Abstract
Purpose
The objective was to study whether positive surgical margins (PSMs) predict biochemical recurrence (BCR) in all patients without adjuvant therapy after radical prostatectomy (RP).
Materials and Methods
We retrospectively reviewed the medical records of patients who underwent RP for prostate cancer at Veterans Health Service Medical Center from 2005 to 2011. BCR was defined by a prostate-specific antigen (PSA) value ≥0.2 ng/mL. The clinicopathological factors of the PSM group were compared with those of the negative surgical margin (NSM) group, and the predictive impact of a PSM for BCR-free survival were evaluated. In addition, we analyzed the prognostic difference for BCR-free survival between solitary and multiple PSMs.
Results
A PSM was noted in 167 patients (45.5%). BCR was reported in 101 men in total (27.5%). The BCR-free survival rate of the PSM group was lower than that of the NSM group (p<0.001). In a multivariate analysis for the total patients, PSM was significantly associated with BCR-free survival (p<0.001). After stratification by pathological T stage, Gleason score (GS), and preoperative PSA value, PSM was significantly predictive for BCR-free survival in men with pT2 and/or GS ≤6 or 7 and/or a PSA value <10 or 10-20 ng/mL (all p<0.05). Multiple PSMs were more predictive of BCR-free survival than was a solitary PSM (p=0.001).
Conclusions
A PSM is a significant predictor of postoperative BCR in patients with pT2 and/or GS ≤7 and/or preoperative PSA <20 ng/mL. Multiple PSMs are considered a stronger prognostic factor for prediction of BCR than is a solitary PSM.
Keywords: Prostatectomy, Prostatic neoplasms, Recurrence
INTRODUCTION
In Korea, prostate cancer (PCa) is the fifth most common malignancy in men and the incidence has been rising steadily [1]. Therefore, the choice of proper treatment of PCa is very important.
Radical prostatectomy (RP) is a reasonable treatment option for patients with localized PCa and for selected patients with locally advanced PCa [2-4].
In previously published studies, which reported various outcomes, a high preoperative prostate-specific antigen (PSA) level, a high Gleason score (GS), high pathological stage, seminal vesicle invasion, large tumor volume, or positive surgical margin (PSM) could predict disease recurrence after RP [5-11]. However, not all patients with these predictive factors experience disease recurrence. Therefore, most urologists are concerned about whether adjuvant treatment is needed after RP.
A PSM is a relatively frequent finding in pathological reports following RP, and the incidence of PSMs ranges from 10% to 60% despite meticulous surgical technique [10-15]. Most investigators define a PSM as extension of the tumor to the inked cut surface of the resected specimen [16]. Therefore, a PSM may suggest the presence of residual tumor cells in the surgical bed, implying that local treatment with surgery has failed. Until recently, however, the im pact of a PSM on oncologic outcomes, especially biochemical recurrence (BCR), was not clear.
Thus, we investigated whether a PSM predicts BCR in patients who did not receive adjuvant therapy before BCR. We also analyzed the impact of a PSM on the risk of BCR, stratifying patients by clinicopathological factors. In addition, we analyzed the prognostic difference for BCR-free survival between subgroups with a PSM at a single site or at two or more sites.
MATERIALS AND METHODS
1. Patient selection and follow-up
We retrospectively reviewed the medical records of patients who underwent RP for PCa at Veterans Health Service Medical Center from 2005 to 2011. All patients underwent RP by an open retropubic approach. Low-risk patients (clinical T1c or T2a stage; GS, 6; and PSA, <10 ng/mL) underwent the conventional nerve-sparing procedure. The men who had received neoadjuvant therapy or adjuvant therapy before an appearance of BCR were excluded from the analyses. All RP specimens were coated with ink, sectioned at 3-4 mm intervals, analyzed by a single pathologist, and processed by using the Stanford technique. When at least 1 cell of PCa extended to the ink-coated surface, the resection margin was considered positive. The patients were followed for more than 1 year postoperatively. Finally, 367 patients were included in our analyses. Follow-up visits were scheduled at 1 and 3 months after RP and then at 3-month intervals. BCR was defined by a serum PSA value ≥0.2 ng/mL.
