Abstract
We investigated the effects of fatigue produced by timed maximal voluntary contraction (MVC) of the index finger of the right hand on performance in MVC and accurate cyclic force production tasks in right-handed young (Young group) and strength-matched elderly (Elderly group) participants. We hypothesized that, before fatigue, the Elderly group would show weaker force-stabilizing synergies and smaller adaptive changes in the synergy index during fatigue. Synergies were defined as covaried adjustments of neural commands to fingers (finger modes) across trials that stabilize total force. Fatigue caused a significant reduction in the MVC, which was larger in the Young group compared with the Elderly group for both fatigued finger (index finger) and four fingers (index, middle, ring, and little fingers pressing together). Indexes of finger enslaving (lack of individuation) increased with fatigue in both groups. The index of force-stabilizing synergies was similar for the two groups before fatigue, while its increase with fatigue was significantly larger in the Elderly group compared with the Young group. We infer that changes in the indexes of finger interaction (enslaving) and coordination (synergy) with age seem to be correlated with changes in muscle strength. This correlation may be causally related to the progressive death of neurons at different levels of the neuromotor hierarchy. The surprisingly large changes in the synergy index with fatigue in older adults suggest that, by itself, aging does not necessarily lead to impairment in synergic control. Strength training may be a method to avoid age-related decrement in finger interaction and coordination.
Keywords: fatigue, aging, elderly, hand, synergy, coordination
muscle fatigue is a cause of major concern among the elderly (17, 33), and it has been defined as a transient reduction in the peak force, power, or other measures of performance during exercise (4, 15, 22, 29, 70). Within the framework of this definition, many studies have shown that muscles in older adults are weaker but either similar or more resistant to fatigue in isometric tasks than young adults (5, 12, 25, 29, 33, 34, 44). This has been partially attributed to the progressive death of α-motoneurons innervating fast-twitch fibers that later become reinnervated and incorporated into slower motor units (29, 51); to a smaller decline (in the elderly population) in motor unit firing rates (54); and to a smaller decrease in intracellular pH and less accumulated inorganic phosphate and H2PO4− in older adults (33) during fatigue.
In contrast to the above-mentioned findings, some studies have shown that, despite a higher resistance to fatigue, older adults show increased within-trial motor output variability (21, 68) during sustained contractions. This has been partially attributed to a higher variability in the motor unit discharge rate (6, 14, 43). Other studies have shown that, for nonfatiguing tasks, older adults show larger trial-by-trial variability (7) and poor bimanual coordination (27). To the best of our knowledge, there are no studies that have investigated the effects of local muscle fatigue on multielement coordination in the elderly population. The present study aims to address this question.
We study multielement coordination in motor tasks that involve redundant sets of elements using the principle of abundance and the idea of motor synergies (37), and the computational apparatus associated with the uncontrolled manifold (UCM) hypothesis (39, 56). According to the idea of synergies, the neural controller acts in a space of elemental variables, organizes in that space a subspace (UCM) corresponding to a desired value of a performance variable to which all the elemental variables contribute, and acts to limit variance across trials to that subspace. If the variance of the elemental variables within the UCM (VUCM) is larger than the variance orthogonal to the UCM (VORT), we conclude that there is a motor synergy stabilizing that particular performance variable.
Previously, it has been shown that aging is associated with weaker multifinger synergies stabilizing total force (23, 31, 47) and also a counterintuitive drop in the index of unintended force production by noninstructed fingers of the hand (enslaving; Refs. 31, 58). The lower enslaving in older adults correlated with the lower maximum voluntary force [maximum voluntary contraction (MVC)] (59). To disentangle the likely correlation between the force-producing ability and indexes of finger interaction and coordination, in the current study we selected a group of healthy older adults whose MVC force was comparable to that in our group of younger adults. Our first hypothesis was that, compared with young adults, strength-matched older adults would show a smaller drop in MVC during fatigue. Our second hypothesis was that the before-fatigue index of enslaving would be similar for the two groups, and enslaving would show similar changes in the two groups during fatigue (cf. Refs. 61, 62).
Previous studies on young healthy adults have shown that goal-relevant features of performance in a motor task that involves a redundant set of elements are relatively preserved after a fatiguing exercise by one of the elements (8, 9, 19, 28, 60, 62). This was associated with an increase in the index of synergy (ΔV) stabilizing the task-relevant performance variables. Our third hypothesis was that older adults would show lower ΔV before fatigue and smaller adaptive changes (i.e., smaller increase in the ΔV) with fatigue. This hypothesis has been motivated by reports of age-related decrements in sensorimotor adaptation (3, 18, 42, 57) and in corticomotor plasticity (53).
METHODS
Participants.
Eight young (mean age 25.8 ± 3.3 yr, 5 men; Young) and eight older (mean age 74.4 ± 4.5 yr, 4 men; Elderly) adults participated in the study. All participants were right-handed (self-reported). The elderly participants were recruited from a local retirement community and passed a screening process that involved a cognition test, a depression test, a quantitative sensory test, and a general neurological examination. The mean weight and height of the Young group was 73.3 ± 14.95 kg and 171.5 ± 8.72 cm, respectively. The mean weight and height for the Elderly group was 78.3 ± 13.28 kg and 168.7 ± 18.08 cm, respectively. The hand length and width for the two groups were 7.10 ± 0.28, 3.14 ± 0.12, and 6.90 ± 0.56, 3.23 ± 0.27 cm, respectively. All of the participants were healthy with no known history of neurological or motor disorders. Hand length was measured as the distance from the tip of the distal phalanx of digit 3 to the distal crease of the wrist with the hand in a neutral flexion extension pose. Hand width was measured between the lateral aspects of the index (I) and little (L) finger metacarpophalangeal joints. None of the participants had a history of long-term involvement in hand or finger activities, such as typing and playing musical instruments. All participants gave informed consent, according to the procedures approved by the Office for the Research Protections of the Pennsylvania State University, University Park, PA.
Experimental setup.
Four 6 degrees of freedom (DOF) strain-gauge force sensors (Nano-17, ATI Industrial Automation, Garner, NC) were used in the study. The sensors were placed under the index (I), middle (M), ring (R), and L fingers. Each sensor was covered with sandpaper (300 grit) to increase friction and prevent increased skin moisture during fatigue from affecting the sensors. All of the sensors were placed within slots in a panel that allowed the movement of the sensors in the sagittal plane of the participant, but not along the frontal plane. The participants determined the most comfortable location for each sensor, and the sensors were moved accordingly. The fixed distance between the centers of the sensors in the latero-medial direction was 3.0 cm. The analog signals of the normal force from the four force sensors were collected and digitized with a 12-bit analog-digital converter (PCI-6031 and PCI-6033, National Instruments, Austin, TX) with the help of a customized LabVIEW program (LabVIEW 10.0, National Instruments, Austin, TX). The sampling frequency was set at 200 Hz, and before each trial the signals from the sensors were zeroed.
