Abstract
Fluctuating forces imposed on the airway smooth muscle due to breathing are believed to regulate hyperresponsiveness in vivo. However, recent animal and human isolated airway studies have shown that typical breathing-sized transmural pressure (Ptm) oscillations around a fixed mean are ineffective at mitigating airway constriction. To help understand this discrepancy, we hypothesized that Ptm oscillations capable of producing the same degree of bronchodilation as observed in airway smooth muscle strip studies requires imposition of strains larger than those expected to occur in vivo. First, we applied increasingly larger amplitude Ptm oscillations to a statically constricted airway from a Ptm simulating normal functional residual capacity of 5 cmH2O. Tidal-like oscillations (5–10 cmH2O) imposed 4.9 ± 2.0% strain and resulted in 11.6 ± 4.8% recovery, while Ptm oscillations simulating a deep inspiration at every breath (5–30 cmH2O) achieved 62.9 ± 12.1% recovery. These same Ptm oscillations were then applied starting from a Ptm = 1 cmH2O, resulting in approximately double the strain for each oscillation amplitude. When extreme strains were imposed, we observed full recovery. On combining the two data sets, we found a linear relationship between strain and resultant recovery. Finally, we compared the impact of Ptm oscillations before and after constriction to Ptm oscillations applied only after constriction and found that both loading conditions had a similar effect on narrowing. We conclude that, while sufficiently large strains applied to the airway wall are capable of producing substantial bronchodilation, the Ptm oscillations necessary to achieve those strains are not expected to occur in vivo.
Keywords: airway smooth muscle, intact airways, bronchodilation, bronchoprotection, asthma
when periodic length oscillations mimicking tidal breathing and deep inspirations (DIs) are applied to airway smooth muscle (ASM) during or after excitation, the increase in ASM force is less than would otherwise have occurred, if the excitatory stimulus were applied while the ASM was static (13, 21, 61). This observation led to the notion that periodic length fluctuations perturb the binding of myosin to actin (14, 33, 38, 48), or cause remodeling of the contractile apparatus (19, 30, 50), thereby limiting the resultant tension that the ASM can generate. With regard to asthma, the isolated ASM studies motivated the conjecture that the mechanism behind the beneficial effect of DIs in preventing airway hyperresponsiveness (AHR) is intricately tied to stretching of the ASM during breathing (13, 17, 29, 48). By extrapolation, these ASM strip level studies led to the hypothesis that, in asthmatic subjects, conditions such as wall remodeling or the presence of a milieu of inflammatory mediators could all conspire to limit tidal stretch of the ASM, resulting in chronically stiffer and more contractile airways (15–17). Consistent with these notions is that DIs in healthy subject are far more effective at dilating constricted airways (bronchodilation) (9, 41, 53) than they are in asthmatic subjects (8, 12, 25). There is also evidence that a DI can protect against future constriction (bronchoprotection) (26, 37, 54, 55) in healthy but not asthmatic subjects (26), and prohibition of DIs results in amplified reactivity in healthy subjects (58).
While the whole lung data are tantalizingly consistent with the isolated ASM studies, there are multiple complex mechanisms at the level of the whole organ that can come into play when examining how a DI alters the reactivity of a branching airway system embedded in lung parenchyma (3, 10). Consequently, one cannot unambiguously conclude from whole lung data that the bronchoprotection and bronchodilation distinctive behaviors are strictly the result of how stretch on the ASM embedded within the airway wall differs. In this study, we attempt to explicitly establish how behavior from isolated ASM strip studies translates to the whole airway. Recently, several groups have examined the impact of fluctuating stress and strain applied to isolated airways (31, 32, 43, 44, 46) or airways residing in lung slices (34). LaPrad et al. (31) showed in intact bovine airways that transmural pressure (Ptm) fluctuations associated with tidal breaths and DIs imposed around a constant mean Ptm of 5 cmH2O were relatively ineffective at reversing narrowing. Noble et al. (44) found similar results in intact human airways. In both of these studies, the mean Ptm was kept constant (5 cmH2O) for all oscillation amplitudes to isolate the role of dynamics and eliminate the confounding effects of airway dilation due to increased mean static load, which occurs during normal breathing patterns. Lavoie et al. (34) showed, in precision-cut lung slices, that stretches mimicking DIs with greater than 30% area strain were required to elicit substantial reversal of bronchoconstriction. However, it is not possible to explicitly translate fluctuations of lung slices to physiological forces associated with in vivo airways. The effect of large-amplitude strains applied from a constant Ptm has not been tested in the intact airway configuration.
In this study, we hypothesized that the strain imposed on the ASM during dynamic forcing of a constricted intact airway is a critical determinant of the degree of bronchodilation, and that strains sufficient to significantly ablate the constriction are likely not typically achieved in situ. We quantified the bronchodilatory effects of imposing tidal-like [5-cmH2O peak-to-peak (p-p) amplitude] up through large (25-cmH2O p-p amplitude) Ptm oscillations on intact airways from a Ptm mimicking functional residual capacity (FRC) (5 cmH2O) and then again in a separate set of airways from a reduced Ptm (1 cmH2O) to allow for larger strains per cycle. Additionally, we assessed the bronchoprotective effect of Ptm oscillations by comparing the impact of applying Ptm oscillations before and after constriction to applying the same Ptm oscillations only after constriction.
We demonstrate that bronchodilation of the intact bovine airway following constriction is strain-amplitude dependent. Ptm oscillations simulating tidal breathing (5–10 cmH2O) were ineffective at reversing airway constriction. To induce a recovery greater than 50%, Ptm oscillations mimicking a DI at every breath (5–30 cmH2O) were required. Reducing the FRC to a Ptm of 1 cmH2O resulted in twice the reversal of bronchoconstriction for any given amplitude of Ptm oscillations, but the corresponding strains were much larger than those expected in vivo. Additionally, we found that, while large Ptm oscillations do have a modest bronchodilatory effect, they do not provide an additional bronchoprotective effect when applied before and during constriction. Overall, the degree of recovery from constriction is linearly dependent on the strain one can impose during force-induced oscillations of the airway wall, but the Ptm oscillations necessary to produce the strain that result in substantial bronchodilation are not expected to occur in vivo.
METHODS
Intact Airway Segment Preparation
Bovine lungs were obtained from a local slaughterhouse immediately after death and placed on ice (Research 87, Bolyston, MA). The main-stem bronchus (generation 10–17, ∼35 mm long) was dissected free of parenchyma, and side branches were ligated. Cannulas were tied at each end, and the airway was placed in a tissue bath with gassed (95% O2, 5% CO2) and heated (37°C) Krebs solution (in mM: 121 NaCl; 5.4 KCl; 1.2 MgSO4; 25 NaHCO3; 5.0 sodium morpholinopropanesulphonic acid; 11.5 glucose; 2.5 CaCl2, pH = 7.4). The airway was stretched to 110–120% of its resting length to mimic airway lengthening during tidal breathing and held fixed throughout the experiment (27). Tissue viability was confirmed with both electric field stimulation and acetylcholine (ACh; 10−5 M), as previously described (27, 31, 32).
Ultrasound Intact Airway System
A custom-built system was utilized to obtain real-time measurements of luminal radius and wall thickness along the length of the airway, while Ptm oscillations could be delivered with a computer-controlled syringe pump, as described previously (31). Briefly, a portable ultrasound system (Terason t3000) linear array transducer (12L5-V) was partially submerged in the tissue bath and mounted over the longitudinal axis of the intact airway. Automated edge detection developed in MATLAB 7.14, R2012a (MathWorks, Natick, MA) was used to determine the location of airway walls in images. Then the luminal radius and wall thickness in pixels were converted to millimeters using the embedded length scale and a correction algorithm to account for the speed of sound and ultrasound pulse width (31). Luminal radius was quantified at each location along the airway's length and then expressed as the mean value.