2. Patient grouping and statistical analysis
Clinical data (age, preoperative PSA value, prostate weight, and BCR status) and pathological data (pathological T stage, pathological GS, and surgical margin status) were collected in our database. The patients were divided into two groups that were stratified by surgical margin status: the PSM group and the negative surgical margin (NSM) group. The clinicopathological factors of the PSM group were compared with those of the NSM group by use of independent sample t-tests and chi-square analysis. The BCR-free survival rates of the two groups were estimated by Kaplan-Meier survival analysis, and the survival curves were compared by the log-rank test. The predictive impact of a PSM for BCR-free survival of the total patients was evaluated by use of multivariate Cox proportional hazard regression models. These multivariate Cox analyses were also performed for subgroups stratified by pathological T stage (pT2, pT3a-b, and pT3c), GS (≤6, 7, and ≥8), and preoperative PSA level (<10, 10-20, and ≥20 ng/mL). In addition, the PSM group was separated into subgroups of a solitary (single site) PSM and multiple (two or more sites) PSMs. The clinicopathological factors of the two groups were compared and the BCR-free survival curves were made by the above-mentioned methods. Also, we analyzed the prognostic difference in BCR-free survival between the solitary and multiple PSM groups by using the multivariate Cox model. All statistical tests were performed by using the IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
Among a total of 367 patients, 167 patients (45.5%) had PSMs. BCR was reported in 101 men in total (27.5%). The median follow-up period was 22 months (interquartile range [IQR], 8 to 41). The patients in the PSM group had a higher preoperative PSA value (p=0.002), pathological T stage (p<0.001), GS (p<0.001), and BCR rate (47.3% vs. 11.0% in the PSM and NSM groups, respectively; p<0.001) and lower prostate weight (p<0.001) than did the patients in the NSM group (Table 1).
TABLE 1.
Clinicopathological factors in the NSM and PSM groups
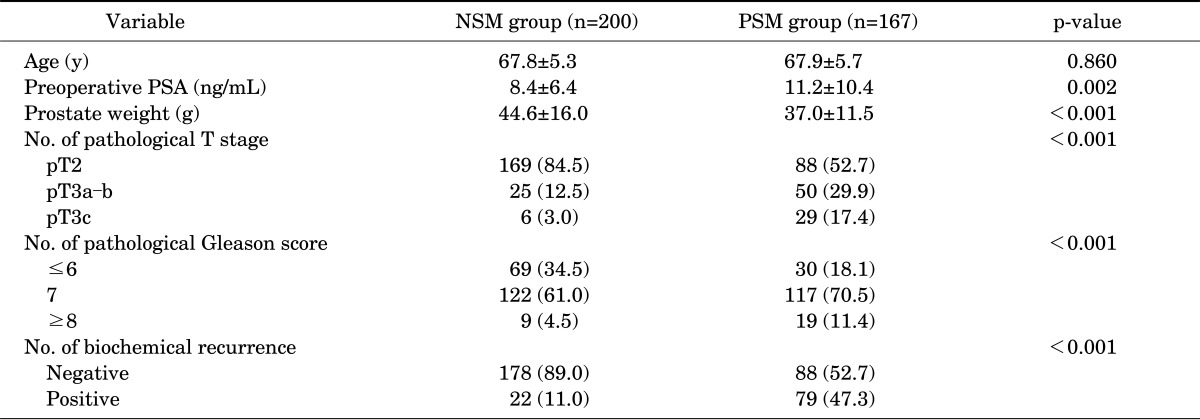
Values are presented as mean±standard deviation or number (%).
NSM, negative surgical margin; PSM, positive surgical margin; PSA, prostate-specific antigen.