During the experiment, the participants sat in a chair facing the test table with their upper arms at ∼45° of abduction in the coronal plane and 45° of flexion in the sagittal plane. The elbow was flexed at ∼135°. The right forearm rested on a wooden board that housed the sensors (see Fig. 1A) and was strapped to the wooden board with three sets of Velcro straps. The left forearm rested on the laps of the participants. A custom-fit support object was placed underneath the palm of the right hand to help maintain a constant configuration of the hand and fingers while the participants performed the experiment. The participant's hand formed a dome-like structure with the metacarpophalangeal joint flexed at 20° and all interphalangeal joints slightly flexed as well. Participants were asked to select comfortable thumb positions during the experiment. A computer monitor was located 0.65 m away from the participant. The monitor displayed the task (described below in Procedures).
Fig. 1.
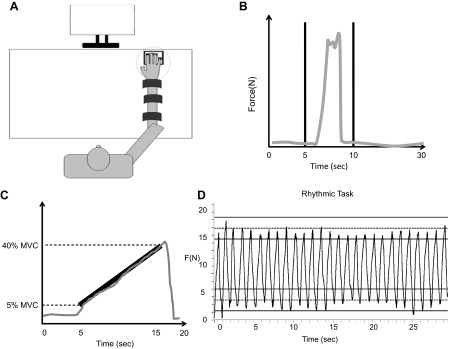
A: a schematic representation of the experimental setup. B: the computer feedback provided to the participants for the maximum voluntary contraction (MVC) tasks. The participants were asked to produce their maximum force within a 5-s window (vertical black lines). C: the computer feedback provided to the participants for the single-finger ramp tasks. The black line was shown as a template to the participants. D: the computer feedback provided to the participants for the one-finger and four-finger rhythmic tasks. The participants were asked to accurately produce forces (F) between the two dashed lines and use the solid lines as buffer zones.
Procedures.
The experiment was conducted in a single session. There were four different tasks in the experiment. The first task was maximal force production trials (henceforth referred to as MVC trials). The second task was called single-finger ramp task. These trials were conducted to measure the unintentional force production in one-finger tasks by the noninstructed fingers. The third task was called one-finger rhythmic task, and this task was performed only by the I finger. The last task was called four-finger rhythmic task, and all four fingers of the right hand performed this task.
Before the participants started the experiment, they were familiarized with the protocols for all the four tasks. Familiarization trials were repeated for all of the different experimental tasks (except MVC trials for which only one practice trial was performed by participants) until the participant had acquired a reasonable level of consistency and accuracy. Typically the participants performed 1–2 trials for the ramp task and 8–12 trials for each of the rhythmic tasks.
MVC trials.
The participants performed MVC trials by each of the four fingers, I, M, R, L, and all four fingers together (IMRL). The participants were given visual feedback of their performance and were instructed to produce maximum force with the instructed finger [between the two vertical lines (separated by 5 s) in Fig. 1B] and to let go when they were unable to produce any extra force. Each trial was 30 s long, but the participants were only asked to produce their MVC within the 5-s time interval separated by the vertical lines. During the trial, they were asked to keep the uninstructed fingers on the sensors. The order of the five MVC trials was randomized across participants, and they were given a 2-min rest between trials.
Single-finger ramp trials.
For this task, the participants were shown a template on the screen (see Fig. 1C) and were asked to reproduce the template by producing force with the instructed finger. The template showed a linear ramp from 5% MVC to 40% MVC over a 10-s period (the total trial duration was 20 s). They were also asked to simultaneously ensure that the uninstructed fingers were placed on the sensors during the trial. Participants were instructed not to pay attention to any force production by the other three fingers, and no feedback on forces produced by these fingers was provided. The data from these trials were used to compute the enslaving matrix E (described in Data processing and the appendix). There was one trial for each finger, as the instructed finger and the order of these trials were randomized across participants. A 15-s rest was given to participants between trials.
Rhythmic tasks.
The next two tasks involved rhythmic accurate force production with one finger (I) and four fingers (IMRL). The computer screen showed two horizontal targets placed at 10 and 30% of MVC. The task was to change force profile in a smooth, sinelike fashion in such a way that the crests and troughs lied within the target range specified by two horizontal lines at 10 ± 3 and 30 ± 3% of MVC (see Fig. 1D). Each trial was 30 s long. The participants were also required to do this at a pace set by a metronome that produced audible “ticks” at a frequency of 0.9 Hz. This frequency was set based on earlier studies that showed 0.9 Hz to be a comfortable frequency for young participants (20). The elderly participants also found this frequency comfortable. Three trials were repeated in a block for both the one-finger as well as the four-finger task. There were 15-s intervals between trials within a task and 2- to 3-min intervals between tasks. The order of these two blocks was randomized across participants.
Fatigue protocol and during-fatigue trials.
The participants were given a 5- to 10-min break after the completion of the before-fatigue trials. Participants performed a 1-min fatiguing exercise with the I finger at MVC, followed by a 20-s refatiguing exercises after each trial to avoid recovery from fatigue. At all times, the participants were verbally encouraged to produce as high forces as possible. This protocol has been used in our laboratory's previous studies (11, 49, 61, 62). Before and during the fatiguing exercise, the participants were reminded to try not to involve noninstructed fingers of the hand and to avoid excessive muscle co-contraction (stiffening the hand). For each participant, the during-fatigue trials within a condition were conducted in the same order as the before-fatigue trials. For the during-fatigue trials, the single-finger ramp tasks (5% MVC to 40% MVC over a 10-s period) and rhythmic tasks (10 ± 3 and 30 ± 3% of MVC) were scaled to the MVC forces that were produced by the participants after performing the fatiguing exercise(s).
Data processing.
The data were processed using MATLAB (MATLAB version 7.10.0. Natick, MA; The MathWorks, 2010). The data were filtered using a fourth-order zero-lag Butterworth filter with a cutoff frequency of 10 Hz.
Peak MVC force task.
Peak MVC force was measured at the instant when the combined force of the instructed fingers peaked.
Single-finger ramp task.
The single-finger ramp tasks were performed to compute the unintended force produced by the nontask fingers. This phenomenon has been termed enslaving (13, 32, 46, 73). To account for the phenomenon, we convert the force data matrix into force modes. Force modes are hypothetical elemental variables that can be modified by the controller one at a time. We first computed the enslaving matrices (E) for each participant by using the single-finger ramp trials. The method of computation of E has been previously described in detail in several publications (31, 62, 63) and is also described in the appendix. The index of enslaving “E” was computed as the sum of the off-diagonals of E. E was used to compute the time changes in the vector of hypothetical commands to fingers (force modes, m) based on the forces produced by each finger:
| (1) |
where m and f are force mode and force vectors, respectively; subscript i denotes a finger (I, M, R, or L), and subscript t denotes a time point.
Rhythmic force production tasks.
For the one-finger and four-fingers trials, we rejected data from the first and last 3 s of each trial to remove edge effects within a trial. The total force data (FTOT) were segmented into the periods of force increase (force-up) and force decrease (force-down). To compute the root mean square error (RMSE) for the rhythmic task, the quasi-sinusoidal force profiles were aligned and averaged for the force-up and force-down segments separately. Only those segments where the force crossed the midline were considered for analysis. In addition, we rejected segments where the start or end of segment was >15% MVC away from the target or <20% of a cycle away from the metronome tick. The accepted segments were then resampled into 100 points using cubic spline interpolation. RMSE and variance of force were computed about the mean at each of the resampled points and then averaged over the 100 points for both the force-up and force-down half-cycles. In this paper, only the force-up cycles are analyzed and presented. The RMSE was computed for the FTOT, whereas the variance of force was computed for the forces produced by each finger. For across-participants comparisons, RMSE was normalized by 20% (mean value of the sinusoidal cycle) of MVC (IMRL), and variance was normalized by the square of 20% of MVC (IMRL).
