Abstract
Human immunodeficiency virus type 1 (HIV-1) infection occurs in the central nervous system and causes a variety of neurobehavioral and neuropathological disorders. Both microglia, the residential macrophages in the brain, and astrocytes are susceptible to HIV-1 infection. Unlike microglia that express and utilize CD4 and chemokine coreceptors CCR5 and CCR3 for HIV-1 infection, astrocytes fail to express CD4. Astrocytes express several chemokine coreceptors; however, the involvement of these receptors in astrocyte HIV-1 infection appears to be insignificant. In the present study using an expression cloning strategy, the cDNA for the human mannose receptor (hMR) was found to be essential for CD4-independent HIV-1 infectivity. Ectopic expression of functional hMR rendered U87.MG astrocytic cells susceptible to HIV-1 infection, whereas anti-hMR serum and hMR-specific siRNA blocked HIV-1 infection in human primary astrocytes. In agreement with these findings, hMR bound to HIV-1 virions via the abundant and highly mannosylated sugar moieties of HIV-1 envelope glycoprotein gp120 in a Ca2+-dependent fashion. Moreover, hMR-mediated HIV-1 infection was dependent upon endocytic trafficking as assessed by transmission electron microscopy, as well as inhibition of viral entry by endosomo- and lysosomotropic drugs. Taken together, these results demonstrate the direct involvement of hMR in HIV-1 infection of astrocytes and suggest that HIV-1 interaction with hMR plays an important role in HIV-1 neuropathogenesis.
Astrocytes, often identified by glial fibrillary acidic protein expression, constitute a majority of the cells in the brain and are essential for maintaining homeostasis in the brain and, hence, normal brain activities. A number of different and quite diverse functions have been attributed to astrocytes. These include secretion of neurotrophic factors, regulation of the interstitial pH, uptake and metabolism of neurotransmitters, antioxidant defense via scavenging and transforming oxygen free radicals into nontoxic species, modulation of neuronal signals, being an essential structural component of the blood-brain barrier, and participating in immune responses through production and secretion of cytokines, proteases, protease inhibitors, adhesion molecules, and extracellular matrix components that are key mediators of immunity and inflammation (for recent reviews, see references 6, 13, and 58). Although it is important to note that several of the functions listed above are still controversial, the highly dynamic and reciprocal relationship between astrocytes and neurons suggests that dysfunction of astrocytes could contribute to the pathogenesis of neurological diseases.
Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) occurs in a majority of patients with AIDS and causes a variety of neurological dysfunctions, such as memory loss and motor control deficits (60). Microglia and/or macrophages are the major target cells for HIV-1 infection in the CNS (39). However, HIV-1 infection of astrocytes has also been well documented in pediatric patients and, to a lesser extent, in adult patients, as well as in in vitro cell cultures (63, 76, 81). The unique features of HIV-1 infection of astrocytes, i.e., CD4-independent viral entry and nonproductive viral replication (8, 27, 70), have made astrocytes an excellent model for studying molecular mechanisms of CD4-independent HIV-1 entry and regulation of HIV-1 replication. Moreover, the absolute large number of astrocytes in the brain and their extremely important roles in this organ strongly support the notion that HIV infection of astrocytes contributes to HIV-associated neuropathogenesis.
Much progress has been made in terms of the mechanisms of nonproductive HIV-1 replication in astrocytes. Evidence has accumulated that the inability of astrocytes to sustain HIV-1 gene expression is a combined result of entry and postentry restrictions in the viral life cycle (for a review, see reference 7). One of the restrictions is inadequate Rev function (34, 45). Recent studies have shown that the block in Rev function results from a lower level of constitutive expression of Sam68 protein in astrocytes (43), a molecule essential for Rev function (42). However, the other unique feature of HIV-1 infection of astrocytes, i.e., CD4-independent viral entry, still remains undefined. Unlike microglia that express CD4 and chemokine coreceptors CCR5 and CCR3 for HIV infection (28), astrocytes do not have a detectable level of CD4 receptor expression, and HIV-1 infection of the non-CD4-bearing astrocytes is not blocked by anti-CD4 monoclonal antibodies or soluble CD4 (27, 78). A number of reports have demonstrated the expression of chemokine receptors in astrocytes. CCR1, CXCR2, and CXCR4 have been detected on both murine and human astrocytes, and CXCR4 expression can be significantly upregulated in response to interleukin-1β (73, 80). The HIV-1 coreceptor CCR5 has recently been shown to be expressed in astrocytes in the hippocampus and cerebellum (68). Although CCR5 and CXCR4 have been shown to be utilized for simian immunodeficiency virus and HIV-2 viral entry in the absence of CD4 expression (17, 32), the significance of those chemokine receptors in HIV-1 infection of human astrocytes seems to be, at most, minimal (69). In the present study, we demonstrate that the human mannose receptor (hMR) serves as the HIV-1 receptor for CD4-independent infection of astrocytes. The identification of hMR as a CD4-independent HIV-1 receptor in astrocytes may provide new clues for developing therapeutics targeted at the HIV-brain interaction and the potential HIV-1 reservoir in the brain, as well as for understanding mechanisms of other CD4-independent HIV-1 infection.
MATERIALS AND METHODS
Cells and transfections.
U87.MG, U138.MG, U373.MG, NIH 3T3, ampho-phoenix, and 293T cells were purchased from the American Tissue Culture Collection (Manassas, Va.). U87.CD4.CXCR4 cells and U87.CD4.CCR5 cells were obtained from the NIH AIDS Reagents Program, donated by D. Littman. All of the cells listed were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum at 37°C with 5% CO2. U87.MR cell lines that stably express hMR were obtained by transfection of a hMR expression cassette pcDNA3.hMR (see below) in U87.MG cells, followed by G418 selection (200 μg/ml). Several U87.MR cell lines were obtained, and U87.MR clone 2-8 was used throughout the studies unless stated otherwise. Normal human astrocytes were obtained from Clonetics (Walkersville, Md.) or prepared as described previously (46). Immunofluorescence staining was performed for each lot to assure a purity of ≥98% glial acidic fibrillary protein (GFAP)-positive cells, i.e., astrocytes. Human peripheral blood mononuclear cells (PBMCs) were isolated and cultured as previously described (42). Cell transfections were performed by the standard calcium phosphate precipitation method.
Plasmids.
HIVΔenv.GFP, HIVΔenv.Luc, HXB2.env, YU-2.env, and vesicular stomatitis virus protein G (VSV-G) expression plasmids were described elsewhere (28). To obtain the full-length and functional hMR cDNA, human fetal brain Marathon-Ready cDNA (Clontech, Palo Alto, Calif.) was used as the template and amplified by using a High-Fidelity Advantage cDNA PCR kit (Clontech) and primers 5′-AGGTACCATGAGGCTACCCCTGCTCCTGGTT-3′ (the KpnI site is underlined) and 5′-ATTTGCGGCCGCCTAGATGACCGAGTGTTCATTCTG-3′ (the NotI site is underlined) with a PCR program of one cycle of 94°C for 30 s, 30 cycles of 94°C for 5 s and 68°C for 4 min, and one cycle of 68°C for 7 min. The primers were designed according to the published sequence (18). Amplified DNA was then gel purified, digested with KpnI and NotI restriction enzymes, and ligated into pcDNA3 (Invitrogen, Carlsbad, Calif.) linearized by these same enzymes. The resulting plasmid was designated pcDNA3.hMR (see the text for details). For hMR small interfering RNA (siRNA) retroviral expression vector, the oligonucleotides 5′-GCAGCTTGCAACCAGGATGCCTATAGTGAGTCGTATTAC-3′ and 5′-TCGGCATCCTGGTTGCAAGCCTATAGTGAGTCGTATTAC-3′, which targets the 5′ cDNA of hMR, were synthesized, annealed, and cloned into the pMSCV-puro backbone as previously described (23).
Double immunofluorescence staining.
For paraffin-embedded brain sections, the two-step parallel double immunofluorescence staining method was used. Briefly, after deparaffinization, hydration, and antigen retrieval (33), the sections were blocked in phosphate-buffered saline (PBS) containing 2% normal serum and 1% bovine serum albumin (BSA) for 30 min at room temperature, followed by incubation with the mixture of mouse anti-human GFAP (1:100) and goat anti-hMR serum (1:50) diluted in blocking solution for 1 h at room temperature, followed by the mixture of the fluorescein isothiocyanate (FITC)-conjugated donkey anti-goat (1:200) and phycoerythrin-conjugated rabbit anti-mouse (1:100) secondary antibodies in PBS for 30 min at room temperature. All incubations were carried out under humidified conditions, and slides were washed three times between steps with PBS. Finally, sections were counterstained in PBS containing 3 nM DAPI (4′,6′-diamidino-2-phenylindole), mounted with antifade aqueous mounting medium, and observed by using a Zeiss LSM-510 confocal microscope with a ×40 objective lens and appropriate filters. Omission of the primary antibodies in parallel staining was included as a control, and no nonspecific staining was noted. For cultured cells, a similar staining protocol was used, except for that a 30-min fixation in 4% paraformaldehyde and a 15-min permeabilization in 0.2% Tween 20 (to allow intracellular staining for GFAP) were added to substitute for the initial deparaffinization, hydration, and antigen retrieval steps. These cells from the double immunofluorescence staining were analyzed by fluorescence-activated cell sorting (FACS) or observed by using the confocal microscope.
Human fetal brain expression cDNA library and expression cloning.
A human fetal brain cDNA library was constructed in the backbone of a retroviral vector pMX provided by T. Kitamura (56). Total RNA was purchased from Life Technologies, Inc. (Gaithersburg, Md.) and used for cDNA synthesis. EcoRI linkers were ligated onto both ends of the cDNA for preparation of the cDNA library. The cDNA library was determined to contain cDNA inserts of an average size of 1.8 kb and more than 2 × 106 independent clones with more than 95% harboring cDNA inserts. High-titer retroviruses (more than 3 × 106 PFU/ml) of the cDNA library were produced by using a transient retrovirus packaging system (57) and used to transduce U87.MG cells. Transduced cells were challenged with the HIV-green fluorescence protein (GFP) reporter viruses (see below) containing the YU-2 envelope and then enriched for GFP-positive and CD4-negative cells by immunofluorescence staining for CD4 expression and FACS. Genomic DNA from GFP-positive and CD4-negative cells was extracted by using a genomic isolation kit (Promega, Madison, Wis.). cDNA inserts were PCR amplified from genomic DNA and directly sequenced. To ascertain the production of a high titer of retroviruses and higher transduction efficiency, both of which are very critical for representative expression of all cDNAs in the retroviral cDNA library, we also constructed and included pMX.GFP in the experiments to optimize the cloning protocol.
