Abstract
Obesity is a primary risk factor for the development of obstructive sleep apnea in humans, but the impact of obesity on central sleep apnea is less clear. Given the comorbidities associated with obesity in humans, we developed techniques for long-term recording of diaphragmatic EMG activity and polysomnography in obese mice to assess breathing patterns during sleep and to determine the effect of obesity on apnea generation. We hypothesized that genetically obese ob/ob mice would exhibit less variability in breathing across the 24-h circadian cycle, be more prone to central apneas, and be more likely to exhibit patterns of increased diaphragm muscle activity consistent with obstructive apneas compared with lean mice. Unexpectedly, we found that obese mice exhibited a greater circadian impact on respiratory rate and diaphragmatic burst amplitude than lean mice, particularly during rapid eye movement (REM) sleep. Central apneas were more common in REM sleep (42 ± 17 h−1) than non-REM (NREM) sleep (14 ± 5 h−1) in obese mice (P < 0.05), but rates were not different between lean and obese mice in either sleep state. Even after experimentally enhancing central apnea generation by acute withdrawal of hypoxic chemoreceptor activation during sleep, central apnea rates remained comparable between lean and obese mice. Last, we were unable to detect patterns of diaphragmatic burst activity suggestive of obstructive apnea events in obese mice. In summary, obesity does not predispose mice to increased occurrence of central or obstructive apneas during sleep, but does lead to a more pronounced circadian variability in respiration.
Keywords: central sleep apnea, circadian variability, control of breathing, obesity, obstructive sleep apnea
sleep has the potential to impact breathing across a spectrum from natural circadian variation to discrete pathological pauses characterized as apneas. In humans, obesity is a primary risk factor for development of apneas (39), specifically obstructive apneas (9), with resultant and established downstream sequelae that include cardiovascular, metabolic, and cognitive impairment and associated increased mortality (11, 17, 19, 24). Rodents have proved excellent models of apnea sequelae and provided important information on the pathways linking apnea to organ dysfunction and pathological outcomes (3, 8, 16, 26, 28). In contrast, despite a wide availability of genetically manipulated or experimentally induced models of obesity (2, 14, 36), few studies have used obese rodents to study the pathogenesis of sleep-related breathing abnormalities.
Studies of apnea pathogenesis in rodents are predominantly focused on central sleep apnea (6, 15, 18, 27). Central sleep apnea, unlike obstructive sleep apnea where increasing respiratory efforts occur in the presence of an obstructed upper airway, results from periodic and complete cessation of the major respiratory pump muscles (i.e., predominantly diaphragm in mice). Mice and rats exhibit central sleep apnea in either of two forms, classified as spontaneous or post-sigh (18). The vast majority of rodent apnea studies have utilized whole body plethysmography (e.g., 10, 12, 18, 38), which is a technique constrained by numerous assumptions and is technically difficult to combine with electrical polysomnographic signals to assess sleep and breathing over significant periods of time in a natural and undisturbed environment. Although a small number of studies in rodents have assessed sleep and breathing more directly with chronic implantation of diaphragmatic EMG electrodes (25, 27, 29, 30, 33), no study to date has simultaneously measured sleep and diaphragm EMG activity in obese mice for extended periods spanning a full circadian cycle.
The overall goal of our study was to determine the impact of obesity on respiratory control and development of apneas in mice during sleep using customized chronically implanted diaphragmatic EMG electrodes combined with standard polysomnographic electrodes. Breathing patterns were studied in both lean and obese mice across an undisturbed 24-h period as well as in response to acute withdrawal of hypoxic chemoreceptor activation during sleep. We hypothesized that compared with lean mice, genetically obese ob/ob mice, with previously described tachypnea, would exhibit less variability in breathing across the 24-h circadian cycle. Furthermore, obese mice would be more prone to central apneas on room air and with acute chemoreceptor withdrawal, as well as more likely to exhibit patterns of diaphragmatic burst activity consistent with obstructive apneas.
METHODS
Animals and Ethical Approval
Seven obese C57BL/6J-Lepob male mice (ob/ob; 50.2 ± 1.7 g) and 7 C57BL/6J male mice (26.3 ± 1.0 g) aged 10–12 wk from Jackson Laboratory (Bar Harbor, ME) were used in the study. The study was approved by the University of Pittsburgh Animal Use and Care Committee and complied with the American Physiological Society guidelines. For all surgical procedures, anesthesia was induced and maintained by using 1–2% isoflurane administered through a face mask. At the completion of experiments, animals were euthanized by cervical dislocation in accordance with our established protocols.
Procedures
Surgical preparation.
Mice were instrumented with chronically implanted polysomnographic electrodes and diaphragm electromyographic (EMG) electrodes for simultaneous determination of sleep/wake state and breathing. Implantation of diaphragm EMG electrodes was based on modifications of techniques described previously (33). Under sterile conditions, a 1.5-cm incision was made in the right subcostal region, and the abdominal cavity was exposed. The skin and soft tissue were retracted upward, and the liver was held down with a surgical gauze pad. Two diaphragm EMG loop electrodes (customized at 6.5 cm in length, 0.125 mm diameter, Teflon insulated except for the terminal loops; E363/8-Loop; Plastics One, Roanoke, VA) were sutured with 8–0 prolene to the right hemi-diaphragm approximately 0.5 cm apart, without penetrating the thoracic cavity. The electrodes were tunneled subcutaneously to the base of the skull and the abdominal incision was closed. Next, electroencephalographic (EEG; E363/1, Plastics One) and nuchal EMG electrodes (E363/76, Plastics One) were implanted as described previously (22, 34). In brief, a midline incision was made to expose the skull and nuchal muscles. After clearing the fascial layer, three holes were drilled through the skull (left frontal region and bilateral parietal regions) for placement of three EEG electrodes, which were fastened via small screws. One EMG electrode was sutured flat onto the left nuchal region for detection of nuchal muscle activity. All six electrodes were fixed with dental acrylic into a pedestal (MS363, Plastics One) at the base of the skull.
