Abstract
Cervical cancer cells express high-risk human papillomavirus (HPV) E6 and E7 proteins. When both HPV oncogenes are repressed in HeLa cervical carcinoma cells, the dormant p53 and retinoblastoma (Rb) tumor suppressor pathways are activated, and the cells undergo senescence in the absence of apoptosis. When the E6 gene is repressed in cells that continue to express an E7 gene, the p53 pathway, but not the Rb pathway, is activated, and both senescence and apoptosis are triggered. To determine the role of p53 signaling in senescence or apoptosis after repression of HPV oncogenes, we introduced a dominant-negative allele of p53 into HeLa cells. Dominant-negative p53 prevented senescence and apoptosis when E6 alone was repressed but did not inhibit senescence when both E6 and E7 were repressed. To determine whether reduced telomerase activity was involved in senescence or apoptosis after E6 repression, we generated HeLa cells stably expressing an exogenous hTERT gene, which encodes the catalytic subunit of telomerase. Although these cells contained markedly elevated telomerase activity and elongated telomeres, hTERT expression did not prevent senescence and apoptosis when E6 alone was repressed. These results demonstrate that when the Rb tumor suppressor pathway is inactivated by the E7 protein, E6 repression activates p53 signaling, which in turn is required for growth inhibition, senescence, and apoptosis. Thus, sustained inactivation of the p53 pathway by the E6 protein is required for maintenance of the proliferative phenotype of HeLa cervical carcinoma cells.
Cervical carcinoma is initiated by infection with a high-risk human papillomavirus (HPV) type, usually HPV type 16 (HPV16) or HPV18, and gene transfer studies have identified the E6 and E7 genes as the major HPV oncogenes (72). The E6 and the E7 proteins modulate cellular proteins that regulate the cell cycle (reviewed in references 44 and 49). In cooperation with the cellular protein E6-AP, the E6 protein binds to the tumor suppressor protein, p53, and targets it for accelerated degradation. The E6 protein also induces expression of human telomerase (hTERT), the catalytic protein subunit of telomerase, the enzyme that maintains the ends of chromosomes. The E7 protein binds to the active, hypophosphorylated form of p105Rb and other members of the retinoblastoma (Rb) family of tumor suppressor proteins, resulting in the destabilization of Rb family members and loss of Rb/E2F complexes that repress transcription of genes required for cell cycle progression. Cyclin-dependent kinases (Cdk) can phosphorylate p105Rb, resulting in the disruption of Rb/E2F complexes, and p21, a transcriptional target of p53, can inhibit Cdk activity. The E6 and E7 proteins have additional effects on these tumor suppressor pathways (e.g., E7 can inhibit p21 action [21, 40, 56]), as well as p53- and Rb-independent activities (e.g., E6 can bind and destabilize a number of PDZ-containing proteins [22]).
During carcinogenesis, normal cells overcome their inability to grow indefinitely. The inexorable loss of replicative potential as normal human cells are passaged serially in culture is known as replicative senescence, an irreversible, nonproliferative but viable cellular state characterized by growth factor-resistant growth arrest, specific morphological changes including cell enlargement and flattening, increased autofluorescence, and elevated senescence-associated β-galactosidase (SAβ-Gal) activity (8, 58). Escape from replicative senescence and immortalization of cultured human keratinocytes requires reactivation of telomerase and inactivation of the Rb and p53 pathways (15, 42, 54). Coexpression of high-risk HPV E6 and E7 genes can also induce immortalization of keratinocytes, but HPV-immortalized cells are not tumorigenic, implying that additional mutations are required for malignant progression.
Even though HPV gene expression is not sufficient for tumorigenesis, studies utilizing the papillomavirus E2 proteins demonstrate that continuous expression of the viral oncogenes is required for the proliferation of cervical carcinoma cell lines. The papillomavirus E2 proteins are viral regulatory proteins that bind to the HPV major early promoter and inhibit transcription of the E6 and E7 genes (55, 62). Infection of HeLa cervical carcinoma cells with an simian virus 40 (SV40)-based viral vector that expresses the bovine papillomavirus (BPV) E2 protein represses expression of the integrated HPV18 E6 and E7 genes (24, 37). BPV E2-mediated repression of E6 and E7 expression in several cervical carcinoma cell lines, as well as in HPV-immortalized human keratinocytes, rapidly activates the p53 and Rb pathways and inhibits telomerase activity (13, 18, 24, 26, 36, 37, 43, 47, 50). p53 induction is followed by transcriptional induction of downstream target genes, including p21 and mdm2. Induction of the growth-inhibitory, hypophosphorylated form of p105Rb results in transcriptional repression of several E2F-responsive genes such as cyclin A and cdc25A due to the assembly of inhibitory Rb/E2F complexes and their recruitment to promoters containing E2F sites (50, 69). Under these conditions, cellular DNA synthesis is suppressed and the cells rapidly and uniformly acquire a phenotype that is indistinguishable from that of cells that have undergone replicative senescence (26, 43, 67). In these experiments, the E2 protein exerts no effect on the Rb or p53 pathways, telomerase activity, or cell proliferation when both E6 and E7 are constitutively expressed in HeLa cells (11, 19, 24) although in some situations papillomavirus E2 proteins can elicit HPV-independent effects, including apoptosis (12-14, 20, 57, 65). HPV18 E6/E7 RNA interference can also induce HeLa cells to undergo senescence (32). These results indicate that proliferation of cervical cancer cell lines requires continuous expression of the E6 and E7 genes.
To explore the role of the individual HPV oncogenes in cervical cancer cells, we separately expressed the HPV16 E6 or E7 gene in HeLa cells, thereby generating HeLa/16E6 and HeLa/16E7 cells, respectively (11). E2 expression represses the endogenous HPV18 genes in the cells, but not the HPV16 genes, which are expressed from a promoter that does not respond to the E2 protein. In this way, we can, in effect, repress HPV E6 alone, by introducing the E2 protein into HeLa/16E7 cells, or HPV E7 alone by introducing the E2 protein into HeLa/16E6 cells. When the E7 gene is specifically repressed in HeLa/16E6 cells, the Rb pathway but not the p53 pathway was activated, and senescence occurred in ca. 95% of the cells. In contrast, when the E6 gene is specifically repressed in HeLa/16E7 cells, the p53 but not the Rb pathway was activated. In these cells, senescence occurred less efficiently (about two-thirds of the cells senesce), and many cells continue to proliferate. After about 1 week, ca. 50% of the proliferating cells undergo apoptosis, a nonviable state characterized by a number of features, including annexin V binding and TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) positivity. Repression of either E6 or E7 resulted in a marked reduction of telomerase activity.
We are interested in determining the cellular signaling pathways responsible for the phenotypes caused by HPV repression in HeLa cells. In many situations, p53 is a key mediator of senescence and apoptosis. Indeed, overexpression of p53 can induce these phenotypes in a variety of cell types (see, for example, reference 61 and 70). However, when both E6 and E7 are repressed, p53 does not appear to be required for induced senescence. First, senescence is efficiently induced by E7 repression in HeLa/16E6 cells even though activation of the p53 pathway is prevented by constitutive expression of the HPV16 E6 gene (11). Second, the E2 protein repressed HPV30 E6/E7 expression and induced senescence in the absence of p21 induction in HT-3 cells, a cervical carcinoma cell line that contains HPV30 DNA and that harbors only transactivation-defective p53 (26, 50). Taken together, these results suggest that HPV E7 repression can activate a p53-independent pathway that results in senescence.
Telomere status and telomerase activity are also involved in senescence. Telomere shortening is an important trigger for replicative senescence in primary cells (2, 63), and keratinocyte immortalization requires activation of telomerase (15, 42). Although HeLa cells express telomerase and display stable telomere length, the average length of their telomeres is quite short (5, 23). To assess the role of telomeres in induced senescence, we introduced the catalytic protein subunit of hTERT into HeLa cells, resulting in greatly elevated telomerase activity and long telomeres (23). Despite these lengthened telomeres, the cells still efficiently and rapidly underwent senescence in response to E2-mediated repression of both HPV E6 and E7. Thus, once expression of the HPV E6/E7 genes is extinguished, senescence does not appear to be initiated by short telomeres.