Preparation of cell lysates and Western blot analysis.
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and then harvested with a cell harvester. Cell pellets were suspended in 2 volumes of whole-cell lysis buffer (10 mM NaHPO4, 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 0.2% sodium azide, 0.5% sodium deoxycholate, 0.004% sodium fluoride, 1 mM sodium orthovanadate), followed by incubation on ice for 10 min. Whole-cell lysates were obtained by centrifugation and removal of the cell debris. Cell lysates (25 μg of protein) were electrophoretically separated on SDS-8% polyacrylamide gel electrophoresis (PAGE) and analyzed by immunoblotting. The blots were first probed with goat anti-hMR serum (1:400) and then with a horseradish peroxidase-conjugated donkey anti-goat secondary antibody, and visualized with the ECL system (Amersham Biosciences Corp., Piscataway, N.J.).
mBSA uptake assay.
The functionality of hMR expressed in U87.MR cells was assessed by hMR-mediated endocytosis assay using FITC-labeled mannosylated BSA (FITC-mBSA; E. Y. Laboratories, La Jolla, Calif.), a specific hMR ligand (30). Briefly, cells were grown in 24-well plates. Before the assay, cells were washed twice with DMEM without serum; added with 200 μl of DMEM containing 10% BSA, 5 mM CaCl2, and FITC-mBSA at concentrations as indicated; and incubated at 37°C for 30 min to allow internalization of FITC-mBSA. After 30 min, unbound FITC-mBSA was removed by extensive washing with ice-cold DMEM containing 0.5% BSA, followed by PBS, and then fixed with 4% paraformaldehyde. Internalized FITC-mBSA was assessed by FACS analysis. In ligand inhibition experiments, competitor ligands were added at the initial FITC-mBSA ligand-binding step and maintained throughout internalization.
Preparation and infection of pseudotyped HIV-1 reporter viruses.
HIV-1 viruses pseudotyped with different envelope proteins were prepared as previously described (28). Briefly, 293T cells (2 × 106 cells per 10-cm plate) were transfected with 20 μg of HIV-1 reporter plasmids (HIVΔenv.GFP or HIVΔenv.Luc) and 4 μg of HXB2.env, YU-2.env, or VSV-G expression plasmid by the calcium phosphate precipitation method. Cell culture supernatants were collected 48 h after change of the transfection medium, filtered, and saved as virus stock. For infection, U87.MG and its derivatives were plated at a density of 5 × 104 cells/well in a 24-well plate and allowed to grow for at least 24 h. The cells were infected with 100 ng of gagp24 HIV-GFP or HIV-Luc reporter viruses in the presence of 8 μg of Polybrene/ml. After 2 h, the viruses were removed, and the cells were extensively washed and replaced with fresh medium. The cells were allowed to grow for 48 h before they were harvested and assayed for HIV-1 viral entry by FACS for GFP expression or by luciferase assay for the luciferase gene expression (28).
p24 enzyme-linked immunosorbent assay (ELISA) of HIV-1 replication in primary human astrocytes and PBMCs.
Human primary astrocytes (5 × 105 cells/well in a six-well plate) and PBMCs (5 × 105 cells/well in a six-well plate) were infected with 100 ng of gagp24 recombinant HIV-1 NL4-3 viruses for 2 h in the presence of 8 μg of Polybrene/ml. After 2 h, the viruses were removed, and the cells were extensively washed and then placed in cell culture medium. Cell culture supernatants were collected at different times as indicated and assayed for HIV-1 replication by using a p24 ELISA kit (Perkin-Elmer, Boston, Mass.) that has a lower limit of detection of 4.3 pg/ml. In the antibody blocking experiments, cells were treated with Leu3A or its immunoglobulin G (IgG) isotype control (20 μg/ml; Becton Dickinson, Paramus, N.J.) or with goat anti-human MR serum or preimmune serum (1:50) at 37°C for 30 min before infection and throughout the infection. Recombinant CD4 protein (10 μg/ml; NIH AIDS Reagents Program [donated by N. Schuelke]) was incubated with viruses at room temperature for 1 h before infection.
Binding assay of HIV-1 virions and recombinant envelope glycoprotein gp120 to human primary astrocytes and hMR.
Human primary astrocytes, U87.MG cells, or U87.MR cells were grown on a six-well plate and incubated with 100 ng of gagp24 HIV-GFP viruses on ice for 2 h in the absence or presence of anti-MR blocking antibodies or MR ligand inhibitors as indicated. After 2 h of incubation, the viruses were removed, and the cells were thoroughly washed with the culture medium, harvested, lysed, and assayed for HIV p24 antigen by ELISA (Perkin-Elmer). For gp120 binding, recombinant gp120 and its nonglycosylated derivative from HIV-1 SF2 isolate (obtained from the NIH AIDS Reagents Program [donated by Chiron Corp.]) were iodinated with Na125I (Amersham Bioscience, Inc.) by using Pierce's Iodo-Beads according to the protocol provided. Free Na125I was removed with a PD10 column (Amersham Biosciences, Inc.). The specific activity of protein iodination was typically between 2,200 and 2,500 cpm/ng of protein. U87.MR cells were plated in a 24-well plate at a density of 2 × 105 cells/well and allowed to grow overnight. 125I-labeled proteins were added in a volume of 250 μl of ligand-binding buffer (DMEM containing 5 mM CaCl2 and 0.5 mM MgCl2), followed by incubation on ice for 2 h. After the removal of unbound 125I-labeled protein through repeated washes with PBS, cells were harvested and assayed for protein binding by using a gamma counter. For Ca2+ depletion, cells were incubated with 125I-labeled proteins in the ligand-binding buffer containing no CaCl2 but 20 mM EGTA.
Transmission electron microscopy.
U87.MR cells and human primary astrocytes were grown on Thermanox coverslips (Nalge Nunc International, Rochester, N.Y.) in 10-cm culture dishes. After 1 day in culture, the plates were removed from the incubator and chilled on ice for 30 min. After 30 min, HIV viruses were added and incubated on ice for an additional 30 min. The culture plates were then placed back in a 37°C incubator, and samples on coverslips were taken at 0, 5, 10, 30 and 60 min. The coverslips were rinsed in PBS and fixed in 0.1 M phosphate buffer containing 2% paraformaldehyde and 2% glutaraldehyde for at least 1 h. The coverslips were rinsed again in PBS and then postfixed in 0.1 M phosphate buffer containing 1% osmium tetroxide for 1 h. After a brief rinse in PBS, the cells were dehydrated by using a graded series of ethanol (ETOH) solutions: 70% ETOH for 10 min, 95% ETOH for 10 min, and 100% ETOH three times for 5 min for each step. The cells were infiltrated by using the Embed 812 resin (Electron Microscopy Sciences, Fort Washington, Pa.) in 100% ETOH overnight. After infiltration in pure resin for 1 h, the coverslips were cut to appropriate sizes and embedded in flat embedding molds by using fresh resin. The resin was allowed to polymerize overnight at 60°C in the oven. The blocks were trimmed and first cut into 1-mm thick sections with glass knives and then into 90-nm thin sections with a diamond knife. Sections were collected onto copper grids and stained with uranyl acetate and lead citrate prior to being viewed with a Phillips EM-400 transmission electron microscope.
RESULTS
CD4-, CCR5-, and CXCR4-independent HIV-1 entry into astrocytes.
Consistent with studies from other groups (8, 27, 77), we did not detect CD4 expression either inside or on the surface of primary human fetal astrocytes (data not shown). To ascertain that HIV-1 infection of astrocytes occurs via a CD4-independent pathway, we performed the antibody-blocking experiments. Human primary astrocytes were preincubated with Leu3A, a CD4 neutralizing antibody prior to infection by the YU-2 envelope protein-pseudotyped HIV-Luc reporter viruses. Astrocyte infection was not blocked by anti-Leu3A antibody (Fig. 1A), whereas this antibody successfully blocked infection of U87.CD4.CCR5 cells (Fig. 1B). We then tested the possibility that HIV-1 used galactosyl ceramide (GalCer) (26, 50) or major chemokine receptors CCR5 and CXCR4 as primary receptors for astrocyte infection. Antibodies to GalCer or CCR5, as well as macrophage inflammatory protein 1β (MIP-1β) that binds to CCR5, failed to block HIV-1 infection of astrocytes (Fig. 1A). Similarly, we also tested whether CXCR4 was involved in entry of HIV-1 Luc reporter viruses pseudotyped with HXB2 envelope protein into astrocytes with anti-CXCR4 neutralizing antibody and SDF-1. Both treatments showed little effect on the viral entry (data not shown). Failure to block HIV-1 infection of astrocytes with neutralizing antibodies specific for CD4, GalCer, CCR5, and CXCR4 and with chemokines specific for CCR5 and CXCR4 suggests that HIV-1 infection of astrocytes is mediated through alternative mechanism(s).
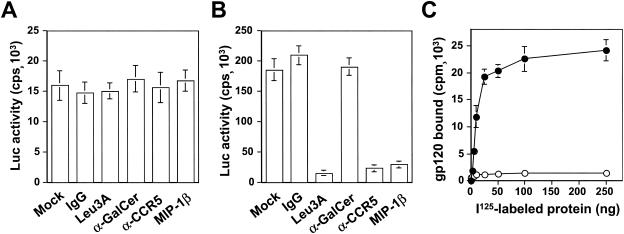
FIG. 1.
