Abstract
Molecular subtyping of breast cancer may provide additional prognostic information regarding patient outcome. However, its clinical significance remains to be established. In this study, the main aims were to discover whether reclassification of breast cancer into molecular subtypes provides more precise information regarding outcome compared to conventional histopathological grading and to study breast cancer-specific survival in the different molecular subtypes. Cases of breast cancer occurring in a cohort of women born between 1886 and 1928 with long-term follow-up were included in the study. Tissue microarrays were constructed from archival formalin-fixed, paraffin-embedded tissue from 909 cases. Using immunohistochemistry and in situ hybridisation as surrogates for gene expression analyses, all cases were reclassified into the following molecular subtypes: Luminal A; Luminal B (HER2−); Luminal B (HER2+); HER2 subtype; Basal phenotype; and five negative phenotype. Kaplan–Meier survival curves and Cox proportional hazards models were used in the analyses. During the first 5 years after diagnosis, there were significant differences in prognosis according to molecular subtypes with the best survival for the Luminal A subtype and the worst for HER2 and five negative phenotype. In this historic cohort of women with breast cancer, differences in breast cancer-specific survival according to subtype occur almost exclusively amongst the histopathological grade 2 tumours. From 5 years after time of diagnosis until the end of follow-up, there appears to be no difference in survival according to molecular subtype or histopathological grade.
Keywords: Breast cancer, Molecular subtype, Histopathological grade, Tissue microarray, Breast cancer-specific survival, Prognosis
Introduction
Breast cancer is the most common cancer and leading cause of cancer-related death amongst women worldwide [13, 35]. The disease is heterogeneous in its histopathology, therapeutic response, metastatic patterns and outcome. Current treatment guidelines are based on histopathological grading, tumour size, lymph node-, hormone receptor-, human epidermal growth factor receptor 2 (HER2)- and proliferation (Ki67) status. More recently, gene expression analyses using c-DNA microarray technology have provided a deeper understanding of the complexity of breast cancer. Perou et al. [30] describe four molecular subtypes: Luminal-like, HER2 enriched, Basal-like and Normal-like. More recent publications have confirmed these subtypes with some modifications and it has been shown that molecular subtypes also differ in their response to treatment and outcome [4, 8]. Molecular subtyping with immunohistochemistry (IHC) and in situ hybridisation (ISH) as surrogates for gene expression analyses makes it possible to study large numbers of archival breast cancer cases with long-term follow-up.
Histopathological grade is a well-established prognostic factor [3, 12, 32]. Recent studies confirm the importance of grading in breast cancer prognostication, although grading systems based on gene expression, such as the Gene expression grade index (GGI), have recently emerged [7, 32, 37]. Molecular subtyping may provide additional information on patient outcome, but consensus has yet to be reached regarding IHC or ISH markers that could be used as surrogates for gene expression analyses [17]. Most surrogate markers used for subtyping are available in clinical practice today, but it remains to document the benefits of a new classification prior to implementation.
The aims of this study were to discover whether reclassification of breast tumours into molecular subtypes provides more information regarding outcome compared to conventional histopathological grading and to study breast cancer-specific survival (BCSS) for molecular subtypes over time. To achieve this, a cohort of breast cancer cases with long-term follow-up was reclassified into molecular subtypes. Most of the markers examined are widely used, such as oestrogen receptor (ER), progesterone receptor (PR), HER2 and Ki67. In addition, cytokeratin 5 (CK5) and epithelial growth factor receptor 1 (EGFR) were included [2, 6]. The patients in this population experienced breast cancer in a time period or at an age where adjuvant treatment after surgery was rarely an option and the disease thus had a near-natural course.
Materials and methods
Study population
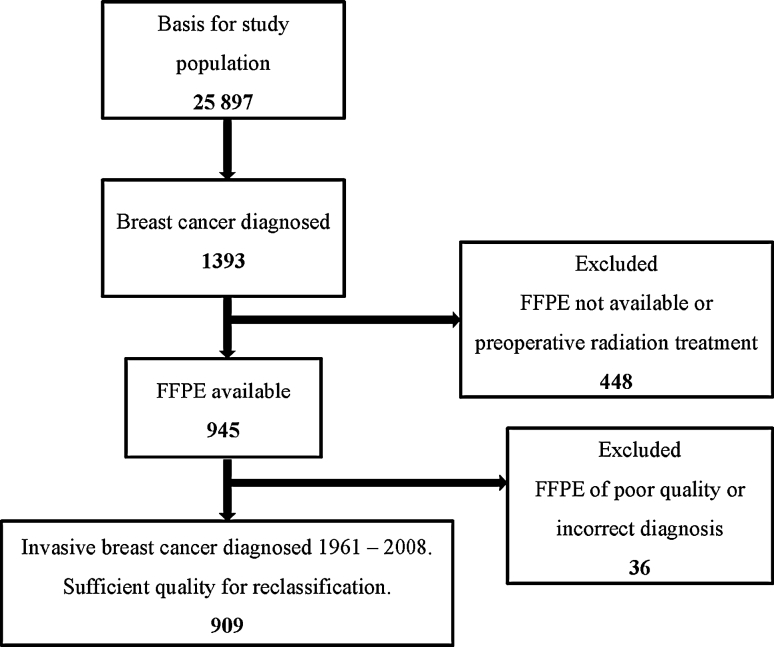
Between 1956 and 1959, 25,897 women in the Norwegian county of Nord-Trøndelag, born between 1886 and 1928, were invited to participate in a screening programme for early diagnosis of breast cancer [22, 29]. The screening comprised a clinical examination and a questionnaire focussed on reproductive history. Data were linked with the Norwegian Cancer Registry and the Cause of Death Registry of Norway. In all, 1,393 new cases of breast cancer occurred between 1961 and 2008. Most of these were analysed at the Department of Pathology, St. Olav’s Hospital, Trondheim University Hospital, Norway. A total of 448 cases were excluded from the study. For the remaining 945 cases, formalin-fixed, paraffin-embedded (FFPE) tissue was available and 909 were of sufficient quality for reclassification into molecular subtypes (see Fig. 1).
Fig. 1.
