Abstract
Locomotor patterns are generally very consistent but also contain a high degree of adaptability. Motor adaptation is a short-term type of learning that utilizes this plasticity to alter locomotor behaviors quickly and transiently. In this study, we used a variation of an adaptation paradigm in order to test whether explicit information as well as the removal of the visual error signal after adaptation could improve retention of a newly learned walking pattern 24 h later. On two consecutive days of testing, participants walked on a treadmill while viewing a visual display that showed erroneous feedback of swing times for each leg. Participants were instructed to use this feedback to monitor and adjust swing times so they appeared symmetric within the display. This was achieved by producing a novel interlimb asymmetry between legs. For both legs, we measured adaptation magnitudes and rates and immediate and 24-h retention magnitudes. Participants showed similar adaptation on both days but a faster rate of readaptation on day 2. There was complete retention of adapted swing times on the increasing leg (i.e., no evidence of performance decay over 24 h). Overall, these findings suggest that the inclusion of explicit information and the removal of the visual error signal are effective in inducing full retention of adapted increases in swing time over a moderate (24 h) interval of time.
Keywords: motor adaptation, motor learning, walking, savings, human
human walking is highly stereotyped, with a very consistent rhythmic pattern of flexor and extensor muscle activity that generates forward stepping in healthy individuals. However, this pattern is also inherently plastic and is modified in response to varying environmental and physical demands and behavioral goals (Drew 1988; Forssberg et al. 1975; Hiebert et al. 1996; Whelan et al. 1995). Locomotor adaptation is a type of short-term learning that capitalizes on this plasticity to quickly alter feedforward motor commands for walking (Blanchette et al. 2012; Blanchette and Bouyer 2009; Dietz et al. 1994; Fortin et al. 2009; Gordon et al. 1995; Reisman et al. 2007, 2009; Reynolds and Bronstein 2003; Savin et al. 2010). When a perturbation is applied (often a novel load or force but can be any change to the walking pattern, the environment, or the sensory feedback experienced during walking), the walking pattern is altered to a degree and direction proportional to the perturbation. The subsequent mismatch between the expected and actual sensorimotor information generates an error signal in the nervous system that drives adaptation (Ito 2000; Shadmehr et al. 2010; Wolpert and Kawato 1998), such that with repeated exposure to the perturbation the erroneous walking pattern is gradually corrected (i.e., adapted). After adaptation, when the perturbation is removed, the walking pattern is initially altered again, this time in the direction opposite from the original shift induced by the perturbation. This erroneous pattern is termed the negative aftereffect and is used as an indicator of storage of new feedforward motor commands (Shadmehr and Mussa-Ivaldi 1994; Weiner et al. 1983).
Several studies have suggested that locomotor adaptation might be a feasible tool to facilitate gait rehabilitation in patients with neurological conditions, such as stroke or spinal cord injury, in which negative aftereffects, such as altered interlimb symmetry or elevated toe clearance, might be beneficial (Reisman et al. 2007, 2009; Savin et al. 2013; Yen et al. 2012). However, recall that motor adaptation by definition produces only a transient change in behavior; new motor patterns generated during the adaptation period are immediately replaced by negative aftereffects once the perturbing signal is removed and the baseline condition restored. Likewise, the negative aftereffects also quickly “wash out” during the deadaptation period as the nervous system readjusts to the absence of the perturbation. Attempts to make aftereffects longer-lasting by repeated exposure to the adaptive condition have been shown to be only moderately successful (Blanchette et al. 2012). Therefore, given the transient nature of aftereffects, traditional locomotor adaptation paradigms may have limited practical value in real-world rehabilitation settings.
Another way in which traditional motor adaptations differ from typical rehabilitation practices is that motor adaptations are thought to work through relatively implicit mechanisms (Martin et al. 1996; Mazzoni and Krakauer 2006; Shadmehr et al. 2010). In general, participants are usually not instructed to think about the perturbation and are often unaware of the effects it has on their movements. In some cases, participants are unaware that a perturbation even exists. On the other hand, during locomotor rehabilitation patients are usually explicitly aware of the intended movement modifications and are instructed to make conscious efforts to achieve them. It was recently shown that during a visuomotor adaptation requiring participants to adjust reaching direction in response to rotated visual feedback of hand position, explicit information on the presence of the rotation enhanced the initial and final performance levels after switching between two different rotation settings (Imamizu et al. 2007). Similarly, it was also shown that explicit information may improve long-term (24 h) retention of adapted movements in certain circumstances (Osu et al. 2004).
We wondered whether a modified locomotor adaptation paradigm could be developed that avoided washout of the newly learned motor pattern while also capitalizing on potentially additive benefits of explicit information in order to enhance retention. To our knowledge, this possibility has never been tested. To that end, we developed a locomotor adaptation paradigm that uses erroneous visual feedback to alter temporal aspects of interlimb symmetry (specifically, swing times). When the new walking pattern is achieved, the visual feedback is removed so that no visual signal is available to drive deadaptation. Because of the removal of this error signal, along with the inclusion of explicit knowledge of the perturbation, we expected that aftereffects would not occur, and instead participants would continue walking with the adapted pattern. Specifically, we hypothesized that participants would use adaptive trial-and-error learning mechanisms to achieve a novel swing time asymmetry in response to a single 15-min exposure to a visual perturbation. We predicted that participants would produce negative aftereffects after adaptation if the visual information was not removed and was instead restored so that it accurately represented participants' movements. In addition, we predicted that performance of the newly learned pattern would not immediately degrade after adaptation if we combined removal of the visual feedback with the provision of explicit information. Furthermore, we hypothesized that under these conditions the newly learned walking asymmetry would be retained over a 24-h interval and that relearning during a second exposure would occur faster than learning during the initial exposure (i.e., significant savings).
METHODS
Participants.
Nine healthy adults (8 women, 1 man) with an average age of 34.3 ± 13.4 yr (mean ± SD) participated in experiment 1, and six healthy adults (4 women, 2 men) with an average age of 23.6 ± 3.1 yr participated in experiment 2. All subjects had no known orthopedic, neurological, or cardiovascular conditions. This study was approved by the University of Iowa Institutional Review Board, and all procedures were in compliance with the Declaration of Helsinki. All participants signed a written informed consent form.
Experiment 1: main experiment.