Although p53 and telomerase are not required for senescence when both E6 and E7 are repressed, it is possible that redundant or overlapping cellular pathways are activated when both viral oncogenes are repressed. Therefore, roles for p53 and telomere length in senescence and apoptosis may be revealed when the E6 and the E7 genes are repressed individually. Accordingly, we sought to test the hypothesis that p53 signaling mediates senescence or apoptosis after repression of E6 alone in the HeLa/16E7 cells. Because cells that escape senescence after E6 repression continue to proliferate in the face of reduced telomerase activity, we also hypothesized that the resulting shortened telomeres may eventually fall below a certain threshold length and trigger the delayed onset of apoptosis in these cells. The experiments reported here demonstrate that p53 is in fact required for senescence and apoptosis after repression of E6 alone. In contrast, our experiments did not reveal a role of telomerase in these activities.
MATERIALS AND METHODS
Cells and viruses.
HeLa/LXSN-1 and HeLa/16E7-3 cell lines and the empty hygromycin resistance vector, pRVY, were described previously (11). These cell lines were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum, penicillin-streptomycin, and 10 mM HEPES (pH 7.3) (DME10) supplemented with 500 μg of G418/ml. pBABE-Hygro-TERT retroviral DNA carrying the hTERT gene was obtained from K. Rundell (Northwestern University). pRVY-p53CTF was constructed by digesting pLXSN-p53DD (28) (obtained from K. Münger, Harvard Medical School) with SpeI and BamHI, and the p53CTF insert was cloned into pRVY retroviral DNA. Retrovirus stocks were prepared in gp2-293 cells and used to infect HeLa/LXSN-1 and HeLa/16E7-3 cells as previously described (11), and stable cell lines were expanded from individual colonies after 2 weeks of selection in DME10 containing G418 and hygromycin B. HeLa/16E6-16E7 cells were constructed by sequential infection of HeLa/sen2 cells with RVY-HPV16 E6 and LXSN-HPV16 E7 (obtained from D. Galloway, Fred Hutchinson Cancer Research Center) (31) and selection. Cell lines carrying both drug resistance markers were maintained in medium containing 500 μg of G418 and 100 μg of hygromycin B/ml.
High-titer stocks of the SV40/BPV type 1 (BPV) recombinant virus expressing the BPV-1 E2 protein were prepared, the titers were determined, and the stocks were used to infect cells at a multiplicity of infection of 20, as described previously (50). Infected cells were maintained in the absence of drug selection with fresh medium every 3 days for the duration of the experiment.
Telomere length.
Telomere length was measured as described previously (23). Briefly, genomic DNA was digested with RsaI and HinfI and resolved by electrophoresis in a 0.7% agarose gel. After transfer to a Nytran membrane, telomeres were detected by hybridization to a 32P-5′-end-labeled (TTAGGG)3 telomere-specific oligonucleotide.
Telomerase activity.
Cells were seeded at 2 × 105 per 60 mm dish and the next day mock infected or infected with E2 virus. After 2 and 6 days, the cells were harvested and stored at −80°C. The samples were analyzed for telomerase activity by using a TRAPeze telomerase detection kit (Intergen, Purchase, N.Y.) according to the manufacturer's instructions for radioisotopic detection. The reaction products were resolved by nondenaturing polyacrylamide gel electrophoresis and quantitated by using a Storm 840 PhosphorImager (Molecular Dynamics, Inc.). No activity was detectable if samples were heat inactivated prior to analysis.
Western and Northern blots.
Cells were lysed with TRIzol reagent (Life Technologies, Inc., Bethesda, Md.) 2 days after mock infection or infection with the E2 virus, and total cellular RNA and protein extracts were prepared as described previously (25). For the analysis of p53CTF, 2 days after mock infection or infection with the E2 virus, cells were lysed in modified EBC buffer as described previously (11).
Portions (5 or 10 μg) of total protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot analyses were performed as described previously (11) with antibodies specific for the following proteins: wild-type p53 (Ab-6 from Oncogene Sciences, Boston, Mass., or 15801A from Pharmingen, San Diego, Calif.), p53CTF (Ab-1 from Oncogene Sciences), p21 (SC-6246 from Santa Cruz Biotechnology, Santa Cruz, Calif.), p105Rb (554136 from Pharmingen), and cyclin A (gift of H. Zhang, Yale University). After being washed, the membranes were incubated with species-specific horseradish peroxidase-conjugated donkey antibody (Jackson ImmunoResearch, West Grove, Pa.). Membranes were incubated with ECL+ (Amersham, Little Chalfont, United Kingdom), and the signals were detected by using Hyperfilm (Amersham).
For Northern blot analysis, 4 or 5 μg of total RNA was denatured, resolved by electrophoresis on a 1% formaldehyde-agarose gel, transferred to a Nytran Supercharge membrane (Schleicher & Schuell, Keene, N.H.), and cross-linked to the membrane by UV irradiation with a Stratalinker (Stratagene, La Jolla, Calif.). The immobilized RNA was detected by hybridization to random prime-labeled probes made by using the Strip-EZ DNA kit (Ambion, Inc., Austin, Tex.), and the signal was detected with a PhosphorImager. Sequential hybridizations were performed after stripping the previous probe from the membrane.
DNA synthesis assay.
Cellular DNA synthesis assays were performed in quadruplicate 2 days after mock infection or infection with the E2 virus. Cells were labeled for 4 h in medium containing 1.5 μCi of [3H]thymidine (ICN)/ml, and the incorporation of acid-insoluble thymidine into DNA was measured as described previously (24).
Autofluorescence and annexin V binding.
A total of 2 × 105 or 5 × 105 cells were seeded into 100-mm-diameter dishes and the next day were mock infected or infected with the E2 virus, respectively. The assays were performed 6 days after infection, as described previously (11).
SAβ-Gal assay.
Cells (1 × 103 or 5 × 104) were seeded into six-well plates and the next day were mock-infected or infected with the E2 virus, respectively. Nine days later, the cells were stained at pH 6.0 with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as described previously (17).
RESULTS
Introduction of a dominant-negative p53 gene into HeLa cells constitutively expressing HPV16 E7.
Repression of the HPV E6 protein in cells constitutively expressing the E7 protein activates the p53 pathway but not the Rb pathway and triggers both senescence and delayed apoptosis. We utilized a dominant-negative version of p53 to determine whether p53 function is required for senescence and apoptosis when the E6 protein is repressed. p53CTF, which is a dominant-negative form of p53 lacking amino acids 14 to 301 (designated p53DD in reference 59), was introduced into HeLa/16E7-3 cells, which constitutively express the HPV16 E7 protein. Retroviruses consisting of either the empty vector (RVY) or the vector with the p53CTF gene were used to infect HeLa/16E7-3 or control HeLa/LXSN-1 cells, and individual hygromycin-resistant colonies were expanded to generate HeLa/16E7-RVY, HeLa/16E7-p53CTF, and HeLa/LXSN-p53CTF cell lines. A recombinant SV40-derived viral vector was then used to introduce the BPV E2 gene acutely into these cells. The E2 protein specifically represses transcription of the endogenous HPV18 E6 and E7 genes, but the retroviral long terminal repeat driving expression of the HPV16 E7 or the p53CTF gene is not repressed by the E2 protein. Thus, E2 expression in effect represses the E6 gene alone in these cells.
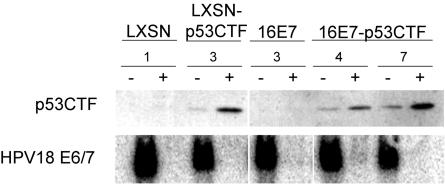
These cell lines were analyzed to confirm expression of dominant-negative p53. Protein extracts were prepared from the cells 2 days after mock infection or infection with the E2 virus, and expression of the truncated dominant-negative p53CTF was analyzed by Western blotting (Fig. 1). p53CTF was expressed only in cells transduced with the retrovirus carrying the p53CTF gene. In the transduced cells, there was little expression of p53CTF in the absence of E2 expression, presumably because the endogenous HPV18 E6 gene destabilizes p53CTF in addition to full-length p53. Expression of the E2 protein induced the expression of p53CTF in these cell lines due to repression of the endogenous HPV18 E6 gene (see below) and the loss of the E6-mediated destabilization of p53. HeLa/16E7-p53CTF-7 cells express higher levels of p53CTF than do HeLa/16E7-p53CTF-4 cells.
FIG. 1.