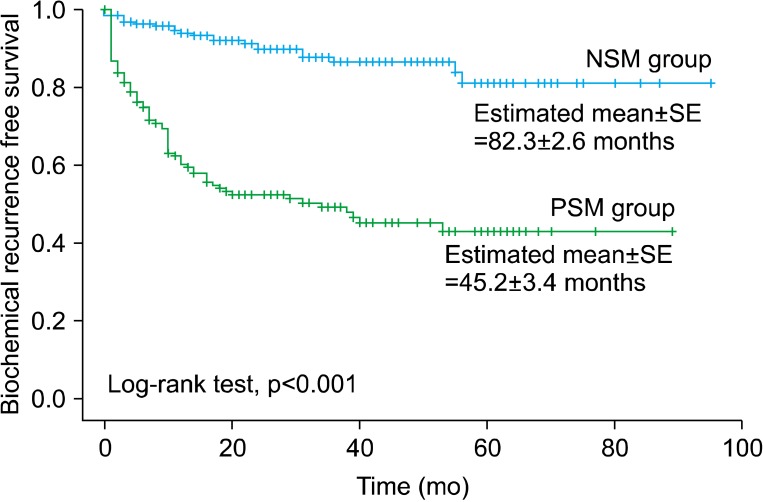
Kaplan-Meier survival analysis with log-rank test showed that the estimated values of mean (±standard error) time for BCR-free survival were 82.3 (±2.6) months in the NSM group and 45.2 (±3.4) months in the PSM group, and the BCR-free survival rates were significantly different between the two groups (p<0.001) (Fig. 1).
FIG. 1.
Biochemical recurrence-free survival curve stratified by the surgical margin status (NSM group vs. PSM group). NSM, negative surgical margin; PSM, positive surgical margin; SE, standard error.
In the multivariate Cox proportional hazard regression for total patients, a PSM was significantly associated with BCR-free survival (hazard ratio [HR], 3.48; p<0.001). In the multivariate analyses performed after stratification by pathological T stage (pT2, pT3a-b, and pT3c), GS (≤6, 7, and ≥8), and preoperative PSA level (<10, 10-20, and ≥20 ng/mL), a PSM was significantly predictive for BCR-free survival in men with pT2 (HR, 3.81; p<0.001) and/or GS ≤6 (HR, 5.46; p=0.010) or 7 (HR, 3.50; p<0.001) and/or PSA value <10 (HR, 4.29; p<0.001) or 10-20 ng/mL (HR, 3.13; p=0.033). However, in the analyses of other stratified groups, such as the patients with pT3, GS ≥8, and preoperative PSA value ≥20 ng/mL, PSM was not a statistically significant predictor of BCR-free survival (all p>0.05) (Table 2).
TABLE 2.
Multivariate analyses for the prediction of biochemical recurrence-free survival by Cox proportional hazard regression models
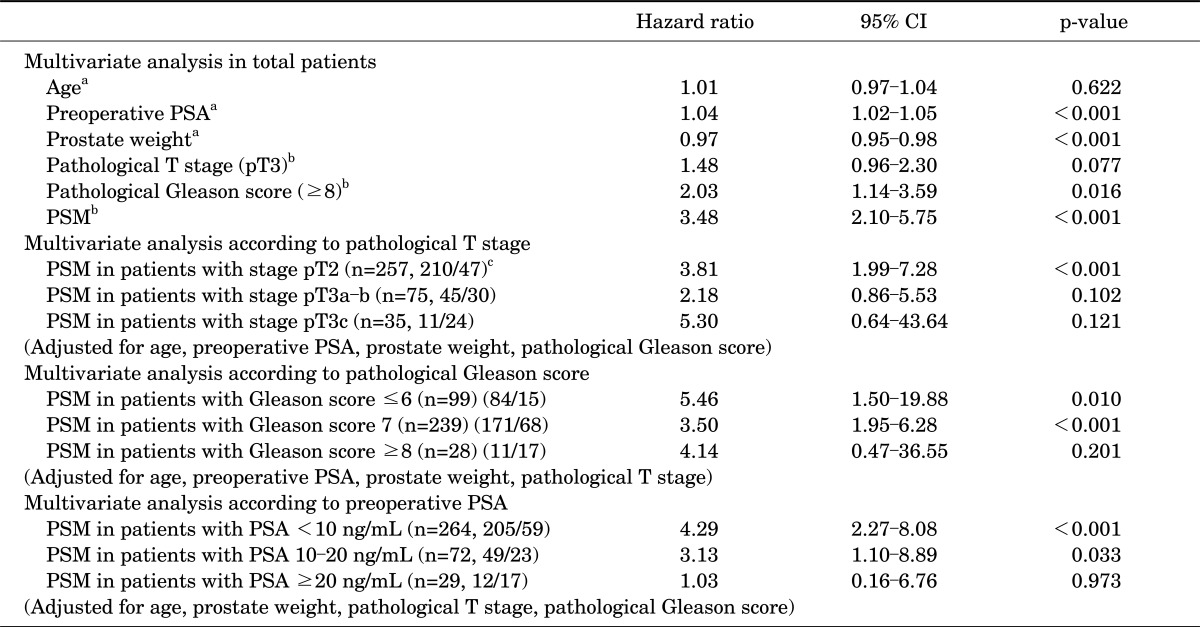
CI, confidence interval; PSA, prostate-specific antigen; PSM, positive surgical margin.
