Abstract
We tested the hypothesis that reduced nitric oxide (NO) bioavailability contributes to the attenuated peak and total vasodilation following single-muscle contractions in older adults. Young (n = 10; 24 ± 2 yr) and older (n = 10; 67 ± 2 yr) adults performed single forearm contractions at 10, 20, and 40% of maximum during saline infusion (control) and NO synthase (NOS) inhibition via NG-monomethyl-l-arginine. Brachial artery diameters and velocities were measured using Doppler ultrasound and forearm vascular conductance (FVC; in ml·min−1·100 mmHg−1) was calculated from blood flow (ml/min) and blood pressure (mmHg). Peak and total vasodilator responses [change (Δ) in FVC from baseline] were attenuated in older adults at all intensities (P < 0.05). NOS inhibition reduced the peak ΔFVC at 10% (88 ± 12 vs. 52 ± 9 ml·min−1·100 mmHg−1), 20% (125 ± 13 vs. 83 ± 13 ml·min−1·100 mmHg−1), and 40% (207 ± 26 vs. 133 ± 20 ml·min−1·100 mmHg−1) in young subjects, (P < 0.05 for all) and in older adults at 10% (59 ± 5 vs. 47 ± 7 ml·min−1·100 mmHg−1, P < 0.05) and 20% (88 ± 9 vs. 68 ± 9 ml·min−1·100 mmHg−1, P < 0.05), but not 40% (128 ± 12 vs. 105 ± 11 ml·min−1·100 mmHg−1, P = 0.11). The relative (%) reduction in peak ΔFVC due to NOS inhibition was greater in young vs. older adults at 20% (−36 ± 5 vs. −23 ± 5%, P < 0.05) and 40% (−35 ± 6 vs. −16 ± 7%, P < 0.05). The reduction in the total vasodilator response (area under the curve) with NOS inhibition was also greater in young vs. older adults at all intensities. Our data suggest that contraction-induced rapid vasodilation is mediated in part by NO, and that the contribution of NO is greater in young adults.
Keywords: aging, vasodilation, nitric oxide, muscle contraction
the mechanisms associated with increases in skeletal muscle blood flow during activity (i.e., exercise hyperemia) have been under investigation for more than 130 yr. In general, skeletal muscle blood flow increases in proportion to the metabolic demands of the tissue. Throughout this investigation period, the majority of studies in humans concerned with vascular control during exercise have focused primarily on blood flow responses in contracting muscles during steady-state submaximal exercise. However, the mechanisms for initiating a hyperemic response at the onset of exercise may differ from those that maintain it. At the onset of exercise, there is a rapid increase in blood flow to active skeletal muscle. Indeed, muscle blood flow increases within the first 1–2 s after the completion of a single-muscle contraction, and the magnitude of this response is graded with contraction intensity (6, 8, 9, 16, 58). Examining the blood flow response to a single-muscle contraction allows assessment of the local mechanisms underlying exercise hyperemia without the confounding effects of subsequent contractions (15). The increase in blood flow immediately following a single-muscle contraction is thought to be due to local vasodilation (32), although the exact mechanisms are unclear. Nitric oxide (NO) is a fast-acting vasodilator substance that can be generated from both the endothelium and skeletal muscle cells during contraction. Therefore, NO is an appealing candidate that might explain some of the rapid vasodilator response following a single-muscle contraction. Along these lines, Brock and colleagues (6) demonstrated that the peak and total blood flow response following a muscle contraction are reduced during NO synthase (NOS) inhibition, thus suggesting a role of NO in the hyperemic response.
Evidence from both humans (8, 9, 38) and experimental animals (2, 3, 34) suggests that rapid vasodilation and increases in blood flow after a muscle contraction are attenuated with aging. Interestingly, aging is also associated with a reduction in NO bioavailability and decreased endothelial-dependent (NO-mediated) vasodilation (12, 19, 33, 54, 55). Moreover, alterations in NO bioavailability have been implicated in the age-related reductions in skeletal muscle blood flow during dynamic exercise (11, 17, 52). However, whether alterations in NO-mediated mechanisms contribute to the attenuated rapid vasodilator response after a single-muscle contraction in older adults is unknown.
With this information as background, we tested the hypothesis that the contribution of NO to the rapid vasodilator response would be reduced in older adults. Specifically, we examined whether the reduction in the rapid vasodilator response after a single forearm contraction during NOS inhibition was greater in young compared with older adults. Additionally, we used bolus intra-arterial infusions of acetylcholine (ACh) and sodium nitroprusside (NTP) to mimic single forearm contractions and determined how aging affects the rapid endothelial and vascular smooth muscle vasodilator responses, respectively.
METHODS
Subjects.
A total of 10 young (age range 18–32 yr) and 10 older (age range 61–73 yr) healthy subjects volunteered to participate in the study. Subjects completed written, informed consent, underwent a standard screening, and were healthy, nonobese (body mass index ≤ 30 kg/m2), nonsmokers, not taking any vasoactive medications, and were sedentary to moderately active. One young subject was taking Pantaprazole (proton pump inhibitor) to treat gastroesophageal reflux (withheld for a minimum of 3 days before study), and two older subjects were taking Synthroid to treat hypothyroidism (withheld 3 days before study). Six subjects (one young and five older) reported taking a daily vitamin. Studies were performed after an overnight fast and refraining from exercise and caffeine for at least 24 h. Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (43). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by the Institutional Review Board and were performed according to the Declaration of Helsinki.
Arterial catheterization.
A 20-gauge, 5-cm (model RA-04020, Arrow International, Reading, PA) catheter was placed in the brachial artery of the experimental arm under aseptic conditions after local anesthesia (2% lidocaine) for administration of study drugs. The catheter was connected to a three-port connector in series, as previously described in detail (21). One port was linked to a pressure transducer positioned at heart level (model PX600F, Edwards Lifescience, Irvine, CA) to allow measurement of arterial pressure and was continuously flushed (3 ml/h) with saline with a stop-cock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration.
Heart rate and systemic blood pressure.
Heart rate was recorded via continuous three-lead ECG. A pressure transducer connected to the arterial catheter measured beat-to-beat blood pressure (Cardiocap/5, Datex-Ohmeda, Louisville, CO).
Forearm blood flow.
Brachial artery mean blood velocity and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole at rest (before contraction) and 45 s postcontraction. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute (ml/min).
Single-muscle contractions.