Analysis of multifinger synergies.
The analysis of multifinger synergies stabilizing the FTOT for the four-finger tasks was performed within the framework of the UCM hypothesis (56). According to the UCM hypothesis, the neural controller works in a space of elemental variables and creates in that space a subspace corresponding to a desired value of a particular performance variable. The four-dimensional space of finger modes can be divided into two subspaces, one corresponding to a fixed value of the FTOT (the UCM, three-dimensional space) and the other leading to changes in the FTOT (VORT, one-dimensional). The amount of trial-by-trial VUCM and VORT were computed per DOF. A synergy index, ΔV, was computed reflecting the relative amount of VUCM and VORT in the total variance (VTOT), also per DOF [VTOT = 0.25 × (3VUCM + VORT)]. The computational methods for computing force-stabilizing synergies are described in detail in the appendix.
Statistics.
The data are presented in the paper and Figs. 2–6 as means ± SE. The study had a three-factor design with fatigue (before fatigue and during fatigue as two levels) and group as the fixed factors (Young and Elderly as levels), and participants as a random factor. Participants were nested within the factor group. Repeated-measures (RM) ANOVAs were used to test hypotheses on the effects of fatigue and group. A linear-mixed model for RM was fit to the data. Diagnostic tests were performed on the data, and they were checked for normality (Shapiro-Wilk test) and heteroscedasticity (Levene's test). Appropriate transformations were made to the data before the ANOVA tests were performed wherever necessary. The level of significance was chosen at α = 0.05. All statistical tests were performed in SPSS 20.0.
Fig. 2.
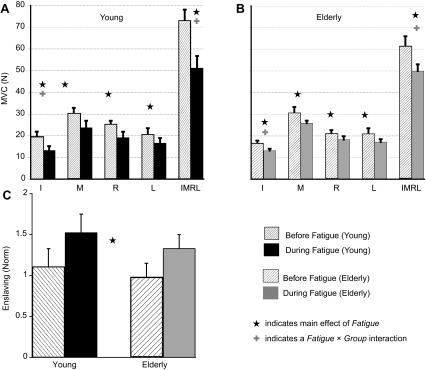
A: average maximum forces produced by the index (I), middle (M), ring (R), little (L), and four-finger (IMRL) combinations of the Young participants in the MVC tasks. B: average maximum forces produced by the Elderly participants. C: index of enslaving (E) for the Young and Elderly groups. Values are means ± SE. ★, Main effect of fatigue; +, fatigue × group interaction.
Fig. 6.
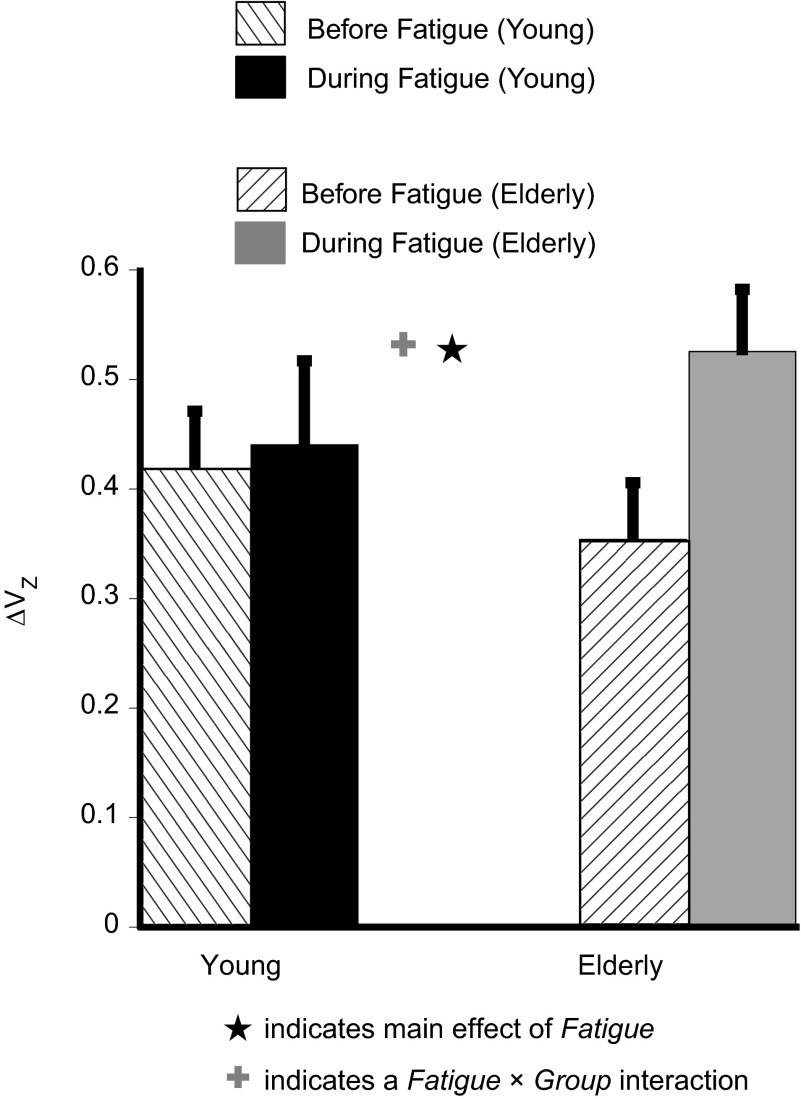
Average Z-transformed index (ΔVZ) of the force-stabilizing synergy for the rhythmic IMRL tasks. ΔVZ increased for both groups during fatigue, but the increase was significantly larger for the Elderly group. ★, Main effect of fatigue; +, fatigue × group interaction.
RESULTS
Before fatigue, there were no differences between the two groups for most of the variables of interest. Fatigue caused a larger decrease in the maximal voluntary force produced by the I finger, MVC (I), and by all four fingers together, MVC (IMRL); a larger increase in the error index during accurate force production task by the I finger, RMSE (I); a smaller increase in VUCM; and a smaller increase in the ΔV for the Young group compared with the Elderly group. None of the other effects induced by fatigue were statistically different between the two groups.
Effects of fatigue on maximal force and enslaving.
Before fatigue, there were no differences in the MVC and index of enslaving, E, between the two groups. The fatiguing exercise led to a significant drop in MVC of both one-finger and four-finger tasks. Five separate ANOVAs were performed on the MVC of each finger combination (I, M, R, L, and IMRL) with fatigue and group as factors. On average, fatigue led to a 27% decrease in MVC (I) for both the groups. The two-way RM ANOVA showed a main effect of fatigue [F(1,14) = 74.53, P < 0.001] and a fatigue × group interaction [F(1,14) = 5.5, P < 0.05]. Since the two groups had similar MVCs before fatigue, post hoc t-tests confirmed that the drop in MVC (I) was significantly greater for the Young group (∼32.5%, see Fig. 2A) compared with the Elderly group (∼21%, Fig. 2B). MVC of the M, R, and L fingers dropped by ∼18–20% for both groups during fatigue. The two-way RM ANOVAs showed significant main effects of fatigue for MVC (M), MVC (R), and MVC (L). No other effects were significant. For the four-finger MVC task, MVC (IMRL) dropped by ∼25% on average during fatigue. A two-way RM ANOVA on MVC (IMRL) with fatigue and group as factors showed a main effect of fatigue [F(1,14) = 71.08, P < 0.001] and a fatigue × group interaction [F(1,14) = 6.9, P < 0.05]. Post hoc t-tests revealed that the drop for the Young group (∼30%) was significantly greater than that for the Elderly group (∼18%).