Study population
Specimen characteristics
Pathology reports and FFPE tissue from all cases were retrieved from the archives of the department of pathology. In cases with recurrent disease or second or multiple primary breast cancer, only the first primary tumour was included. New 4-μm-thick full-face sections were cut from representative paraffin blocks from tumours and lymph node metastases and stained with haematoxylin–erythrosine–saffron (HES). Forty cases comprised only core biopsies or small tissue fragments unsuitable for tissue microarray (TMA). From these, serial sections were made. The HES-stained sections were reviewed under a microscope independently by two experienced pathologists (OAH, AMB) and classified according to histopathological type and grade according to the World Health Organization Classification of Tumours [23] and the Nottingham grading system [12, 33]. Any discrepancies in grade or type were discussed and consensus reached. In cases where tumour size was missing in the pathology report, size was measured in millimetres on the glass slide. Only cases with a measurement of the whole tumour in the pathology report and/or measurement of the full diameter on the glass slide were registered. All other cases were classified as size uncertain [n = 268 (29.5 %)].
TMA construction
TMA blocks were made using the Tissue Arrayer MiniCore® 3 with TMA Designer2 software (Alphelys). Areas of interest in the HES sections were marked by a pathologist. Three 1-mm-diameter tissue cores were extracted from peripheral regions of the tumour in the FFPE blocks and inserted into TMA recipient blocks. From the TMA blocks, 4-μm sections were cut and stained. IHC was done with antibodies for ER, PR, HER2(CB11), CK5, Ki67 and EGFR in addition to HES staining. In addition, HER2 status was also examined by chromogenic in situ hybridization (CISH).
Assay methods
Sections were mounted on Superfrost+glass slides, dried at 37 °C overnight and stored at −20 °C. All sections were stained within 12 weeks of sectioning. The slides were heated to 60 °C for 2 h. Pre-treatment was performed in a PT Link, Pre-Treatment Module for Tissue Specimens (Dako) with buffer (High pH Target Retrieval Solution K8004) at 97 °C for 20 min. All sections were immunostained for ER, PR, HER2 (CB11), CK5 and Ki67 in a DakoCytomation Autostainer Plus (Dako). For visualization, the Dako REAL™ EnVision™ Detection System was used with Peroxidase/DAB+, Rabbit/Mouse, code K5007. EGFR was immunostained using EGFR pharmDx™ for autostainer, code K1494. See Table 1 for sources and dilutions of primary antibodies. Negative controls were included in each staining run. CISH was used to visualize the HER2 gene (red chromagen) and chromosome 17 (blue chromagen) using the dual colour probe kit HER2 CISH pharmDx™ Kit, code 109 (Dako). Two of the steps in the CISH procedure were modified slightly. The incubation time for red chromogen solution was increased from 10 to 15 min, and the dilution of haematoxylin was increased from 1:5 to 1:7.
Table 1.
Sources and dilutions of primary antibodies
| Antibody | Clone | Manufacturer | Concentration of antibody | Dilution |
|---|---|---|---|---|
| ER | SP1 | Cell marque | 33 mg/ml | 1:100 |
| PR | 16 | Novocastra | 360 mg/l | 1:400 |
| HER2 | CB11 | Novocastra | 3.9 g/l | 1:640 |
| Ki67 | MIB1 | Dako | 35 mg/l | 1:100 |
| CK5 | XM26 | Novocastra | 50 mg/l | 1:100 |
| EGFR | 2-18C9 | Dako | Ready to use | No dilution |
Scoring and reporting
All HES- and IHC-stained slides were digitalized using the tissue scanner Ariol™ SL-50 3.3 Scan system and analysis station (Genetix) at 5× and 20× magnification. Expression of ER, PR, HER2 (CB11), CK5, Ki67 and EGFR was evaluated using the Ariol review station. The images were viewed and subjectively scored by two persons independently. HER2 gene amplification status was annotated under a bright field microscope. All cases were evaluated by at least one pathologist. Any discrepancies were discussed and consensus reached.
Classification of each marker
ER and PR were positive when ≥1 % of the tumour cells showed positive nuclear staining [19]. For Ki67, a total of 500 tumour nuclei were examined. Cases with ≥15 % positive nuclei were classified as Ki67 high and <15 % as Ki67 low [16].
HER2 was assessed using both IHC and CISH [11]. For HER2 IHC, the CB11 clone [31, 43] was used and the Herceptest (Dako) guidelines for interpretation were used with a membrane-staining score ranging from 0 to +3. HER2 IHC was considered negative when the score was 0 or +1, positive when +3 and borderline when +2. Since the preanalytical treatment of the samples was unknown, the results of HER2 IHC were only used in cases where CISH was unsuccessful. In IHC (+2) and unsuccessful CISH (18 cases), the corresponding IHC was revised by two authors (AMB and MJE) and reclassified as either +1(14 cases) or +3(4 cases).
The HER2 gene was considered amplified if the gene to chromosome ratio was ≥2.0 [1, 34]. A minimum of 20 non-overlapping nuclei with signals for both chromosome and gene were assessed.
For CK5, a staining index (SI) was estimated. Staining intensity was graded as 0 (no staining), 1 (weak), 2 (moderate) and 3 (strong). The proportion of positive staining cells was scored as 1 (<10 %), 2 (10–50 %) and 3 (>50 %). The score for intensity multiplied by proportion is the SI [14, 26]. In this study, the results were considered to be negative when SI was 0–1 and positive when the SI was 2–9. For EGFR, membranous staining was scored according to the guidelines in the Dako PharmDx kit and a SI was calculated when this was combined with the proportion of cells showing positive staining resulting in a SI as described above.
Classification of molecular subtypes
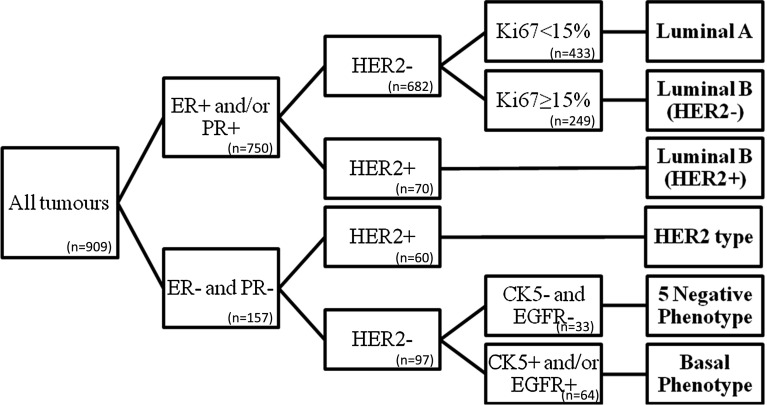
Using the six biomarkers, the tumours were then classified in molecular subtypes: Luminal A, Luminal B (HER2−), Luminal B (HER2+), HER2 subtype five negative phenotype (5NP) and Basal-like phenotype (BP) (Fig. 2).