a:Age, preoperative PSA, and prostate weight are continuous variables. b:Pathological T stage, pathological Gleason score, and surgical margin are categorical variables (referent categories : pT2, ≤7, and negative finding, respectively). c:(A/B): A is the number of patients without biochemical recurrence and B is the number of patients with biochemical recurrence.
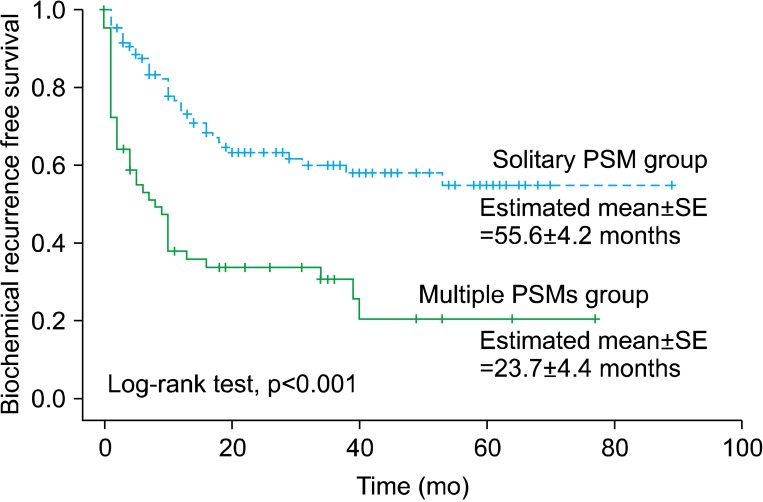
In a further analysis according to the number of PSMs, among a total of 167 patients with PSMs, a solitary PSM and multiple PSMs were noted in 105 and 62 patients, respectively. The group with multiple PSMs had a higher preoperative PSA value (p=0.017), pathological T stage (p=0.013), GS (p=0.018), and BCR rate (66.1% vs. 36.2% in the groups with multiple and a solitary PSM, respectively; p<0.001) than did the solitary PSM group (Table 3). The estimated values of mean (±standard error) time for BCR-free survival were 55.6 (±4.2) months in the solitary PSM group and 23.7 (±4.4) months in the multiple PSM group, and the difference in BCR-free survival rates between the two groups was statistically significant (p<0.001) (Fig. 2). In a multivariate Cox proportional hazard regression for patients with PSMs, multiple PSMs showed significance for prediction for BCR-free survival (HR, 2.32; p=0.001) (Table 4).
TABLE 3.