Experimental Protocols
Two protocols were tested. Protocol 1 was designed to examine the effects of increasingly larger Ptm oscillations from either a simulated normal or reduced FRC on dilation of a constricted airway. Protocol 2 was designed to explore the effect of large p-p amplitude Ptm oscillations when applied before and during a constriction compared with the same large p-p amplitude Ptm oscillations imposed to a statically constricted airway.
Protocol 1: Increasing Ptm oscillation amplitude above constant FRC.
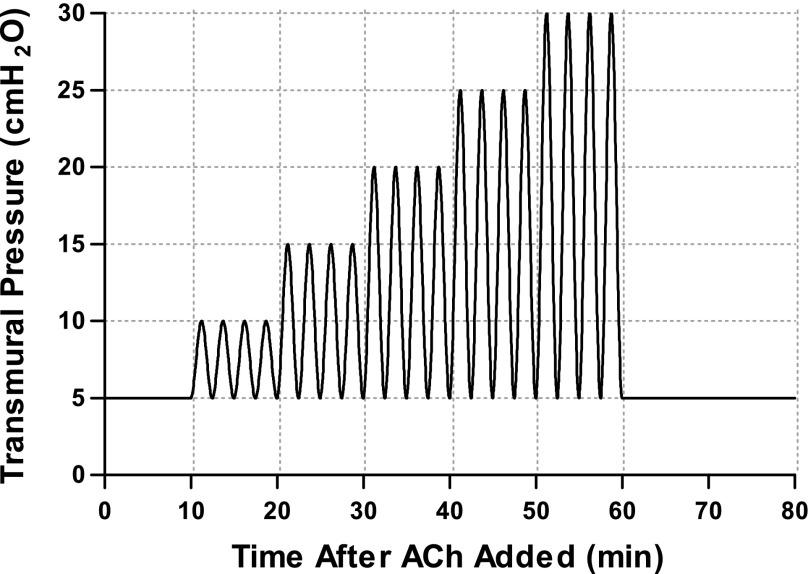
Airways were exposed to a moderate dose of ACh (10−5 M) against a constant Ptm simulating a normal FRC (Ptm = 5 cmH2O) for 10 min. Following static constriction, sinusoidal Ptm oscillations (0.2 Hz) were applied, whose p-p amplitude increased every 10 min (5, 10, 15, 20, and 25 cmH2O) (Fig. 1). The smallest oscillations (5–10 cmH2O) simulated the pressures of tidal breathing, while the largest oscillations (5–30 cmH2O) represented breathing to total lung capacity (TLC) on every breath. After the largest oscillation amplitude, the airway was again held at a constant Ptm, simulating FRC for 20 min. The airway was then allowed to relax in fresh Krebs solution for 60 min, and the protocol was repeated. The same experiment was then repeated on a separate set of airways, but the FRC was reduced from a Ptm of 5 cmH2O to 1 cmH2O. This was designed to probe the effect of larger cyclic strains since the airway is more compliant in this range of Ptm.
Fig. 1.
Schematic of protocol 1. Intact airways were constricted under a static transmural pressure (Ptm; 5 cmH2O) for 10 min, followed by Ptm oscillations (frequency = 0.2 Hz) applied above a simulated functional residual capacity (FRC) (Ptm = 5 cmH2O) of increasing peak-to-peak (p-p) magnitude from 5 to 25 cmH2O in increments of 5 cmH2O, and finally 20 min of static Ptm. The experiment was repeated in a separate set of airways with a reduced FRC (Ptm = 1 cmH2O) (not shown). ACh, acetylcholine.
Protocol 2: Bronchodilatory vs. bronchoprotective effect of large Ptm oscillations.
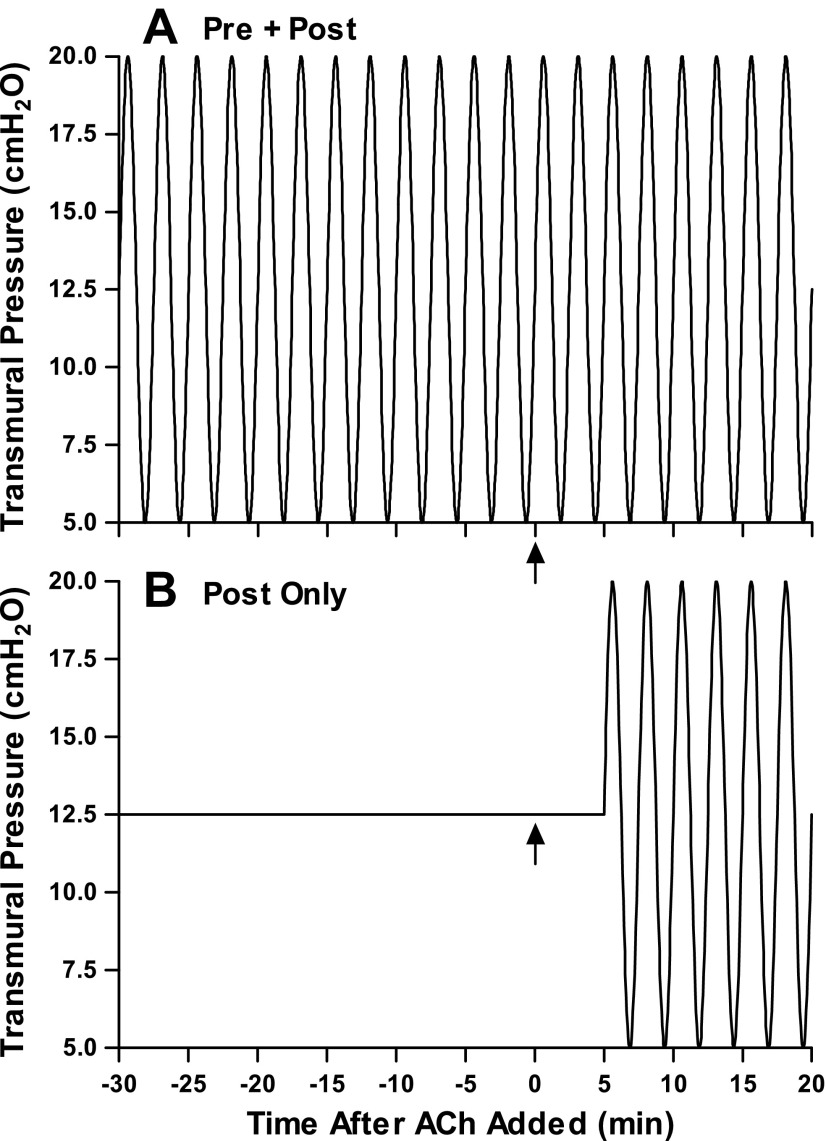
This protocol examined the degree to which large (15-cmH2O p-p amplitude) breathing frequency fluctuations in Ptm applied before and during constrictions were able to reduce the degree of constriction compared with oscillations imposed only after static constriction. We chose to examine large oscillations because previously studies have shown that tidal oscillations (p-p amplitude of 10 cmH2O or less) have failed to elicit a sustained bronchodilatory effect (31, 44). Airways were constricted with a moderate dose of ACh (10−5 M), while imposing one of two loading conditions in random order: Pre + Post (Fig. 2A), where large p-p amplitude Ptm oscillations (5–20 cmH2O) were imposed for 30 min immediately before and during the duration of the constriction (20 min); and Post Only (Fig. 2B), where the airway was constricted against a constant Ptm (12.5 cmH2O) for 5 min (Static loading condition), and then large Ptm oscillations (5–20 cmH2O) were applied for the remaining 15 min. After the conclusion of the first condition, the Krebs solution was replaced in the tissue bath, the airway was allowed to relax for 60 min, and the airway was constricted under the other loading condition.
Fig. 2.
Schematic of protocol 2. Intact airways were constricted twice in random order. A: Pre + Post: intact airways were exposed to large Ptm oscillations (5–20 cmH2O, 0.2 Hz) for 30 min before addition of ACh (10−5 M) and throughout the duration (20 min) of the constriction. B: Post Only: intact airways were constricted with the same dose of ACh under a static Ptm for 5 min (Static loading condition), and then large Ptm oscillations (5–20 cmH2O) were applied for the next 15 min. Arrows indicate the time at which ACh was added to the bath.
