Abstract
Polymorphisms in the gene for the α5 nicotinic acetylcholine receptor (nAChR) subunit are associated with vulnerability to nicotine addiction. However, the underlying normal functions of α5-containing nAChRs in the brain are poorly understood. Striatal dopamine (DA) transmission is critical to the acquisition and maintenance of drug addiction and is modulated strongly by nicotine acting at heteromeric β2-containing (β2*) nAChRs. We explored whether α5 subunits, as well as α4, α6, and β3 subunits, participate in the powerful regulation of DA release probability by β2* nAChRs in nucleus accumbens (NAc) core and in dorsal striatum [caudatoputamen (CPu)]. We detected evoked dopamine release using fast-scan cyclic voltammetry at carbon-fiber microelectrodes in striatal slices from mice with deletions of α4, α5, α6, or β3 subunits. We show that the nAChR subtypes that dominantly regulate dopamine transmission depend critically upon α5 subunits in the dorsal CPu in α4α5(non-α6)β2-nAChRs but not in NAc core, where α4α6β2β3-nAChRs are required. These data reveal the distinct populations of nAChRs that govern DA transmission in NAc core versus dorsal CPu. Furthermore, they indicate that α5 subunits are critical to the regulation of DA transmission by α4β2* nAChRs in regions of striatum associated with habitual and instrumental responses (dorsal CPu) rather than pavlovian associations (NAc).
Introduction
There has been longstanding interest in the nicotinic acetylcholine receptor (nAChR) subtypes that participate in nicotine addiction. Receptors containing α4 and β2 subunits are well known to be involved in nicotine's cellular and reinforcing effects. For example, deletion of either α4 or β2 subunits prevents nicotine-induced changes in DA neuron excitability and nicotine self-administration in rodents (Picciotto et al., 1998; Maskos et al., 2005; Pons et al., 2008). Recently, genome-wide association studies have identified that polymorphisms in nAChR genes in gene clusters for other subunits, α5/α3/β4 and α6/β3, are associated with tobacco addiction (Thorgeirsson et al., 2008; Saccone et al., 2009; Bierut, 2010). Allelic variation and expression levels of α5 subunits have been particularly strongly associated, across independent studies (Saccone et al., 2009; Wang et al., 2009; Bierut, 2010). The α5 subunits, like β3 subunits, are accessory subunits that are known to modify the properties of α4- or α6-containing nAChRs respectively (Tumkosit et al., 2006; Grady et al., 2010; Kuryatov et al., 2011), but the roles of α5-containing (α5*) or β3* nAChRs in normal brain function and in nicotine action in situ are poorly understood.
Recently, α5* nAChRs in the habenulo-interpeduncular pathway have been shown to regulate nicotine intake (Fowler et al., 2011; Frahm et al., 2011). However, nicotine dependence also critically involves striatal dopamine (DA). Mesostriatal DA neurons express a diverse array of subunits (α3-7, β2-4), and it has been suggested that up to six possible types of β2* nAChRs are available in striatal DA axons for striatal ACh and nicotine to modulate DA transmission, namely, α4β2, α4α5β2, α4α6β2β3, α4α6β2, α6β2β3, and α6β2 (Picciotto et al., 1998; Champtiaux et al., 2003; Exley and Cragg, 2008; Gotti et al., 2010; Exley et al., 2011). Striatal β2* nAChRs respond to ACh released from striatal cholinergic interneurons to gate the dynamic probability of DA release and govern its sensitivity to the frequency of activity in DA axons (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004), which DA neurons change to signal information about reinforcers, or other salient stimuli and their cues (Schultz, 1998; Matsumoto and Hikosaka, 2009). It has been shown that the striatal nAChRs that regulate DA release probability differ in sensorimotor-associated striatum [caudatoputamen (CPu)] versus limbic-associated striatum [nucleus accumbens (NAc)], in particular that α4(non-α6) nAChRs are dominant in CPu but α4α6* nAChRs are dominant in NAc (Exley et al., 2008, 2011). Since the nAChR subunit stoichiometries identified in striatum suggest that α6* and α5* nAChRs are mutually exclusive populations, these data suggest in turn that any function for α5 subunits in regulating DA release might also covary with striatal regions and be precluded in NAc. Here, by using mice with deletions for α4, α5, α6, or β3 subunits we defined the subunit compositions of the α4/α6β2* nAChRs that dynamically gate DA release probability in NAc core and in CPu.