Expression of dominant-negative p53 and its effect on HPV E6 and E7 gene expression. At 2 days after infection with the E2 virus (+) or mock infection (−), protein and RNA were harvested from HeLa/LXSN-1, HeLa/LXSN-p53CTF-3, and HeLa/16E7-3 cells, as well as from two HeLa/16E7-p53CTF cell lines, as indicated. In the top panel, 5 μg of protein of each sample was resolved by gel electrophoresis, transferred to a membrane, and probed with antibodies specific for p53CTF. In the bottom panel, 5 μg of RNA of each sample was resolved by gel electrophoresis, transferred to a membrane, and hybridized to a probe specific for the HPV18 E6/E7 region.
To determine the effects of the E2 protein in cells expressing p53CTF, we first tested whether expression of the E2 protein repressed the endogenous HPV18 E6 and E7 genes. Cell lines were either mock infected or infected with the E2 virus, and RNA was prepared 2 days later and analyzed for HPV16 and HPV18 E6/E7 gene expression by Northern blotting. As expected, expression of the endogenous HPV18 E6 and E7 genes was substantially repressed by E2 expression in all of the cell lines (Fig. 1; similar results were obtained for HeLa/16E7-RVY cells). In contrast, expression of the exogenous HPV16 E7 gene was not repressed by the E2 protein (data not shown). Thus, dominant-negative p53 had no effect on HPV gene expression.
Effect of dominant-negative p53 tumor suppressor pathways in HeLa cells constitutively expressing HPV16 E7.
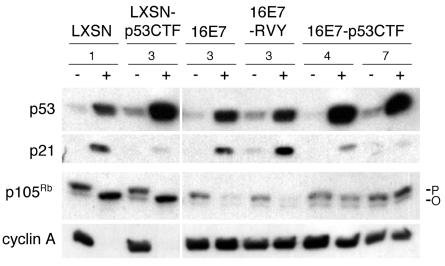
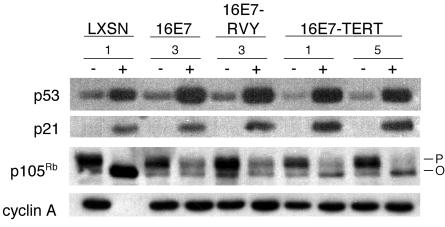
Expression of the E2 protein in the HeLa/16E7-3 cells leads to activation of the p53 pathway due to the repression of HPV18 E6, whereas continued expression of HPV16 E7 prevents activation of the Rb pathway (11). Western blotting was used to assess the activity of the p53 and Rb tumor suppressor pathways (Fig. 2). Full-length, wild-type p53 was expressed in all of the cell lines, and as expected, its expression was markedly induced by E2-mediated E6 repression. Full-length p53 is induced to a greater extent in cells expressing p53CTF, possibly because this dominant-negative form inhibits p53-mediated expression of mdm2 (see below), which stimulates p53 degradation. To test whether p53CTF was functioning as a dominant-negative inhibitor of the p53 pathway, we examined the expression of p21, the product of a p53-responsive gene. Expression of the E2 protein in cell lines lacking p53CTF resulted in a marked increase in p21 levels, reflecting the increased abundance of wild-type p53 in response to E6 repression. E2-mediated induction of p21 was reduced in cells expressing dominant-negative p53, despite the elevation of p53 itself by E6 repression. Induction of p21 was impaired to a greater extent in HeLa/E7-p53CTF clone 7 than in clone 4, a finding consistent with the higher levels of p53CTF in the former cells. Similarly, p53CTF blunted E2-mediated increases in p21 mRNA and in mdm2 protein and mRNA (data not shown). These results indicate that p53CTF functioned as a dominant-negative protein and inhibited the induction of p53-responsive genes after HPV18 E6 repression.
FIG. 2.
Effect of dominant-negative p53 expression on the p53 and Rb pathways. At 2 days after infection with the E2 virus (+) or mock infection (−), protein was harvested from HeLa/LXSN-1, HeLa/LXSN-p53CTF-3, HeLa/16E7-3, and HeLa/16E7-RVY-3 cells, as well as from two HeLa/16E7-p53CTF cell lines, as indicated. 5 μg or 10 μg (for cyclin A) of each sample was resolved by gel electrophoresis, transferred to a membrane, and probed with antibodies specific for wild-type p53, p21, p105Rb, or cyclin A, as indicated. The hyperphosphorylated and hypophosphorylated forms of p105Rb are indicated by P and O, respectively.
The status of the Rb pathway was also assessed by Western blotting (Fig. 2, bottom two panels). In the absence of the E2 protein, all of the cell lines expressed similar levels of p105Rb, which was predominantly hyperphosphorylated. In the cell lines without a transduced HPV16 E7 gene, expression of the E2 protein led to a marked increase in the levels of hypophosphorylated p105Rb, due to repression of the endogenous HPV18 E7 gene and subsequent loss of the E7-mediated destabilization of p105Rb. In cell lines harboring a constitutively expressed HPV16 E7 gene, the E2 protein did not induce the accumulation of hypophosphorylated p105Rb. Similarly, in HeLa cells lacking HPV16 E7, the E2 protein repressed expression of the E2F-responsive cyclin A gene as a result of activation of the Rb pathway, but cyclin A was not repressed by the E2 protein in cells constitutively expressing HPV16 E7, confirming that the constitutively expressed E7 protein prevented activation of the Rb pathway. p53CTF did not effect the level of hypophosphorylated p105Rb or the expression of cyclin A whether or not the E7 protein was expressed.
The abundance of the hyperphosphorylated form of p105Rb provided further insight into p53 activity. When both E6 and E7 were repressed in HeLa/LXSN cells, the level of hyperphosphorylated p105Rb was reduced. This reduction was not prevented by p53CTF in the HeLa/LXSN-p53CTF cells, presumably because E7 repression reduced the expression of cdc25A and cyclin A, which are required for Cdk activity. In contrast, in cells constitutively expressing HPV16 E7 and p53CTF, hyperphosphorylated p105Rb persisted despite repression of E6, and this effect was more pronounced in the HeLa/16E7-p53CTF-7 cells which express more of this dominant-negative form of p53. These results imply that in cells constitutively expressing HPV16 E7, E2-mediated loss of p105Rb hyperphosphorylation is p53 dependent, presumably due to p21-mediated inhibition of Cdk activity. Taken together, with the analysis of the expression of p53-responsive genes, these results demonstrate that expression of dominant-negative p53 inhibited activation of the p53 pathway in response to E6 repression.
Effect of dominant-negative p53 on E2-induced growth inhibition, senescence and apoptosis in HeLa cells constitutively expressing HPV16 E7.
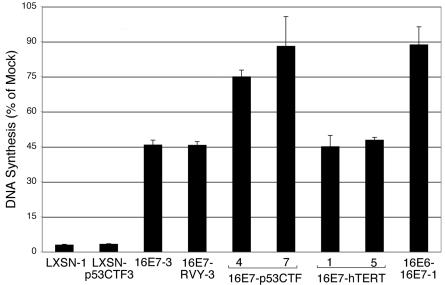
Repression of the HPV18 E6 and E7 genes in HeLa cells causes virtually all of the cells to undergo senescence, whereas constitutive expression of HPV16 E7 allows approximately one-third of the cells to escape senescence and continue proliferating (11). Incorporation of thymidine was used to determine the effect of dominant-negative p53 on this response (Fig. 3). In HeLa/LXSN-1 and HeLa/LXSN-p53CTF cells, which do not constitutively express HPV16 E7, the E2 protein caused a profound inhibition of DNA synthesis whether or not p53CTF was expressed. In contrast, in cells constitutively expressing HPV16 E7, p53CTF provided substantial protection against E2-mediated growth inhibition. In the absence of p53CTF, DNA synthesis was inhibited ca. 55% in HeLa/16E7-3 cells, whereas in the HeLa/16E7-p53CTF-7 cells, in which the p53 pathway is inactivated, DNA synthesis was essentially resistant to E2-mediated inhibition, similar to the situation where the E6 gene itself is constitutively expressed (HeLa/16E6-16E7 cells). Partial protection against growth inhibition was displayed by the HeLa/E7-p53CTF-4 cells, in which p53CTF provides partial inhibition of p53 function. DNA synthesis was inhibited to a greater extent in the p53CTF-4 cells compared to the p53CTF-7 cells in five independent experiments. Thus, p53CTF largely eliminated the E2-mediated inhibition of DNA synthesis in cells constitutively expressing HPV16 E7.