Housing and signal detection.
After recovery from surgery, a connector cable was placed onto the head pedestal and attached to a low-friction mercury swivel allowing unrestricted movement of the tethered mouse. The mouse was housed with free access to water, food, and bedding in an individual cage (STANK/WF; Instech Solomon, Plymouth Meeting, PA) that was contained within a larger chamber to maintain sound insulation and control of the 12:12-h light/dark cycle (BRS/LVE, Laurel, MD). The polysomnographic leads and diaphragm EMG leads exited out of the top of the chamber and connected to amplifiers.
A pen recorder (Grass Instruments, Quincy, MA) was used to amplify and filter EEG (0.3–35 Hz), nuchal EMG (10–75 Hz), and diaphragmatic EMG (300–30,000 Hz) activity. Data from the amplifier were sampled at 1,500 Hz and converted to digital format (DI-200 data acquisition board, Dataq Instruments, Akron, OH) and stored with Windaq/200 acquisition software (Dataq Instruments).
Protocols
Circadian protocol.
After a 3- to 5-day undisturbed acclimation period, a 24-h continuous recording of the polysomnogram and diaphragm EMG activity was obtained for each mouse (Fig. 1A: sample tracing). Data from the study were sampled according to the criteria detailed below to assess variation in breathing and apnea generation during sleep across the circadian cycle.
Fig. 1.
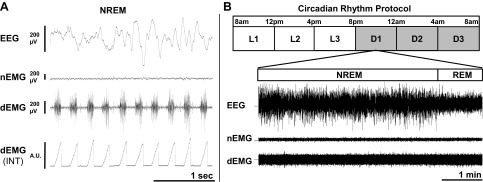
Sample recording and schematic of the study timeline for the circadian protocol of sleep and breathing. A: a sample recording of electroencephalographic (EEG), nuchal electromyographic (nEMG), and diaphragmatic EMG (dEMG) signals in a lean mouse during non-REM (NREM) sleep. The diaphragmatic EMG signal was rectified and integrated (INT) to provide burst amplitude for each breath (arbitrary units: A.U.). B: for the circadian rhythm protocol, sleep and breathing data were recorded continuously for 24 h and sampled for a 5-min period of NREM sleep followed by a 1-min period of REM sleep during each of six 4-h time bins (L = light period; D = dark period).
Acute withdrawal of hypoxic chemoreceptor activation protocol.
The day after the circadian protocol was completed the animals underwent an acute chemoreceptor stimulation and withdrawal protocol to experimentally enhance breathing variability and apnea induction during sleep. The protocol was conducted between 11:00 am and 3:00 pm and timed to coincide with the middle of the light/sleeping period, using an adaptation of the protocol described by Han et al. (12) in awake mice. The mouse was placed in a customized cage designed for rapid changes in gas delivery [see Polotsky et al. (22) for details] at 9:00 am allowing time for the animal to adapt to the new environment before beginning the protocol.
An automated gas control delivery system regulated the timing and flow of room air, N2, O2, or CO2 into the customized cage as previously described (22). Flow rates for all gases into the customized cage were set at 8 l/min to minimize any mechanical disturbances the animal may experience with the switch between different gases. At this flow rate of 8 l/min, animals remained asleep approximately 25% of the time during the transitions between different gases.
Animals were exposed to two different 10-min protocols, each repeated 12 successive times in an order determined by block design between different animals. In the first protocol, animals were exposed to 12 cycles of 6 min of room air, 3 min of 10% O2 + 5% CO2, and 1 min of 100% O2. In the second protocol, animals were exposed to 12 cycles of 6 min of room air, 3 min of 10% O2, and 1 min of 100% O2. The two protocols were delivered consecutively with a 6-min room air exposure period between the last cycle of the first protocol and the first cycle of the second protocol. The ambient fraction of inspired O2 (FiO2) was monitored at the level of the mouse using an oxygen analyzer (Vacu-med Model 17620, Ventura, CA) and the transition from an FiO2 of 0.10 to 1.00 took a maximum of 12 s and reached a hyperoxic level of 0.50 within 3 s.
Analyses
Circadian protocol.
The data from the 24-h polysomnogram were divided into six successive 4-h time bins (Fig. 1B). Within each time bin, the last sleep cycle in which the animal had at least 5 min of non-REM (NREM) sleep followed immediately by at least 1 min of REM sleep was used for the analyses. NREM sleep was determined based on visual identification of high-amplitude, low-frequency delta waves (0.5–4.0 Hz) combined with low nuchal EMG activity. REM sleep was identified visually by the presence of low-amplitude, higher frequency theta waves (6–9 Hz) associated with reduced delta wave activity and low nuchal EMG activity (4). The six periods of NREM and REM sleep were used for analyses of breathing variability, apnea rates, and diaphragm minute activity (see below for details) during sleep across the circadian period.