For the experimental trials, brief forearm contractions were performed with a handgrip device at 10, 20, and 40% of the subject's maximal voluntary contraction (MVC), determined at the beginning of each experiment. The weight was lifted 4–5 cm over a pulley for a single, 1-s forearm muscle contraction. Subjects were instructed to contract and relax on verbal command issued from laboratory personnel. Each contraction was visually observed by laboratory personnel to ensure the proper timing of contraction. Two minutes of relaxation were given between each contraction to allow continuous measures of forearm hemodynamics postcontraction. Workload intensity was randomized within each condition, and each contraction intensity was performed in duplicate to calculate an average response for each subject for a given condition. The average weight used for the young subjects was 4.1 ± 0.5, 8.2 ± 1.1, and 16.5 ± 2.2 kg for 10, 20, and 40% MVC, respectively. The average weight used for the older subjects was 3.4 ± 0.3, 6.8 ± 0.7, and 13.6 ± 1.4 kg for 10, 20, and 40% MVC, respectively (P = 0.22–0.27 compared with young subjects).
Pharmacological infusions.
NG-monomethyl-l-arginine (l-NMMA; NOS inhibitor; Bachem, Switzerland) was infused at a loading dose of 5 mg/min for 5 min and then at a maintenance dose of 1 mg/min for the remainder of the study. This dose of l-NMMA has been shown to effectively attenuate the forearm vasodilatory response to exogenous ACh (10). The immediate peak vasodilator responsiveness to the endothelium-dependent and -independent vasodilators ACh (4 μg/dl of limb volume) and NTP (1.0 μg/dl of limb volume) were assessed using 2-ml intra-arterial bolus infusions of each respective drug.
Experimental protocol.
Each subject completed a single study day, which consisted of single forearm contractions during saline (control) and l-NMMA administration. Contraction intensity (10, 20, and 40% MVC) was performed in duplicate and randomized within each condition. Thus each subject performed a total of 12 single forearm contraction trials during the study [6 contractions (2 at each intensity) × 2 conditions]. Each trial consisted of 2 min of rest, followed by a single forearm contraction. Brachial artery velocity and hemodynamics were measured during the rest period and for 45-s postcontraction. Additionally, intra-arterial bolus infusions of ACh and NTP were performed. This pharmacological approach was used as follows: 1) to confirm effective NOS inhibition; and 2) in an attempt to create a rapid vasodilator response that resembled what is observed following a single-muscle contraction. That is, we tried to create a brief stimulus to elicit a vasodilator response that mimicked the transient rapid nature that occurs following a brief forearm contraction. Furthermore, the bolus infusions of ACh and NTP were used to gain insight into the endothelial and vascular smooth muscle contributions to the rapid vasodilator responses. Each bolus infusion was completed in 1–2 s. Bolus infusions were performed in duplicate and consisted of 2 min of rest, followed by a rapid infusion of either ACh or NTP. Brachial artery velocity and hemodynamics were measured during the rest period and for 90-s post-rapid infusion. Bolus infusions were performed during saline (control) and l-NMMA administration. Due to the long half-life of l-NMMA, NOS inhibition trials were always performed last. A rest period of 15 min was allowed between conditions.
Data analysis and statistics.
Data were collected at 250 Hz, stored on a computer, and analyzed off-line with signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform, and heart rate was determined from the electrocardiogram. Baseline FBF and MAP represent an average of the last 30-s of the resting time period before each muscle contraction and were used to quantify the hyperemic response. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100 and expressed as ml·min−1·100 mmHg−1. To account for baseline changes (Δ) in FBF and FVC with l-NMMA, the flow and vasodilator responses following muscle contraction or bolus drug infusion (ACh and NTP) were adjusted (i.e., postcontraction or infusion value − baseline value) and expressed as ΔFBF and ΔFVC. Of particular interest to this study, the peak and total FBF and FVC responses were analyzed between conditions and age groups. Total FBF and FVC were defined as the area under the curve after respective baseline values were subtracted for a given flow or conductance curve (28, 42). To further investigate the contribution of NO in the age-related changes in contraction-induced rapid vasodilator responses (ΔFVC), we compared the relative (%) reductions during l-NMMA administration between age groups.
All values are expressed as means ± SE. ANOVA was used to analyze baseline differences between age groups. Baseline MAP, FBF, and FVC were compared via repeated-measures ANOVA to detect differences between age groups and across conditions. To determine the effect of age (group) and NOS inhibition (condition) on the hyperemic and vasodilator response to single forearm contractions, differences in the peak ΔFBF and ΔFVC and total FBF and FVC at each contraction intensity (10, 20, and 40% MVC) were determined via repeated-measures ANOVA. Additional two-way repeated-measures ANOVA were performed to examine rapid endothelial and vascular smooth muscle vasodilator responses to ACh and NTP within each condition (saline vs. l-NMMA) and between age groups. Appropriate post hoc analysis determined where statistical differences occurred. When significance was detected, Tukey's post hoc analysis was used to identify differences between groups. Statistical difference was set a priori at P < 0.05.
RESULTS
Subject characteristics are summarized in Table 1. Young and older subjects were of similar height, weight, and body mass index (P > 0.05 for all). Additionally, young and older subjects exhibited similar forearm volume and MVC (P > 0.05 for both). Older subjects demonstrated a greater MAP, total cholesterol, and high- and low-density lipoprotein than their younger counterparts (P < 0.05).
Table 1.
Subject characteristics
| Variable | Young | Older |
|---|---|---|
| Age, yr | 24 ± 2 | 67 ± 2* |
| Men/women | 5/5 | 5/5 |
| Height, cm | 174 ± 3 | 170 ± 3 |
| Weight, kg | 72 ± 5 | 72 ± 6 |
| BMI, kg/m2 | 23.5 ± 1.1 | 24.7 ± 1.1 |
| FAV, ml | 879 ± 71 | 841 ± 96 |
| MVC, kg | 41 ± 5 | 34 ± 4 |
| MAP, mmHg | 97 ± 2 | 106 ± 2* |
| Total cholesterol, mmol/l | 3.8 ± 0.2 | 4.9 ± 0.2* |
| LDL, mmol/l | 2.1 ± 0.2 | 2.9 ± 0.2* |
| HDL, mmol/l | 1.2 ± 0.1 | 1.7 ± 0.2* |
| Triglycerides, mmol/l | 1.0 ± 0.2 | 1.1 ± 0.1 |
Values are means ± SE. BMI, body mass index; FAV, forearm volume; MVC, maximal voluntary contraction; MAP, mean arterial pressure; LDL, low-density lipoprotein, HDL, high-density lipoprotein.
P < 0.05 vs. young.
Hyperemic and vasodilator responses to single forearm contractions in young and older adults.