Fig. 2.
Classification algorithm for molecular subtyping
Statistical analyses
All women were followed from the date of breast cancer diagnosis to the date of death from breast cancer, death from any other cause or to the end of follow-up (December 31, 2010), whichever came first. BCSS according to molecular subtypes and histopathological grade was estimated using Kaplan–Meier methods and compared by log-rank tests. Cox proportional hazards models were used to estimate risk of death from breast cancer adjusted for age (5-year intervals), stage (in five categories: stage I–IV and unknown) at diagnosis according the data from the Cancer Registry [21] and time period of diagnosis (10-year intervals). Hazard ratios (HR) were calculated with 95 % confidence intervals (CI) for two time periods: first 5 years after diagnosis and from 5 years after diagnosis and onwards (conditional on surviving the first 5 years). Cox analyses of the first 5 years were stratified by histopathological grade. Statistical analyses were carried out using Stata version 12.1 IC for Windows (Stata Corp.). This study complies with the REMARK reporting recommendations for tumour marker studies [25].
Ethics
The study was approved by the Regional Committee for Medical and Health Sciences Research Ethics (REK, Midt-Norge, ref. nr: 836/2009) and dispensation from the requirement of patient consent was granted.
Results
Description of the population
In all, 909 cases were included. Mean age at diagnosis was 72.5 years (SD 10.7; range 41–102). Only 12.5 % were <60 years and 58.9 % were 60–79 years. Most tumours were 2–5 cm in diameter (43.2 %), but for 29.5 %, tumour size was unknown or uncertain. At the end of the observation period, 359 (39.5 %) had died of breast cancer, 390 (42.9 %) of other causes and 160 (17.6 %) were still alive. Median follow-up was 6.4 years [interquartile range (IQR) 10.0 years]. See Table 2 for patient and tumour data.
Table 2.
Descriptive statistics for the 909 breast cancer cases
| Luminal A | Luminal B (HER2−) | Luminal B (HER2+) | HER2 type | 5 Negative phenotype | Basal phenotype | Total | |
|---|---|---|---|---|---|---|---|
| Number (%) | 433 (47.6) | 249 (27.4) | 70 (7.7) | 60 (6.6) | 33 (3.6) | 64 (7) | 909 |
| Mean age at diagnosis (SD) | 73.9 (9.9) | 71.9 (10.9) | 69 (11.4) | 67.3 (11.6) | 75.9 (11.1) | 71.7 (11.3) | 72.5 (10.7) |
| Median years of follow-up after diagnosis (IQR) | 7.4 (9.3) | 7.0 (11.1) | 5.2 (12.5) | 3.2 (8.1) | 3.4 (8.5) | 5.1 (9.1) | 6.4 (10.0) |
| Tumour grade (%) | |||||||
| 1 | 91 (21.0) | 20 (8.0) | 2 (2.9) | 0 | 0 | 4 (6.3) | 117 (12.9) |
| 2 | 297 (68.6) | 120 (48.2) | 33 (47.1) | 10 (16.7) | 21 (63.6) | 7 (10.9) | 488 (53.7) |
| 3 | 45 (10.4) | 109 (43.8) | 35 (50.0) | 50 (83.3) | 12 (36.4) | 53 (82.8) | 304 (33.4) |
| Histopathological type (%) | |||||||
| Ductal | 299 (69.1) | 182 (73.1) | 57 (81.4) | 47 (78.3) | 14 (42.4) | 37 (57.8) | 636 (70.0) |
| Lobular | 68 (15.7) | 35 (14.1) | 6 (8.6) | 1 (1.7) | 12 (36.4) | 2 (3.1) | 124 (13.6) |
| Tubular | 4 (0.9) | 0 | 0 | 0 | 0 | 0 | 4 (0,4) |
| Mucinous | 31 (7.2) | 8 (3.2) | 3 (4.3) | 0 | 0 | 1 (1.6) | 43 (4.7) |
| Papillary | 19 (4.4) | 7 (2.8) | 3 (4.3) | 1 (1.7) | 0 | 2 (3.1) | 32 (3.5) |
| Medullary | 0 | 6 (2.4) | 0 | 6 (10.0) | 2 (6.1) | 7 (10.9) | 21 (2.3) |
| Metaplastic | 0 | 1 (0.4) | 0 | 2 (3.3) | 1 (3.0) | 9 (14.1) | 13 (1.4) |
| Other | 12 (2.8) | 10 (4.0) | 1 (1.4) | 3 (5.0) | 4 (12.1) | 6 (9.4) | 36 (4.0) |
| Tumour sizea (%) | |||||||
| <2 | 94 (21.7) | 50 (20.1) | 12 (17.1) | 4 (6.7) | 3 (9.1) | 6 (9.4) | 169 (18.6) |
| 2–5 | 193 (44.6) | 97 (39.0) | 27 (38.6) | 21 (35.0) | 18 (54.5) | 37 (57.8) | 393 (43.2) |
| >5 | 35 (8.1) | 19 (7.6) | 7 (10.0) | 13 (21.7) | 2 (6.1) | 3 (4.7) | 79 (8.7) |
| Uncertain | 111 (25.6) | 83 (33.3) | 24 (34.3) | 22 (36.7) | 10 (30.3) | 18 (28.1) | 268 (29.5) |
| Lymph node invasiona | |||||||
| Yes | 129 (29.8) | 82 (32.9) | 25 (35.7) | 32 (53.3) | 15 (45.5) | 27 (42.2) | 310 (34.1) |
| No (≥5 nodes or SNBb) | 142 (32.8) | 66 (26.5) | 24 (34.3) | 15 (25.0) | 5 (15.2) | 21 (32.8) | 273 (30.0) |
| No (<5 nodes examined) | 123 (28.4) | 84 (33.7) | 20 (28.6) | 9 (15.0) | 10 (30.3) | 11 (17.2) | 257 (28.3) |
| Uncertain | 39 (9.0) | 17 (6.8) | 1 (1.4) | 4 (6.7) | 3 (9.1) | 5 (7.8) | 69 (7.6) |
| Stagec | |||||||
| I | 238 (55.0) | 123 (49.4) | 29 (41.4) | 23 (38.3) | 15 (45.5) | 27 (42.2) | 455 (50.1) |
| II | 157 (36.3) | 90 (36.1) | 28 (40.0) | 27 (45.0) | 14 (42.4) | 30 (46.9) | 346 (38.1) |
| III | 23 (5.3) | 17 (6.8) | 3 (4.3) | 7 (11.7) | 3 (9.1) | 4 (6.3) | 57 (6.3) |
| IV | 13 (3.0) | 17 (6.8) | 8 (11.4) | 3 (5.0) | 1 (3.0) | 3 (4.7) | 45 (5.0) |
| Unknown | 2 (0.5) | 2 (0.8) | 2 (2.9) | 0 | 0 | 0 | 6 (0.7) |
aHistologically confirmed
bSentinel node biopsy
cCancer Registry of Norway, combined clinical and histological stage
Histopathological characteristics
Of the 909 tumours, 12.9 % were grade 1, 53.7 % grade 2 and 33.4 % grade 3. The histopathological types were as follows: ductal: 70.0 %; lobular: 13.6 %; and other special types: 16.4 %. All cases were reclassified into molecular subtypes based on assessment of ER, PR, HER2, Ki67, CK5 and EGFR. Table 2 shows distribution of histopathological types and grades for each molecular subtype. Table 3 shows the number of positive cases of each marker.