Interaction of HIV-1 with astrocytes. Human primary astrocytes (A) and U87.CD4.CCR5 cells (B) were plated in a six-well plate at a density of 5 × 105 cells/well 1 day before infection. On the day of infection, the cells were incubated with anti-CD4 Leu3A antibody (20 μg/ml), anti-GalCer antibody (20 μg/ml), anti-CCR5 antibody (20 μg/ml), or 500 ng of MIP-1β/ml for 3 h. The cells were then infected with 100 ng of gagp24 HIV-Luc reporter viruses pseudotyped with YU-2 envelope protein and allowed to grow for 48 h before harvest for the Luc activity assay. Compared to human primary astrocytes, only one-tenth of the lysates from U87.CD4.CCR5 cells was used in the Luc assay. (C) For HIV-1 gp120 binding, human primary astrocytes were incubated with 125I-labeled gp120 protein (•) or 125I-labeled BSA (○) at the indicated concentrations on ice for 30 min. Unbound proteins were removed, followed by extensive washes with prechilled regular cell culture medium. The cells were then harvested and processed for measuring gp120 binding by using a gamma scintillation counter
Two possible alternatives exist by which HIV-1 infection of astrocytes is likely to occur. One is nonspecific adsorption of virion particles by astrocytes leading to CD4-independent entry of HIV-1 into astrocytes. The other is via a specific receptor(s) that is expressed on the surface of astrocytes, the identity of which remains unknown. To distinguish between these two possibilities, we characterized HIV-1 envelope glycoprotein gp120 binding to human primary astrocytes. The rationale was that specific interaction of gp120 with cell surface receptor(s) would be expected for the receptor-mediated HIV-1 entry but not for nonspecific absorption-mediated uptake of virion particles. Thus, we labeled gp120 protein with Na125I, incubated 125I-labeled gp120 with human primary astrocytes at 4°C, and determined the binding kinetics for gp120 protein on these cells. 125I-labeled BSA was included as a control. The results showed saturable binding of gp120 to human primary astrocytes with a calculated Kd of 43 nM (Fig. 1C), suggesting that there were specific sites (non-CD4 receptors) for gp120 binding. As expected, there was little BSA binding to these cells.
Identification of hMR as a receptor for HIV-1.
To isolate the putative HIV-1 receptors from astrocytes, we adopted and modified a highly efficient retrovirus cDNA library expression cloning approach that has been successfully used to isolate cDNA with a specific phenotypic function, including receptors for simian immunodeficiency viruses and HIV-1 (15). We constructed a human fetal brain cDNA library in a retroviral vector pMX (56) and expressed the human fetal brain cDNA library within the CD4-negative astroglial cell line U87.MG, which we showed to be refractory to HIV-1 infection (data not shown). Human fetal brain cDNA library-expressing U87.MG cells were then challenged with HIV-GFP reporter viruses pseudotyped with YU-2 envelope protein. We then used FACS to enrich GFP-positive and CD4-negative cells, which were immediately subcloned and expanded. Initially, we obtained 21 cell clones. Subsequent infection showed that only five of these clones were reproducibly infectible by HIV-1. Isolation of cDNA from these five clones showed a common cDNA insert ca. 2.7 kb in length, and sequence analysis further revealed that the cDNA was identical to the open reading frame of the hMR at the 3′ terminus, which contained the entire 3′-untranslated region (711 bp), the cytoplasmic domain (132 bp), the transmembrane domain (81 bp), and the extracellular domain (∼1.8 kb).
hMR was initially cloned in 1990 from macrophages (18) and placenta (74). However, we encountered a great deal of difficulty in cloning the full-length and functional hMR and propagating a mammalian hMR expression vector (generously provided by A. Ezkorwitz). The difficulty was found to be due to multiple sequence repeats present in the hMR cDNA and inadvertent recombination between the hMR cDNA and the genome of Escherichia coli used in the cDNA cloning and plasmid preparation (Y. Liu and J. He, unpublished results). To alleviate recombination of the hMR cDNA with the E. coli genome, various conditions, including different competent bacteria, bacterial culture temperatures, and incubation times were tested. The integrity of hMR cDNA was first screened by in vitro T7-coupled transcription-translation (Promega), electrophoresis of hMR protein on SDS-6% PAGE, and Western blot analysis with goat anti-hMR serum (generously provided by P. Stahl). The cDNA clones that showed a corresponding hMR molecular mass of 165 kDa on SDS-PAGE and immunoreactivity with anti-hMR antibody upon Western blot analysis were then subjected to direct sequencing in its entirety. The results showed that pcDNA3.hMR containing the full-length hMR cDNA can reproducibly be obtained by using STBL competent cells (Life Technologies, Inc., Gaithersburg, Md.) under the culture conditions of 30°C and 200 rpm for approximate 15 h. Cell surface expression and functionality of human MR were then validated by FACS with anti-hMR monoclonal antibody clone 19.2 (Pharmingen, San Diego, Calif.) and hMR-mediated endocytosis assay of FITC-conjugated mBSA (see below).
Expression of functional hMR in astrocytes and HIV-1 infection.
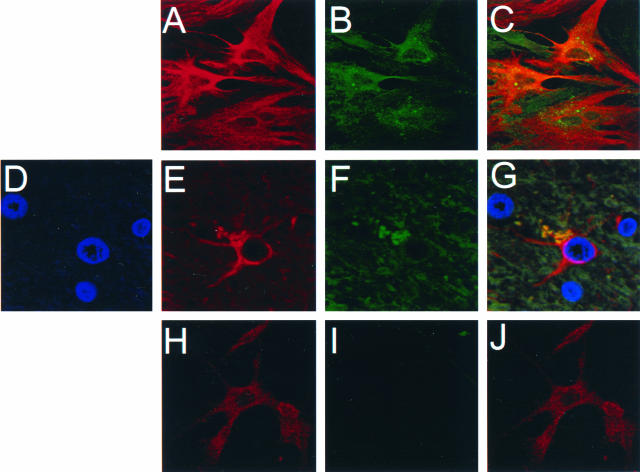
hMR mainly functions in molecular scavenging and host defense through hMR-mediated endocytosis and phagocytosis pathways (for a review, see reference 59). Our expression cloning results suggest a potential role of hMR in HIV-1 infection of astrocytes. Although MR has been shown to be expressed in murine astrocytes (10), no direct evidence is available as to whether hMR is expressed on human astrocytes. Thus, we first determined hMR expression in human primary astrocytes, which have been shown to be susceptible to HIV-1 infection (25, 29, 63, 76, 81). Double immunofluorescence staining showed that hMR was expressed in human primary astrocytes (Fig. 2A to C). To ascertain that the hMR expression noted in human primary astrocytes was not due to in vitro cell manipulation, double immunofluorescence staining was also performed in normal human brain tissues. hMR was detected on astrocytes in the brain, particularly in astrocyte-abundant regions, such as the cerebellum and cortex (Fig. 2D to G). However, little hMR expression was expressed in U87.MG cells (Fig. 2H to J).
FIG. 2.
Expression of hMR in human primary astrocytes and in astrocytes of human normal brain tissues. Human primary astrocytes (A to C), human normal brain tissues (D to G), and U87.MG cells (H to J) were stained with primary antibodies against astrocyte-specific GFAP and hMR, followed by appropriate secondary antibodies. Expression of hMR and GFAP was determined by confocal microscopy. (A, E and H) GFAP staining; (B, F, and I) MR staining; (D) DAPI counterstaining for nuclei; (C and J) colocalization of GFAP and MR staining; (G) colocalization of GFAP, MR, and nuclei in brain tissues. Parallel staining containing isotype-matched IgGs was also performed, and no nonspecific staining was observed.
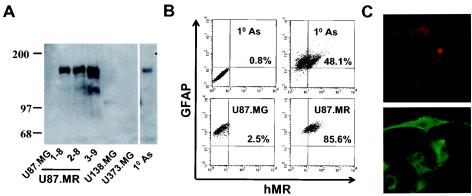
The apparent association of hMR expression with HIV-1 infectivity in human primary astrocytes prompted us to further examine the roles of hMR in HIV-1 infection. We determined whether hMR expression would make these cells susceptible to HIV-1 infection. To this end, we introduced the full-length hMR cDNA into U87.MG cells. Several cell clones, designated U87.MR, were generated and shown to stably express hMR at comparable levels and the expected molecular mass of 165 kDa (Fig. 3A). These clones were also shown to be relatively homogeneous in terms of hMR expression (Fig. 3B). In addition, hMR protein was detected in human primary astrocytes, but not in U87.MG, as well as in two other HIV-1 nonpermissive human astroglial cells U138.MG and U373.MG, by Western blotting (Fig, 3A). Double immunofluorescence staining showed that in human primary astrocytes more than 98% cells were GFAP positive; of these, ca. 50% were also hMR positive (Fig. 3B). To examine whether hMR stably expressed in U87.MR cells traffics to the cell surface, a characteristic consistent with a potential role in viral uptake, we also performed confocal microscopy on these cells. hMR expression was indeed localized on the plasma membrane of these cells (Fig. 3C).
FIG. 3.
Expression and functionality of hMR in U87.MR cells. (A) hMR expression by Western blot analysis. Cell lysates (20 μg of protein) from U87.MG cells, U87.MR clones 1-8, 2-8, and 3-9, U138.MG cells, U373.MG cells, and human primary astrocytes (10 As) were separated by electrophoresis on SDS-6% PAGE and blotted onto Hybon-P membrane. hMR expression was detected by using goat anti-hMR serum (1:400) as the primary antibody and horseradish peroxidase-conjugated donkey anti-goat secondary antibody (1:2,000). (B) hMR expression in human primary astrocytes as determined by FACS. Human primary astrocytes, as well as U87.MG and U87.MR (clone 2-8) cells, were double immunofluorescence stained and then analyzed by FACS. Parallel isotype-matched IgG staining was performed on all cells; no nonspecific staining was observed, and only isotype IgG staining on human primary astrocytes was shown as a representative (upper left quadrate in panel B). (C) hMR expression in U87.MR cells by confocal microscopy. Similar double immunofluorescence staining was performed on U87.MR cells, and then the cells were observed under a Zeiss confocal microscope. Top, GFAP staining; bottom, hMR staining.
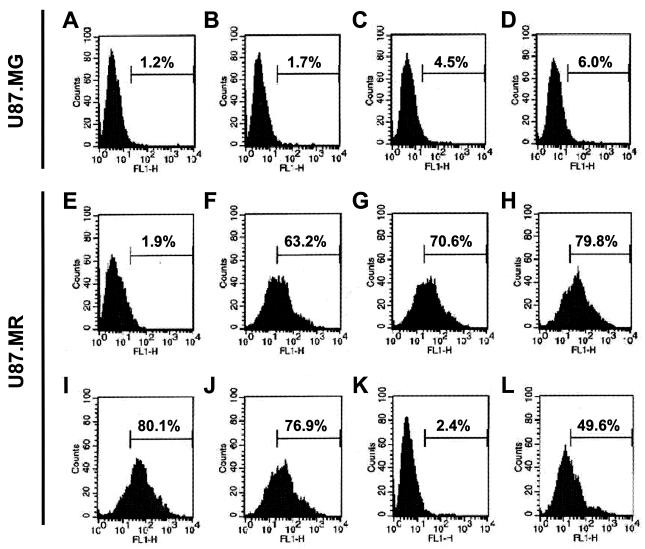
We next determined whether the hMR stably expressed in U87.MR cells is biologically functional. We performed a hMR-mediated internalization assay with FITC-mBSA, a well-characterized hMR ligand (31). FITC-mBSA was added to U87.MR cells, and uptake of FITC-mBSA was determined by FACS. Uptake of FITC-mBSA into U87.MR cells was 1.9, 63.2, 70.6, and 79.8% when FITC-mBSA was added at concentrations of 0, 0.5, 1, and 2 μg/ml, respectively, and no further increase of FITC-mBSA uptake was noted at 5 μg/ml (Fig. 4). In contrast, there was little FITC-mBSA uptake in U87.MG cells at all concentrations tested. To confirm the specificity of hMR-mediated uptake, U87.MR cells were preincubated with hMR ligand antagonists, including yeast mannan (1) and d-mannose (31), prior to the addition of FITC-mBSA. As expected, yeast mannan and d-mannose decreased FITC-mBSA uptake from 79.8% to 2.4 and 49.6%, respectively, whereas an unrelated disaccharide, d-galactose, had little effect (Fig. 4). Taken together, these results demonstrate that U87.MR cells express the full-length and biologically functional hMR.