Clinicopathological factors of the solitary PSM group and multiple PSMs group
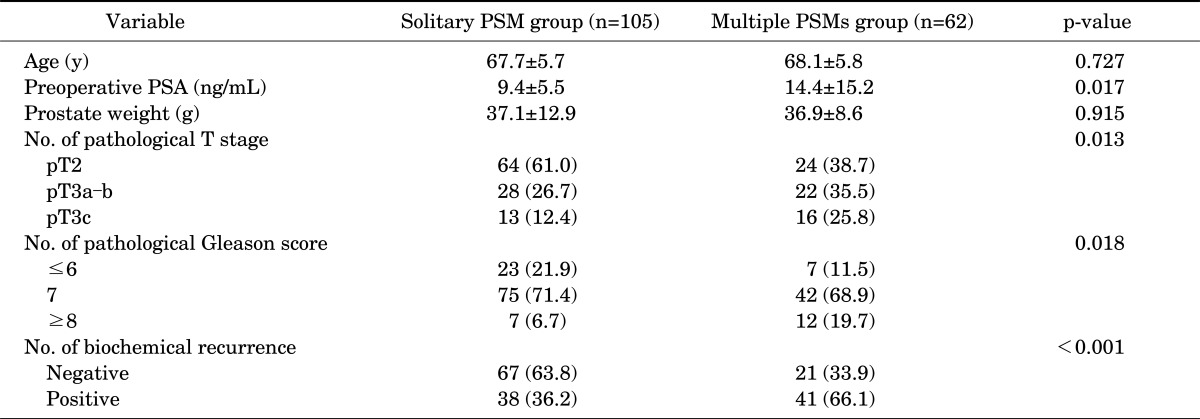
Values are presented as mean±standard deviation or number (%).
Solitary PSM, positive surgical margin on a single site; Multiple PSMs, positive surgical margins on two or more sites; PSA, prostate-specific antigen.
FIG. 2.
Biochemical recurrence-free survival curve stratified by the surgical margin status (solitary PSM group vs. multiple PSMs group). Solitary PSM, positive surgical margin on a single site; Multiple PSMs, positive surgical margins on two or more sites; SE, standard error.
TABLE 4.
Multivariate analysis for the prediction of biochemical recurrence-free survival by Cox regression models (solitary PSM group and multiple PSMs group, n=167)
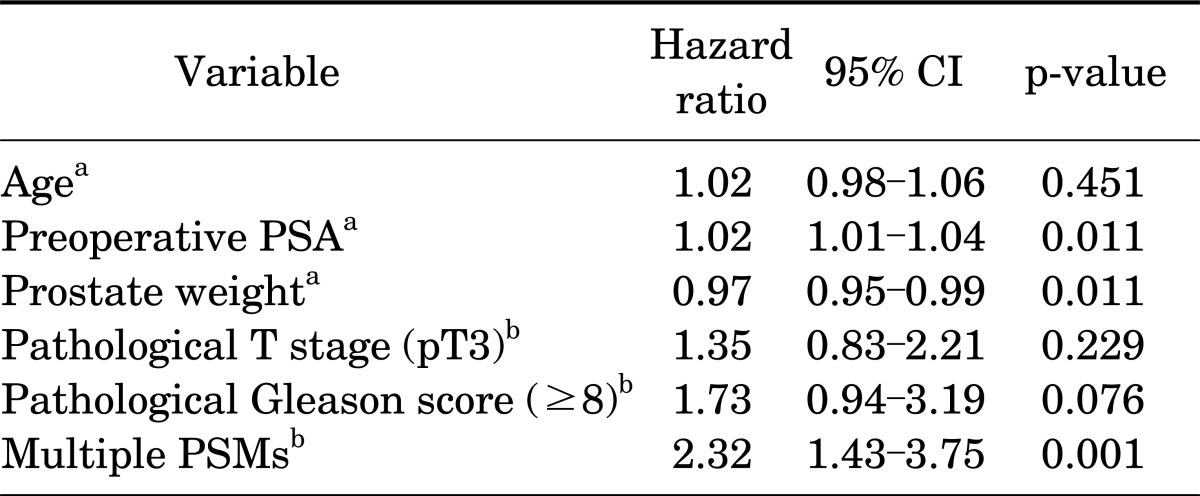
Solitary PSM, positive surgical margin on a single site; Multiple PSMs, positive surgical margins on two or more sites; CI, confidence interval; PSA, prostate-specific antigen.
a:Age, preoperative PSA, and prostate weight are continuous variables. b:Pathological T stage, pathological Gleason score, and surgical margin are categorical variables (referent categories: pT2, ≤7, and 1 PSM, respectively).