Materials and Methods
Slice preparation and voltammetry.
Coronal striatal slices (0.98–1.18 mm anterior to bregma) (Franklin and Paxinos, 2008), 300 μm thick, were prepared using described methods (Exley et al., 2008, 2011) from brains of adult male mice (C57BL/6J strain) of wild-type (Charles River), or α6−/−, α4−/−, α5−/− or β3−/− mice. Knock-out mice have been described previously (Marubio et al., 1999; Champtiaux et al., 2002; Cui et al., 2003; Salas et al., 2003). They have normal gross phenotypes and were backcrossed with a wild-type C57BL/6J line for a minimum of 10 generations (as recommended by the Banbury Conference on Genetic Background in Mice, 1997) to ensure similar genetic backgrounds.
Extracellular dopamine concentration ([DA]o) was monitored at 32°C using fast-scan cyclic voltammetry with 7–8 μm carbon-fiber microelectrodes (tip length ∼50–100 μm) and a Millar voltammeter (Julian Millar, Barts and London School of Medicine and Dentistry, London, UK) as described previously (Exley et al., 2008, 2011). In brief, the scanning voltage was a triangular waveform (−0.7 to +1.3 V vs Ag/AgCl) at a scan rate of 800 V/s, with a frequency of 8 Hz. Evoked electrochemical currents were attributable to DA by potentials for peak oxidation and reduction currents seen for applied DA (+500/600 and −250 mV respectively) (see Figs. 1b, 3b). Electrodes were calibrated post hoc with 2 μm DA in experimental media.
Figure 1.
α6, β3, and α4 subunits required for nAChR regulation of DA release probability in NAc core. a, Mean peak [DA]o ± SEM versus frequency (four pulses) in control (drug-free) conditions (circles), with α-CtxMII (diamonds) or with α-CtxMII plus DHβE (triangles), n = 9. Three-way ANOVA for drug, frequency, and genotype: significant interactions (p < 0.001). Drug effects within genotypes: *p < 0.05, **p < 0.01, ***p < 0.001 (vs control), two-way ANOVA with Bonferroni post hoc t tests. b, Typical evoked DA voltammograms (unscaled).
Figure 3.
Versatility in subunit sufficiency but critical role for α5 subunits in nAChR regulation of DA release in CPu. a, Mean peak [DA]o ± SEM versus frequency (4p) in control conditions (circles), with α-CtxMII (diamonds) or α-CtxMII with DHβE (triangles), n = 9. Three-way ANOVA for drug, frequency, and genotype: significant interactions (p < 0.001). Symbols indicate drug effects within genotypes: *p < 0.05, **p < 0.01, ***p < 0.001 (vs control); †††p < 0.001 (vs α-CtxMII), two-way ANOVA with Bonferroni post hoc t test. b, Typical evoked DA voltammograms (unscaled).
DA release was evoked by a local bipolar concentric electrode (25 μm diameter, Pt/Ir; FHC). Stimulus pulses (200 μs duration) were generated at the lowest current (0.5 mA) that generated maximal DA release with a single pulse. Release was Ca2+ dependent and TTX sensitive (data not shown), and was not modulated by glutamate or GABA antagonists (Threlfell et al., 2010).
Experimental design and analysis.
Stimuli were repeated at 2.5 min intervals to ensure consistent release. Stimuli were either single pulses (1p) or trains of four pulses (4p) at frequencies ranging from “tonic” (1–10 Hz) to “phasic” burst frequencies (≥15–40 Hz) of DA neurons that signal salient events in vivo (e.g., reward-predicting stimuli) (Schultz, 1986; Hyland et al., 2002; Bayer and Glimcher, 2005). The highest frequency (100 Hz) is particularly useful for probing changes in DA release probability (Rice and Cragg, 2004; Threlfell et al., 2010). CPu recording sites were located centrally in the dorsal quartile of CPu; those in NAc were ventral to the anterior commissure in the NAc core.