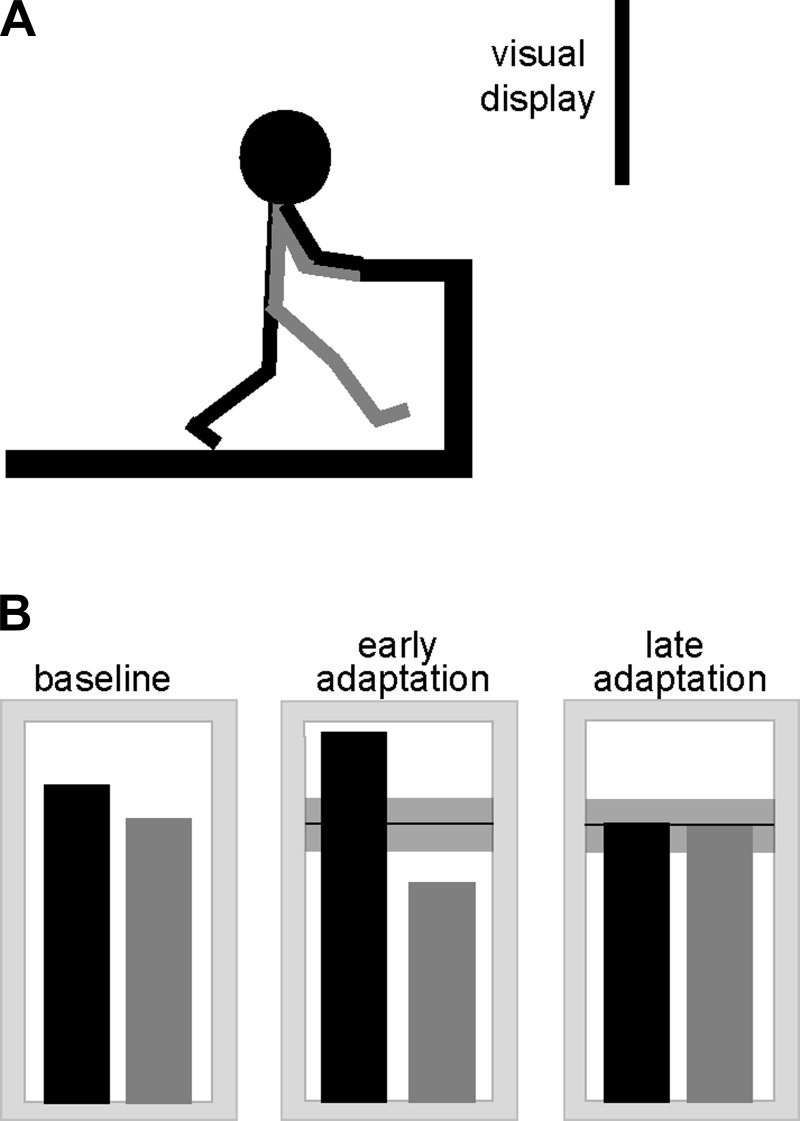
Participants visited the laboratory for two consecutive days of treadmill testing. Day 2 testing was always 20–28 h after day 1 testing. On both visits, participants walked comfortably and continuously on a treadmill at a self-selected but constant speed (range 0.89–1.34 m/s). All participants held onto two support side-rails while walking. Day 1 consisted of baseline (3 min), adaptation (15 min), and retention (15 min) periods. Day 2 consisted of an initial 24-h retention period (3 min) followed by the same three periods exactly as in day 1. In front of the treadmill, a projected image displayed red and blue vertical bars, which served as a real-time graphical representation of each leg's swing time during walking. The real-time visual display was created with API software provided by Northern Digital (Waterloo, ON, Canada) and was linked with a custom program written in Visual C++ (Microsoft, Redmond, WA). The display showed visual feedback continuously (updated at ∼60 Hz). A red bar represented the swing time for the right leg, and a blue bar represented the swing time for the left leg. Each of these bars began to grow in height at the time of toe off of its respective limb and stopped growing at the time of that limb's heel strike. During baseline periods, the two bars corresponded to participants' actual swing times and therefore appeared grossly symmetric in height (since these subjects all had symmetric walking patterns). During adaptation a horizontal gray target zone was added to the display, and the speed of rise of the red and blue bars was adjusted so that one bar grew at a speed that was 15% faster than its speed during baseline while the other bar grew at a speed that was 15% slower than its speed during baseline. Ultimately, this created bar heights that were 15% increased on one side and 15% decreased on the other side, presenting participants with visual feedback of apparently asymmetric swing times across legs. Through motor adaptation, participants were expected to adjust to the altered visual information, so that they would eventually produce relatively equal bar heights on the right and left as viewed through the feedback of the visual display. To achieve this, they had to adopt a new asymmetry in actual swing times between right and left legs. During all retention periods (day 1, 24 h, and day 2), the visual feedback was removed and participants were instructed to maintain the new asymmetry. See Fig. 1 for an illustration of the experimental setup.
Fig. 1.
A: illustration of the experimental setup. Participants walked on a treadmill while viewing the visual display. B: illustration of what the visual display might show for a typical participant at 3 time points: baseline (left), early adaptation (center), and late adaptation (right). A blue bar (shown here in black) represented swing time of the left leg, and a red bar (here gray) represented swing time of the right leg. Although the illustration shows the display at a single point in time (heel strike), the display actually showed participants' leg performance in real time. Thus 1 bar was constantly moving at all times. During early adaptation, bars appear asymmetric because participants initially continued to walk symmetrically, yet, as participants' walking became more asymmetric in response to the visual feedback, the bars began to appear more symmetric in height (late adaptation).
Participants were given specific instructions and information during each testing period. During baseline, participants were told to walk comfortably and to ignore the bars rising and falling on the screen in front of them. During adaptation, participants were told that “the red bar represents the amount of time your right leg is in the air, and the blue bar represents the amount of time your left leg is in the air. Try to bring both bars to the center of the gray target zone. In order to do this, you need to increase the amount of time one leg is in the air, and decrease the amount of time the other leg is in the air.” One minute prior to the beginning of the retention period, participants were instructed to “try and remember exactly what you are doing to get the bars into the target zone. In about a minute, the bars will disappear and you should continue walking with this same new pattern.” Participants were given no further instructions during this period. On day 2, during the 24-h retention period, participants were instructed to “walk with the pattern you learned yesterday.” Then, when participants entered the baseline phase on day 2, they were instructed to “walk normally.” Participants were also encouraged to walk with their normal gait pattern over the 24-h interval between day 1 and day 2 testing.
Experiment 2: control experiment.
To ensure that the visually guided learning task in experiment 1 altered walking parameters by updating predictive motor commands (i.e., was a true motor adaptation), we performed a separate experiment in which participants experienced a washout period instead of the retention period as in experiment 1. For this experiment, participants reported to the laboratory for 1 day of testing only. Participants walked comfortably at a self-selected speed (range 0.94–1.3 m/s) for 3 min (baseline phase) and then underwent the same perturbation and received the same explicit instructions as participants in experiment 1 (adaptation phase). After adaptation, the visual display remained on the screen but was switched back to its unperturbed (null) state. Thus the bars once again accurately represented participants' swing times. Participants were instructed to continue to bring both bars to the center of the gray target zone but were given no indication that the visual display was no longer perturbed and were not instructed to try and maintain the adapted pattern. Experiment 2 was designed to test for negative aftereffects and thus ensure that participants stored new predictive commands to adjust their walking to the altered visual display.
For both experiments 1 and 2, the direction of asymmetry was counterbalanced between participants, so that approximately half experienced the faster rising bar on the right and the slower rising bar on the left, and the other half experienced the opposite perturbation. Throughout the text, we will refer to the leg for which the visual feedback of swing time was increased in speed as the “decreasing” leg (because appropriate adaptation would be to decrease swing times on that leg) and the leg for which the visual feedback was decreased in speed as the “increasing” leg, even in testing periods in which walking and the visual feedback were symmetric (i.e., no increasing or decreasing sides).
Data collection.
