Abstract
A contributing factor to increased peripheral resistance seen during hypertension is an increased production of endothelium-derived contractile factors (EDCFs). The main EDCFs are vasoconstrictor prostanoids, metabolites of arachidonic acid (AA) produced by Ca2+-dependent cytosolic phospholipase A2 (cPLA2) following phosphorylation (at Ser505) mediated by extracellular signal-regulated kinase (ERK1/2) and cyclooxygenase (COX) activations. Although endoplasmic reticulum (ER) stress has been shown to contribute to pathophysiological alterations in cardiovascular diseases, the relationship between ER stress and EDCF-mediated responses remains unclear. We tested the hypothesis that ER stress plays a role in EDCF-mediated responses via activation of the cPLA2/COX pathway in the aorta of the spontaneously hypertensive rat (SHR). Male SHR and Wistar-Kyoto rats (WKY) were treated with ER stress inhibitor, tauroursodeoxycholic acid or 4-phenlybutyric acid (TUDCA or PBA, respectively, 100 mg·kg−1·day−1 ip) or PBS (control, 300 μl/day ip) for 1 wk. There was a decrease in systolic blood pressure in SHR treated with TUDCA or PBA compared with control SHR (176 ± 3 or 181 ± 5, respectively vs. 200 ± 2 mmHg). In the SHR, treatment with TUDCA or PBA normalized aortic (vs. control SHR) 1) contractions to acetylcholine (ACh), AA, and tert-butyl hydroperoxide, 2) ACh-stimulated releases of prostanoids (thromboxane A2, PGF2α, and prostacyclin), 3) expression of COX-1, 4) phosphorylation of cPLA2 and ERK1/2, and 5) production of H2O2. Our findings demonstrate a novel interplay between ER stress and EDCF-mediated responses in the aorta of the SHR. Moreover, ER stress inhibition normalizes such responses by suppressing the cPLA2/COX pathway.
Keywords: EDCF, cPLA2, ERK1/2, cyclooxygenase, endoplasmic reticulum stress
hypertension is a leading risk factor for cardiovascular-related morbidity and mortality. A contributing factor to sustained hypertension is increased vascular tone, which is partially controlled by endothelium-derived factors. There is a balance of endothelium-derived relaxing factors (EDRFs), endothelium-derived hyperpolarizing factors (EDHFs), and endothelium-derived contracting factors (EDCFs). Alterations in the balance of these factors can contribute to changes in blood pressure (12, 27, 44). An accumulating body of evidence has suggested that during hypertension there is an increased production of EDCFs and a decreased production of EDRFs and EDHFs. Indeed, there are several reports suggesting that the increased EDCF productions and responses are due to the increased activities/expression of cyclooxygenases (COXs) and increased reactive oxygen species (ROS) production in hypertensive arteries (53, 55). In the vasculature, COX-1 and COX-2 metabolism of arachidonic acid (AA) results in the formation of endoperoxides, which can be processed by enzymes to form vasoactive prostanoids, prostaglandin (PG)E2, PGD2, PGF2α, prostacyclin (PGI2), and thromboxane A2 (TXA2) (11, 55). Therefore, therapies aimed at normalizing the imbalance may be an important target for hypertensive treatment.
The release of AA, which is a substrate for COXs, plays a crucial role in the production of EDCFs (68). After stimulation of membrane receptors, AA release is primarily mediated by the rate-limiting phospholipase A2 (PLA2) family (2). The mammalian PLA2 family consists of a 140-kDa Ca2+-dependent secreted PLA2, an 85-kDa Ca2+-dependent cytosolic PLA2 (cPLA2), and the Ca2+-independent PLA2 (iPLA2). The regulation of cPLA2 is important in understanding how AA release and metabolism contribute to the production of EDCFs during hypertension. The activation of cPLA2 occurs following a rise in cytosolic calcium (Ca2+) levels and by phosphorylation of serine 505 by the mitogen-activated protein (MAP) kinase, extracellular signal-regulated kinase (ERK1/2) (3, 6). However, the mechanism through which ERK1/2 becomes activated to phosphorylate cPLA2 during hypertension is still unclear.
The endoplasmic reticulum (ER) is a multifunctional intracellular organelle (30). The ER is easily perturbed by a variety of cellular stresses, which leads to the misfolding and aggregation of proteins within the lumen of the ER, causing ER stress (18, 29). When ER stress occurs, the ER initiates a complex cellular signaling network known as the unfolded protein response (UPR). The UPR acts through three transmembrane stress sensors, pancreatic ER kinase (PKR)-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6), to reestablish ER and cellular homeostasis. Prolonged activation of the PERK pathway of the UPR has been shown to activate ERK1/2 (31). Chronic ER stress has recently been shown to contribute to major pathophysiological changes in cardiovascular diseases (19). Pharmacological inhibitors of ER stress, such as chemical chaperones that act through improving folding capacity and stabilizing protein conformation, have been shown to ameliorate many cardiovascular pathologies (16, 21, 51). How chemical chaperones might affect the cPLA2 and AA pathway-related responses including EDCF-mediated contractile responses during hypertension is unknown.
During hypertension increased ER stress occurs in the vasculature. Given that ER stress can lead to the phosphorylation of ERK1/2 and that ERK1/2 is known to phosphorylate cPLA2, identifying molecular mechanisms through which ER stress and ERK1/2 are involved in EDCF-mediated signaling could give us important insights into understanding pathological changes that occur during hypertension. In the present study, we tested the hypothesis that the suppression of ER stress could normalize the EDCF-mediated responses in the spontaneously hypertensive rat (SHR).
MATERIALS AND METHODS
Animals and blood pressure measurement.
Adult male Wistar-Kyoto (WKY) rats and SHR, 15–16 wk old (Harlan Laboratories, Indianapolis, IN), were used in these studies. Rats were housed in a 12:12-h light-dark cycle with standard rat chow and water ad libitum. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were reviewed and approved by the Institutional Animals Care and Use committee of the Georgia Regents University.