To assess the effects of frequency and drug, data were obtained from a population of sites where frequency and drug were varied at each site (see Figs. 1, 3). To assess the effects of nicotine (see Figs. 2, 4), a simplified protocol of two types of stimuli was used (single pulse or a four-pulse, 100 Hz train), and data were obtained from multiple recording locations each sampled only once. Data are means ± SEM, and the sample size, n, is the number of observations. The number of animals for each dataset is three or more. Effects of genotype, nAChR drugs, and/or region during stimuli of varying frequency were analyzed by three-way ANOVA and Holm–Sidak post hoc multiple-comparison t tests. The effects of each nAChR drug during stimuli of varying frequency in a given genotype were subsequently analyzed by two-way ANOVA and Bonferroni post hoc multiple-comparison t tests. Statistical analyses were performed using Sigmaplot11 and GraphPad Prism 4.
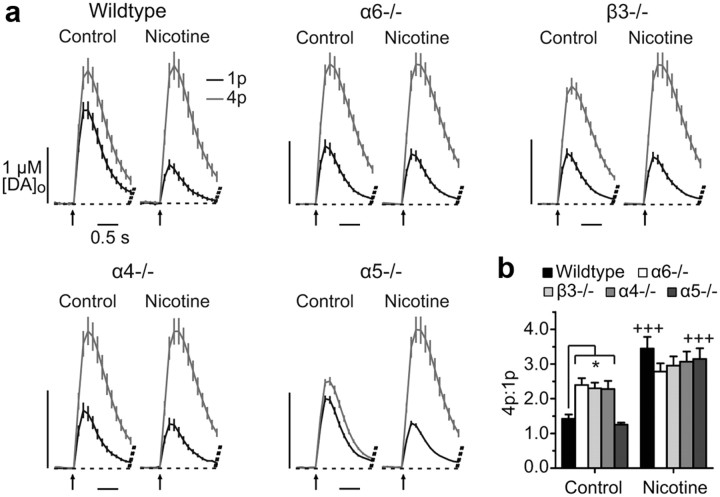
Figure 2.
Nicotine modulation of DA release probability lost after deletion of α6, β3, and α4 subunits. a, Mean profiles of [DA]o ± SEM evoked by 1p or 4p/100 Hz in NAc core in drug-free controls or nicotine (500 nm), n = 16–32. b, Ratios of [DA]o evoked by 4p/100 Hz versus 1p in controls and nicotine (500 nm). Enhanced 4p/1p release in α6−/−, β3−/−, and α4−/− versus wild type is not modified by nicotine. Two-way ANOVA with Bonferroni post hoc t tests.*p < 0.01 (vs wild type), +++p < 0.001 (nicotine vs control).
Figure 4.
Nicotine modulation of DA release probability in CPu after subunit deletion. a, Mean profiles of [DA]o ± SEM evoked by 1p or 4p/100 Hz in CPu. Nicotine-mediated changes in [DA]o were not affected by subunit deletion, n = 16–32. b, Ratios of [DA]o evoked by 4p/100 Hz versus 1p in control or nicotine (500 nm) where subunit deletion did not alter the 4p/1p release from wild type. Two-way ANOVA with Bonferroni post hoc t tests. +++p < 0.001 (nicotine vs control).
Drugs.
α-conotoxin MII (α-CtxMII) was synthesized as previously (Cartier et al., 1996) and used at concentrations which have no effects at non-α6/α3 nAChRs (Cartier et al., 1996) but maximally inhibit the α-CtxMII-sensitive component of striatal DA release (Exley et al., 2008, 2011). Deletion of α6 subunits eliminates α-CtxMII binding (Champtiaux et al., 2003) and, as expected, eliminated effects of α-CtxMII (30–100 nm) on evoked DA release in α6−/− mice (1p/4p stimuli, data not shown), and thus the effect of α-Ctx-MII was not explored further in α6−/− mice. Nicotine (tartrate) and dihydro-β-erythroidine (DHβE) were from Tocris Bioscience, and other reagents were from Sigma-Aldrich.