Table 3.
The number of positive cases for each marker
| Marker | No. of positive (%) | Not possible to interpret |
|---|---|---|
| ER | 749 (82.4) | 2 (0.2 %) |
| PR | 521 (57.3) | 1 (0.1 %) |
| HER2 | 130 (14.3) | 0 |
| Ki67 | 406 (44.7) | 1 (0.1 %) |
| CK5 | 164 (18.0) | 0 |
| EGFR | 64 (7.0) | 0 |
Distribution of molecular subtypes
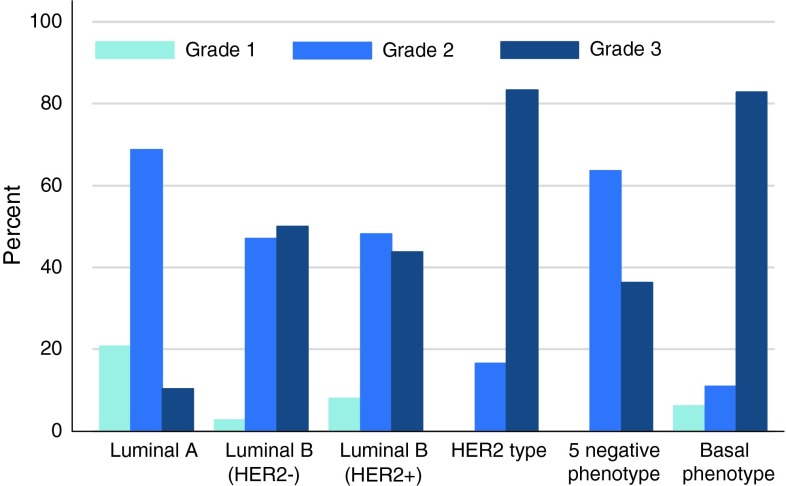
The distribution of subtypes was as follows: Luminal A: 47.6 %; Luminal B (HER2−): 27.4 %; Luminal B (HER2+): 7.7 %; HER2 subtype: 6.6 %; 5NP: 3.6 %; and BP: 7.0 %. See Table 2. Mean age at diagnosis was 72.8 (SD 10.5) for women with luminal tumours and 70.9 (SD 11.8) for non-luminal tumours. Luminal A had the highest proportion of grades 1 and 2 (Fig. 3). Only HER2 subtype and BP comprised a higher proportion of grade 3 than grade 2. Grade 1 was not found in HER2 and 5NP subtypes. The Luminal B subtypes had very similar distribution of grades despite differences in other characteristics.
Fig. 3.
Distribution of grade in percent according to subtype
Breast cancer-specific survival, molecular subtypes and histopathological grade
Luminal A subtype had the best survival, closely followed by Luminal B (HER2−) with 5-year BCSS higher than 75 %. The HER2 and 5NP subtypes had the poorest prognosis, with 5-year survival around 50 %. Of the triple-negative cases, BP had a better prognosis than 5NP. BP and Luminal B (HER2+) were similar in terms of 5-year survival (Fig. 4).
Fig. 4.
Kaplan–Meier plot. Breast cancer-specific survival according to molecular subtypes. P-value from log-rank test of differences in BCSS was <0.0001
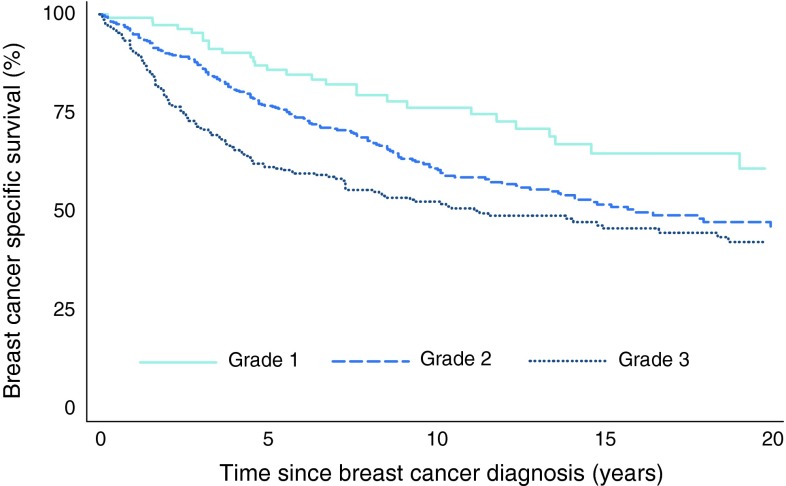
Figure 5 shows BCSS according to histopathological grade for up to 20 years of follow-up. Adjustment for age did not substantially influence the curves, but after adjustment for stage, survival for grade 1 tumours was improved (data not shown).