Under control conditions, baseline FBF and FVC did not differ with age (Table 2). MAP was consistently higher across all contraction trials in older subjects (P < 0.05). Peak hyperemic (ΔFBF) and vasodilator (ΔFVC) responses were observed at the fourth to fifth cardiac cycle postcontraction in both young and older adults for each relative workload (Fig. 1). Older adults demonstrated reduced peak hyperemic and vasodilator responses at all workloads (P < 0.05; Fig. 2). Moreover, total (area under the curve for 30 cardiac cycles postcontraction) hyperemic and vasodilator responses were attenuated in older adults at all workloads (P < 0.05; Fig. 3).
Table 2.
Baseline (resting) hemodynamics under each condition
| Young |
Older |
|||||||
|---|---|---|---|---|---|---|---|---|
| FBF, ml/min | FVC, ml/min | MAP, mmHg | Heart rate, beats/min | FBF, ml/min | FVC, ml/min | MAP, mmHg | Heart rate, beats/min | |
| Control | ||||||||
| 10% MVC | 66 ± 10 | 67 ± 11 | 98 ± 2 | 61 ± 2 | 72 ± 8 | 68 ± 8 | 105 ± 2* | 63 ± 2 |
| 20% MVC | 66 ± 10 | 68 ± 11 | 97 ± 2 | 61 ± 2 | 72 ± 6 | 67 ± 6 | 104 ± 3* | 63 ± 2 |
| 40% MVC | 66 ± 8 | 66 ± 8 | 98 ± 2 | 61 ± 2 | 67 ± 6 | 63 ± 6 | 104 ± 2* | 63 ± 2 |
| l-NMMA | ||||||||
| 10% MVC | 47 ± 8† | 45 ± 8† | 103 ± 3† | 59 ± 2 | 44 ± 4† | 40 ± 4† | 113 ± 2*† | 61 ± 2 |
| 20% MVC | 41 ± 7† | 41 ± 7† | 103 ± 3† | 60 ± 2 | 42 ± 6† | 37 ± 5† | 113 ± 1*† | 61 ± 2 |
| 40% MVC | 45 ± 8† | 44 ± 8† | 103 ± 3† | 60 ± 2 | 43 ± 5† | 38 ± 4† | 113 ± 2*† | 62 ± 2 |
Values are means ± SE. l-NMMA, NG-monomethyl-l-arginine; FBF, forearm blood flow; FVC, forearm vascular conductance.
P < 0.05 vs. young.
P < 0.05 vs. control at respective MVC.
Fig. 1.
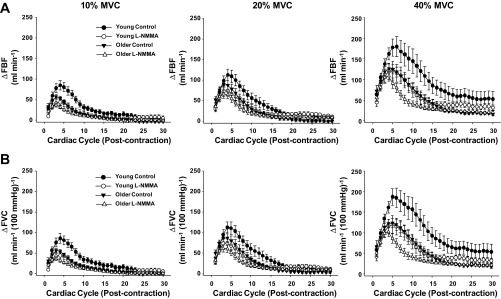
Hyperemic [change (Δ) in forearm blood flow (FBF); A] and vasodilator [Δforearm vascular conductance (FVC); B] responses over 30 cardiac cycles following single forearm contractions at 10, 20, and 40% maximal voluntary contraction (MVC). l-NMMA, NG-monomethyl-l-arginine.
Fig. 2.
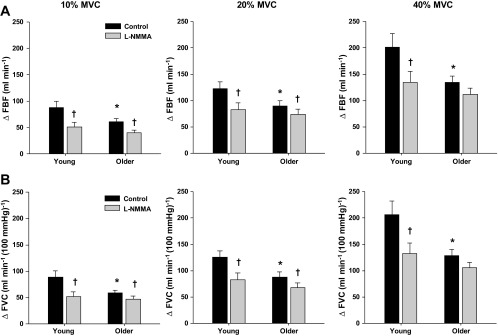
Peak hyperemic (ΔFBF; A) and peak vasodilator (ΔFVC; B) responses to single-muscle contractions at 10, 20, and 40% MVC during saline (control) and l-NMMA administration in young and older adults. Under control conditions, peak hyperemic and vasodilator responses were attenuated in older adults at all contraction intensities. NO synthase inhibition (l-NMMA) blunted the peak hyperemic and vasodilator responses at all contraction intensities in young adults and at 10 and 20% MVC in older adults. *P < 0.05 vs. young. †P < 0.01 vs. control.
Fig. 3.
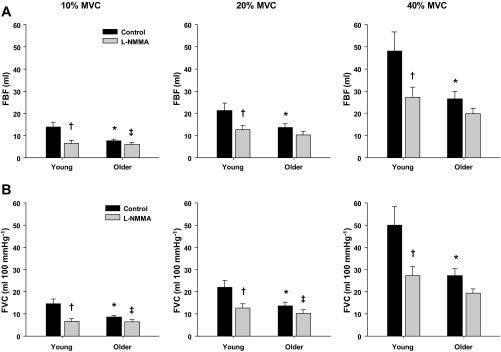
Total hyperemic (A) and vasodilator (B) responses (area under the curve) to single-muscle contractions at 10, 20, and 40% MVC during saline (control) and l-NMMA administration in young and older adults. Under control conditions, total hyperemic and vasodilator responses were attenuated in older adults at all contraction intensities. NO synthase inhibition (l-NMMA) blunted the total hyperemic and vasodilator responses at all contraction intensities in young adults. NO synthase inhibition only blunted the total hyperemic response at 10% MVC and the total vasodilator response at 10% and 20% MVC in older adults. *P < 0.05 vs. young. †P < 0.01 vs. control. ‡P < 0.05 vs. control.
