PTTV-AOS was unknown, and the timing between OFT wall motion and blood pressure was also uncertain, so that comparison with measured velocities was used to determine PTTV-AOS and to synchronize wall motion and blood pressure (ΦMI and ΦV). We found that to reproduce measured blood flow velocities, PTTV-AOS had to be ∼10% of the cardiac cycle (our present results show a PTTV-AOS of ∼12%), and Φ between maximal inlet OFT wall expansion and peak systolic ventricular pressure was ∼20% of the cardiac cycle (our present results also show Φ of ∼20%), with maximal wall expansion occurring before ventricular peak pressure (as in our present results; see Fig. 10A). Simultaneous ECG-pressure and ECG-OCT data were not available at the time we performed these previous studies; thus, those results were reached independently. Furthermore, pressure peak and wall motion relationships in the OFT were consistent with previous pressure-volume measurements in the chicken embryo heart (19), and PTTV-AOS was consistent with the reported depolarization speed (conductance speed) in the OFT wall (22). The data thus far indicate that pressure wave propagation in the OFT is the result of blood flow interacting with an actively contracting myocardium wall; otherwise (i.e., passive wall), maximum pressure would be coincident with maximum OFT wall expansion. Active contraction of the OFT myocardium may aid in the increase of systolic blood pressure.
Our observations show that the chick ventricle contracts and expands almost synchronously, whereas wall motion in the OFT is more peristaltic-like. This observation is in agreement with other studies (1, 27, 30), which explained the difference between ventricle and OFT contraction as the result of higher conduction speeds in the ventricle aided by trabecular structures and the onset of development of the cardiac conduction system. Our synchronized OFT wall motion-pressure data show that as myocardial cells start passive relaxation after peak wall contraction, high blood pressure could facilitate OFT wall expansion.
Pulse Wave Propagation in Banded HH18 Chick Embryos
After 2 h of OFT banding, several hemodynamic changes occur that affect pulse wave propagation in the chick embryonic cardiovascular system (see Table 1), and these changes strongly depend on band tightness. Banding increases ventricular, AOS, and DA blood pressures in OTB embryos (both diastolic and peak systolic; see Fig. 5) and also increases ΦV (see Fig. 6). However, banding decreases PTT in the OFT, as evidenced by both PTTV-AOS and PTTMI-MO (see Figs. 6 and 11). These immediate hemodynamic alterations (within a few minutes to a few hours) are of particular importance, as they provide the initial mechanical stimuli for cardiac cells to adjust to the new hemodynamic environment.
Fig. 11.
NPs in the OFT versus band tightness obtained from OCT images. Data were acquired from 8 OTB embryos with band tightness between 35% and 50% and 2 OTB embryos with band tightness between 10% and 30%. Data from 0% band tightness corresponds to CON embryos (n = 8), shown as the average NP ± SD.
The increase in embryonic blood pressure is a consequence of cardiac afterload introduced by the band. The dependence of ventricular blood pressure on band tightness was expected: the tighter the band, the larger the resistance to blood flow through the OFT, and thus the larger the increase in ventricular pressure. Since both ventricular diastolic and systolic pressures increased after banding, it is reasonable to expect an increase in blood pressure in the rest of the chick circulation as a result of traveling pressure waves (39, 40). Within 2 h of banding, the increase in blood pressure is not significant until band tightness reaches ∼30% (see Fig. 5). Beyond 30% tightness, however, blood pressure depends nonlinearly on band tightness.
The increased in ΦV could be due to a combination of wave reflection and cardiomyocyte contraction events. The band restricts motion of the OFT wall, creating a region of high impedance and a wave reflection site. It is possible that when the pressure wave originating in the ventricle reaches the band site, wave reflection (back to the ventricle) causes a time delay in the ventricular pressure peak. As band tightness increases, the reflected portion of the coming pressure wave should also increase, possibly explaining the increase in ΦV and its dependence on band tightness. Moreover, cardiomyocytes generally exhibit an inverse relationship between generated force and contraction velocity. Thus, it is also possible that the afterload introduced by banding increases the time for full myocardial force generation after depolarization, increasing ΦV, as also suggested in Ref. 20. A combination of pulse reflection and a slower cardiomyocyte force generation could therefore contribute to the larger ΦV observed after banding.