The Optotrak motion capture system (Certus system, NDI, Waterloo, ON, Canada) was used to record walking performance continuously (all strides) throughout all testing periods. Infrared markers were placed on 12 anatomical landmarks: the head of the fifth metatarsal, the lateral malleolus, lateral joint space of the knee, greater trochanter, iliac crest, and acromion process, bilaterally. Position data were sampled at 100 Hz.
Data analysis.
Position data were analyzed with custom-written MATLAB programs (MathWorks, Natick, MA). Data were low-pass filtered at 8 Hz. Heel strike was defined as the time at which the lateral malleolus marker was most anterior, and toe off was defined as the time at which the fifth metatarsal marker was most posterior (Noble and Prentice 2006). Because participants were explicitly instructed to modify swing time and because swing time information was the only information available within the visual display, swing time was the primary measurement. Swing time was defined as the time from toe off until the next heel strike on the same leg. We also measured two secondary features of gait. First, we measured the spatial variable forward foot placement, to determine whether participants achieved swing time changes by simply holding the leg in the air for more or less time or by also altering where the foot was placed. This was calculated as the anterior-posterior distance between the ankle and hip markers of the same leg at the time of heel strike. In addition, we measured step length, which was defined as the anterior-posterior distance between the two ankle markers during double support. We chose to analyze step length because, although it is measured in spatial terms, it has been shown that step length changes can occur through alterations to either purely spatial or purely temporal features of gait or some combination of the two (Malone and Bastian 2010). Last, we also measured cadence (the number of steps per minute), stride length (the anterior-posterior distance between the ankle marker position at the time of heel strike and at the time of toe off), and stride time (the duration of each stride) and used these as global indicators of whether or not other features of the locomotor pattern were altered.
In addition to the real swing time data, we also calculated swing times in terms of the visual error produced during adaptation. Swing time errors were measured as the difference between the actual swing times produced and the exact swing times required by the visual feedback display. We converted error measures to a percentage by dividing these values by the actual swing times produced, which gave the results in terms of a percentage difference. For example, a swing time error of +15% would indicate no correction in response to the (+15% perturbed) visual feedback. Because there was no visual feedback display during retention periods (experiment 1), there were no swing time error measures for those time periods.
For each participant, we averaged all gait variables on each leg over several key epochs: late baseline, early adaptation, late adaptation, early retention, and late retention (both days). Early epochs consisted of the first 5 strides of that particular period, whereas late epochs consisted of the last 40 strides of that particular period. Note that for experiment 2, retention periods were replaced by washout periods. Day 2 (experiment 1 only) contained an additional epoch, late 24-h retention.
For experiment 1, we also calculated magnitudes of adaptation and immediate retention on days 1 and 2 and magnitude of 24-h retention on day 2, for each leg individually. Formulae for these calculations were
where LA refers to late adaptation, LB refers to late baseline, and LR refers to late retention (either immediate retention or 24-h retention). The numerator for each equation provides the percent change in swing time relative to late baseline performance. The denominator of ±15 (−15 for the decreasing leg, +15 for the increasing leg) provides a further normalization to the total amount of correction expected if adaptation and retention were complete. Thus values of +100% would indicate full adaptation and full retention. Magnitudes of 24-h retention were normalized to day 1 late baseline performance.
We also calculated the rate of adaptation combined across both legs, for days 1 and 2 of experiment 1 only. To reduce stride-to-stride variability, swing times for both legs were binned by every five strides over the entire adaptation period for each participant. Then we subtracted swing times between the two legs (increasing leg minus decreasing leg) at each binned data point, which gave values representing swing time interlimb asymmetry throughout the adaptation period. We averaged these interlimb asymmetry values over the last 40 strides (8 bins) of the adaptation period and used this as the measure of the final level of interlimb asymmetry obtained by each participant. We identified the time point at which interlimb asymmetry first approached this plateau point [i.e., the first bin period in which the interlimb asymmetry value reached within 1 SD of the final level of interlimb asymmetry (averaged over the last 40 strides) at the end of adaptation and maintained it for 15 consecutive strides (3 bins)]. We then took the reciprocal of this value as a measure of rate of adaptation. Thus, as rate of adaptation increased, the number of strides taken until the plateau of interlimb asymmetry was reached decreased.
Statistical analyses.
Statistical analyses were completed with STATISTICA (StatSoft, Tulsa, OK). For experiments 1 and 2, separate one-way repeated-measures analysis of variance (ANOVA) was used to compare swing time errors across epochs for each leg individually. Furthermore, for experiment 1 only, separate one-way repeated-measures ANOVA was used to compare swing times, step lengths, and forward foot placement across each epoch for each leg individually. Factorial ANOVA, with factors “leg” and “time point,” was used to compare adaptation and retention magnitudes. Finally, a paired t-test was used to compare rates of adaptation between days 1 and 2. For the ANOVAs, when a significant main effect was detected, post hoc analyses were completed with Tukey's honestly significant difference test. For all statistical tests, we used α < 0.05 as our criterion for statistical significance.
RESULTS
General patterns of adaptation.
All participants began with a grossly symmetric gait pattern during the day 1 baseline period. In general, participants were able to alter the swing time of each limb in opposite directions over the course of adaptation. This produced an obvious “limp” to the gait pattern, in which more time was spent in swing on one leg compared with the other. Interestingly, the amount of swing time adaptation achieved by the increasing leg was always greater than that achieved by the decreasing leg. Our examination of other global measures of walking (cadence, stride length, and stride time) showed no differences in any of these features during adaptation, on either day 1 or day 2 (difference between early and late adaptation, paired t-tests: P > 0.373 for all).
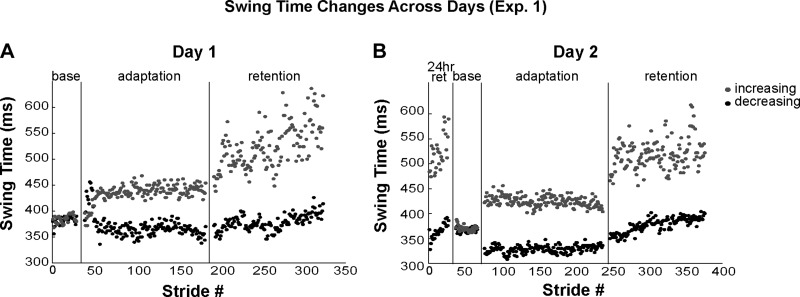
Figure 2 shows walking performance on a stride-by-stride basis from a representative participant in experiment 1. On day 1, the participant gradually learned the swing time asymmetry during adaptation and fully maintained this asymmetry during immediate retention. In fact, not only was there no evidence of decay of the newly learned asymmetry, the participant actually exaggerated the adapted asymmetry during retention. Recall that no visual feedback was available during this period. Interestingly, the exaggeration of the asymmetry was primarily achieved by further increasing swing times on the increasing leg. On day 2, performance during the 24-h retention period was similar to performance at the end of day 1 retention (i.e., full 24-h retention). This participant was then able to immediately return to her normal gait pattern during day 2 baseline walking and then quickly reacquire the asymmetric stepping pattern during day 2 adaptation.
Fig. 2.