DISCUSSION
PSMs can result from inadvertent capsular incision into otherwise organ-confined tumors and artifacts induced by tissue processing as well as by failure to excise extraprostatic extensions of PCa [16-18]. Previous studies have shown that the incidence of PSMs ranges from 10% to 60% even though surgeons performed RP carefully [10-15]. Our data showed that a PSM was noted in 45.5% of cases, and this result is comparable to previous data.
The impact of a PSM on disease recurrence after RP remains controversial. Most previous studies found that a PSM was a prognostic parameter for postoperative BCR or disease progression of PCa [5,10-12,14,15,17,19]. In contrast with the above studies, several other studies demonstrated that a PSM was not associated with BCR or disease progression [20,21]. In our study, BCR was reported in 27.5% of the patients in total and the BCR rate of the PSM group (47.3%) was higher than that of the NSM group (11.0%). Furthermore, the mean time for BCR-free survival was shorter in the PSM group (45.2±3.4 months) than in the NSM group (82.3±2.6 months, p<0.001) in the Kaplan-Meier survival analysis with log-rank test. A PSM was significantly associated with BCR-free survival (HR, 3.48; p<0.001) in the multivariate Cox proportional hazard regression models.
Generally, the fact that a PSM is an independent predictive factor for postoperative BCR means that immediate adjuvant or early salvage treatment is needed after RP. However, there is a dilemma about selecting optimal patients who need adjuvant or salvage treatment because the prognostic value of PSMs can vary according to the clinicopathological factors. In addition, many, if not most, patients with PSMs never experience BCR; thus, adjuvant or salvage treatment will probably result in overtreatment.
Corcoran et al. [10] examined the impact of PSMs on the risk of BCR stratified by the risk of occult metastatic disease (low risk [PSA, <10 ng/mL; pT2 stage; and GS, ≤6], intermediate risk [PSA, 10-20 ng/mL; pT2 stage; and/or GS, 7], and high risk, [PSA, >20 ng/mL; pT3-4 stage; GS, 8-10). They suggested that the presence of a PSM had a significant impact on the risk of recurrence only in intermediate-risk disease and that this represented the need for immediate adjuvant or early salvage radiation therapy. By contrast, for high- and low-risk disease, the risk of recurrence was driven by intrinsic tumor biology, and the presence of a PSM had little impact on outcome.
Ploussard et al. [11] investigated the impact of a PSM as an independent predictor of BCR and the need for salvage therapy after RP by using univariate and multivariate analyses. In their study, PSM was significantly predictive for PSA failure (HR, 2.6; p<0.001) and the need for salvage therapy (HR, 2.9; p<0.001) and after stratification by pathological stage and GS, PSM was significantly predictive for PSA failure in pT2 (HR, 3.81; p<0.001), pT3a (HR, 2.09; p=0.001), and/or GS ≤7 cancers (HR, 3.52; p<0.001), whereas the impact of a PSM did not reach significance in pT3b (HR, 1.46; p=0.196), pT4 (HR, 2.17; p=0.061), and/or GS ≥8 cancers (HR, 1.41; p=0.115). They concluded that PSMs were associated with poor prognosis in terms of BCR-free survival and the need for salvage therapy. However, such a distinction between negative or positive margin cancers seemed to appear clinically less relevant in locally advanced disease with seminal vesicle invasion or high GS ≥8 owing to the predominant significance of these two indicators of poor prognosis for prediction of PSA failure. Similarly, our results showed that the PSM was significantly predictive for BCR-free survival in men with pT2 (HR, 3.81; p<0.001) and/or GS ≤6 (HR, 5.46; p=0.010) or 7 (HR, 3.50; p<0.001), and/or PSA value <10 (HR, 4.29; p<0.001) or 10-20 ng/mL (HR, 3.13; p=0.033). In patients with pT3, GS ≥ 8, and preoperative PSA value ≥20 ng/mL, PSM was not a statistically significant predictor for BCR-free survival (all p>0.05).