Rats were anesthetized with isoflurane (5% initially and then maintained at 2.5% in 100% oxygen) via a nose cone for blood withdrawal and euthanasia. The anesthesia was monitored through toe pinch and ocular touch, and no reaction was taken as a confirmation of proper anesthesia. The thoracic aorta was rapidly excised, placed in a 4°C cold physiological salt solution (PSS) containing (mM) 130 NaCl, 14.9 NaHCO3, 4.7 KCl, 1.18 KH2PO4, 1.18 MgSO4·7H2O, 1.56 CaCl2·2H2O, 0.026 EDTA, and 5.5 glucose, and cleaned of adventitial tissue.
Systolic blood pressure was measured in nonanesthetized animals 24 h before death by tail cuff using a RTBP1001 blood pressure system at the end of the treatment (Kent Scientific). Values were then averaged within group from a reading of 10 cycles from each group.
Pharmacological inhibition of ER stress.
Animals were divided into six groups. Each WKY and SHR group received intraperitoneal (ip) injections of phosphate-buffered saline (PBS; 300 μl) for control or ER stress inhibitors, tauroursodeoxycholic acid or 4-phenylbutyric acid (TUDCA or PBA, respectively; 100 mg·kg−1·day−1) for a period of 1 wk.
Vascular functional studies.
Aortic ring segments from the proximal end of the thoracic aorta (2 mm in length) were carefully dissected in cold (4°C) PSS and mounted onto an isometric myograph (model 610 DMT), and tension was recorded by a PowerLab 8/SP data acquisition system (ADInstruments). After determination of optimal tension through the use of length tension analysis, the aortic rings were placed at a passive force of 30 mN and allowed to equilibrate for 45 min in PSS at 37°C with continuous bubbling with 5% CO2 and 95% O2 to maintain the pH of the PSS around 7.4–7.5 (15). Arterial integrity was assessed through stimulation of vessels with 120 mM KCl. The presence of the endothelium was assessed by contracting the ring with phenylephrine (PE; 1 μM), followed by acetylcholine (ACh; 10 μM) stimulation. A relaxation response to ACh stimulation was taken as evidence of intact endothelium. Concentration-response curves to ACh (10 nM to 100 μM), AA (100 nM to 10 μM), and tert-butyl hydroperoxide (t-BOOH; 1 μM to 1 mM) were performed in aortic rings after 30-min incubation with the nitric oxide synthase inhibitor NG-nitro-l-arginine (l-NNA; 100 μM).
Isolation of aortic vascular smooth muscle cells.
Vascular smooth muscle cells (VSMCs) were isolated from the thoracic aorta of male WKY rats and SHR (15–16 wk) using an explant technique previously described (46). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO-BRL, Gaithersburg, MD) and supplemented with fetal bovine serum (10%; Thermo Scientific, Logan, UT) and streptomycin/penicillin (100 U/ml; Invitrogen, Grand Island, NY). To characterize the VSMCs, immunoreactivity assays were performed. The cells stained positive for α-smooth muscle actin and calponin, markers of VSMCs, and stained negative for von Willebrand factor VIII and platelet endothelial cell adhesion molecule (PECAM-1), markers for endothelial cells. Before treatment with ER stress inhibitors, third-passage cells were incubated in serum-free DMEM (24 h) before treatment with TUDCA or PBA (500 μM, 24 h). After treatment, protein was extracted from cells by using M-PER lysis buffer (Roche, Mannheim, Germany) with protease inhibitors, and protein lysate was used for Western blot analysis.
Western blot analysis.
Proteins (20 μg) were extracted from individual endothelium-intact or endothelium-denuded aorta from each of the experimental groups. They were separated by gel electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% instant milk in Tris-buffered saline solution with Tween (TBS-T) for 1 h at room temperature. Membranes were incubated with primary antibodies in 5% bovine serum album in TBS-T overnight at 4°C. The following antibodies were used: BAX (1:1,000), BCL-2 (1:1,000), GRP78 (1:2,000), PDI (1:2,000), phospho-cPLA2 (1:1,000), cPLA2 (1:1,000), COX-2 (1:2,500), p-ERK1/2 (1:2,000), and total ERK1/2 (1:2,000) from Cell Signaling Technology (Danvers, MA); CHOP (1:200) from Santa Cruz Biotechnology (Santa Cruz, CA); and COX-1 (1:2,000) from BD Transduction Laboratory (San Jose, CA). The membranes were then washed thoroughly and incubated with horseradish peroxidase-coupled secondary IgG antibody (1:5,000–10:000) for 1 h at room temperature. After thorough washing, bound antibodies were visualized by enhanced chemiluminescence using FlourChem E (Protein Simple, Santa Clara, CA) and quantified by density profile extraction (Un-Scan It, Orem, UT). Results were then normalized to β-actin protein expression (1:40,000; Sigma-Aldrich, St. Louis, MO) and expressed as arbitrary units.
Release of prostanoids.
Aortic endothelium-intact rings (2 mm in length) from each of the four groups were placed for 30 min in siliconized tubes containing 500 μl of PSS containing 100 μM l-NNA and either indomethacin (10 μM) or vehicle (water) and then placed in a 37°C water bath. ACh (10 μM) or vehicle (water) was then added to the tubes for 15 min. Next, aortic rings were removed, dry weight was recorded, and tubes were flash frozen in liquid nitrogen and stored at −80°C for later analysis. Thromboxane B2(TXB2), 6-keto-PGF1α, and PGF2α release from aortic rings were analyzed using a commercially available enzyme immunoassay kit (Cayman Chemical).
H2O2 production measurement with Amplex red.