Walking performance from a representative participant in experiment 1 during day 1 (A) and day 2 (B). Each data point represents swing time on 1 leg during 1 stride. Black dots represent the decreasing leg; gray dots represent the increasing leg. Vertical lines separate the different experimental periods. base, Baseline period; 24 hr ret, 24-h retention period.
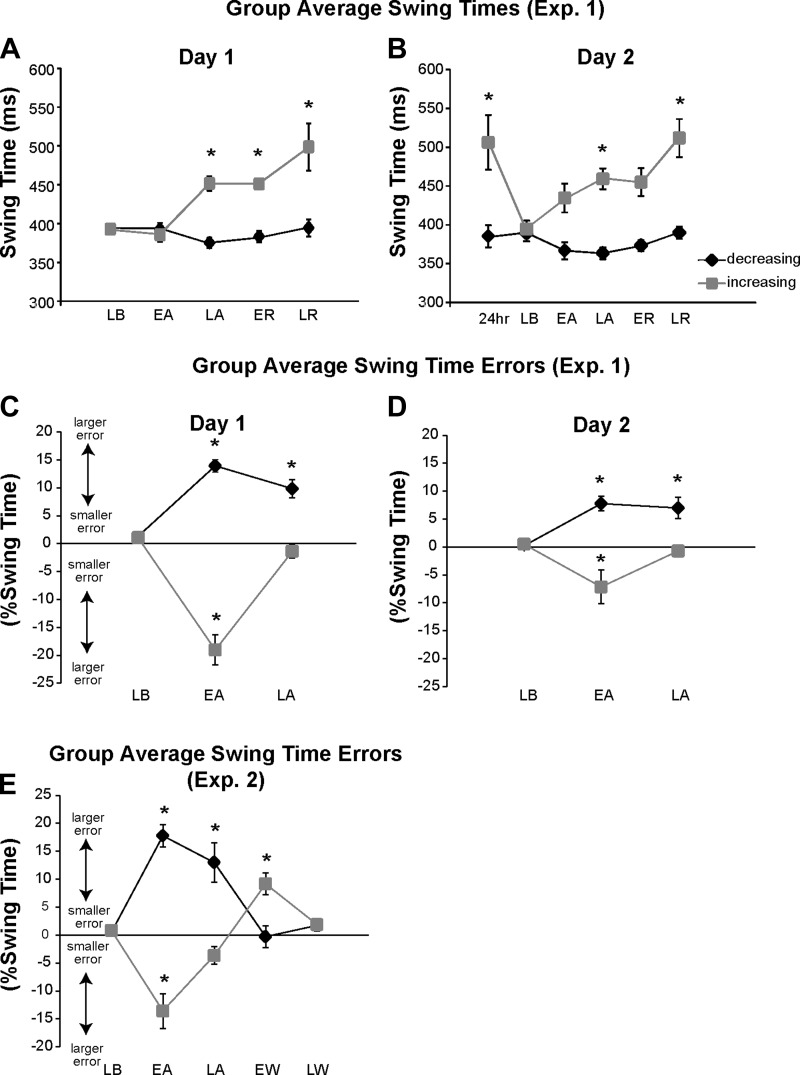
Average swing time performance over all key epochs for all participants in experiment 1 is shown in Fig. 3, A and B. On day 1, the decreasing leg did not alter performance significantly across epochs (main effect of epoch: P = 0.192), but the increasing leg did (main effect of epoch: P < 0.001). On the increasing leg, increased swing times emerged at late adaptation and were maintained throughout early and late retention (post hoc, difference from late baseline for each: P < 0.042 for all). On day 2, the decreasing leg again showed only relatively small changes in swing time, which did not reach significance (main effect of epoch: P = 0.054). However, the increasing leg did show adaptive changes (main effect of epoch: P < 0.001). Specifically, 24-h retention swing times for the increasing leg were significantly greater than those seen during baseline (post hoc: P < 0.001), and these increased swing times once again emerged by late adaptation (post hoc, difference from late baseline: P < 0.042). These differences were maintained, and even exaggerated, throughout the retention period (post hoc, difference between late baseline and late retention: P < 0.001).
Fig. 3.
A and B: experiment 1 group average swing times during all key epochs of day 1 (A) and day 2 (B). C and D: experiment 1 swing time errors during day 1 (C) and day 2 (D). E: experiment 2 swing time errors. Note that for C–E the y-axis “%Swing Time” refers to the swing time error measurement (the difference between the swing times required by the visual display and the actual swing times produced and normalized to the actual swing times). For example, a +15% error would indicate no correction of swing times on the increasing leg. For all, black diamonds represent the decreasing leg and gray squares represent the increasing leg. Error bars show ±1 SE. Asterisks indicate significant differences from the late baseline epoch based on post hoc analyses. LB, late baseline; EA, early adaptation; LA, late adaptation; ER, early retention; LR, late retention; 24hr, late 24-h retention; EW, early washout; LW, late washout.
Figure 3, C–E, show group swing time errors for experiments 1 (Fig. 3, C and D) and 2 (Fig. 3E). Recall that these error measures depict the degree to which swing times approached the desired ±15% change indicated by the visual display and no visual errors are available during experiment 1 retention periods. For experiment 1 on day 1 (Fig. 3C), both legs altered their swing time errors over time (main effect of epoch for both legs: P < 0.001). The decreasing leg showed significant errors during early adaptation compared with baseline (post hoc: P < 0.001), but these errors were not fully corrected for by late adaptation (post hoc, difference from baseline: P < 0.001; difference from early adaptation: P = 0.072). The increasing leg also showed large errors during early adaptation (post hoc, difference from late baseline: P < 0.001), and these errors were restored to baseline levels by late adaptation (post hoc, difference from late baseline: P = 0.579; difference from early adaptation: P < 0.001). On day 2, similar patterns of error were observed in both legs (main effect of epoch: P < 0.016 for both; post hoc, difference between late baseline and early adaptation for both legs: P < 0.020; post hoc, difference between late baseline and late adaptation: P < 0.013 for decreasing leg and P = 0.879 for increasing leg; difference between early and late adaptation: P > 0.900 for decreasing leg and P < 0.05 for increasing leg). Also, when comparing error magnitudes across days, errors during early adaptation were significantly less on day 2 than on day 1 for the increasing leg (factorial ANOVA, significant day × leg interaction: P < 0.001; post hoc, day 1 early adaptation vs. day 2 early adaptation: P < 0.01).
In experiment 2 (Fig. 3E), errors during the adaptation period were similar in both legs compared with the errors in experiment 1, day 1. That is, swing time errors were significantly increased during early adaptation (main effect of epoch: P < 0.001 for both legs; post hoc, difference between early adaptation and late baseline: P < 0.001 for both legs) and then fully corrected for on the increasing leg but not significantly corrected for on the decreasing leg by late adaptation (post hoc, difference between late adaptation and late baseline: P < 0.003 for decreasing leg and P = 0.270 for increasing leg). Recall that experiment 2 was intended to test whether the current paradigm would produce negative aftereffects during a washout period that included the presentation of unperturbed (null) visual feedback. Post hoc testing showed that for the increasing leg there were significantly increased errors in the opposite direction during early washout compared with baseline performance (post hoc: P < 0.008), and these errors returned to baseline levels by late retention (post hoc, difference between late baseline and late retention: P = 0.987). For the decreasing leg, error size during early washout was not different from late baseline levels (post hoc: P = 0.993).