The before-fatigue enslaving for the two groups was also similar; the enslaving values for the Elderly group was 0.98 ± 0.16 and for the Young group 1.10 ± 0.2. Enslaving increased during fatigue (by ∼35%, see Fig. 2C), and the increase was similar for both groups. The two-way RM ANOVA model showed a main effect of fatigue [F(1,14) = 24.8, P < 0.001]. No other effects were significant.
Effects of fatigue on accuracy of rhythmic task performance.
In general, error about the mean performance for the one-finger task, RMSE (I), seemed larger than the error for the four-finger task, RMSE (IMRL) (∼15%, but not significant, see Fig. 3). Before fatigue, RMSE (I) and RMSE (IMRL) also appeared to be larger for the Elderly group than the Young group by ∼25 and 30%, respectively. However, t-tests did not reveal any significant differences between the two groups.
Fig. 3.
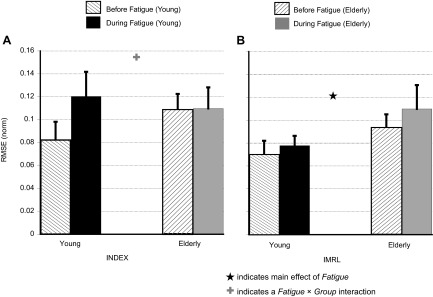
Indexes of performance (root mean square error, RMSE) in the I finger (A) and IMRL (B) tasks. Fatigue had no effect on RMSE (IMRL) but increased RMSE (I). The increase in RMSE (I) was larger for the Young group. Values are means ± SE. ★, Main effect of fatigue; +, fatigue × group interaction.
A three-way RM ANOVA with fatigue, group, and task (I and IMRL as levels) and RMSE as the dependent variables showed a main effect of fatigue [F(1,13.5) = 6.25, P < 0.05], task [F(1,13.8) = 13.4, P < 0.01] and a fatigue × group × task [F(1,29.4) = 5.1, P < 0.05] interaction. Since there was a three-way interaction and a main effect for task, we performed two-way RM ANOVAs on RMSE (I) and RMSE (IMRL) separately. A two-way RM ANOVA on RMSE (I) with fatigue and group as factors showed a significant fatigue × group interaction [F(1,14) = 5.3, P < 0.05]. The main effect of fatigue approached significance [F(1,14) = 3.6, P = 0.08]. Post hoc t-tests showed that fatigue led to a large increase in RMSE (I) (see Fig. 3A) for the Young group (0.08 ± 0.01 to 0.12 ± 0.02, increase of ∼50%), while there was only a minimal change for the Elderly group (0.10 ± 0.01 to 0.11 ± 0.01). For RMSE (IMRL), a two-way RM ANOVA with fatigue and group as factors only showed a significant main effect of fatigue [F(1,14) = 4.86, P < 0.05]. Since RMSE (IMRL) was similar between the two groups before fatigue, this indicates that the changes during fatigue in RMSE (IMRL) were not statistically different between the two groups (see Fig. 3B). RMSE (IMRL) increased by ∼10% (from 0.07 ± 0.01 to 0.077 ± 0.008) in the Young group and from 0.09 ± 0.01 to 0.11 ± 0.02 in the Elderly group.
Effects of fatigue on force sharing and variance of finger forces in the four-finger rhythmic task.
Force sharing was defined as the ratio of the force produced by the individual digit to the FTOT in the four-finger rhythmic task. Before fatigue, there were no differences in the force-sharing patterns of all of the four fingers between the two groups. On average, the share of the force produced by the I finger was 0.2, M finger was 0.4 and the R finger was 0.25. A two-way RM multivariate ANOVA on the Z-transformed force shares (force shares of I, M, and R fingers as dependent variables) showed no effects of either fatigue or group.
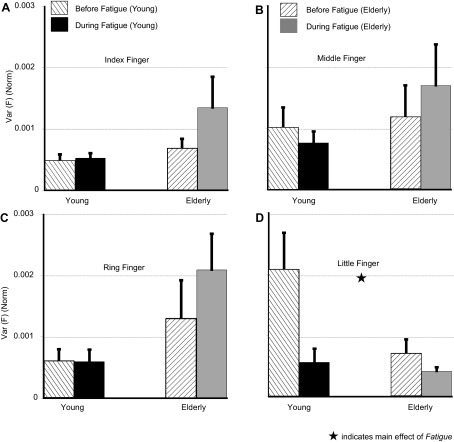
Before fatigue, there were no statistically significant differences in the variance of the force produced by the I finger [Var(I)], M finger [Var(M)], and R finger [Var(R)] between the two groups (see Fig. 4). The variance of the force produced by the L finger [Var(L)] was ∼50% smaller for the Elderly group (P < 0.05). Only the difference for Var(L) reached statistical significance (P < 0.01).
Fig. 4.
Force variance [Var(F); normalized to squared MVC of individual fingers] of individual fingers, averaged over the task interval, for the I (A), M (B), R (C), and L (D) fingers for the rhythmic IMRL tasks. Fatigue only increased the variance of force for the I finger (P = 0.08) and decreased it for the L finger. Values are means ± SE. ★, Main effect of fatigue.
Fatigue seemed to increase Var(I) for the Young group by ∼10% (0.0004 ± 0.0001 to 0.0005 ± 0.00008) and for the Elderly group by ∼90% (0.0006 ± 0.0001 to 0.001 ± 0.0005). A two-way RM ANOVA on Var(I) showed a main effect of group [F(1,14) = 5.65, P < 0.05], and the effect of fatigue approached significance [F(1,14) = 3.4, P = 0.08]. The lack of fatigue × group interaction showed that the increase in Var(I) were not statistically different between the two groups, even though the average magnitude of increase for the Elderly group was larger than that for the Young group. Fatigue had no significant effect on Var(M) and Var(R) (see Fig. 4, B and C). In contrast, fatigue decreased Var(L) in both groups by ∼ 60% (see Fig. 4D), from 0.0023 ± 0.0006 to 0.00063 ± 0.0002 in the Young group and from 0.0005 ± 0.0001 to 0.0004 ± 0.00006 in the Elderly group. The main effects of fatigue [F(1,11.8) = 8.4, P < 0.05] and group [F(1,11.67) = 5.4, P < 0.05] were significant.
Effects of fatigue on multifinger synergies.
We analyzed patterns of across-trial covariation among hypothetical commands to fingers (finger modes) by using the framework of the UCM hypothesis (56) for the four-finger rhythmic tasks. We used an index of covariation as an index of a force-stabilizing ΔV. Variance in the finger mode space was computed across trials for each data point and represented as the sum of two variance components: one that did not affect FTOT values (VUCM) and the other that did (VORT). Both indexes, VUCM and VORT, were quantified per dimension in the corresponding spaces to compute an ΔV (see Eqs. A8 and A9 in the appendix).