FIG. 3.
Effect of dominant-negative p53 and hTERT on cellular DNA synthesis. At 2 days after infection with the E2 virus or mock infection, HeLa/LXSN-1, HeLa/LXSN-p53CTF-3, HeLa/16E7-3, HeLa/16E7-RVY-3, HeLa/16E6-16E7-1 cells, as well as two HeLa/16E7-p53CTF cell lines and two HeLa/16E7-TERT cell lines, were assayed in quadruplicate for incorporation of [3H]thymidine. For each cell line, the data are displayed as the percentage of [3H]thymidine incorporation by the infected cells compared to mock-infected cells. The results of a representative experiment are shown in which the average of replicate samples are shown with error bars representing one standard deviation of the mean. Similar results were obtained in multiple independent experiments.
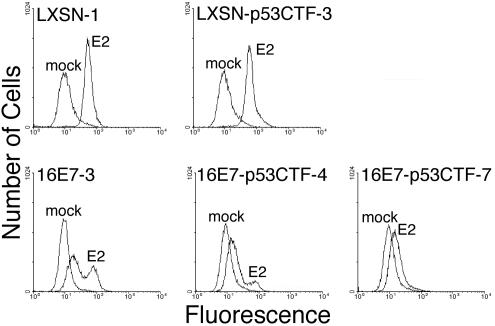
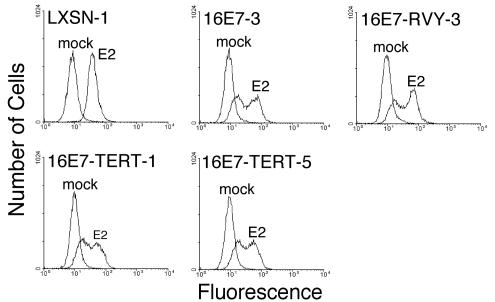
To determine the effect of dominant-negative p53 on cellular senescence, we measured autofluorescence in cells that were mock infected or infected with the E2-expressing virus. After 6 days, flow cytometry was used to measure the intrinsic fluorescence of the cells (Fig. 4). The E2 protein induced a dramatic and uniform increase in fluorescence in both the HeLa/LXSN and HeLa/LXSN-p53CTF cells, indicative of efficient induction of senescence in these cells. Thus, when both E6 and E7 were repressed, senescence did not require p53 function. As reported previously, E2 expression caused the HeLa/16E7 cells to display a more complex fluorescence shift consisting of two populations of cells, one of which is highly fluorescent. This complex pattern is due to the coexistence of proliferating, senescing, and apoptotic cells in the culture. p53CTF impaired the E2-mediated generation of this highly fluorescent cell population in cells constitutively expressing HPV16 E7; rather, infected HeLa/16E7-p53CTF cells primarily displayed autofluorescence that was only slightly elevated compared to uninfected, proliferating cells. In addition, in some experiments, a small population of infected HeLa/16E7-p53CTF-4 cells displayed high autofluorescence. These results suggest that dominant-negative p53 severely impaired E2-mediated induction of senescence and apoptosis in cells constitutively expressing HPV16 E7.
FIG. 4.
Effect of dominant-negative p53 expression on autofluorescence. HeLa/LXSN-1, HeLa/LXSN-p53CTF-3, and HeLa/16E7-3 cells, as well as two HeLa/16E7-p53CTF cell lines, were assayed for autofluorescence by flow cytometry at 6 days after infection with the E2 virus or mock infection.
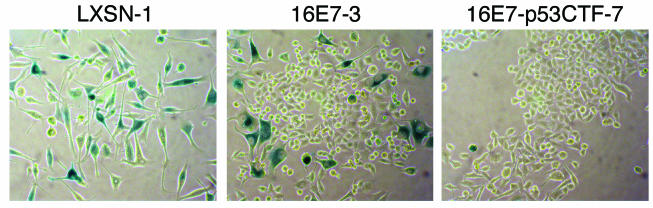
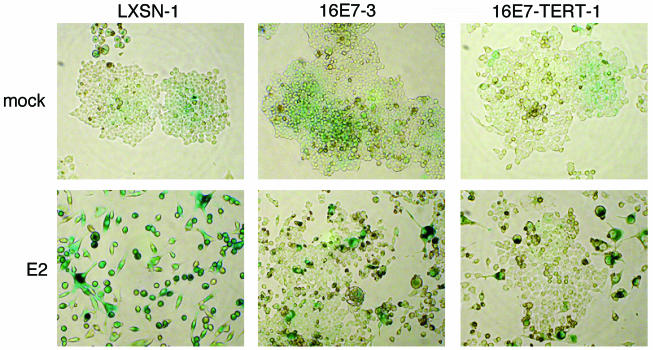
As another marker of senescence, SAβ-Gal activity was determined by incubating the cells with the chromogenic substrate X-Gal at pH 6.0, followed by bright-field microscopy 9 days after mock infection or infection with the E2 virus (Fig. 5 and data not shown). All of the mock-infected cell lines formed colonies that showed faint background staining. After E2 expression, HeLa/LXSN-1 cells displayed a flattened morphology and intense blue staining indicative of SAβ-Gal activity and senescence. As previously reported (11), the E2-infected HeLa/16E7 culture contained cells displaying a flattened morphology and intense blue staining, interspersed with numerous proliferating colonies that did not stain, indicating that a fraction of these cells underwent senescence. Strikingly, the E2 protein did not induce the HeLa/16E7-p53CTF cells to display blue staining or senescent morphology; rather, the cells formed colonies that did not stain for SAβ-Gal activity. These results demonstrate that expression of dominant-negative p53 in cells constitutively expressing the HPV16 E7 protein prevented E2-mediated induction of SAβ-Gal activity or morphological evidence of senescence.
FIG. 5.
Effect of dominant-negative p53 expression on SAβ-Gal activity. HeLa/LXSN-1, HeLa/16E7-3, and HeLa/16E7-p53CTF-7 cells were stained for SAβ-Gal activity 9 days after infection with the E2 virus. Cells were photographed with bright-field optics.
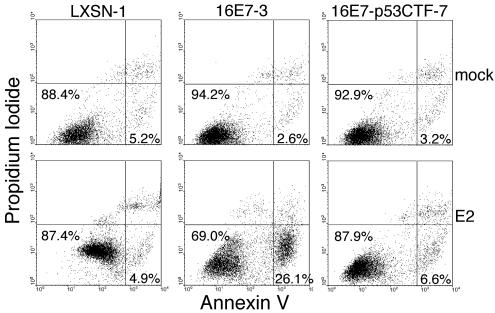
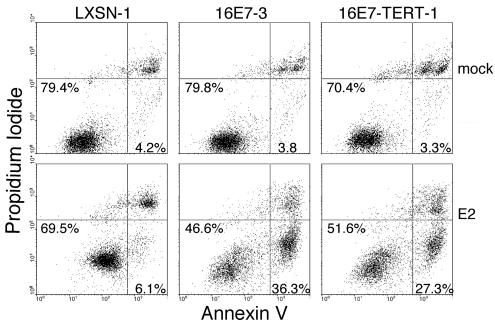
After infection with the E2 virus, many HeLa/16E7 cells undergo apoptosis after a delay of approximately one week. To examine the effect of dominant-negative p53 on apoptosis, nonpermeabilized cells were stained with fluorescent annexin V and propidium iodide and then analyzed by flow cytometry (Fig. 6). The membranes of proliferating and senescing cells are intact, and so these cells take up neither dye and are visualized in the lower left quadrant of a two-dimensional plot. Early apoptotic cells stain with annexin but are impermeable to propidium iodide and therefore are located in the lower right quadrant. Mock-infected cells showed low levels of apoptosis. The E2 protein did not significantly increase the percentage of apoptotic cells in the control HeLa/LXSN-1 culture, although it did induce increased fluorescence in both channels due to autofluorescence that accompanies senescence. In contrast, the E2 protein induced a 10-fold increase in the percentage of apoptotic cells in the HeLa/16E7-3 culture compared to mock-infected cells. Strikingly, p53CTF markedly inhibited E2-mediated induction of apoptosis or senescence in cells constitutively expressing HPV16 E7. In the experiments shown in Fig. 6, the E2 protein induced a modest twofold increase in apoptosis in HeLa/16E7-p53CTF-7 cells, but additional experiments did not reveal increased apoptosis even at later times (data not shown). Therefore, apoptosis that is induced by E6 repression is p53 dependent. Taken together, the results presented here demonstrate that expression of a dominant-negative form of p53 severely impaired senescence and apoptosis after E6 repression.