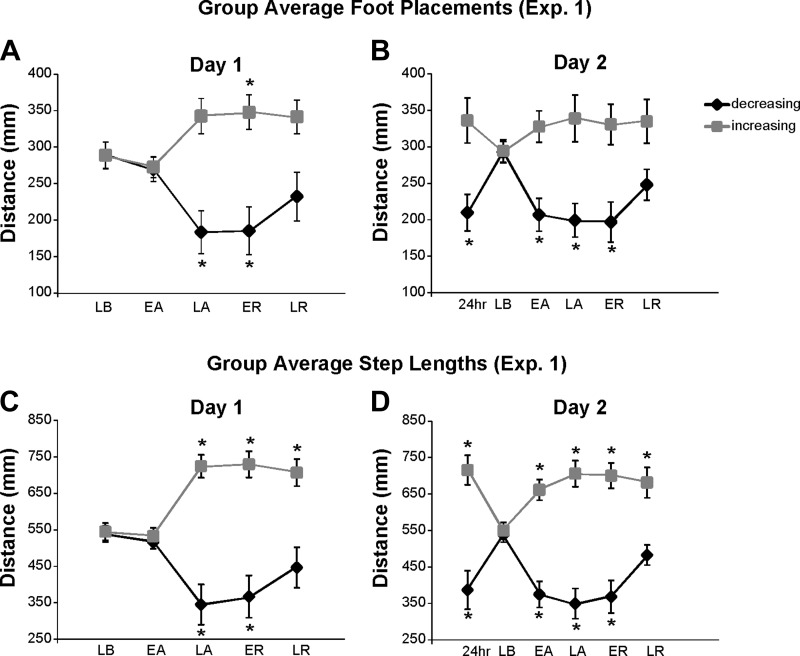
Figure 4 shows group results for forward foot placement and step length across all key epochs in experiment 1. Unlike swing time, these measures generally showed more bilateral adaptation. The ANOVA revealed changes to forward foot placement in both legs on day 1 (main effect of epoch: P < 0.001 for both; Fig. 4A). The decreasing leg foot placement was significantly reduced by late adaptation (post hoc, difference from late baseline: P < 0.001), and this reduction was maintained during early retention (post hoc, difference from late baseline: P < 0.001). The increasing leg showed a trend toward a significant increase in forward foot placement at late adaptation (post hoc, difference from late baseline: P = 0.054), which reached significance at early retention only (post hoc, difference from late baseline: P < 0.03 for early retention and P = 0.061 for late retention). On day 2 (Fig. 4B), the ANOVA revealed significant alteration of forward foot placement on the decreasing leg only (main effect of epoch: P < 0.001 for decreasing leg, P = 0.139 for increasing leg). On this leg, adjustment of foot placement was maintained during 24-h retention (post hoc, difference from late baseline: P < 0.001), reemerged during early adaptation, and was maintained through early retention (post hoc, difference of early and late adaptation and early retention from late baseline: P < 0.001 for all).
Fig. 4.
A and B: experiment 1 group average forward foot placements during all key epochs of day 1 (A) and day 2 (B). C and D: experiment 1 group average step lengths during all key epochs of day 1 (C) and day 2 (D). For all, black diamonds represent the decreasing leg and gray squares represent the increasing leg. Error bars show ±1 SE. Asterisks indicate significant differences from the late baseline epoch based on post hoc analyses.
For step length (Fig. 4, C and D), results were very similar to forward foot placement. There were significant alterations in step length on both days and for both legs (main effects of epoch: P < 0.001 for both). For both legs, these changes emerged at late adaptation on day 1 (Fig. 4C; post hoc, difference from late baseline: P < 0.001 for both legs) and persisted through early (post hoc, difference from late baseline: P < 0.002 for both legs) or late (post hoc, difference from late baseline, increasing leg only: P < 0.001) retention. On day 2 (Fig. 4D), measures during the 24-h retention epoch revealed bilateral maintenance of adjustments to step length (post hoc, difference from late baseline: P < 0.001 for both legs). Subsequent step length changes on day 2 were similar to those seen on day 1, except that the asymmetry emerged earlier than it did on day 1 (post hoc, difference between early adaptation and late baseline: P < 0.001 for both legs).
Magnitudes of adaptation and retention (experiment 1).
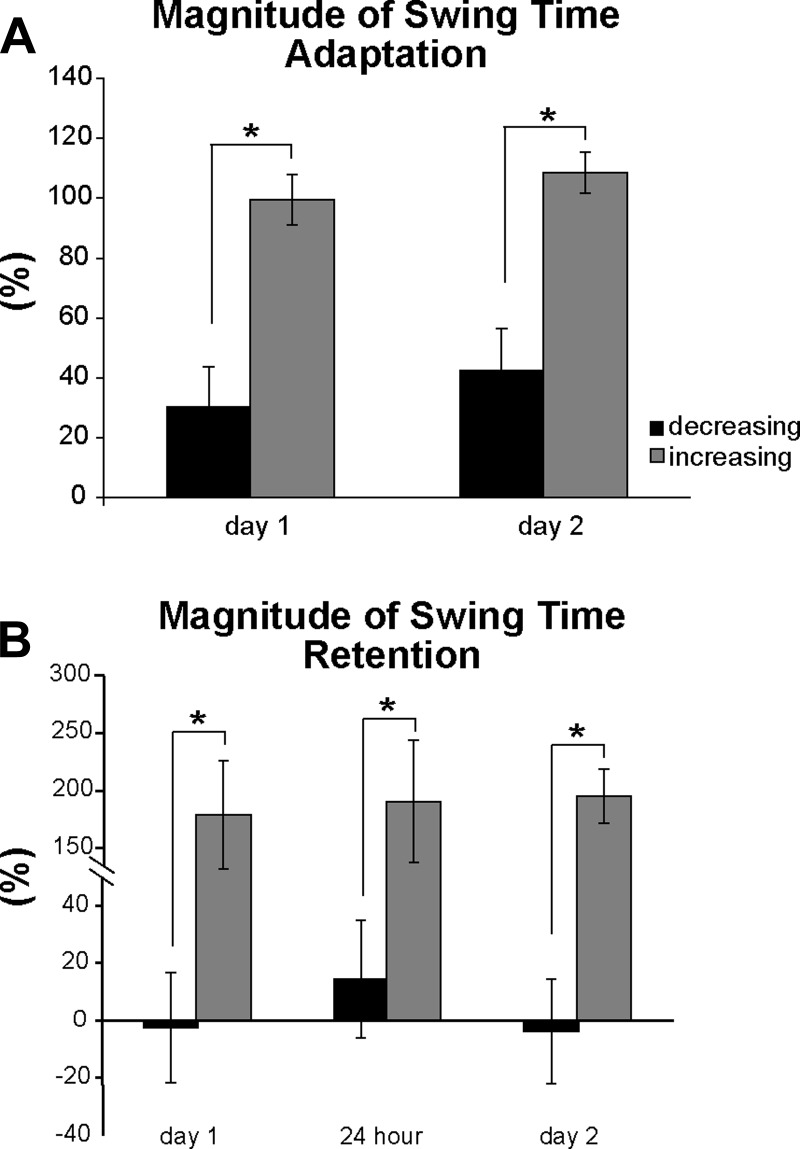
Average magnitudes of adaptation are shown in Fig. 5A. Adaptation magnitudes were similar between days (main effect of time point: P = 0.182), but the decreasing leg always showed reduced adaptation magnitudes compared with the increasing leg (main effect of leg: P < 0.001). On the increasing leg swing time adaptation was always complete, or ∼100% [day 1: 99.4 ± 8.5%, day 2: 108.6 ± 6.8% (mean ± SE)], but on the decreasing leg adaptation magnitudes were 30.3 ± 13.5% on day 1 and 42.5 ± 14.2% on day 2. There was no significant leg × time point interaction (P = 0.848).