H2O2 production from the aortic rings of the six experimental groups was measured using an Amplex red H2O2 assay kit (Invitrogen) according to the manufacturer's protocol. After removal of adventitial tissue, aortic rings were equilibrated in Krebs-HEPES buffer containing (in mM) 20 HEPES, 119 NaCl, 4.6 KCl, 1.0 MgSO4·7H2O, 0.15 Na2HPO4, 0.4 KH2PO4, 5 NaHCO3, 1.2 CaCl2, and 5.5 dextrose (1 h, 37°C, pH 7.4). After equilibration, the rings were incubated in the dark with Amplex red working solution (100 μM, 1 h, 37°C). The supernatant was transferred to a 96-well plate, and the plate was read at an absorbance of 560 nM. To determine H2O2 concentrations, a standard curve of H2O2 was set up on the same 96-well plate and the standard curve was incubated with Amplex red working solution for the same time in the dark as the aortic rings (1 h, 37°C). Total protein concentration was determined with bicinchoninic acid assay methods and used for normalization.
Statistical analysis.
Results are means ± SE, where n represents the number of rats used in the experiments. Contractions were recorded as changes in millinewtons (mN) from baseline and normalized to 120 mM KCl contraction. Concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 5.0; GraphPad Software). Statistical significance was set at P < 0.05. Statistical analysis was performed using one-way ANOVA with Bonferroni analysis for multiple comparisons.
Chemicals.
Acetylcholine chloride, indomethacin, l-NNA, phenylephrine hydrochloride, t-BOOH, and PBA were purchased from Sigma-Aldrich. TUDCA was purchased from Calbiochem/Millipore (Billerica, MA).
RESULTS
General parameters.
No significant differences were observed in the systolic blood pressure from control WKY rats (122 ± 2 mmHg, n = 9) vs. TUDCA- or PBA-treated WKY rats (124 ± 6 or 127 ± 5 mmHg, n = 6–7, respectively). High systolic blood pressure seen in control SHR (200 ± 2 mmHg, n = 8) was reduced when SHR were treated with either TUDCA or PBA (176 ± 3 or 181 ± 5 mmHg, n = 7–9, respectively). There were no significant changes in body weight between groups (WKY: 314 ± 5 g, n = 9; WKY TUDCA: 320 ± 5 g, n = 6; WKY PBA: 319 ± 3 g, n = 7; SHR: 308 ± 8 g, n = 8; SHR TUDCA: 312 ± 6 g, n = 7; SHR PBA: 310 ± 6.1 g, n = 9). These results suggest that ER stress induction may contribute to increases in blood pressure in the SHR.
Expression of ER stress markers.
To investigate whether ER stress is increased in the aorta of the SHR and whether our treatment with ER stress inhibitors could effectively suppress ER stress, we examined the following markers of ER stress: GRP78, an ER chaperone (Fig. 1A), PDI, an ER protein disulfide isomerase (Fig. 1B), and CHOP, a proapoptotic protein (Fig. 1C) (29). These proteins are upregulated following ER stress (35). All three ER stress markers were upregulated in aortas from the control SHR compared with control WKY rats. There were no differences in expression of ER stress markers in either the WKY rats or the SHR treated with TUDCA or PBA. These results provide evidence that treatment with TUDCA or PBA effectively inhibited ER stress in the aorta of the SHR.
Fig. 1.
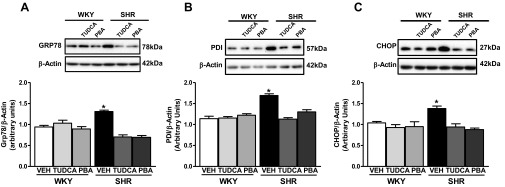
Treatment with endoplasmic reticulum (ER) stress inhibitors tauroursodeoxycholic acid (TUDCA) or 4-phenlybutyric acid (PBA) can normalize ER stress marker expression in the aorta from spontaneously hypertensive rats (SHR). Top, representative Western blot images of GRP78 (A), protein disulfide isomerase (PDI; B), or C/EBP homologous protein (CHOP; C) and β-actin. Bottom, corresponding graphs showing the relative expression measured using densitometry analysis with values normalized to β-actin. Data are means ± SE (n = 6). *P < 0.05 vs. control Wistar-Kyoto (WKY) rats. Veh, vehicle.
ACh-induced endothelium-dependent contractile responses and AA-induced contraction.
It has been shown that endothelium-dependent contractions induced by ACh are increased in the aorta of the SHR compared with the aorta of the WKY rat (17, 33, 34). To investigate how ER stress might influence ACh-induced endothelium-dependent contraction, we examined an ACh-induced concentration-response curve (10 nM to 100 μM) in the presence of l-NNA (100 μM) (Fig. 2A). ACh-induced contraction elicited a greater contraction in the aorta from the control SHR compared with control WKY rat, and, interestingly, this increase was abolished in the SHR treated with either TUDCA or PBA (Fig. 2A). Treatment with either TUDCA or PBA had no effect on ACh-induced contractions in the aorta from the WKY rat.
Fig. 2.
Treatment with ER stress inhibitors TUDCA or PBA can attenuate increased acetylcholine (ACh) and arachidonic acid (AA)-induced contraction in the aorta of the SHR. Aortic rings were incubated with NG-nitro-l-arginine (l-NNA; 30 min, 100 μM) before ACh (10 nM to 100 μM; A) or AA (100 nM to 10 μM; B) concentration-response curves were performed. Data are means ± SE (n = 6–8). *P < 0.05 vs. control WKY rats. †P < 0.05 vs. control SHR.
Similar effects were seen when a concentration-response curve (100 nM to 10 μM) was performed using AA in the presence of l-NNA (100 μM). The aortic contraction to AA was significantly greater in the control SHR than WKY rat but was attenuated when the SHR were treated with either PBA or TUDCA (Fig. 2B). Treatment with either TUDCA or PBA had no effect on AA-induced contractions in the aorta from the WKY rat.
ACh-induced prostanoid release.