FIG. 6.
Effect of dominant-negative p53 expression on apoptosis. At 6 days after infection with the E2 virus (bottom panels) or mock infection (top panels), HeLa/LXSN-1, HeLa/16E7-3 and HeLa/16E7-p53CTF-7 cells were assayed by flow cytometry for annexin V binding and propidium iodide uptake. The percentages of cells in the proliferating-senescing quadrant (lower left) and the apoptotic quadrant (lower right) are shown.
Introduction of the hTERT gene into HeLa cells constitutively expressing HPV16 E7.
We also tested whether the senescence or apoptosis that occurs with the specific repression of the E6 protein is due to short telomeres or to E2-mediated reduction in telomerase activity. For this purpose, we first generated HeLa/16E7 cells that stably overexpress the catalytic subunit of hTERT from a heterologous promoter. Retroviruses comprised of the empty vector RVY or a vector containing the hTERT gene were used to infect HeLa/16E7-3 cells, and individual hygromycin-resistant colonies were expanded to generate HeLa/16E7-RVY and HeLa/16E7-TERT cell lines, respectively.
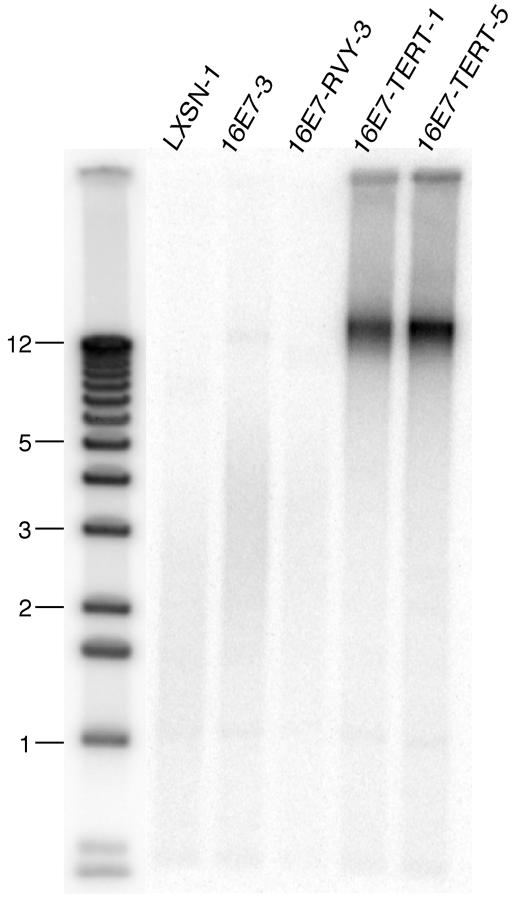
These stable cell lines were analyzed to confirm that the transduced hTERT gene extended telomere length. Genomic DNA was digested with restriction endonucleases that do not cut within the telomeric repeat and analyzed by Southern blotting with a radiolabeled telomere repeat-specific oligonucleotide. In all cell lines lacking exogenous hTERT, telomeres are 1.5-4 kb in length, far shorter than the length at which primary keratinocytes senesce, and difficult to visualize. In contrast, cells carrying a transduced hTERT gene showed a dramatic increase in average telomere length in comparison to the parental HeLa/16E7-3 cells, as assessed by slow electrophoretic mobility and increased intensity of the signal generated with the telomere repeat probe (Fig. 7 and data not shown). We estimated that the length of telomeres in the HeLa/16E7-TERT clones was ca. 15 kb, similar to the telomere length in early-passage primary human keratinocytes.
FIG. 7.
Effect of hTERT expression on telomere length. DNA was purified from the indicated cell lines, digested with HinfI plus RsaI, and subjected to electrophoresis and Southern blotting with a telomere repeat-specific probe to determine telomere length. Sizes (in kilodaltons) of marker fragments are shown at the left.
To determine the effects of the transduced hTERT gene on telomerase activity, we used an in vitro telomerase repeat amplification protocol assay to measure telomerase activity in extracts prepared from cells 2 days after mock infection or 2 or 6 days after infection with the E2 virus. As expected, the cell lines transduced with the hTERT gene exhibited substantially higher telomerase activity than the nontransduced cells (data not shown). In all cell lines, E2 expression resulted in a reduction in telomerase activity in comparison to mock-infected cells, but cell lines expressing a transduced hTERT gene expressed more telomerase activity 6 days after E2 infection than did the mock-infected cells without the hTERT gene (data not shown).
Effect of hTERT on HPV gene expression and tumor suppressor pathways in HeLa cells constitutively expressing HPV16 E7.
Expression of the HPV18 and HPV16 genes was assessed by Northern blotting (data not shown). In all of the cell lines, the E2 protein dramatically repressed the expression of endogenous HPV18 E6 and E7 genes. Furthermore, as expected, HPV16 E7 mRNA was detected in the cell lines containing a transduced E7 gene, and its level was not affected by E2 or hTERT expression. Thus, expression of the endogenous HPV18 genes and the exogenous HPV16 E7 gene was not affected by the transduced hTERT gene or the resulting elevated telomerase activity and extended telomeres.
We also tested whether the constitutive expression of hTERT affected activation of the p53 and Rb tumor suppressor pathways. Protein extracts were prepared 2 days after mock infection or infection with the E2 virus and analyzed by Western blotting (Fig. 8). In the absence of E2 expression, there was little expression of p53 or p21 in any of the cell lines, and all cell lines displayed similar patterns of p105Rb and cyclin A expression, indicating that the p53 and Rb pathways were not activated by hTERT expression. As expected, expression of the E2 protein and E6 repression markedly induced expression of both p53 and p21 in all of the cell lines (Fig. 8, top two panels). Expression of the E2 gene caused a marked increase in the level of hypophosphorylated p105Rb in the control HeLa/LXSN-1 cells but not in the cell lines constitutively expressing HPV16 E7 (Fig. 8, third panel). Similarly, the E2 protein repressed expression of cyclin A in the HeLa/LXSN-1 cells, indicating that the Rb pathway was activated in these cells, but cyclin A was not repressed in cells harboring a constitutively expressed HPV16 E7 gene (Fig. 8, bottom panel). The level of hyperphosphorylated p105Rb was decreased by the E2 protein in all of the cell lines. Notably, the presence of the transduced hTERT gene did not affect the expression of p53, p21, p105Rb, or cyclin A or their responses to the E2 protein. Therefore, in cells constitutively expressing HPV16 E7, E2-mediated activation of the p53 and Rb tumor suppressor pathways was not affected by increased telomerase activity and extended telomeres.
FIG. 8.
Effect of hTERT expression on the p53 and Rb pathways. At 2 days after infection with the E2 virus (+) or mock infection (−), protein was harvested from HeLa/LXSN-1, HeLa/16E7-3, and HeLa/16E7-RVY-3 cells, as well as from two HeLa/16E7-TERT cell lines, as indicated. Then, 5 μg of each sample was resolved by gel electrophoresis, transferred to a membrane, and probed with antibodies specific for wild-type p53, p21, p105Rb, or cyclin A, as indicated. The hyperphosphorylated and hypophosphorylated forms of p105Rb are indicated by P and O, respectively.
Effect of hTERT on E2-induced growth inhibition, senescence, and apoptosis in HeLa cells containing a constitutively expressed copy of HPV16 E7.
We then examined the effect of the transduced hTERT gene on the phenotypic response to the E2 protein. The results of a representative thymidine incorporation experiment to measure cellular DNA synthesis are shown in Fig. 3. As noted above, HeLa/16E7-3 cells were partially protected from the growth inhibition caused by the E2 protein, incorporating ca. 45% as much thymidine as mock-infected cells. Constitutive expression of the hTERT gene did not provide further protection against E2-mediated inhibition of DNA synthesis. Thus, increased telomerase activity and extended telomeres did not prevent E2-induced growth inhibition in HeLa cells constitutively expressing the HPV16 E7 protein.