The faster wave propagation in the OFT of banded embryos is consistent with an “artificial” stiffening of the OFT wall due to the presence of the band, a surgical suture that restricts wall motion. As the wall becomes stiffer, the speed of pressure wave propagation should increase, with a larger increase corresponding to tighter banding, which correspond to a stiffer, less-compliant wall. OTB thus affects not only blood pressure but also pressure wave propagation and, therefore, the peristaltic-like patterns of OFT wall motion.
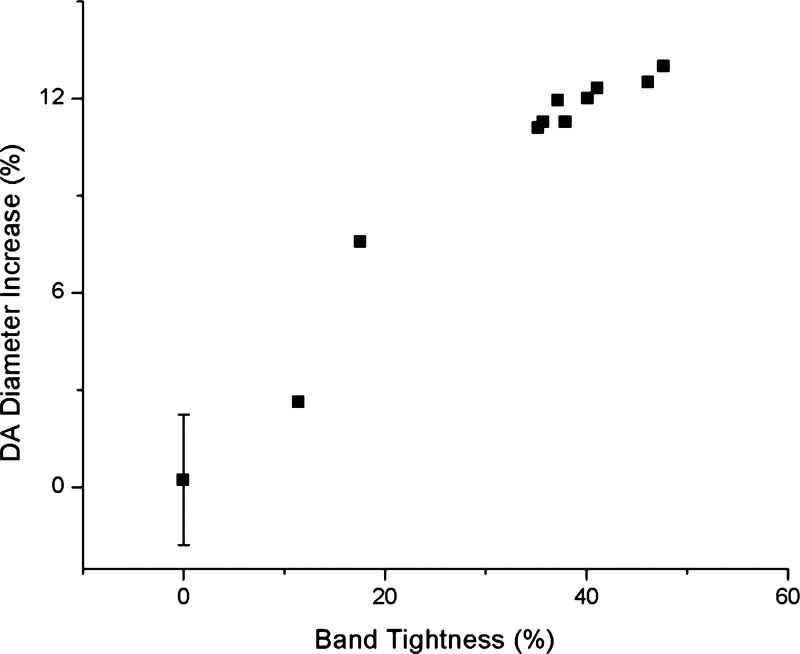
While pressure wave propagation was faster in the OFT of OTB embryos, propagation from the AOS to DA was slightly slower. The increase in PTTAOS-DA could be attributed to an increase in DA diameter after banding (see Fig. 12). From OCT measurements of DA dimensions, DA luminal radius was 240 ± 13 μm before OTB and dilated to 264 ± 17 μm (n = 8) 2 h after OTB (P < 0.01). This increase in diameter is likely caused by either a mechanical change (blood pressure increase that expands the DA walls) or a biochemical change (release of nitric oxide, which dilates the DA walls) or a combination of these. The observed blood pressure increase in the DA after banding could explain, at least partially, the increase in DA diameter. In CON and NL embryos, DA wall radius changes by ∼20 μm after cyclic pressure variations (ΔP ∼ 0.8 mmHg). Note, however, that DA wall excursions are very difficult to quantify using OCT due to contrast, and thus the oscillation data are approximate. After OTB, when diastolic pressure increases by ∼0.25 mmHg, the DA radius increases by ∼20 μm, more than expected due to pressure increase alone. While a more careful study is needed to determine the causes of DA increase in diameter after OTB, our data thus far suggest that an increase in pressure alone does not explain the increase in DA diameter after OTB. Nitric oxide release (potentially from the banded region of the OFT, which is subjected to larger shear stresses after OTB) in combination with the blood pressure increase could explain the relatively large increase in DA diameter observed after 2 h of OTB.
Fig. 12.
DA diameter increase after 2 h of banding versus band tightness.