Although there were no significant age-related reductions in MVC or differences in the weight used for each relative intensity between groups, we examined whether the attenuated hyperemic and vasodilator responses (ΔFBF and ΔFVC) to single-muscle contractions observed in older adults persisted after normalizing for workload (i.e., flow and/or conductance per 100 g of weight lifted). Utilizing this approach revealed similar results as those reported above. Under control conditions, older adults still demonstrated reduced peak hyperemic (1.86 ± 0.21 vs. 2.26 ± 0.19, 1.37 ± 0.15 vs. 1.57 ± 0.12, and 1.01 ± 0.09 vs. 1.29 ± 0.16 ml·min−1·100 g of weight lifted−1) and vasodilator (1.79 ± 0.21 vs. 2.26 ± 0.19, 1.29 ± 0.14 vs. 1.60 ± 0.12, and 0.98 ± 0.10 vs. 1.32 ± 0.15 ml·min−1·100 mmHg−1·100 g of weight lifted−1) responses at 10, 20, and 40% MVC, respectively, compared with their young counterparts (P < 0.05). Additionally, the total hyperemic (0.24 ± 0.03 vs. 0.37 ± 0.02, 0.19 ± 0.03 vs. 0.28 ± 0.04, and 0.20 ± 0.02 vs. 0.31 ± 0.06 ml/100 g of weight lifted) and vasodilator (0.27 ± 0.03 vs. 0.39 ± 0.06, 0.20 ± 0.02 vs. 0.28 ± 0.04, and 0.20 ± 0.02 vs. 0.32 ± 0.06 ml·100 mmHg−1·100 g of weight lifted−1) responses remained lower in older compared with young adults at 10, 20, and 40% MVC, respectively, after correcting for workload (P < 0.05).
Effect of NOS inhibition (via l-NMMA) on hyperemic and vasodilator responses to single forearm contractions.
l-NMMA reduced baseline FBF and FVC and increased MAP in both young and older adults (P < 0.05; Table 2). The time to reach a peak vasodilator response was achieved at the fourth cardiac cycle postcontraction in both young and older adults for each relative workload. At 40% MVC, NOS inhibition resulted in a faster time to reach a peak vasodilator response in all subjects (P < 0.05; main effect of l-NMMA). In young adults, NOS inhibition reduced the peak and total hyperemic and vasodilator responses at all contraction intensities (P < 0.01; Figs. 2 and 3, respectively). Peak hyperemic and vasodilator responses were reduced during l-NMMA at 10 and 20% MVC in the older adults (P < 0.01; Fig. 2). Furthermore, the total hyperemic response (Fig. 3A) at 10% MVC and the total vasodilator response (Fig. 3B) at 10 and 20% MVC were reduced in the older adults during the l-NMMA trials (P < 0.05). The age-associated differences observed under control conditions for peak and total responses were no longer present during NOS inhibition (Figs. 1–3). The relative reduction in peak vasodilator responses during the NOS inhibition trials (via l-NMMA) were substantially greater in young compared with older adults at 20 and 40% MVC (P < 0.05; Fig. 4A). Additionally, NOS inhibition resulted in a greater relative reduction of the total vasodilator responses in young compared with older adults at all contraction intensities (P < 0.05; Fig. 4B).
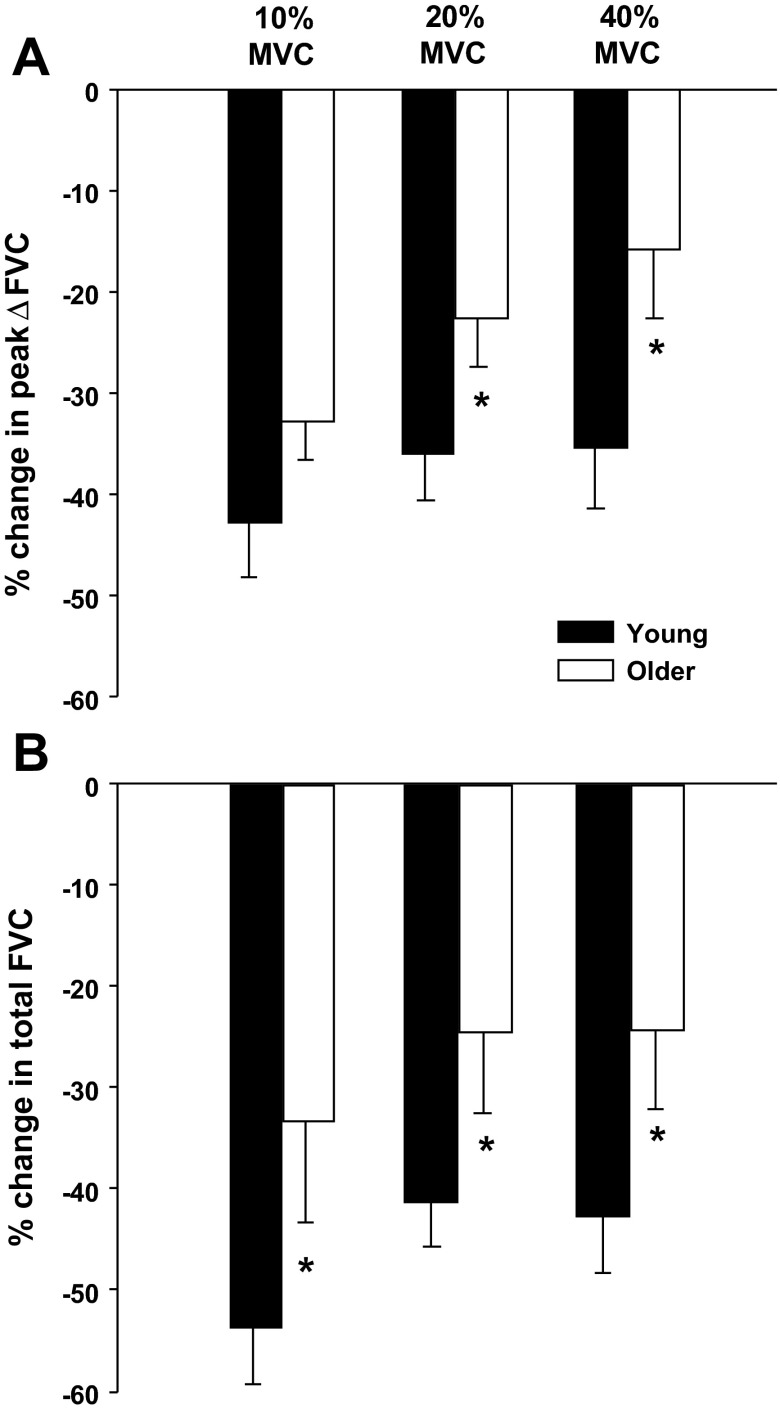
Fig. 4.
Relative reduction (%) in peak (A) and total (B) vasodilator responses to single forearm contractions at 10, 20, and 40% MVC during l-NMMA administration compared with saline (control) condition in young and older adults. NO synthase inhibition (l-NMMA) reduced peak vasodilator responses to a greater extent in young compared with older adults at 20 and 40% MVC. NO synthase inhibition reduced total vasodilator responses to a greater extent in young compared with older adults at all contraction intensities. *P < 0.05 vs. young.
Vasodilator responses to rapid infusion of ACh in young and older adults.