To determine the effect of the hTERT gene on senescence, we examined autofluorescence (Fig. 9) and SAβ-Gal activity (Fig. 10 and data not shown). The E2 protein induced the HeLa/LXSN-1 and the HeLa/16E7-3 cells to undergo the characteristic fluorescence shifts described in an earlier section. In cells constitutively expressing the HPV16 E7 protein, the fluorescence profiles of E2-infected cells were not affected by the transduced hTERT gene. Staining for SAβ-Gal activity revealed that the E2 protein induced the appearance of enlarged, flat blue cells interspersed with proliferating colonies in both the HeLa/16E7-3 cells and HeLa/16E7-TERT cell lines. Taken together, these results suggested that increased telomerase activity and extended telomeres did not prevent E2-mediated senescence in cells constitutively expressing the HPV E7 protein.
FIG. 9.
Effect of hTERT expression on autofluorescence. HeLa/LXSN-1, HeLa/16E7-3, and HeLa/16E7-RVY-3 cells, as well as two HeLa/E7-TERT cell lines, were assayed for autofluorescence by flow cytometry 6 days after infection with the E2 virus or mock infection.
FIG. 10.
Effect of hTERT expression on SAβ-Gal activity. HeLa/LXSN-1, HeLa/16E7-3, HeLa/16E7-TERT-2-1 cells were stained for SAβ-Gal activity 9 days after mock infection (top panels) or infection with the E2 virus (bottom panels). Cells were photographed with bright-field optics.
We used annexin V binding and flow cytometry to determine the effect of the hTERT gene on apoptosis (Fig. 11 and data not shown). E2 expression induced a similar level of apoptosis in the HeLa/16E7-TERT cells and the parental HeLa/16E7-3 cells. Thus, increased telomerase activity and extended telomeres did not prevent E2-induced apoptosis in cells constitutively expressing the HPV16 E7 protein.
FIG. 11.
Effect of hTERT expression on apoptosis. Six days after infection with the E2 virus (bottom panels) or mock infection (top panels), HeLa/LXSN-1, HeLa/16E7-3, HeLa/16E7-TERT-2-1 cells were assayed by flow cytometry for annexin V binding and propidium iodide uptake. The percentages of cells in the proliferating-senescing quadrant (lower left) and the apoptotic quadrant (lower right) are shown.
DISCUSSION
Cervical carcinoma cells provide a unique opportunity to determine the status of dormant growth inhibitory pathways in cancer cells and to assess the ability of these pathways to impose growth control upon reactivation. Combined repression of both of the HPV oncogenes in cervical carcinoma cells results in activation of the p53 and Rb tumor suppressor pathways and induction of cellular growth arrest and senescence. When the HPV18 E6 gene is repressed in HeLa cells that constitutively express the E7 protein, the p53 pathway is activated and telomerase activity declines, but the Rb pathway is not activated (11). This results in a complex cellular phenotype in which initially approximately two-thirds of the cells senesce and the remaining cells proliferate. Many of these proliferating cells undergo apoptosis approximately 1 week later. Because the Rb pathway is not activated when the E6 protein is repressed in cells that constitutively express the E7 protein, senescence and apoptosis must occur by a pathway independent of Rb signaling. The goal of the present study was to determine whether p53 signaling or reduced telomerase activity is required for senescence and apoptosis when HPV E6 alone is repressed.
We tested the ability of a dominant-negative form of p53 to inhibit senescence and apoptosis induced by E6 repression in cells constitutively expressing the HPV16 E7 protein. Dominant-negative p53 impaired signaling by the p53 pathway, as assessed by the reduced ability of E6 repression to induce expression of p53-responsive genes and, in cells expressing HPV16 E7, to prevent p105Rb hyperphosphorylation. Strikingly, elimination of p53 signaling largely prevented both senescence and apoptosis after E6 repression. Thus, continuous inactivation of the p53 pathway by the E6 protein is required for maintenance of the proliferative state of HeLa cells. The experiments reported here were conducted in HeLa cells only. However, ongoing E6 expression in the great majority of cervical cancers and cancer-derived cell lines suggests that continuous E6-mediated p53 degradation may be a general requirement for the maintenance of the transformed phenotype in cervical cancer cells.
Wells et al. (67) previously reported that the E2 protein induced senescence in HeLa cells via Rb- and p21-dependent pathways. However, both E6 and E7 were repressed in that study, a situation that complicates analysis of the cellular signaling mechanisms responsible for the observed phenotypes. We have shown here and previously (11) that the E6 and E7 proteins control distinct aspects of the biochemical and physiological phenotype of cervical carcinoma cells. By dissecting the separate contributions of the E6 and E7 proteins, we have identified a specific requirement for E6-mediated inactivation of the p53 pathway in maintaining the transformed state of cervical cancer cells.
Our results indicate that the signaling events responsible for growth inhibition, senescence, and apoptosis induced by repression of the E6 protein alone required an intact p53 pathway. This conclusion is reinforced by the finding that the protection from senescence correlated with the level of p53CTF expression and the extent of inhibition of p53 signaling. The slight residual growth inhibitory effects of E6 repression in HeLa/16E7-p53CTF cells presumably reflected incomplete inhibition of p53 signaling by p53CTF, but we have not ruled out the alternative explanation that p53-independent signaling played a minor role. The senescence and delayed apoptosis induced by the E2 protein in HeLa cells constitutively expressing HPV16 E7 is prevented by coexpression of HPV16 E6 (11). Thus, in this setting p53-dependent senescence and apoptosis required HPV E6 repression. This phenotype is distinct from p53-independent apoptosis that can be elicited by transfected E2 genes (13). This later effect does not require HPV repression but rather can be mediated by an intrinsic proapoptotic activity of the HPV18 E2 protein (12).
Interference with p53 signaling in HeLa cells has differing effects on senescence depending on whether both the E6 gene and the E7 gene are repressed or whether E6 alone is repressed. When both genes were repressed, senescence efficiently occurred whether or not p53 signaling is impaired, indicating that a p53-independent pathway can induce senescence in this setting. This is consistent with our previous findings that repression of the E7 gene alone can induce senescence without activation of the p53 pathway and that HPV30 repression can induce senescence in HT-3 cells, which lack a functional p53 pathway (11, 26). On the other hand, when the E6 gene alone was repressed, p53 signaling was required for senescence. Thus, repression of HPV gene expression mobilizes both p53-dependent and p53-independent pathways that can result in senescence.
p53 was induced either when both E6 and E7 were repressed or when E6 alone was repressed, but apoptosis occurred only in the latter situation. Therefore, repression of the E7 protein prevented p53-mediated apoptosis, possibly by inducing senescence, which can provide a protective effect against apoptosis (64). In other systems, Rb signaling can protect against p53-dependent apoptosis (3, 29, 33, 35, 48), suggesting that E7 repression may prevent apoptosis in HeLa cells by activating the Rb pathway. In fact, it has been reported that delivery of the p53 gene into HPV16-containing cervical carcinoma cell lines induced apoptosis, which was inhibited by coexpression of the gene encoding p105Rb (38).
p53-dependent senescence and apoptosis occurred after repression of the E6 protein alone in the HeLa/16E7 cells, even though Rb did not accumulate in a hypophosphorylated form, and E2F-regulated genes were not repressed. Therefore, the p53-dependent signaling cascade that results in senescence and apoptosis upon repression of the E6 gene does not appear to involve p21-mediated accumulation of hypophosphorylated Rb and repression of E2F-responsive genes. Similarly, expression of p53 or p21 can induce growth arrest and apoptosis in cells lacking Rb (1, 16, 45). The ability of p53 to induce p53-independent senescence and apoptosis might depend on other p53-regulated genes implicated in these processes, such as BAX or PERP (4, 46).
Telomere shortening can initiate replicative senescence in primary cells (2, 63), and telomerase expression in conjunction with inactivation of the Rb pathway is required for immortalization of primary keratinocytes (15, 42, 54). However, overexpression of hTERT and telomere lengthening is not sufficient to prevent senescence when the E6 protein is repressed, even though the E7 protein maintains the Rb pathway in an inactive state. Similarly, expression of hTERT in normal human fibroblasts does not prevent premature senescence induced by activated Ha-ras or other stress (27, 66). Thus, senescence initiated by activation of tumor suppressor pathways in cancer cells and premature senescence induced by various treatments of primary cells may be under different control than replicative senescence.