Fig. 5.
Kaplan–Meier plot. Breast cancer-specific survival according to grade. P-value from log-rank test of differences in BCSS was 0.0001
Risk of death from breast cancer
Table 4 shows risk of death from breast cancer according to molecular subtype and histopathological grade. During the first 5 years, grades 2 and 3 had a poorer prognosis compared to grade 1 with HR 3.8 (95 % CI 2.14–6.75) for grade 3 and HR 1.97 (95 % CI 1.11–3.51) for grade 2. In the same time period, the hormone receptor-negative and/or HER2-positive subtypes had the poorest prognoses compared to Luminal A. Particularly poor prognoses were shown for the HER2 subtype [HR 4.24 (95 % CI 2.79–6.42)] and 5NP [HR 3.34, (95 % CI 1.91–5.82)]. After 5 years, neither grade nor molecular subtype showed any clear association with survival. Adjustment for age had no impact on the results, and adjustment for stage only slightly attenuated risk estimates.
Table 4.
Risk of death from breast cancer according to molecular subtype and histopathological grade
| No. of cases | Deaths from breast cancer | HR 95 % CI unadjusted | HR 95 % CI adjusted for age | HR 95 % CI adjusted for stage | ||||
|---|---|---|---|---|---|---|---|---|
| Histopathological grade, follow-up first 5 years after diagnosis | ||||||||
| 1 | 117 | 13 | 1.00 | 1.00 | 1.00 | |||
| 2 | 488 | 101 | 1.97 | 1.11–3.51 | 1.95 | 1.09–3.48 | 1.47 | 0.82–2.64 |
| 3 | 304 | 110 | 3.80 | 2.14–6.75 | 3.74 | 2.10–6.66 | 3.12 | 1.75–5.55 |
| 909 | 224 | |||||||
| Histopathological grade, follow-up from 5 years after diagnosisa | ||||||||
| 1 | 78 | 18 | 1.00 | 1.00 | 1.00 | |||
| 2 | 291 | 83 | 1.37 | 0.82–2.29 | 1.29 | 0.77–2.16 | 1.21 | 0.72–2.02 |
| 3 | 153 | 34 | 0.98 | 0.55–1.74 | 0.94 | 0.53–1.67 | 0.90 | 0.51–1.60 |
| 522 | 135 | |||||||
| Molecular subtype, follow-up first 5 years after diagnosis | ||||||||
| Luminal A | 433 | 73 | 1.00 | 1.00 | 1.00 | |||
| Luminal B (HER2−) | 249 | 56 | 1.42 | 1.01–2.02 | 1.42 | 1.00–2.02 | 1.29 | 0.92–1.84 |
| Luminal B (HER2+) | 70 | 25 | 2.33 | 1.48–3.67 | 2.36 | 1.48–3.74 | 2.11 | 1.33–3.33 |
| HER2 | 60 | 32 | 4.24 | 2.79–6.42 | 4.39 | 2.86–6.72 | 3.72 | 2.44–5.65 |
| 5 Negative phenotype | 33 | 15 | 3.34 | 1.91–5.82 | 3.18 | 1.81–5.61 | 3.17 | 1.81–5.53 |
| Basal phenotype | 64 | 23 | 2.43 | 1.52–3.89 | 2.38 | 1.48–3.82 | 2.39 | 1.48–3.82 |
| 909 | 224 | |||||||
| Molecular subtype, follow-up from 5 years after diagnosisa | ||||||||
| Luminal A | 271 | 69 | 1.00 | 1.00 | 1.00 | |||
| Luminal B (HER2−) | 148 | 44 | 1.15 | 0.79–1.68 | 1.21 | 0.82–1.77 | 1.15 | 0.80–1.69 |
| Luminal B (HER2+) | 36 | 10 | 0.81 | 0.41–1.57 | 0.88 | 0.44–1.73 | 0.92 | 0.46–1.83 |
| HER2 | 23 | 4 | 0.66 | 0.24–1.80 | 0.71 | 0.26–1.96 | 0.66 | 0.24–1.82 |
| 5 Negative phenotype | 12 | 3 | 0.84 | 0.27–2.68 | 0.89 | 0.27–2.90 | 0.94 | 0.30–3.01 |
| Basal phenotype | 32 | 5 | 0.58 | 0.23–1.43 | 0.62 | 0.25–1.55 | 0.58 | 0.23–1.46 |
| 522 | 135 | |||||||
HR hazard ratio, CI confidence interval
aConditional on surviving the first 5 years
Table 5 shows risk of death from breast cancer the first 5 years after diagnosis according to molecular subtype for grade 2 and 3. For grade 2, the HR for HER2 subtype compared to Luminal A was 6.62 (95 % CI 2.82–15.57), and adjustment for age and stage did not substantially influence the estimate. In grade 3, there was no clear difference in risk of death from breast cancer according to molecular subtype. Since 12 of the 13 patients who died of grade 1 tumours had Luminal A tumours, HRs were not calculated. Adjustment for time period of diagnosis did not change the results (not shown).
Table 5.