Acute withdrawal of hypoxic chemoreceptor activation protocol.
The 12 successive cycles of room air, hypoxia/hypercapnia, and hyperoxia were visually scored for sleep using the above criteria. Any cycles that exhibited contiguous sleep without evidence of arousals (based on 5-s scoring epochs) during the transition to, and throughout the period of hyperoxia, were included in analyses (2.9 ± 0.2 cycles per mouse for each of the two stimuli). The first 15 breaths after the animal was exposed to hyperoxia were analyzed for breathing variability, apnea rates, and diaphragm burst amplitude (see below for details) and compared with the final 15 breaths of room air breathing immediately before exposure to hypoxia/hypercapnia.
Breathing variability.
The diaphragm EMG signal was rectified and each breath was identified by a peak detector (CODAS software, Dataq Instruments). The respiratory rate was determined by the inverse of the peak-to-peak interval between breaths. Breathing variability was assessed by the coefficient of variation of respiratory rate (SD/mean respiratory rate).
Central apneas.
Adapting the criteria of Nakamura et al. (18), central apneas were defined as a cessation of diaphragm EMG signal for greater than two basal respiratory cycles during either NREM or REM sleep. For the circadian protocol the basal respiratory cycle was defined as the average cycle length across the 5-min period of NREM sleep. For the acute withdrawal of hypoxic chemoreceptor activation protocol the basal respiratory cycle was defined as the final 15 breaths of room air breathing immediately before exposure to hypoxia/hypercapnia.
Central apneas were classified as post-sigh if the preceding breath had a diaphragm burst at least 25% above the average burst amplitude during the prior four breaths. All remaining central apneas not preceded by a sigh were classified as spontaneous.
Augmented and sequential inspiratory activity consistent with obstructive apneas.
A recent paper by Hernandez et al. (13) showed evidence of airflow limitation associated with an increase in respiratory movement in obese mice. We utilized their representative tracing of respiratory movement (see Fig. 7A of Ref. 13), which showed a series of sequential breaths with increasing respiratory effort to 50% above the baseline period, and applied a similar criteria to our diaphragm EMG recordings to identify breathing patterns consistent with obstructive apneas. We defined a potential obstructive event as at least four consecutive breaths with increased diaphragm EMG burst amplitude above the average amplitude of the four “baseline” preceding breaths, with at least one of the four breaths achieving a 50% or greater increase relative to baseline.
Fig. 7.
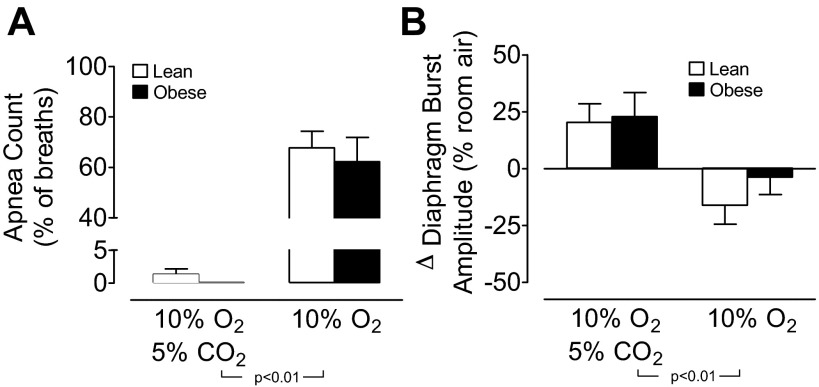
The mean ± SE for apnea count (as a percentage of first 15 breaths during hyperoxia) (A) and change in diaphragm burst amplitude (as percent of average during room air) (B) during the first 15 breaths of 100% O2 based on previous stimulation with either combined 10% O2 and 5% CO2 or 10% O2 alone in lean and obese mice (n = 5 per group). Statistical differences were determined by two-way ANOVA.
Periodic breathing.
Adapting the criteria of Han et al. (12) in awake mice, periodic breathing was defined as at least three cyclic groupings of breaths separated by periods of spontaneous apnea as detailed above.
Diaphragm burst amplitude and minute activity.
The beginning of a diaphragm EMG burst period was defined visually as the time from the origin of five consecutive multiunit spikes that were greater than background activity during the preceding expiratory pause. The end of the diaphragm EMG burst period was defined visually as the time at the end point of five consecutive multiunit spikes that were greater than background during the subsequent expiratory pause. The raw signal was rectified and integrated between the beginning and end of each diaphragm EMG burst period and reported in arbitrary units. For all statistical analyses the diaphragm burst amplitude was normalized to 1) the overall average for all breaths analyzed in the circadian protocol and 2) the average of the final 15 breaths of room air breathing immediately before exposure to hypoxia/hypercapnia in the acute withdrawal of hypoxic chemoreceptor activation protocol. The term minute activity was used as an approximation for minute ventilation (respiratory rate multiplied by diaphragm burst amplitude) as previously described (37).
Statistical Analyses
All results are presented as means ± SE. Statistical differences between lean and obese mice across the circadian cycle, or in response to hyperoxia, were determined by two-way analysis of variance (ANOVA) with repeated measures. Statistical significance between individual means was determined by Newman-Keuls post hoc analyses.