For both protocols, ultrasound images were acquired every 20 s during static Ptm and videos (30 s at 8 frames/s) were captured every 60 s during Ptm oscillations. Quasi-static Ptm-radius curves were recorded (105 s at 4 frames/s) at the very beginning (relaxed) and immediately before washout (constricted) of each constriction to establish volume history and measure the global mechanical properties of the airway by exposing the airway to slow linear ramps (−15 to 30 cmH2O, 1 cmH2O/s). The third complete cycle was recorded.
Bronchodilation and Strain Calculation
The imposed Ptm oscillations resulted in strain of the airway wall. In protocol 1, strain was defined as the change in mean luminal radius from the statically constricted state (RC) to end inspiration (REI), divided by the airway's baseline mean luminal radius (RB) and averaged over six cycles.
In protocol 2, the strain was defined as the change in radius from end expiration (Ptm = 5 cmH2O) to end-inspiration (Ptm = 20 cmH2O), normalized by the relaxed radius at end-expiration.
Bronchodilation due to Ptm oscillations was quantified in protocol 1 as the percent recovery from RC back to the RB. Measurements were taken at end-expiration (REE), which remained at a constant pressure (5 cmH2O) for all oscillation amplitudes.
In protocol 2, the steady-state percent constriction from baseline was calculated after 20 min of constriction during the Pre + Post and Post Only loading conditions. These values were compared with the amount of constriction after 5 min of static constriction during the Post Only constriction (Static loading condition).
Data and Statistical Analysis
All data are expressed as means ± SD, and n represents the number of airways. One-way repeated-measures ANOVA was used to determine the effect of loading condition (oscillation amplitude in protocol 1; Static, Pre + Post, and Post Only conditions in protocol 2) on airway reactivity and strain amplitudes. In protocol 2, paired t-tests were used to test for significant differences in mean values. Statistical significance was defined as P < 0.05.
RESULTS
Protocol 1: Bronchodilation Due to Ptm Oscillations From FRC
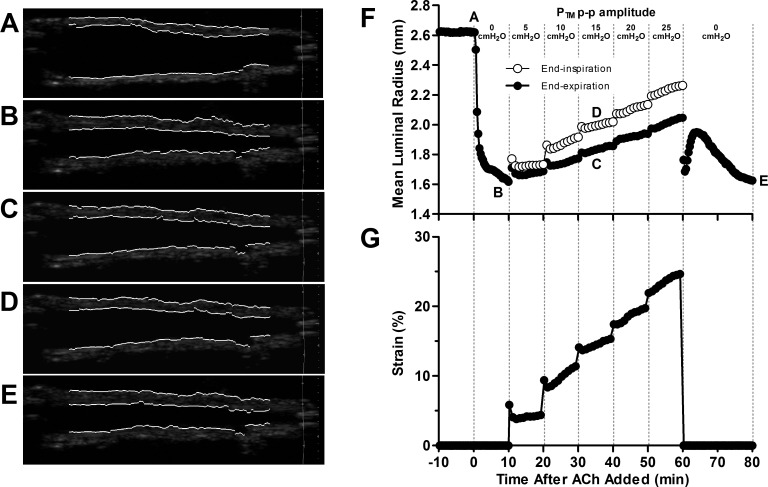
Airways were exposed to increasing p-p amplitude Ptm oscillations ranging from 5 cmH2O (mimicking tidal breathing) up to 25 cmH2O (mimicking a DI every breath) to test the bronchodilatory effect of Ptm oscillations. Two sets of experiments were performed: one with the Ptm oscillations applied above a Ptm simulating a normal FRC (5 cmH2O), and one with a reduced FRC (Ptm = 1 cmH2O). Figure 3, F and G, shows the mean luminal radii and strain amplitudes of a representative airway from protocol 1 when the oscillations were applied above a normal FRC (Ptm = 5 cmH2O). The luminal radius is plotted at end-expiration (Ptm = 5 cmH2O, black circles) and end-inspiration (open circles). Important time points are labeled on the luminal radius plot, and corresponding processed ultrasound images of the intact airway are shown in Fig. 3, A–E. The airway narrowed by 38% of its baseline radius (A) after static constriction (B). Consistent with previous results (31), Ptm oscillations simulating tidal breathing resulted in modest dilation (7%), despite 4% strain. Each increase in oscillation amplitude resulted in greater strains and further bronchodilation. For example, Ptm oscillations with p-p amplitude of 15 cmH2O imposed strains of 15% at end-inspiration (Fig. 3D) and resulted in 24% recovery back to baseline. Nevertheless, even the largest Ptm oscillations resulted in only 43% recovery. On cessation of the oscillations, the airway narrowed back to its initial constricted radius almost immediately, then dilated some, but within 20 min the preoscillation radius was reestablished in steady state (Fig. 3E). For the airways oscillated from a Ptm = 1 cmH2O, the degree of bronchodilation achievable exceeded those observed from airways oscillated from Ptm = 5 cmH2O, and higher amplitude Ptm oscillations completely ablated the bronchoconstriction.
Fig. 3.
Representative trace of airway's mean luminal radius (F) and strain amplitude (G), along with processed ultrasound images (A–E) corresponding to five distinct points along these curves during protocol 1, with Ptm oscillations applied above a Ptm simulating a normal FRC. At position A, the intact airway is in a relaxed state at its baseline radius. A moderate dose of ACh (10−5 M) was then added to bath, and the airway narrows for 10 min to its statically constricted radius at position B. Ptm oscillations of increasing p-p amplitude (5–25 cmH2O) were then applied. The mean luminal radius at a Ptm corresponding to FRC (5 cmH2O, ●) and end-inspiration (○) were extracted from the ultrasound images. Representative images are shown during 15-cmH2O Ptm p-p amplitude oscillations at end-expiration (position C, Ptm = 5 cmH2O) and end-inspiration (position D, Ptm = 20 cmH2O). Following the largest amplitude oscillation, the perturbations were stopped, and the airway was held statically for 20 min, returning to its final steady-state radius at position E. Small tidal Ptm oscillations (5-cmH2O p-p amplitude) provide minimal bronchodilation, despite 4% strain. The airway dilates more as the Ptm p-p amplitude and strains increase.
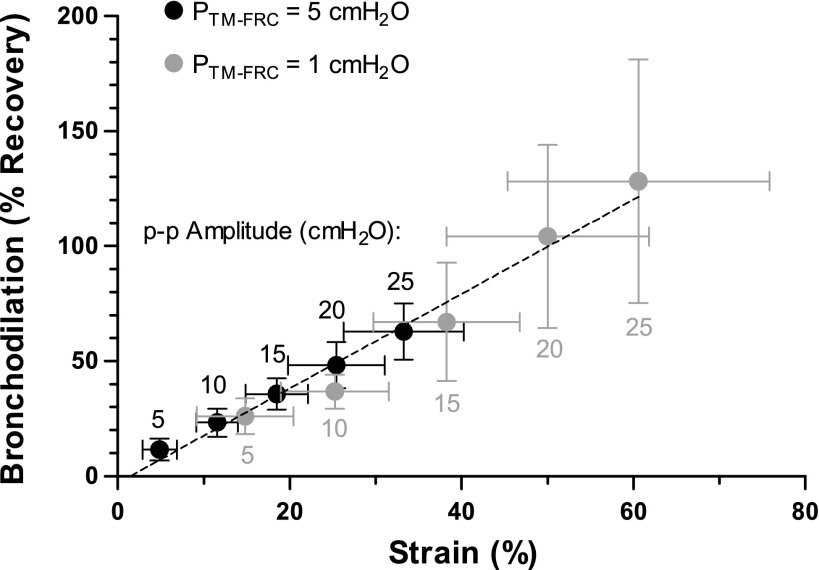
Figure 4 summarized the pooled results from all airways in protocol 1 and shows the impact of different sized Ptm oscillations on the imposed strain to the airways and recovery from the statically constricted state. When the FRC was set at physiological Ptm of 5 cmH2O (n = 5, black circles), tidal-like oscillations (p-p amplitude = 5 cmH2O) caused only a small dilatory impact (11.6 ± 4.8% recovery), despite strain amplitudes of 4.9 ± 2.0%. Excursions from FRC up to TLC (p-p amplitude = 25 cmH2O) on every breath were necessary to achieve bronchodilation of greater than 50% (62.9 ± 12.1% recovery). Reducing the FRC to a Ptm of 1 cmH2O (n = 4, gray circles) resulted in approximately twice the amount of strain imposed to the airway wall for any given p-p amplitude Ptm oscillation. This increased strain to the airway wall resulted in a proportional increase in bronchodilation and the same relationship between recovery and strain holds for both the normal and reduced FRC experiments (R2 = 0.99). Tidal-like oscillations from an FRC of 1 cmH2O resulted in 14.8 ± 5.6% strains to the airway wall and resulted in 26.1 ± 7.9% recovery, while excursions with p-p amplitudes of 20 cmH2O or larger resulted in full recovery back to the unconstricted radius, but this required strains of 50.0 ± 11.8%. The regression line fit to the means of both data sets (dashed black line) suggests there is a threshold strain of ∼1.5% required to achieve any bronchodilation.