Risk of death from breast cancer according to molecular subtype for each histopathological grade
| Number of cases | Deaths from breast cancer | HR 95 % CI Unadjusted | HR 95 % CI Adjusted for age | HR 95 % CI Adjusted for stage | ||||
|---|---|---|---|---|---|---|---|---|
| Molecular subtype, follow-up first 5 years after diagnosis: grade 2 | ||||||||
| Luminal A | 297 | 45 | 1.00 | 1.00 | 1.00 | |||
| Luminal B (HER2−) | 120 | 24 | 1.45 | 0.88–2.38 | 1.50 | 0.91–2.48 | 1.33 | 0.81–2.19 |
| Luminal B (HER2+) | 33 | 12 | 2.67 | 1.41–5.04 | 2.97 | 1.54–5.70 | 2.29 | 1.20–4.38 |
| HER2 | 10 | 6 | 6.62 | 2.82–15.57 | 7.81 | 3.18–19.18 | 5.64 | 2.36–13.51 |
| 5 Negative Phenotype | 21 | 11 | 4.68 | 2.42–9.06 | 3.91 | 1.97–7.76 | 4.42 | 2.26–8.64 |
| Basal Phenotype | 7 | 3 | 3.39 | 1.05–10.92 | 2.56 | 0.75–8.69 | 3.35 | 1.03–10.85 |
| 488 | 101 | |||||||
| Molecular subtype, follow-up first 5 years after diagnosis: grade 3 | ||||||||
| Luminal A | 45 | 16 | 1.00 | 1.00 | 1.00 | |||
| Luminal B (HER2–) | 109 | 31 | 0.73 | 0.40–1.34 | 0.73 | 0.39–1.36 | 0.57 | 0.31–1.04 |
| Luminal B (HER2+) | 35 | 13 | 1.00 | 0.48–2.09 | 0.96 | 0.45–2.05 | 0.85 | 0.41–1.79 |
| HER2 | 50 | 26 | 1.60 | 0.86–2.99 | 1.60 | 0.84–3.05 | 1.21 | 0.64–2.29 |
| 5 Negative Phenotype | 12 | 4 | 0.90 | 0.30–2.70 | 0.87 | 0.28–2.64 | 0.94 | 0.31–2.82 |
| Basal Phenotype | 53 | 20 | 1.07 | 0.55–2.06 | 1.07 | 0.54–2.11 | 0.86 | 0.44–1.68 |
| 304 | 110 | |||||||
Follow-up first 5 years after diagnosis. HRs were not calculated for histopathological grade 1 because 12 of the 13 patients who died of grade 1 tumour had Luminal A tumour
Amongst HER2-positive cases, the hazard ratio for the HER2 subtype compared to Luminal B (HER2+) was 1.8 (95 % CI 1.07–3.05) (not shown in table).
Discussion
In this long-term follow-up of breast cancer patients, the HER2 and 5NP subtypes showed the poorest prognosis during the first 5 years after diagnosis. After 5 years, BCSS did not significantly differ amongst the six molecular subtypes. However, the numbers of 5-year survivors in these two groups are low. The patients came from a cohort of women with breast cancer who lived through a time period with limited access to adjuvant treatment. However, 192 women would have qualified for antihormonal treatment according to the treatment guidelines operative at the time of diagnosis. None were qualified for treatment with trastuzumab. Kaplan–Meier BCSS estimates for patients with ER-positive tumours who may have received treatment and those who did not qualify for treatment do not differ significantly (data not shown).
During the first 5 years of follow-up, differences in survival according to subtype occurred almost exclusively amongst patients with grade 2 tumours. Grade 2 was significantly associated with poorer survival for all subtypes except Luminal B (HER2−).
These results support the findings of others that hormone receptor status defines two groups within HER2-positive breast cancer with differing BCSS [42]. The HER2 subtype had the poorest 5-year survival of all subtypes, whereas the Luminal B (HER2+) subgroup had a substantially better 5-year survival, supporting the significance of ER status in determining survival. It has been shown that, despite problems associated with crosstalk between ER and HER2, Luminal B (HER2+) benefits from antihormonal treatment [20]. The hazard ratio for the HER2 subtype compared to Luminal B (HER2+) would appear to confirm this.
To predict response to endocrine therapy, the cutoff for ER was previously set at 10 % positive staining nuclei [28]. In accordance with current guidelines, the cutoff is now set at ≥1 % [19]. In this study, 24 cases showed ER-positive staining in ≥ 1 < 10 % of tumour cell nuclei and were classified as Luminal. A majority (16 cases) were Luminal B, and in the Luminal B (HER2+) subtype, they accounted for 9 % of cases. Deyarmin et al. [10] have suggested that the classification of ER-low tumours as Luminal may be inappropriate. These cases exert little or no influence on the results of the Kaplan–Meier and Cox analyses in the present study.
Classification of breast cancer into molecular subtypes with surrogate markers for gene expression is widely used. In 2010, Blows et al. [4] published a large collaborative analysis that showed survival for different subtypes, where the subtyping in all the 12 included studies was done by IHC. These methods are more accessible and affordable than gene profile studies and can be applied to archival FFPE tissue. The St. Gallen Consensus Discussion in 2011 opened for molecular subtyping of breast cancer using ER, PR, HER2 and Ki67/grade, all factors already in clinical use, though the cutoff for Ki67 is still controversial [18]. The panel did not support the incorporation of EGFR or CK 5/6, thus the basal phenotype and the five negative phenotype were classified as ‘triple negative’ [15, 17]. Discussion is ongoing regarding which markers are best suited for the classification of molecular subtypes.
In the present study, 5-year survival was better for BP compared to 5NP. This is in contrast with the findings of others [4, 6, 40]. The 5NP subtype had poorer prognosis despite the fact that it comprised a higher proportion of histological grade 2 tumours. Validation studies will reveal whether or not this finding is consistent. This may be a group that would have benefited from adjuvant treatment as offered today.
Histopathological grade, tumour size and lymph node status are strong prognostic factors and are well established in clinical practice. Reduced long-term survival is associated with higher grade [4, 36, 44]. In the present study, high grade was associated with non-luminal subtypes. However, the prognostic value of the different factors may vary with time after diagnosis [24]. Since the risk of relapse and death is the highest during the first 5 years, particularly for ER-negative disease [27, 41], two periods of time were analysed separately in this study: the first 5 years after diagnosis and the subsequent years. Even after many years, there is some risk of breast cancer recurrence. Interestingly, in this cohort, there are no differences in survival according to subtypes for those who have survived the first 5 years. Further research may reveal whether adjuvant treatment modifies this tendency.
Histopathological grade 1 tumours are associated with the best prognosis, whereas grade 3 tumours are associated with the poorest prognosis. Grade 2 tumours comprise a more heterogeneous group where the majority has an intermediate prognosis, but some cases may exhibit similarity with grades 1 and 3 [7, 32]. The same applies in this study. It is possible to classify grade 2 tumours into low risk and high risk of recurrence using the GGI which is based on analysis of 97 genes [37]. A 3-gene proliferation score using PCR assay to identify TOP2A, FOXM1 and MKI67 has similar prognostic value as GGI and might be easier to implement [39]. However, the present study shows that it is possible to obtain significant additional information of prognostic value by using already implemented or readily accessible tests, and this may be of value in prognostication of grade 2 tumours.