Before fatigue, there were no significant differences in the variance components VUCM and VORT between the two groups, while VUCM >> VORT (by ∼190%). A three-way RM ANOVA on the variance values with fatigue, group, and variance type (VUCM and VORT as levels) as factors showed a main effect of fatigue [F(1,21.73) = 12.13, P < 0.01], variance type [F(1,33.05) = 79.25, P < 0.001], and a fatigue × group × variance type [F(1,34.32) = 8.3, P < 0.01] interaction. Since there were no before-fatigue differences in VUCM and VORT between the two groups, this implied that fatigue affected the two variance components differently across the two groups. Therefore, we analyzed the effects of fatigue on the two variance components (VUCM and VORT) separately.
A two-way RM ANOVA for VUCM showed a main effect of fatigue [F(1,13.28) = 7.05, P < 0.05] and a fatigue × group interaction [F(1,13.28) = 4.69, P < 0.05]. Post hoc tests showed that the increase in VUCM was significantly larger for the Elderly group. Fatigue increased VUCM for the Young group by ∼225% and for the Elderly group by ∼400% (see Fig. 5A). VORT also increased during fatigue for both the groups by ∼65%, but there were no statistical differences between the groups (see Fig. 5B). A two-way RM ANOVA on VORT only showed a main effect of fatigue [F(1,12.6) = 5.8, P < 0.05]. No other effects were significant.
Fig. 5.
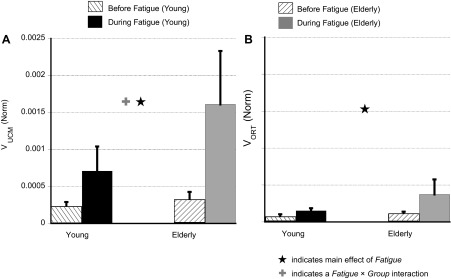
Two components of variance in the finger mode space computed for the rhythmic IMRL tasks. A: variance of the elemental variables within the uncontrolled manifold (VUCM) reflects the amount of variance that did not affect total force. B: variance orthogonal to the uncontrolled manifold (VORT) reflects the amount of variance that affected total force. Note the larger increase in VUCM (than VORT) during fatigue for both the groups. Values are means ± SE. ★, Main effect of fatigue; +, fatigue × group interaction.
Before fatigue, there were no differences in the ΔV between the two groups (see Fig. 6). Both groups showed an increase in the ΔV during fatigue. A two-way RM ANOVA with Z-transformed ΔV as the dependent variable showed a main effect of fatigue [F(1,14) = 8.99, P < 0.01] and a fatigue × group [F(1,14) = 5.4, P < 0.05] interaction. Post hoc tests showed that fatigue caused a larger increase in Z-transformed ΔV for the Elderly group (∼50%) compared with the Young group (∼7%) (see Fig. 6).
DISCUSSION
Our results generally confirmed the first two hypotheses, but not the third hypothesis, formulated in the Introduction. Our first hypothesis was that, compared with young adults, strength-matched older adults would show a smaller drop in MVC during fatigue. Indeed, we observed a significantly smaller drop in the MVC during the one-finger task performed by the exercised (I) finger and by all four fingers together. The second hypothesis was that the before-fatigue index of enslaving would be similar for the two groups, and enslaving would show similar changes in the two groups during fatigue. While older subjects, on average, showed somewhat smaller enslaving indexes (cf. Ref. 58), these differences did not reach significance; fatigue produced similar changes (an increase) in the enslaving indexes in the two groups. The third hypothesis was that older adults would show lower ΔV before fatigue and smaller adaptive changes in these indexes with fatigue. In fact, older adults showed larger changes in the ΔV with fatigue.
One of the main differences between the findings of the present study and previous aging studies (31, 47, 59) is that we found no major differences in the MVC, enslaving, RMSE, and force-stabilizing synergies between the two groups before fatigue. This was because of the selection criteria for older subjects. In this study, we recruited strength-matched participants to eliminate the possible strength-related confound (cf., the “strength-dexterity trade-off” in Ref. 59) and focus only on the effects of age on indexes of finger interaction and coordination and their changes with fatigue.
Are the elderly more fatigue resistant than young adults?
The results from our present study showed smaller drops in MVC of the fatigued finger (I) and the four fingers (IMRL) for the Elderly group compared with the Young group. These results are similar and consistent with other previous studies that have shown longer endurance times for the elderly population during fatigue in an isometric task (29, 30, 34, 72). Another recent study by Walker and colleagues (69) has shown that older men are more resistant to central fatigue during maximal strength exercises. These findings have led to a commonly held belief that, for isometric tasks, muscles in older adults are more fatigue resistant than in young adults (reviewed in Ref. 1). The higher fatigue resistance in the elderly has been associated with a preferential atrophy of type II fibers (35, 40) and a preferential loss of fast motor units (16) with aging. Our data corroborate the general idea that the elderly are more fatigue resistant than young adults (though this may be due to other reasons, see Limitations of the present study).
Do the elderly show larger changes in ΔV because they are more fatigue resistant?
There have been no studies comparing changes in synergies to graded fatigue, leading to MVC drops of different magnitude. Based on the idea that the synergy changes are adaptive to fatigue (62), we expect these changes to be larger for conditions with stronger fatigue effects (estimated as the amount of MVC drop due to fatigue). This was expected because, in one of our previous studies, we have shown that a fatiguing exercise performed by the I finger of one hand leads to a drop in the MVC of the fingers of the same hand, as well as the nonexercised hand (63). The drop in MVC was larger in the exercised hand. The dissimilar drops in MVC of the exercised as well as nonexercised hands indicated that the neural pathways associated with both of the hands were affected during fatigue, and that there was possibly a decrease in the central drive during fatigue. However, when the participants performed unimanual rhythmic force production task during fatigue (same task as the present study), the exercised hand showed an increase in RMSE, VUCM, and VORT, whereas it seemed that there was only an increase in VUCM for the nonexercised hand (although this increase was smaller in magnitude and did not reach statistical significance). Hence, the larger MVC drop in the Young group in the present study is expected to lead to larger synergy changes. Our result, however, is opposite. The two groups showed similar increase in VORT during fatigue, but we observed larger changes in VUCM and the ΔV in the Elderly. This observation suggests that the differences between the two groups in the effects of fatigue on the ΔV were not due to the differences in the MVC drop. We discuss some potential mechanisms below.
Changes in synergic control with age and fatigue.
Typical everyday movements involve more elements (joints, muscles, digits, etc.) compared with the number of constraints associated with everyday tasks. This is referred to as the problem of motor redundancy (2). There are two ways to perform such actions: element based and synergic. Element-based actions are associated with selection of particular combinations of elemental variables (those produced by the elements), for example, those that optimize a certain cost function (cf., Refs. 10, 50). Synergic control is associated with creating links among elemental variables such that they can show different values as long as these values covary to stabilize task-related performance variables to which they all contribute (36). Many everyday actions performed by young adults are associated with synergies stabilizing potentially important performance variables (reviewed in Refs. 38, 39).