Fig. 4.
Impact of strain imposed by each Ptm p-p amplitude on percent recovery of a statically constricted airway. Ptm oscillations applied above an FRC of 1 cmH2O resulted in about twice the strain and bronchodilation as the same sized Ptm oscillation imposed above a normal FRC of 5 cmH2O. Degree of recovery from a statically constricted airway is proportional to strain imposed by the Ptm oscillations (R2 = 0.99).
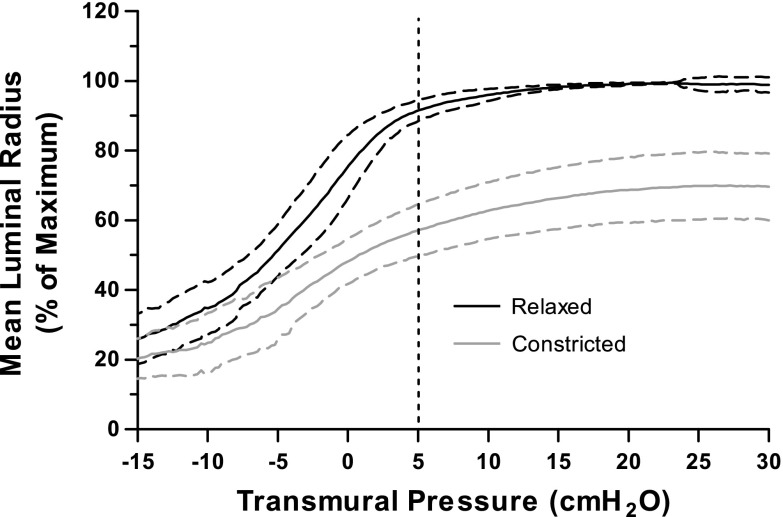
The mechanical properties of the airway were quantified over the entire physiological range of Ptm with quasi-static Ptm-radius curves. Figure 5 displays the deflation curves of the mean inner radius (normalized to each airway's relaxed maximum radius) for all of the airways studied in this paper before and after constriction. An important feature is that the lower end of the typical breathing operating range corresponds to a Ptm of 5 cmH2O. For both the relaxed and constricted state, the curves suggest that the airways are quite stiff above 5 cmH2O, thereby preventing large strains from being imposed, even with large Ptm oscillations. In the relaxed airway at a Ptm simulating FRC (5 cmH2O), the luminal radius is 88.7 ± 3.0% of its maximum radius and increases to 96.0 ± 1.7% at a Ptm of 10 cmH2O. For the constricted airway, the luminal radius at a Ptm simulating FRC (5 cmH2O) is 57.2 ± 7.4% of its maximum relaxed radius. With inflation to TLC, the airway reaches 70.1 ± 9.6% of its relaxed radius at TLC. The airway is compliant below a Ptm of 5 cmH2O, and, therefore, reducing FRC to a value of 1 cmH2O allowed the imposition of significantly greater strains imposed for a given p-p amplitude Ptm oscillation for both the relaxed and constricted states.
Fig. 5.
Quasi-static deflation Ptm-radius curves at the very beginning of each protocol (relaxed, black) and after constriction, just before washout of the ACh (10−5 M) (constricted, gray). Airways were normalized by the radius achieved at total lung capacity (30 cmH2O) in the relaxed state and then averaged. Curves are mean (solid lines) ± SD (dashed lines).
Protocol 2: Bronchodilation and Bronchoprotection of Large Ptm Oscillations
In protocol 1, it was found that Ptm oscillation from 5–20 cmH2O results in significant dilation (36% recovery). In protocol 2, airways were either exposed to large Ptm oscillations before and during constriction (Pre + Post) or only following constriction against a static load (Post Only). The goal was to evaluate whether 1) relaxed airway softens and/or dilates when exposed to large Ptm oscillations; and 2) if reactivity is significantly lessened if Ptm oscillations are imposed both before and after an airway is constricted, compared with holding the airway statically until after constriction. If so, then the Ptm oscillations also imbue a protective effect.
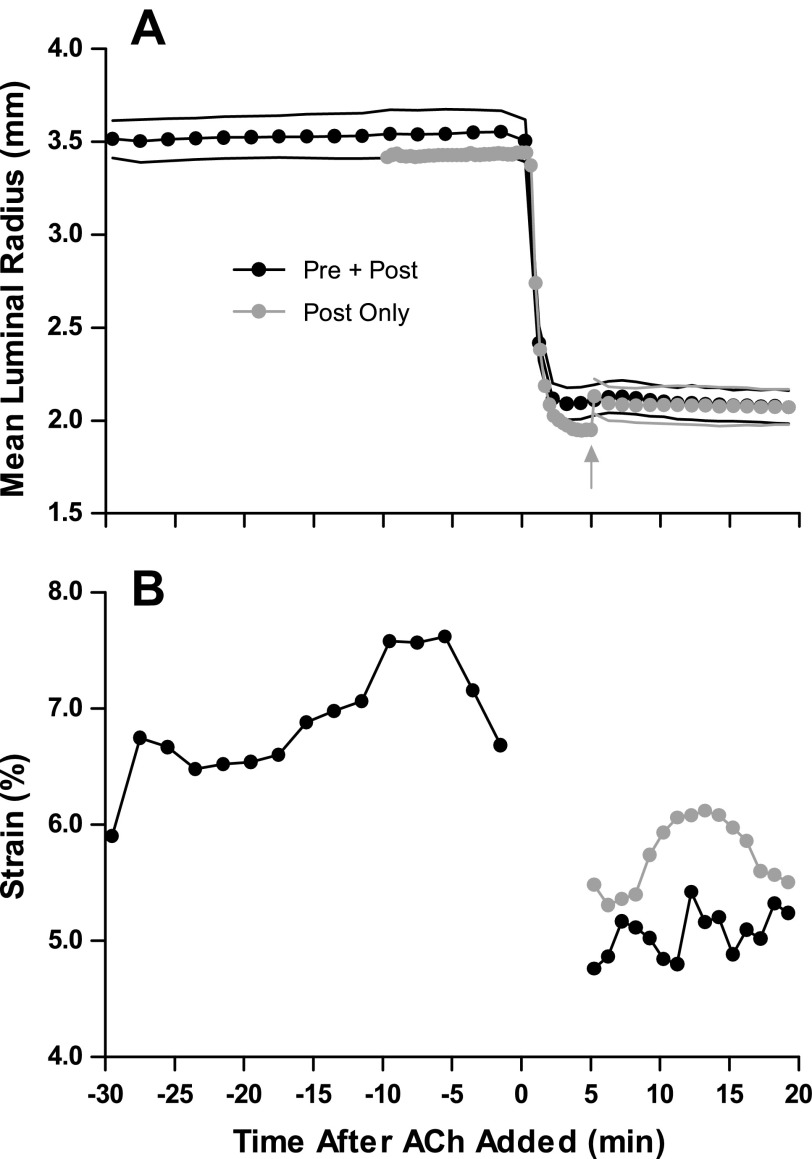
Figure 6 depicts mean inner radius (A) and strain (B) from an example single airway from protocol 2. In the Pre + Post condition, 30 min of large Ptm oscillations (5–20 cmH2O) did not result in significant dilation or decrease in stiffness, as measured by strain. This was consistent for all airways studied (time t = 0 min: 5.3 ± 1.0% strain, t = 30 min: 5.5 ± 0.9% strain, P = 0.52). The mean luminal radius at baseline (at Ptm = 12.5 cmH2O) was similar for both loading conditions (Post Only: 2.90 ± 0.43 mm vs. Pre + Post: 2.96 ± 0.46 mm, P = 0.12). For the Post Only condition, the airway initially constricted to a greater degree (gray arrow in Fig. 6, Static loading condition) than when the airway was exposed to Ptm oscillations for the entire duration (Pre + Post condition). However, the Ptm oscillations were able to dilate the airway to the same level in steady-state as the Pre + Post constriction. In this airway, the strain was slightly larger in the Post Only condition, but this was not a consistent result (P = 0.36).