FIG. 4.
Functionality of hMR in U87.MR cells. U87.MG cells (A to D) and U87.MR cells (E to L) were incubated at 37°C with FITC-mBSA at concentrations of 0 (A and E), 0.5 (B and F), 1 (C and G), 2 (D and H), and 5 (I to L) μg/ml in the absence of competitors (A to I) or in the presence of 3 mg of d-galactose/ml (J), 3 mg of yeast mannan/ml (K), or 3 mg of d-mannose/ml (L). Unbound FITC-mBSA was then removed from the cell culture medium by extensive washing with prechilled regular cell culture medium, and the cells were fixed in 4% paraformaldehyde. hMR function was determined by FACS analysis for the internalization of FITC-mBSA into the cells.
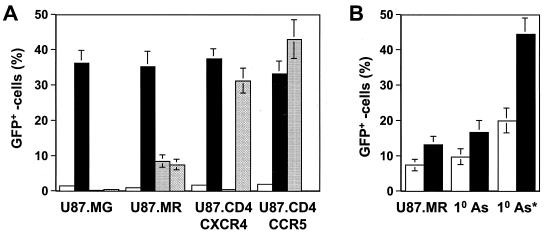
We then evaluated HIV-1 infection of U87.MR cells. U87.MR cells were infected with HIV-GFP reporter viruses pseudotyped without an envelope glycoprotein or with HIV-1 envelope protein YU-2, HXB2, or VSV-G, and viral entry was determined by FACS for GFP expression. We included U87.MG cells, U87.CD4.CCR5 cells, and U87.CD4.CXCR4 cells as controls in the experiments. HIV-GFP pseudotyped with YU-2 and HXB2 envelopes gave rise to 7.9 and 6.3% GFP positive, respectively, but few GFP-positive cells in U87.MG cells (Fig. 5A). As expected, HIV-GFP pseudotyped with VSV-G-infected all cells and at a comparable infection efficiency, whereas HIV-GFP pseudotyped with YU-2 envelope only infected U87.CD4.CCR5 cells, and HIV-GFP pseudotyped with HXB2 envelope only infected U87.CD4.CXCR4 cells. In addition, U87.MR cells infected with either YU-2 or HXB2 envelope-pseudotyped viruses exhibited a mean GFP fluorescence intensity at least 10-fold lower than that of VSV-G-pseudotyped infected cells, U87.CD4.CCR5 cells infected with YU-2-pseudotyped viruses, or U87.CD4.CXCR4 cells infected with HXB2-pseudotyped viruses.
FIG. 5.
hMR expression and HIV-1 infection. (A) HIV-1 infection of hMR-expressing U87.MR cells. U87.MG cells, U87.MR cells, U87.CD4.CXCR4 cells, and U87.CD4.CCR5 cells were infected with 100 ng of gagp24 HIV-GFP reporter viruses pseudotyped with VSV-G envelope protein (closed bar), YU-2 envelope protein (dotted bar), HXB2 envelope protein (hatched bar), or pcDNA3 control (no env, open bar) at 37°C for 2 h in the presence of 8 μg of Polybrene/ml. After 2 h, the viruses were removed, and the cells were washed with regular cell culture medium. The cells were allowed to grow for 48 h and then harvested for the HIV-1 infection assay and FACS analysis. HIV-1 infection was expressed as the percentage of GFP-positive cells. (B) Effects of virus input and hMR density on HIV-1 infection. Human primary astrocytes were labeled with FITC-mBSA, sorted out by FACS for the top 5% higher hMR-expressing cells, and infected with 100 ng (open bar) or 400 ng (closed bar) gagp24 HIV-GFP reporter viruses pseudotyped with HIV-1 YU-2 envelope protein. Presorted human primary astrocytes and U87.MR cells were also included. 10 As, human primary astrocytes; 10 As*, the top 5% higher hMR-expressing human primary astrocytes.
To determine whether the low infection efficiency was due to the low virus input, we increased the virus input to 400 ng of gagp24 in the infection. The higher virus input resulted in 14.3% infection of U87.MR cells (Fig. 5B), whereas 100 ng of gagp24 HIV-GFP viruses only infected 9.7% of human primary astrocytes; this level increased to 17.3% when 400 ng of gagp24 viruses was used. These results showed that the disparity between the percentage of hMR-positive cells and HIV-1 infection efficiency in both U87.MR cells and human primary astrocytes was not due to insufficient HIV-1 viral input. We then determined whether the number of hMR receptors on each cell, i.e., the receptor density, would affect HIV-1 infection efficiency. To this end, we took advantage of the heterogeneity of hMR expression in human primary astrocytes (Fig. 3B), labeled these cells with FITC-conjugated mannosylated BSA, a hMR ligand (Fig. 4), and sorted out by FACS the higher hMR-expressing cells (top 5%). We then infected these cells with HIV-GFP reporter viruses. Surprisingly, these higher hMR-expressing cells showed a greatly improved infection efficiency, i.e., 21% at the amount of 100 ng of gagp24 input viruses and 45% at the amount of 400 ng of gagp24 input viruses (Fig. 5B). Taken together, these results suggest that the density of hMR on the cells is critical for successful hMR-mediated HIV-1 entry.
Direct involvement of hMR in HIV-1 infection of human primary astrocytes.
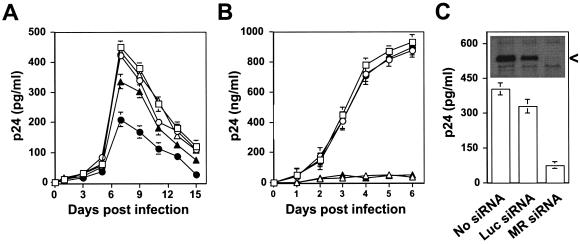
We next determined whether hMR plays a direct role in HIV-1 infection of human primary astrocytes. We took advantage of a goat anti-hMR serum that has been shown to react with hMR (Fig. 3A) and block hMR-mediated endocytic function (61) and examined the effects of this serum on HIV-1 infection of astrocytes. Primary human fetal astrocytes were preincubated with goat anti-hMR serum (1:50 dilution) prior to infection with HIV-1 NL4-3 viruses, and HIV-1 replication was monitored by an ELISA for p24 production in the cell culture supernatants. We also included human PBMCs and a neutralization monoclonal antibody to human CD4, Leu3A, in these experiments. Treatment with goat anti-hMR serum decreased maximal p24 production, i.e., HIV-1 viral replication, in primary astrocytes by approximately 60% compared to mock treatment (Fig. 6A). No apparent decrease in p24 production were detected in the supernatants of astrocytes treated with goat preimmune serum, anti-CD4 Leu3A antibody, or the IgG isotype control. In contrast, anti-CD4 Leu3A antibody completely blocked HIV-1 p24 production in PBMCs infected with NL4-3 viruses, whereas other treatments, such as goat anti-hMR serum, goat preimmune serum, and IgG isotype control, showed little effects (Fig. 6B). The lack of neutralization of HIV-1 infection of astrocytes with anti-CD4 Leu3A antibody was further tested by using soluble recombinant CD4 protein, which has been shown to block HIV-1 infection by binding to the HIV-1 envelope gp120 and thereby preventing HIV-1 virus from binding to the CD4 receptor of HIV-1. As expected, preincubation of recombinant CD4 protein with HIV-1 NL4-3 viruses exhibited complete inhibition of the HIV-1 p24 production in the supernatant of PBMCs (Fig. 6B) but, surprisingly, also exhibited 25% inhibition in astrocytes (Fig. 6A).
FIG. 6.
Neutralization of HIV-1 infection of human primary astrocytes by goat anti-human mannose receptor antibody. Human primary astrocytes (A) and PBMCs (B) were infected with 100 ng of gagp24 HIV-1 NL4-3 viruses at 37°C for 2 h in the presence of goat preimmune serum (1:50, ○), goat anti-hMR serum (1:50, •), anti-human CD4 monoclonal antibody Leu3A (20 μg/ml, ▵), isotype-matched IgG control antibody (10 μg/ml, ⋄), or recombinant CD4 protein (10 μg/ml, ▴). HIV-1 NL4-3 viruses were prepared by transfection of pNL4-3 plasmid DNA in 293T cells similarly to the envelope protein-pseudotyped HIV reporter viruses. All infections were performed in the presence of 8 μg of Polybrene/ml. Fresh antibodies were added every other day when the cell culture supernatants were collected. HIV-1 replication was monitored by measuring p24 production in the culture supernatants by using a p24 ELISA kit according to the manufacturer's instructions. (C) Inhibition of HIV-1 replication in human primary astrocytes by MR siRNA. Retroviruses carrying MR siRNA were prepared by transfection of pMSCV-puro MR siRNA DNA in ampho-phoenix cells and used to transduce human primary astrocytes. Transduced cells were then infected with HIV-1 NL4-3 viruses, as well as for hMR expression by Western blot analysis (insert). At day 7 after infection, the culture supernatant was collected for HIV-1 replication assay by a p24 ELISA kit. MSCV-puro viruses containing no siRNA DNA and Luc siRNA DNA targeting the luciferase gene were also included as controls. Puromycin gene expression was used to determine the virus titers and transduction efficiency, which were comparable. <, MR protein.