This study contributes to the understanding of breast cancer heterogeneity partly because of the unique nature of the study population. These women lived in a time before birth control pills and hormone replacement therapy at menopause were available, and they had not undergone organized mammography screening. Furthermore, due to age and time period, they had limited postoperative treatment and thus we come as close to the natural course of the disease as possible. One drawback in this study is the relatively high age of the cohort and the results must be considered in light of this fact. This may explain the relatively high proportion of grade 2 tumours and the slightly lower proportion of HER2-positive tumours [38]. Another weakness may be the IHC estimation of HER2 where standardized preanalytical conditions were unattainable, thus precluding a semiquantitative estimation of protein expression. Despite this, there was full correlation between IHC and CISH in 587 cases. 13 cases were IHC + 3, but showed chromosome 17 polysomy with ratios <2.0. Two cases scored +3, but no changes in chromosome or gene copy number. For the same reason, false-positive and -negative results may have occurred for the other biomarkers. However, the distribution of subtypes is comparable to that of other studies [4, 5, 9]. All laboratory tests were carried out under standardized conditions and their interpretation together with complete revision of the histopathological diagnoses, type and grade was done within the context of this study according to present-day guidelines. By adding two markers to identify the basal phenotype to the set of markers in clinical use, it was possible to subdivide triple-negative cases into BP and the 5NP. In this study, these two subtypes had significantly differing BCSS. Molecular tests such as GGI are promising in terms of clinical benefit, but so far the documented benefit is complementary to histopathological methods [32]. Similarly, molecular subtyping using surrogate markers may provide important additional information for selected subgroups of breast cancer patients.
Acknowledgements
The study has received financial support from the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, The Research Council of Norway and the Cancer Fund, St. Olav’s Hospital, Trondheim University Hospital, Norway. The authors thank the Department of Pathology and Medical Genetics, St. Olav’s Hospital, for making the archives available for the study and the Cancer Registry of Norway for providing the patient data. Medical scientist Åse Kristin Skain Hansen and biomedical scientists Borgny Ytterhus, Nina Sandberg and Linda Anita Dyrnes have made invaluable contributions to the logistical and laboratory aspects of the study.
Conflict of interest
The authors declare that they have no competing interests.
Abbreviations
- BCSS
Breast cancer-specific survival
- BP
Basal phenotype
- CI
Confidence intervals
- CISH
Chromogenic in situ hybridization
- CK5
Cytokeratin 5
- EGFR
Epithelial growth factor receptor 1
- ER
Oestrogen receptor
- FFPE
Formalin-fixed, paraffin-embedded
- GGI
Gene expression grade index
- HER2
Human epidermal growth factor receptor 2
- HES
Haematoxylin–erythrosin–saffron
- HR
Hazard ratio
- IHC
Immunohistochemistry/immunohistochemical
- PR
Progesterone receptor
- 5NP
Five negative phenotype
- SI
Staining index
- TMA
Tissue microarray
Contributor Information
M. J. Engstrøm, Email: Monica.j.engstrom@ntnu.no
S. Opdahl, Email: signe.opdahl@ntnu.no
A. I. Hagen, Email: anne.irene.hagen@stolav.no
P. R. Romundstad, Email: pal.romundstad@ntnu.no
L. A. Akslen, Email: Lars.Akslen@k1.uib.no
O. A. Haugen, Email: olav.haugen@ntnu.no
L. J. Vatten, Email: lars.vatten@ntnu.no
A. M. Bofin, Email: anna.bofin@ntnu.no
References
- 1.Bartlett JM, Starczynski J, Atkey N, Kay E, O’Grady A, Gandy M, Ibrahim M, Jasani B, Ellis IO, Pinder SE, Walker RA. HER2 testing in the UK: recommendations for breast and gastric in situ hybridisation methods. J Clin Pathol. 2011;64:649–653. doi: 10.1136/jcp.2011.089847. [DOI] [PubMed] [Google Scholar]
- 2.Bhargava R, Beriwal S, McManus K, Dabbs DJ. CK5 is more sensitive than CK5/6 in identifying the “basal-like” phenotype of breast carcinoma. Am J Clin Pathol. 2008;130:724–730. doi: 10.1309/AJCP3KFF1LTYWQIY. [DOI] [PubMed] [Google Scholar]
- 3.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldarella A, Puliti D, Crocetti E, Bianchi S, Vezzosi V, Apicella P, Biancalani M, Giannini A, Urso C, Zolfanelli F, Paci E. Biological characteristics of interval cancers: a role for biomarkers in the breast cancer screening. J Cancer Res Clin Oncol. 2012 doi: 10.1007/s00432-012-1304-1. [DOI] [PubMed] [Google Scholar]
- 6.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury N. Histopathological and genomic grading provide complementary prognostic information in breast cancer: a study on publicly available datasets. Pathol Res Int. 2011;2011:890938. doi: 10.4061/2011/890938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawood S, Hu R, Homes MD, Collins LC, Schnitt SJ, Connolly J, Colditz GA, Tamimi RM. Defining breast cancer prognosis based on molecular phenotypes: results from a large cohort study. Breast Cancer Res Treat. 2011;126:185–192. doi: 10.1007/s10549-010-1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawson SJ, Duffy SW, Blows FM, Driver KE, Provenzano E, LeQuesne J, Greenberg DC, Pharoah P, Caldas C, Wishart GC. Molecular characteristics of screen-detected vs symptomatic breast cancers and their impact on survival. Br J Cancer. 2009;101:1338–1344. doi: 10.1038/sj.bjc.6605317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyarmin B, Kane JL, Valente AL, van Laar R, Gallagher C, Shriver CD, Ellsworth RE. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann Surg Oncol. 2013;20:87–93. doi: 10.1245/s10434-012-2588-8. [DOI] [PubMed] [Google Scholar]