Results
We probed the identities of the striatal β2* nAChRs through which endogenous ACh regulates DA transmission in NAc core and in CPu. We assessed the effect of the deletion of subunits α4, α5, α6, or β3 on the regulation of DA release evoked by brief trains of a broad spectrum of frequencies (5–100 Hz). We combined this approach with the application of the α6-selective antagonist α-CtxMII and then the broad-spectrum β2-antagonist DHβE to explore the role of first α6* nAChRs and subsequently non-α6 nAChRs (which in striatum are α4* nAChRs). By thus combining individual subunit deletions with the effects of pharmacological antagonists, we can deduce the nAChRs subunits that are responsible for ACh control of DA release.
In NAc core, in wild-type mice in control (drug-free) conditions, peak evoked extracellular DA concentration ([DA]o) was only weakly sensitive to frequency (Fig. 1a), following a bell-shaped curve, as reported previously (Exley et al., 2008, 2011). These observations are consistent with high initial DA release probability and accompanying short-term depression (Rice and Cragg, 2004; Zhang and Sulzer, 2004). In NAc core in wild-type mice as shown previously, α-CtxMII (30 nm) reduced [DA]o evoked by low frequencies (≤10 Hz) but promoted frequency sensitivity, which increased [DA]o evoked by high frequency (100 Hz) (Exley et al., 2008, 2011). Subsequent application of the broad-spectrum nAChR antagonist DHβE (1 μm) did not further modify release (Fig. 1a), consistent with the dominant role of α6β2*-nAChRs in NAc shown previously (Exley et al., 2008, 2011). In mice with deletions of α6, β3, or α4 subunits, peak evoked [DA]o values in NAc core were modified compared with wild type (three-way ANOVAs, genotype × frequency × drug treatment, pairwise interactions, p < 0.001). Release showed greater frequency sensitivity even in drug-free conditions (two-way ANOVA, frequency × genotype, p < 0.001). This was due to the significant loss of nAChR control of DA release: the effects of α-CtxMII and DHβE were markedly attenuated, causing only slight additional changes to [DA]o (Fig. 1a). In α5 nulls by contrast, the frequency sensitivity of DA release in drug-free conditions followed a bell-shaped curve unlike the other null mice. α-CtxMII strongly increased frequency sensitivity with bidirectional effects on evoked [DA]o, as seen in wild-type mice (Fig. 1a). These data suggest that the β2* nAChRs regulating DA release in NAc core depend strongly on α6, α4, and β3 subunits, but less so on α5 subunits, suggesting that the α4α6αβ2β3 nAChR in NAc core is the dominant nAChR. The minor effects of nAChR antagonists seen in α6-, β3-, or α4-null mice suggest only minor roles for any α4(non-α6), α6(nonβ3), or α6(non-α4) nAChRs.
We tested in NAc core whether the effects of nicotine on DA release showed similar subunit dependence, using a simplified protocol to compare the ratio of DA release by a single pulse versus 4p at 100 Hz, for which nAChR effects are most marked (Exley et al., 2008, 2011). Nicotine (500 nm) has been shown previously to desensitize β2* nAChRs with the same outcome as nAChR antagonism (Zhou et al., 2001; Rice and Cragg, 2004; Zhang and Sulzer, 2004): consistent with these data, nicotine in wild types reduced 1p release, which promoted the sensitivity of [DA]o to activity (4p/100 Hz vs 1p) (Fig. 2). However, in α6-, α4-, or β3-nulls, but not α5-nulls, the ratios of [DA]o evoked by 4p (100 Hz) versus 1p were elevated compared with wild-type mice in drug-free conditions (Fig. 2b, as seen in experiments for Fig. 1), and were not enhanced further by nicotine (500 nm). This ratio was enhanced by nicotine in α5-nulls (Fig. 2).