RESULTS
Obese Mice Exhibit Increased Rate and Depth of Breathing During Sleep in the Dark Period
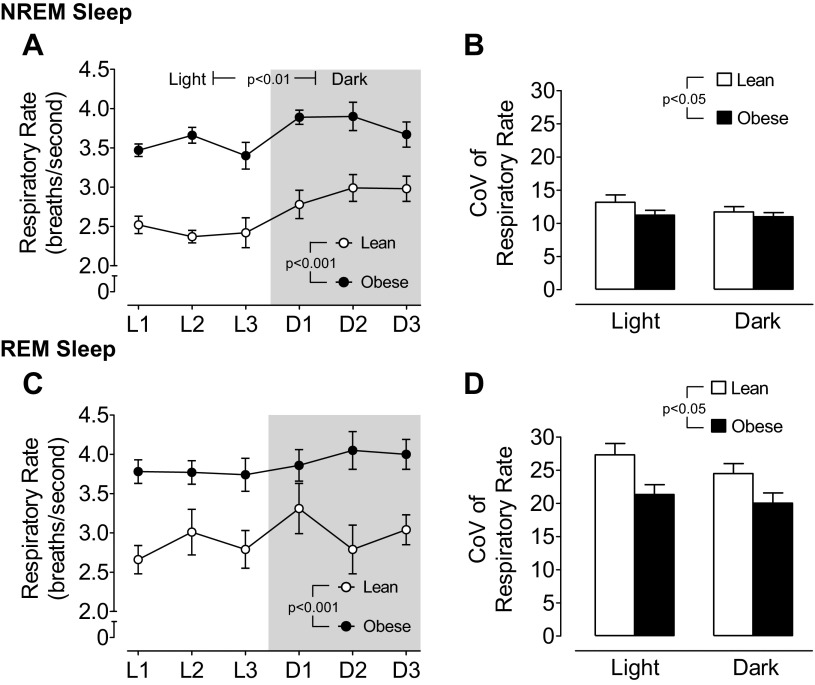
A sample trace of sleep and breathing in a lean mouse is shown in Fig. 1, A and B. During the course of the 24-h circadian cycle, obese mice exhibited a significant elevation in respiratory rate that was approximately 25% higher than in lean mice in both NREM and REM sleep (Fig. 2, A and C). A circadian variation in respiratory rate was evident in both lean and obese mice during NREM sleep (P < 0.01), with higher rates exhibited during the dark period compared with the light period (Fig. 2A). In contrast, during REM sleep there was no significant difference in respiratory rate between the light and the dark period for either lean or obese mice (P = 0.17 for obese mice). Against a background of higher basal respiratory rate, obese mice demonstrated significantly less variability in respiratory rate (P < 0.05) compared with lean mice (Fig. 2, B and D).
Fig. 2.
Circadian variation in mean ± SE for respiratory rate and coefficient of variation (CoV) of respiratory rate during NREM sleep in lean and obese mice (A and B; n = 7 per group) and during REM sleep in lean and obese mice (C and D; n = 7 per group). Statistical differences were determined by two-way ANOVA and the CoV for respiratory rate was greater during REM sleep compared with NREM sleep in both lean and obese mice (P < 0.001).
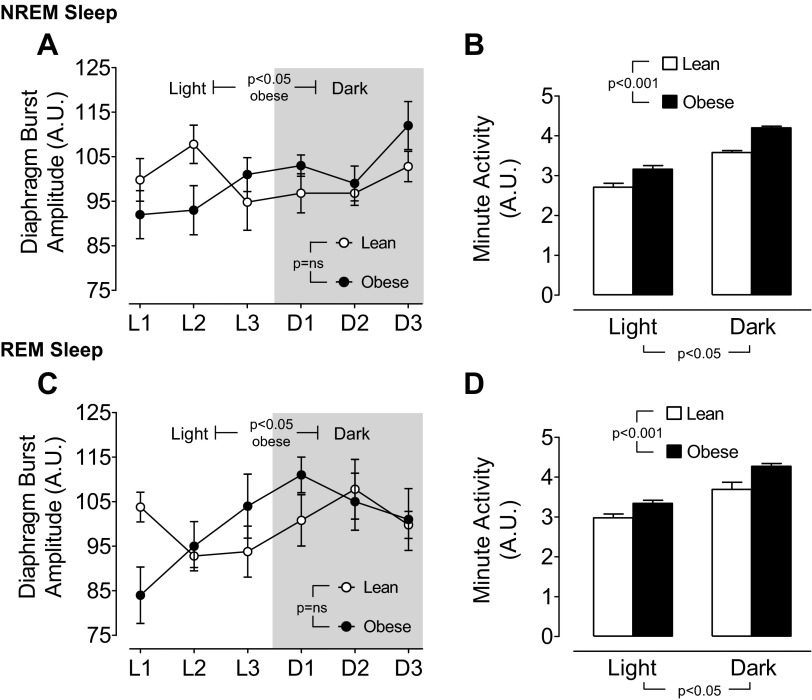
Obese mice exhibited an increased diaphragm burst amplitude during both NREM and REM sleep in the dark period relative to the light period, whereas there was no circadian impact on diaphragm burst amplitude in lean mice (Fig. 3, A and C). Minute activity (respiratory rate × diaphragm burst amplitude) was greater in obese compared with lean mice, as well as in the dark period compared with the light period during both NREM and REM sleep (Fig. 3, B and D).
Fig. 3.
Circadian variation in mean ± SE diaphragm burst amplitude and minute activity during NREM sleep in lean and obese mice (A and B; n = 7 per group) and during REM sleep in lean and obese mice (C and D; n = 7 per group). Diaphragm burst amplitude was normalized for each animal as percent of the average across the full circadian cycle. Minute activity = diaphragm burst amplitude × the respiratory rate. Statistical differences were determined by two-way ANOVA.