Several studies have reported that the extent of margin positivity correlates with disease recurrence after RP. Mauermann et al. [15] evaluated the influence of a solitary PSM and multiple PSMs on important clinical endpoints. In their multivariate analyses, multiple PSMs were a stronger predictive factor (HR, 2.08; p<0.001) for BCR than was a solitary PSM (HR, 1.71; p=0.001). Therefore, patients with multiple PSMs were most likely to receive salvage RT. Stephenson et al. [22] analyzed the predictive usefulness of several subclassifications of PSMs. According to their results, the number of PSMs was a significant predictor of BCR, with multiple PSMs having a worse prognosis than a solitary PSM (HR, 1.4; p=0.002). However, other studies reported different results. Marks et al. [23] evaluated the prognostic significance of the linear extent of margin positivity in a series of 174 consecutive PSM specimens and concluded that the extent of margin positivity was not predictive of BCR (HR, 1.00; p=0.97). In our study, BCR-free survival showed a statistically significant difference between the solitary PSM group (55.6 months) and the multiple PSM group (23.7 months, p<0.001), and multiple PSMs showed significance for prediction of BCR-free survival (HR, 2.32; p=0.001).
Despite some benefit of immediate adjuvant RT for the management of patients with a PSM, most urologists have experienced difficulty determining the further management (adjuvant or salvage therapy) of patients with PSMs. As we stated above, our results suggest some criteria for further treatment of patients with PSMs. If a PSM is found in patients with pT2 and/or GS ≤7 and/or a preoperative PSA value <20 ng/mL who have undergone RP, adjuvant therapy is worth taking under active consideration, although the pathological T stage, GS, and preoperative PSA level do not have a high risk of recurrence or progression. Furthermore, multiple PSMs can be regarded as a predominant parameter for worse prognosis than a solitary PSM as well as other clinicopathological factors (Table 4), whereupon adjuvant treatment can be applied to men with this pathological outcome.
There may be several limitations to our study. The inclusion or exclusion criteria for adjuvant therapy did not coincide among all clinicians owing to a retrospective approach. However, we believe that this limitation did not prohibit us from drawing conclusions because all patients who underwent an adjuvant treatment were excluded and the same criterion for BCR was applied in all cases. Also, we could not analyze the impact of the PSM location, which might affect BCR. In addition, the small number of subjects and the relatively short follow-up period in the present study may be challengeable to some degree. A long-term, prospective and randomized trial with extensive cases is needed to found a definitive clinical recommendation.
CONCLUSIONS
The prognostic value of PSMs and the decision of whether to proceed with adjuvant or salvage therapy in patients with a PSM remain controversial. According to our results, the PSM is a significant predictor of postoperative BCR in patients with pT2 and/or GS ≤7 and/or a preoperative PSA value <20 ng/mL. Adjuvant therapy for patients with these clinicopathological features is worth taking under active consideration, although the pathological T stage, GS, and preoperative PSA level do not have a high risk of re currence or progression. Also, multiple PSMs are considered to be a stronger prognostic factor for BCR than is a solitary PSM; therefore, adjuvant treatment could be applied to these men.
Footnotes
The authors have nothing to disclose.