Fig. 6.
Representative trace of airway's mean luminal radius (A) and strain amplitude (B) during protocol 2. A: circles represent luminal radius at 12.5 cmH2O, while the black line below and above the circles represents the end-expiratory (5 cmH2O) and end-inspiratory (20 cmH2O) radii, respectively. The airway constricts to a similar steady-state radius under both loading conditions (Pre + Post: black, Post Only: gray). The gray arrow depicts where the oscillations of the Post Only condition commence (Static loading condition). B: the strain in the Pre + Post loading condition remains constant before activation with ACh and decreases slightly after activation, suggesting a stiffer airway. In this airway, the strain was greater in the Post Only loading condition. Strain data are omitted during the first 5 min after the addition of ACh in the Pre + Post condition as the airway is constricting.
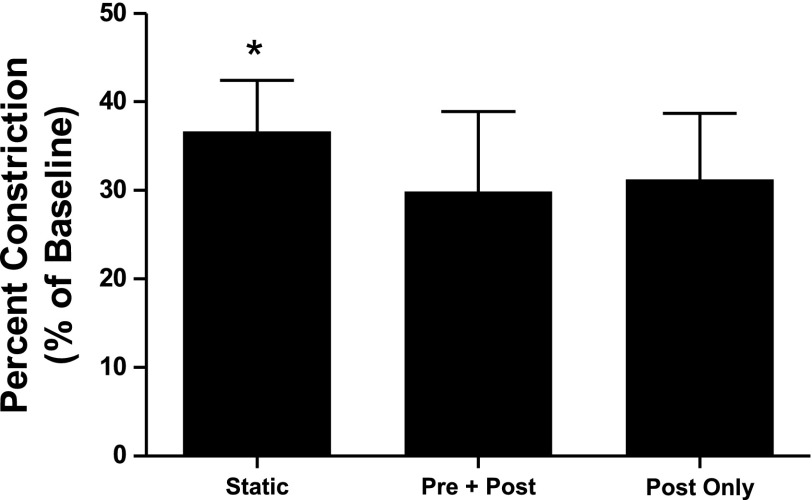
Figure 7 compares the degree of bronchoconstriction under the three loading conditions (n = 5). Imposing large Ptm oscillations resulted in less bronchoconstriction compared with a statically constricted airway, regardless of whether the Ptm oscillations were applied throughout the entire protocol (Pre + Post vs. Static, P = 0.004), or only after static constriction (Post Only vs. Static, P = 0.014). This is consistent with the results from protocol 1. However, there was no change in reactivity between the Pre + Post and Post Only conditions (P = 0.44). These results indicate that large Ptm oscillations have a modest bronchodilatory effect, but do not provide bronchoprotection.
Fig. 7.
Airways constricted to a significantly greater degree when no oscillations were imposed (Static) than when large oscillations (5–20 cmH2O) were applied (Pre + Post and Post Only) (*P < 0.05). However, whether the oscillations were applied exclusively after the constriction (Post Only) or before and after the constriction (Pre + Post) had no effect on the final amount of constriction (P = 0.44).
The mean strain imposed by the large oscillations from 5 to 20 min after the addition of ACh was the same for the two conditions (Post Only: 4.7 ± 0.8% vs. Pre + Post: 5.4 ± 1.4%, P = 0.36). This implies that an absence of oscillations during constriction did not modulate the compliance of the airway between Ptm of 5 and 20 cmH2O.
DISCUSSION
In recent years, a major focus has been examining how the dynamic mechanical environment of the lung impacts airway responsiveness in health and disease (42). In vivo, DIs in normal lungs are capable of reversing imposed bronchoconstriction (bronchodilation) (9, 41, 53) and attenuating future bronchoconstriction (bronchoprotection) (26, 37, 54, 55), but both of these effects are diminished or completely absent in asthmatic subjects (8, 12, 25, 26). Furthermore, prohibition of DIs during bronchial challenge of healthy subjects increases their responsiveness (58). The mechanisms behind bronchodilation and bronchoprotection are unknown, but are often attributed to the cyclic stretch of the ASM embedded within airway wall with tidal breathing and DI (13, 16, 17). This hypothesis is supported by studies showing that length oscillations before (61) and during (13, 21) activation of isolated tracheal ASM strips result in reduced force production, although there is also evidence that mechanical strain might have a relatively small effect on ASM force generation (49), or perhaps even enhance ASM contractility (36). These studies have led to the hypothesis that periodic fluctuations perturb the binding of myosin to actin (14, 33, 38, 48), or result in remodeling of the contractile apparatus (19, 30, 50) and, therefore, limit ASM tension generation and airway narrowing. Investigators have subsequently conjectured that the forces associated with breathing and DIs are crucial regulators that prevent AHR in vivo.
While these conjectures conveyed a sensible potential pathway for how the dynamics of the ASM (or lack thereof) could be an important modulator of AHR, ultimately they had to be tested and quantified at the intact airway level. Recent studies of intact airways now cast substantial doubt and confusion as to how or even if the sensitivity of ASM force generation to imposed realistic Ptm oscillations are relevant to explain reactivity at the whole airway level (31, 32, 43, 44, 46). In this study, we extended the results of LaPrad et al. (31, 32) and Noble et al. (43, 44, 46) by examining Ptm oscillations from FRC, as is typically imposed in situ. We used an integrated system with real-time ultrasound imaging to quantify the effect of small and large Ptm oscillations from a Ptm corresponding to either a normal or a reduced FRC on the reactivity and mechanical properties of an intact airway. Our results show that bronchodilation of the bovine intact airway is strain-amplitude dependent, but, to induce a recovery greater than 50%, Ptm oscillations mimicking a DI at every breath were required when applied above an FRC of 5 cmH2O, and the recovery was not sustained once the oscillations seized. Reducing the FRC to a Ptm of 1 cmH2O resulted in an approximate doubling in the magnitude of imposed strain and corresponding recovery for any given Ptm oscillation amplitude. In addition, while large Ptm oscillations (5–20 cmH2O) have a modest bronchodilatory effect, they do not provide additional bronchoprotection when imposed before constriction of an intact airway.
Our laboratory has previously shown that small, tidal-like Ptm oscillations (p-p amplitude of 10 cmH2O or less) imposed around a fixed mean Ptm corresponding to FRC are not effective at mitigating airway constriction (31). In protocol 1, we wondered if we would observe the bronchodilatory effect by applying larger Ptm oscillations from a constant Ptm, ranging from tidal-like up through mimicking a DI at every breath. Indeed, we showed that breathing-induced bronchodilation is strain-amplitude dependent (Fig. 4), but normal tidal breathing occurs at pressures much lower than those required to sufficiently strain the airway wall to achieve more than 50% recovery.