Fig. 5.
Magnitude of swing time adaptation (A) and magnitude of swing time retention (B). Black bars represent the decreasing leg; gray bars represent the increasing leg. Error bars show ±1 SE. Asterisks indicate significant differences from the ANOVA, main effect of leg.
Average magnitudes of day 1, 24-h, and day 2 retention are shown in Fig. 5B. Similar to adaptation magnitudes, retention magnitudes were similar across all time points (no main effect of time point: P = 0.856) and no leg × time point interaction existed (P = 0.896). Also similar to the adaptation magnitudes, the increasing leg always showed a large degree of retention, even after 24 h, whereas the decreasing leg retention values were small (main effect of leg: P < 0.001). Retention magnitudes for the increasing leg always exceeded 100%, whereas, on average, retention magnitudes for the decreasing leg were near zero. This reflects what is observed in the representative participant stride-by-stride data (Fig. 2) and the group swing time results (Fig. 3, A and B) from experiment 1: participants frequently overcompensated when visual information was not available and exaggerated the swing time asymmetry by further increasing swing time on the increasing leg.
Adaptation rates (experiment 1).
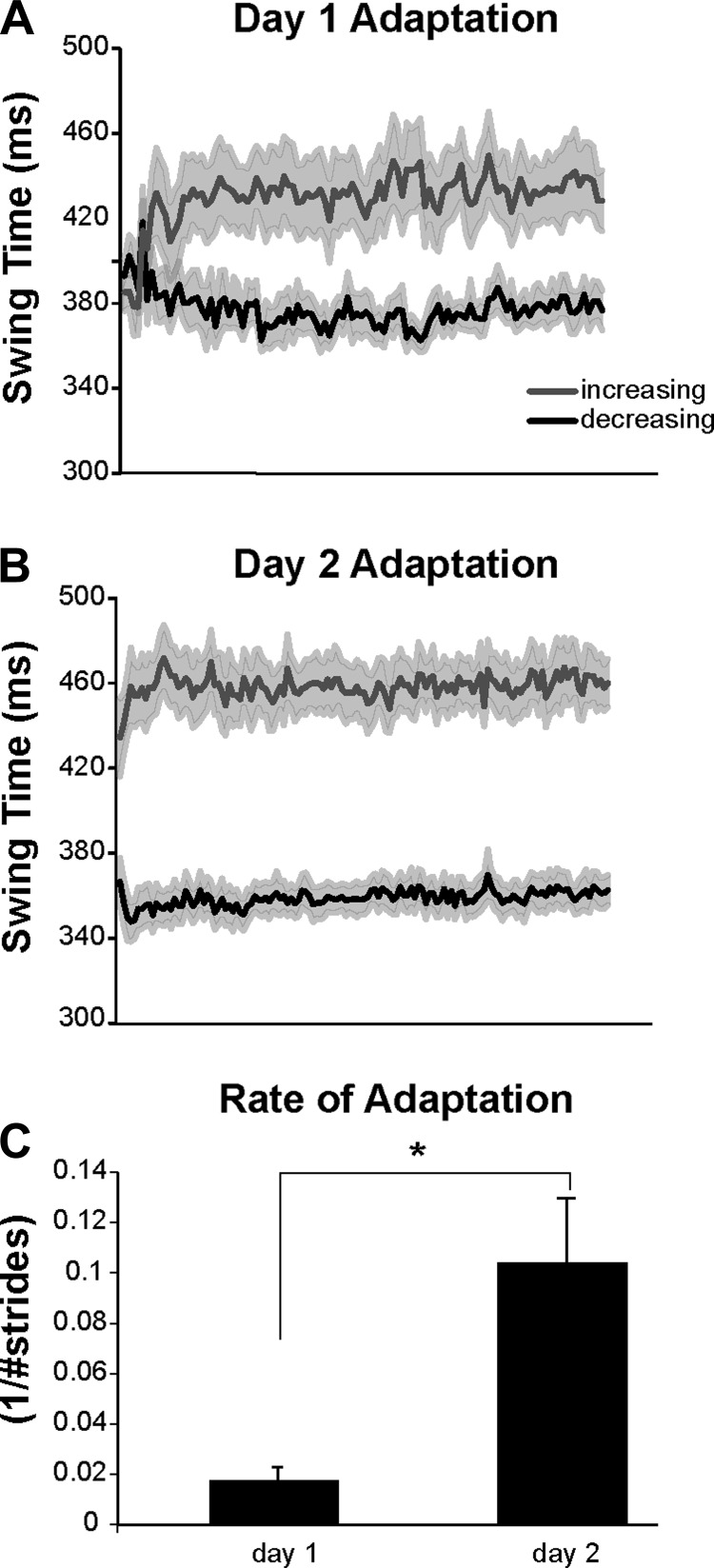
Figure 6, A and B, show the complete adaptation curve, averaged over all participants, during days 1 (Fig. 6A) and 2 (Fig. 6B) of experiment 1. Savings, or a faster rate of adaptation on day 2 compared with day 1, can be visualized through the steeper slope in swing time values during day 2 versus day 1 as performance reaches its plateau. Figure 6C shows average rates of adaptation of swing time interlimb asymmetry. The adaptation rate was significantly increased on day 2 compared with day 1 (P < 0.018), demonstrating that participants in experiment 1 show significant savings of the locomotor adaptation.
Fig. 6.
A and B: group-averaged plots of the entire adaptation period, shown stride by stride for the increasing and decreasing legs during day 1 (A) and day 2 (B). Gray lines represent the increasing leg; black lines represent the decreasing leg. Shading, ±1 SE. C: group averages of swing time interlimb symmetry adaptation rates. Error bars, ±1 SE. Asterisk indicates the significant difference between days.