Apnea Rates Are Comparable Between Lean and Obese Mice
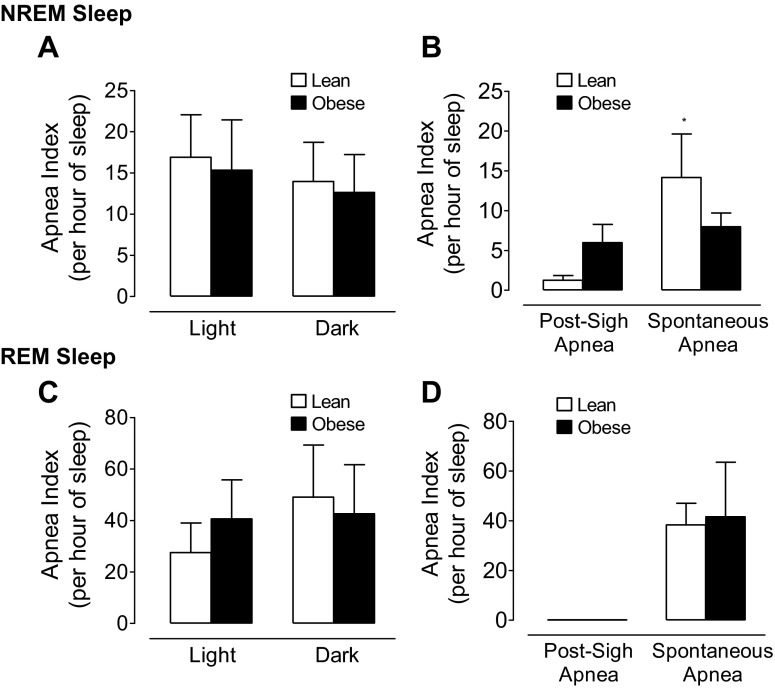
Despite differing degrees of breathing variability in lean and obese mice, there was no difference in central apnea rates between strains during either NREM or REM sleep (Fig. 4, A and C). Central apnea rates during REM sleep were significantly higher and exhibited greater between-animal variability compared with NREM sleep. In both lean and obese mice there was no evidence of a circadian impact on apnea rates during NREM or REM sleep. There was a differential expression of the type of central apnea exhibited, with spontaneous apneas being more common than post-sigh apneas (P < 0.005; Fig. 4, B and D). In REM sleep, all apneas were classified as spontaneous for lean and obese mice (Fig. 4D).
Fig. 4.
Mean ± SE for central apnea index during light and dark periods in lean and obese mice during NREM sleep (A; n = 7 per group) and during REM sleep (C; n = 7 per group). The mean ± SE for post-sigh and spontaneous apnea index in lean and obese mice during NREM sleep (B; n = 7 per group) and during REM sleep (D; n = 7 per group). Statistical differences were determined by two-way ANOVA, and the central apnea index was significantly greater in REM sleep compared with NREM sleep in lean and obese mice (P < 0.005). By one-way ANOVA, lean mice showed a strong trend (*P = 0.056) for a higher spontaneous apnea rate compared with the post-sigh apnea during NREM sleep.
Based on a predefined criteria of diaphragmatic EMG burst pattern, there was little evidence of obstructive apneic events occurring in lean or obese mice. In the data sampled from the selected NREM/REM cycles in the 24-h circadian rhythm protocol, there was only one sequence of diaphragmatic EMG burst pattern that could be considered consistent with an obstructive event, and that occurred in a lean mouse. A comprehensive analysis of the full 24-h period in two mice from each strain revealed only one potential obstructive event in a lean mouse during NREM sleep and no evidence of obstructive events in the obese mice. Due to the high variability in respiratory rate and burst amplitude during REM sleep, the analysis of diaphragmatic EMG burst pattern was restricted to NREM sleep only.
Acute Withdrawal of Chemoreceptor Input Increases Apnea Rate in Lean and Obese Mice
Representative tracings of diaphragm EMG burst activity during hypoxic chemoreceptor withdrawal in NREM sleep are shown in Fig. 5 for lean and obese mice. Exposure to 10% O2 or combined 10% O2 + 5% CO2 resulted in an increase in respiratory rate and diaphragmatic EMG burst amplitude (Fig. 5, challenge column) in both strains. Rapid withdrawal of the hypoxic stimulus with exposure to 100% O2 induced significant breathing instability, and the effect was greater in animals with prior exposure to 10% O2 compared with prior exposure to 10% O2 + 5% CO2. Despite the marked irregularity of breathing with the switch to 100% O2, there was no evidence of periodic breathing in lean or obese mice during NREM based on the pattern of diaphragm EMG burst activity.
Fig. 5.
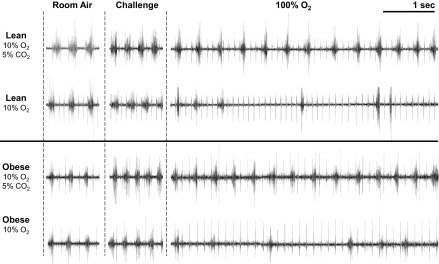
Representative diaphragmatic EMG tracings from one lean (upper tracings) and one obese mouse (lower tracings) during NREM sleep. Steady-state breathing periods are shown during room air and in response to 10% O2 alone or combined 10% O2 and 5% CO2. The first 5 s of breathing with the rapid transition to 100% O2 show an increase in breathing variability that is more marked with previous exposure to 10% O2 than to combined 10% O2 and 5% CO2. Note, the smaller amplitude, higher frequency pattern is due to EKG.