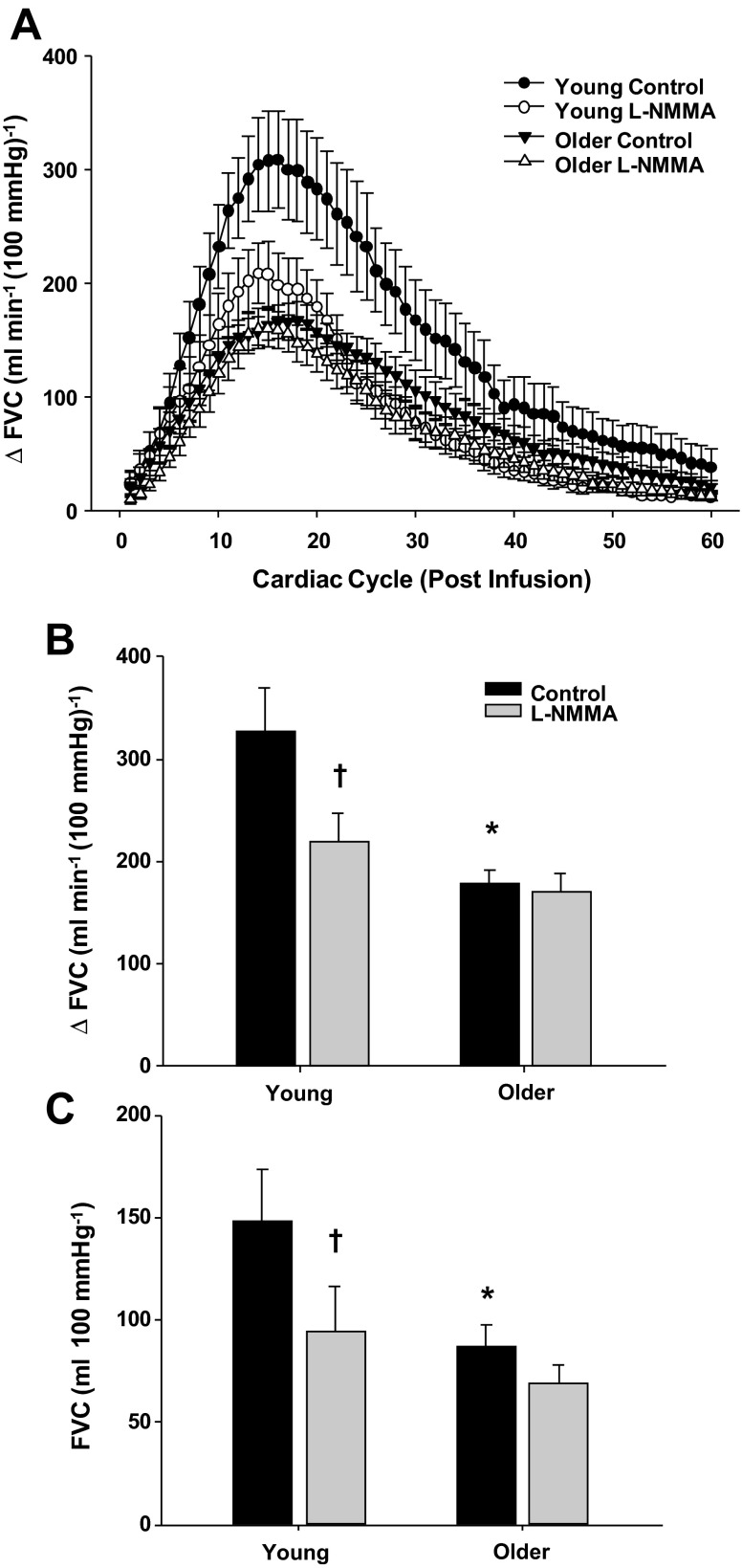
Figure 5A demonstrates the rapid vasodilator response over 60 cardiac cycles to bolus infusions of ACh in young and older adults under control (saline) and NOS inhibition (l-NMMA) conditions. Under control conditions, peak vasodilator responses to ACh were observed at the 16th cardiac cycle following the infusion of ACh in both young and older adults. Older adults demonstrated a blunted peak and total vasodilator response to ACh compared with their young counterparts (P < 0.05; Fig. 5, B and C). NOS inhibition did not alter the timing of peak vasodilation in either age group. However, the peak and total vasodilator responses to ACh during l-NMMA administration were reduced in young (P < 0.05) but not in older adults (P > 0.05) (Fig. 5, B and C).
Fig. 5.
Vasodilator responses to bolus infusions of acetylcholine (ACh) during saline (control) and l-NMMA administration in young and older adults. A: rapid vasodilator responses (ΔFVC) over 60 cardiac cycles after bolus infusion of ACh. Peak (B) and total (C) vasodilator responses to ACh were attenuated in older adults under control conditions. NO synthase inhibition (l-NMMA) blunted the peak and total vasodilator responses to ACh in young adults (B and C). *P < 0.05 vs. young. †P < 0.05 vs. control.
Vasodilator responses to rapid infusion of NTP in young and older adults.