We (26, 38) and others (8, 54) have demonstrated that the overproduction, as well as changed expression, of prostanoids or prostanoid-producing or -converting enzymes contributes to an enhanced EDCF response in various disease states. To investigate whether the suppression of ER stress could reduce the endothelium-dependent prostanoid release, we examined the ACh (10 μM)-stimulated release of the stable TXA2 metabolite TXB2 (Fig. 3A), a stable form of prostacyclin, 6-keto-PGF1α (Fig. 3B), and PGF2α (Fig. 3C) in aortic rings from all groups in the presence of l-NNA (100 μM), as well as in the presence or absence of the nonspecific COX inhibitor indomethacin (10 μM). There were no significant differences in the basal release of prostanoids TXB2, 6-keto-PGF1α, or PGF2α between any of the groups. ACh-induced prostanoid release was not significantly different in the control WKY compared with the TUDCA- or PBA-treated WKY rats (data not shown). On stimulation with ACh, control SHR aortic rings released significantly greater amounts of all prostanoids compared with aortic rings from control WKY rats, as well as TUDCA- or PBA-treated SHR. Indomethacin completely abolished the ACh-stimulated release of prostanoids in all groups.
Fig. 3.

Treatment with ER stress inhibitors TUDCA or PBA can normalize endothelium-derived prostanoid release in the aorta of the SHR. Thromboxane B2 (TXB2; A), 6-keto-prostaglandin F1α (6-keto-PGF1α; B), and PGF2α (C) release was measured in aortic rings incubated in physiological salt solution (37°C, 500 μl) with or without l-NNA (30 min, 100 μM) and stimulated with ACh (15 min, 10 μM) in the presence or absence of indomethacin (10 μM). Data are means ± SE (n = 5–7). *P < 0.05 vs. control WKY rats.
Protein expression of COXs, cPLA2 phosphorylation, and ERK1/2 phosphorylation.
To investigate mechanisms underlying the suppressive effects of ER stress inhibition on EDCF productions and/or responses, we first measured the protein expression of COXs (Fig. 4). Protein expression of COX-1 was significantly higher in the aorta of the control SHR group compared with the aorta of the control WKY rats. Interestingly, this was attenuated in the aorta of the SHR treated with either TUDCA or PBA (Fig. 4A). There were no significant differences in aortic COX-1 expression between control WKY rats and TUDCA- or PBA-treated WKY rats. On the other hand, the aortic protein expression of COX-2 did not alter among all groups (Fig. 4B).
Fig. 4.
Treatment with ER stress inhibitors TUDCA or PBA can attenuate enhanced expression of cyclooxygenase (COX)-1 in the aorta from SHR. Top, representative Western blot images from endothelium-intact aortic homogenates (A and B), endothelium-denuded aortic homogenates (C and D), and aortic vascular smooth muscle cell (VSMC) homogenates (E and F) from WKY rats or SHR treated with vehicle (PBS), TUDCA (500 μM, 24 h), or PBA (500 μM, 24 h) showing expression of COX-1 (A, C, and E) and COX-2 (B, D, and F). Bottom, corresponding graphs showing the relative expression measured using densitometry analysis with values normalized to β-actin. Data for A–D are means ± SE (n = 6–9). Data for E and F are means ± SE from aortic VSMC isolated from 6 rats/group with experiments performed in duplicate. *P < 0.05 vs. control WKY rats.
To identify in which cell type COX-1 expression is upregulated, we analyzed COX-1/2 protein expression from denuded aorta of WKY and SHR treated with or without TUDCA or PBA. Removal of the endothelium from the aorta of the control SHR abolished the increase in COX-1 protein expression we observed in intact vessels. There was no difference in the expression of COX-1 or COX-2 in the control WKY or in WKY and SHR treated with TUDCA or PBA from that in the control SHR (Fig. 4, C and D).
To confirm the results we saw in denuded vessels, we isolated VSMCs from the aorta of WKY rats and SHR. Protein expression of either COX-1 (Fig. 4E) or COX-2 (Fig. 4F) was not significantly different in VSMCs from WKY or SHR treated or not with TUDCA or PBA. This suggests that the upregulation of COX-1 in the aorta of the SHR is most likely in the endothelial cells, which has been previously shown (60).
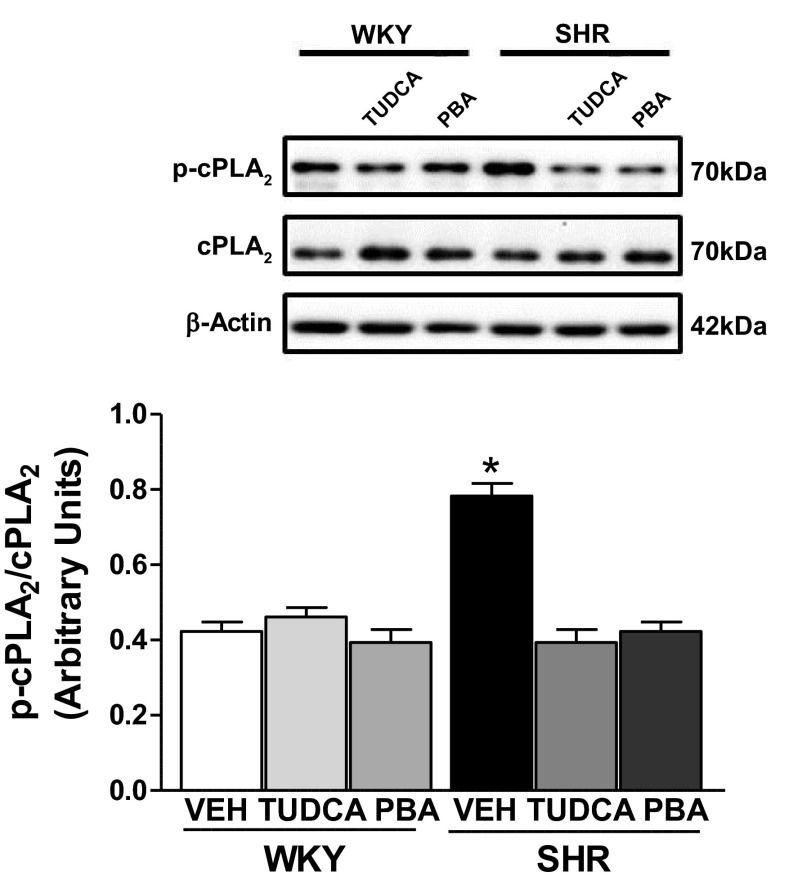
The activation of cPLA2 occurs following a rise in cytosolic calcium on receptor stimulation and by phosphorylation at Ser505 by ERK 1/2 (3, 6), and we recently suggested that the enhanced EDCF production was associated with increased phosphorylation of cPLA2 (26). Therefore, we next measured the level of phosphorylated cPLA2 (Fig. 5). In the aorta from control SHR, cPLA2 phosphorylation levels were increased compared with those in control WKY rats. Interestingly, the phosphorylation levels were greatly suppressed in the aorta of the SHR by TUDCA or PBA treatment (Fig. 5). Total cPLA2 levels did not change among groups. Therefore, these results imply that the suppressive effects of ER stress inhibition on EDCF release/responses are due not only to suppression of COX-1 (downstream component of AA metabolites) expression but also to reduction of cPLA2 activity (upstream component of AA metabolites).