To highlight the effect of rapid withdrawal of the hypoxic stimulus on breathing instability, the sequential breathing interval and diaphragm burst amplitude are plotted for an individual lean and obese mouse (Fig. 6, A–H). For both mice, over half the initial breaths that occurred with the switch from the 10% O2 stimulus to 100% O2 were classified as apneas, whereas prior exposure to the 10% O2 + 5% CO2 stimulus did not result in any apneas with the switch to 100% O2. Average data for apnea rates resulting from the switch to 100% O2 are shown in Fig. 7A. Apnea rates were high (more than 60% of breaths) with the switch from 10% O2 to 100% O2, and were comparable between lean and obese mice. However, with the switch from 10% O2 + 5% CO2 to 100% O2, apneas were effectively absent in both strains of mice.
Fig. 6.
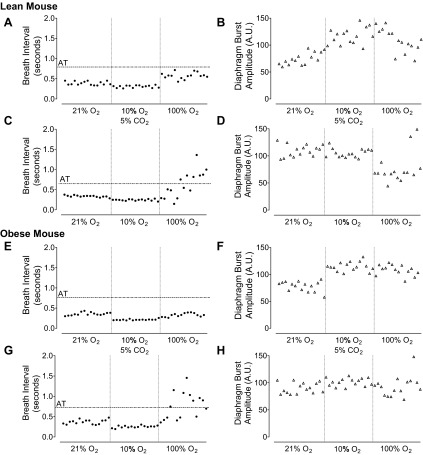
Breath interval and diaphragm burst amplitude plotted in one lean (A–D) and one obese mouse (E–H) for 15 sequential breaths during steady-state breathing periods of room air breathing and in response to 10% O2 alone or combined 10% O2 and 5% CO2. The first 15 breaths after the rapid transition to 100% O2 are shown in the right hand third of each figure and demonstrate greatest variability for breath interval with prior exposure to 10% O2. For breath interval the apnea threshold (AT) is depicted as horizontal line marked at two times the average breath interval during 21% O2.
In contrast to the marked breathing instability that occurred with the switch from 10% O2 to 100% O2, diaphragm burst amplitude was relatively unaffected by hyperoxia in lean and obese mice (Fig. 6, D and H; Fig. 7B, two right hand bars). In animals exposed to 10% O2 + 5% CO2, the resulting increase in diaphragm burst amplitude was maintained above basal room air levels throughout the period of exposure to 100% O2 (Fig. 6, B and F; Fig. 7B, two left hand bars). Taken together, these data show that the maintenance of breathing stability during sleep is most susceptible to the acute withdrawal of hypoxic drive and that the response is independent of the presence or absence of obesity.
DISCUSSION
The ability to simultaneously assess diaphragmatic EMG activity and polysomnography across the circadian cycle in mice allows us to make several unique observations on the impact of obesity on respiratory control and breathing stability in mice during sleep. Obese mice exhibited a distinct circadian increase in respiratory rate in NREM sleep as well as diaphragmatic burst amplitude during NREM and REM sleep during the dark period. In contrast, lean mice only showed a circadian increase in respiratory rate during NREM sleep, but overall their breathing variability was greater than obese mice. Despite the greater breathing variability in lean mice, the occurrence of central apneas was comparable between the strains, with spontaneous apneas more common than post-sigh apneas, particularly during REM sleep. Acute withdrawal of hypoxic chemoreceptor activation during sleep induced a very high rate of central apneas, although no evidence of periodic breathing, which again was not different between lean and obese mice. We were unable to detect any convincing evidence of patterns of diaphragmatic burst activity suggestive of obstructive apnea events in either lean or obese mice. Taken together, our data demonstrate that being obese, and leptin deficient, does not predispose mice to development of central or obstructive apneic events, but does lead to greater circadian influence on breathing frequency and depth despite already elevated basal respiratory rates. One implication of our study is that leptin-deficient obesity is not sufficient to induce central apnea during sleep, but rather the presence of other precipitating factors such as cerebrovascular disease, cardiovascular disease, or hypoventilatory conditions are likely necessary.
A Circadian Variability in Breathing is Prominent in Obese Mice
A major goal of our study was to assess the impact of obesity on breathing stability over the 24-h circadian period while maintaining the animals in a natural, undisturbed environment. Stephenson et al. (32) have previously measured circadian variation in breathing in lean rats by combining whole body plethysmography and polysomnography. Similar to our results in lean mice, they observed an increase in minute ventilation during the dark period that was due to changes in respiratory rate but not tidal volume. We now extend these findings to show that despite the presence of a high basal respiratory rate, obese mice exhibit a robust circadian rhythm in breathing frequency and diaphragm EMG burst amplitude during NREM sleep. Moreover, the circadian rhythmicity in depth of breathing remained present even during autonomically unstable periods of REM sleep. These data suggest that it may be overly simplistic to ascribe higher respiratory rates during NREM sleep in lean or obese mice to increased activity and body temperature during periods of wakefulness in the dark or active period. Genetically obese ob/ob mice are considerably less active than their lean littermates and have a comparable rate of heat production (although less if corrected for body weight) (7) indicating that activity and metabolic factors are unlikely to account for the pronounced circadian variability in breathing during sleep associated with obesity. We have previously demonstrated that leptin is a respiratory stimulant (20) and putatively the absence of leptin in the ob/ob mice may make them more responsive to underlying circadian factors that can impact breathing, potentially including variation in clock genes. We would predict, based on our previous demonstration of leptin as a potent respiratory stimulus (20), that administration of leptin would reduce the circadian variability in ob/ob mice and the response would be comparable to C57BL/6J mice with diet-induced obesity, which exhibit high circulating leptin.