After E6 repression, cells that escaped senescence underwent p53-dependent apoptosis after a delay of approximately 1 week. Similarly, inhibition of telomerase in cancer cells can induce delayed apoptosis (30, 34, 71). However, E6 repression still activated the p53 pathway in cells transduced with the hTERT gene, and senescence and apoptosis still occurred. Thus, short telomeres were not required for induction of p53 or for p53-mediated senescence and apoptosis when E6 alone was repressed, just as short telomeres were not required for senescence when E6 and E7 were repressed together (23). It is possible that E6 repression induced the ongoing accumulation of cellular damage in the cells that escaped senescence, triggering apoptosis after several days once this damage exceeded a certain threshold. For example, p53 can induce the generation of reactive oxygen species, and the resulting oxidative damage may trigger apoptosis (39, 53).
Senescence did not occur in HeLa/16E6 cells unless the E7 gene is repressed, and senescence and apoptosis did not occur in the HeLa/16E7 cells unless the E6 gene is repressed. Moreover, repression of E6 was not sufficient to induce apoptosis, because apoptosis did not occur when both E7 and E6 are repressed. Thus, the cellular response to repression of the HPV oncogenes appears to be dictated by the balance between E6 and E7 expression and not by the absolute expression level of either gene. It may be possible to harness p53-mediated apoptosis as a therapeutic approach in virus-infected cells and cervical cancer cells by selective inhibition of E6 expression or activity. Indeed, the induction of apoptosis in cervical carcinoma cells by peptides that bind the E6 protein or by RNAs that inhibit E6 expression is consistent with this scenario (6, 7, 9).
The biological role of the E6 protein in the papillomavirus life cycle is unclear. Many viruses have evolved activities to inhibit apoptosis that occurs in response to virus infection and viral gene expression (see, for example, reference 10). In cultured cells and in transgenic mice, the E7 protein has proapoptotic activity which is inhibited by E6 expression and by disruption of the p53 gene (41, 51, 52, 60, 68). It is possible that an important activity of the E6 protein during the virus life cycle and carcinogenesis is to impair p53-mediated apoptosis that would otherwise occur in response to E7 expression.
Acknowledgments
We thank Kathleen Rundell, Denise Galloway, Hui Zhang, and Karl Münger for essential reagents, Edward Goodwin and George Miller for advice and support, and Jan Zulkeski for assistance in preparing the manuscript.
L.M. was supported by a Dermatology Training Grant from the National Institutes of Health (AR07016). This study was supported by a grant from the National Cancer Institute (CA16038).
REFERENCES
- 1.Alexander, K., and P. W. Hinds. 2001. Requirement for p27KIP1 in retinoblastoma protein-mediated senescence. Mol. Cell. Biol. 21:3616-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allsopp, R. C., and C. B. Harley. 1995. Evidence for a critical telomere length in senescence human fibroblasts. Exp. Cell Res. 219:130-136. [DOI] [PubMed] [Google Scholar]
- 3.Almasan, A., Y. Yin, R. E. Kelly, E. Y. Lee, A. Bradley, W. Li, J. R. Bertino, and G. M. Wahl. 1995. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F-responsive genes, and apoptosis. Proc. Natl. Acad. Sci. USA 92:5436-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attardi, L. D., E. E. Reczek, C. Cosmas, E. G. Demicco, M. E. McCurrach, S. W. Lowe, and T. Jacks. 2000. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 14:704-718. [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan, T. M., A. Englezou, M. A. Dunham, and R. R. Reddel. 1998. Telomere length dynamics in telomerase-positive immortal human cell populations. Exp. Cell Res. 239:370-378. [DOI] [PubMed] [Google Scholar]
- 6.Butz, K., C. Denk, A. Ullmann, M. Scheffner, and F. Hoppe-Seyler. 2000. Induction of apoptosis in human papillomavirus-positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc. Natl. Acad. Sci. USA 97:6693-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butz, K., T. Ristriani, A. Hengstermann, C. Denk, M. Scheffner, and F. Hoppe-Seyler. 2003. siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 22:5938-5945. [DOI] [PubMed] [Google Scholar]
- 8.Campisi, J. 1997. The biology of replicative senescence. Eur. J. Cancer 33:703-709. [DOI] [PubMed] [Google Scholar]
- 9.Cho, C. W., H. Poo, Y. S. Cho, M. C. Cho, K. A. Lee, S. J. Lee, S. N. Park, I. K. Kim, Y. K. Jung, Y. K. Choe, Y. I. Yeom, I. S. Choe, and D. Y. Yoon. 2002. HPV E6 antisense induces apoptosis in CaSki cells via suppression of E6 splicing. Exp. Mol. Med. 34:159-166. [DOI] [PubMed] [Google Scholar]
- 10.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 7:546-554. [DOI] [PubMed] [Google Scholar]
- 11.DeFilippis, R. A., E. C. Goodwin, L. Wu, and D. DiMaio. 2003. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J. Virol. 77:1551-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demeret, C., A. Garcia-Carranca, and F. Thierry. 2003. Transcription-independent triggering of the extrinsic pathway of apoptosis by human papillomavirus 18 E2 protein. Oncogene 22:168-175. [DOI] [PubMed] [Google Scholar]
- 13.Desaintes, C., C. Demeret, S. Goyat, M. Yaniv, and F. Thierry. 1997. Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J. 16:504-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desaintes, C., S. Goyat, S. Garbay, M. Yaniv, and F. Thierry. 1999. Papillomavirus E2 induces p53-independent apoptosis in HeLa cells. Oncogene 18:4538-4545. [DOI] [PubMed] [Google Scholar]
- 15.Dickson, M. A., W. C. Hahn, Y. Ino, V. Ronfard, J. Y. Wu, R. A. Weinberg, D. N. Louis, F. P. Li, and J. G. Rheinwald. 2000. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diller, L., J. Kassel, C. E. Nelson, M. A. Gryka, G. Litwak, M. Gebhardt, B. Bressac, M. Ozturk, S. J. Baker, B. Vogelstein, and S. H. Friend. 1990. p53 functions as a cell cycle control protein in osteosarcomas. Mol. Cell. Biol. 10:5772-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimri, G. P., X. Lee, G. Basile, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowhanick, J. J., A. A. McBride, and P. M. Howley. 1995. Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol. 69:7791-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis, D. A., S. I. Schmid, and P. M. Howley. 2000. Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J. Virol. 74:2679-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frattini, M. G., S. D. Hurst, H. B. Lim, S. Swaminathan, and L. A. Laimins. 1997. Abrogation of a mitotic checkpoint by E2 proteins from oncogenic human papillomaviruses correlates with increased turnover of the p53 tumor suppressor protein. EMBO J. 16:318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardiol, D., C. Kuhne, B. Glaunsinger, S. S. Lee, R. Javier, and L. Banks. 1999. Oncogenic human papillomavirus E6 proteins target the discs large tumor suppressor for proteasome-mediated degradation. Oncogene 18:5487-5496. [DOI] [PubMed] [Google Scholar]
- 23.Goodwin, E. C., and D. DiMaio. 2001. Induced senescence in HeLa cervical carcinoma cells containing elevated telomerase activity and extended telomeres. Cell Growth Differ. 12:525-534. [PubMed] [Google Scholar]
- 24.Goodwin, E. C., and D. DiMaio. 2000. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc. Natl. Acad. Sci. USA 97:12513-12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodwin, E. C., L. K. Naeger, D. E. Breiding, E. J. Androphy, and D. DiMaio. 1998. Transactivation-competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J. Virol. 72:3925-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin, E. C., E. Yang, C.-J. Lee, H.-W. Lee, D. DiMaio, and E.-S. Hwang. 2000. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 97:10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbunova, V., A. Seluanov, and O. M. Pereira-Smith. 2002. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J. Biol. Chem. 277:38540-38549. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb, E., R. Haffner, T. von Ruden, E. F. Wagner, and M. Oren. 1994. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 13:1368-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas Kogan, D. A., S. C. Kogan, D. Levi, P. Dazin, Tang, A., Y. K. T. Fung, and M. A. Israel. 1995. Inhibition of apoptosis by the retinoblastoma gene product. EMBO J. 14:461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn, W. C., S. A. Stewart, M. W. Brooks, S. G. York, E. Eaton, A. Kurachi, R. L. Beijersbergen, J. H. M. Knoll, M. Meyerson, and R. A. Weinberg. 1999. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 5:1164-1170. [DOI] [PubMed] [Google Scholar]