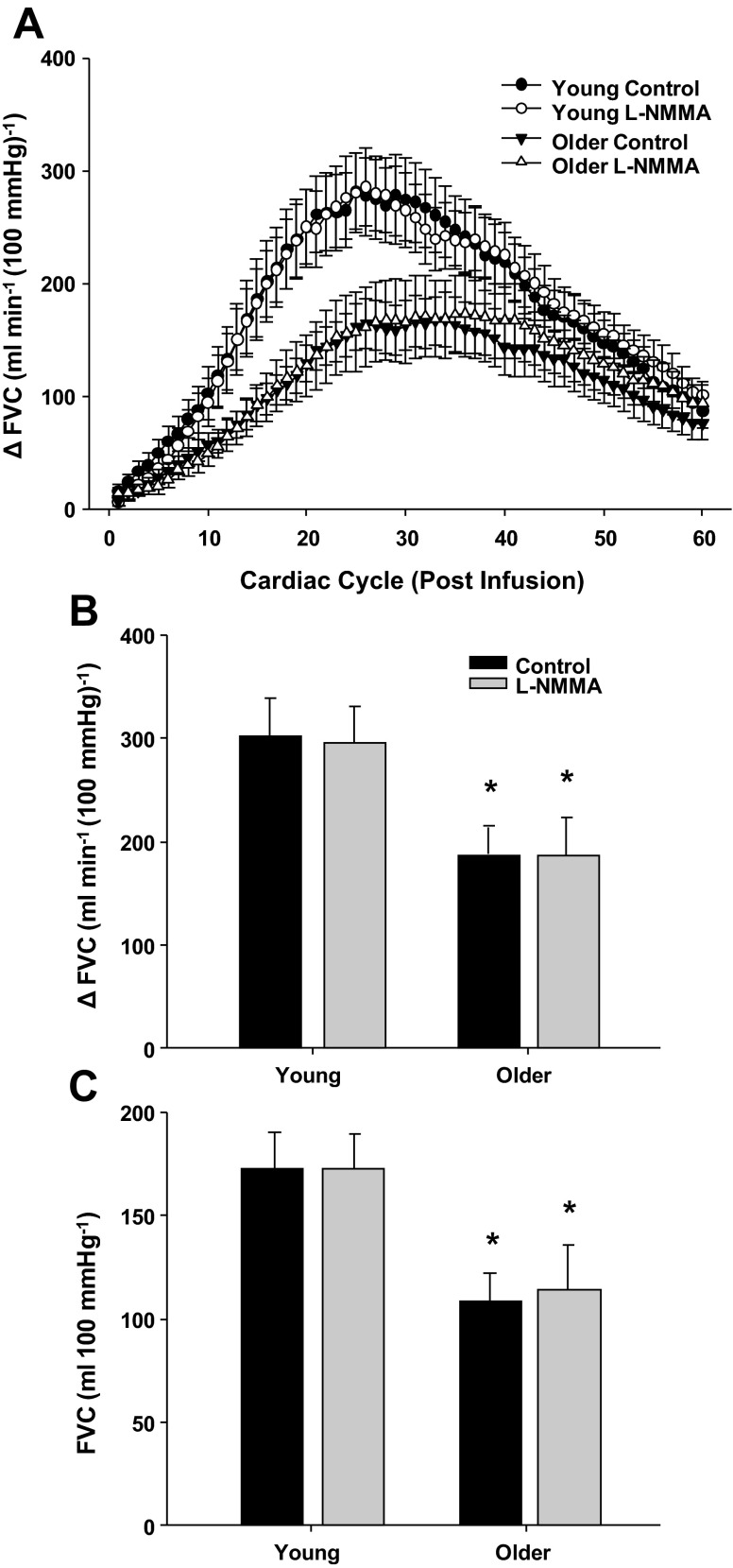
Figure 6A demonstrates the rapid vasodilator response over 60 cardiac cycles to bolus infusions of NTP in young and older adults under control (saline) and NOS inhibition (l-NMMA) conditions. Under control conditions, the time to reach a peak vasodilator response was slower in older compared with young adults (30 ± 2 vs. 25 ± 2 cardiac cycles, P < 0.05). Older adults also demonstrated a blunted peak and total vasodilator response to NTP (P < 0.05; Fig. 6, B and C). NOS inhibition did not alter the timing of peak vasodilation in either age group. Moreover, NOS inhibition did not change the peak and total vasodilator responses to NTP in either age group (Fig. 6, B and C).
Fig. 6.
Vasodilator responses to bolus infusions of sodium nitroprusside (NTP) during saline (control) and l-NMMA administration in young and older adults. A: rapid vasodilator responses (ΔFVC) over 60 cardiac cycles after bolus infusion of NTP. Peak (B) and total (C) vasodilator responses to NTP were attenuated in older adults under control and l-NMMA conditions. Peak and total vasodilator responses to NTP were unaffected by l-NMMA in both age groups. *P < 0.05 vs. young.
DISCUSSION
This is the first study to examine the role of NO in the age-related reduction in skeletal muscle contraction-induced rapid vasodilation in humans. Our present findings suggest that 1) NO contributes to the peak and total hyperemic and vasodilator responses after a single-muscle contraction at all intensities in young adults (Figs. 1–3); 2) the contribution of NO to the hyperemic and vasodilator responses is less in older adults and appears to diminish with increasing contraction intensity (Fig. 4); and 3) both rapid endothelial and vascular smooth muscle responsiveness are impaired with aging (Figs. 5 and 6). Our finding that NOS inhibition blunts the hyperemic response after a single forearm contraction in young adults is in agreement with previous work by Brock et al. (6). However, when comparing the relative changes in flow during NOS inhibition between the two studies at moderate contraction intensities (∼20% MVC), we observed approximately a twofold greater reduction in the peak (−36 vs. −17%) and total (−41 vs. −26%) hyperemic response, thus suggesting the contribution of NO to contraction-induced rapid hyperemia and vasodilation might be greater in young adults than previously thought.
NO and age-related reductions in contraction-induced rapid vasodilation.
The primary novel finding of the present study is that, although NO appears to contribute to the rapid hyperemic and vasodilator responses after a single-muscle contraction at mild to moderate intensities (10–20% MVC) in older adults, the contribution is substantially less than that observed in young adults (Fig. 4). The diminished role of NO in the contraction-induced vasodilation is likely due to less bioavailable NO in older adults. However, the age-related impairment in the vasodilator response to a muscle contraction might also be due in part to a diminished responsiveness to NO, as evidenced by the attenuated and delayed vasodilation to bolus infusions of NTP in older adults (Fig. 6). Theoretically, age-related reductions in NO bioavailability can be a result of changes in endothelial NOS (eNOS) expression and/or activity of the enzyme. To date, evidence to support this idea is unclear as eNOS expression and/or activation from arterial tissue in experimental animals has shown to be decreased, increased, or unchanged with aging (13, 26, 49, 59). Moreover, recent evidence from vascular endothelial cells obtained from the brachial artery of humans suggests that eNOS protein expression tends to be greater, and the activation of eNOS is significantly increased, in older compared with young adults (24). The increased activation of eNOS with age has been postulated to be a compensatory mechanism for low NO bioavailability in older adults (24).
Age-related reductions in NO bioavailability may also be a result of increased oxidative stress and an enhanced scavenging of NO. Along these lines, measures of endothelial function are inversely related to circulating markers of oxidative stress (29, 31). Additionally, acute administration of antioxidants (e.g., ascorbic acid) improves endothelial-dependent vasodilation (30, 54, 60) and more recently has been shown to increase muscle blood flow during continuous dynamic forearm exercise in older adults (38). However, acute administration of intra-arterial ascorbic acid failed to improve the rapid hyperemic and vasodilator response to single-muscle contractions in older adults (38). Therefore, it is unclear if the reductions in NO-mediated vasodilation following single-muscle contractions observed in the older adults of the present study are due to oxidative stress related mechanisms.
Alternative role of NO in the rapid vasodilation.