To further ascertain that hMR is directly involved in HIV-1 infection of astrocytes, we downmodulated the constitutive hMR expression in human primary astrocytes and determined its effects on HIV-1 infection of these cells. We utilized the retrovirus-mediated siRNA technology to silence the constitutive hMR expression in human primary astrocytes. hMR siRNA expression efficiently downmodulated hMR expression in these cells, as demonstrated by Western blotting (Fig. 6C, inset). Meanwhile, p24 production, i.e., HIV-1 replication in these hMR siRNA-expressing cells was inhibited by ca. 80% (Fig. 6C). As noted in most siRNA studies, expression of an unrelated siRNA, such as Luc siRNA, also downmodulated hMR expression to some extent and, as a result, showed some effects on HIV-1 replication. Nevertheless, the inhibition of HIV-1 replication by Luc siRNA was considerably lower. Taken together, these results further support the notion that hMR is directly involved in HIV-1 infection of human astrocytes.
Direct binding of HIV-1 virions with hMR.
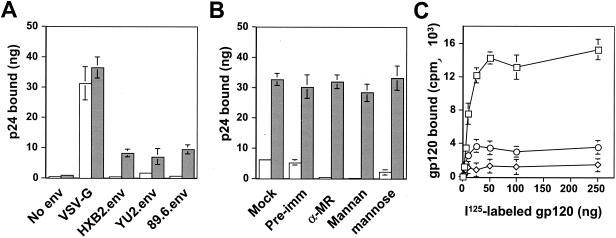
Our studies showed specific binding of HIV-1 envelope glycoprotein gp120 to astrocytes (Fig. 1C) and a direct role for hMR in HIV-1 infection of human primary astrocytes (Fig. 5 and 6). Thus, we next determined whether gp120 in the context of whole HIV-1 virions would bind to hMR. We performed a virus capture assay with U87.MR cells. HIV-1 viruses pseudotyped with VSV-G, HIV-1 YU-2, HXB2, or 89.6 envelope protein were added to U87.MR cells and incubated at 4°C for 30 min. Binding of HIV-1 virions to hMR was assessed by measuring p24 (viruses) captured on U87.MR or U87.MG cells with a p24 ELISA kit. In agreement with the results obtained from direct HIV-1 infection, HIV-1 pseudotyped with YU-2, HXB2, or 89.6 envelope exhibited similar binding to U87.MR cells (Fig. 7A). There was little detectable binding of HIV-1 to U87.MG cells and also little binding of HIV-1 pseudotyped with no envelope proteins to either U87.MG or U87.MR cells. In contrast, HIV-1 pseudotyped with VSV-G envelope protein showed, as expected, indiscriminate and stronger binding to both U87.MG and U87.MR cells (Fig. 7A).
FIG. 7.
Interaction of HIV-1 viruses and gp120 with hMR. (A) Binding of HIV-1 viruses to hMR. U87.MG cells (□) and U87.MR cells ( ) grown in a 96-well plate were prechilled on ice for 30 min, followed by the addition of 100 ng of gagp24 HIV-GFP viruses pseudotyped with VSV-G, HXB2, YU-2, and 89.6 envelope proteins, or without envelope protein (no env) were then added. The cells were allowed to incubate with the viruses for an additional 30 min. After 30 min, the viruses were removed and the cells were washed extensively with prechilled regular culture medium. The cells were then lysed with a 1% NP-40-containing buffer, and the lysates were processed to determine HIV-1 binding by using the p24 ELISA kit. (B) Inhibition of HIV-1 binding to human mannose receptor by hMR antibody and ligand antagonists. U87.MR cells were incubated with HIV-GFP viruses pseudotyped with HXB2 envelope protein (□), or VSV-G envelope protein ( ), as stated above, in the presence of goat preimmune serum (1:50), goat anti-hMR serum (1:50), and 3 mg of yeast mannan or d-mannose/ml. HIV-1 binding was determined as described above. (C) Direct binding of HIV-1 gp120 proteins to hMR. U87.MR cells were incubated with 125I-labeled gp120 protein (□) or 125I-labeled nonglycosylated gp120 protein (⋄) at concentrations as indicated, in the presence of 20 mM EGTA (○), on ice for 30 min. Unbound proteins were removed, followed by an extensive wash with prechilled regular cell culture medium. The cells were then harvested and processed for measuring gp120 binding by using a gamma scintillation counter.
To further ascertain that the binding of HIV-1 virions to U87.MR cells is mediated by hMR expressed on U87.MR cells, HIV-1 virions pseudotyped with HXB2 envelope protein were incubated with U87.MR cells in the presence of goat anti-hMR serum, or hMR ligand antagonists yeast mannan and d-mannose. HIV-1 virions pseudotyped with VSV-G envelope protein, mock treatment, and goat preimmune serum were also included as controls in these experiments. Preincubation with both goat anti-hMR serum and yeast mannan completely abolished binding of HIV-1 virions to U87.MR cells, whereas d-mannose preincubation showed only 34 to 39% binding of HIV-1 virions to U87.MR cells compared to the mock treatment control (Fig. 7B). In contrast, HIV-1 virions pseudotyped with VSV-G envelope protein showed no detectable change in any of the treatments, since VSV-G interacts with phospholipids, the putative VSV receptors that are ubiquitously and abundantly present in all mammalian cells (71). These results taken together demonstrate that HIV-1 binds to U87.MR in a hMR-dependent (specific) manner and are consistent with our previous results that hMR functions as a CD4-independent HIV-1 receptor in astrocytes.
hMR belongs to the large C-type lectin superfamily, and its ligand binding is mediated by mannosylated and/or mannose-rich glycan moieties present in its ligands (59). Biochemical characterization of HIV-1 surface glycoprotein gp120 has shown that 11 of 24 glycan side chains of gp120 are either completely mannosylated or highly mannosylated (5, 41, 51, 52). To determine whether the binding of HIV-1 to hMR is also mediated by the mannosylated carbohydrate moieties of gp120 protein, recombinant gp120 and nonglycosylated gp120 proteins were 125I-labeled and then added to and incubated with U87.MR cells. The direct binding of 125I-labeled proteins to U87.MR was examined. Similar to these results obtained from gp120 binding to primary astrocytes, as stated earlier (Fig. 1C), recombinant native gp120 exhibited saturable binding to U87.MR cells with a calculated Kd of 68 nM (Fig. 7C). However, recombinant nonglycosylated gp120 protein only showed a basal level of binding to U87.MR cells. In addition, we also tested whether Ca2+ is required for binding of gp120 binding to hMR. We included 20 mM EGTA in the cell culture medium and throughout the binding experiments for Ca2+ depletion. The results showed that addition of EGTA almost completely abolished recombinant native gp120 binding to U87.MR cells (Fig. 7C). These results demonstrate that HIV-1 binding to hMR involves the mannose-containing glycan side chains present in HIV-1 surface envelope gp120 protein and that the binding is Ca2+ dependent and provide biochemical evidence to support the specific nature of HIV-1 and hMR interaction.
hMR-mediated endocytosis of HIV-1 into astrocytes.
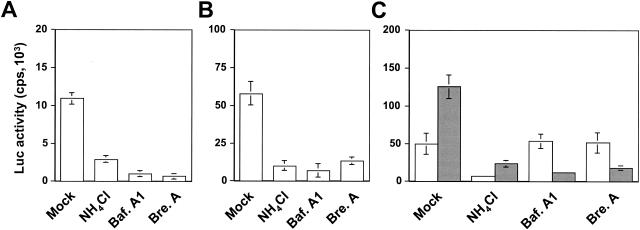
Binding of hMR to its ligands is known to be followed by internalization of the ligands by hMR-mediated endocytosis (59). To determine whether hMR-mediated HIV-1 infection also occurs via the hMR-mediated endocytic pathway, we determined the effects of several agents that act upon lysosomal enzymes, endosome acidification, and early-to-late endosome transition on HIV-1 infection of astrocytes. U87.MR cells were infected with HIV-Luc reporter viruses pseudotyped with HXB2 envelope protein in the presence of NH4Cl, which affects endosome acidification, bafilomycin A, an inhibitor of vacuolar proton ATPase (3), and brefeldin A, a fungal antibiotic which causes early endosomes to form a tubular network and prevents early-to-late endosome transition (44). Infection of U87.MR cells was determined by measuring Luc reporter gene expression. HIV-Luc reporter viruses pseudotyped with VSV-G envelope protein that has been shown to infect target cells via endocytosis (2) were also included as a control. NH4Cl, bafilomycin A1, and brefeldin A inhibited Luc gene expression by 63, 84, and 87%, respectively, in U87.MR cells infected with HIV-Luc reporter viruses pseudotyped with HXB2 envelope protein (Fig. 8A). Comparable inhibitory effects were obtained in U87.MR infected with HIV-Luc pseudotyped with VSV-G envelope protein, although the absolute infection efficiency was significantly higher (Fig. 8B). To ascertain that the inhibitory effects obtained are not due to nonspecific and adverse effects of these drugs on HIV-1 viruses or are not other unknown hMR expression-induced effects, we also examined effects of these drugs on the membrane fusion-mediated HIV-1 infection with U87.CD4.CXCR4 cells. U87.CD4.CXCR4 cells were infected with HIV-Luc reporter viruses pseudotyped with HXB2 envelope protein in the presence of each of these drugs. We also included the HIV-Luc viruses pseudotyped with VSV-G envelope protein in the experiments. As expected, NH4Cl, which is known to raise the pH in the extracellular microenviroment and thereby inhibit low-pH-dependent membrane fusion process (12), inhibited Luc gene expression by 85% in U87.CD4.CXCR4 cells infected with HIV-Luc viruses pseudotyped with HXB2 envelope protein, whereas both bafilomycin A1 and brefeldin A had little effects in the same cells infected by the same viruses (Fig. 8C). In contrast, all three drugs showed inhibitory effects in U87.CD4.CXCR4 cells infected with HIV-Luc viruses pseudotyped with VSV-G envelope protein (Fig. 8). Taken together, these results suggest that hMR-mediated HIV-1 infection of astrocytes is endocytic in nature.
FIG. 8.
hMR-mediated endocytosis of HIV-1 viruses. U87.MR cells were infected with 100 ng of gagp24 HIV-Luc reporter viruses pseudotyped with HXB2 envelope protein (A) or VSG-G envelope protein (B) at 37°C for 2 h in the presence of 10 μM NH4Cl, 150 nM bafilomycin A1 (Baf.A1), or 5 μg of brefeldin A (Bre.A)/ml. After 2 h, the viruses were removed, and the cells were washed with the regular cell culture medium. The cells were allowed to grow for 48 h and then harvested for measuring Luc enzymatic activity for HIV-1 infection. (C) U87.CD4.CXCR4 cells were similarly infected with HIV-Luc reporter viruses pseudotyped with HXB2 (□) or VSV-G ( ) envelope protein in the presence of different reagents, and HIV-1 infection was determined as described above. For VSV-G pseudotyped virus infection, only 1/20 of the lysates was used for Luc enzymatic activity assay, whereas only one-fifth of the lysates was used for HXB2 pseudotyped HIV-1 infection of U87.CD4.CXCR4 cells.