The Impact of Obesity on Generation of Central Apneas
The central apnea rates in our study, based on diaphragmatic EMG activity, differ from that described previously by Nakamura et al. (18) in 129/Sv mice. Despite using identical classification techniques, we report much higher rates of central apnea during REM sleep and a higher proportion of spontaneous, compared with post-sigh, apneas during NREM sleep. Two main differences between our study and that of Nakamura et al. (18) were that their study was conducted over a shorter 6-h period and breathing was assessed with whole body plethysmography. It is possible that post-sigh apneas are overestimated by whole body plethysmography due to insensitivity in differentiating movement-related artifacts from respiratory-induced fluctuations in the pressure signal. Alternatively, strain differences between C57BL/6J and 129/Sv mice may have contributed to a disparity in apnea rates between the studies. Assuming spontaneous apneas dominate in both NREM and REM sleep, it could be concluded that central apnea in mice has a common genesis across sleep states. Nevertheless, there is concurrence between the studies that lean mice do exhibit a significant and clinically relevant number of central apneas during NREM and REM sleep.
Obesity, in contrast to our a priori hypothesis, did not predispose to an increased occurrence of central apneas during NREM or REM sleep compared with lean wild-type mice. Despite the greater propensity for obese mice to maintain a circadian variation in respiratory rate and diaphragm EMG burst amplitude, there was no difference in apnea rates or the proportion of spontaneous compared with postsigh apnea generation between lean and obese mice in either sleep state. The absence of a circadian impact on apnea generation in lean and obese mice is somewhat surprising given that apnea rates in lean rats are reported to be influenced by the time of day (31) and human apnea patterns change across the night (5), although the human studies are largely based on obstructive apnea events. Nevertheless, we can conclude from our study in mice that obesity does not induce higher rates of central apnea, suggesting if the finding applies in humans then other precipitating factors such as cardiovascular disease, altitude exposure, impaired respiratory control, or obstructive events dominate central apnea generation in the clinical setting.
Do Mice Exhibit Periodic Breathing?
In mice with chemically induced hyperpnea, a rapid change to hyperoxia produces high rates of central apnea. As expected, hyperoxia produced less apneas in animals switching from stimulation with 10% O2 + 5% CO2 compared to 10% O2 alone, consistent with a maintenance of hypercapnic drive during the 30-s period of acute withdrawal of hypoxic activation of the peripheral chemoreceptors. The inability of ob/ob mice to generate more central apneas than lean mice with the rapid switch to hyperoxia likely reflects 1) the greater importance of the peripheral chemoreceptors vs. central chemoreceptors in apnea generation; and 2) leptin-deficient obese mice exhibit normal hypoxic ventilatory responsiveness (35). Moreover, the pattern of apnea we observed did not show evidence of any form of periodic breathing in either lean or obese mice during NREM and REM sleep. Our data contrast a previous study reporting that acute exposure to hyperoxia can induce periodic breathing in awake C57BL/6J mice (12). Although there may be technical differences in the rate at which hyperoxia was induced, it is likely that the presence of wakefulness vs. sleep was the major factor between studies. Awake animals, perceiving the rapid change in the inspired gas, may exhibit a startle response resulting in an abrupt sensation of breathing followed by a subsequent burst of rapid breaths leading to a pattern simulating periodic breathing reported in humans. If true, this suggests that, as for the discussion above indicating obesity per se does not lead to higher rates of central apnea, precipitating factors or underlying pathology may be necessary to generate periodic breathing.
Do Obese Mice Exhibit Obstructive Apnea?
Although rodents are primarily used as models of central sleep apnea, obese mice do exhibit abnormalities in upper airway soft tissue structure (1, 21) and their hypercapnic ventilatory response is depressed during sleep (23). The potential predisposition of obese mice to airflow obstruction led to a recent study noninvasively measuring airflow and whole body plethysmography in the NZO mouse (13). Data demonstrated periods of airflow limitation in the NZO mouse and an isolated physiological trace from one mouse showed a surrogate measure of increased respiratory effort occurring during a period of flow limitation. Because we directly measured respiratory activity of the diaphragm in mice, we utilized the criteria of increasing sequential inspiratory activity (based on Figure 7A of Ref. 13) to determine if comparable patterns of diaphragm EMG activity occur in the ob/ob mouse, and if so, how common were they? Apart from two isolated episodes in lean mice, we did not find diaphragmatic EMG patterns during NREM sleep which met the criteria suggestive of airflow limitation in obese mice. Due to the inherent irregularity of breathing in REM sleep our observations were limited to NREM sleep only. Thus we are unable to report data supportive of obstructive apneas in obese mice, and further studies are required to simultaneously and directly measure airflow and respiratory effort, particularly in REM sleep, which represents the most vulnerable sleep state for upper airway collapse in humans.