In a recent study, we demonstrated that α-adrenergic blockade via phentolamine augments hyperemic and vasodilator responses after a single-muscle contraction in older adults (9). This finding could be interpreted that an enhanced α-adrenergic tone contributes to the age-related reductions in contraction-induced rapid vasodilation. However, the improvement in rapid vasodilation observed in older adults during phentolamine administration could also suggest the age-related differences are a result of impairments in functional sympatholysis in the vascular beds of contracting skeletal muscle, as observed during dynamic exercise (22). Evidence in young adults suggests that functional sympatholysis occurs after a single-muscle contraction (18). Although the mechanisms for functional sympatholysis have not been fully elucidated, NO has been shown to inhibit sympathetic vasoconstriction in contracting skeletal muscle of experimental animals and humans (14, 51, 56, 57). Taken together with our present data, a decreased NO bioavailability with aging might be linked to a decreased ability to blunt sympathetic vasoconstriction in older adults and thus contribute to the attenuated rapid vasodilator responses to muscle contractions in older adults.
Single vs. dynamic muscle contractions and the influence of aging.
To date, the majority of studies comparing muscle blood flow and vascular control in exercising young and older humans have demonstrated that the control of blood flow to dynamically contracting skeletal muscle is altered with normal aging during submaximal forearm and leg exercise (36, 38, 39, 46–48), which has been attributed in part to less NO-mediated vasodilation in older adults (52). However, it should be noted that other studies have failed to identify any age-associated differences in the hyperemic and vasodilator response during or immediately following dynamic forearm exercise (25, 35, 40). If blood flow under steady-state conditions is maintained with aging, it could be argued that the impairment in NO-mediated vasodilation following a single-muscle contraction is compensated for by other vasodilator mechanisms as exercise continues. Thus the physiological importance of an impaired rapid vasodilator response might appear insignificant. However, we would argue that daily activities with which older adults often have difficulty are commonly characterized by short bursts of activity that require rapid adjustments in blood flow, and, therefore, any impairment in rapid vasodilation can be considered physiologically relevant.
Pharmacological induced rapid vasodilation: effect of aging.
In the present study, we used rapid bolus infusions (2 ml intra-arterial push within 1–2 s) of ACh and NTP as a novel approach to help pharmacodissect and further examine whether the blunted contraction-induced responses with aging were due to altered endothelial (ACh) or vascular smooth muscle (NTP) responsiveness. The magnitude of endothelial-dependent vasodilation via continuous ACh administration has consistently been shown to be blunted with aging (1, 11, 19, 38, 54, 55). Furthermore, evidence in isolated skeletal muscle resistance arterioles from rats indicates that, in addition to a reduced magnitude of vasodilation in response to ACh, aging also impairs the dynamics and timing of vasodilation, in part, through the endothelium (4). In the present study, rapid infusion of ACh produced a relatively rapid rise in flow that promptly decays over time following infusion. Of particular interest to the current study, the rapid and total vasodilator responses to ACh were 1) significantly less in older adults and 2) were only sensitive to NOS inhibition in young adults (Fig. 5). These observations related to age-related differences and the impact of NOS inhibition are very similar to what was observed during single-muscle contractions and supports the idea that age-related endothelial dysfunction and/or decreased NO bioavailability contribute in part to the reduced contraction-induced rapid vasodilation.
The older subjects in the present study also demonstrated a blunted and delayed vasodilator response to bolus infusions of the endothelium-independent agonist NTP (Fig. 6). The substantially lower vasodilator responses to NTP observed in the present study were somewhat surprising to us, as a large majority of the studies in humans indicate that the steady-state vascular smooth muscle responsiveness to continuous infusions of NTP is preserved with aging (19, 20, 23, 27, 37, 55). However, it should be noted that others have reported some degree of impairment in NTP-induced vasodilation with aging (1, 38, 45). Irrespective of these discrepancies in the literature, this is the first study to quantify the magnitude and timing of vasodilation in response to a rapid bolus infusion of NTP in humans. As seen in Fig. 6, the responses to NTP were slower and more prolonged than those observed during the ACh infusion (Fig. 5). Additionally, the vasodilator responses to NTP in young and older adults were unaffected during NOS inhibition. When considering the timing of the vasodilator response as well as the lack of change with NOS inhibition, the attenuated vascular smooth muscle responsiveness to NTP observed in the older subjects might not be related to the age-associated reductions in the rapid vasodilator responses to single-muscle contractions. However, since some degree of NTP-mediated vasodilation occurs within the timeframe in which contraction-induced peak responses appear (i.e., 4–5 cardiac cycles), it may be possible that alterations in smooth muscle responsiveness contribute to some extent to the age-related reductions in vasodilation following a single-muscle contraction. Lastly, we cannot rule out the possibility that some of the age-related differences in the responses to pharmacological stimulation or muscle contraction may be attributed to structural adaptations in the vascular smooth muscle of older adults. Along these lines, remodeling of resistance arteries that is associated with aging and certain pathologies (e.g., hypertension) can bring about alterations in vascular function and ultimately affect the regulation of blood flow in response to muscle contraction or other stimuli (41).
Experimental considerations.
Basal leg blood flow has been shown to be positively related to leg muscle mass and inversely related to age (44). Additionally, aging is often associated with decreases in skeletal muscle mass and strength (53), both of which might contribute to lower exercise blood flows in older adults. Lean muscle mass was not measured in this study, and we cannot be certain that potential differences in muscle mass contributed to the attenuated vasodilator responses in the older adults. However, lean muscle mass in the forearm has been shown to be similar between young and older adults (8). Moreover, the forearm volume, MVC, and relative contraction intensities were not different between the age groups in the present study. Taken together, the age-related attenuation in contraction-induced rapid vasodilation is not likely explained by differences in forearm muscle mass.
In the present study, we quantified the hyperemic and vasodilator responses to single-muscle contractions as an absolute change from baseline (resting) values. This approach is similar to our previous study, which examined the influence of α-adrenergic vasoconstriction in the contraction-induced rapid vasodilation (9). However, prior studies from other groups examining the rapid vasodilator response to single-muscle contractions have expressed the magnitude of the response as a relative (%) change from baseline (5, 8, 38). This analytic approach has demonstrated that aging is associated with a blunted rapid vasodilation (8, 38). Our approach of expressing the flow and vasodilator response to a single-muscle contraction as an absolute change above baseline (i.e., postcontraction value − baseline value) supports the findings that age-related decrements in rapid vasodilation exist.