Older adults showed impaired synergic control in a variety of tasks, including accurate force production tasks with a redundant set of digits (31, 47). These findings, in combination with the documented decrease in the enslaving index with age (58, 59), have led to a hypothesis that aging is associated with a progressive switch from predominantly synergic control back to element-based control [“back-to-elements” hypothesis (31)]. These changes could be causally related to the documented progressive death of neurons with age in supraspinal structures (24, 66, 67).
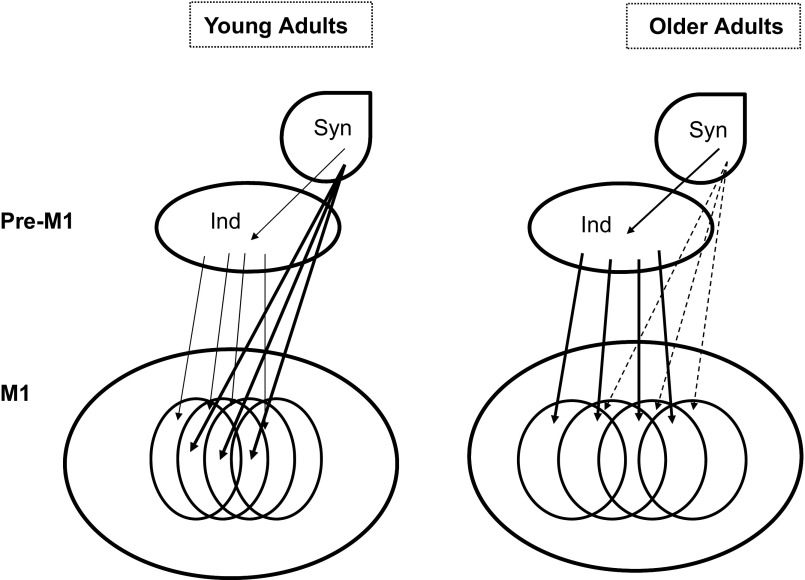
The idea is illustrated in Fig. 7. It assumes two hierarchically higher levels that define an input into the primary motor cortex (M1). The level of multifinger control [synergic (Syn)] can be based on learned patterns of multifinger involvement (cf. the idea of “cortical piano” of Ref. 55). These patterns facilitate multifinger synergies but contribute to higher enslaving in individual finger tasks. In older persons, death of neurons at the Syn level can lead to loss of necessary projections to M1; as a result, multifinger actions become element-based via the hierarchically lower level [individual (Ind)]. This leads to better finger individuation and impaired synergies.
Fig. 7.
An illustration of the “back-to-elements” idea using a cartoon of a hypothetical hierarchy with two levels that are defined inputs into the primary motor cortex (M1). Young adults can control individual fingers (Ind), but these projections (thin arrows) are overpowered by the strong synergic control (Syn; thick arrows). In older adults, the trend is reversed: Ind projections become relatively stronger, resulting in better finger individuation (lower enslaving), while Syn control is weaker.
Earlier studies of the effects of fatigue on multifinger synergies in young adults resulted in an unexpected finding: synergies became stronger during fatigue (60, 62, 63). These adaptive changes allowed multielement actions to preserve relatively high accuracy when some of the elemental variables showed increased variability during fatigue (60, 62). In our present study, we also observed smaller effects of fatigue on accuracy of the performance of the four-finger task compared with the single-finger task in the Young group (Fig. 3). The “back-to-elements” hypothesis suggests that older persons would not have a comparable ability to strengthen synergies because of the overall impairment in synergic control. Our results argue against this prediction: in fact, older persons showed significantly higher changes of multifinger synergies with fatigue (Fig. 6). These changes were associated with a very large increase in the amount of “good variability” (VUCM, see Fig. 5). Note that large amounts of VUCM cannot be predicted by an optimization of a cost function and is a sign of strong synergic control (cf. Ref. 71). This very unexpected result is discussed in the next subsection.
Is strength a defining factor in age-related changes in motor coordination?
There is a striking contrast between the results of our tests before fatiguing exercise and those reported earlier. Several earlier studies reported low MVC forces, lower enslaving indexes, and lower ΔV in older adults compared with younger adults (47, 58). In our studies, none of the mentioned indexes showed a significant difference between the young and older adults. We believe that the cause for the seeming inconsistency is the selection of older subjects in the present study who were not only in an overall very good health, but were very close to the younger group in their strength.
Several group comparisons of the indexes of finger interaction and coordination have shown consistent correlations with indexes of maximal voluntary force production (31, 59). In addition, a study of the effects of finger strength training in older adults have documented parallel changes in MVC indexes, enslaving, and indexes of multifinger force-stabilizing synergies (48). In that study, strength training also produced a significant improvement in the performance of the participants in the dexterity test (pegboard test). Taken together, these findings suggest that there may be a common underlying factor leading to parallel changes in strength and coordination indexes.
We suggest that the rate of neuronal death at different levels of the central nervous system with age may be such a factor. Progressive death of α-motoneurons in combination with the processes of muscle fiber denervation and reinnervation (51) is a likely important contributor to the loss of muscle mass and MVC force in older adults. On the other hand, if a similar process takes place in supraspinal structures (24, 66), it may be expected to lead to lower enslaving and weaker synergies as in the “back-to-elements” hypothesis (31). The selection of our subjects based on their preserved MVC might result in a subgroup of older adults in whom the age-related neuronal degeneration processes were slower compared with the general elderly population. This could lead to the mentioned differences in the results of our prefatigue tests compared with the earlier studies. These results also suggest that strength training may be a method to avoid age-related decrement in finger interaction and coordination (31, 58, 59). The study by Walker and colleagues (69) has compared the effects of constant vs. variable external training on young and old individuals. Based on the results from that study and our study, it seems that the elderly might benefit more from maximal strength training protocols for improving strength, as well as coordination.
The unexpectedly large increase in the ΔV after the fatiguing exercise in older adults may result from several factors. First, there is a possibility that the decreased projections in Fig. 7, underlying the impaired synergic control in older persons, could reflect not anatomical but functional impairment; then, under certain challenging conditions, these projections may start to function, thus creating more room for improvement of the ΔV. It is also possible that older adults showed larger modifications of the sharing pattern from trial to trial and from cycle to cycle while searching for a more comfortable way to perform the task under the conditions of fatigue. Younger adults could find a new, “optimal” way of performing the task quicker (cf. delayed adaptive changes in older adults Refs. 18, 57) and then show only spontaneous changes in the sharing pattern reflected in VUCM. The much larger increase in VUCM in older adults could reflect two factors, the spontaneous changes in the sharing pattern (as in younger adults) and continuing search for more comfortable ways to perform the task. A natural next step to test this hypothesis would be to perform a larger cross-sectional study of older adults with varying degrees of age-related strength reduction.
Limitations.