We explored the nAChR subunits responsible for ACh control of DA release in CPu. In CPu wild types, as in NAc, [DA]o was only weakly sensitive to frequency (Fig. 3a), following a bell-shaped curve, as reported previously (Exley et al., 2008). However, there were key differences to NAc core in effects of genotypes and nAChR drugs (three-way ANOVAs: region × frequency × genotype interactions, p < 0.001; region × frequency × drug treatment interactions in wild types, p < 0.01). In CPu, unlike in NAc, α4(non-α6)β2* nAChRs strongly govern DA release probability, and α6* nAChRs appear to have a minor role (Exley et al., 2008). As shown previously, in wild types, α-CtxMII (30 nm) had only small effect compared with DHβE (1 μm) on frequency sensitivity of release (Fig. 3a). Surprisingly however, subunit deletion had no significant effects on the net nAChR regulation of frequency sensitivity of DA release (two-way ANOVA, frequency × genotype, p > 0.05). In drug-free conditions across genotypes, frequency sensitivity remained limited and was significantly promoted by the broad-spectrum antagonist DHβE (Fig. 3a). However, the effects of α-CtxMII varied with genotype (pairwise post hoc t tests, p < 0.001), revealing roles for key individual subunits and substitution of subtypes that maintain net nAChR control after deletion. Deletion of α6 subunits eliminated α-CtxMII effects (data not shown) and indicated that non-α6 nAChRs (i.e., α4* nAChRs) are entirely sufficient to support nAChR control of DA. Deletion of β3 subunits, however, eliminated the modest α-CtxMII effects (Fig. 3a), indicating that the minor α6-dependent component seen in wild types results from α6β3(β2)* nAChRs. After deletion of either α4 or α5 subunits, the substantial remaining nAChR control was entirely α-CtxMII sensitive (Fig. 3a). These data indicate first that while α6 nAChRs do not normally play a major role, the α6β3(β2) nAChRs can be functional in CPu under appropriate conditions, substituting for the normally dominant α4(non-α6)β2* nAChRs. Second, they indicate that normally dominant α4β2* control depends critically on the availability of α5 subunits, presumably through the α4α5β2 nAChRs, which are known to be present (Gotti et al., 2010).
Subunit knockout in CPu had similar impact on the net effects of nicotine. In all knockouts, the ratios of [DA]o evoked by 4p (100 Hz) versus 1p were not different compared with wild types in drug-free conditions, and were significantly enhanced by nicotine (500 nm) (Fig. 4).
Discussion
We show that control of DA transmission by ACh and nicotine in striatum is dominated by mutually exclusive populations of nAChRs: α4α6β2β3 nAChRs in NAc core and α4α5β2 nAChRs in dorsal CPu. We show also a difference in the necessity versus sufficiency for α4, α5, α6, or β3 subunits in the nAChR regulation of DA release in CPu versus NAc core.
These data corroborate findings that α4α6β2* nAChRs are necessary for ACh and nicotine to regulate DA release probability in NAc (Exley et al., 2011), and reveal further that this control codepends on β3 subunits, through presumably α4α6β2β3 nAChRs. We find evidence for only minor roles for other subtypes after subunit deletions [i.e., the α4/α5(non-α6), α6(nonβ3), or α6(non-α4) nAChRs], but, given the α-CtxMII sensitivity of release under normal conditions, only the α6* nAChRs of these could be expected to contribute, if any. A role for α4α6β2β3 nAChRs in NAc core is supported by the presence of this receptor within striatum (Gotti et al., 2010) and by reductions in α-CtxMII-sensitive striatal DA release and nAChR binding after α4 or β3 subunit deletion (Champtiaux et al., 2002; Salminen et al., 2005, 2007). The dominance of this nAChR type is, however. slightly surprising given that it has been suggested to account for only 14% of nAChRs here (Gotti et al., 2010). This disparity emphasizes that it is difficult to infer the function of nAChR subtypes in regulating DA transmission from nAChR expression levels alone (Exley et al., 2008). It could be argued that the protocol used here, which drives DA release in slices during discrete, subsecond but synchronous activation of DA and ACh release, better demonstrates the function of particular nAChR subtypes over others. However, the same protocol in the adjacent striatal region, CPu, reveals a different subunit dependence.
In dorsal CPu, we show that, in contrast to NAc core, no single type of subunit (of α4-α6, β3) is necessary to support nAChR regulation of DA release by ACh/nicotine. Rather, alternative nAChR subtypes become sufficient. We show in particular after deletion of individual subunits, that either α4(non-α6) or α6(non-α4) nAChRs can operate and be sufficient, that α6 function requires β3, and that α4 function critically requires α5. Together, these data indicate that the nAChR subtypes that govern DA release probability in CPu are substitutable, unlike in NAc core, and that while α6(non-α4)β2β3-nAChRs can regulate DA release, DA in CPu is normally dominantly regulated by α4(non-α6)β2-nAChRs, which require an α5 subunit (i.e., α4α5β2 nAChRs).