To mimic breathing in vivo in protocol 1, we applied increasing Ptm oscillations from a constant Ptm simulating FRC. In our laboratory's previous study (31), Ptm oscillations were applied around a mean Ptm (5 cmH2O) to eliminate the confounding effects of airway dilation due to increased mean static load, which occurs during normal breathing patterns. That study mimicked the protocols of ASM strip studies (33, 48) and was designed to explicitly test the mechanism of dynamically equilibrium ASM proposed by those studies. By applying oscillations above a constant Ptm in this study, we were able to apply realistic strains and probe the bronchodilatory effect of Ptm oscillations in the operating range that occurs in vivo. To account for the increase in mean Ptm with oscillation amplitude, we evaluated bronchodilation at the troughs of the oscillations, which remained constant throughout the experiments.
Our intact airway system provides a unique way to monitor the luminal radius and wall thickness of an isolated airway in response to agonists and perturbations in Ptm, which mimic the pressures of breathing in vivo. In the lung, airways are embedded within the parenchyma, and the size of the airway is controlled by the transpulmonary pressure (Ptp). Tidal breathing oscillations in vivo are typically from a Ptp of 5 cmH2O at end-expiration (FRC) up to 8 or 10 cmH2O at end-inspiration, and, at TLC, Ptp is increased up to 30 cmH2O. At the end of exhalation or inhalation, when flow is zero, Ptm is equal to Ptp, and, therefore, oscillations in Ptm similar to the perturbations of Ptp experienced in the whole lung were applied to our intact airways to mimic breathing.
The strains applied to the airway due to Ptm oscillations in this study are consistent with changes in airway size due to Ptp fluctuations measured in vivo. Strain was defined in a similar manner, as shown in previous in vivo (8, 62) and in vitro (43, 44) studies, as the change in radius from the statically constricted radius up to the end-expiration radius and normalized by the baseline radius. Imaging studies in vivo have suggested that, for airways of similar size as the ones studied in this experiment (human generation 4), the airway's radius increases by between 8.5% (62) and 16% (8) during inflation from FRC to TLC in a relaxed lung, as measured by anatomical optical coherence tomography and high-resolution computed tomography, respectively. The deflation relaxed quasi-static Ptm-radius curve (Fig. 5) suggests an average radius increases in radius of 8.6% from 5 to 30 cmH2O. For the constricted lung, in vivo measurements show inflation from FRC to TLC cause an increase in radius of 14% (8), consistent with our measurements of a 13% increase. When the Ptm fluctuations are applied dynamically, as occurs during breathing, the strain imposed increases. For example, tidal breathing from FRC of 5 cmH2O resulted in an average of 12% radius strain, while excursion from FRC to TLC with every breath resulted in 33% strain. These values are consistent with strains experience in isolated human airways by imposing a DI (22%) (44).
In addition to mimicking Ptm oscillations from a normal FRC of 5 cmH2O, protocol 1 also explored the effect of reducing the FRC to 1 cmH2O to a range of Ptm where the airway is more compliant. The strains applied to the airway from a reduced FRC were about twice as large for any given p-p amplitude Ptm oscillations, which resulted in about twice as much bronchodilation. When we combined both data sets, we found an intriguing phenomenon, namely that there may be a universal relationship between strain amplitude and degree of bronchodilation achievable (see Fig. 4). Hence, while indeed (following from isolated ASM studies) one can ablate the constriction by straining the airway periodically, the amount of strain necessary to do so are way above those occurring during tidal breathing and even deep breaths.
Our results are consistent with the recent work of Lavoie and colleagues (34), who studied expansion of human lung slices. The control parameter of these precision-cut lung slices is the depth of the indenter, which is then converted to an area strain, and it is unclear whether the resulting area strains relate to Ptm oscillations. Nevertheless, in the Lavoie system, they explicitly tried to compare small to larger oscillations and, like our protocol, imposed these from the FRC-like state. In particular, a circular indenter was used to stretch precision-cut lung slices at six levels of airway luminal fluctuation from simulated tidal breathing (2% area strain) up to a full DI at every breath (30% area strain). Similar to our present findings, only the largest oscillations resulted in substantial reversal of bronchoconstriction, and the reversal was not sustained when the oscillations were stopped (34).
In vivo studies examining the bronchodilatory effect of tidal breathing with the occasional DI have suggested that healthy subject can reverse bronchoconstriction by ∼60% (40, 55), while some have suggested full, sustained reversal from a single DI (25). Our results indicate that Ptm oscillations simulating taking a DI with every breath are necessary to generate a similar level of bronchodilation, and that this reversal is not sustained when the Ptm oscillations are stopped. During normal breathing, DIs are only taken once every 6 min (4), so, for a DI to be an effective bronchodilator, the effect must be sustained over a similar time frame. During a conventional bronchoconstrictor challenge, a person is likely capable of exerting larger pressures to overcome the greater impedance of bronchoconstriction. However, this study suggests that one might have to increase the pressure in the lung up to a Ptp corresponding to TLC for the breathing oscillations to be effective.
In this study, a single dose of ACh (10−5 M) was used to simulate a moderate exposure to an agonist (31). The amount of bronchodilation of a particular size of oscillation is likely inversely correlated with the degree of constriction, because the more constricted an airway becomes, the stiffer it will be and the less strains will be imposed by the same Ptm fluctuations (46, 49). Indeed, Lavoie et al. (34) showed in lung slices that greater reversal occurred when bronchoconstriction was least severe. It would be interesting to investigate the relationship between severity of constriction and the bronchodilatory power of Ptm oscillations in intact airways. Our laboratory (31) has previously shown that, for normal breathing patterns, there was no dependence on severity of constriction on the effectiveness of the Ptm oscillations in mitigating bronchoconstriction of intact airways. In human isolated airways, Noble et al. (44) showed that a DI results in greater initial reversal of narrowing when constricted with a lower dose of ACh, but also re-narrows quicker, and within 12 s after the DI the reversal is similar for all concentrations of ACh. However, it is unclear if this relationship would change with the larger Ptm oscillations applied in this study.
Several studies have shown that the magnitude of the bronchodilatory effect is strain-amplitude dependent (1, 11, 33, 34, 46, 57). However, it remains unclear how much the ASM is strained within the airway wall in vivo and how much strain is necessary to result in significant and sustained bronchodilation. The airway wall is a complex system whose mechanical properties are captured globally by the Ptm-radius curve (see Fig. 5). The nature of airway mechanics is such that, in the pressure range relevant in vivo, tidal breathing (5–10 cmH2O) only results in 5% strains (see Fig. 4). The bend of the curve typically occurs between 5 and 10 cmH2O, and an additional increase in pressure will only result in a few additional percentages of dilation. This characteristic is consistent with human airway expansion with lung inflation in vivo over several generations measured using high-resolution computed tomography (7) and anatomical optical coherence tomography (62). Relaxed airways are highly distensible up to Ptp of 5–7 cmH2O, but further increases in pressure up to 30 cmH2O result in no further distention (7). Intact airway studies of different species (20, 23, 31, 51), as well as excised lungs (22, 24), have shown similar pressure-radius relationships. Moreover, after excitation, the maximum radius achievable by the contracted airway even at a Ptm of 30 cmH2O is 30% less that at baseline (see Fig. 5). This means that, even if Ptm oscillations simulating a DI are imposed with every breath, the ASM can never be stretched to the absolute lengths achievable at baseline. This is apparent from Fig. 3, which shows that, after constriction, even 25-cmH2O p-p amplitude Ptm fluctuations only stretch the airway to 2.2 mm, whereas its resting radius at 5 cmH2O was 2.6 mm. In short, once the ASM causes the airway to constrict, the entire intact airway system becomes locally and globally stiffer, and imposition of our Ptm oscillations can no longer create substantive strains of this system. In addition, the strain imposed to the ASM layer is smaller than the measured luminal strain as a result of the airway's cylindrical geometry. If the ASM becomes uncoupled from the other wall structures (39), or if extracellular matrix stiffness is significantly less than the stiffness of the ASM (47), the ASM strain might be reduced further. Taken together, our results suggest that, while physiologically achievable Ptm oscillations are capable of imposing some dilation, they are not capable of imposing the necessary ASM strains that would result in the same level of bronchodilation observed in vivo.