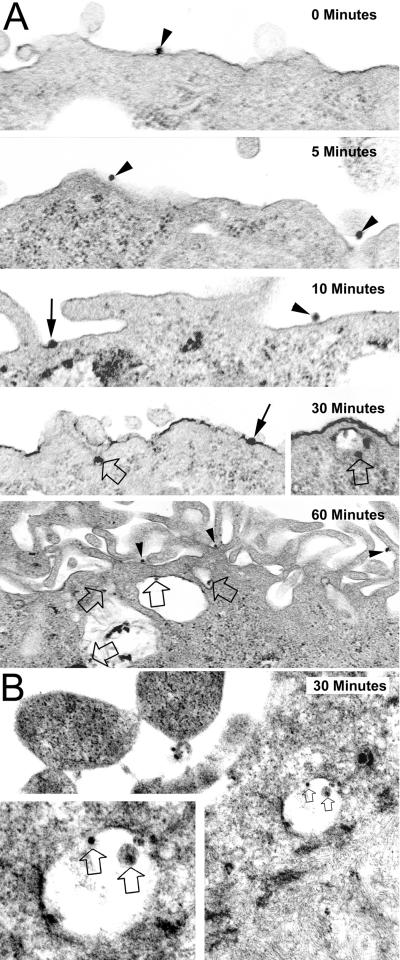
To further ascertain the role of hMR as HIV-1 receptor for astrocyte and the endocytic nature of hMR-mediated HIV-1 entry into astrocytes, we performed transmission electron microscopy to directly visualize the hMR-mediated uptake of HIV virions. U87.MR cells and human primary astrocytes grown on Thermanox coverslips were prechilled on ice for 30 min, followed by the addition of HIV-Luc viruses pseudotyped with YU-2 envelope protein. We first incubated the cells with viruses at 4°C for 30 min to allow virus binding to the cells and then at 37°C for different lengths of time to allow virus internalization, which was monitored by transmission electron microscopy. We also included in the experiments HIV-Luc viruses pseudotyped with VSV-G envelope protein, which has been shown to infect cells through endocytosis (2). The results showed that, like HIV-Luc viruses pseudotyped with VSV-G envelope protein (data not shown), HIV-Luc viruses pseudotyped with YU-2 envelope protein bound to the outer surface of the plasma membrane at early times (0, 5, and 10 min), followed by localization within endosome at 30 min and beyond (Fig. 9). These data provide additional evidence to support the importance of hMR-mediated endocytosis in HIV-1 infection of astrocytes.
FIG. 9.
Electron transmission microscopy of hMR-mediated HIV-1 endocytosis. U87.MR cells (A) or human primary astrocytes (B) were prechilled on ice for 30 min. After 30 min, HIV-GFP viruses pseudotyped with YU-2 envelope protein were added, followed by incubation with the cells for additional 30 min. The cells were then transferred back to 37°C and kept at 37°C for 0, 5, 10, 30, and 60 min. The locations of viruses on or inside U87.MR cells were visualized by electron microscopy. Arrowheads, viruses bound to the membrane; solid arrows, viruses fused with the membrane; open arrows, viruses located inside endosomes. Magnifications for U87.MR cells are 48,000 for 0 min, 34,000 for 5 min, 36,500 for 10 min, 30,000 for 30 min, 36,500 for the 30-min inset, and 22,000 for 60 min; magnifications for the human primary astrocytes are 22,000 for 30 min and 50,000 for the 30-min inset.
DISCUSSION
Using the expression cloning strategy, we have successfully isolated from a human fetal brain cDNA library a cDNA that was associated with HIV-1 infectivity of astrocytes. The cDNA was found to be identical to hMR. Differential hMR expression among HIV-1 infection-refractory astroglial cell lines, such as U87.MG, U373.MG, and U138.MG, and HIV-1 infection-permissive human primary astrocytes (Fig. 2 and 3) prompted us to further characterize the roles of hMR in HIV-1 infection of astrocytes. Ectopic expression of the full-length hMR conferred HIV-1 infection susceptibility to U87.MG cells in a single-round HIV-1 infection assay (Fig. 5). Direct involvement of hMR in HIV-1 infection of astrocytes was further supported by the inhibition of HIV-1 infection in human primary astrocytes by anti-hMR serum and by hMR-specific siRNA (Fig. 6). In agreement with this newly identified function of hMR, hMR exhibited carbohydrate- and Ca2+-dependent binding to HIV-1 virions and HIV-1 surface glycoprotein gp120 (Fig. 7). Further analysis showed that HIV-1 viruses were internalized into astrocytes through hMR-mediated endocytosis (Fig. 8 and 9). Taken together, these results demonstrate for the first time that hMR is able to function as an HIV-1 receptor for astrocyte infection, and also suggest that the receptor-mediated endocytosis may represent a new CD4-independent HIV-1 entry mechanism into its target cells.
HIV-1 infection typically requires coexpression of the primary HIV-1 receptor CD4 and HIV-1 chemokine coreceptor CCR5 or CXCR4. Based on use of CCR5 or CXCR4, HIV-1 strains that use CXCR4 are grouped as T-tropic, those that use CCR5 as M-tropic, and those that use both CXCR4 and CCR5 as dualtropic (for a review, see reference 4). The present study showed that hMR expression alone was sufficient to allow HIV-1 infection in the absence of CD4 (Fig. 5). This is in agreement with results indicating that a neutralizing monoclonal antibody, anti-CD4 Leu3A, failed to block HIV-1 infection of human primary astrocytes (Fig. 6A) (27, 78). Nevertheless, consistent with studies demonstrating a higher-affinity binding of CD4 to gp120 protein (67) and partial overlapping of the binding domain of CD4 on gp120 (75) with the mannosylated glycan side chains within the domain, preincubation of recombinant CD4 protein with HIV-1 viruses partially inhibited HIV-1 infection of human primary astrocytes (Fig. 6A), suggesting that coexpression of CD4 and hMR physically impedes hMR interaction with HIV-1 viruses and may subsequently diminish hMR function as the HIV-1 receptor on some cells such as monocytes and dendritic cells. Both T-tropic and M-tropic viruses were able to infect hMR-expressing astrocytes in the absence of HIV-1 chemokine coreceptors CCR5 and CXCR4 (Fig. 5). HIV-1 viruses pseudotyped with HIV-1 envelope protein 89.6, which uses both CCR5 and CXCR4 bound to hMR similarly to HIV-1 viruses pseudotyped with either HXB2 envelope protein or YU-2 envelope protein (Fig. 7A). Similar results have been obtained from studies with chemokines specific for both CXCR4 and CCR5 that fail to inhibit HIV-1 replication in astrocytes (69), and direct infection of astrocytes with both HIV-1 virus strains (16). The ability of hMR to be utilized by both T- and M-tropic HIV-1 viruses corroborated our results from the virus capture assay and the direct gp120 binding assay indicating that the interaction between hMR and HIV-1 viruses was mediated by the carbohydrate moiety of HIV-1 gp120 protein (Fig. 7C), and our results with neutralizing antibodies targeted at the V3 region of gp120 protein showing that preincubation of the V3 neutralizing antibodies with HIV-1 viruses had no effect on HIV-1 infection of hMR-expressing U87.MR cells and human primary astrocytes (data not shown).
hMR cDNA was initially cloned in 1990 (18, 74) and encodes 1,456 amino acids with a short signal peptide at the N terminus, a cysteine-rich domain, a fibronectin type II repeat, eight lectin-like carbohydrate recognition domains, a transmembrane domain, and a very short cytoplasmic tail (59). hMR is expressed in tissue-differentiated macrophages, endothelial cells, epithelial cells, and dendritic cells, with defined functions in molecular scavenging and host defense through hMR-mediated endocytosis and phagocytosis (59). Our results showed that hMR was expressed in human primary astrocytes and normal human brain tissues but not in astroglial cell lines (Fig. 2 and 3). Studies have shown that hMR is expressed in primary astrocytes, but is developmentally and spatially regulated (9, 10) and also highly regulated by anti- and proinflammatory cytokines (10, 53, 64). Interestingly, we observed that hMR was rapidly downregulated in primary astrocytes after HIV-1 infection and also that there was a brief increase in hMR expression concomitant with activation of HIV-1 replication in HIV-1 latently infected astrocytes when they were treated with cytokines, including tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, or interleukin-1β (data not shown). hMR expression has recently been shown to be downmodulated in HIV-1-infected patients (35, 36), and the hMR promoter has been shown to be negatively regulated by HIV-1 Tat protein expression (11). Thus, it is conceivable that downmodulation of hMR expression after HIV-1 infection may also contribute to the nonproductive viral replication in astrocytes.
Previous studies have demonstrated specific HIV-1 gp120 binding sites on the surface of astrocytes (46). Interestingly, immunofluorescence staining with the sera raised against the uncharacterized gp120-binding molecule showed some reactivity to U87.MR cells (J. He and A. Nath, unpublished results), suggesting that the unknown molecule(s) may be related to hMR or a member of C-type lectin superfamily. The other possibility is that additional molecules on astrocytes are also involved in gp120 binding and HIV-1 entry (24, 46). The higher binding affinity of gp120 to human primary astrocytes (Kd 43 nM, Fig. 1C) than U87.MR cells (Kd 68 nM, Fig. 7C) also supports the existence of additional molecules for HIV-1 infection of astrocytes.