Surprisingly, whereas PTTV-AOS and PTTAOS-DA changed after OTB, PTTV-DA remained approximately the same (within measurement accuracy) as that in CON embryos. While banding increased PTTAOS-DA along the OFT, it also decreased PTTV-AOS (see Figs. 8A and 9), which in magnitude just compensates the increased PTTAOS-DA caused by OTB (Fig. 8C). Moreover, banding does not significantly change the relationship between OFT wall motion and blood pressure oscillations (see Fig. 10). Peak blood pressure occurs in both cases around the time that the myocardium wall is most contracted. These results suggest that after OTB, mechanical and biochemical adaptations ensure the preservation of cardiac function.
Conclusions
We have successfully acquired simultaneous ECG-pressure as well as simultaneous ECG-OCT data in stage HH18 chicken embryos and used these data to synchronize cardiovascular events. Blood pressure and pressure wave propagation were significantly affected by banding and strongly depended on band tightness. Furthermore, the increased time elapsed between cardiac depolarization and peak ventricular systolic pressure with increased band tightness suggests that cardiomyocyte force generation is slowed by banding and points to a possible reflection of pressure waves at the band site. Our results further show that banding affects the entire embryonic circulation, and thus growth and remodeling in response to altered hemodynamics, which have been shown to lead to cardiac malformations, are likely not confined to the heart. OFT growth and remodeling in response to banding are likely not only the result of pressure changes and increased shear stress but also of altered pressure wave propagation that affects strains and strain gradient distributions.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant R01-HL-094570 and National Science Foundation Grant DBI-1052688.
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of grant-giving bodies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.S. and S.R. conception and design of research; L.S. performed experiments; L.S. and S.H. analyzed data; L.S. prepared figures; L.S. drafted manuscript; L.S., S.G., S.H., and S.R. edited and revised manuscript; S.G., M.T.H., K.L.T., and S.R. interpreted results of experiments; S.R. approved final version of manuscript.
REFERENCES
- 1. Boucek RJ, Murphy WP, Paff GH. Electrical and mechanical properties of chick embryo heart chambers. Circ Res 7: 787–793, 1959 [DOI] [PubMed] [Google Scholar]
- 2. Cain JR, Abbott UK, Rogallo VL. Heart rate of the developing chick embryo. Proc Soc Exp Biol Med 126: 507–510, 1967 [DOI] [PubMed] [Google Scholar]
- 3. Chabert S, Taber LA. Intramyocardial pressure measurements in the stage 18 embryonic chick heart. Am J Physiol Heart Circ Physiol 282: H1248–H1254, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP, Moorman AF. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol 223: 266–278, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Clark EB, Hu N. Developmental hemodynamic changes in the chick embryo from stage 18 to 27. Circ Res 51: 810–815, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Clark EB, Hu N, Dummett JL, Vandekieft GK, Olson C, Tomanek R. Ventricular function and morphology in chick embryo from stages 18 to 29. Am J Physiol Heart Circ Physiol 250: H407–H413, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ. Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am J Physiol Heart Circ Physiol 257: H55–H61, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Clark EB, Hu N, Rosenquist GC. Effect of conotruncal constriction on aortic-mitral valve continuity in the stage 18, 21 and 24 chick embryo. Am J Cardiol 53: 324–327, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation 17: 164–178, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol 21: 1361–1367, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Gan L, Miocic M, Doroudi R, Selin-Sjogren L, Jern S. Distinct regulation of vascular endothelial growth factor in intact human conduit vessels exposed to laminar fluid shear stress and pressure. Biochem Biophys Res Commun 272: 490–496, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res 96: 1291–1298, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 195: 231–272, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res 80: 473–481, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65: 1665–1670, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Hu N, Keller BB. Relationship of simultaneous atrial and ventricular pressures in stage 16–27 chick embryos. Am J Physiol Heart Circ Physiol 269: H1359–H1362, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Jones EA, Baron MH, Fraser SE, Dickinson ME. Measuring hemodynamic changes during mammalian development. Am J Physiol Heart Circ Physiol 287: H1561–H1569, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Keller BB, Hu N, Serrino PJ, Clark EB. Ventricular pressure-area loop characteristics in the stage 16 to 24 chick embryo. Circ Res 68: 226–231, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Keller BB, Yoshigi M, Tinney JP. Ventricular-vascular uncoupling by acute conotruncal occlusion in the stage 21 chick embryo. Am J Physiol Heart Circ Physiol 273: H2861–H2866, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Lin IE, Taber LA. Mechanical effects of looping in the embryonic chick heart. J Biomech 27: 311–321, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Liu A, Rugonyi S, Pentecost JO, Thornburg KL. Finite element modeling of blood flow-induced mechanical forces in the outflow tract of chick embryonic hearts. Comput Struct 85: 727–738, 2007 [Google Scholar]
- 23. Liu A, Yin X, Shi L, Li P, Thornburg KL, Wang R, Rugonyi S. Biomechanics of the chick embryonic heart outflow tract at HH18 using 4D optical coherence tomography imaging and computational modeling. PLos One 7: e40869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134: 3317–3326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lucitti JL, Visconti R, Novak J, Keller BB. Increased arterial load alters aortic structural and functional properties during embryogenesis. Am J Physiol Heart Circ Physiol 291: H1919–H1926, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Ma Z, Liu A, Yin X, Troyer A, Thornburg K, Wang RK, Rugonyi S. Measurement of absolute blood flow velocity in outflow tract of HH18 chicken embryo based on 4D reconstruction using spectral domain optical coherence tomography. Biomed Opt Express 1: 798–811, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paff GH, Boucek RJ. Simultaneous electrocardiograms and myograms of the isolated atrium. Anat Rec 142: 73–79, 1962 [DOI] [PubMed] [Google Scholar]
- 28. Rugonyi S, Shaut C, Liu A, Thornburg K, Wang RK. Changes in wall motion and blood flow in the outflow tract of chick embryonic hearts observed with optical coherence tomography after outflow tract banding and vitelline-vein ligation. Phys Med Biol 53: 5077–5091, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254: 238–252, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Sedmera D, Reckova M, Bigelow MR, Dealmeida A, Stanley CP, Mikawa T, Gourdie RG, Thompson RP. Developmental transitions in electrical activation patterns in chick embryonic heart. Anat Record Part A 280A: 1001–1009, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Stekelenburg-de Vos S, Ursem NT, Hop WC, Wladimiroff JW, Gittenberger-de Groot AC, Poelmann RE. Acutely altered hemodynamics following venous obstruction in the early chick embryo. J Exp Biol 206: 1051–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Taber LA. Biomechanics of cardiovascular development. Annu Rev Biomed Eng 3: 1–25, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Taber LA. Biomechanics of growth, remodeling, and morphogenesis. Appl Mech Rev 48: 487–545, 1995 [Google Scholar]
- 34. Taber LA, Lin IE, Clark EB. Mechanics of cardiac looping. Dev Dyn 203: 42–50, 1995 [DOI] [PubMed] [Google Scholar]
- 35. Tobita K, Schroder EA, Tinney JP, Garrison JB, Keller BB. Regional passive ventricular stress-strain relations during development of altered loads in chick embryo. Am J Physiol Heart Circ Physiol 282: H2386–H2396, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Tomanek RJ, Hu N, Phan B, Clark EB. Rate of coronary vascularization during embryonic chicken development is influenced by the rate of myocardial growth. Cardiovasc Res 41: 663–671, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Tomlins PH, Wang RK. Theory, developments and applications of optical coherence tomography. J Phys D Appl Phys 38: 2519–2535, 2005 [Google Scholar]
- 38. Yoshigi M, Ettel JM, Keller BB. Developmental changes in flow-wave propagation velocity in embryonic chick vascular system. Am J Physiol Heart Circ Physiol 273: H1523–H1529, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Yoshigi M, Keller BB. Characterization of embryonic aortic impedance with lumped parameter models. Am J Physiol Heart Circ Physiol 273: H19–H27, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Yoshigi M, Keller BB. Linearity of pulsatile pressure-flow relations in the embryonic chick vascular system. Circ Res 79: 864–870, 1996 [DOI] [PubMed] [Google Scholar]