Variation between CPu and NAc core in substitutability of alternative nAChR subtypes after individual subunit deletion, is likely to occur at the level of nAChR formation and/or functional coupling rather than through compensatory upregulation in expression levels of alternate subunits, because there are no reported changes in transcript levels of alternate subunits after deletion of individual subunits (Champtiaux et al., 2002, 2003; Cui et al., 2003; Salas et al., 2003; Grady et al., 2007). Variation in such mechanisms between CPu and NAc is not altogether surprising given that these axons derive from different parent DA neurons, in substantia nigra pars compacta (SNc) versus ventral tegmental area (VTA), respectively. However, the mechanisms that could differentiate receptor trafficking and functional coupling in CPu versus NAc have yet to be identified. We have, however, previously shown that after deletion of either α4 or α6 subunits, the lack of “rescue” of net nAChR control of DA release by other subunits in NAc is not due to ACh levels being below some threshold that is required for nAChR activation: inhibitors of ACh-esterase, which promote ACh levels, do not restore any nAChR control by remaining subunits (Exley et al., 2011).
In striatum, the α5 subunit has been shown to form a receptor with α4 and β2 subunits of similar properties to the “high sensitivity” α4β2 nAChR (Grady et al., 2010). It would not have been predicted, however, that the α5α4β2 nAChR accounts for the entire population of the α-CtxMII-resistant regulation of DA release in CPu, as shown here: coimmunoprecipitation studies suggest that ∼40% of non-α6 (i.e., α4) nAChRs do not contain α5 (Gotti et al., 2010).
In conclusion, we have identified that discrete nAChR populations govern the regulation of DA release probability in NAc core versus CPu. We reveal that α5 subunits are essential to α4* nAChR function in CPu but not in NAc core, regions respectively associated with instrumental and habitual behaviors versus pavlovian associations. The function of α5* nAChRs in regulating activity in DA neurons within VTA or SNc is not yet resolved, but the functions of nAChRs we show at axon terminals suggest that modifications to α5 function might modify the risk of nicotine dependence through a dysregulation of behaviors encoded at the level of dorsal striatum.
Footnotes
This work was supported by Medical Research Council UK Grant G0700932, Parkinson's UK Grant G1103, and NIH National Institute on Drug Abuse Grant P30 DA015663.
References
- Banbury Conference on Genetic Background in Mice. Mutant mice and neuroscience: recommendations concerning genetic background. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 2005;47:129–141. doi: 10.1016/j.neuron.2005.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24-25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C, Clementi F, Moretti M, Rossi FM, Le Novère N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–2166. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux JP, Maskos U, Cragg SJ, Faure P. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibañez-Tallon I. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Ed 3. New York: Elsevier; 2008. [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci. 2010;40:91–95. doi: 10.1007/s12031-009-9263-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloëz-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol. 2003;63:1059–1066. doi: 10.1124/mol.63.5.1059. [DOI] [PubMed] [Google Scholar]
- Salminen O, Whiteaker P, Grady SR, Collins AC, McIntosh JM, Marks MJ. The subunit composition and pharmacology of alpha-conotoxin MII-binding nicotinic acetylcholine receptors studied by a novel membrane-binding assay. Neuropharmacology. 2005;48:696–705. doi: 10.1016/j.neuropharm.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of {alpha}-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Schultz W. Responses of midbrain dopamine neurons to behavioral trigger stimuli in the monkey. J Neurophysiol. 1986;56:1439–1461. doi: 10.1152/jn.1986.56.5.1439. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, Stacey SN, Bergthorsson JT, Thorlacius S, Gudmondsson J, Johsson T, Jakobsdottir M, Saemundsdottir J, Olafsdottir O, Gudmundsson LJ, Bjornsdottir G, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumkosit P, Kuryatov A, Luo J, Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol Pharmacol. 2006;70:1358–1368. doi: 10.1124/mol.106.027326. [DOI] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, et al. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]