One of the main conclusions from our study is that strength training may assist in avoiding age-related decrements in finger interaction and coordination. However, we should interpret these results with some caution. One confounding factor of the study could be a subpar effort from the elderly participants during the fatiguing exercise. This is very unlikely, because we provided similar encouragement to the two groups as we have provided in all of our previous fatigue studies and expected that both groups of participants would respond to the encouragement in a similar way. The similar changes in RMSE (IMRL), VORT, and larger changes in VUCM and the ΔV with fatigue in the Elderly group provide indirect support for our assumption that the older adults produced at least as much effort as the younger subjects. Another limitation was the relatively moderate group size (8 participants each), which could be the cause of a few close-to-significant effects. A third possible confound for our present study could be similarities in cognitive abilities of the two groups. Studies have shown that physical fitness is associated with slower cognitive decline in older adults and motor functioning (26, 52). Most of our elderly and young participants were involved in recreational fitness activities, and it is likely that they also had similar cognitive and motor functions. Our rhythmic tasks were fairly demanding, and they require concentration to maintain both spatial as well as temporal accuracy. Our task is definitely more difficult to perform during fatigue. In such tasks, an interaction between fatigue and cognitive functions (41) cannot be ruled out and needs further examination.
According to the earlier studies, a fatigue-induced increase in variance of force produced by the fatigued finger could be expected (61, 62). However, that was not the case in the present study. We think that the difference could be due to the slightly different experimental design. In all previous studies, the participants trained on all the tasks on day 1 and then returned the following day to perform the actual study. This was done to minimize the effects of learning within a session. In the present study, we could not repeat the previous design and compromised by performing the experiment on a single day, while giving more trials for training to both groups. However, this design might lead to data affected by a combination of fatigue and learning within the session. It seems plausible that the young adults learned the task better than the elderly, resulting in the relatively reduced variance of the force produced by the individual digits during fatigue. This possible interaction of the effects of fatigue and learning is a study limitation. One final possible limitation/confound is related to results of force-tracking experiments in older subjects using visual feedback that showed greater force variability within a trial due, apparently, to specific deficits involving visual control of force (45, 64, 65). We think, however, that this factor was unlikely to play a major role in our main tests because of two factors. First, the synergy analysis was performed across trials. So, possible age-related differences in the time series structure were not expected to have strong effects on our outcome variables. Second, our task was not truly a force-tracking task, because the visual feedback only showed the two force zones between which the force had to oscillate.
GRANTS
The study was supported in part by National Institutes of Health Grants AG-018751, NS-035032, and AR-048563.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.S., V.M.Z., and M.L.L. conception and design of research; T.S. performed experiments; T.S. analyzed data; T.S. and M.L.L. interpreted results of experiments; T.S. prepared figures; T.S. and M.L.L. drafted manuscript; T.S., V.M.Z., and M.L.L. edited and revised manuscript; T.S., V.M.Z., and M.L.L. approved final version of manuscript.
APPENDIX
The data from the cyclic force production task were analyzed within the framework of the UCM hypothesis. To recapitulate, the space of elemental variables (commands to individual fingers, finger modes) is divided into two subspaces: one corresponding to the desired value of the performance variable (FTOT, the UCM, three-dimensional space), and the other that corresponds to changes in the FTOT (VORT, ORT, one-dimensional). Furthermore, variance across trials is compared within the two subspaces (per dimension); if significantly more variance lies within the UCM, then a conclusion is drawn that a multielement synergy stabilizes the FTOT.
Due to the phenomenon of enslaving, individual finger forces covary positively. To analyze the task-specific patterns of covariation, forces have to be converted into finger modes (it is assumed that the controller can hypothetically change finger modes one at a time). This was done using the corresponding enslaving matrix, E. Single-finger ramp trials were used to generate E for each participant. For each single-finger trial, a linear regression of the forces produced by individual fingers against the FTOT produced by all of the four fingers over a 8-s time interval in the middle of the ramp was computed. The regression coefficients were used to construct an enslaving matrix for each participant as follows:
| (A1) |
where bi,j is the slope of the regression equation when finger i force was regressed on the sum of all four finger forces, where finger j is the task finger. Computation of the enslaving matrix E, allowed for a conversion of the force data f into finger modes by using the equation m = E−1f, where m is the mode vector.
In the IMRL task, changes in the value of the FTOT can be written as a function of the changes in the modes dm = [dmi dmm dmr dml]T, where T signifies a matrix transpose.
| (A2) |
We used force modes, m, as elemental variables. The FTOT was the performance variable. The UCM is defined by an orthogonal set of eigenvectors in mode space ei that do not change the FTOT, i.e.,
| (A3) |
These directions can be found by taking the null space of the Jacobian of this transformation ([1 1 1 1] E). The mean-free modes were projected onto these directions and summed to give
| (A4) |
for a four-finger task, n = 4 is the number of DOF of the m vector, and p = 1 the number of DOF of the performance variable (FTOT). The component orthogonal to the null space is given by
| (A5) |
The amount of variance per DOF parallel to (or within) the UCM is then given by:
| (A6) |
This is the variance that does not change the FTOT. Similarly, the amount of variance per DOF VORT is given by
| (A7) |
Variance across trials was computed for each time sample and compared within the two subspaces, VUCM (or VGOOD) and VORT (or VBAD) after normalization per DOF. We interpret VUCM > VORT as a multifinger synergy stabilizing the FTOT. A ΔV was computed reflecting the relative amount of VUCM and VORT in the VTOT. All variance indexes are computed per DOF.