References
- 1.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian PJ, Boorjian SA, Bossi A, Briganti A, Heidenreich A, Freedland SJ, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012;61:1096–1106. doi: 10.1016/j.eururo.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Grubb RL, Kibel AS. High-risk localized prostate cancer: role of radical prostatectomy. Curr Opin Urol. 2010;20:204–210. doi: 10.1097/MOU.0b013e3283384101. [DOI] [PubMed] [Google Scholar]
- 4.Sundi D, Jeong BC, Lee SB, Han M. Optimizing the management of high-risk, localized prostate cancer. Korean J Urol. 2012;53:815–820. doi: 10.4111/kju.2012.53.12.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001;165:119–125. doi: 10.1097/00005392-200101000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Ro YK, Lee S, Jeong CW, Hong SK, Byun SS, Lee SE. Biochemical recurrence in Gleason score 7 prostate cancer in korean men: significance of the primary Gleason grade. Korean J Urol. 2012;53:826–829. doi: 10.4111/kju.2012.53.12.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein JI, Partin AW, Potter SR, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: prognostic stratification based on pathologic parameters. Urology. 2000;56:283–288. doi: 10.1016/s0090-4295(00)00640-3. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BA, Shappell SB, Chang SS, Wells N, Farnham SB, Smith JA, Jr, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2006;97:1169–1172. doi: 10.1111/j.1464-410X.2006.06148.x. [DOI] [PubMed] [Google Scholar]
- 9.Uhlman MA, Sun L, Stackhouse DA, Caire AA, Polascik TJ, Robertson CN, et al. Tumor volume, tumor percentage involvement, or prostate volume: which is predictive of prostate-specific antigen recurrence? Urology. 2010;75:460–466. doi: 10.1016/j.urology.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran NM, Hovens CM, Metcalfe C, Hong MK, Pedersen J, Casey RG, et al. Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease. BJU Int. 2012;110:821–827. doi: 10.1111/j.1464-410X.2011.10868.x. [DOI] [PubMed] [Google Scholar]
- 11.Ploussard G, Agamy MA, Alenda O, Allory Y, Mouracade P, Vordos D, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naïve patients. BJU Int. 2011;107:1748–1754. doi: 10.1111/j.1464-410X.2010.09728.x. [DOI] [PubMed] [Google Scholar]
- 12.Chalfin HJ, Dinizo M, Trock BJ, Feng Z, Partin AW, Walsh PC, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012;110:1684–1689. doi: 10.1111/j.1464-410X.2012.11371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.So BK, Choi JD, Lee SY, Kim HS, Park SY, Seo SI. Experience of 100 laparoscopic radical prostatectomies performed by a single surgeon: an analysis of surgical and functional outcomes. Korean J Urol. 2011;52:517–523. doi: 10.4111/kju.2011.52.8.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh JJ, Hong SK, Byun SS, Choe G, Lee SE. Prognostic significance of positive surgical margins after radical prostatectomy among pT2 and pT3a prostate cancer. Urol Oncol. 2013;31:595–600. doi: 10.1016/j.urolonc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Mauermann J, Fradet V, Lacombe L, Dujardin T, Tiguert R, Tetu B, et al. The Impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013;64:19–25. doi: 10.1016/j.eururo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998;160:299–315. [PubMed] [Google Scholar]
- 17.Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 5: surgical margins. Mod Pathol. 2011;24:48–57. doi: 10.1038/modpathol.2010.155. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996;23:651–663. doi: 10.1016/s0094-0143(05)70343-8. [DOI] [PubMed] [Google Scholar]
- 19.Eminaga O, Hinkelammert R, Titze U, Abbas M, Eltze E, Bettendorf O, et al. The presence of positive surgical margins in patients with organ-confined prostate cancer results in biochemical recurrence at a similar rate to that in patients with extracapsular extension and PSA≤10 ng/ml. Urol Oncol. 2013 Feb 19; doi: 10.1016/j.urolonc.2012.11.021. [Epub]. http://dx.doi.org/10.1016/j.urolonc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 21.Vis AN, Schroder FH, van der Kwast TH. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006;50:258–265. doi: 10.1016/j.eururo.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Stephenson AJ, Wood DP, Kattan MW, Klein EA, Scardino PT, Eastham JA, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009;182:1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Marks RA, Koch MO, Lopez-Beltran A, Montironi R, Juliar BE, Cheng L. The relationship between the extent of surgical margin positivity and prostate specific antigen recurrence in radical prostatectomy specimens. Hum Pathol. 2007;38:1207–1211. doi: 10.1016/j.humpath.2007.01.006. [DOI] [PubMed] [Google Scholar]