HIV-1 gp120 protein is one of the highly glycosylated viral proteins, and the carbohydrate moieties take up more than 50% of its total molecular weight (22). Biochemical characterization of the carbohydrate component of gp120 protein has shown that almost half of the glycan side chains present in gp120 are completely mannosylated or mannose-rich (5, 41, 51, 52). Indeed, an earlier study has demonstrated that the glycan side chains of gp120-mediated interaction do not require CD4 (38). The low efficiency of hMR-mediated HIV-1 infection (Fig. 5A) and the low HIV-1 replication in primary astrocytes (Fig. 6A) were apparently due to a low level of hMR expression in these cells, since enrichment of higher hMR-expressing cells in human primary astrocytes resulted in a reasonably higher infection efficiency (Fig. 5B). Moreover, the results further showed that HIV-1 binding to hMR on astrocytes is followed by hMR-mediated endocytosis (Fig. 8 and 9). Endocytosis has long been proposed as a general HIV-1 entry pathway (19, 20, 47) and as the HIV-1 entry pathway in astrocytes (24, 25). In general, ligands that are taken into cells by receptor-mediated endocytosis are trafficked to early sorting endosomes where many ligands are dissociated from their receptors and then rapidly trafficked to late endosomes and then lysosomes for degradation (48, 49, 65). Thus, the extremely low efficiency of hMR-mediated HIV-1 infection and the extremely low GFP reporter gene expression in hMR-expressing cells, i.e., at least 10-fold lower than that of cells expressing CD4 and CCR5 or CD4 and CXCR4 (Fig. 5A), and the extremely low HIV-1 replication in primary astrocytes, i.e., >1,000-fold lower than that of human PBMCs (Fig. 6), may also suggest that only a small percentage of the HIV-1 virions taken up by hMR-mediated pathway are able to escape from degradation within the endosome/lysosomes and proceed to the typical postentry pathway of HIV-1 infection, such as reverse transcription, nuclear translocation of proviral DNA and integration, and viral gene expression. The inefficiency of hMR-mediated HIV-1 infection may also contribute to the fact that the significance of hMR-mediated HIV-1 infection has never been appreciated in cells such as macrophages, in which CD4 and CCR5 are overwhelmingly utilized for HIV-1 infection (54).
Nevertheless, hMR is not the only C-type lectin receptor that is involved in HIV-1 infection through the lectin-like carbohydrate recognition domains. DC-SIGN, another member of the same large C-type lectin superfamily, has been identified to bind to HIV-1 gp120 and to transmit HIV-1 viruses from dendritic cells to T cells (14, 21). A very recent study has shown that direct binding of DC-SIGN to HIV-1 viruses can also lead to internalization of HIV-1 viruses (37). Interestingly, both hMR and DC-SIGN have been shown to be expressed on dendritic cells, but the relative expression levels are highly dependent on the stages of differentiation of dendritic cells (79). Thus, it is tempting to speculate that whether dendritic cells are directly infected with HIV-1 viruses or only serve as an intermediary for HIV-1 transmission may depend on differential expression of these two receptors on dendritic cells at various stages of differentiation.
Astrocytes occupy ∼20% of the cell volume of the gray matter, and their processes are found around synapses and in close apposition to the nodes of Ranvier, axon tracts, capillaries, and the blood-brain barrier (6, 13, 58). hMR interaction with some of its ligands has been shown to activate secretion of lysosomal enzymes, expression of tumor necrosis factor, interleukin-12, matrix metalloproteinase-9, and signal-regulated protein kinases (40, 55, 62, 66, 72). Therefore, in addition to the adverse effects of hMR-mediated HIV-1 entry into astrocytes, it is very likely that hMR-mediated intracellular signaling induced by HIV-1 gp120 binding may contribute, to an even more significant extent, to astrocyte dysfunction, for example, through glutamate metabolism and Ca2+ signaling, and eventually to AIDS neuropathogenesis. The relationship between HIV-1 induced hMR-mediated signaling in astrocytes and astrocyte dysfunction merits further investigation. In addition, identification of hMR as an HIV-1 receptor raises the possibility that astrocytes located at the blood-brain barrier serve as the initial route of HIV-1 transmission from the periphery to the CNS and also supports the notion that astrocytes represent a potential HIV-1 reservoir in the CNS. Therefore, the present study may provide new drug targets for treating and preventing HIV-1 infection of the CNS.
Acknowledgments
We thank Alan Ezekovitz for the hMR expression plasmid phMR, Philip Stahl for goat anti-hMR serum, Greg Hannon for the pMSCV-puro retroviral siRNA expression plasmid, George Sandusky for paraffin-embedded normal human brain tissues, Caroline Miller for assistance with the electron microscopy studies, and Yan Xiao for performing the double immunofluorescence staining. We also thank Richard Haak for assistance in preparing the manuscript and Hal Broxmeyer and Arun Srivastava for critical reading of the manuscript.
This study was supported by grants R01NS39804 and R01MH65158 (to J.J.H.) from the NIH and a research award from the Ralph W. and Grace M. Showalter Trust Foundation (to J.J.H.).
REFERENCES
- 1.Achord, D. T., F. E. Brot, and W. S. Sly. 1977. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem. Biophys. Res. Commun. 77:409-415. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blass, and R. Fuchs. 1998. Effcets of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 5.Biller, M., A. Bolmstedt, A. Hemming, and S. Olofsson. 1998. Simplified procedure for fractionation and structural characterisation of complex mixtures of N-linked glycans, released from HIV-1 gp120 and other highly glycosylated viral proteins. J. Virol. Methods 76:87-100. [DOI] [PubMed] [Google Scholar]
- 6.Bouzier-Sore, A. K., M. Merle, P. J. Magistretti, and L. Pellerin. 2002. Feeding active neurons: (re)emergence of a nursing role for astrocytes. J. Physiol. (Paris) 96:273-282. [DOI] [PubMed] [Google Scholar]
- 7.Brack-Werner, R. 1999. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 8.Brack-Werner, R., A. Kleinschmidt, A. Ludvigsen, et al. 1992. Infection of human brain cells by HIV-1: restricted virus production in chronically infected human glial cell lines. AIDS 6:273-285. [PubMed] [Google Scholar]
- 9.Burudi, E. M., and A. Regnier-Vigouroux. 2001. Regional and cellular expression of the mannose receptor in the post-natal developing mouse brain. Cell Tissue Res. 303:307-317. [DOI] [PubMed] [Google Scholar]
- 10.Burudi, E. M., S. Riese, P. D. Stahl, and A. Regnier-Vigouroux. 1999. Identification and functional characterization of the mannose receptor in astrocytes. Glia 25:44-55. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell, R. L., B. S. Egan, and V. L. Shepherd. 2000. HIV-1 Tat represses transcription from the mannose receptor promoter. J. Immunol. 165:7035-7041. [DOI] [PubMed] [Google Scholar]
- 12.Carr, C. M., C. Chaudhry, and P. S. Kim. 1997. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 94:14306-14313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, Y., and R. A. Swanson. 2003. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23:137-149. [DOI] [PubMed] [Google Scholar]
- 14.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, H. K., D. Unutmaz, V. N. KewalRamani, and D. R. Littman. 1997. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388:296-300. [DOI] [PubMed] [Google Scholar]
- 16.Di Rienzo, A. M., F. Aloisi, A. C. Santarcangelo, C. Palladino, E. Olivetta, D. Genovese, P. Verani, and G. Levi. 1998. Virological and molecular parameters of HIV-1 infection of human embryonic astrocytes. Arch. Virol. 143:1599-1615. [DOI] [PubMed] [Google Scholar]
- 17.Edinger, A. L., C. Blanpain, K. J. Kunstman, S. M. Wolinsky, M. Parmentier, and R. W. Doms. 1999. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J. Virol. 73:4062-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezekowitz, R. A., K. Sastry, P. Bailly, and A. Warner. 1990. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 172:1785-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fackler, O. T., and B. M. Peterlin. 2000. Endocytic entry of HIV-1. Curr. Biol. 10:1005-1008. [DOI] [PubMed] [Google Scholar]
- 20.Fredericksen, B. L., B. L. Wei, J. Yao, T. Luo, and J. V. Garcia. 2002. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 76:11440-11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 22.Geyer, H., C. Holschbach, G. Hunsmann, and J. Schneider. 1988. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 263:11760-11767. [PubMed] [Google Scholar]
- 23.Hammond, S. M., E. Bernstein, D. Beach, and G. J. Hannon. 2000. An RNA-directed nuclease mediates posttranscriptional gene silencing in Drosophila cells. Nature 404:293-296. [DOI] [PubMed] [Google Scholar]
- 24.Hao, H. N., F. C. Chiu, L. Losev, K. M. Weidenheim, W. K. Rashbaum, and W. D. Lyman. 1997. HIV infection of human fetal neural cells is mediated by gp120 binding to a cell membrane-associated molecule that is not CD4 nor galactocerebroside. Brain Res. 764:149-157. [DOI] [PubMed] [Google Scholar]
- 25.Hao, H. N., and W. D. Lyman. 1999. HIV infection of fetal human astrocytes: the potential role of a receptor-mediated endocytic pathway. Brain Res. 823:24-32. [DOI] [PubMed] [Google Scholar]
- 26.Harouse, J. M., S. Bhat, S. L. Spitalnik, L. M., K. Stefano, D. H. Silberberg, and F. Gonzalez-Scarano. 1991. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science 253:320-323. [DOI] [PubMed] [Google Scholar]
- 27.Harouse, J. M., C. Kunsch, H. T. Hartle, M. A. Laughlin, J. A. Hoxie, B. Wigdahl, and F. Gonzalez-Scarano. 1989. CD4-independent infection of human neural ells by human immunodeficiency virus type 1. J. Virol. 63:2527-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Busciglio, X. Yang, W. Hofmann, W. Newman, C. R. Mackay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are coreceptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 29.He, J., C. M. deCastro, G. R. Vandenbark, J. Busciglio, and D. Gabuzda. 1997. Astrocyte apoptosis induced by HIV-1 transactivation of the c-kit protooncogene. Proc. Natl. Acad. Sci. USA 94:3954-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoppe, C. A., and Y. C. Lee. 1983. The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. Effect of the sugar density. J. Biol. Chem. 258:14193-14199. [PubMed] [Google Scholar]