| (A8) |
Given the number of DOF in each space, VTOT = 0.25 × (3VUCM + VORT). Hence the ΔV ranges between 1.33 (all variance is within the UCM) and −4 (all variance is in the orthogonal subspace). For statistical analysis, the ΔV values were transformed using a Fisher's z-transformation adapted to the boundaries of ΔV
| (A9) |
REFERENCES
- 1. Avin KG, Law LAF. Age-related differences in muscle fatigue vary by contraction type: a meta-analysis. Phys Ther 91: 1153–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein NA. The Co-Ordination and Regulation of Movements. Oxford, UK: Pergamon, 1967 [Google Scholar]
- 3. Buch ER, Young S, Contreras-Vidal JL. Visuomotor Adaptation in normal aging. Learn Mem 10: 55–63, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cairns SP, Knicker AJ, Thompson MW, Sjøgaard G. Evaluation of models used to study neuromuscular fatigue. Exerc Sport Sci Rev 33: 9–16, 2005 [PubMed] [Google Scholar]
- 5. Chan KM, Raja AJ, Strohschein FJ, Lechelt K. Age-related changes in muscle fatigue resistance in humans. Can J Neurol Sci 27: 220–228, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Christou EA. Aging and variability of voluntary contractions. Exerc Sport Sci Rev 39: 77–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christou EA, Poston B, Enoka JA, Enoka RM. Different neural adjustments improve endpoint accuracy with practice in young and old adults. J Neurophysiol 97: 3340–3350, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Côté JN, Feldman AG, Mathieu PA, Levin MF. Effects of fatigue on intermuscular coordination during repetitive hammering. Motor Control 12: 79–92, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Côté JN, Mathieu PA, Levin MF, Feldman AG. Movement reorganization to compensate for fatigue during sawing. Exp Brain Res 146: 394–398, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Crowninshield RD, Brand RA. A physiologically based criterion of muscle force prediction in locomotion. J Biomech 14: 793–801, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Danion F, Li ZM, Zatsiorsky VM, Latash ML. The effect of fatigue on multifinger co-ordination in force production tasks in humans. J Physiol 523: 523–532, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ditor DS, Hicks A. The effect of age and gender on the relative fatigability of the human adductor pollicis muscle. Can J Physiol Pharmacol 78: 781–790, 2000 [PubMed] [Google Scholar]
- 13. Duinen Hv, Gandevia SC. Constraints for control of the human hand. J Physiol 589: 5583–5593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Evans WJ, Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Faulkner J, Brooks S. Muscle fatigue in old animals–unique aspects of fatigue in elderly humans. In: Fatigue–Neural and Muscular Mechanisms, edited by Gandevia S, Enoka R, McComas A, Stuart D, Thomas C. New York: Plenum, 1995, p. 471–480 [PubMed] [Google Scholar]
- 18. Fernández-Ruiz J, Hall C, Vergara P, Diaz R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Brain Res Cogn Brain Res 9: 223–226, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Forestier N, Nougier V. The effects of muscular fatigue on the coordination of a multijoint movement in human. Neurosci Lett 252: 187–190, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Friedman J, Skm V, Zatsiorsky VM, Latash ML. The sources of two components of variance: an example of multifinger cyclic force production tasks at different frequencies. Exp Brain Res 196: 263–277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69: 2108–2115, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1790, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Gorniak SL, Zatsiorsky VM, Latash ML. Manipulation of a fragile object by elderly individuals. Exp Brain Res 212: 505–516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henderson G, Tomlinson BE, Gibson PH. Cell counts in human cerebral cortex in normal adults throughout life using an image analysing computer. J Neurol Sci 46: 113–136, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Hicks AL, Cupido CM, Martin J, Dent J. Muscle excitation in elderly adults: the effects of training. Muscle Nerve 15: 87–93, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hillman CH, Weiss EP, Hagberg JM, Hatfield BD. The relationship of age and cardiovascular fitness to cognitive and motor processes. Psychophysiology 39: 303–312, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Hu X, Newell KM. Aging, visual information, and adaptation to task asymmetry in bimanual force coordination. J Appl Physiol 111: 1671–1680, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Huffenus AF, Amarantini D, Forestier N. Effects of distal and proximal arm muscles fatigue on multi-joint movement organization. Exp Brain Res 170: 438–447, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Hunter SK, Critchlow A, Enoka RM. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol 99: 890–897, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Hunter SK, Rochette L, Critchlow A, Enoka RM. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve 31: 730–740, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Kapur S, Zatsiorsky VM, Latash ML. Age-related changes in the control of finger force vectors. J Appl Physiol 109: 1827–1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Keen DA, Fuglevand AJ. Common input to motor neurons innervating the same and different compartments of the human extensor digitorum muscle. J Neurophysiol 91: 57–62, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Kent-Braun JA. Skeletal muscle fatigue in old age: whose advantage? Exerc Sport Sci Rev 37: 3–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93: 1813–1823, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand 104: 129–136, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Latash ML. The bliss (not the problem) of motor abundance (not redundancy). Exp Brain Res 217: 1–5, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Latash ML. Motor synergies and the equilibrium-point hypothesis. Motor Control 14: 294–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Latash ML, Scholz JP, Schöner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev 30: 26–31, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Latash ML, Scholz JP, Schöner G. Toward a new theory of motor control. Motor Control 11: 276–308, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15-to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 41. Lorist MM, Kernell D, Meijman TF, Zijdewind I. Motor fatigue and cognitive task performance in humans. J Physiol 545: 313–319, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McNay EC, Willingham DB. Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem 4: 411–420, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2559, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Narici M, Bordini M, Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol 71: 1277–1281, 1991 [DOI] [PubMed] [Google Scholar]
- 45. Ofori E, Samson JM, Sosnoff JJ. Age-related differences in force variability and visual display. Exp Brain Res 203: 299–306, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Ohtsuki T. Inhibition of individual fingers during grip strength exertion. Ergonomics 24: 21–36, 1981 [DOI] [PubMed] [Google Scholar]
- 47. Olafsdottir H, Zhang W, Zatsiorsky VM, Latash ML. Age-related changes in multifinger synergies in accurate moment of force production tasks. J Appl Physiol 102: 1490–1501, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Olafsdottir HB, Zatsiorsky VM, Latash ML. The effects of strength training on finger strength and hand dexterity in healthy elderly individuals. J Appl Physiol 105: 1166–1178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park J, Singh T, Zatsiorsky VM, Latash ML. Optimality versus variability: effect of fatigue in multi-finger redundant tasks. Exp Brain Res 216: 591–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prilutsky BI, Zatsiorsky VM. Optimization-based models of muscle coordination. Exerc Sport Sci Rev 30: 32–38, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rice CL. Muscle function at the motor unit level: consequences of aging. Top Geriatr Rehabil 15: 70–82, 2000 [Google Scholar]
- 52. Rikli RE, Edwards DJ. Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Res Q Exerc Sport 62: 61–67, 1991 [DOI] [PubMed] [Google Scholar]
- 53. Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol 107: 1874–1883, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Rubinstein S, Kamen G. Decreases in motor unit firing rate during sustained maximal-effort contractions in young and older adults. J Electromyogr Kinesiol 15: 536–543, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol 86: 2125–2143, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999 [DOI] [PubMed] [Google Scholar]
- 57. Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull 70: 337–346, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol 95: 1361–1369, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol 94: 259–270, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Singh T, Latash ML. Effects of muscle fatigue on multi-muscle synergies. Exp Brain Res 214: 335–350, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Singh T, SK MV, Zatsiorsky VM, Latash ML. Adaptive increase in force variance during fatigue in tasks with low redundancy. Neurosci Lett 485: 201–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh T, SK MV, Zatsiorsky VM, Latash ML. Fatigue and motor redundancy: adaptive increase in finger force variance in multi-finger tasks. J Neurophysiol 103: 2990–3000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh T, Zatsiorsky VM, Latash ML. Effects of fatigue on synergies in a hierarchical system. Hum Mov Sci 31: 1379–1398, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sosnoff JJ, Newell KM. Aging, visual intermittency, and variability in isometric force output. J Gerontol B Psychol Sci Soc Sci 61: P117–P124, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Sosnoff JJ, Newell KM. Are visual feedback delays responsible for aging-related increases in force variability? Exp Aging Res 33: 399–415, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol 21: 530–539, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Thomas CK, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve 33: 21–41, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol 94: 903–912, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Walker S, Peltonen H, Avela J, Häkkinen K. Neuromuscular fatigue in young and older men using constant or variable resistance. Eur J Appl Physiol 113: 1069–1079, 2013 [DOI] [PubMed] [Google Scholar]
- 70. Williams CA, Ratel S. Human Muscle Fatigue. London: Routledge, 2009 [Google Scholar]
- 71. Yang JF, Scholz JP, Latash ML. The role of kinematic redundancy in adaptation of reaching. Exp Brain Res 176: 54–69, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yassierli Nussbaum MA, Iridiastadi H, Wojcik LA. The influence of age on isometric endurance and fatigue is muscle dependent: a study of shoulder abduction and torso extension. Ergonomics 50: 26–45, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Zatsiorsky VM, Li ZM, Latash ML. Coordinated force production in multi-finger tasks: finger interaction and neural network modeling. Biol Cybern 79: 139–150, 1998 [DOI] [PubMed] [Google Scholar]