Fig. 5.
Treatment with ER stress inhibitors TUDCA or PBA can normalize phosphorylation of the Ca2+-dependent cytosolic phospholipase A2 (cPLA2) in aorta from SHR. Top, representative Western blot images of phospho(p)-cPLA2, total cPLA2, and β-actin. Bottom, corresponding graphs showing the relative expression measured using densitometry analysis with phosphorylated-to-total values normalized to β-actin. Data are means ± SE (n = 6). *P < 0.05 vs. control WKY rats.
Since there are reports suggesting that the activation of ERK1/2 led to increased AA release via cPLA2 activation in endothelial cells (22, 25), we next investigated whether ER stress inhibition could suppress the ERK 1/2 activity (Fig. 6). The increased phosphorylation of ERK1/2 in the aorta of the control SHR was attenuated in the SHR treated with either TUDCA or PBA (Fig. 6). Neither ER stress inhibitor had any effect on phosphorylation of ERK 1/2 in the aorta of the WKY rats.
Fig. 6.
Treatment with ER stress inhibitors TUDCA or PBA can normalize phosphorylation of extracellular signal-regulated kinase (ERK1/2). Top, representative Western blot images of p-ERK1/2, total ERK1/2, and β-actin. Bottom, corresponding graphs showing the relative expression measured using densitometry analysis with phosphorylated-to-total values normalized to β-actin. Data are means ± SE (n = 6). *P < 0.05 vs. control WKY rats.
Oxidative stress-induced contraction and H2O2 production.
Another important modulator of EDCF-mediated responses is ROS (53, 55, 62). Oxidative stress induced by t-BOOH can alter vascular reactivity and is associated with the COX pathway (14, 37). Moreover, t-BOOH is a membrane-permeant oxidant capable of inducing contraction in a variety of experimental models. To investigate whether the inhibition of ER stress could suppress t-BOOH-mediated responses and ROS production, we examined the effect of t-BOOH (1 μM to 1 mM) on the aortic contractile response and the aortic H2O2 production (Fig. 7). No significant differences were observed in the t-BOOH-induced contraction in the aorta of the control WKY rats compared with that in the aorta of WKY rats treated with either TUDCA or PBA; however, the contraction elicited by t-BOOH was significantly elevated in the aorta from the control SHR compared with that in control WKY rats, and this was normalized in the aorta from the SHR treated with either TUDCA or PBA (Fig. 7A). As shown in Fig. 7B, ER stress inhibition decreased H2O2 production from aortic rings of the SHR treated with either TUDCA or PBA compared with control SHR, and there were no differences in H2O2 production in the aortic rings of the WKY rats treated with either TUDCA or PBA compared with control WKY rats (Fig. 7B).
Fig. 7.
Treatment with ER stress inhibitors TUDCA or PBA can attenuate the increased tert-butyl hydroperoxide (t-BOOH)-induced contraction and H2O2 production in the aorta of the SHR. A: aortic rings were incubated with l-NNA (30 min, 100 μM) and a concentration-response curve to t-BOOH (1 μM to 1 mM) was performed. B: H2O2 production was measured using Amplex red and normalized to protein concentration. Data are means ± SE (n = 5–8). Symbols represent the results from 2-way ANOVA. *P < 0.05 vs. control WKY rats. † P < 0.05 vs. control SHR.
Protein expression of apoptotic markers.
Apoptosis of vascular cells may modify vascular contraction. A link between ER stress and apoptosis contributes to the pathology of many cardiovascular diseases. Recently, studies have demonstrated that treatment with the ER stress inhibitors TUDCA or PBA improves myocardial dysfunction and atherosclerosis through the attenuation of apoptosis (4, 9). Aortic protein expression of the proapoptotic marker BAX was enhanced in the control SHR compared with the control WKY (Fig. 8A). The increased aortic BAX expression was attenuated in the SHR treated with either TUDCA or PBA, and there were no differences in BAX expression between the control WKY and WKY treated with either TUDCA or PBA. Aortic protein expression of the antiapoptotic marker BCL-2 was reduced in the control SHR compared with the control WKY and was restored in the SHR treated with either TUDCA or PBA (Fig. 8B). There were no differences in the aortic BCL-2 expression between the control WKY and WKY treated with either TUDCA or PBA.
Fig. 8.