The generation 10–17 bovine airway is similar in size (luminal radius of 2–3 mm) and global mechanical properties to a generation 4 human airway (62). The main-stem bronchus of the bovine lung tapers as it goes deeper into the lung, as shown in the longitudinal ultrasound images in Fig. 3, A–E. Our laboratory (31) has previously explored longitudinal heterogeneity during constriction and found that an airway constricts relatively homogenously along its length to the dose of ACh administered in this study. The airways studied do contain some cartilage content, but are able to fully close when exposed to a high-enough dose of ACh, suggesting the cartilage did not substantially limit contractile responses.
In protocol 2, we tested whether large-amplitude Ptm oscillations also have a bronchoprotective effect in the intact airway. The first major result from protocol 2 was that large Ptm oscillations applied to a relaxed bovine airway do not result in dilation or softening of the airway. In relaxed human airways, pressure oscillations simulating tidal breathing and DIs result in minor dilation, which can be attributed to reversal of intrinsic ASM tone (44). These results are inconsistent with isolated, inactivated ASM cell studies, which show that stretch results in a prompt decrease in the cell stiffness through cytoskeleton fluidization (28, 29, 60). The reason for this discrepancy is unclear and might be because the rate of cross-bridge attachment and detachment are similar in a relaxed ASM (13). Perhaps a simpler explanation is that even the baseline relaxed airway operates near the maximum of its Ptm-radius curve. Physiological Ptm oscillations might simply not create the strains necessary for the intact airway's ASM to alter its contractile response.
Based on ASM strip and cell experiments, investigators have hypothesized that, if imposed force fluctuations become too small, the ASM will become increasingly stiffer as a result of decreased cross-bridge cycling and/or reduced cytoskeleton fluidization (29). The airway then collapses into a statically equilibrated state with the ASM in a frozen latch state that is so stiff that tidal breathing and DI can no longer perturb actin-myosin cross bridges. If the above mechanism held, the ASM of the intact airway for the Post Only condition in protocol 2 would establish latched cross-bridges during the first 5 min of static constriction, resulting in a stiffer airway. The large Ptm oscillations would, therefore, result in decreased strain of the ASM and, therefore, less of a bronchodilatory effect. In the Pre + Post condition, the large Ptm oscillations during constriction should inhibit the airway from reaching a latch state and therefore constrict less than both the Static and Post Only conditions. However, the large Ptm oscillations did not modulate the stiffness of the airway during constriction and did not provide any additional bronchoprotective effect. Airways exposed to Ptm oscillations before and during constriction exhibited the same level of reactivity as airways that were exposed to Ptm oscillations after static constriction (see Fig. 7). Note that, while some of the comparisons in protocol 2 are of borderline significance due to a small sample size, any difference, significant or not, would not correspond to a functionally important difference.
Protocol 2 was designed to test whether Ptm oscillations before and during constrictions were able to reduce the degree of constriction compared with Ptm oscillations only after static constriction. In a living human, the Pre + Post loading condition is closer to what actually occurs in that one breathes constantly as agonist stimulation occurs. Hence, we tested for whether realistic dynamic forcing before and during a stimulation would reduce the reactivity. To assess what happens if Ptm oscillations are applied only before constriction starts, a third “Pre Only” could have been tested, but was omitted because it does not simulate realistic conditions. Our data explicitly compare whether Ptm oscillations constantly present (Pre + Post) are protective compared with no Ptm oscillations until after static constriction (Post Only).
This study is the first to directly examine the effects of pressure oscillations on subsequent airway narrowing and extends the work of Noble et al. (45), who examined the bronchoprotective effect in intact airways by tracking changes in active isovolumetric force generated following a period of pressure fluctuations. Consistent with our present results, they showed that pressure oscillations resulted in no beneficial bronchoprotection. They also observed an unexpected modest increase in active pressure, which did not translate into a change in airway narrowing in our study (45).
While one leading hypothesis explaining the beneficial effect of a DI is related to the effect stretch has on ASM cross-bridge dynamics (14, 33, 38, 48), several other potential mechanisms have been proposed as well (56). Furthermore, some human studies have suggested that the bronchoprotection and bronchodilation involve distinct physiological mechanisms (55). In particular, the release of relaxant factors, such as prostaglandins or atrial natriuretic peptide, through stretch-activated neural or hormonal pathways may be the mechanism in which DI can protect against future bronchoconstriction (5, 52, 59). Functional changes to the ASM due to chronic inflammation could potentially inhibit these stretch-induced mechanisms in asthma (18). Alternatively, stretch-activated surfactant release could also be responsible for the bronchoprotective effect of the DI (2, 35, 63). Our group has also provided some evidence that prohibition of DIs in healthy subjects might simply cause atelectasis, which amplifies reactivity (6).
In conclusion, our study examined the bronchodilatory and bronchoprotective effects at the level of the intact airway. Breathing-induced bronchodilation is strain-amplitude dependent, and there appears to be a universal relationship between strain amplitude and degree of bronchodilation achievable. While it is indeed possible to ablate constriction of an airway by straining it periodically, as has been shown in isolated ASM studies, the amplitude of Ptm oscillations and corresponding strains necessary are much larger than those that occur during normal breathing patterns. Furthermore, while large Ptm oscillations do provide a modest bronchodilatory effect, the bronchoprotective effect is not present at the level of the intact airway. These results challenge the current ASM cellular hypotheses and suggest other mechanisms might dominate what controls AHR in vivo.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-096797.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.C.H., H.P., and K.R.L. conception and design of research; B.C.H. performed experiments; B.C.H. analyzed data; B.C.H., H.P., and K.R.L. interpreted results of experiments; B.C.H. prepared figures; B.C.H. drafted manuscript; B.C.H., H.P., and K.R.L. edited and revised manuscript; B.C.H., H.P., and K.R.L. approved final version of manuscript.