It has been argued that expressing data as a percent change in flow or conductance is the most appropriate approach when differences in resting vascular tone exist (7). When the present data are expressed as a relative (%) change in FVC from baseline, there is no difference between control (saline) and NOS inhibition (l-NMMA) trials in the young subjects. However, using the percent change in FVC in older adults would suggest that the rapid vasodilation is significantly enhanced during l-NMMA infusions compared with saline trials at 20 and 40% MVC (189 ± 12 vs. 131 ± 11% and 279 ± 11 vs. 212 ± 19%, respectively; P < 0.01). The paradoxical increase in percent change vasodilation during l-NMMA in the older adults might simply be explained by the lower denominator used in the equation of percent differences. Using the absolute changes to quantify the hyperemic and vasodilator responses (ΔFBF and ΔFVC) in the present study accounts for the baseline (resting) values and minimizes the potential confound that a smaller denominator might present. Furthermore, changes in absolute flow closely reflect the metabolic activity of the contracting muscle (50). Expressing the absolute rapid vasodilator responses normalized to the metabolic activity (i.e., workload performed) in the present study supports the idea of age-related impairments in the contraction-induced rapid vasodilation, as well as the role of NO in the response. Of particular interest to the present study, when we examined the changes in the peak and total ΔFBF and ΔFVC during the l-NMMA trials (compared with saline conditions), both the absolute and relative (Fig. 4) reductions were substantially greater in the young compared with older adults, which suggests a decreased role of NO in the rapid vasodilator response following a single forearm contraction in older adults. Therefore, we chose to report the absolute change in muscle blood flow and conductance from baseline and believe these values most represent the rapid vasodilator responses to single-muscle contractions.
As stated above, we attempted to pharmacologically mimic the rapid vasodilator response to single-muscle contractions and assess the endothelium-dependent and -independent components of the response. However, the time to peak vasodilation occurred substantially later during both bolus ACh (∼16 cardiac cycles) and NTP (∼25 and 30 cardiac cycles for young and older adults, respectively) infusions compared with single-muscle contractions (∼4–5 cardiac cycles). The differences in the temporal pattern of each response are likely due to the drugs being infused upstream from the resistance arterioles of the forearm (site of action), whereas the vasodilator response originates immediately within the forearm during a muscle contraction. Additionally, the delayed vasodilator responses to ACh and NTP may also be due to issues related to drug distribution within the forearm. Nonetheless, the age-related impairments in the dynamics of vasodilation during bolus ACh infusion were similar to single-muscle contractions, and NOS inhibition attenuated rapid vasodilation to a similar degree between conditions for each respective age group.
Conclusions.
To our knowledge, this is the first study to demonstrate that the age-related impairments in contraction-induced rapid vasodilation are, in part, due to alterations in endothelial function and blunted NO signaling in healthy older adults. When considered with our previous data (9), a decreased NO bioavailability with aging may contribute to the blunted rapid vasodilation through one of two ways: 1) less direct vasodilation via cGMP-induced smooth muscle cell relaxation; and/or 2) decreased ability to blunt sympathetic vasoconstriction (e.g., functional sympatholysis). Impairments in either of these mechanisms can limit blood flow distribution and oxygen delivery within the active muscle and ultimately lead to functional limitations. Regardless of the exact role of NO in the rapid vasodilator response to muscle contraction, interventions aimed at increasing NO bioavailability might prove to be useful in reversing the age-related decline in muscle blood flow during exercise, particularly at the onset of muscle contractions.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Research Grants HL-105467 (to D. P. Casey) and HL-46493 (to M. J. Joyner) and by Clinical and Translational Science Award UL1 TR000135. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.C. and M.J.J. conception and design of research; D.P.C., B.G.W., S.M.R., J.L.T., and M.J.J. performed experiments; D.P.C., B.G.W., and J.L.T. analyzed data; D.P.C., B.G.W., S.M.R., J.L.T., and M.J.J. interpreted results of experiments; D.P.C. prepared figures; D.P.C. drafted manuscript; D.P.C., B.G.W., S.M.R., J.L.T., and M.J.J. edited and revised manuscript; D.P.C., B.G.W., S.M.R., J.L.T., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to the study volunteers for participation. We also thank Essa Mohamed, Pam Engrav, Shelly Roberts, and Sarah Wolhart for technical assistance.
REFERENCES
- 1. Al-Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol 109: 201–206, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bearden SE. Advancing age produces sex differences in vasomotor kinetics during and after skeletal muscle contraction. Am J Physiol Regul Integr Comp Physiol 293: R1274–R1279, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Behnke BJ, Delp MD. Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol 108: 14–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blain GM, Limberg JK, Mortensen GF, Schrage WG. Rapid onset vasodilatation is blunted in obese humans. Acta Physiol (Oxf) 205: 103–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev 29: 159–163, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey DP, Walker BG, Curry TB, Joyner MJ. Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 589: 1477–1488, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res 83: 279–286, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clifford PS, Tschakovsky ME. Rapid vascular responses to muscle contraction. Exerc Sport Sci Rev 36: 25–29, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964 [DOI] [PubMed] [Google Scholar]
- 17. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol 93: 555–560, 2002 [DOI] [PubMed] [Google Scholar]
- 19. DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol 542: 255–262, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol 105: 1359–1363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297: H425–H432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587: 3271–3285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elvebak RL, Eisenach JH, Joyner MJ, Nicholson WT. The function of vascular smooth muscle phosphodiesterase III is preserved in healthy human aging. Clin Transl Sci 3: 239–242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol 571: 661–668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franzoni F, Ghiadoni L, Galetta F, Plantinga Y, Lubrano V, Huang Y, Salvetti G, Regoli F, Taddei S, Santoro G, Salvetti A. Physical activity, plasma antioxidant capacity, and endothelium-dependent vasodilation in young and older men. Am J Hypertens 18: 510–516, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol 557: 1013–1020, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol 474: 353–360, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. J Physiol 588: 4017–4027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Limberg JK, Evans TD, Pegelow DF, Eldridge MW, Sebranek JJ, Proctor LT, Schrage WG. Heterogeneous vascular responses to hypoxic forearm exercise in young and older adults. Eur J Appl Physiol 112: 3087–3095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology 24: 45–57, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 300: 230–235, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I. Lack of age-related decreases in basal whole leg blood flow in resistance-trained men. J Appl Physiol 99: 1384–1390, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell 9: 304–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 97: 13818–13823, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Short KR, Nair KS. Muscle protein metabolism and the sarcopenia of aging. Int J Sport Nutr Exerc Metab 11, Suppl: S119–S127, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A 95: 15090–15095, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004 [DOI] [PubMed] [Google Scholar]
- 59. van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]