Treatment with ER stress inhibitors TUDCA or PBA attenuated proapoptotic marker expression and restored antiapoptotic marker expression in the aorta of the SHR. Top, representative Western blot images of BAX (A) or BCL-2 (B) and β-actin. Bottom, corresponding graphs showing the relative expression measured using densitometry analysis with values normalized to β-actin. Data are means ± SE (n = 5–8). *P < 0.05 vs. control WKY rats.
DISCUSSION
We have observed that ER stress contributes to pathophysiological changes in the vasculature during hypertension. The major findings of this study are 1) ER stress contributes to increased COX-1 expression and prostanoid release by enhancing phosphorylation of ERK1/2 and cPLA2 at Ser505, contributing to the enhanced EDCF response in the aorta of the SHR; 2) ER stress inhibition prevented ROS-stimulated enhanced contraction and ROS production; and 3) the chemical chaperones TUDCA or PBA lower blood pressure in the SHR (Fig. 9). Inhibition of ER stress is likely to play an increasingly important role in the area of pharmacological drugs targeting cardiovascular diseases (39).
Fig. 9.
Schematic diagram showing the possible signaling pathways through which ER stress mediates enhanced endothelium-derived contractile factor (EDCF) contractions in the aorta of the SHR. We suggest that this enhancement is because 1) ER stress contributes to increased COX-1 expression and prostanoid release by enhancing phosphorylation of ERK 1/2 and cPLA2 at Ser505, contributing to the enhanced EDCF response in the aorta of the SHR, 2) ER stress inhibition prevents reactive oxygen species (ROS)-stimulated enhanced contraction and ROS production, and 3) chemical chaperones TUDCA or PBA lower blood pressure in the SHR. EC, endothelial cell; SMC, smooth muscle cell.
The phosphorylation of cPLA2 by ERK1/2 plays an important role in prostanoid release and EDCF-mediated responses during hypertension. In the present experiments, ERK1/2 and cPLA2 phosphorylation was greater in the aorta of the SHR compared with the control WKY rats. ER stress inhibition with either TUDCA or PBA attenuated the enhanced phosphorylation of both ERK1/2 and cPLA2 in the aorta of the SHR. These results suggest that ER stress plays an important role in the activation of ERK1/2 and cPLA2 during hypertension. Indeed, ER stress induction leads to the activation of ERK1/2 in various cells (31). Moreover, direct inhibition of ERK1/2 has also been shown to improve endothelial function and attenuate enhanced contractility in the mesenteric arteries of the SHR (58). There has been an increasing amount of evidence demonstrating important roles for MAP kinase family members p38 and JNK, as well as nuclear factor-κB, in ER stress-induced apoptosis and inflammation (24, 32). However, it is not known whether they are involved in ER stress-mediated changes of cPLA2 phosphorylation and EDCF responses of the aorta of the SHR.
The exact molecular mechanisms by which COX-1 expression is regulated in the aorta of the SHR are unknown; however, various cellular signaling pathways that are activated during hypertension may be involved. We found that ER stress inhibition led to the normalization of COX-1 protein expression, and there were no differences in the expression of COX-2 among all groups. This may then contribute to the abolishment of ACh-induced contractions we observed in the aorta of the SHR treated with either TUDCA or PBA. This is in line with work showing that EDCF responses are abolished in aorta from COX-1 knockout mice but maintained in the aorta of COX-2 knockout animals, demonstrating the preferential importance of COX-1 enzymatic activity in the aorta (52). Interestingly, COX-2-derived PGF2α has been demonstrated to mediate EDC in the aorta of aging hamsters (64) and renovascular hypertensive rats (57). Additionally, EDC were enhanced following the upregulation of COX-2 via p38 MAPK in the mouse aorta following bone morphogenic protein stimulation (65). Further research will be required to focus on how ER stress may influence the regulation or expression of COX-1 in the aorta of the SHR. Since the cellular localization of COX-1 is at the ER (45, 59), the suppression of COX-1 expression/activity may be attributable to the normalization of ER function by treatment with ER stress inhibitors. Furthermore, Cho et al. (5) found that following ER stress there is an upregulation of activating transcription factor 4, which can bind the COX-2 promoter, leading to COX-2 upregulation during hepatic inflammation following hepatitis B infection. Whether COX-1 has an ER stress response element in its promoter region of endothelial cells is unknown.
In the endothelium, the activation of PLA2 and release of AA from cellular phospholipids leads to the release of prostanoids (22, 25, 63). It has been reported that the release of prostanoids is enhanced in the aorta of the SHR compared with the WKY rats (56). We found that the enhanced ACh-induced prostanoid release of TXB2, 6-keto-PGF1α, and PGF2α observed in aortic rings of the SHR was abolished when the animals were treated with either TUDCA or PBA. We speculate that this abolishment could be due synergistically to the effects of the decrease in cPLA2 phosphorylation and COX-1 expression. Furthermore, preincubation with indomethacin completely abolished the ACh-induced prostanoid release in all groups, demonstrating the importance of COX enzymes in the production of prostanoids. These findings are supported by our previous study demonstrating that in a type 2 diabetic rat model, losartan treatment lead to an inhibition of prostanoid release from the mesenteric arteries via an inhibition of cPLA2/COX pathway (26).
A link and possible cross talk between ER stress and oxidative stress has been studied and could be another mechanism underlying the beneficial effects of ER stress inhibition (36). Oxidative stress enhances EDCF-mediated responses (53, 61), and it has been observed in the mesenteric arteries of the SHR that H2O2 causes increased contractions through the production of TXA2 (13). Tian et al. (57) found that H2O2 can stimulate the release of PGF2α in a renovascular model of hypertension. In the present study, we found that treatment with ER stress inhibitors led to a decrease in t-BOOH-induced contraction in the aorta of the SHR, as well as a decrease in H2O2 production. Indeed, previous reports (1, 28, 47) and the present data suggest that these chemical chaperone ER stress inhibitors decrease oxidative stress, and therefore the decrease in EDCF-mediated contractions may be partially mediated by their antioxidative properties. This is supported by previous studies demonstrating that the suppression of oxidative stress by various treatments could reduce (normalize) the EDCF-mediated responses (28, 40, 43). Moreover, Wong et al. (62) demonstrated that the improvement of EDCF-mediated response by vitamin D treatment is due to reduced expression of COX-1 and production of ROS in the SHR. However, it remains unclear whether these pharmacological inhibitors have direct effects on antioxidant cellular pathways. Further investigation will be needed to elucidate these actions.