DISCUSSION
In this study, we demonstrated that a novel visual error-based locomotor adaptation that utilizes explicit instructions and avoidance of a washout period induces significant immediate and 24-h retention of adapted swing time on the increasing leg (experiment 1). We also found significant savings over days. However, if the visual display is switched back to its unperturbed (null) state after adaptation (during the washout period), participants produce negative aftereffects on the increasing leg (experiment 2), indicating that the adaptive changes to swing time seen in both experiments can likely be attributed to the storage of new, predictive motor commands (Martin et al. 1996; Morton and Bastian 2006). This indicates that the adaptation observed here is similar to other locomotor adaptation paradigms (Blanchette et al. 2012; Fortin et al. 2009; Gordon et al. 1995; Gordon and Ferris 2007; Houldin et al. 2012; Noble and Prentice 2006; Savin et al. 2010; Torres-Oviedo and Bastian 2012; Yen et al. 2012). What is novel here is that participants in experiment 1 demonstrated full retention on the increasing leg immediately after adaptation and after 24 h, without any substantial washout of the newly learned walking pattern. To our knowledge, this study is the first demonstration of a visually driven error-based locomotor adaptation task that produces full retention of the adapted walking pattern 24 h later.
Unexpectedly, there were major differences in the behavior of the increasing and decreasing legs. The decreasing leg showed little to no change in swing times over the course of adaptation and no significant retention within or between days (experiment 1), nor did it show an aftereffect (experiment 2). We think the reason for this is because it is physically much more demanding to decrease swing time than it is to increase swing time. That is, participants must quickly pull the decreasing leg forward so that it can advance on the treadmill in a significantly shortened period of time. There is a finite limit to the amount that swing time can decrease without also preventing the forward advancement of that leg on the treadmill. We think we inadvertently reached that limit by asking participants to decrease swing time by 15%. Specifically, participants average swing times were ∼394 ms during baseline. This would result in the visual display showing symmetry during adaptation if the decreasing leg achieved swing times of ∼335 ms. It has been shown that when swing times drop to ∼345 ms, healthy individuals typically make a switch from walking to running (Prilutsky and Gregor 2001). Therefore, swing times within this range likely represent a mechanical or energetic transition point. Notably, similar limits to a decrease in swing time have been observed previously in a different locomotor adaptation paradigm (Savin et al. 2010). In contrast, increasing swing time only requires that participants hold their leg in the air longer during swing phase. Because the adaptive changes to swing time on the decreasing leg were so minimal, it is expected that the aftereffects and retention magnitudes would be similarly reduced on that side. Thus, because of the physiological limit to decreasing swing times, the findings for aftereffects and retention magnitudes in this study apply solely to the increasing leg.
Interestingly, however, this does not mean that adaptation did not occur bilaterally. Despite the lack of significant changes in swing time on the decreasing leg, participants still demonstrated bilateral and oppositely directed changes in forward foot placement and step length during adaptation. Note that we could not measure these spatial variables in terms of visual error because the visual display only represented swing time and did not provide any information on step length or foot placement. Therefore, we cannot calculate adaptation and retention magnitudes for these measures, nor can we measure aftereffects in terms of movement error. Still, the forward foot placement measure suggests that participants achieved alterations in swing time in part by adjusting the placement of their feet (see Fig. 4), even though adjustment of spatial features of gait was not a requirement of the walking task. Therefore, altering spatial characteristics of gait may have facilitated changes in temporal gait features, even if those temporal changes were rather small on the decreasing leg. For example, taking a shorter step would assist with shortening swing time without having to increase swing speed as much. It is also possible that, by taking a shorter step on the decreasing leg, participants were able to unload the increasing leg and thus begin swing phase earlier in time, which would also lengthen the duration of swing time on that side. This possibility might be more readily observed if we had only perturbed the increasing leg. The fact that spatial changes were not required to successfully alter swing times and restore symmetry within the visual display may explain why, in some cases, it appeared that the adjustment of step lengths and forward foot placement tapered off during late retention periods. It may be that participants altered spatial gait parameters in order to facilitate changes in swing time and that later in the retention periods further optimization occurred in order to allow swing time to be modified without also extensively altering spatial parameters. Interestingly, these spatial parameters tended to trend back toward baseline levels at these same instances (late retention periods of days 1 and 2). It is possible that this trending back toward baseline is a result of participants increasing their forward foot placement during adaptation and early retention but then switching to an alternate strategy of simply holding the leg in the air longer, without significant forward translation, during late retention.
We hypothesized that participants would show full retention of adapted swing times (experiment 1) for two reasons. First, during conventional adaptation tasks participants utilize a sensory prediction error to adjust future movements based on past performance (Tseng et al. 2007). When a perturbation is applied, an error signal representing the difference between expected and actual sensory feedback is perceived, which drives adaptation of movement. When the perturbing stimulus is removed, a new, oppositely directed error is perceived, which then drives deadaptation. Here, participants used a visual error signal to adjust feedforward commands for stepping. The sensory prediction error is the mismatch between the expected visual information and the perturbed visual information provided in the display. After adaptation, we avoided the situation in which unperturbed visual information itself would generate a new error signal (as occurred in experiment 2) by completely removing the visual display (experiment 1). This removal of the potential for a new error signal is a major distinction between this study and other adaptation studies, although it is somewhat similar to error-clamping used in other adaptation tasks, in which, regardless of the true movement error, errors are artificially represented as being equal to zero (Criscimagna-Hemminger and Shadmehr 2008; Schmuelof et al. 2012). It is important to note that, despite the use of a visual error signal, the visual system per se may not be particularly important for the long-term locomotor retention observed here. Rather, we think it is the removal of the error signal (regardless of its modality) that is the major contributor.
The second reason we expected participants to show full retention is because they were given explicit instructions about the erroneous visual information. In a study where participants were required to reach in two visually rotated conditions, Imamizu et al. (2007) provided participants with explicit information about the nature of the perturbation. Participants who received explicit instruction performed better during the first few trials each time the rotational condition changed compared with participants who did not receive explicit instruction. Here, after learning the new walking pattern, all participants easily switched from the newly learned pattern back to their normal pattern. Evidence for this was seen during day 2 at the transition from the 24-h retention period to the baseline period; we observed that all participants returned to their normal gait symmetry within the first three strides of baseline walking. Participants also walked with their normal gait pattern immediately after completing day 1 testing and did not appear to require a washout phase to retrieve this pattern. However, after baseline walking on day 2, participants did not immediately switch back to the full asymmetry, but they did reacquire it much more rapidly than on day 1 (Fig. 6). In light of these results and previous findings (Imamizu et al. 2007), we think that explicit information provided during adaptation may recruit additional cognitive learning mechanisms, and that these may facilitate a longer duration of retention. This is almost certainly the factor that led to the overretention observed in the increasing leg, in which participants exaggerated the asymmetry on that side, presumably to ensure their success when the visual feedback was not present.
How do the full (100%+) retention values observed in this study compare to other, traditional locomotor adaptations? Recently, Blanchette et al. (2012) examined retention over several days of repeated exposure to a locomotor adaptation. The paradigm in this previous study was similar to that presented here; however, it did not include explicit cuing or removal of the perturbing error signal. These authors found that the most significant reduction in error occurred between days 1 and 2, and amounted to ∼60% (our estimate, calculated by subtracting reported day 2 initial error from day 1 initial error and dividing by day 1 initial error). It is important to clarify that retention was measured as reduced error upon initial exposure to the perturbation over days. Measuring retention in the context of the perturbation can cause inflation of the true amount of retention due to additional learning that takes place when participants are reexposed to the perturbation (Schmidt and Lee 2011). Our measure of retention was taken from an initial bout of walking in the absence of the perturbing stimulus, such that it represents participants' ability to voluntarily recall the adapted walking pattern when asked to do so. To our knowledge, this form of retention has never been examined previously in the context of locomotor adaptation.