- 31.Hoppe, C. A., and Y. C. Lee. 1982. Stimulation of mannose-binding activity in the rabbit alveolar macrophage by simple sugars. J. Biol. Chem. 257:12831-12834. [PubMed] [Google Scholar]
- 32.Hoxie, J. A., C. C. LaBranche, M. J. Endres, J. D. Turner, J. F. Berson, R. W. Doms, and T. J. Matthews. 1998. CD4-independent utilization of the CXCR4 chemokine receptor by HIV-1 and HIV-2. J. Reprod. Immunol. 41:197-211. [DOI] [PubMed] [Google Scholar]
- 33.Kim, B. O., Y. Liu, Y. Ruan, Z. C. Xu, L. Schantz, and J. J. He. 2003. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 162:1693-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinschmidt, A., M. Neumann, C. Moller, V. Erfle, and R. Brack-Werner. 1994. Restricted expression of HIV1 in human astrocytes: molecular basis for viral persistence in the CNS. Res. Virol. 145:147-153. [DOI] [PubMed] [Google Scholar]
- 35.Koziel, H., Q. Eichbaum, B. A. Kruskal, P. Pinkston, R. A. Rogers, M. Y. Armstrong, F. F. Richards, R. M. Rose, and R. A. Ezekowitz. 1998. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J. Clin. Investig. 102:1332-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koziel, H., B. A. Kruskal, R. A. Ezekowitz, and R. M. Rose. 1993. HIV impairs alveolar macrophage mannose receptor function against Pneumocystis carinii. Chest 103:111S-112S. [DOI] [PubMed] [Google Scholar]
- 37.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T-cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 38.Larkin, M., R. A. Childs, T. J. Matthews, S. Thiel, T. Mizuochi, A. M. Lawson, J. S. Savill, C. Haslett, R. Diaz, and T. Feizi. 1989. Oligosaccharide-mediated interactions of the envelope glycoprotein gp120 of HIV-1 that are independent of CD4 recognition. AIDS 3:793-798. [DOI] [PubMed] [Google Scholar]
- 39.Lee, S. C., W. C. Hatch, W. Liu, C. F. Brosnan, and D. W. Dickson. 1993. Productive infection of human fetal microglia in vitro by HIV-1. Ann. N. Y. Acad. Sci. 693:314-316. [DOI] [PubMed] [Google Scholar]
- 40.Lefkowitz, D. L., K. Mills, A. Castro, and S. S. Lefkowitz. 1991. Induction of tumor necrosis factor and macrophage-mediated cytotoxicity by horseradish peroxidase and other glycosylated proteins: the role of enzymatic activity and LPS. J. Leukoc. Biol. 50:615-623. [DOI] [PubMed] [Google Scholar]
- 41.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 42.Li, J., Y. Liu, B. O. Kim, and J. J. He. 2002. Direct participation of Sam68, the 68-kilodalton Src-associated protein in mitosis, in the CRM1-mediated Rev nuclear export pathway. J. Virol. 76:8374-8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, J., Y. Liu, I. W. Park, and J. J. He. 2002. Expression of exogenous Sam68, the 68-kilodalton SRC-associated protein in mitosis, is able to alleviate impaired Rev function in astrocytes. J. Virol. 76:4526-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippinocott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and K. R. D. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanisms for regulating organelle structure and membrane traffic. Cell 67:601-616. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig, E., F. C. Silberstein, J. van Empel, V. Erfle, M. Neumann, and R. Brack-Werner. 1999. Diminished rev-mediated stimulation of human immunodeficiency virus type 1 protein synthesis is a hallmark of human astrocytes. J. Virol. 73:8279-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma, M., J. D. Geiger, and A. Nath. 1994. Characterization of a novel binding site for the human immunodeficiency virus type 1 envelope protein gp120 on human fetal astrocytes. J. Virol. 68:6824-6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsh, M., and A. Pelchen-Matthews. 2000. Endocytosis in viral replication. Traffic. 1:525-532. [DOI] [PubMed] [Google Scholar]
- 49.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575-625. [DOI] [PubMed] [Google Scholar]
- 50.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat. Med. 8:150-156. [DOI] [PubMed] [Google Scholar]
- 51.Mizuochi, T., M. W. Spellman, M. Larkin, J. Solomon, L. J. Basa, and T. Feizi. 1988. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem. J. 254:599-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizuochi, T., M. W. Spellman, M. Larkin, J. Solomon, L. J. Basa, and T. Feizi. 1988. Structural characterization by chromatographic profiling of the oligosaccharides of human immunodeficiency virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese hamster ovary cells. Biomed. Chromatogr. 2:260-270. [DOI] [PubMed] [Google Scholar]
- 53.Montaner, L. J., R. P. da Silva, J. Sun, S. Sutterwala, M. Hollinshead, D. Vaux, and S. Gordon. 1999. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-γ or IL-10. J. Immunol. 162:4606-4613. [PubMed] [Google Scholar]
- 54.Nguyen, D. G., and J. E. Hildreth. 2003. Involvement of macrophage mannose receptor in the binding and transmission of HIV by macrophages. Eur. J. Immunol. 33:483-493. [DOI] [PubMed] [Google Scholar]
- 55.Ohsumi, Y., and Y. C. Lee. 1987. Mannose-receptor ligands stimulate secretion of lysosomal enzymes from rabbit alveolar macrophages. J. Biol. Chem. 262:7955-7962. [PubMed] [Google Scholar]
- 56.Onishi, M., S. Kinoshita, Y. Morikawa, A. Shibuya, J. Phillips, L. L. Lanier, D. M. Gorman, G. P. Nolan, A. Miyajima, and T. Kitamura. 1996. Applications of retrovirus-mediated expression cloning. Exp. Hematol. 24:324-329. [PubMed] [Google Scholar]
- 57.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perea, G., and A. Araque. 2002. Communication between astrocytes and neurons: a complex language. J. Physiol. (Paris) 96:199-207. [DOI] [PubMed] [Google Scholar]
- 59.Pontow, S. E., V. Kery, and P. D. Stahl. 1992. Mannose receptor. Int. Rev. Cytol. 137B:221-244. [DOI] [PubMed] [Google Scholar]
- 60.Price, R. W., B. Brew, J. Sidtis, M. Rosenblum, A. C. Scheck, and P. Cleary. 1988. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 239:586-592. [DOI] [PubMed] [Google Scholar]
- 61.Prigozy, T. I., P. A. Sieling, D. Clemens, P. L. Stewart, S. M. Behar, S. A. Porcelli, M. B. Brenner, R. L. Modlin, and M. Kronenberg. 1997. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6:187-197. [DOI] [PubMed] [Google Scholar]
- 62.Puig-Kroger, A., M. Relloso, O. Fernandez-Capetillo, A. Zubiaga, A. Silva, C. Bernabeu, and A. L. Corbi. 2001. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood 98:2175-2182. [DOI] [PubMed] [Google Scholar]
- 63.Ranki, A., M. Nyberg, V. Ovod, M. Haltia, I. Elovaara, R. Raininko, H. Haapasalo, and K. K. 1995. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001-1008. [DOI] [PubMed] [Google Scholar]
- 64.Raveh, D., B. A. Kruskal, J. Farland, and R. A. Ezekowitz. 1998. Th1 and Th2 cytokines cooperate to stimulate mannose-receptor-mediated phagocytosis. J. Leukoc. Biol. 64:108-113. [PubMed] [Google Scholar]
- 65.Riezman, H., P. G. Woodman, G. v. Meer, and M. Marsh. 1997. Molecular mechanisms of endocytosis. Cell 91:731-738. [DOI] [PubMed] [Google Scholar]
- 66.Rivera-Marrero, C. A., W. Schuyler, S. Roser, J. D. Ritzenthaler, S. A. Newburn, and J. Roman. 2002. Mycobacterium tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 282:L546-L555. [DOI] [PubMed] [Google Scholar]
- 67.Robey, F. A., T. Harris-Kelson, M. Robert-Guroff, D. Batinic, B. Ivanov, M. S. Lewis, and P. P. Roller. 1996. A synthetic conformational epitope from the C4 domain of HIV Gp120 that binds CD4. J. Biol. Chem. 271:17990-17995. [DOI] [PubMed] [Google Scholar]
- 68.Rottman, J. B., K. P. Ganley, K. Williams, L. Wu, C. R. Mackay, and D. J. Ringler. 1997. Cellular localization of the chemokine receptor CCR5: correlation to cellular targets of HIV-1 infection. Am. J. Pathol. 151:1341-1351. [PMC free article] [PubMed] [Google Scholar]
- 69.Sabri, F., E. Tresoldi, M. Di Stefano, S. Polo, M. C. Monaco, A. Verani, J. R. Fiore, P. Lusso, E. Major, F. Chiodi, and G. Scarlatti. 1999. Nonproductive human immunodeficiency virus type 1 infection of human fetal astrocytes: independence from CD4 and major chemokine receptors. Virology 264:370-384. [DOI] [PubMed] [Google Scholar]
- 70.Saito, Y., L. R. Sharer, L. G. Epstein, J. Michaels, M. Mintz, M. Louder, K. Golding, T. A. Cvetkovich, and B. M. Blumberg. 1994. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology 44:474-481. [DOI] [PubMed] [Google Scholar]
- 71.Schlegel, R., M. C. Willingham, and I. H. Pastan. 1982. Saturable binding sites for vesicular stomatitis virus on the surface of Vero cells. J. Virol. 43:871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibata, Y., W. J. Metzger, and Q. N. Myrvik. 1997. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 159:2462-2467. [PubMed] [Google Scholar]
- 73.Tanabe, S., M. Heesen, I. Yoshizawa, M. A. Berman, Y. Luo, C. C. Bleul, T. A. Springer, K. Okuda, N. Gerard, and M. E. Dorf. 1997. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J. Immunol. 159:905-911. [PubMed] [Google Scholar]
- 74.Taylor, M. E., J. T. Conary, M. R. Lennartz, P. D. Stahl, and K. Drickamer. 1990. Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J. Biol. Chem. 265:12156-12162. [PubMed] [Google Scholar]
- 75.Thomas, E. K., J. N. Weber, J. McClure, P. R. Clapham, M. C. Singhal, M. K. Shriver, and R. A. Weiss. 1988. Neutralizing monoclonal antibodies to the AIDS virus. AIDS 2:25-29. [DOI] [PubMed] [Google Scholar]
- 76.Tornatore, C., R. Chandra, J. R. Berger, and E. O. Major. 1994. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481-487. [DOI] [PubMed] [Google Scholar]
- 77.Tornatore, C., K. Meyers, W. Atwood, K. Conant, and E. Major. 1994. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J. Virol. 68:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tornatore, C., A. Nath, K. Amemiya, and E. O. Major. 1991. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1β. J. Virol. 65:6094-6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turville, S. G., P. U. Cameron, A. Handley, G. Lin, S. Pohlmann, R. W. Doms, and A. L. Cunningham. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975-983. [DOI] [PubMed] [Google Scholar]
- 80.Vallat, A. V., U. De Girolami, J. He, A. Mhashilkar, W. Marasco, B. Shi, F. Gray, J. Bell, C. Keohane, T. W. Smith, and D. Gabuzda. 1998. Localization of HIV-1 coreceptors CCR5 and CXCR4 in the brain of children with AIDS. Am. J. Pathol. 152:167-178. [PMC free article] [PubMed] [Google Scholar]
- 81.Wiley, C. A., R. D. Schrier, J. A. Nelson, P. W. Lampert, and M. B. Oldstone. 1986. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc. Natl. Acad. Sci. USA 83:7089-7093. [DOI] [PMC free article] [PubMed] [Google Scholar]