- 31.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall, A. H. S., and K. A. Alexander. 2003. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J. Virol. 77:6066-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haupt, Y., S. Rowan, and M. Oren. 1995. p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene 10:1563-1571. [PubMed] [Google Scholar]
- 34.Herbert, B.-S., A. E. Pitts, S. I. Baker, S. E. Hamilton, W. E. Wright, J. W. Shay, and D. R. Corey. 1999. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc. Natl. Acad. Sci. USA 96:14276-14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howes, K. A., N. Ransom, D. S. Papermaster, J. G. Lasudry, D. M. Albert, and J. J. Windle. 1994. Apoptosis or retinoblastoma: alternative fates of photoreceptors expressing the HPV-16 E7 gene in the presence or absence of p53. Genes Dev. 8:1300-1310. (Erratum, 8:1738.) [DOI] [PubMed] [Google Scholar]
- 36.Hwang, E.-S., L. K. Naeger, and D. DiMaio. 1996. Activation of the endogenous p53 growth inhibitory pathway in HeLa cervical carcinoma cells by expression of the bovine papillomavirus E2 gene. Oncogene 12:795-803. [PubMed] [Google Scholar]
- 37.Hwang, E.-S., D. J. Riese II, J. Settleman, L. A. Nilson, J. Honig, S. Flynn, and D. DiMaio. 1993. Inhibition of cervical carcinoma cell line proliferation by introduction of a bovine papillomavirus regulatory gene. J. Virol. 67:3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ip, S. M., T.-G. Huang, W. S. B. Yeung, and H. Y. S. Ngan. 2001. pRb-expressing adenovirus Ad5-Rb attenuates the p53-induced apoptosis in cervical cancer cell lines. Eur. J. Cancer 37:2475-2483. [DOI] [PubMed] [Google Scholar]
- 39.Johnson, T. M., Z.-X. Yu, V. J. Ferrans, R. A. Lowenstein, and T. Finkel. 1996. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 93:11848-11852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones, D. L., R. M. Alani, and K. Munger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, D. L., D. A. Thompson, and K. Munger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 42.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Kleingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 43.Lee, C. J., E. J. Suh, H. T. Kang, J. S. Im, S. J. Um, J. S. Park, and E. S. Hwang. 2002. Induction of senescence-like state and suppression of telomerase activity through inhibition of HPV E6/E7 gene expression in cells immortalized by HPV16 DNA. Exp. Cell Res. 277:173-182. [DOI] [PubMed] [Google Scholar]
- 44.Mantovani, F., and L. Banks. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874-7887. [DOI] [PubMed] [Google Scholar]
- 45.Marcellus, R. C., J. G. Teodoro, R. Charbonneau, G. C. Shore, and P. E. Branton. 1996. Expression of p53 in Saos-2 osteosarcoma cells induces apoptosis which can be inhibited by Bcl-2 or the adenovirus E1B-55 kDa protein. Cell Growth Differ. 7:1643-1650. [PubMed] [Google Scholar]
- 46.McCurrach, M. E., T. M. F. Connor, C. M. Knudson, S. J. Korsmeyer, and S. W. Lowe. 1997. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 94:2345-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon, M. S., C. J. Lee, S. J. Um, J. S. Park, J. M. Yang, and E. S. Hwang. 2001. Effect of BPV1 E2-mediated inhibition of E6/E7 expression in HPV16-positive cervical carcinoma cells. Gynecol. Oncol. 80:168-175. [DOI] [PubMed] [Google Scholar]
- 48.Morgenbesser, S. D., B. O. Williams, T. Jacks, and R. A. DePinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371:72-74. [DOI] [PubMed] [Google Scholar]
- 49.Munger, K., J. R. Basile, S. Duensing, A. Eichten, S. L. Gonzalez, M. Grace, and V. L. Zacny. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888-7898. [DOI] [PubMed] [Google Scholar]
- 50.Naeger, L. K., E. C. Goodwin, E.-S. Hwang, R. A. DeFilippis, H. Zhang, and D. DiMaio. 1999. Bovine papillomavirus E2 protein activates a complex growth-inhibitory program in p53-negative HT-3 cervical carcinoma cells that includes repression of cyclin A and cdc25A phosphatase genes and accumulation of hypophosphorylated retinoblastoma protein. Cell Growth Differ. 10:413-422. [PubMed] [Google Scholar]
- 51.Pan, H., and A. E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 8:1285-1299. [DOI] [PubMed] [Google Scholar]
- 52.Pan, H., and A. E. Griep. 1995. Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 9:2157-2169. [DOI] [PubMed] [Google Scholar]
- 53.Polyak, K., Y. Xia, J. L. Zweier, K. W. Kinzler, and B. Vogelstein. 1997. A model for p53-induced apoptosis. Nature 389:300-305. [DOI] [PubMed] [Google Scholar]
- 54.Rheinwald, J. G., W. C. Hahn, M. R. Ramsey, J. Y. Wu, Z. Guo, H. Tsao, M. DeLuca, C. Catricala, and K. M. O'Toole. 2002. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol. Cell. Biol. 22:5157-5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romanczuk, H., F. Thierry, and P. M. Howley. 1990. Mutations analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J. Virol. 64:2849-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruesch, M. N., and L. A. Laimins. 1997. Initiation of DNA synthesis by human papillomavirus E7 oncoproteins is resistant to p21-mediated inhibition of cyclin E-cdk2 activity. J. Virol. 71:5570-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Perez, A.-M., S. Soriano, A. R. Clarke, and K. Gaston. 1997. Disruption of the human papillomavirus type 16 E2 gene protects cervical carcinoma cells from E2F-induced apoptosis. J. Gen. Virol. 78:3009-3018. [DOI] [PubMed] [Google Scholar]
- 58.Sedivy, J. M. 1998. Can ends justify the means?: telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA 95:9078-9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaulian, E., A. Zauberman, D. Ginsberg, and M. Oren. 1992. Identification of a minimal transforming domain of p53: negative dominance through abrogation of sequence-specific DNA binding. Mol. Cell. Biol. 12:5581-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoppler, H., M. Conrad Stoppler, E. Johnson, C. M. Simbulan-Rosenthal, M. E. Smulson, S. Iyer, D. S. Rosenthal, and R. Schlegel. 1998. The E7 protein of human papillomavirus type 16 sensitizes primary human keratinocytes to apoptosis. Oncogene 17:1207-1214. [DOI] [PubMed] [Google Scholar]
- 61.Sugrue, M. M., D. Y. Shin, S. W. Lee, and S. A. Aaronson. 1997. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA 97:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thierry, F., and M. Yaniv. 1987. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 6:3391-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 64.Wang, E. 1995. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55:2284-2292. [PubMed] [Google Scholar]
- 65.Webster, K., J. Parish, M. Pandya, P. L. Stern, A. R. Clarke, and K. Gaston. 2000. The human papillomavirus (HPV) 16 E2 protein induces apoptosis in the absence of other HPV proteins and via a p53-dependent pathway. J. Biol. Chem. 275:87-94. [DOI] [PubMed] [Google Scholar]
- 66.Wei, S., W. Wei, and J. M. Sedivy. 1999. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 59:1539-1543. [PubMed] [Google Scholar]
- 67.Wells, S. I., D. A. Francis, A. Y. Karpova, J. J. Dowhanick, J. D. Benson, and P. M. Howley. 2000. Papillomavirus E2 induces senescence in HPV-positive cells via pRB- and p21CIP-dependent pathways. EMBO J. 19:5762-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White, A. E., E. M. Livanos, and T. D. Tlsty. 1994. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 8:666-677. [DOI] [PubMed] [Google Scholar]
- 69.Wu, L., E. C. Goodwin, L. K. Naeger, E. Vigo, K. Galaktionov, K. Helin, and D. DiMaio. 2000. E2F-Rb complexes assemble and inhibit cdc25A transcription in cervical carcinoma cells following repression of human papillomavirus oncogene expression. Mol. Cell. Biol. 20:7059-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yonish-Rouach, E., D. Resnitzky, J. Loten, L. Sachs, A. Kimchi, and M. Oren. 1991. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352:345-347. [DOI] [PubMed] [Google Scholar]
- 71.Zhang, X., V. Mar, W. Zhou, L. Harrington, and M. O. Robinson. 1999. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.zur Hausen, H. 1999. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin. Cancer Biol. 9:405-411. [DOI] [PubMed] [Google Scholar]