REFERENCES
- 1. Ansell TK, Noble PB, Mitchell HW, West AR, Fernandes LB, McFawn PK. Effects of simulated tidal and deep breathing on immature airway contraction to acetylcholine and nerve stimulation. Respirology 14: 991–998, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bates JHT, Maksym GN. Mechanical determinants of airways hyperresponsiveness. Crit Rev Biomed Eng 39: 281–296, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol 19: 195–198, 1964 [DOI] [PubMed] [Google Scholar]
- 5. Berry EM, Edmonds JF, Wyllie H. Release of prostaglandin E2 and unidentified factors from ventilated lungs. Br J Surg 58: 189–192, 1971 [DOI] [PubMed] [Google Scholar]
- 6. Black LD, Henderson AC, Atileh H, Israel E, Ingenito EP, Lutchen KR. Relating maximum airway dilation and subsequent reconstriction to reactivity in human lungs. J Appl Physiol 96: 1808–1814, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Brown RH, Mitzner W. Effect of lung inflation and airway muscle tone on airway diameter in vivo. J Appl Physiol 80: 1581–1588, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 163: 994–1001, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Brusasco V, Crimi E, Barisione G, Spanevello A, Rodarte JR, Pellegrino R. Airway responsiveness to methacholine: effects of deep inhalations and airway inflammation. J Appl Physiol 87: 567–573, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Brusasco V, Pellegrino R. Complexity of factors modulating airway narrowing in vivo: relevance to assessment of airway hyperresponsiveness. J Appl Physiol 95: 1305–1313, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Duggan CJ, Chan J, Whelan AJ, Berend N. Bronchodilatation induced by deep breaths in relation to transpulmonary pressure and lung volume. Thorax 45: 930–934, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol 50: 1079–1086, 1981 [DOI] [PubMed] [Google Scholar]
- 13. Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 156: 1752–1759, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 159: 959–967, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 81: 2703–2712, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J 12: 1252–1256, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Fredberg JJ. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol 106: 615–624, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Fryer AD, Jacoby DB. Effect of inflammatory cell mediators on M2 muscarinic receptors in the lungs. Life Sci 52: 529–536, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Gunst SJ, Meiss RA, Wu MF, Rowe M. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol Cell Physiol 268: C1267–C1276, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Gunst SJ, Mitzner W. Mechanical properties of contracted canine bronchial segments in vitro. J Appl Physiol 50: 1236–1247, 1981 [DOI] [PubMed] [Google Scholar]
- 21. Gunst SJ. Contractile force of canine airway smooth muscle during cyclical length changes. J Appl Physiol 55: 759–769, 1983 [DOI] [PubMed] [Google Scholar]
- 22. Hahn HL, Watson A, Wilson AG, Pride NB. Influence of bronchomotor tone on airway dimensions and resistance in excised dog lungs. J Appl Physiol 49: 270–278, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Hyatt RE, Flath RE. Influence of lung parenchyma on pressure-diameter behavior of dog bronchi. J Appl Physiol 21: 1448–1452, 1966 [DOI] [PubMed] [Google Scholar]
- 24. Hyatt RE, Rodarte JR, Wilson TA. Effect of increased static lung recoil on bronchial dimesions of excised lungs. J Appl Physiol 39: 429–433, 1975 [DOI] [PubMed] [Google Scholar]
- 25. Jensen A, Atileh H, Suki B, Ingenito EP, Lutchen KR. Selected contribution: airway caliber in healthy and asthmatic subjects: effects of bronchial challenge and deep inspirations. J Appl Physiol 91: 506–515, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol 89: 711–720, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Khangure SR, Noble PB, Sharma A, Chia PY, McFawn PK, Mitchell HW. Cyclical elongation regulates contractile responses of isolated airways. J Appl Physiol 97: 913–919, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Krishnan R, Park CY, Lin YC, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, Butler JP, Fredberg JJ. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLos One 4: e5486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krishnan R, Trepat X, Nguyen TTB, Lenormand G, Oliver M, Fredberg JJ. Airway smooth muscle and bronchospasm: fluctuating, fluidizing, freezing. Respir Physiol Neurobiol 163: 17–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuo KH, Wang L, Paré PD, Ford LE, Seow CY. Myosin thick filament lability induced by mechanical strain in airway smooth muscle. J Appl Physiol 90: 1811–1816, 2001 [DOI] [PubMed] [Google Scholar]
- 31. LaPrad AS, Szabo TL, Suki B, Lutchen KR. Tidal stretches do not modulate responsiveness of intact airways in vitro. J Appl Physiol 109: 295–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LaPrad AS, West AR, Noble PB, Lutchen KR, Mitchell HW. Maintenance of airway caliber in isolated airways by deep inspiration and tidal strains. J Appl Physiol 105: 479–485, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Latourelle J, Fabry B, Fredberg JJ. Dynamic equilibration of airway smooth muscle contraction during physiological loading. J Appl Physiol 92: 771–779, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am J Respir Crit Care Med 186: 225–232, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu M, Tanswell AK, Post M. Mechanical force-induced signal transduction in lung cells. Am J Physiol Lung Cell Mol Physiol 277: L667–L683, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Maksym GN, Deng L, Fairbank NJ, Lall CA, Connolly SC. Beneficial and harmful effects of oscillatory mechanical strain on airway smooth muscle. Can J Physiol Pharmacol 83: 913–922, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Malmberg P, Larsson K, Sundblad BM, Zhiping W. Importance of the time interval between FEV1 measurements in a methacholine provocation test. Eur Respir J 6: 680–686, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Mijailovich SM, Butler JP, Fredberg JJ. Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys J 79: 2667–2681, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitchell HW, Gray PR. Uncoupling in the wall of the cartilaginous bronchus of the pig produced by smooth muscle contraction. Pulm Pharmacol 9: 29–34, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Moore BJ, Verburgt LM, King GG, Paré PD. The effect of deep inspiration on methacholine dose-response curves in normal subjects. Am J Respir Crit Care Med 156: 1278–1281, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Nadel JA, Tierney DF. Effect of a previous deep inspiration on airway resistance in man. J Appl Physiol 16: 717–719, 1961 [DOI] [PubMed] [Google Scholar]
- 42. Noble PB, Ansell TK, James AL, McFawn PK, Mitchell HW. Airway Smooth Muscle Dynamics and Hyperresponsiveness: In and outside the Clinic. J Allergy (Cairo) 2012: 157047, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Noble PB, Jones RL, Cairncross A, Elliot JG, Mitchell HW, James AL, McFawn PK. Airway narrowing and bronchodilation to deep inspiration in bronchial segments from subjects with and without reported asthma. J Appl Physiol 114: 1460–1471, 2013 [DOI] [PubMed] [Google Scholar]
- 44. Noble PB, Jones RL, Needi ET, Cairncross A, Mitchell HW, James AL, McFawn PK. Responsiveness of the human airway in vitro during deep inspiration and tidal oscillation. J Appl Physiol 110: 1510–1518, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Noble PB, McFawn PK, Mitchell HW. Intraluminal pressure oscillation enhances subsequent airway contraction in isolated bronchial segments. J Appl Physiol 96: 1161–1165, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Noble PB, McFawn PK, Mitchell HW. Responsiveness of the isolated airway during simulated deep inspirations: effect of airway smooth muscle stiffness and strain. J Appl Physiol 103: 787–795, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Noble PB, West AR, McLaughlin a R, Armstrong JJ, Becker S, McFawn PK, Williamson JP, Eastwood PR, Hillman DR, Sampson DD, Mitchell HW. Airway narrowing assessed by anatomical optical coherence tomography in vitro: dynamic airway wall morphology and function. J Appl Physiol 108: 401–411, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol 37: 264–272, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pascoe CD, Seow CY, Paré PD, Bossé Y. Decrease of airway smooth muscle contractility induced by simulated breathing maneuvers is not simply proportional to strain. J Appl Physiol 114: 335–343, 2013 [DOI] [PubMed] [Google Scholar]
- 50. Pratusevich VR, Seow CY, Ford LE. Plasticity in canine airway smooth muscle. J Gen Physiol 105: 73–94, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramchandani R, Shen X, Gunst SJ, Tepper RS. Comparison of elastic properties and contractile responses of isolated airway segments from mature and immature rabbits. J Appl Physiol 95: 265–271, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Said SI, Kitamura S, Yoshida T, Preskitt J, Holden LD. Humoral control of airways. Ann N Y Acad Sci 221: 103–114, 1974 [DOI] [PubMed] [Google Scholar]
- 53. Salerno FG, Pellegrino R, Trocchio G, Spanevello A, Brusasco V, Crimi E. Attenuation of induced bronchoconstriction in healthy subjects: effects of breathing depth. J Appl Physiol 98: 817–821, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med 162: 910–916, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med 163: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Scichilone N, Pyrgos G, Kapsali T, Anderlind C, Brown R, Permutt S, Togias A. Airways hyperresponsiveness and the effects of lung inflation. Int Arch Allergy Immunol 124: 262–266, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol 83: 1202–1208, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 96: 2393–2403, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol 24: 55–72, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Trepat X, Deng L, An SS, Navajas D, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal physical responses to stretch in the living cell. Nature 447: 592–595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang L, Paré PD, Seow CY. Effects of length oscillation on the subsequent force development in swine tracheal smooth muscle. J Appl Physiol 88: 2246–2250, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Williamson JP, McLaughlin RA, Noffsinger WJ, James AL, Baker VA, Curatolo A, Armstrong JJ, Regli A, Shepherd KL, Marks GB, Sampson DD, Hillman DR, Eastwood PR. Elastic properties of the central airways in obstructive lung diseases measured using anatomical optical coherence tomography. Am J Respir Crit Care Med 183: 612–619, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]