- 11.Di Palma S, Collins N, Bilous M, Sapino A, Mottolese M, Kapranos N, Schmitt F, Isola J. A quality assurance exercise to evaluate the accuracy and reproducibility of chromogenic in situ hybridisation for HER2 analysis in breast cancer. J Clin Pathol. 2008;61:757–760. doi: 10.1136/jcp.2007.053850. [DOI] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 14.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, Trudel M, Akslen LA. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 15.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: Summary of the Consensus Discussion. Breast Care (Basel) 2011;6:136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiu S, Michiels S, Andre F, Cortes J, Denkert C, Di Leo A, Hennessy BT, Sorlie T, Sotiriou C, Turner N, Van de Vijver M, Viale G, Loi S, Reis-Filho JS. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 working group statement. Ann Oncol. 2012;23:2997–3006. doi: 10.1093/annonc/mds586. [DOI] [PubMed] [Google Scholar]
- 19.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi N, Niikura N, Yamauchi H, Nakamura S, Ueno NT. Adding hormonal therapy to chemotherapy and trastuzumab improves prognosis in patients with hormone receptor-positive and human epidermal growth factor receptor 2-positive primary breast cancer. Breast Cancer Res Treat. 2013;137:523–531. doi: 10.1007/s10549-012-2336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofvind S, Lee CI, Elmore JG. Stage-specific breast cancer incidence rates among participants and non-participants of a population-based mammographic screening program. Breast Cancer Res Treat. 2012;135:291–299. doi: 10.1007/s10549-012-2162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kvåle G, Heuch I, Eide G. A prospective study of reproductive factors and breast cancer. Am J Epidemiol. 1987;126:831–841. doi: 10.1093/oxfordjournals.aje.a114720. [DOI] [PubMed] [Google Scholar]
- 23.Lakhani SR, Ellis I, Schnitt SJ, Tan PH, Van de Vijver M, World Health Organization (eds) (2012) WHO Classification of Tumours of the Breast. International Agency for Research on Cancer (IARC), Lyon
- 24.Leong AS, Zhuang Z. The changing role of pathology in breast cancer diagnosis and treatment. Pathobiology. 2011;78:99–114. doi: 10.1159/000292644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK) Breast Cancer Res Treat. 2006;100:229–235. doi: 10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 26.Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Frequency of the basal-like phenotype in African breast cancer. APMIS. 2007;115:1391–1399. doi: 10.1111/j.1600-0463.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 27.Norton N, Perez EA. How relevant is hormone receptor status in the context of outcome to HER2-positive breast cancer? Breast Cancer Res. 2013;15:101. doi: 10.1186/bcr3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa Y, Moriya T, Kato Y, Oguma M, Ikeda K, Takashima T, Nakata B, Ishikawa T, Hirakawa K. Immunohistochemical assessment for estrogen receptor and progesterone receptor status in breast cancer: analysis for a cut-off point as the predictor for endocrine therapy. Breast Cancer. 2004;11:267–275. doi: 10.1007/BF02984548. [DOI] [PubMed] [Google Scholar]
- 29.Opdahl S, Alsaker MD, Janszky I, Romundstad PR, Vatten LJ. Joint effects of nulliparity and other breast cancer risk factors. Br J Cancer. 2011;105:731–736. doi: 10.1038/bjc.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 31.Purdie CA, Jordan LB, McCullough JB, Edwards SL, Cunningham J, Walsh M, Grant A, Pratt N, Thompson AM. HER2 assessment on core biopsy specimens using monoclonal antibody CB11 accurately determines HER2 status in breast carcinoma. Histopathology. 2010;56:702–707. doi: 10.1111/j.1365-2559.2010.03533.x. [DOI] [PubMed] [Google Scholar]
- 32.Rakha EA, Reis-Filho JS, Baehner F, Dabbs DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Richardson AL, Schnitt SJ, Schmitt FC, Tan PH, Tse GM, Badve S, Ellis IO. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12:207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robbins P, Pinder S, de Klerk N, Dawkins H, Harvey J, Sterrett G, Ellis I, Elston C. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 34.Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 35.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 36.Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, Nordgren H, Farmer P, Praz V, Haibe-Kains B, Desmedt C, Larsimont D, Cardoso F, Peterse H, Nuyten D, Buyse M, Van de Vijver MJ, Bergh J, Piccart M, Delorenzi M. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 38.Syed BM, Green AR, Paish EC, Soria D, Garibaldi J, Morgan L, Morgan DA, Ellis IO, Cheung KL. Biology of primary breast cancer in older women treated by surgery: with correlation with long-term clinical outcome and comparison with their younger counterparts. Br J Cancer. 2013;108:1042–1051. doi: 10.1038/bjc.2012.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szekely B, Iwamoto T, Szasz AM, Qi Y, Matsuoka J, Symmans WF, Tokes AM, Kulka J, Swanton C, Pusztai L. A 3-gene proliferation score (TOP-FOX-67) can re-classify histological grade-2. ER-positive breast cancers into low- and high-risk prognostic categories. Breast Cancer Res Treat. 2013 doi: 10.1007/s10549-013-2475-4. [DOI] [PubMed] [Google Scholar]
- 40.Tischkowitz M, Brunet JS, Begin LR, Huntsman DG, Cheang MC, Akslen LA, Nielsen TO, Foulkes WD. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaz-Luis I, Ottesen RA, Hughes ME, Marcom PK, Moy B, Rugo HS, Theriault RL, Wilson J, Niland JC, Weeks JC, Lin NU. Impact of hormone receptor status on patterns of recurrence and clinical outcomes among patients with human epidermal growth factor-2-positive breast cancer in the National Comprehensive Cancer Network: a prospective cohort study. Breast Cancer Res. 2012;14:R129. doi: 10.1186/bcr3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaz-Luis I, Winer EP, Lin NU. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann Oncol. 2013;24:283–291. doi: 10.1093/annonc/mds286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker RA, Bartlett JM, Dowsett M, Ellis IO, Hanby AM, Jasani B, Miller K, Pinder SE. HER2 testing in the UK: further update to recommendations. J Clin Pathol. 2008;61:818–824. doi: 10.1136/jcp.2007.054866. [DOI] [PubMed] [Google Scholar]
- 44.Warwick J, Tabar L, Vitak B, Duffy SW. Time-dependent effects on survival in breast carcinoma: results of 20 years of follow-up from the Swedish two-county study. Cancer. 2004;100:1331–1336. doi: 10.1002/cncr.20140. [DOI] [PubMed] [Google Scholar]