Chemical chaperones, such as TUDCA and PBA, are safe pharmacological agents with pleiotropic effects and have been used to inhibit ER stress cardiovascular diseases such as heart failure, stroke, atherosclerosis, and kidney disease; however, little is known about what effect these chaperones have in hypertension (7, 10, 48, 49). The exact mechanisms triggering ER stress in hypertension are unclear, but they likely involve multiple cellular stresses, including inflammation, oxidative stress, and tissue ischemia (19, 42, 50, 66). We have shown in the present study that the inhibition of ER stress through the treatment with chemical chaperones in the aorta of the SHR led to 1) a reduction in the blood pressure in the SHR, 2) normalization of ACh-, AA-, and t-BOOH-induced contractions, 3) decreased phosphorylation of ERK1/2 and cPLA2, 4) decreased COX-1 expression, 5) decreased oxidative stress, and 6) decreased proapoptotic marker expression while normalizing antiapoptotic marker expression. Together, the present data suggest that ER stress inhibition normalizes EDCF-mediated contractions and oxidative stress via attenuation of ERK 1/2 and cPLA2 activity.
However, limitations to our study are that the finding of the normalization of EDCF responses in TUDCA- or PBA-treated SHR could be secondary to the systemic administration of these inhibitors acting in the central nervous system, leading to the reduction in blood pressure. Young et al. (67) found that direct inhibition of ER stress in the subfornical organ (SFO) alone blunts hypertension. It is possible that systemic administration of PBA and TUDCA could lead to a reduction in blood pressure due to the reduction of ER stress in the SFO (23, 67). Taurine, the amino acid that can be conjugated in vivo with ursodeoxycholic acid to form TUDCA, has been shown to decrease blood pressure in the SHR linked to normalization of the renin-angiotensin system in the brain (41). At this time it is unclear what the primary effect of TUDCA or PBA is, and therefore further research will be needed to focus and look at local effects, as well as time course changes of blood pressure and endothelial function in SHR.
In conclusion, our study demonstrates that suppression of ER stress normalizes EDCF-mediated contractions in the aorta of the SHR due to reduced cPLA2/COX signaling pathways such as reduced cPLA2 phosphorylation, reduced COX-1 expression, and reduced oxidative stress. Therefore, our studies should stimulate further interest in how targeting ER stress could be a therapeutic target for the treatment of hypertension.
GRANTS
This study was supported by National Institutes of Health Grants HL71138 and DK83685 and by the Society for Women's Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.M.S., T.M., and R.C.W. conception and design of research; K.M.S. performed experiments; K.M.S. analyzed data; K.M.S., T.M., and R.C.W. interpreted results of experiments; K.M.S. prepared figures; K.M.S. drafted manuscript; K.M.S., T.M., and R.C.W. edited and revised manuscript; K.M.S., T.M., and R.C.W. approved final version of manuscript.
REFERENCES
- 1. Abebe W, Mozaffari MS. Role of taurine in the vasculature: an overview of experimental and human studies. Am J Cardiovasc Dis 1: 293–311, 2011 [PMC free article] [PubMed] [Google Scholar]
- 2. Bonventre JV. Phospholipase A2 and signal transduction. J Am Soc Nephrol 3: 128–150, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Borsch-Haubold AG, Bartoli F, Asselin J, Dudler T, Kramer RM, Apitz-Castro R, Watson SP, Gelb MH. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J Biol Chem 273: 4449–4458, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol 50: 107–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Cho HK, Cheong KJ, Kim HY, Cheong J. Endoplasmic reticulum stress induced by hepatitis B virus X protein enhances cyclo-oxygenase 2 expression via activating transcription factor 4. Biochem J 435: 431–439, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Clark JD, Schievella AR, Nalefski EA, Lin LL. Cytosolic phospholipase A2. J Lipid Mediat Cell Signal 12: 83–117, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Cunard R, Sharma K. The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol 300: F1054–F1061, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doroudi R, Gan LM, Sjögren LS, Jern S. Effects of shear stress on eicosanoid gene expression and metabolite production in vascular endothelium as studied in a novel biomechanical perfusion model. Biochem Biophys Res Commun 269: 257–264, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15: 1383–1391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15: 1383–1391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feletou M, Huang Y, Vanhoutte PM. Vasoconstrictor prostanoids. Pflügers Arch 459: 941–950, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989 [PubMed] [Google Scholar]
- 13. Gao YJ, Lee RM. Hydrogen peroxide induces a greater contraction in mesenteric arteries of spontaneously hypertensive rats through thromboxane A2 production. Br J Pharmacol 134: 1639–1646, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Cohen EC, Marin J, Diez-Picazo LD, Baena AB, Salaices M, Rodriguez-Martinez MA. Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J Pharmacol Exp Ther 293: 75–81, 2000 [PubMed] [Google Scholar]
- 15. Giachini FR, Lima VV, Carneiro FS, Tostes RC, Webb RC. Decreased cGMP level contributes to increased contraction in arteries from hypertensive rats: role of phosphodiesterase 1. Hypertension 57: 655–663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol 44: 453–459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146: 834–845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 8: 1391–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Gotoh T, Endo M, Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int J Inflam 2011: 259462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res 107: 1185–1197, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Gudmundsdottir IJ, Halldorsson H, Magnusdottir K, Thorgeirsson G. Involvement of MAP kinases in the control of cPLA2 and arachidonic acid release in endothelial cells. Atherosclerosis 156: 81–90, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Hasty AH, Harrison DG. Endoplasmic reticulum stress and hypertension–a new paradigm? J Clin Invest 122: 3859–3861, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Houliston RA, Pearson JD, Wheeler-Jones CP. Agonist-specific cross talk between ERKs and p38mapk regulates PGI2 synthesis in endothelium. Am J Physiol Cell Physiol 281: C1266–C1276, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Ishida K, Matsumoto T, Taguchi K, Kamata K, Kobayashi T. Mechanisms underlying altered extracellular nucleotide-induced contractions in mesenteric arteries from rats in later-stage type 2 diabetes: effect of ANG II type 1 receptor antagonism. Am J Physiol Heart Circ Physiol 301: H1850–H1861, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Kagota S, Yamaguchi Y, Nakamura K, Kunitomo M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen Pharmacol 34: 201–209, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Kassan M, Galan M, Partyka M, Saifudeen Z, Henrion D, Trebak M, Matrougui K. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler Thromb Vasc Biol 32: 1652–1661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 22: 193–201, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lavoie C, Roy L, Lanoix J, Taheri M, Young R, Thibault G, Farah CA, Leclerc N, Paiement J. Taking organelles apart, putting them back together and creating new ones: lessons from the endoplasmic reticulum. Prog Histochem Cytochem 46: 1–48, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem 280: 21763–21772, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Lin WC, Chuang YC, Chang YS, Lai MD, Teng YN, Su IJ, Wang CC, Lee KH, Hung JH. Endoplasmic reticulum stress stimulates p53 expression through NF-kappaB activation. PLoS One 7: e39120, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luscher TF, Diederich D, Weber E, Vanhoutte PM, Buhler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension 11: 573–578, 1988 [DOI] [PubMed] [Google Scholar]
- 34. Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 8: 344–348, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18: 716–731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 293: H1480–H1490, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 295: H1165–H1176, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107: 1071–1082, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol 31: 1124–1132, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Okamoto K, Tabei R, Fukushima M, Nosaka S, Yamori Y. Further observations of the development of a strain of spontaneously hypertensive rats. Jpn Circ J 30: 703–716, 1966 [DOI] [PubMed] [Google Scholar]
- 42. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C, Marie JC, Pouzet C, Samadi M, Elbim C, O'Dowd Y, Bens M, Vandewalle A, Gougerot-Pocidalo MA, Lizard G, Ogier-Denis E. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension 31: 1047–1060, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Ren Y, Walker C, Loose-Mitchell DS, Deng J, Ruan KH, Kulmacz RJ. Topology of prostaglandin H synthase-1 in the endoplasmic reticulum membrane. Arch Biochem Biophys 323: 205–214, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50: 172–186, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sener G, Ozer Sehirli A, Ipçi Y, Cetinel S, Cikler E, Gedik N, Alican I. Taurine treatment protects against chronic nicotine-induced oxidative changes. Fundam Clin Pharmacol 19: 155–164, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Song XJ, Yang CY, Liu B, Wei Q, Korkor MT, Liu JY, Yang P. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int J Med Sci 8: 564–572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Srinivasan K, Sharma SS. Sodium phenylbutyrate ameliorates focal cerebral ischemic/reperfusion injury associated with comorbid type 2 diabetes by reducing endoplasmic reticulum stress and DNA fragmentation. Behav Brain Res 225: 110–116, 2011 [DOI] [PubMed] [Google Scholar]
- 50. Sukumaran V, Watanabe K, Veeraveedu PT, Gurusamy N, Ma M, Thandavarayan RA, Lakshmanan AP, Yamaguchi K, Suzuki K, Kodama M. Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiac inflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci 7: 154–167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res 107: 839–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol 46: 761–765, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, Vanhoutte PM. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol 151: 15–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics 32: 409–418, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Tang EH, Vanhoutte PM. Prostanoids and reactive oxygen species: team players in endothelium-dependent contractions. Pharmacol Ther 122: 140–149, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Tang EH, Vanhoutte PM. Prostanoids and reactive oxygen species: team players in endothelium-dependent contractions. Pharmacol Ther 122: 140–149, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Tian XY, Wong WT, Leung FP, Zhang Y, Wang YX, Lee HK, Ng CF, Chen ZY, Yao X, Au CL, Lau CW, Vanhoutte PM, Cooke JP, Huang Y. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin F(2alpha) impairs endothelial function in renovascular hypertensive rats. Antioxid Redox Signal 16: 363–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Touyz RM, Deschepper C, Park JB, He G, Chen X, Neves MF, Virdis A, Schiffrin EL. Inhibition of mitogen-activated protein/extracellular signal-regulated kinase improves endothelial function and attenuates Ang II-induced contractility of mesenteric resistance arteries from spontaneously hypertensive rats. J Hypertens 20: 1127–1134, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38: 97–120, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol 586: 5295–5304, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Wong MS, Delansorne R, Man RY, Svenningsen P, Vanhoutte PM. Chronic treatment with vitamin D lowers arterial blood pressure and reduces endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol 299: H1226–H1234, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin 31: 1095–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M, Huang Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res 104: 228–235, 2009 [DOI] [PubMed] [Google Scholar]
- 65. Wong WT, Tian XY, Chen Y, Leung FP, Liu L, Lee HK, Ng CF, Xu A, Yao X, Vanhoutte PM, Tipoe GL, Huang Y. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res 107: 984–991, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Xin W, Lu X, Li X, Niu K, Cai J. Attenuation of endoplasmic reticulum stress-related myocardial apoptosis by SERCA2a gene delivery in ischemic heart disease. Mol Med 17: 201–210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhou MS, Nishida Y, Chen QH, Kosaka H. Endothelium-derived contracting factor in carotid artery of hypertensive Dahl rats. Hypertension 34: 39–43, 1999 [DOI] [PubMed] [Google Scholar]