Summary and Implications
Circadian impact on respiratory rate and diaphragm EMG amplitude is more pronounced in obese mice and even persists in unstable breathing periods during REM sleep. Thus, in the presence of baseline tachypnea, obese individuals may be more reliant on increases in tidal volume to adjust to the changing metabolic demands across the circadian cycle. Whereas obesity impacted circadian respiratory demands, there was no evidence that obesity worsened the rate or characteristics of central apneic events occurring during NREM and REM sleep. Moreover, obesity in mice did not induce patterns of diaphragmatic EMG burst activity suggestive of the presence of obstructive apnea. The implications of our findings are that if we can find pathologic interventions or transgenic alterations in mice that increase central apneas, or develop techniques to experimentally manipulate the upper airway of obese mice to induce airway obstruction, we will gain valuable insight into the underlying pathogenesis of central and obstructive apneas in the clinical setting.
GRANTS
This study was supported in part by National Institutes of Health Grant T32 HL-082610.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.M.D., A.L.M., P.J.S., and C.P.O. conception and design of research; E.M.D. performed experiments; E.M.D., L.W.L., and C.P.O. analyzed data; E.M.D., L.W.L., A.L.M., P.J.S., and C.P.O. interpreted results of experiments; E.M.D. prepared figures; E.M.D. drafted manuscript; E.M.D., L.W.L., A.L.M., P.J.S., and C.P.O. edited and revised manuscript; E.M.D., L.W.L., A.L.M., P.J.S., and C.P.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lanping Guo for assistance with surgical techniques.
REFERENCES
- 1. Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med 179: 158–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brockmann GA, Bevova MR. Using mouse models to dissect the genetics of obesity. Trends Genet 18: 367–376, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol 99: 2028–2035, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Campen MJ, Tagaito Y, Jenkins TP, Smith PL, Schwartz AR, O'Donnell CP. Phenotypic differences in the hemodynamic response during REM sleep in six strains of inbred mice. Physiol Genomics 11: 227–234, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest 106: 1695–1701, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Christon J, Carley DW, Monti D, Radulovacki M. Effects of inspired gas on sleep-related apnea in the rat. J Appl Physiol 80: 2102–2107, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Dauncey MJ, Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Q J Exp Physiol 72: 549–559, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Fletcher EC, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992 [DOI] [PubMed] [Google Scholar]
- 9. Fogel RB, Malhotra A, Dalagiorgou G, Robinson MK, Jakab M, Kikinis R, Pittman SD, White DP. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep 26: 150–155, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Friedman L, Haines A, Klann K, Gallaugher L, Salibra L, Han F, Strohl KP. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol 97: 1787–1795, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics 102: 616–620, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol 92: 1133–1140, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Hernandez AB, Kirkness JP, Smith PL, Schneider H, Polotsky M, Richardson RA, Hernandez WC, Schwartz AR. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol 112: 671–680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered 41: 317–318, 1950 [DOI] [PubMed] [Google Scholar]
- 15. Koo BB, Strohl KP, Gillombardo CB, Jacono FJ. Ventilatory patterning in a mouse model of stroke. Respir Physiol Neurobiol 172: 129–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, Polotsky VY. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 97: 698–706, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365: 1046–1053, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Nakamura A, Fukuda Y, Kuwaki T. Sleep apnea and effect of chemostimulation on breathing instability in mice. J Appl Physiol 94: 525–532, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. J Am Med Assoc 283: 1829–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 20. O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Polotsky M, Elsayed-Ahmed AS, Pichard L, Harris CC, Smith PL, Schneider H, Kirkness JP, Polotsky V, Schwartz AR. Effects of leptin and obesity on the upper airway function. J Appl Physiol 112: 1637–1643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polotsky VY, Rubin AE, Balbir A, Dean T, Smith PL, Schwartz AR, O'Donnell CP. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med 7: 7–16, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Polotsky VY, Smaldone MC, Scharf MT, Li J, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol 96: 991–998, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE, Sleep Heart Health Study. I Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Real C, Popa D, Seif I, Callebert J, Launay JM, Adrien J, Escourrou P. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep 30: 1295–1302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res 52: 449–453, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol Regul Integr Comp Physiol 259: R282–R287, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sherrey JH, Megirian D. Respiratory EMG activity of the posterior cricoarytenoid, cricothyroid and diaphragm muscles during sleep. Respir Physiol 39: 355–365, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Steenland HW, Kim SS, Zhuo M. GluR3 subunit regulates sleep, breathing and seizure generation. Eur J Neurosci 27: 1166–1173, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Stephenson R, Horner RL. The effect of time of day on apnoea index in the sleeping rat. Respir Physiol Neurobiol 154: 351–355, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Stephenson R, Liao KS, Hamrahi H, Horner RL. Circadian rhythms and sleep have additive effects on respiration in the rat. J Physiol 536: 225–235, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sunderram J, Semmlow J, Thakker-Varia S, Bhaumik M, Hoang-Le O, Neubauer JA. Heme oxygenase-1-dependent central cardiorespiratory adaptations to chronic hypoxia in mice. Am J Physiol Regul Integr Comp Physiol 297: R300–R312, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol 91: 2758–2766, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Tankersley C, Kleeberger S, Russ B, Schwartz A, Smith P. Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81: 716–723, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Taylor BA, Wnek C, Schroeder D, Phillips SJ. Multiple obesity QTLs identified in an intercross between the NZO (New Zealand obese) and the SM (small) mouse strains. Mamm Genome 12: 95–103, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol 110: 1299–1310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol 162: 117–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]