A few caveats to these results must be mentioned. First, our paradigm does not distinguish whether explicit knowledge or the removal of the visual error signal that drives deadaptation is the primary reason for near-full 24-h retention. The full retention seen on the increasing leg may be attributable to the explicit knowledge alone, the removal of the visual display alone, or some combination of the two. Further testing is required to differentiate these possibilities. Second, the enhanced retention we observed could also be due to the specific nature of the task, such as its level of difficulty. Our task may not have been extremely novel for every participant; some people have probably walked in a similar (limping) manner at some point in their lives. However, it is very unlikely that any participant ever previously walked with as much asymmetry as was indicated by the visual display here (30%), and none had any previous exposure to a visually driven locomotor adaptation. We observed that most participants found the task very challenging, and many reported that it required significant amounts of both physical and cognitive resources. Therefore, we do not think the paradigm was retained so well merely because of its simplicity but rather because of the involvement of explicit information and the removal of the visual error after adaptation. Third, in order for there to be any future applications to rehabilitation, it must be determined whether the newly learned walking pattern transfers to overground walking, and to what extent. Also, this paradigm has not yet been tested in any patient populations, and it remains to be seen whether patients with neurological injury would respond to this task in the same way that healthy participants do. Indeed, there have been some reports of explicit knowledge interfering with upper extremity motor adaptation in patients with cerebral stroke (Boyd and Winstein 2003, 2004). Therefore, careful testing of this paradigm in patients is necessary.
In conclusion, this study demonstrates that a visually driven locomotor adaptation task using explicit instructions can induce full retention of adapted swing times. These retention findings differ from previous reports of retention in that participants were able to perform the newly learned walking pattern accurately even when the erroneous visual information was not available. Additionally, retention levels did not degrade over time, indicating that this form of adaptation induces a novel motor behavior that is well-retained and easily recalled after 24 h. The paradigm presented here also induced significant savings upon reexposure to the perturbing environment. Taken together, these findings point toward the efficacy of additional explicit knowledge and avoidance of aftereffects after adaptation to enhance the retention of newly learned locomotor behaviors in healthy individuals.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grant R21 NS-067189 and the Foundation for Physical Therapy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.H., A.S.H., and S.M.M. performed experiments; S.J.H., A.S.H., S.-C.T., and S.M.M. analyzed data; S.J.H. and S.M.M. interpreted results of experiments; S.J.H. prepared figures; S.J.H. drafted manuscript; S.J.H., A.S.H., S.-C.T., and S.M.M. edited and revised manuscript; S.J.H., A.S.H., S.-C.T., and S.M.M. approved final version of manuscript; S.-C.T. and S.M.M. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Andrew Couch for his assistance with programming portions of the visual display and Tara Hackney and Kelly Meyn for their assistance with data collection and analysis.
Present addresses: S.-C. Tseng, School of Physical Therapy, Texas Woman's University, 6700 Fannin St., Houston, TX 77030 (e-mail: stseng@twu.edu); A. S. Hanson, Iowa Specialty Hospital (formerly Wright Medical Center), 1316 South Main St., Clarion, IA 50545 (e-mail: angela.hanson@iaspecialty.com).
REFERENCES
- Blanchette A, Bouyer LJ. Timing-specific transfer of adapted muscle activity after walking in an elastic force field. J Neurophysiol 102: 568–577, 2009 [DOI] [PubMed] [Google Scholar]
- Blanchette A, Moffet H, Roy JS, Bouyer LJ. Effects of repeated walking in a perturbing environment: a 4-day locomotor learning study. J Neurophysiol 108: 275–284, 2012 [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther 83: 976–989, 2003 [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem 11: 388–396, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci 28: 9610–9618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101: 513–520, 1994 [DOI] [PubMed] [Google Scholar]
- Drew T. Motor cortical cell discharge during voluntary gait modification. Brain Res 457: 181–187, 1988 [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res 85: 103–107, 1975 [DOI] [PubMed] [Google Scholar]
- Fortin K, Blanchette A, McFadyen BJ, Bouyer LJ. Effects of walking in a force field for varying durations on aftereffects and on next day performance. Exp Brain Res 199: 144–155, 2009 [DOI] [PubMed] [Google Scholar]
- Gordon CR, Fletcher WA, Melvill Jones G, Block EW. Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res 102: 540–545, 1995 [DOI] [PubMed] [Google Scholar]
- Gordon KE, Ferris DP. Learning to walk with a robotic ankle exoskeleton. J Biomech 40: 2636–2644, 2007 [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75: 1126–1137, 1996 [DOI] [PubMed] [Google Scholar]
- Houldin A, Chua R, Carpenter MG, Lam T. Limited interlimb transfer of locomotor adaptations to a velocity-dependent force field during unipedal walking. J Neurophysiol 108: 943–952, 2012 [DOI] [PubMed] [Google Scholar]
- Imamizu H, Sugimoto N, Osu R, Tsutsui K, Sugiyama K, Wada Y, Kawato M. Explicit contextual information selectively contributes to predictive switching of internal models. Exp Brain Res 181: 395–408, 2007 [DOI] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res 886: 237–245, 2000 [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103: 1954–1962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996 [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble JW, Prentice SD. Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res 169: 482–495, 2006 [DOI] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci 7: 111–112, 2004 [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Gregor RJ. Swing- and support-related muscle actions differentially trigger human walk-run and run-walk transitions. J Exp Biol 204: 2277–2287, 2001 [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair 23: 735–744, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The broken escalator phenomenon. Aftereffect of walking onto a moving platform. Exp Brain Res 151: 301–308, 2003 [DOI] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Morton SM. Bilateral adaptation during locomotion following a unilaterally applied resistance to swing in nondisabled adults. J Neurophysiol 104: 3600–3611, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin DN, Tseng SC, Whitall J, Morton SM. Poststroke hemiparesis impairs the rate but not magnitude of adaptation of spatial and temporal locomotor features. Neurorehabil Neural Repair 27: 24–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics, 2011, p. 397–398 [Google Scholar]
- Schmuelof L, Huang VS, Haith AM, Delnicki RJ, Mazzoni P, Krakauer JW. Overcoming motor “forgetting” through reinforcement of learned actions. J Neurosci 32: 14617–14621, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010 [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J Neurophysiol 107: 346–356, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YQ, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007 [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallet M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33: 766–772, 1983 [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Hiebert GW, Pearson KG. Plasticity of the extensor group I pathway controlling the stance to swing transition in the cat. J Neurophysiol 74: 2782–2787, 1995 [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw 11: 1317–1329, 1998 [DOI] [PubMed] [Google Scholar]
- Yen SC, Schmit BD, Landry JM, Roth H, Wu M. Locomotor adaptation to resistance during treadmill training transfers to overground walking in human SCI. Exp Brain Res 216: 473–482, 2012 [DOI] [PubMed] [Google Scholar]